Sorption Behavior of Organic Pollutants on Biodegradable and Nondegradable Microplastics: pH Effects
Abstract
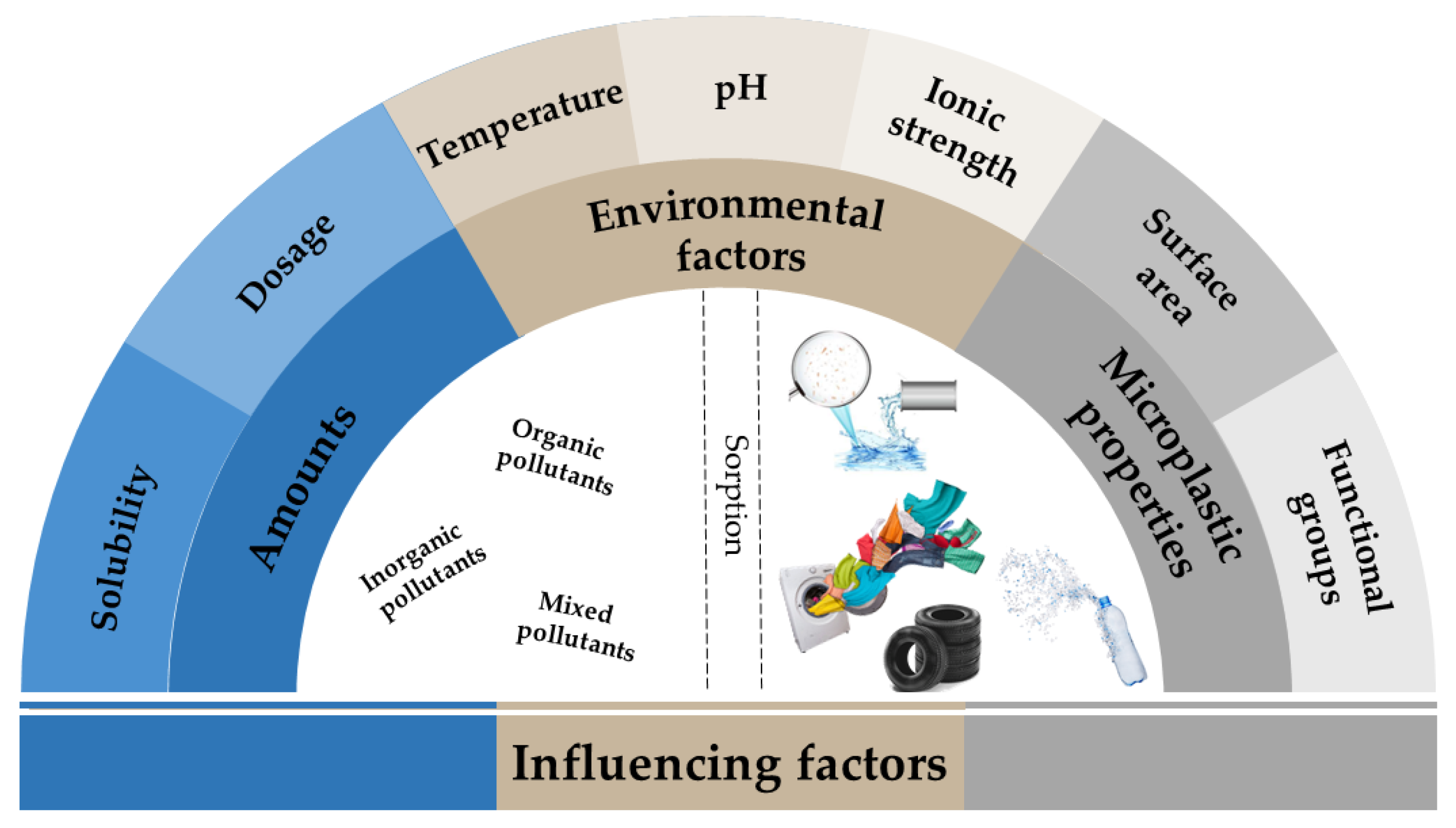
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Batch Adsorption Experiments
2.3. Analytical Procedure, Quality Assurance and Quality Control
3. Results and Discussion
3.1. Determination of Point of Zero Charge
3.2. pH Effect on Sorption Affinity of Organic Pollutants on MPs
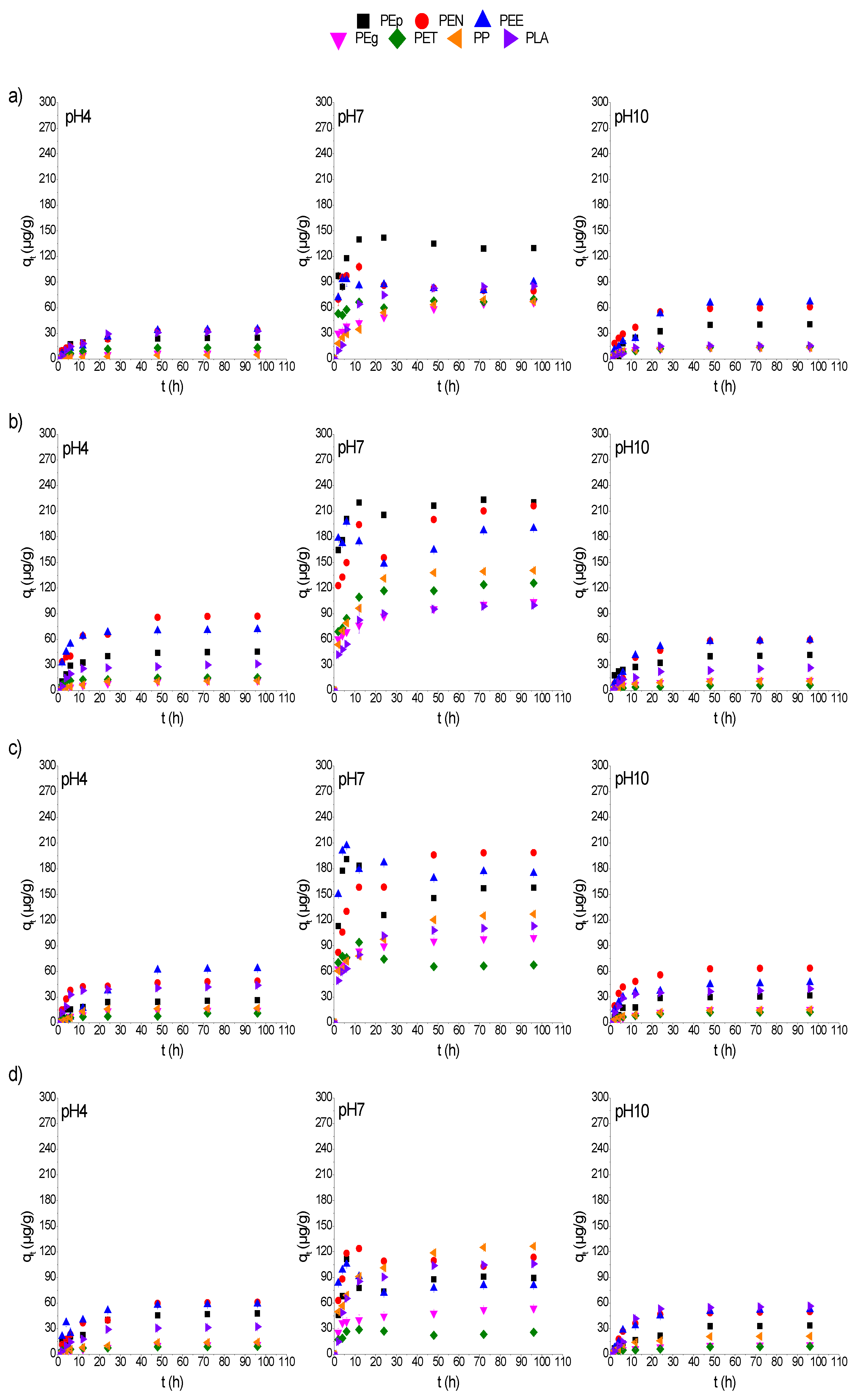
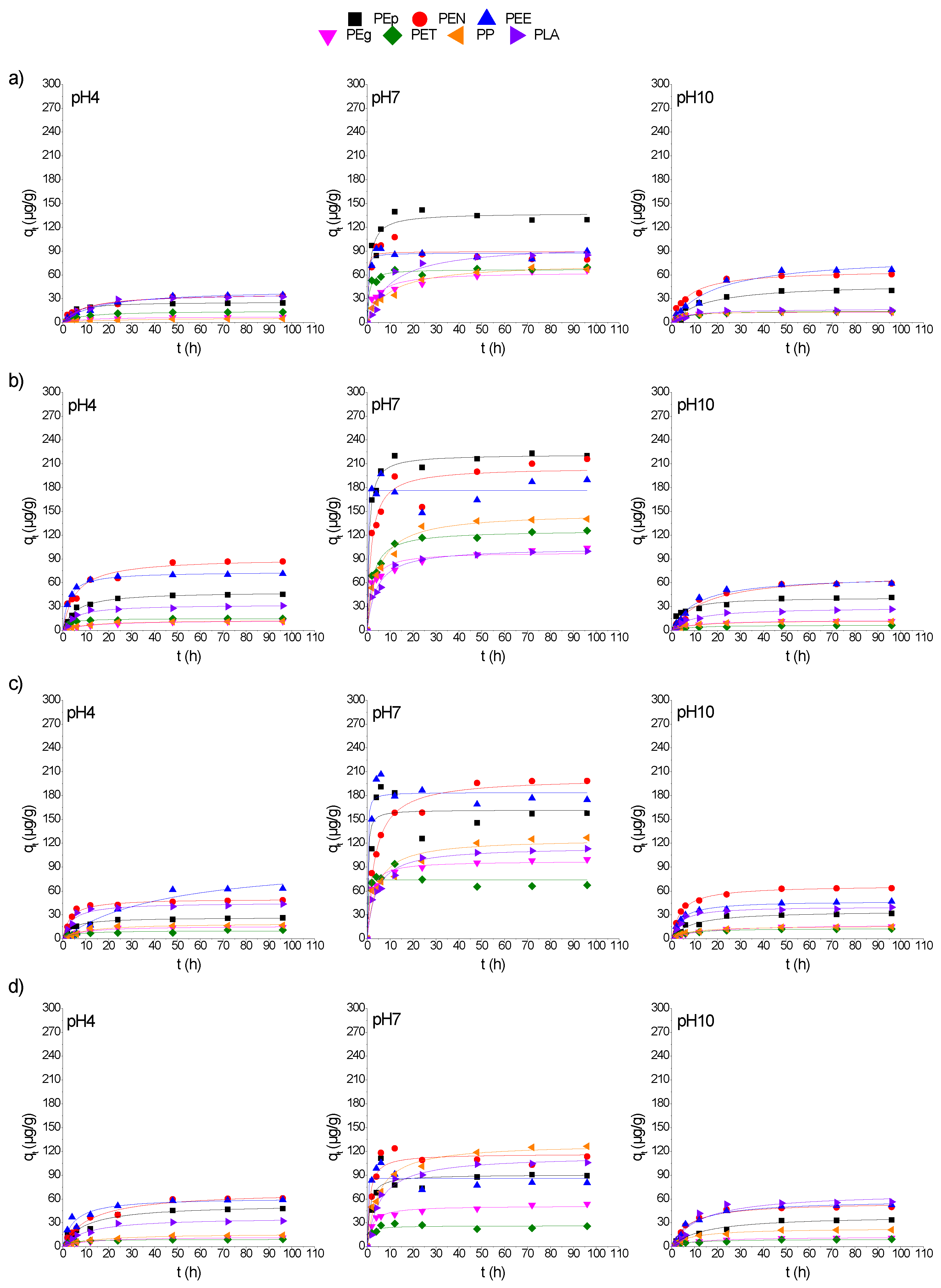
3.3. The pH Effect on Adsorption Kinetics of CPs on MPs
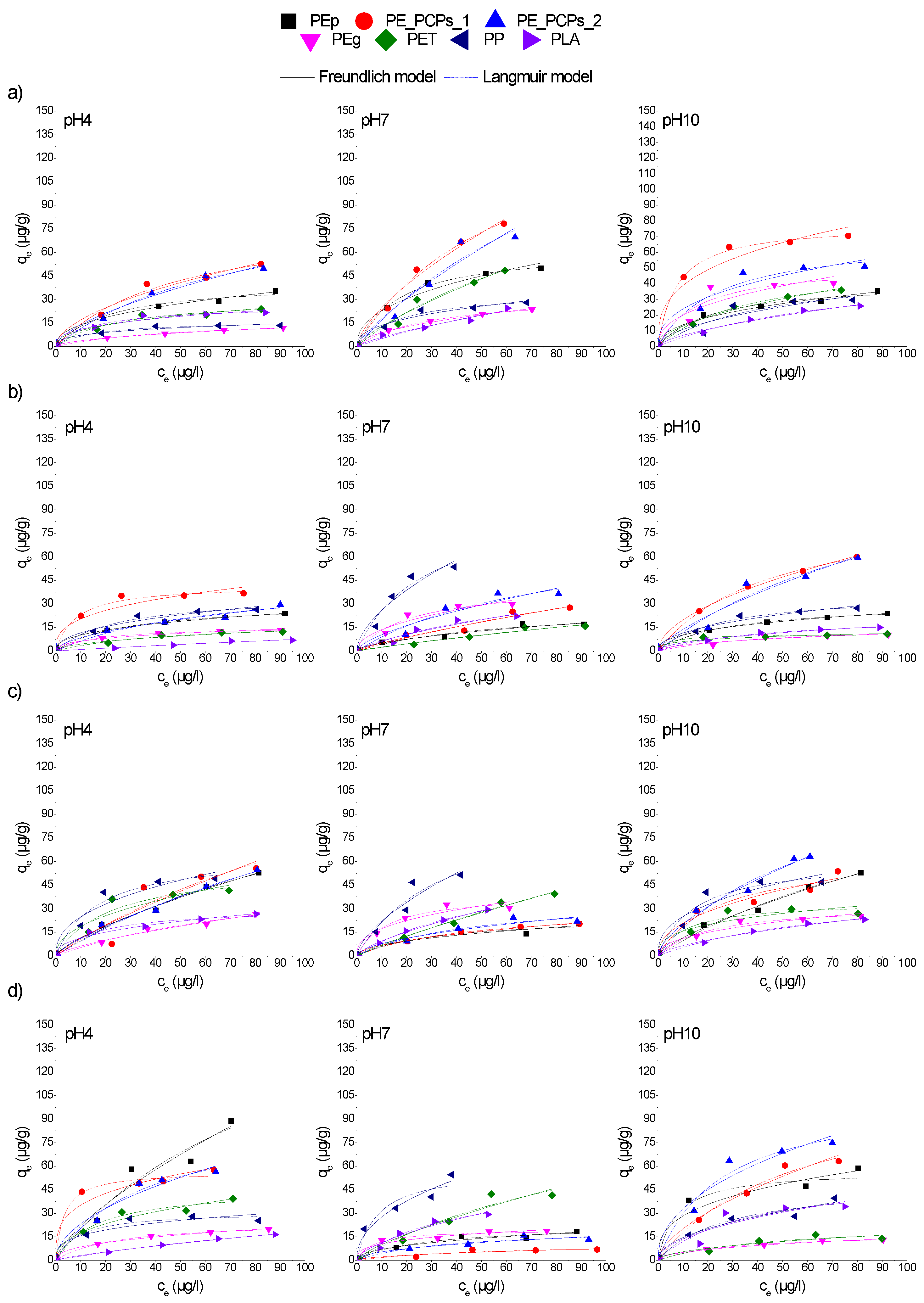
3.4. The pH Effect on Adsorption Isotherms of CPs on MPs
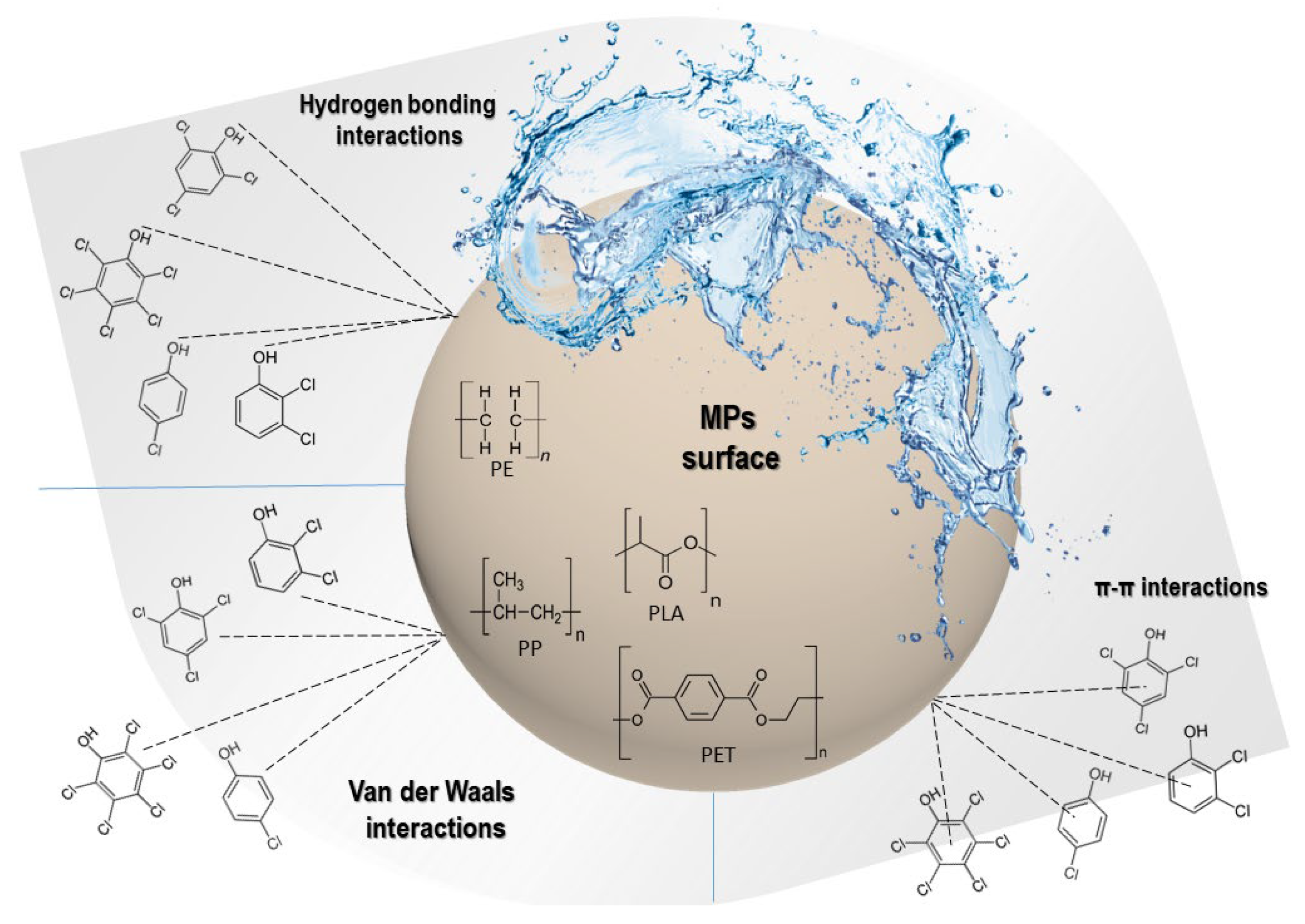
3.5. Possible Adsorption Mechanism between the CPs and MPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoang, T.C. Plastic Pollution: Where Are We Regarding Research and Risk Assessment in Support of Management and Regulation? Integr. Environ. Assess. Manag. 2022, 18, 851–852. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Puckowski, A.; Cwięk, W.; Mioduszewska, K.; Stepnowski, P.; Białk-Bielińska, A. Sorption of Pharmaceuticals on the Surface of Microplastics. Chemosphere 2021, 263, 127976. [Google Scholar] [CrossRef] [PubMed]
- Plastic Europe. Plastics—The Facts 2022; Plastic Europe: Brussels, Belgium, 2022. [Google Scholar]
- Luo, H.; Liu, C.; He, D.; Xu, J.; Sun, J.; Li, J.; Pan, X. Environmental Behaviors of Microplastics in Aquatic Systems: A Systematic Review on Degradation, Adsorption, Toxicity and Biofilm under Aging Conditions. J. Hazard. Mater. 2022, 423, 126915. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zheng, H.; Xiang, H.; Fan, J.; Jiang, H. The Surface Degradation and Release of Microplastics from Plastic Films Studied by UV Radiation and Mechanical Abrasion. Sci. Total Environ. 2022, 838, 156369. [Google Scholar] [CrossRef] [PubMed]
- Vujić, M.; Vasiljević, S.; Rocha Santos, T.; Agbaba, J.; Čepić, Z.; Radonić, J.; Tubić, A. Improving of an Easy, Effective and Low-Cost Method for Isolation of Microplastic Fibers Collected in Drying Machines Filters. Sci. Total Environ. 2023, 892, 164549. [Google Scholar] [CrossRef]
