Microbial Diversity of the Produced Waters from the Oilfields in the Republic of Tatarstan (Russian Federation): Participation in Biocorrosion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oilfield Water and Deposit Sampling
2.2. Chemical Analyses of Water
2.3. Enrichment of Microbes
2.4. Experimental Setup for Biocorrosion
2.5. Bacterial 16S rRNA Gene Amplicon Sequencing and Bioinformatic Analysis
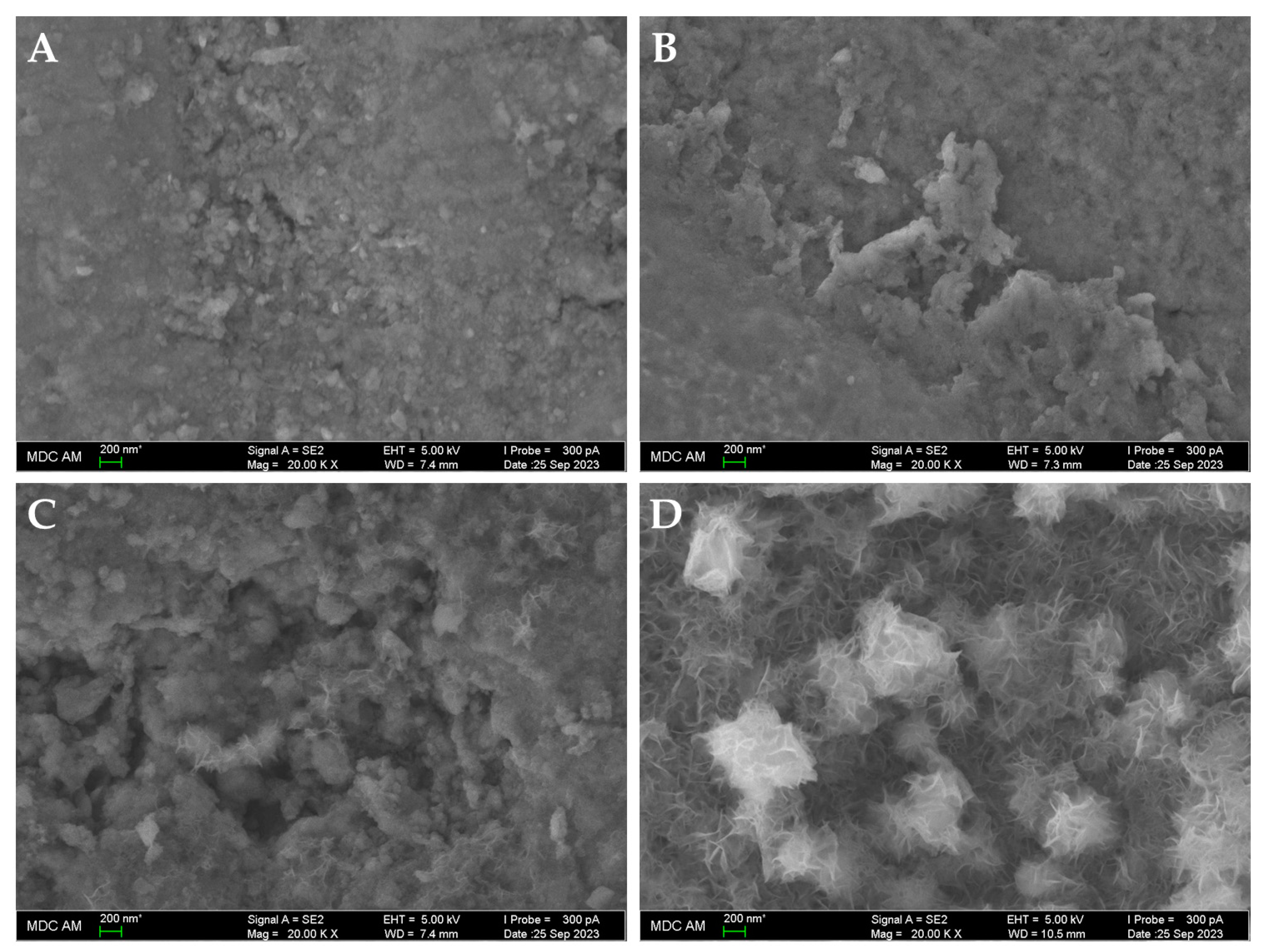
2.6. Scanning Electron Microscopy (SEM) and Elemental Analysis
3. Results and Discussion
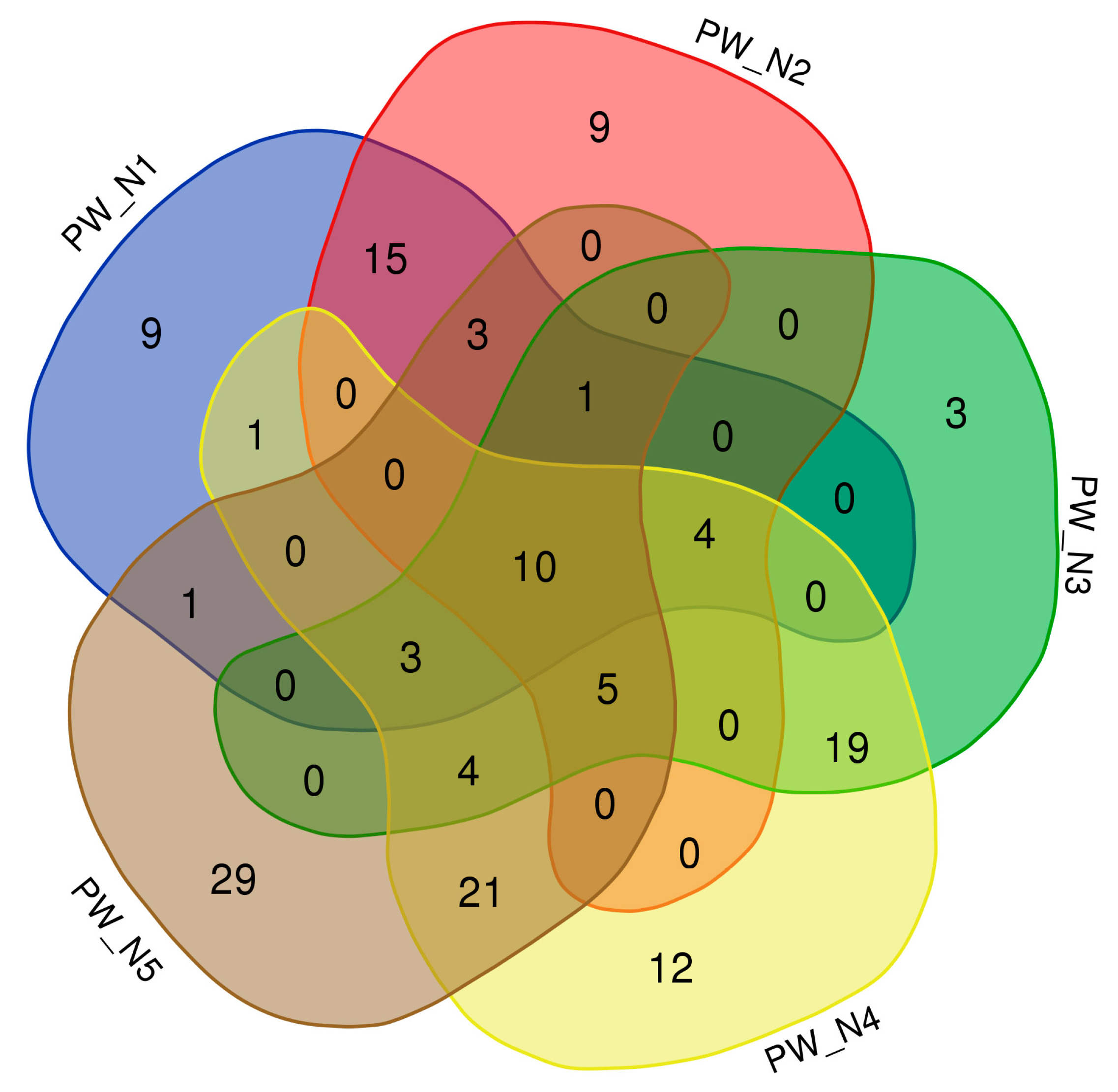
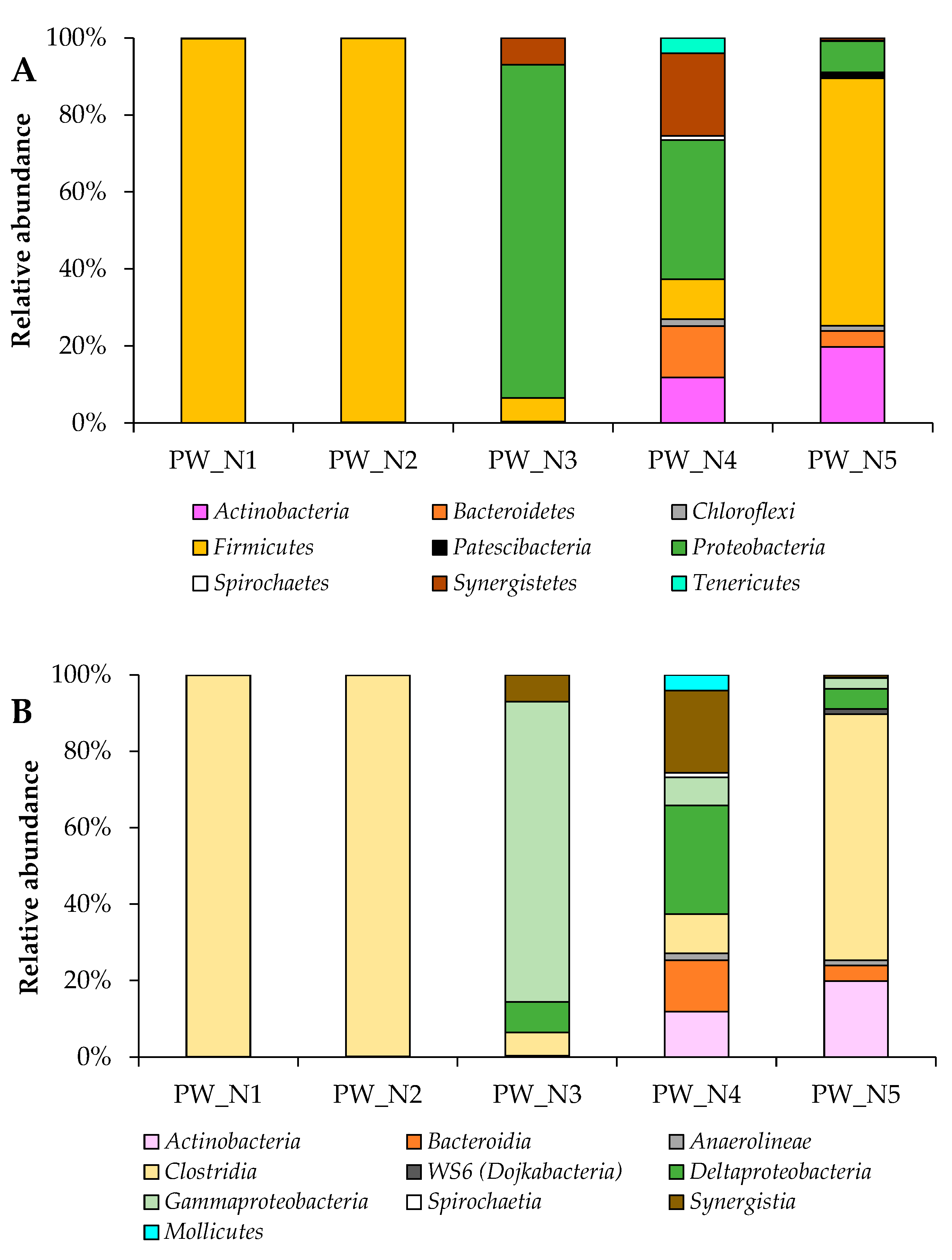
3.1. Taxonomic Distribution of Bacterial Communities from Oil Wells near Nurlat
3.2. Taxonomic Distribution of Bacterial Communities Enriched from Deposits
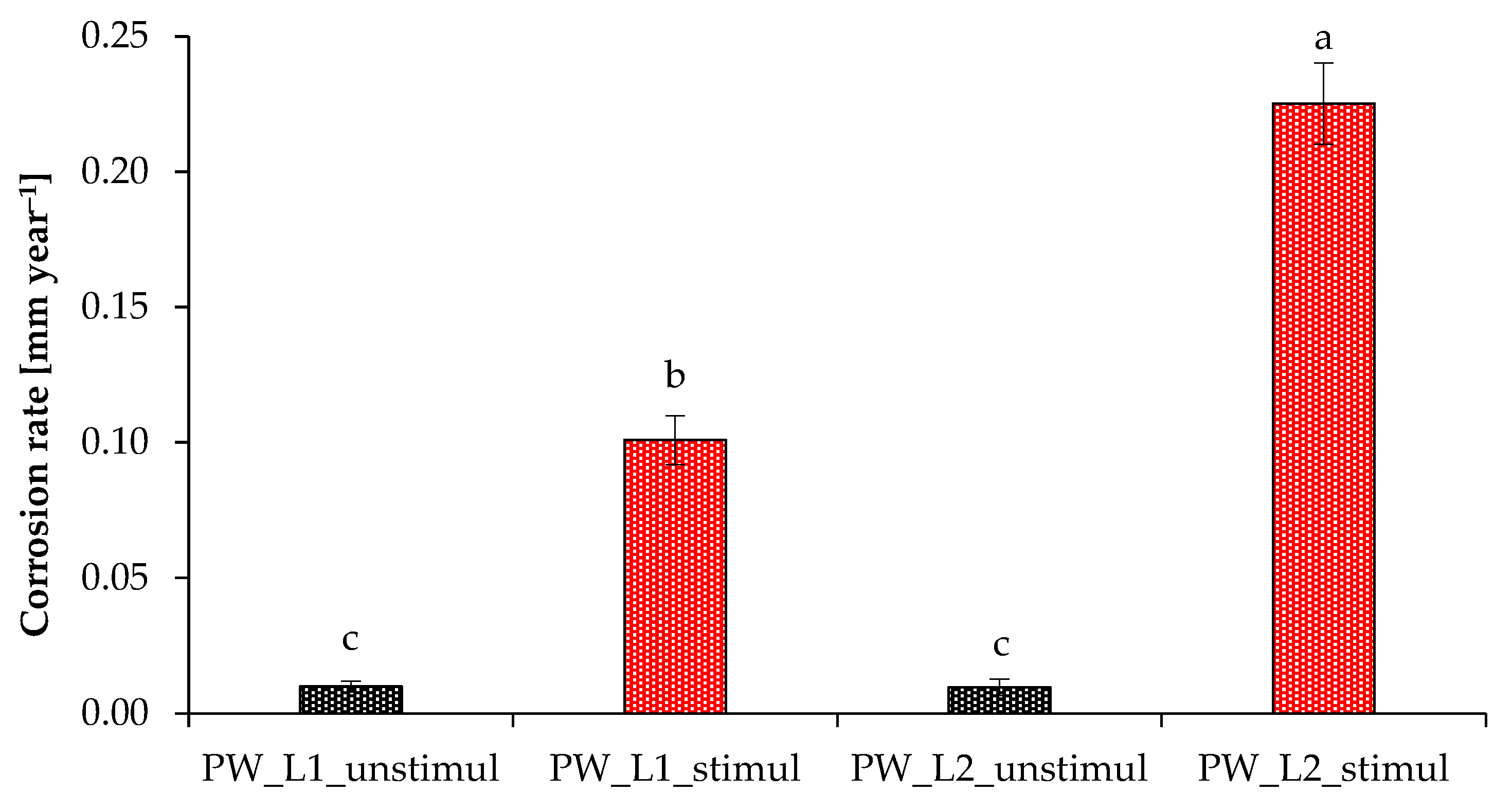
3.3. Corrosive Potential of Microbial Communities
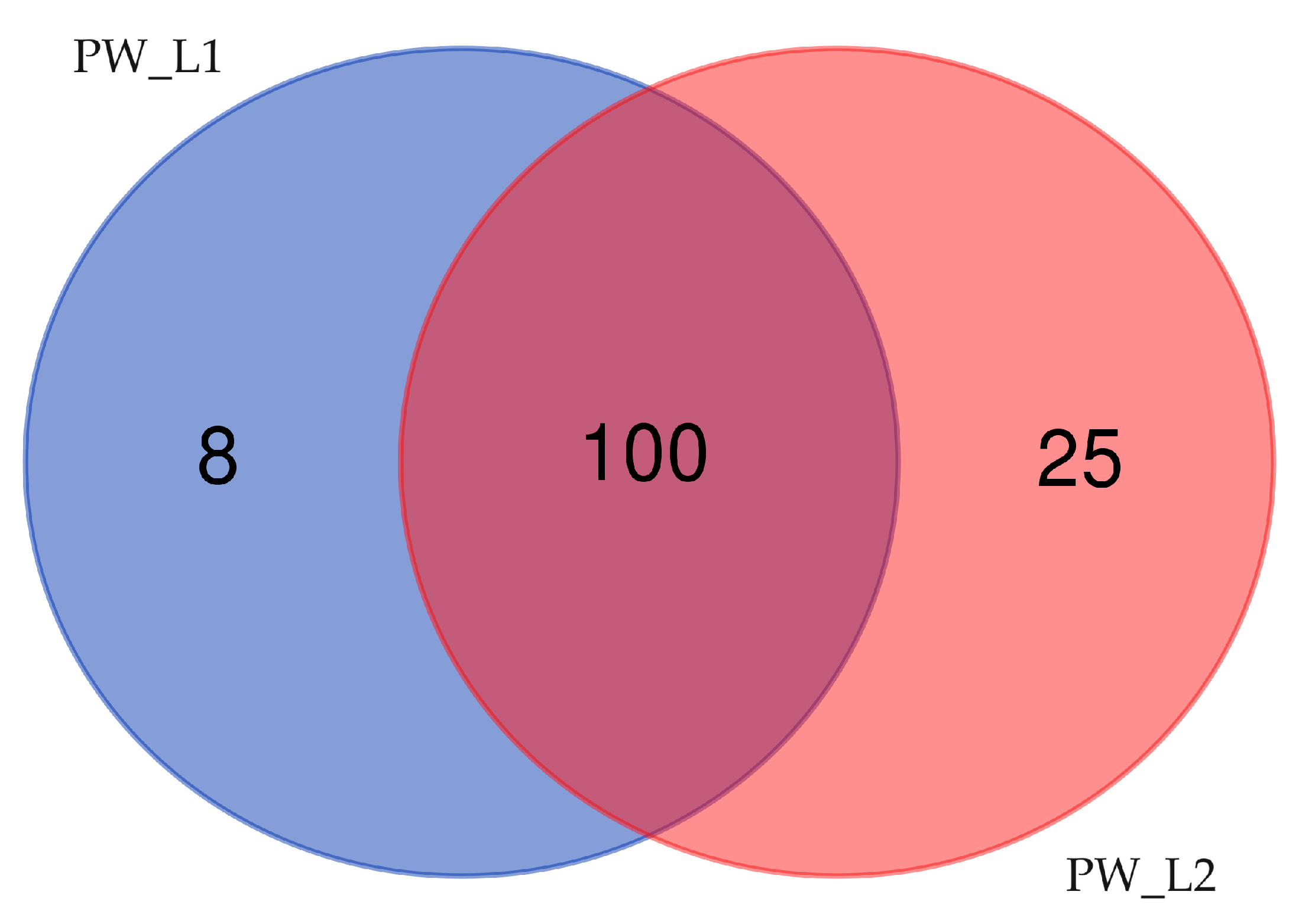
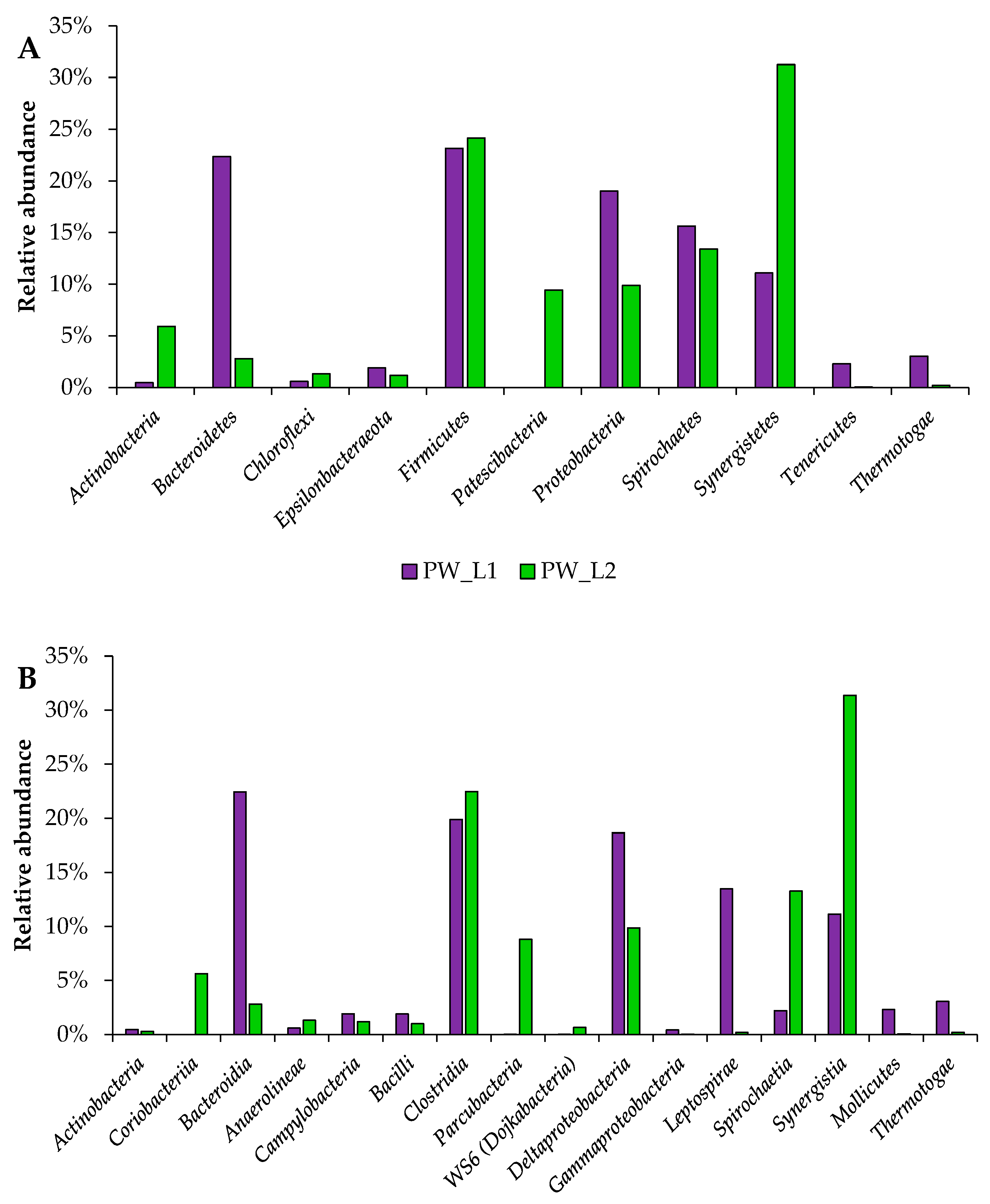
3.4. Taxonomic Distribution of Bacterial Communities from Oil Wells near Leninogorsk
3.5. Function of Microbial Communities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chauhan, P.; Imam, A.; Kanaujia, P.K.; Suman, S.K. Nano-bioremediation: An eco-friendly and effective step towards petroleum hydrocarbon removal from environment. Environ. Res. 2023, 231, 116224. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Y.; Afrin, T.; Huang, Y.; Yodo, N. Sustainable development for oil and gas infrastructure from risk, reliability, and resilience perspectives. Sustainability 2023, 15, 4953. [Google Scholar] [CrossRef]
- Varjani, S.; Joshi, R.; Srivastava, V.K.; Ngo, H.H.; Guo, W. Treatment of wastewater from petroleum industry: Current practices and perspectives. Environ. Sci. Pollut. Res. 2020, 27, 27172–27180. [Google Scholar] [CrossRef] [PubMed]
- Lackner, M.; Hribernig, T.; Lutz, M.; Plank, M.; Putz, K. Extraction of aged hydrocarbons from contaminated soil using plant-oil-in-water emulsions combined with oil/water separation by reusable non-wovens. Appl. Sci. 2022, 12, 6179. [Google Scholar] [CrossRef]
- Lipus, D.; Roy, D.; Khan, E.; Ross, D.; Vikram, A.; Gulliver, D.; Hammack, R.; Bibby, K. Microbial communities in Bakken region produced water. FEMS Microbiol. Lett. 2018, 365, fny107. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rosario, R.; Hildenbrand, Z.L. Produced water treatment and valorization: A techno-economical review. Energies 2022, 15, 4619. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of oilfield produced water treatment technologies. Chemosphere 2022, 298, 134064. [Google Scholar] [CrossRef]
- Ollivier, B.; Alazard, D. The oil reservoir ecosystem. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2259–2269. [Google Scholar]
