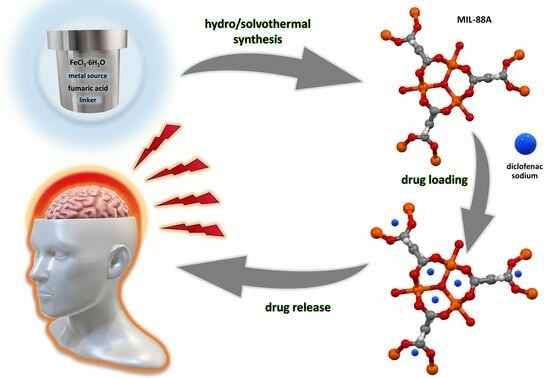
Biocompatible Fe-Based Metal-Organic Frameworks as Diclofenac Sodium Delivery Systems for Migraine Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of MIL-88A Drug Carriers
2.2. Characterization of MIL-88A Carriers
2.2.1. Powder X-ray Diffraction (XRD)
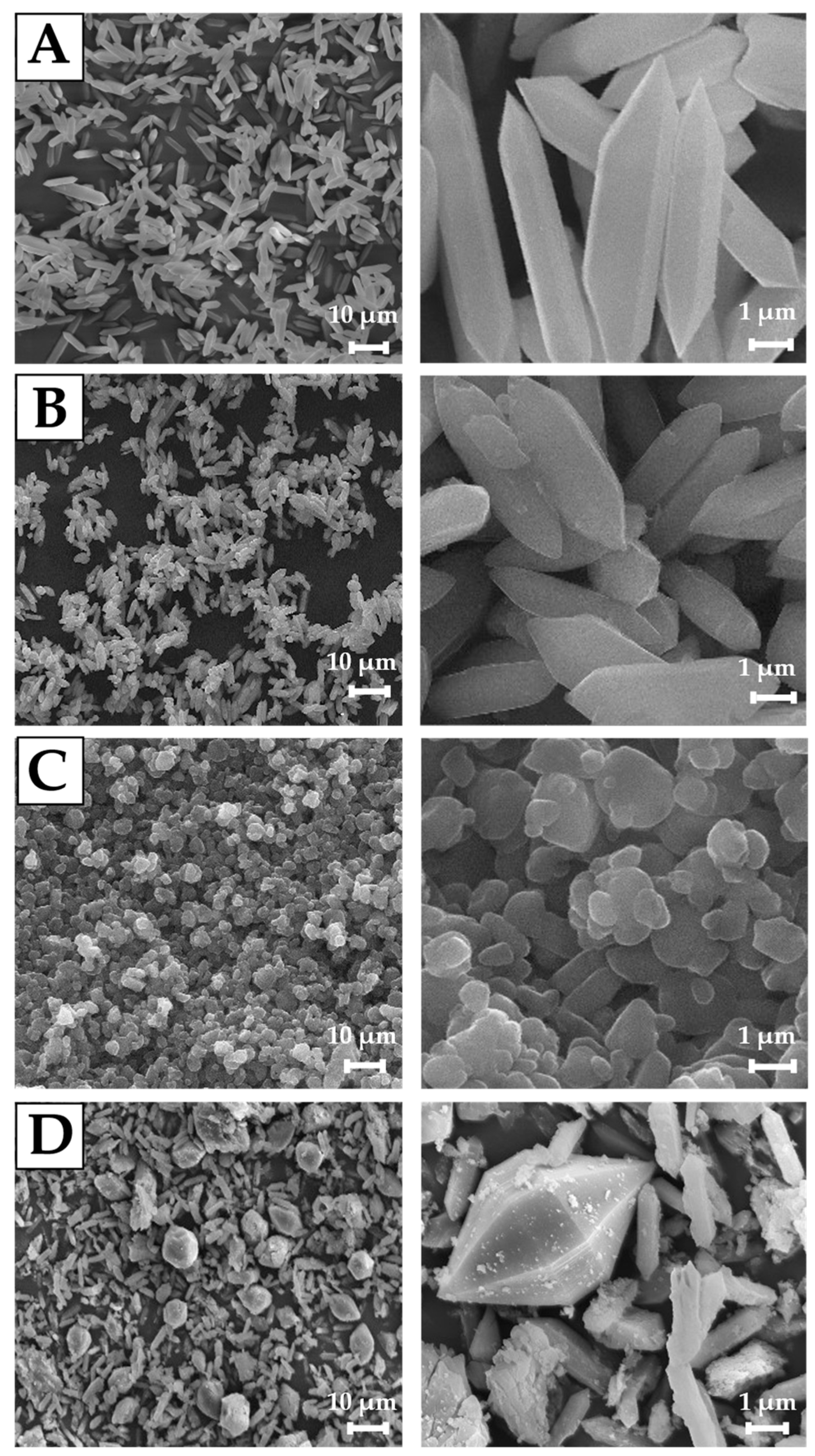
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. Low-Temperature Nitrogen Sorption
2.2.4. Thermogravimetric Analysis (TGA)
2.2.5. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.3. Diclofenac Sodium Adsorption Studies
2.4. Diclofenac Sodium Release Studies
3. Results and Discussion
3.1. Physicochemical Characterization of MIL-88A Carriers
3.2. Adsorption and Release of Diclofenac Sodium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Steiner, T.J.; Stovner, L.J. Global Epidemiology of Migraine and Its Implications for Public Health and Health Policy. Nat. Rev. Neurol. 2023, 19, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chalmer, M.A.; Kogelman, L.J.A.; Callesen, I.; Christensen, C.G.; Techlo, T.R.; Møller, P.L.; Davidsson, O.B.; Olofsson, I.A.; Schwinn, M.; Mikkelsen, S.; et al. Sex Differences in Clinical Characteristics of Migraine and Its Burden: A Population-Based Study. Eur. J. Neurol. 2023, 30, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.F.; Tumminello, A.; Marconi, M.; Gualano, M.R.; Santoro, P.E.; Malorni, W.; Moscato, U. Sex and Gender Differences in Migraines: A Narrative Review. Neurol. Sci. 2022, 43, 5729–5734. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.R.; Rosendale, N. Sex and Gender Considerations in Episodic Migraine. Curr. Pain Headache Rep. 2022, 26, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.S. Diagnosis and Management of Headache: A Review. JAMA—J. Am. Med. Assoc. 2021, 325, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Lachute, C.; Root, M.; Rogers, J.; Richard, M.; Varrassi, G.; Urits, I.; Viswanath, O.; Khater, N.; Kaye, A.D. A Comprehensive Review of Celecoxib Oral Solution for the Acute Treatment of Migraine. Health Psychol. Res. 2022, 10, 34265. [Google Scholar] [CrossRef] [PubMed]
- Rist, P.M.; Buring, J.E.; Cook, N.R.; Kurth, T. Contribution of Migraine to Cardiovascular Disease Risk Prediction. J. Am. Coll. Cardiol. 2023, 81, 2246–2254. [Google Scholar] [CrossRef]
- Geppetti, P.; De Cesaris, F.; Benemei, S.; Cortelli, P.; Cevoli, S.; Pierangeli, G.; Favoni, V.; Lisotto, C.; Usai, S.; Frediani, F.; et al. Self-Administered Subcutaneous Diclofenac Sodium in Acute Migraine Attack: A Randomized, Double-Blind, Placebo-Controlled Dose-Finding Pilot Study. Cephalalgia 2022, 42, 1058–1070. [Google Scholar] [CrossRef]
- Sohail, R.; Mathew, M.; Patel, K.K.; Reddy, S.A.; Haider, Z.; Naria, M.; Habib, A.; Abdin, Z.U.; Razzaq Chaudhry, W.; Akbar, A. Effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Gastroprotective NSAIDs on the Gastrointestinal Tract: A Narrative Review. Cureus 2023, 15, e37080. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted Therapy in Chronic Diseases Using Nanomaterial-Based Drug Delivery Vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Adepu, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 45. [Google Scholar]
