A Bilateral Craniectomy Technique for In Vivo Photoacoustic Brain Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation
- Rats were first anesthetized using 4% isoflurane inhalation followed by maintenance doses of 2–3% isoflurane during surgery.
- Adequate depth of anesthesia was confirmed using both toe and tail pinch maneuvers prior to bringing the animal to the surgical platform.
- The scalp was shaved with hair clippers, and animals were then moved to the stereotactic frame with a heating pad set to normothermic 37 °C to prevent heat loss and hypothermia under anesthesia.
- The head was immobilized in the stereotactic frame to stabilize the skull during drilling, and the head was positioned in slight extension and without any rotation.
- Anesthesia continued through a nosecone secured to the frame. The rat was monitored throughout the procedure for respiratory rate and coloration.
2.2. Procedure: Soft Tissue Dissection and Bony Exposure
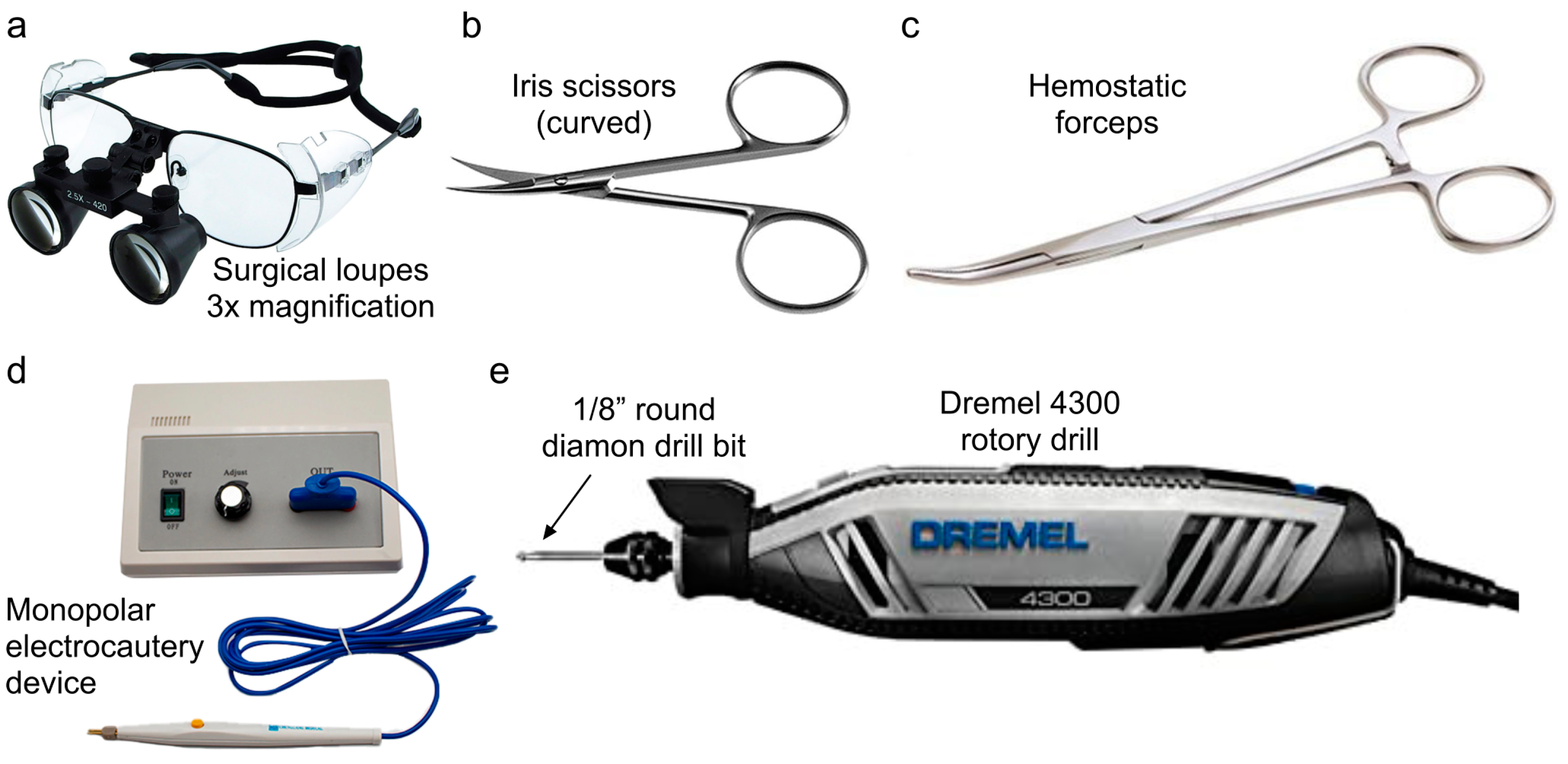
- The remainder of the procedure was performed using magnified surgical loupes with 3× magnification (Designs for Vision, Bohemia, NY, USA).