- ISO/TR 21960:2020; Plastics-Environmental Aspects-State of Knowledge and Methodologies. ISO: Geneva, Switzerland, 2020.
- Stapleton, M.J.; Ansari, A.J.; Hai, F.I. Antibiotic Sorption onto Microplastics in Water: A Critical Review of the Factors, Mechanisms and Implications. Water Res. 2023, 233, 119790. [Google Scholar] [CrossRef]
- Verschoor, J.A.; Kusumawardhani, H.; Ram, A.F.J.; de Winde, J.H. Toward Microbial Recycling and Upcycling of Plastics: Prospects and Challenges. Front. Microbiol. 2022, 13, 821629. [Google Scholar] [CrossRef]
- Guo, X.; Pang, J.; Chen, S.; Jia, H. Sorption Properties of Tylosin on Four Different Microplastics. Chemosphere 2018, 209, 240–245. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption Behavior of Organic Pollutants on Microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between Microplastics, Pharmaceuticals and Personal Care Products: Implications for Vector Transport. Environ. Int. 2021, 149, 106367. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shim, W.J.; Kwon, J.-H.H. Sorption Capacity of Plastic Debris for Hydrophobic Organic Chemicals. Sci. Total Environ. 2014, 470–471, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, M.; Zhang, L.; Wang, K.; Yu, X.; Zheng, Z.; Zheng, R. Sorption Behaviors of Phenanthrene on the Microplastics Identified in a Mariculture Farm in Xiangshan Bay, Southeastern China. Sci. Total Environ. 2018, 628–629, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Wong, C.S.; Chen, D.; Lu, X.; Wang, F.F.; Zeng, E.Y. Interaction of Toxic Chemicals with Microplastics: A Critical Review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, R.; Zhu, X.; Liu, C.; Li, L.; Yu, Z.; Chen, X.; Li, Z.; Yang, Y. Sorption of Tetrabromobisphenol A onto Microplastics: Behavior, Mechanisms, and the Effects of Sorbent and Environmental Factors. Ecotoxicol. Environ. Saf. 2021, 210, 111842. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, L.; Babu Arulmani, S.R.; Yan, J.; Wu, L.; Wu, T.; Zhang, H.; Xiao, T. Adsorption of Different Pollutants by Using Microplastic with Different Influencing Factors and Mechanisms in Wastewater: A Review. Nanomaterials 2022, 12, 2256. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, J.; Razanajatovo, R.M.; Huang, J.; Zheng, L.; Zou, H.; Wang, Z.; Liu, J. Sorption of Selected Pharmaceutical Compounds on Polyethylene Microplastics: Roles of PH, Aging, and Competitive Sorption. Chemosphere 2022, 307, 135561. [Google Scholar] [CrossRef]
- McDougall, L.; Thomson, L.; Brand, S.; Wagstaff, A.; Lawton, L.A.; Petrie, B. Adsorption of a Diverse Range of Pharmaceuticals to Polyethylene Microplastics in Wastewater and Their Desorption in Environmental Matrices. Sci. Total Environ. 2022, 808, 152071. [Google Scholar] [CrossRef]
- Seidensticker, S.; Grathwohl, P.; Lamprecht, J.; Zarfl, C. A Combined Experimental and Modeling Study to Evaluate PH-Dependent Sorption of Polar and Non-Polar Compounds to Polyethylene and Polystyrene Microplastics. Environ. Sci. Eur. 2018, 30, 30. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. The Sorption Kinetics and Isotherms of Sulfamethoxazole with Polyethylene Microplastics. Mar. Pollut. Bull. 2018, 131, 191–196. [Google Scholar] [CrossRef]
- Lončarski, M.; Tubić, A.; Kragulj, I.; Jović, B.M.; Apostolović, T.; Nikić, J.; Agbaba, J. Modeling of Chlorinated Phenols Adsorption on Polyethylene and Polyethylene Terephtalate Microplastic. J. Serbian Chem. Soc. 2020, 85, 697–709. [Google Scholar] [CrossRef]
- Tubić, A.; Lončarski, M.; Maletić, S.; Jazić, J.M.; Watson, M.; Tričković, J.; Agbaba, J.; Molnar Jazić, J.; Watson, M.; Tričković, J.; et al. Significance of Chlorinated Phenols Adsorption on Plastics and Bioplastics during Water Treatment. Water 2019, 11, 2358. [Google Scholar] [CrossRef]
- Wang, T.; Yu, C.; Chu, Q.; Wang, F.; Lan, T.; Wang, J. Adsorption Behavior and Mechanism of Five Pesticides on Microplastics from Agricultural Polyethylene Films. Chemosphere 2020, 244, 125491. [Google Scholar] [CrossRef] [PubMed]