- Chopra, L.; Thakur, K.K.; Chohan, J.S.; Sharma, S.; Ilyas, R.A.; Asyraf, M.R.M.; Zakaria, S.Z.S. Comparative Drug Release Investigations for Diclofenac Sodium Drug (DS) by Chitosan-Based Grafted and Crosslinked Copolymers. Materials 2022, 15, 2404. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Agop, M.; Marinas, I.C.; Zala, A.; Irimiciuc, S.A.; Dobreci, L.; Petrescu, T.C.; Volovat, C. Theoretical Model for the Diclofenac Release from PEGylated Chitosan Hydrogels. Drug Deliv. 2021, 28, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Drug Delivery Applications of Chitin and Chitosan: A Review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Zhao, J.; Lu, Q.; Wang, M.; Sun, B.; Shen, Y.; Liu, H.; Pané, S.; Chen, X.Z.; et al. In Situ Sprayed Nanovaccine Suppressing Exosomal PD-L1 by Golgi Apparatus Disorganization for Postsurgical Melanoma Immunotherapy. ACS Nano 2023, 17, 10637–10650. [Google Scholar] [CrossRef] [PubMed]
- Fekri, M.H.; Soleymani, S.; Mehr, M.R.; Akbari-adergani, B. Synthesis and Characterization of Mesoporous ZnO/SBA-16 Nanocomposite: Its Efficiency as Drug Delivery System. J. Non-Cryst. Solids 2022, 591, 121512. [Google Scholar] [CrossRef]
- Goscianska, J.; Olejnik, A.; Ejsmont, A.; Galarda, A.; Wuttke, S. Overcoming the Paracetamol Dose Challenge with Wrinkled Mesoporous Carbon Spheres. J. Colloid Interface Sci. 2021, 586, 673–682. [Google Scholar] [CrossRef]
- Jusu, S.M.; Obayemi, J.D.; Salifu, A.A.; Nwazojie, C.C.; Uzonwanne, V.; Odusanya, O.S.; Soboyejo, W.O. Drug-Encapsulated Blend of PLGA-PEG Microspheres: In Vitro and In Vivo Study of the Effects of Localized/Targeted Drug Delivery on the Treatment of Triple-Negative Breast Cancer. Sci. Rep. 2020, 10, 14188. [Google Scholar] [CrossRef]
- Rojas, S.; Carmona, F.J.; Maldonado, C.R.; Horcajada, P.; Hidalgo, T.; Serre, C.; Rodriguez, J.A.; Barea, E. Nanoscaled Zinc Pyrazolate Metal-Organic Frameworks as Drug-Delivery Systems. Acta Crystallogr. Sect. A Found. Adv. 2017, 73, C1190. [Google Scholar] [CrossRef]
- Maranescu, B.; Visa, A. Applications of Metal-Organic Frameworks as Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4458. [Google Scholar] [CrossRef] [PubMed]
- Simagina, A.A.; Polynski, M.V.; Vinogradov, A.V.; Pidko, E.A. Towards Rational Design of Metal-Organic Framework-Based Drug Delivery Systems. Russ. Chem. Rev. 2018, 87, 831–858. [Google Scholar] [CrossRef]
- Liu, X.; Liang, T.; Zhang, R.; Ding, Q.; Wu, S.; Li, C.; Lin, Y.; Ye, Y.; Zhong, Z.; Zhou, M. Iron-Based Metal-Organic Frameworks in Drug Delivery and Biomedicine. ACS Appl. Mater. Interfaces 2021, 13, 9643–9655. [Google Scholar] [CrossRef] [PubMed]
- Almáši, M.; Zeleňák, V.; Palotai, P.; Beňová, E.; Zeleňáková, A. Metal-Organic Framework MIL-101(Fe)-NH2 Functionalized with Different Long-Chain Polyamines as Drug Delivery System. Inorg. Chem. Commun. 2018, 93, 115–120. [Google Scholar] [CrossRef]
- Gordon, J.; Kazemian, H.; Rohani, S. MIL-53(Fe), MIL-101, and SBA-15 Porous Materials: Potential Platforms for Drug Delivery. Mater. Sci. Eng. C 2015, 47, 172–179. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Wang, M.; Chen, B.; Zhang, Y.; Sun, Y.; Chen, K.; Du, Q.; Jing, Z.; Jin, Y. Preparation of MIL-88A Micro/Nanocrystals with Different Morphologies in Different Solvents for Efficient Removal of Congo Red from Water: Synthesis, Characterization, and Adsorption Mechanisms. Microporous Mesoporous Mater. 2022, 345, 112241. [Google Scholar] [CrossRef]
- Pang, D.; Wang, C.C.; Wang, P.; Liu, W.; Fu, H.; Zhao, C. Superior Removal of Inorganic and Organic Arsenic Pollutants from Water with MIL-88A(Fe) Decorated on Cotton Fibers. Chemosphere 2020, 254, 126829. [Google Scholar] [CrossRef]