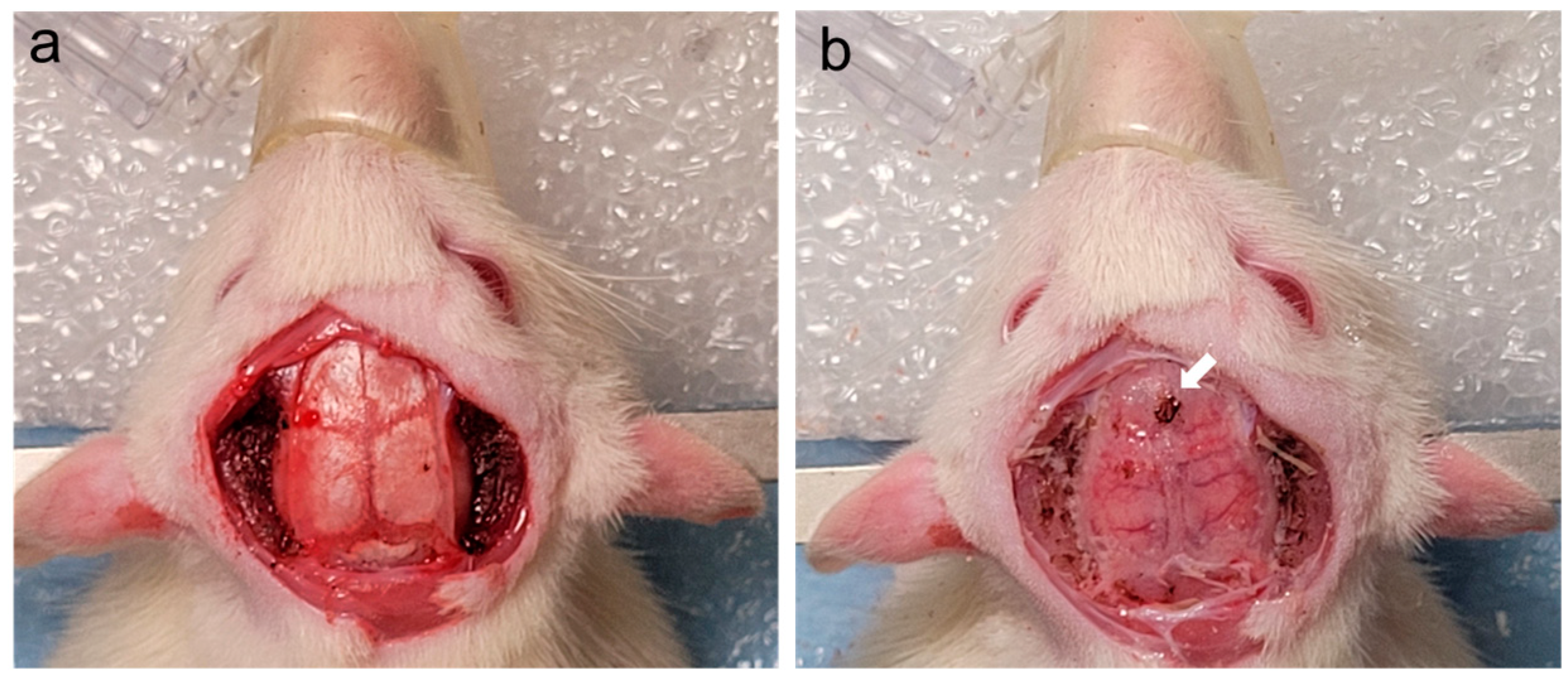
- The scalp was then opened using scissors in a linear fashion following the midline from immediately posterior to the eyes to the nape of the neck (see Figure 2a).
- Connective tissue was bluntly freed using gauze, and the skin was reflected laterally using hemostatic forceps during this process (see Figure 2b).
- Once the connective tissue was fully detached, the scalp was excised in an elliptical fashion.
- The periosteum was dissected from the bone using a cotton-tipped applicator.
- Bony hemostasis was achieved using a portable monopolar electrocautery device.