- Avio, G.C.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants Bioavailability and Toxicological Risk from Microplastics to Marine Mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.G.L.; Sobral, P.; Ferreira, A.M. Organic Pollutants in Microplastics from Two Beaches of the Portuguese Coast. Mar. Pollut. Bull. 2010, 60, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fokkink, R.; Koelmans, A.A. Sorption of Polycyclic Aromatic Hydrocarbons to Polystyrene Nanoplastic. Environ. Toxicol. Chem. 2016, 35, 1650–1655. [Google Scholar] [CrossRef]
- Sørensen, L.; Rogers, E.; Altin, D.; Salaberria, I.; Booth, A.M. Sorption of PAHs to Microplastic and Their Bioavailability and Toxicity to Marine Copepods under Co-Exposure Conditions. Environ. Pollut. 2020, 258, 113844. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Yu, X.; Cai, L.; Wang, J.; Peng, J. Microplastics and Associated PAHs in Surface Water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 2019, 221, 834–840. [Google Scholar] [CrossRef]
- Fan, X.; Zou, Y.; Geng, N.; Liu, J.; Hou, J.; Li, D.; Yang, C.; Li, Y. Investigation on the Adsorption and Desorption Behaviors of Antibiotics by Degradable MPs with or without UV Ageing Process. J. Hazard. Mater. 2021, 401, 123363. [Google Scholar] [CrossRef]
- Zuo, L.Z.; Li, H.X.; Lin, L.; Sun, Y.X.; Diao, Z.H.; Liu, S.; Zhang, Z.Y.; Xu, X.R. Sorption and Desorption of Phenanthrene on Biodegradable Poly(Butylene Adipate Co-Terephtalate) Microplastics. Chemosphere 2019, 215, 25–32. [Google Scholar] [CrossRef]
- Lončarski, M.; Gvoić, V.; Prica, M.; Cveticanin, L.; Agbaba, J.; Tubić, A. Sorption Behavior of Polycyclic Aromatic Hydrocarbons on Biodegradable Polylactic Acid and Various Nondegradable Microplastics: Model Fitting and Mechanism Analysis. Sci. Total Environ. 2021, 785, 147289. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Almeida, C.M.R.; Ramos, S. Adaptation of a Laboratory Protocol to Quantity Microplastics Contamination in Estuarine Waters. MethodsX 2019, 6, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; De-la-Torre, G.E. Sorption of Chemical Contaminants on Degradable and Non-Degradable Microplastics: Recent Progress and Research Trends. Sci. Total Environ. 2021, 757, 143875. [Google Scholar] [CrossRef] [PubMed]