- Huo, J.B.; Yu, G. Layered Double Hydroxides Derived from MIL-88A(Fe) as an Efficient Adsorbent for Enhanced Removal of Lead (II) from Water. Int. J. Mol. Sci. 2022, 23, 14556. [Google Scholar] [CrossRef]
- Fu, H.; Song, X.X.; Wu, L.; Zhao, C.; Wang, P.; Wang, C.C. Room-Temperature Preparation of MIL-88A as a Heterogeneous Photo-Fenton Catalyst for Degradation of Rhodamine B and Bisphenol a under Visible Light. Mater. Res. Bull. 2020, 125, 110806. [Google Scholar] [CrossRef]
- Khandelwal, G.; Maria Joseph Raj, N.P.; Vivekananthan, V.; Kim, S.J. Biodegradable Metal-Organic Framework MIL-88A for Triboelectric Nanogenerator. iScience 2021, 24, 102064. [Google Scholar] [CrossRef]
- Baati, T.; Njim, L.; Neffati, F.; Kerkeni, A.; Bouttemi, M.; Gref, R.; Najjar, M.F.; Zakhama, A.; Couvreur, P.; Serre, C.; et al. In Depth Analysis of the in Vivo Toxicity of Nanoparticles of Porous Iron(Iii) Metal-Organic Frameworks. Chem. Sci. 2013, 4, 1597–1607. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Porous Metal-Organic-Framework Nanoscale Carriers as a Potential Platform for Drug Delivery and Imaging. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef] [PubMed]
- Hirschle, P.; Hirschle, C.; Böll, K.; Döblinger, M.; Höhn, M.; Tuffnell, J.M.; Ashling, C.W.; Keen, D.A.; Bennett, T.D.; Rädler, J.O.; et al. Tuning the Morphological Appearance of Iron(III) Fumarate: Impact on Material Characteristics and Biocompatibility. Chem. Mater. 2020, 32, 2253–2263. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Eubank, J.F.; Wuttke, S.; Xiao, B.; Wheatley, P.S.; Bazin, P.; Lavalley, J.C.; Daturi, M.; Vimont, A.; De Weireld, G.; et al. Nitric Oxide Adsorption and Delivery in Flexible MIL-88(Fe) Metal-Organic Frameworks. Chem. Mater. 2013, 25, 1592–1599. [Google Scholar] [CrossRef]
- Kim, S.N.; Park, C.G.; Huh, B.K.; Lee, S.H.; Min, C.H.; Lee, Y.Y.; Kim, Y.K.; Park, K.H.; Choy, Y. Bin Metal-Organic Frameworks, NH2-MIL-88(Fe), as Carriers for Ophthalmic Delivery of Brimonidine. Acta Biomater. 2018, 79, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, S.; Javanbakht, S.; Heydari, A.; Kazeminava, F.; Gholizadeh, P.; Mahdipour, M.; Shaabani, A. Ultrasound-Assisted Synthesis of MIL-88(Fe) Coordinated to Carboxymethyl Cellulose Fibers: A Safe Carrier for Highly Sustained Release of Tetracycline. Int. J. Biol. Macromol. 2021, 181, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Illes, B.; Wuttke, S.; Engelke, H. Liposome-Coated Iron Fumarate Metal-Organic Framework Nanoparticles for Combination Therapy. Nanomaterials 2017, 7, 351. [Google Scholar] [CrossRef]
- Kołodziejska, J.; Kołodziejczyk, M. Diclofenac in the Treatment of Pain in Patients with Rheumatic Diseases. Reumatologia 2018, 56, 174–183. [Google Scholar] [CrossRef]
- Ortiz, J.A.; Sepúlveda, F.A.; Panadero-Medianero, C.; Murgas, P.; Ahumada, M.; Palza, H.; Matsuhiro, B.; Zapata, P.A. Cytocompatible Drug Delivery Hydrogels Based on Carboxymethylagarose/Chitosan PH-Responsive Polyelectrolyte Complexes. Int. J. Biol. Macromol. 2022, 199, 96–107. [Google Scholar] [CrossRef]
- Amanullah, A.; Upadhyay, A.; Dhiman, R.; Singh, S.; Kumar, A.; Ahirwar, D.K.; Gutti, R.K.; Mishra, A. Development and Challenges of Diclofenac-Based Novel Therapeutics: Targeting Cancer and Complex Diseases. Cancers 2022, 14, 4385. [Google Scholar] [CrossRef]
- Elmas, A.; Akyüz, G.; Bergal, A.; Andaç, M.; Andaç, Ö. Mathematical Modelling of Drug Release. Res. Eng. Struct. Mater. 2020, 6, 327–350. [Google Scholar] [CrossRef]
- Available online: https://www.originlab.com/ (accessed on 2 March 2020).