- The temporalis muscle was then cleared from the underlying temporal bone by detaching the muscle from the temporal ridge using the back of a cotton-tipped applicator or flat dissector.
- Then, the temporalis muscle was excised sharply with either scissors or 15-blade scalpel. Meticulous hemostasis of the muscle was achieved using a battery-powered, high-temperature, fine-tip Bovie monopolar electrocautery (Bovie Medical Corporation, Clearwater, FL, USA) (see Figure 2c).
2.3. Procedure: Bilateral Craniectomies
- The craniotomy commenced using a Dremel 4300 rotary drill with a 225-01 flexible shaft rotary tool attachment.
- The outer layer of bone was thinned using a 1/8” round engraving drill bit, as the cutting action of the drill allowed for quicker removal of bone.
- Bone dust was intermittently cleaned from the field with saline solution, and bony hemostasis was achieved as needed using monopolar electrocautery.
- This thinning process occurred unilaterally by addressing left and right sides separately, encompassing the frontal, parietal, and temporal bones, and sparing the sagittal suture and lambda. The edges of the craniotomy extended from 2–3 mm lateral to the temporal ridge, 2–3 mm anterior to bregma, 0.5–1 mm lateral to sagittal suture, and immediately anterior to lambda.
- Further thinning of the inner layer of bone to the dura was performed using a 1/8” round diamond drill bit, as the rough surface provided additional hemostasis and was less likely to breach the dura.
- The cranial thinning with the high-speed drill was performed with utmost caution and precision to avoid injury to the cortical surface and surrounding structures.
- Once the bilateral craniectomies had been completed through this thinning process, the sagittal suture was addressed, carefully thinning this bone using the 1/8” round diamond drill bit but leaving the inner layer of bone intact to protect the underlying superior sagittal sinus (see Figure 3b).
2.4. Procedure: Brain Visualization
2.5. Hemostasis
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koletar, M.M.; Dorr, A.; Brown, M.E.; McLaurin, J.; Stefanovic, B. Refinement of a chronic cranial window implant in the rat for longitudinal in vivo two–photon fluorescence microscopy of neurovascular function. Sci. Rep. 2019, 9, 5499. [Google Scholar] [CrossRef]
- Chen, J.; Lambo, M.E.; Ge, X.; Dearborn, J.T.; Liu, Y.; McCullough, K.B.; Swift, R.G.; Tabachnick, D.R.; Tian, L.; Noguchi, K.; et al. A MYT1L syndrome mouse model recapitulates patient phenotypes and reveals altered brain development due to disrupted neuronal maturation. Neuron 2021, 109, 3775–3792.e3714. [Google Scholar] [CrossRef]
- Francis-Oliveira, J.; Leitzel, O.; Niwa, M. Are the Anterior and Mid-Cingulate Cortices Distinct in Rodents? Front. Neuroanat. 2022, 16, 914359. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.A.; Lartey, F.M.; Schüler, E.; Rafat, M.; King, G.; Kim, A.; Ko, R.; Semaan, S.; Gonzalez, S.; Jenkins, M.; et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 139, 4–10. [Google Scholar] [CrossRef]
- Matchynski, J.I.; Manwar, R.; Kratkiewicz, K.J.; Madangopal, R.; Lennon, V.A.; Makki, K.M.; Reppen, A.L.; Woznicki, A.R.; Hope, B.T.; Perrine, S.A.; et al. Direct measurement of neuronal ensemble activity using photoacoustic imaging in the stimulated Fos-LacZ transgenic rat brain: A proof-of-principle study. Photoacoustics 2021, 24, 100297. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Muller, A.; Hynynen, K. Ultrasound insertion loss of rat parietal bone appears to be proportional to animal mass at submegahertz frequencies. Ultrasound Med. Biol. 2011, 37, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Kabadi, S.V.; Hilton, G.D.; Stoica, B.A.; Zapple, D.N.; Faden, A.I. Fluid-percussion-induced traumatic brain injury model in rats. Nat. Protoc. 2010, 5, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.E.; Lyeth, B.G.; Povlishock, J.T.; Findling, R.L.; Hamm, R.J.; Marmarou, A.; Young, H.F.; Hayes, R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987, 67, 110–119. [Google Scholar] [CrossRef]