- Wentzel, A.; Lewin, A.; Cervantes, F.J.; Valla, S.; Kotlar, H.K. Deep sub-surface oil reservoirs as poly-extreme habitats for microbial life. A current review. Polyextremophiles 2013, 27, 439–466. [Google Scholar]
- Dong, Y.; Kumar, C.G.; Chia, N.; Kim, P.J.; Miller, P.A.; Price, N.D.; Can, I.K.O.; Flynn, T.M.; Sanford, R.A.; Krapac, I.G.; et al. Halomonas sulfidaeris-dominated microbial community in habits a 1.8 km-deep subsurface Cambrian Sand stone reservoir. Environ. Microbiol. 2014, 16, 1695–1708. [Google Scholar] [CrossRef]
- Tian, H.; Gao, P.; Chen, Z.; Li, Y.; Li, Y.; Wang, Y.; Zhou, J.; Li, G.; Ma, T. Compositions and abundances of sulfate-reducing and sulfur-oxidizing microorganisms in water-flooded petroleum reservoirs with different temperatures in China. Front. Microbiol. 2017, 8, 143. [Google Scholar] [CrossRef]
- Pannekens, M.; Kroll, L.; Müller, H.; Mbow, F.T.; Meckenstock, R.U. Oil reservoirs, an exceptional habitat for microorganisms. New Biotechnol. 2019, 49, 1–9. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Use of microorganisms in the recovery of oil from recalcitrant oil reservoirs: Current state of knowledge, technological advances and future perspectives. Front. Microbiol. 2020, 10, 2996. [Google Scholar] [CrossRef]
- Wu, B.; Xiu, J.; Yu, L.; Huang, L.; Yi, L.; Ma, Y. Research advances of microbial enhanced oil recovery. Heliyon 2022, 8, e11424. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Liu, M.; Liang, Q.; Yang, H.; Li, J.; Yao, Z.; Li, S.; Yan, W. Shifts in bacterial and archaeal community composition in low-permeability oil reservoirs by a nutrient stimulation for enhancing oil recovery. Appl. Sci. 2022, 12, 8075. [Google Scholar] [CrossRef]
- Bi, Y.; Xiu, J.; Ma, T. Application potential analysis of enhanced oil recovery by biopolymer-producing bacteria and biosurfactant-producing bacteria compound flooding. Appl. Sci. 2019, 9, 5119. [Google Scholar] [CrossRef]
- Liang, R.; Grizzle, R.S.; Duncan, K.E.; McInerney, M.J.; Suflita, J.M. Roles of thermophilic thiosulfate-reducing bacteria and methanogenic archaea in the biocorrosion of oil pipelines. Front. Microbiol. 2014, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Magot, M.; Ollivier, B.; Patel, B.K. Microbiology of petroleum reservoirs. Antonie Leeuwenhoek 2000, 77, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Korenblum, E.; Souza, D.B.; Penna, M.; Seldin, L. Molecular analysis of the bacterial communities in crude oil samples from two brazilian offshore petroleum platforms. Int. J. Microbiol. 2012, 2012, 156537. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.Y.; Zhang, X.J.; Song, Z.; Rupert, W.; Gao, G.J.; Guo, S.; Zhao, L.P. Comparison of microbial community compositions of injection and production well samples in a long-term water-flooded petroleum reservoir. PLoS ONE 2011, 6, e23258. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Li, Y.; Zhao, J.Y.; Chi, C.Q.; Huang, L.X.; Dong, H.P.; Wu, X.L. Microbial communities in long-term, water-flooded petroleum reservoirs with different in situ temperatures in the Huabei Oilfield, China. PLoS ONE 2012, 7, e33535. [Google Scholar] [CrossRef]
- Kobayashi, H.; Endo, K.; Sakata, S.; Mayumi, D.; Kawaguchi, H.; Ikarashi, M. Phylogenetic diversity of microbial communities associated with the crude-oil, large-insoluble-particle and formation-water components of the reservoir fluid from a non-flooded high-temperature petroleum reservoir. J. Biosci. Bioeng. 2012, 113, 204–210. [Google Scholar] [CrossRef]
- Bodtker, G.; Thorstenson, T.; Lillebo, B.L.; Thorbjornsen, B.E.; Ulvoen, R.H.; Sunde, E.; Torsvik, T. The effect of long-term nitrate treatment on SRB activity, corrosion rate and bacterial community composition in offshore water injection systems. J. Ind. Microbiol. Biotechnol. 2008, 35, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.M.; Goulart, F.R.V.; Marques, J.M.; Bizzo, H.R.; Blank, A.F.; Groposo, C.; Sousa, M.P.; Volaro, V.; Alviano, C.S.; Moreno, D.S.A.; et al. Growth inhibition of sulfate-reducing bacteria in produced water from the petroleum industry using essential oils. Molecules 2017, 22, 648. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, S.R.G.; Macrae, A.; Peixoto, R.S.; da Costa Rachid, C.T.C.; Mansoldo, F.R.P.; Alviano, D.S.; Fereira, D.F.; de Queiroz Venancio, F.; Fereira, D.F.; Vermelho, A.B. Sulphate-reducing bacterial community structure from produced water of the Periquito and Galo de Campina onshore oilfields in Brazil. Sci. Rep. 2021, 11, 20311. [Google Scholar] [CrossRef] [PubMed]
- Kip, N.; Veen, J.A. The dual role of microbes in corrosion. ISME J. 2015, 9, 542–551. [Google Scholar] [CrossRef]
- Vigneron, A.; Alsop, E.B.; Chambers, B.; Lomans, B.P.; Head, I.M.; Tsesmetzis, N. Complementary microorganisms in highly corrosive biofilms from an offshore oil production facility. Appl. Environ. Microbiol. 2016, 82, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Knisz, J.; Eckert, R.; Gieg, L.M.; Koerdt, A.; Lee, J.S.; Silva, E.R.; Skovhus, T.L.; Stepec, B.A.A.; Wade, S.A. Microbiologically influenced corrosion–more than just microorganisms. FEMS Microbiol Rev. 2023, 47, fuad041. [Google Scholar] [CrossRef] [PubMed]