- Liao, X.; Wang, F.; Wang, F.; Cai, Y.; Yao, Y.; Teng, B.T.; Hao, Q.; Shuxiang, L. Synthesis of (100) Surface Oriented MIL-88A-Fe with Rod-like Structure and Its Enhanced Fenton-like Performance for Phenol Removal. Appl. Catal. B Environ. 2019, 259, 118064. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J. 3D-Superstructured Networks Comprising Fe-MIL-88A Metal-Organic Frameworks under Mechanochemical Conditions. Eur. J. Inorg. Chem. 2019, 2019, 4597–4600. [Google Scholar] [CrossRef]
- Amaro-Gahete, J.; Klee, R.; Esquivel, D.; Ruiz, J.R.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Fast Ultrasound-Assisted Synthesis of Highly Crystalline MIL-88A Particles and Their Application as Ethylene Adsorbents. Ultrason. Sonochem. 2019, 50, 59–66. [Google Scholar] [CrossRef]
- Melchiorre, M.; Lentini, D.; Cucciolito, M.E.; Taddeo, F.; Hmoudah, M.; Di Serio, M.; Ruffo, F.; Russo, V.; Esposito, R. Sustainable Ketalization of Glycerol with Ethyl Levulinate Catalyzed by the Iron(III)-Based Metal-Organic Framework MIL-88A. Molecules 2022, 27, 7229. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wan, J.; Ma, Y.; Wang, Y.; Pu, M.; Guan, Z. Metal-Organic Frameworks MIL-88A with Suitable Synthesis Conditions and Optimal Dosage for Effective Catalytic Degradation of Orange G through Persulfate Activation. RSC Adv. 2016, 6, 112502–112511. [Google Scholar] [CrossRef]
- Walshe, C.A.; Thom, A.J.R.; Wilson, C.; Ling, S.; Forgan, R.S. Controlling the Flexibility of MIL-88A(Sc) Through Synthetic Optimisation and Postsynthetic Halogenation. Chem. Eur. J. 2022, 28, e202201364. [Google Scholar] [CrossRef]
- Butova, V.V.; Aboraia, A.M.; Shapovalov, V.V.; Dzhangiryan, N.A.; Papkovskaya, E.D.; Ilin, O.I.; Kubrin, S.P.; Guda, A.A.; Soldatov, A.V. Iron (II) Fluoride Cathode Material Derived from MIL-88A. J. Alloys Compd. 2022, 916, 165438. [Google Scholar] [CrossRef]
- Chalati, T.; Horcajada, P.; Gref, R.; Couvreur, P.; Serre, C. Optimisation of the Synthesis of MOF Nanoparticles Made of Flexible Porous Iron Fumarate MIL-88A. J. Mater. Chem. 2011, 21, 2220–2227. [Google Scholar] [CrossRef]
- Chameh, B.; Moradi, M.; Hajati, S.; Hessari, F.A.; Kiani, M.A. Morphology Control of Ni Doped Rod like MIL-88A Derived FeS2 Embedded in Nitrogen-Rich Carbon as an Efficient Electrocatalyst for the Oxygen Reduction Reaction. J. Mol. Struct. 2021, 1237, 130329. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, Z.; Chen, J.; Yang, Y.; Ding, C.; Yang, Y.; Wang, Y.; Liu, N.; Wang, L.; Zhang, X. Recent Research Progress and Challenges of MIL-88(Fe) from Synthesis to Advanced Oxidation Process. Surf. Interfaces 2022, 30, 101843. [Google Scholar] [CrossRef]
- Rabeie, B.; Mahmoodi, N.M.; Mahkam, M. Morphological Diversity Effect of Graphene Quantum Dot/MIL88A(Fe) Composites on Dye and Pharmaceuticals (Tetracycline and Doxycycline) Removal. J. Environ. Chem. Eng. 2022, 10, 108321. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, A.; Hou, K.; Liu, M.; Wang, Y.; Song, C.; Zhang, G.; Guo, X. Size- and Morphology-Controlled NH2-MIL-53(Al) Prepared in DMF-Water Mixed Solvents. Dalt. Trans. 2013, 42, 13698–13705. [Google Scholar] [CrossRef] [PubMed]
- El Asmar, R.; Baalbaki, A.; Abou Khalil, Z.; Naim, S.; Bejjani, A.; Ghauch, A. Iron-Based Metal Organic Framework MIL-88-A for the Degradation of Naproxen in Water through Persulfate Activation. Chem. Eng. J. 2021, 405, 126701. [Google Scholar] [CrossRef]
- Hmoudah, M.; El-Qanni, A.; Tesser, R.; Esposito, R.; Petrone, A.; Jung, O.S.; Salmi, T.; Russo, V.; Di Serio, M. Assessment of the Robustness of MIL-88A in an Aqueous Solution: Experimental and DFT Investigations. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2023, 288, 116179. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Wang, M.; Chen, B.; Zhang, Y.; Sun, Y.; Chen, K.; Du, Q.; Wang, Y.; Pi, X.; et al. Efficient Adsorption of Congo Red by Micro/Nano MIL-88A (Fe, Al, Fe-Al)/Chitosan Composite Sponge: Preparation, Characterization, and Adsorption Mechanism. Int. J. Biol. Macromol. 2023, 239, 124157. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Mu, B.; Zhu, Y.; Wang, A. Preparation of Palygorskite/MIL-88A(Fe) Composites for High-Efficient Removal of Congo Red. Appl. Clay Sci. 2023, 242, 107003. [Google Scholar] [CrossRef]