- Klavins, M.; Klavins, L.; Stabnikova, O.; Stabnikov, V.; Marynin, A.; Ansone-Bertina, L.; Mezulis, M.; Vaseashta, A. Interaction between Microplastics and Pharmaceuticals Depending on the Composition of Aquatic Environment. Microplastics 2022, 1, 520–535. [Google Scholar] [CrossRef]
- Gao, L.; Fu, D.; Zhao, J.; Wu, W.; Wang, Z.; Su, Y.; Peng, L. Microplastics Aged in Various Environmental Media Exhibited Strong Sorption to Heavy Metals in Seawater. Mar. Pollut. Bull. 2021, 169, 112480. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Different Partition of Polycyclic Aromatic Hydrocarbon on Environmental Particulates in Freshwater: Microplastics in Comparison to Natural Sediment. Ecotoxicol. Environ. Saf. 2018, 147, 648–655. [Google Scholar] [CrossRef]
- Wu, P.; Cai, Z.; Jin, H.; Tang, Y. Adsorption Mechanisms of Five Bisphenol Analogues on PVC Microplastics. Sci. Total Environ. 2019, 650, 671–678. [Google Scholar] [CrossRef]
- Tubić, A.; Lončarski, M.; Apostolović, T.; Kragulj Isakovski, M.; Tričković, J.; Molnar Jazić, J.; Agbaba, J. Adsorption Mechanisms of Chlorobenzenes and Trifluralin on Primary Polyethylene Microplastics in the Aquatic Environment. Environ. Sci. Pollut. Res. 2021, 28, 59416–59429. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Ho, Y.S. Effect of Temperatures and PH on Methyl Violet Biosorption by Mansonia Wood Sawdust. Bioresour. Technol. 2008, 99, 5411–5417. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. Microplastics Play a Minor Role in Tetracycline Sorption in the Presence of Dissolved Organic Matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef]
- Fotopoulou, K.N.; Karapanagioti, H.K. Surface Properties of Beached Plastic Pellets. Mar. Environ. Res. 2012, 81, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, Quantity and Sorptive Properties of Microplastics Extracted from Cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Güney, A.; Özdilek, C.; Kangal, M.O.; Burat, F. Flotation Characterization of PET and PVC in the Presence of Different Plasticizers. Sep. Purif. Technol. 2015, 151, 47–56. [Google Scholar] [CrossRef]