- Venzlaff, H.; Enning, D.; Srinivasan, J.; Mayrhofer, K.J.J.; Hassel, A.W.; Widdel, F.; Stratmann, M. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 2013, 66, 88–96. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Comparison of the photoautotrophic growth regimens of Chlorella sorokiniana in a photobioreactor for enhanced biomass productivity. Biology 2020, 9, 169. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Sagitov, I.I.; Akhmetova, R.F.; Saleeva, G.T.; Kiassov, A.P.; Gogoleva, N.E.; Shagimardanova, E.I.; Ziganshin, A.M. Comparison of the microbiota and inorganic anion content in the saliva of patients with gastroesophageal reflux disease and gastroesophageal reflux disease-free individuals. BioMed Res. Int. 2020, 2020, 2681791. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Assessment of Chlorella sorokiniana growth in anaerobic digester effluent. Plants 2021, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I. Isolation and purification of sulfate-reducing bacteria. In Microorganisms; IntechOpen: London, UK, 2020. [Google Scholar]
- De Oliveira, E.S.D.; da Costa Pereira, R.F.; de Melo, I.R.; de A. Lima, M.A.G.; Urtiga Filho, S.L. Corrosion behavior of API 5L X80 steel in the produced water of onshore oil recovery facilities. Mater. Res. 2017, 20, 432–439. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Neff, J.; Lee, K.; Deblois, E. Produced water: Overview of composition, fates, and effects. In Produced Water; Lee, K., Neff, J., Eds.; Springer: New York, NY, USA, 2011; pp. 3–54. [Google Scholar]
- Wang, X.; Xu, J.; Sun, C.; Yan, M. Effect of oilfield produced water on corrosion of pipeline. Int. J. Electrochem. Sci. 2015, 10, 8656–8667. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, X.; Hall, R.; Zhang, Y.; Carroll, K.C.; Ramos, F.; Engle, M.A.; Lin, L.; Wang, H.; Sayer, M.; et al. Characterization of produced water and surrounding surface water in the Permian Basin, the United States. J. Hazard. Mater. 2022, 430, 128409. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Jiang, W.; Xu, X.; Wang, H.; Carroll, K.C.; Xu, P.; Zhang, Y. Toxicological characterization of produced water from the Permian Basin. Sci. Total Environ. 2022, 815, 152943. [Google Scholar] [CrossRef]
- Ridley, C.M.; Voordouw, G. Aerobic microbial taxa dominate deep subsurface cores from the Alberta oil sands. FEMS Microbiol. Ecol. 2018, 94, fiy073. [Google Scholar] [CrossRef]
- Sierra-Garcia, I.N.; de Oliveira, V.M. Microbial hydrocarbon degradation: Efforts to understand biodegradation in petroleum reservoirs. In Biodegradation-Engineering and Technology; Chamy, R., Ed.; InTech: London, UK, 2013. [Google Scholar]
- Cai, M.; Nie, Y.; Chi, C.Q.; Tang, Y.Q.; Li, Y.; Wang, X.B.; Liu, Z.S.; Yang, Y.; Zhou, J.; Wu, X.L. Crude oil as a microbial seed bank with unexpected functional potentials. Sci. Rep. 2015, 5, 16057. [Google Scholar] [CrossRef]