- Chen, D.; Chen, S.; Jiang, Y.; Xie, S.; Quan, H.; Hua, L.; Luo, X.; Guo, L. Heterogeneous Fenton-like Catalysis of Fe-MOF Derived Magnetic Carbon Nanocomposites for Degradation of 4-Nitrophenol. RSC Adv. 2017, 7, 49024–49030. [Google Scholar] [CrossRef]
- Bagherzadeh, E.; Zebarjad, S.M.; Hosseini, H.R.M. Morphology Modification of the Iron Fumarate MIL-88A Metal-Organic Framework Using Formic Acid and Acetic Acid as Modulators. Eur. J. Inorg. Chem. 2018, 2018, 1909–1915. [Google Scholar] [CrossRef]
- Medvedev, P.V.; Butova, V.V.; Soldatov, M.A.; Kuzharov, A.A.; Fedorenko, A.G.; Shapovalova (Cherkasova), S.O.; Burachevskaya, O.A.; Gorban, I.E.; Soldatov, A.V. The Influence of Acetic Acid on the Properties of Microporous Metal-Organic Framework MIL-88a at Microfluidic Conditions and Room Temperature. Nanobiotechnol. Rep. 2021, 16, 488–496. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, K.; Zhao, L. A Fenton-like Method Using ZnO Doped MIL-88A for Degradation of Methylene Blue Dyes. RSC Adv. 2020, 10, 39973–39980. [Google Scholar] [CrossRef] [PubMed]
- Ghodsinia, S.S.E.; Eshghi, H.; Mohammadinezhad, A. Synthesis of Double-Shelled Periodic Mesoporous Organosilica Nanospheres/MIL-88A-Fe Composite and Its Elevated Performance for Pb2+ Removal in Water. Sci. Rep. 2023, 13, 8092. [Google Scholar] [CrossRef] [PubMed]
- Alipanah, N.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G. MIL-88A (Fe) Filler with Duplicate Corrosion Inhibitive/Barrier Effect for Epoxy Coatings: Electrochemical, Molecular Simulation, and Cathodic Delamination Studies. J. Ind. Eng. Chem. 2021, 97, 200–215. [Google Scholar] [CrossRef]
- Ashrafi, M.; Farhadi, S. Polyoxometalate Supported on a Magnetic Fe3O4/MIL-88A Rod-like Nanocomposite as an Adsorbent for the Removal of Ciprofloxacin, Tetracycline and Cationic Organic Dyes from Aqueous Solutions. RSC Adv. 2023, 13, 6356–6367. [Google Scholar] [CrossRef] [PubMed]
- Andrew Lin, K.Y.; Chang, H.A.; Hsu, C.J. Iron-Based Metal Organic Framework, MIL-88A, as a Heterogeneous Persulfate Catalyst for Decolorization of Rhodamine B in Water. RSC Adv. 2015, 5, 32520–32530. [Google Scholar] [CrossRef]
- Tan, L.S.; Tan, H.L.; Deekonda, K.; Wong, Y.Y.; Muniyandy, S.; Hashim, K.; Pushpamalar, J. Fabrication of Radiation Cross-Linked Diclofenac Sodium Loaded Carboxymethyl Sago Pulp/Chitosan Hydrogel for Enteric and Sustained Drug Delivery. Carbohydr. Polym. Technol. Appl. 2021, 2, 100084. [Google Scholar] [CrossRef]
- Fayrouz, D.; Abdallah, D.; Aicha, H. Experimental Investigation of Ternary Mixture of Diclofenac Sodium with Pharmaceutical Excipients. Phys. Sci. Rev. 2021, 8, 763–774. [Google Scholar] [CrossRef]
- Kozakevych, R.B.; Bolbukh, Y.M.; Tertykh, V.A. Controlled Release of Diclofenac Sodium from Silica-Chitosan Composites. World J. Nano Sci. Eng. 2013, 03, 69–78. [Google Scholar] [CrossRef]
- Crișan, A.G.; Porfire, A.; Iurian, S.; Rus, L.M.; Lucăcel Ciceo, R.; Turza, A.; Tomuță, I. Development of a Bilayer Tablet by Fused Deposition Modeling as a Sustained-Release Drug Delivery System. Pharmaceuticals 2023, 16, 1321. [Google Scholar] [CrossRef]
- Ptaszkowska-Koniarz, M.; Goscianska, J.; Pietrzak, R. Synthesis of Carbon Xerogels Modified with Amine Groups and Copper for Efficient Adsorption of Caffeine. Chem. Eng. J. 2018, 345, 13–21. [Google Scholar] [CrossRef]
- Troyano, J.; Carné-Sánchez, A.; Pérez-Carvajal, J.; León-Reina, L.; Imaz, I.; Cabeza, A.; Maspoch, D. A Self-Folding Polymer Film Based on Swelling Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2018, 57, 15420–15424. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.B.; Chen, B. Hydrogen-Bonded Organic Frameworks: Chemistry and Functions. Chem 2022, 8, 2114–2135. [Google Scholar] [CrossRef]
- Li, Y.; Sui, J.; Cui, L.S.; Jiang, H.L. Hydrogen Bonding Regulated Flexibility and Disorder in Hydrazone-Linked Covalent Organic Frameworks. J. Am. Chem. Soc. 2023, 145, 1359–1366. [Google Scholar] [CrossRef]
- Kundu, T.; Wahiduzzaman, M.; Shah, B.B.; Maurin, G.; Zhao, D. Solvent-Induced Control over Breathing Behavior in Flexible Metal-Organic Frameworks for Natural-Gas Delivery. Angew. Chem. 2019, 131, 8157–8161. [Google Scholar] [CrossRef]