- Guo, W.; Wang, S.; Wang, Y.; Lu, S.; Gao, Y. Sorptive Removal of Phenanthrene from Aqueous Solutions Using Magnetic and Non-Magnetic Rice Husk-Derived Biochars. R. Soc. Open Sci. 2018, 5, 172382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Chriestie, P.; Luo, Y. Enhanced Adsorption of Oxytetracycline to Weathered Microplastic Polystyrene: Kinetics, Isotherms and Influencing Factors. Environ. Pollut. 2018, 243, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption Behavior of Organic Pollutants and Metals on Micro/Nanoplastics in the Aquatic Environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Liu, G. Sorption Behaviors of Phenanthrene, Nitrobenzene, and Naphthalene on Mesoplastics and Microplastics. Environ. Sci. Pollut. Res. 2019, 26, 12563–12573. [Google Scholar] [CrossRef]
- Czaplicka, M. Sources and Transformations of Chlorophenols in the Natural Environment. Sci. Total Environ. 2004, 322, 21–39. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Igbinosa, E.O. Chlorophenols and Other Related Derivatives of Environmental Concern: Properties, Distribution and Microbial Degradation Processes. Chemosphere 2011, 83, 1297–1306. [Google Scholar] [CrossRef]
- Bakir, A.; O’Connor, I.A.; Rowland, S.J.; Hendriks, A.J.; Thompson, R.C. Relative Importance of Microplastics as a Pathway for the Transfer of Hydrophobic Organic Chemicals to Marine Life. Environ. Pollut. 2016, 219, 56–65. [Google Scholar] [CrossRef]
- Tourinho, P.S.; Kočí, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of Chemical Contaminants to Microplastics: Sorption Mechanisms, Environmental Distribution and Effects on Toxicity and Bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J. Comparative Evaluation of Sorption Kinetics and Isotherms of Pyrene onto Microplastics. Chemosphere 2018, 193, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; D’Almeida, M.; Magueresse, A.; Le Grand, A.; Duval, H.; César, G.; Sire, O.; Bruzaud, S.; Le Tilly, V. Threat of Plastic Ageing in Marine Environment. Adsorption/Desorption of Micropollutants. Mar. Pollut. Bull. 2018, 127, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-Sized Microplastics and Pigmented Particles in Bottled Mineral