- Jiao, Y.; An, L.; Wang, W.; Ma, J.; Wu, C.; Wu, X. Microbial communities and their roles in the Cenozoic sulfurous oil reservoirs in the Southwestern Qaidam Basin, Western China. Sci. Rep. 2023, 13, 7988. [Google Scholar] [CrossRef]
- Sokolova, D.S.; Semenova, E.M.; Grouzdev, D.S.; Bidzhieva, S.K.; Babich, T.L.; Loiko, N.G.; Ershov, A.P.; Kadnikov, V.V.; Beletsky, A.V.; Mardanov, A.V.; et al. Sulfidogenic microbial communities of the uzen high-temperature oil field in Kazakhstan. Microorganisms 2021, 9, 1818. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Ziganshin, A.M. Bacteria in the produced water and wastewater samples from the oil industry. E3S Web Conf. 2023, 462, accepted. [Google Scholar]
- Elumalai, P.; Parthipan, P.; AlSalhi, M.S.; Huang, M.; Devanesan, S.; Karthikeyan, O.P.; Kim, W.; Rajasekar, A. Characterization of crude oil degrading bacterial communities and their impact on biofilm formation. Environ. Pollut. 2021, 286, 117556. [Google Scholar] [CrossRef]
- Javed, M.A.; Stoddart, P.R.; Wade, S.A. Corrosion of carbon steel by sulphate reducing bacteria: Initial attachment and the role of ferrous ions. Corros. Sci. 2015, 93, 48–57. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yuan, J. Influence of produced water with high salinity and corrosion inhibitors on the corrosion of water injection pipe in Tuha oil field. Eng. Fail. Anal. 2014, 45, 225–233. [Google Scholar] [CrossRef]
- Latta, D.E.; Gorski, C.A.; Scherer, M.M. Influence of Fe2+-catalysed iron oxide recrystallization on metal cycling. Biochem. Soc. Trans. 2012, 40, 1191–1197. [Google Scholar] [CrossRef]
- Ziganshin, A.; Ziganshina, E.; Byrne, J.; Gerlach, R.; Struve, E.; Biktagirov, T.; Rodionov, A.; Kappler, A. Fe(III) mineral reduction followed by partial dissolution and reactive oxygen species generation during 2,4,6-trinitrotoluene transformation by the aerobic yeast Yarrowia lipolytica. AMB Express 2015, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Khilyas, I.V.; Ziganshin, A.M.; Pannier, A.J.; Gerlach, R. Effect of ferrihydrite on 2,4,6-trinitrotoluene biotransformation by an aerobic yeast. Biodegradation 2013, 2, 631–644. [Google Scholar] [CrossRef]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef]
- Kryachko, Y.; Dong, X.; Sensen, C.W.; Voordouw, G. Compositions of microbial communities associated with oil and water in a mesothermic oil field. Antonie Leeuwenhoek 2012, 101, 493–506. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Zhou, L.; Mbadinga, S.M.; Yang, S.; Gu, J.; Mu, B. Diversity and composition of sulfate-reducing microbial communities based on genomic DNA and RNA transcription in production water of high temperature and corrosive oil reservoir. Front. Microbiol. 2017, 8, 1011. [Google Scholar] [CrossRef]