- Bui, A.; Guillen, S.G.; Sua, A.; Nguyen, T.C.; Ruiz, A.; Carachure, L.; Weber, M.D.R.; Cortez, A.; Tian, F. Iron-Containing Metal-Organic Framework Thin Film as a Drug Delivery System. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129611. [Google Scholar] [CrossRef] [PubMed]
- Correa-Navarro, Y.M.; Moreno-Piraján, J.C.; Giraldo, L. Competitive Adsorption of Caffeine and Diclofenac Sodium onto Biochars Derived from Fique Bagasse: An Immersion Calorimetry Study. ACS Omega 2022, 8, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Zhang, Q.; Deng, S. Pore Surface Engineering of Covalent Organic Frameworks by Simultaneously Appending Amine Group and Tailoring Pore Size for Efficient Adsorption of Diclofenac Sodium. Chem. Eng. J. 2023, 459, 141561. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Bhowmik, M. Adsorption of Diclofenac Sodium from Aqueous Solution Using Polyaniline as a Potential Sorbent. I. Kinetic Studies. J. Appl. Polym. Sci. 2010, 117, 3615–3622. [Google Scholar] [CrossRef]
- Yuan, R.; Qiu, J.; Yue, C.; Shen, C.; Li, D.; Zhu, C.; Liu, F.; Li, A. Self-Assembled Hierarchical and Bifunctional MIL-88A(Fe)@ZnIn2S4 Heterostructure as a Reusable Sunlight-Driven Photocatalyst for Highly Efficient Water Purification. Chem. Eng. J. 2020, 401, 126020. [Google Scholar] [CrossRef]
- Barczak, M.; Dobrowolski, R.; Borowski, P.; Giannakoudakis, D.A. Pyridine-, Thiol- and Amine-Functionalized Mesoporous Silicas for Adsorptive Removal of Pharmaceuticals. Microporous Mesoporous Mater. 2020, 299, 110132. [Google Scholar] [CrossRef]
- Weidner, E.; Bartczak, P.; Goscianska, J.; Jesionowski, T.; Jaroniec, M.; Ciesielczyk, F. Soft-Templating Synthesis of Mesoporous Alumina Enriched with Lanthana and Its Potential as Diclofenac Delivery System. Microporous Mesoporous Mater. 2023, 351, 112487. [Google Scholar] [CrossRef]
- Lach, J.; Szymonik, A. Adsorption of Diclofenac Sodium from Aqueous Solutions on Commercial Activated Carbons. Desalin. Water Treat. 2020, 186, 418–429. [Google Scholar] [CrossRef]
- Mao, N.; Huang, L.; Shuai, Q. Facile Synthesis of Porous Carbon for the Removal of Diclofenac Sodium from Water. ACS Omega 2019, 4, 15051–15060. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, M.S.; Azha, S.F.; Sellaoui, L.; Badawi, M.; Bonilla-Petriciolet, A.; Ismail, S. Performance and Interactions of Diclofenac Adsorption Using Alginate/Carbon-Based Films: Experimental Investigation and Statistical Physics Modelling. Chem. Eng. J. 2022, 428, 131929. [Google Scholar] [CrossRef]
- Younes, H.A.; Taha, M.; Mahmoud, R.; Mahmoud, H.M.; Abdelhameed, R.M. High Adsorption of Sodium Diclofenac on Post-Synthetic Modified Zirconium-Based Metal-Organic Frameworks: Experimental and Theoretical Studies. J. Colloid Interface Sci. 2022, 607, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Prasetya, N.; Wöll, C. Removal of Diclofenac by Adsorption Process Studied in Free-Base Porphyrin Zr-Metal Organic Frameworks (Zr-MOFs). RSC Adv. 2023, 13, 22998–23009. [Google Scholar] [CrossRef] [PubMed]
- Prasetya, N.; Li, K. MOF-808 and Its Hollow Fibre Adsorbents for Efficient Diclofenac Removal. Chem. Eng. J. 2021, 417, 129216. [Google Scholar] [CrossRef]
- Sharma, A.; Rathore, V.K.; Chakraborty, M. Adsorptive Removal of Diclofenac Sodium from Aqueous Solution by Highly Efficient Metal Organic Framework (UiO-66)/Multi-Walled Carbon Nanotube Composite. Environ. Sci. Pollut. Res. 2023, 1–14. [Google Scholar] [CrossRef]
- Pournara, A.D.; Andreou, E.K.; Armatas, G.S.; Manos, M.J. Zirconium(IV) Metal Organic Frameworks with Highly Selective Sorption for Diclofenac under Batch and Continuous Flow Conditions. Crystals 2022, 12, 424. [Google Scholar] [CrossRef]
- Li, H.Z.; Yang, C.; Qian, H.L.; Yan, X.P. Room-Temperature Synthesis of Ionic Covalent Organic Frameworks for Efficient Removal of Diclofenac Sodium from Aqueous Solution. Sep. Purif. Technol. 2023, 306, 122704. [Google Scholar] [CrossRef]
- Huang, L.; Mao, N.; Yan, Q.; Zhang, D.; Shuai, Q. Magnetic Covalent Organic Frameworks for the Removal of Diclofenac Sodium from Water. ACS Appl. Nano Mater. 2020, 3, 319–326. [Google Scholar] [CrossRef]