Water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, C.; Wang, J. Sorption of Sulfamethoxazole onto Six Types of Microplastics. Chemosphere 2019, 228, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, K.; Huang, X.; Liu, J. Sorption of Pharmaceuticals and Personal Care Products to Polyethylene Debris. Environ. Sci. Pollut. Res. 2016, 23, 8819–8826. [Google Scholar] [CrossRef] [PubMed]
- Hüffer, T.; Weniger, A.K.; Hofmann, T. Sorption of Organic Compounds by Aged Polystyrene Microplastic Particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef]
- Liu, F.; Liu, G.Z.; Zhu, Z.L.; Wang, S.C.; Zhao, F.F. Interactions between Microplastics and Phthalate Esters as Affected by Microplastics Characteristics and Solution Chemistry. Chemosphere 2019, 214, 688–694. [Google Scholar] [CrossRef]
- Gong, W.; Jiang, M.; Han, P.; Liang, G.; Zhang, T.; Liu, G. Comparative Analysis on the Sorption Kinetics and Isotherms of Fipronil on Nondegradable and Biodegradable Microplastics. Environ. Pollut. 2019, 254, 112927. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption Behavior and Mechanism of Hydrophilic Organic Chemicals to Virgin and Aged Microplastics in Freshwater and Seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef]
- Liang, Y.; Ying, C.; Zhu, J.; Zhou, Q.; Sun, K.; Tian, Y.; Li, J. Effects of Salinity, PH, and Cu(II) on the Adsorption Behaviors of Tetracycline onto Polyvinyl Chloride Microplastics: A Site Energy Distribution Analysis. Water 2023, 15, 1925. [Google Scholar] [CrossRef]
- Cui, W.; Hale, R.C.; Huang, Y.; Zhou, F.; Wu, Y.; Liang, X.; Liu, Y.; Tan, H.; Chen, D. Sorption of Representative Organic Contaminants on Microplastics: Effects of Chemical Physicochemical Properties, Particle Size, and Biofilm Presence. Ecotoxicol. Environ. Saf. 2023, 251, 114533. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Li, J.; Deng, S.; Zhang, S. Adsorption of Three Pesticides on Polyethylene Microplastics in Aqueous Solutions: Kinetics, Isotherms, Thermodynamics, and Molecular Dynamics Simulation. Chemosphere 2021, 264, 128556. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Huang, B.; Cao, C.; Liu, X.; Sun, X.; Xiao, L.; Qiu, J.; Luo, Y.; Qian, Q.; Chen, Q. Adsorption–Desorption Behavior of Methylene Blue onto Aged Polyethylene Microplastics in Aqueous Environments. Mar. Pollut. Bull. 2021, 167, 112287. [Google Scholar] [CrossRef] [PubMed]