- Varjani, S.J.; Gnansounou, E. Microbial dynamics in petroleum oilfields and their relationship with physiological properties of petroleum oil reservoirs. Bioresour. Technol. 2017, 245, 1258–1265. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Influence of granular activated carbon on anaerobic co-digestion of sugar beet pulp and distillers grains with solubles. Processes 2020, 8, 1226. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Impact of granular activated carbon on anaerobic process and microbial community structure during mesophilic and thermophilic anaerobic digestion of chicken manure. Sustainability 2022, 14, 447. [Google Scholar] [CrossRef]
- Kim, D.D.; O’Farrell, C.; Toth, C.R.A.; Montoya, O.; Gieg, L.M.; Kwon, T.H.; Yoon, S. Microbial community analyses of produced waters from high-temperature oil reservoirs reveal unexpected similarity between geographically distant oil reservoirs. Microb. Biotechnol. 2018, 11, 788–796. [Google Scholar] [CrossRef]
- Plugge, C.M.; Zhang, W.; Scholten, J.C.; Stams, A.J. Metabolic flexibility of sulfate-reducing bacteria. Front. Microbiol. 2011, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Little, B.J.; Lee, J.S. Microbiologically influenced corrosion. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley & Sons Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Cetin, D.; Aksu, M.L. Corrosion behavior of low-alloy steel in the presence of Desulfotomaculum sp. Corros. Sci. 2009, 51, 1584–1588. [Google Scholar] [CrossRef]
- Bermont-Bouis, D.; Janvier, M.; Grimont, P.A.; Dupont, I.; Vallaeys, T. Both sulfate-reducing bacteria and Enterobacteriaceae take part in marine biocorrosion of carbon steel. J. Appl. Microbiol. 2007, 102, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Philips, J.; Van den Driessche, N.; De Paepe, K.; Prévoteau, A.; Gralnick, J.A.; Arends, J.B.A.; Rabaey, K. A novel Shewanella isolate enhances corrosion by using metallic iron as the electron donor with fumarate as the electron acceptor. Appl. Environ. Microbiol. 2018, 84, e01154-18. [Google Scholar] [CrossRef] [PubMed]
- Salgar-Chaparro, S.J.; Machuca, L.L. Complementary DNA/RNA-based profiling: Characterization of corrosive microbial communities and their functional profiles in an oil production facility. Front. Microbiol. 2019, 10, 2587. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Mulky, L. Microbially influenced corrosion and its control measures: A critical review. J. Bio-Tribo-Corros. 2023, 9, 57. [Google Scholar] [CrossRef]