- Liu, W.; Shen, X.; Han, Y.; Liu, Z.; Dai, W.; Dutta, A.; Kumar, A.; Liu, J. Selective Adsorption and Removal of Drug Contaminants by Using an Extremely Stable Cu(II)-Based 3D Metal-Organic Framework. Chemosphere 2019, 215, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Arabkhani, P.; Javadian, H.; Asfaram, A.; Ateia, M. Decorating Graphene Oxide with Zeolitic Imidazolate Framework (ZIF-8) and Pseudo-Boehmite Offers Ultra-High Adsorption Capacity of Diclofenac in Hospital Effluents. Chemosphere 2021, 271, 129610. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cui, J.; Wu, X.; Zhang, X.; Hu, Q.; Hou, X. Rapid in Situ Microwave Synthesis of Fe3O4@MIL-100(Fe) for Aqueous Diclofenac Sodium Removal through Integrated Adsorption and Photodegradation. J. Hazard. Mater. 2019, 373, 408–416. [Google Scholar] [CrossRef]
- Zhuang, S.; Liu, Y.; Wang, J. Mechanistic Insight into the Adsorption of Diclofenac by MIL-100: Experiments and Theoretical Calculations. Environ. Pollut. 2019, 253, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, S.; Farshchi Tabrizi, F.; Sardarian, A. Efficient Adsorptive Removal of Diclofenac Sodium by Acidified MIL101(Cr): Optimizing the Content of Phosphotungstic Acid, Flow Loop Thin Film Slurry Flat Plate Reactor. J. Porous Mater. 2023, 30, 975–988. [Google Scholar] [CrossRef]
- Goscianska, J.; Marciniak, M.; Pietrzak, R. Ordered Mesoporous Carbons Modified with Cerium as Effective Adsorbents for Azo Dyes Removal. Sep. Purif. Technol. 2015, 154, 236–245. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant Adsorption Isotherms: A Review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef]
- Marciniak, M.; Goscianska, J.; Norman, M.; Jesionowski, T.; Bazan-Wozniak, A.; Pietrzak, R. Equilibrium, Kinetic, and Thermodynamic Studies on Adsorption of Rhodamine B from Aqueous Solutions Using Oxidized Mesoporous Carbons. Materials 2022, 15, 5573. [Google Scholar] [CrossRef]
- Alves, R.C.; Lucena, G.N.; de Farias, R.L.; da Silva, P.B.; da Silva, I.C.; Pavan, F.R.; Chorilli, M.; da Costa Ferreira, A.M.; Galvão Frem, R.C. Copper(II) Biocompatible Coordination Solids as Potential Platforms for Diclofenac Delivery Systems. J. Solid State Chem. 2020, 289, 121479. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, G.; Pickrell, G.R. Silica Nanospheres. Biomed. Ther. Clin. Appl. Bioact. Glas. 2018, 521–544. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal Organic Frameworks as Efficient Adsorbents for Drugs from Wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Ding, H.; Li, B.; Jiang, Y.; Liu, G.; Pu, S.; Feng, Y.; Jia, D.; Zhou, Y. PH-Responsive UV Crosslinkable Chitosan Hydrogel via “Thiol-Ene” Click Chemistry for Active Modulating Opposite Drug Release Behaviors. Carbohydr. Polym. 2021, 251, 117101. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Zhou, Y.; Liu, X.; Kong, L.; Liao, L.; Li, Y.; Liu, M.; Tian, L.; Rao, W.; Lv, G. Effective PH-Responsive Nanocarrier Based on the Anisotropic Surfaces of Halloysite Nanotubes for Controlled Drug Release. Appl. Clay Sci. 2023, 232, 106799. [Google Scholar] [CrossRef]
- Wang, S.-y.; Li, J.; Zhou, Y.; Li, D.-Q.; Du, G.-M. Chemical Cross-Linking Approach for Prolonging Diclofenac Sodium Release from Pectin-Based Delivery System. Int. J. Biol. Macromol. 2019, 137, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Zauska, L.; Bova, S.; Benova, E.; Bednarcik, J.; Balaz, M.; Zelenak, V.; Hornebecq, V.; Almasi, M. Thermosensitive Drug Delivery System SBA-15-PEI for Controlled Release of Nonsteroidal Anti-Inflammatory Drug Diclofenac Sodium Salt: A Comparative Study. Materials 2021, 14, 1880. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, D.; Simeonov, M.; Tzachev, C.; Apostolov, A.; Christov, L.; Vassileva, E. Polyelectrolyte Complexes of Chitosan and Sodium Alginate as a Drug Delivery System for Diclofenac Sodium. Polym. Int. 2022, 71, 668–678. [Google Scholar] [CrossRef]
- Vargas, A.M.; Cipagauta-Ardila, C.C.; Molina-Velasco, D.R.; Ríos-Reyes, C.A. Surfactant-Modified Natural Zeolites as Carriers for Diclofenac Sodium Release: A Preliminary Feasibility Study for Pharmaceutical Applications. Mater. Chem. Phys. 2020, 256, 123644. [Google Scholar] [CrossRef]
- Lucena, G.N.; Alves, R.C.; Abuçafy, M.P.; Chiavacci, L.A.; da Silva, I.C.; Pavan, F.R.; Frem, R.C.G. Zn-Based Porous Coordination Solid as Diclofenac Sodium Carrier. J. Solid State Chem. 2018, 260, 67–72. [Google Scholar] [CrossRef]