- Tubić, A.; Vujić, M.; Gvoić, V.; Agbaba, J.; Vasiljević, S.; Cveticanin, L.; Vukelić, Đ.; Prica, M. Sorption Potential of Microplastics for Azo- and Phthalocyanine Printing Dyes. Dye. Pigment. 2023, 209, 110884. [Google Scholar] [CrossRef]
- Hüffer, T.; Hofmann, T. Sorption of Non-Polar Organic Compounds by Micro-Sized Plastic Particles in Aqueous Solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef]
- Niederer, C.; Schwarzenbach, R.P.; Goss, K.U. Elucidating Differences in the Sorption Properties of 10 Humic and Fulvic Acids for Polar and Nonpolar Organic Chemicals. Environ. Sci. Technol. 2007, 41, 6711–6717. [Google Scholar] [CrossRef]
- McCarty, L.; Lupp, M.; Shea, M. Chlorinated Benzenes in the Aquatic Environment; Ontario Ministry of the Environment: Toronto, ON, Canada, 1984.
- Li, H.; Sheng, G.; Sheng, W.; Xu, O. Uptake of Trifluralin and Lindane from Water by Ryegrass. Chemosphere 2002, 48, 335–341. [Google Scholar] [CrossRef]
- Worch, E. Adsorption Technology in Water Treatment; Walter de Gruyter GmbH & Co KG: Berlin, Gernamy, 2012; ISBN 9783110240221. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vujić, M.; Vasiljević, S.; Nikić, J.; Kordić, B.; Agbaba, J.; Tubić, A. Sorption Behavior of Organic Pollutants on Biodegradable and Nondegradable Microplastics: pH Effects. Appl. Sci. 2023, 13, 12835. https://doi.org/10.3390/app132312835
Vujić M, Vasiljević S, Nikić J, Kordić B, Agbaba J, Tubić A. Sorption Behavior of Organic Pollutants on Biodegradable and Nondegradable Microplastics: pH Effects. Applied Sciences. 2023; 13(23):12835. https://doi.org/10.3390/app132312835
Chicago/Turabian StyleVujić, Maja, Sanja Vasiljević, Jasmina Nikić, Branko Kordić, Jasmina Agbaba, and Aleksandra Tubić. 2023. "Sorption Behavior of Organic Pollutants on Biodegradable and Nondegradable Microplastics: pH Effects" Applied Sciences 13, no. 23: 12835. https://doi.org/10.3390/app132312835