- Mukherjee, M.; Zaiden, N.; Teng, A.; Hu, Y.; Cao, B. Shewanella biofilm development and engineering for environmental and bioenergy applications. Curr. Opin. Chem. Biol. 2020, 59, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, D.; Liu, T.; Zhang, S.; Wang, W.; Yan, L.; Gu, J.D. Growth and genome-based insights of Fe(III) reduction of the high-temperature and NaCl-tolerant Shewanella xiamenensis from Changqing oilfield of China. Front. Microbiol. 2022, 13, 1028030. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.M.; Costa, J.M.; Braga, J.K.; Flynn, T.M.; Brucha, G.; Sancinetti, G.P.; Rodriguez, R.P. Lactate as an effective electron donor in the sulfate reduction: Impacts on the microbial diversity. Environ. Technol. 2021, 43, 3149–3160. [Google Scholar] [CrossRef] [PubMed]
- Magot, M.; Ravot, G.; Campaignolle, X.; Ollivier, B.; Patel, B.K.; Fardeau, M.L.; Thomas, P.; Crolet, J.L.; Garcia, J.L. Dethiosulfovibrio peptidovorans gen. nov., sp. nov., a new anaerobic, slightly halophilic, thiosulfate-reducing bacterium from corroding offshore oil wells. Int. J. Syst. Bacteriol. 1997, 47, 818–824. [Google Scholar] [CrossRef]
- Whitman, W.B. Dethiosulfovibrio. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons Inc.: New York, NY, USA, 2015; pp. 1–4. [Google Scholar]
- Phan, H.C.; Wade, S.A.; Blackall, L.L. Identification of microbes isolated with test kits through culture-dependent and metabarcoding techniques for assessment of microbial corrosion. Curr. Res. Biotechnol. 2022, 4, 129–137. [Google Scholar] [CrossRef]
- Surkov, A.V.; Dubinina, G.A.; Lysenko, A.M.; Glockner, F.O.; Kuever, J. Dethiosulfovibrio russensis sp. nov., Dethisulfovibrio marinus sp. nov. and Dethiosulfovibrio acidaminovorans sp. nov., novel anaerobic thiosulfate- and sulfur-reducing bacteria isolated from “Thiodendron” sulfur mats in different saline environments. Int. J. Syst. Evol. Microbiol. 2001, 51, 327–337. [Google Scholar] [CrossRef]
- Dutra, J.; Garcia, G.; Gomes, R.; Cardoso, M.; Cortes, A.; Silva, T.; Jesus, L.; Rodrigues, L.; Freitas, A.; Waldow, V.; et al. Effective biocorrosive control in oil industry facilities: 16S rRNA gene metabarcoding for monitoring microbial communities in produced water. Microorganisms 2023, 11, 846. [Google Scholar] [CrossRef]
- Qian, Y.; Xu, M.; Deng, T.; Hu, W.; He, Z.; Yang, X.; Wang, B.; Song, D.; Chen, L.; Huang, Y.; et al. Synergistic interactions of Desulfovibrio and Petrimonas for sulfate-reduction coupling polycyclic aromatic hydrocarbon degradation. J. Hazard. Mater. 2021, 407, 124385. [Google Scholar] [CrossRef]
- Grabowski, A.; Tindall, B.J.; Bardin, V.; Blanchet, D.; Jeanthon, C. Petrimonas sulfuriphila gen. nov., sp. nov., a mesophilic fermentative bacterium isolated from a biodegraded oil reservoir. Int. J. Syst. Evol. Microbiol. 2005, 55, 1113–1121. [Google Scholar] [CrossRef]