- Simon, M.A.; Anggraeni, E.; Soetaredjo, F.E.; Santoso, S.P.; Irawaty, W.; Thanh, T.C.; Hartono, S.B.; Yuliana, M.; Ismadji, S. Hydrothermal Synthesize of HF-Free MIL-100(Fe) for Isoniazid-Drug Delivery. Sci. Rep. 2019, 9, 16907. [Google Scholar] [CrossRef]
- Shafiei, F.; Ghavami-Lahiji, M.; Kashi, T.J.; Najafi, F. Drug Release Kinetics and Biological Properties of a Novel Local Drug Carrier System. Dent. Res. J. 2021, 18, 94. [Google Scholar] [CrossRef]
- Paarakh, M.P.; Jose, P.A.; Setty, C.M.; Christoper, G.V. Release Kinetics—Concepts and Applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar] [CrossRef]
- Biswas, G.R.; Bhattacharya, S.; Ghoshal, P.; Majee, S.B. Fabrication of Microsponge as Drug Delivery of an Antihypertensive Drug. Eur. J. Pharm. Med. Res. 2020, 7, 423–430. [Google Scholar]









| Drug | Structure | pKa | Melting Point (°C) | Molecular Weight (g/mol) | Maximum Wavelength (nm) |
|---|---|---|---|---|---|
| Diclofenac sodium |  | 4.15 | 275–277 | 318.1 | 276 |
| Sample | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) | Micropore Area (m2/g) |
|---|---|---|---|---|
| MIL-88A-1 | 10 | 0.05 | 20.30 | - |
| MIL-88A-2 | 292 | 0.21 | 2.83 | 261 |
| MIL-88A-3 | 326 | 0.47 | 5.74 | 279 |
| MIL-88A-4 | 307 | 0.33 | 4.27 | 122 |
| Material | Material Type | Specific Surface Area (m2/g) | Maximum Sorption Capacities (mg/g) | Ref. |
|---|---|---|---|---|
| Pyridine functionalized mesoporous silica | silica | 322 | 352 | [81] |
| Thiol functionalized mesoporous silica | silica | 493 | 48 | |
| Amine functionalized mesoporous silica | silica | 358 | 328 | |
| Al2O3/La2O3 | aluminum oxide | 156 | 82 | [82] |
| F-300 | carbon | 847 | 108 | [83] |
| PC-1000 | carbon | 1236 | 392 | [84] |
| Alginate/carbon films | carbon composite | 35 | 30 | [85] |
| UiO-66-(COOFe)2 | Zr-based MOF | 684 | 860 | [86] |
| MOF-525 | Zr-based MOF | 1955 | 792 | [87] |
| MOF-808 | Zr-based MOF | 1517 | 830 | [88] |
| UiO-66/MWCNT | Zr-based MOF composite | - | 256 | [89] |
| MOR-1 | Zr-based MOF | 1097 | 315 | [90] |
| RT-iCOF | ionic covalent organic framework | 69 | 875 | [91] |
| MCOF-2 | magnetic covalent organic frameworks | 335 | 565 | [92] |
| [Cu(BTTA)]n·2DMF | Cu-based MOF | - | 650 | [93] |
| ZIF-8-NPs | Zn-based MOF | 1568 | 843 | [94] |
| Fe3O4@MIL-100(Fe) | Fe-based MOF | 1245 | 400 | [95] |
| MIL-100 | Fe-based MOF | 1235 | 773 | [96] |
| 12%PTA@MIL101(Cr) | Cr-based MOF composite | 1909 | 413 | [97] |
| MIL-88A-1 | Fe-based MOF | 10 | 2021 | this work |
| Material | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qmax | KL | R2 | KF | 1/n | R2 | |
| MIL-88A-1 | 2070 | 0.049 | 0.793 | 1523 | 0.04 | 0.798 |
| MIL-88A-2 | 1502 | 0.058 | 0.995 | 349 | 0.22 | 0.867 |
| MIL-88A-3 | 905 | 0.027 | 0.967 | 162 | 0.25 | 0.758 |
| MIL-88A-4 | 1255 | 0.075 | 0.773 | 728 | 0.08 | 0.951 |
| Sample | Zero-Order | First-Order | Higuchi | Hixson–Crowell | Korsmeyer–Peppas | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| k0 | R2 | k1 | R2 | kH | R2 | kHC | R2 | kKP | n | R2 | |
| MIL-88A-1 | 7.077 | 0.973 | 0.154 | 0.992 | 24.778 | 0.990 | 0.183 | 0.988 | 33.787 | 0.403 | 0.984 |
| MIL-88A-2 | 8.372 | 0.952 | 0.127 | 0.982 | 25.649 | 0.993 | 0.171 | 0.974 | 19.458 | 0.637 | 0.984 |
| MIL-88A-3 | 6.647 | 0.954 | 0.087 | 0.974 | 18.307 | 0.996 | 0.123 | 0.968 | 16.592 | 0.540 | 0.995 |
| MIL-88A-4 | 13.035 | 0.940 | 0.185 | 0.964 | 30.678 | 0.986 | 0.254 | 0.957 | 23.319 | 0.66 | 0.976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galarda, A.; Goscianska, J. Biocompatible Fe-Based Metal-Organic Frameworks as Diclofenac Sodium Delivery Systems for Migraine Treatment. Appl. Sci. 2023, 13, 12960. https://doi.org/10.3390/app132312960
Galarda A, Goscianska J. Biocompatible Fe-Based Metal-Organic Frameworks as Diclofenac Sodium Delivery Systems for Migraine Treatment. Applied Sciences. 2023; 13(23):12960. https://doi.org/10.3390/app132312960
Chicago/Turabian StyleGalarda, Aleksandra, and Joanna Goscianska. 2023. "Biocompatible Fe-Based Metal-Organic Frameworks as Diclofenac Sodium Delivery Systems for Migraine Treatment" Applied Sciences 13, no. 23: 12960. https://doi.org/10.3390/app132312960
APA StyleGalarda, A., & Goscianska, J. (2023). Biocompatible Fe-Based Metal-Organic Frameworks as Diclofenac Sodium Delivery Systems for Migraine Treatment. Applied Sciences, 13(23), 12960. https://doi.org/10.3390/app132312960