- McIntosh, T.K.; Noble, L.; Andrews, B.; Faden, A.I. Traumatic brain injury in the rat: Characterization of a midline fluid-percussion model. Cent. Nerv. Syst. Trauma 1987, 4, 119–134. [Google Scholar] [CrossRef]
- McIntosh, T.K.; Vink, R.; Noble, L.; Yamakami, I.; Fernyak, S.; Soares, H.; Faden, A.L. Traumatic brain injury in the rat: Characterization of a lateral fluid-percussion model. Neuroscience 1989, 28, 233–244. [Google Scholar] [CrossRef]
- Dixon, C.E.; Clifton, G.L.; Lighthall, J.W.; Yaghmai, A.A.; Hayes, R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 1991, 39, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Drew, P.J.; Shih, A.Y.; Driscoll, J.D.; Knutsen, P.M.; Blinder, P.; Davalos, D.; Akassoglou, K.; Tsai, P.S.; Kleinfeld, D. Chronic optical access through a polished and reinforced thinned skull. Nat. Methods 2010, 7, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.Y.; Mateo, C.; Drew, P.J.; Tsai, P.S.; Kleinfeld, D. A polished and reinforced thinned-skull window for long-term imaging of the mouse brain. J. Vis. Exp. 2012, 61, e3742. [Google Scholar] [CrossRef]
- Dombeck, D.A.; Khabbaz, A.N.; Collman, F.; Adelman, T.L.; Tank, D.W. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 2007, 56, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Mostany, R.; Portera-Cailliau, C. A craniotomy surgery procedure for chronic brain imaging. J. Vis. Exp. 2008, 12, e680. [Google Scholar] [CrossRef]
- Holtmaat, A.; Bonhoeffer, T.; Chow, D.K.; Chuckowree, J.; De Paola, V.; Hofer, S.B.; Hubener, M.; Keck, T.; Knott, G.; Lee, W.C.; et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 2009, 4, 1128–1144. [Google Scholar] [CrossRef] [PubMed]
- Cabrales, P.; Carvalho, L.J. Intravital microscopy of the mouse brain microcirculation using a closed cranial window. J. Vis. Exp. 2010, 45, e2184. [Google Scholar] [CrossRef]
- Zafar, M.; Manwar, R.; Avanaki, K. High-fidelity compression for high-throughput photoacoustic microscopy systems. J. Biophotonics 2022, 15, e202100350. [Google Scholar] [CrossRef]
- Mohsin, Z.; James, I.M.; Rayyan, M.; Seyed, M.R.; Alana, C.C.; Shane, A.P.; Kamran, A. Development of fast photoacoustic microscopy system for small animal brain imaging. In Photons Plus Ultrasound: Imaging and Sensing; SPIE: Bellingham, WA, USA, 2021; p. 116424B. [Google Scholar]
- Zafar, M.; Manwar, R.; McGuire, L.S.; Charbel, F.T.; Avanaki, K. Ultra-widefield and high-speed spiral laser scanning OR-PAM: System development and characterization. J. Biophotonics 2023, 16, e202200383. [Google Scholar] [CrossRef]
- Heo, C.; Park, H.; Kim, Y.T.; Baeg, E.; Kim, Y.H.; Kim, S.G.; Suh, M. A soft, transparent, freely accessible cranial window for chronic imaging and electrophysiology. Sci. Rep. 2016, 6, 27818. [Google Scholar] [CrossRef]
- Scott, B.B.; Brody, C.D.; Tank, D.W. Cellular resolution functional imaging in behaving rats using voluntary head restraint. Neuron 2013, 80, 371–384. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGuire, L.S.; Zafar, M.; Manwar, R.; Charbel, F.T.; Avanaki, K. A Bilateral Craniectomy Technique for In Vivo Photoacoustic Brain Imaging. Appl. Sci. 2023, 13, 12951. https://doi.org/10.3390/app132312951
McGuire LS, Zafar M, Manwar R, Charbel FT, Avanaki K. A Bilateral Craniectomy Technique for In Vivo Photoacoustic Brain Imaging. Applied Sciences. 2023; 13(23):12951. https://doi.org/10.3390/app132312951
Chicago/Turabian StyleMcGuire, Laura S., Mohsin Zafar, Rayyan Manwar, Fady T. Charbel, and Kamran Avanaki. 2023. "A Bilateral Craniectomy Technique for In Vivo Photoacoustic Brain Imaging" Applied Sciences 13, no. 23: 12951. https://doi.org/10.3390/app132312951
APA StyleMcGuire, L. S., Zafar, M., Manwar, R., Charbel, F. T., & Avanaki, K. (2023). A Bilateral Craniectomy Technique for In Vivo Photoacoustic Brain Imaging. Applied Sciences, 13(23), 12951. https://doi.org/10.3390/app132312951