- Ravot, G.; Magot, M.; Fardeau, M.L.; Patel, B.; Thomas, P.; Garcia, J.L.; Ollivier, B. Fusibacter paucivorans gen. nov., sp. nov., an anaerobic, thiosulfate-reducing bacterium from an oil-producing well. Int. J. Syst. Evol. Microbiol. 1999, 49, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Rajbongshi, A.; Gogoi, S.B. A review on anaerobic microorganisms isolated from oil reservoirs. World J. Microbiol. Biotechnol. 2021, 37, 111. [Google Scholar] [CrossRef] [PubMed]
- Dahle, H.; Birkeland, N.K. Thermovirga lienii gen. nov., sp. nov., a novel moderately thermophilic, anaerobic, amino-acid-degrading bacterium isolated from a North Sea oil well. Int. J. Syst. Evol. Microbiol. 2006, 56, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
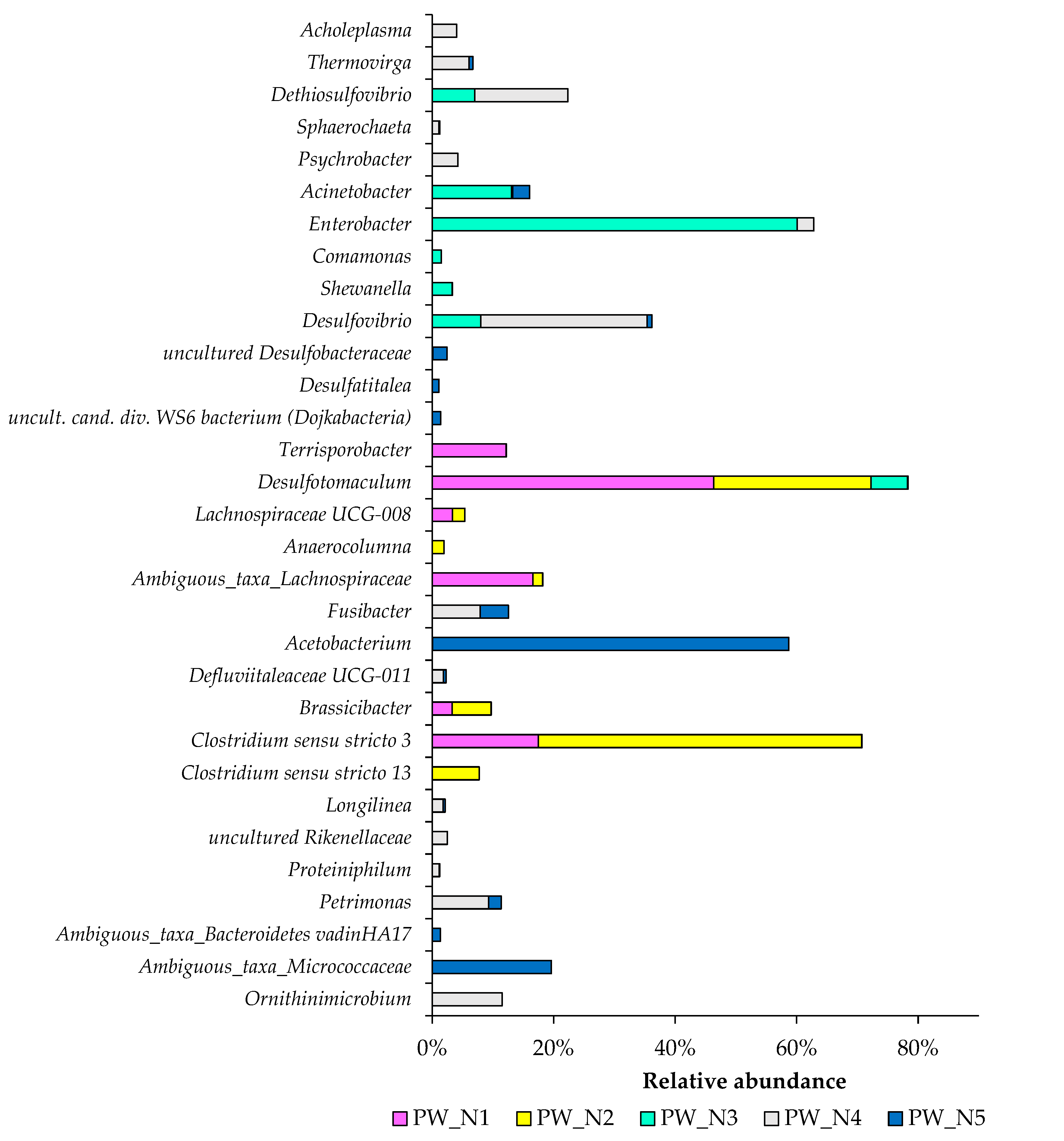
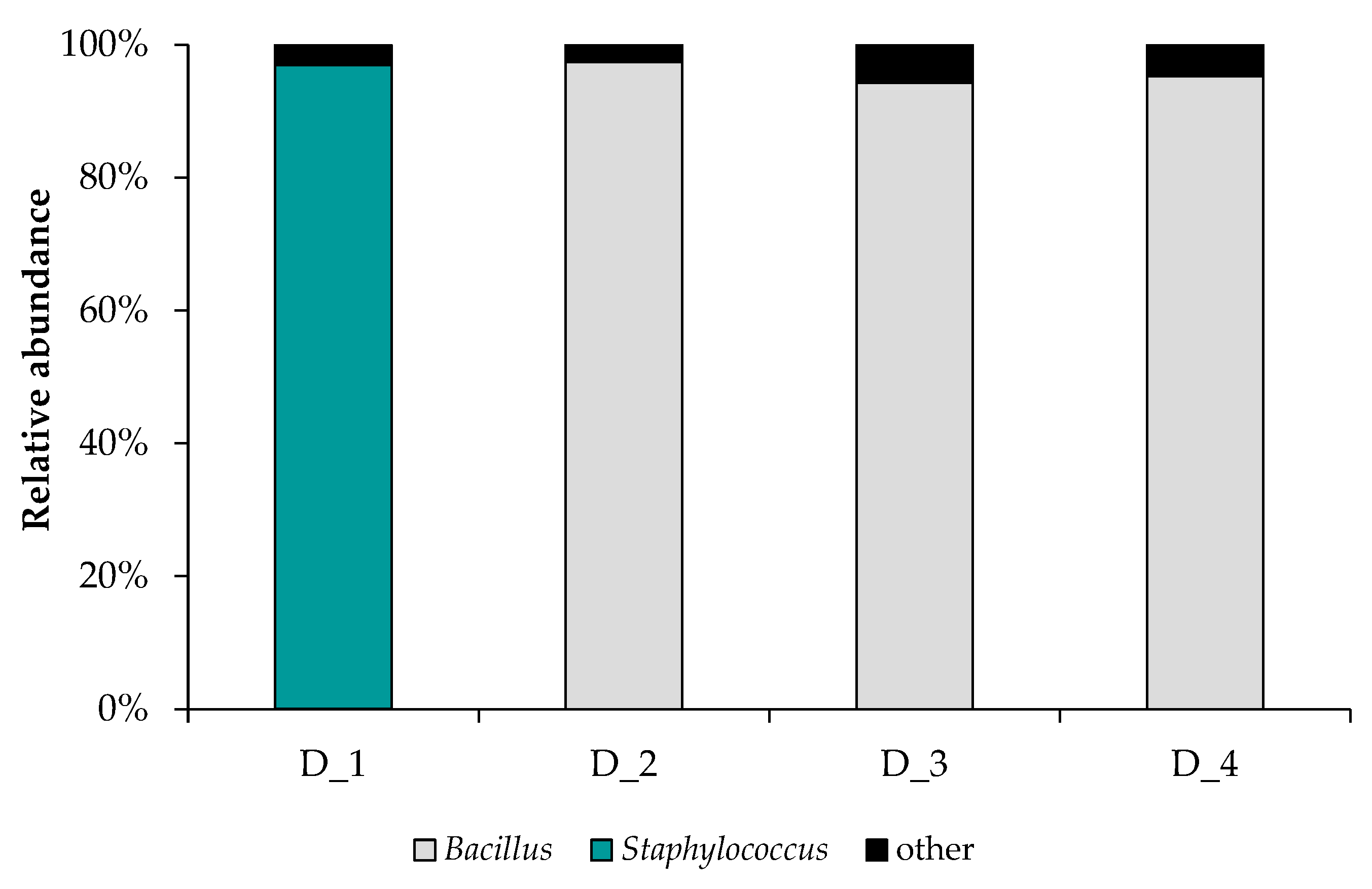
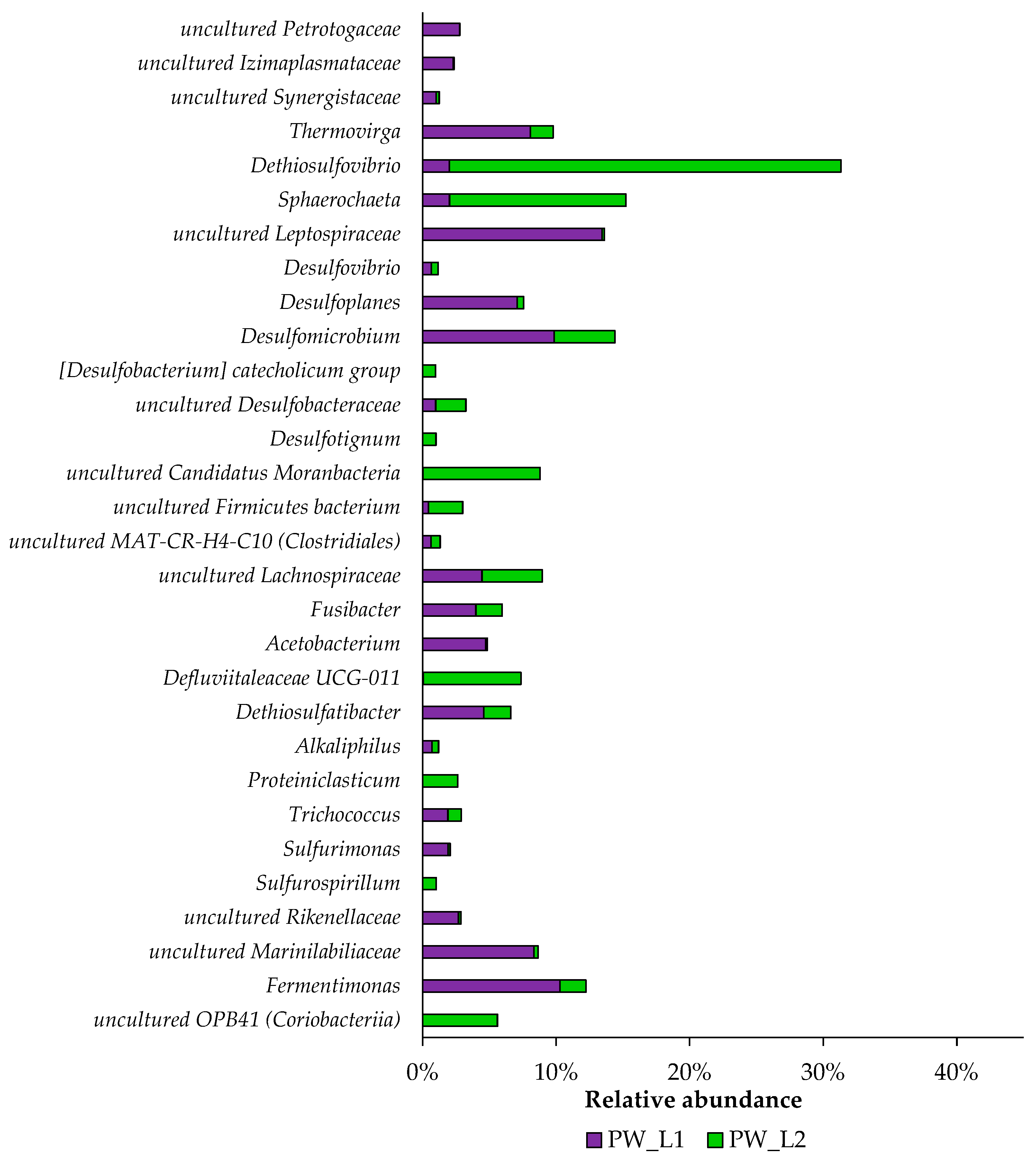
| Sample | OTUs | Chao1 | Shannon | Simpson | PD Whole Tree | Fisher’s Alpha |
|---|---|---|---|---|---|---|
| PW_N1 | 47 | 54 | 2.56 | 0.73 | 3.55 | 5.34 |
| PW_N2 | 47 | 47 | 2.09 | 0.64 | 3.90 | 4.95 |
| PW_N3 | 49 | 51 | 1.81 | 0.39 | 4.35 | 5.28 |
| PW_N4 | 79 | 80 | 3.68 | 0.87 | 7.32 | 9.11 |
| PW_N5 | 77 | 78 | 2.41 | 0.62 | 7.20 | 8.88 |
| Sample | OTUs | Chao1 | Shannon | Simpson | PD Whole Tree | Fisher’s Alpha |
|---|---|---|---|---|---|---|
| D_1 | 41 | 53 | 0.66 | 0.15 | 3.14 | 4.12 |
| D_2 | 72 | 96 | 0.76 | 0.19 | 3.49 | 7.75 |
| D_3 | 74 | 85 | 0.80 | 0.19 | 3.61 | 7.93 |
| D_4 | 56 | 86 | 1.09 | 0.36 | 3.72 | 5.72 |
| Sample | OTUs | Chao1 | Shannon | Simpson | PD Whole Tree | Fisher’s Alpha |
|---|---|---|---|---|---|---|
| PW_L1 | 108 | 114 | 4.16 | 0.87 | 7.28 | 12.30 |
| PW_L2 | 125 | 133 | 4.02 | 0.88 | 8.76 | 14.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziganshina, E.E.; Mohammed, W.S.; Ziganshin, A.M. Microbial Diversity of the Produced Waters from the Oilfields in the Republic of Tatarstan (Russian Federation): Participation in Biocorrosion. Appl. Sci. 2023, 13, 12984. https://doi.org/10.3390/app132412984
Ziganshina EE, Mohammed WS, Ziganshin AM. Microbial Diversity of the Produced Waters from the Oilfields in the Republic of Tatarstan (Russian Federation): Participation in Biocorrosion. Applied Sciences. 2023; 13(24):12984. https://doi.org/10.3390/app132412984
Chicago/Turabian StyleZiganshina, Elvira E., Waleed S. Mohammed, and Ayrat M. Ziganshin. 2023. "Microbial Diversity of the Produced Waters from the Oilfields in the Republic of Tatarstan (Russian Federation): Participation in Biocorrosion" Applied Sciences 13, no. 24: 12984. https://doi.org/10.3390/app132412984
APA StyleZiganshina, E. E., Mohammed, W. S., & Ziganshin, A. M. (2023). Microbial Diversity of the Produced Waters from the Oilfields in the Republic of Tatarstan (Russian Federation): Participation in Biocorrosion. Applied Sciences, 13(24), 12984. https://doi.org/10.3390/app132412984






