A Microalgae Photobioreactor System for Indoor Air Remediation: Empirical Examination of the CO2 Absorption Performance of Spirulina maxima in a NaHCO3-Reduced Medium
Abstract
:1. Introduction
1.1. Background Problems in Indoor CO2 Mitigation
1.2. Microalgae-Based Air Remediation
1.3. Research Question: Spirulina for CO2 Removal and NaHCO3-Reduced Cultivation
1.4. Study Objectives
2. Materials and Methods
2.1. Material and Culture Preparation: Algal Strain, Growth Medium, and Inoculum
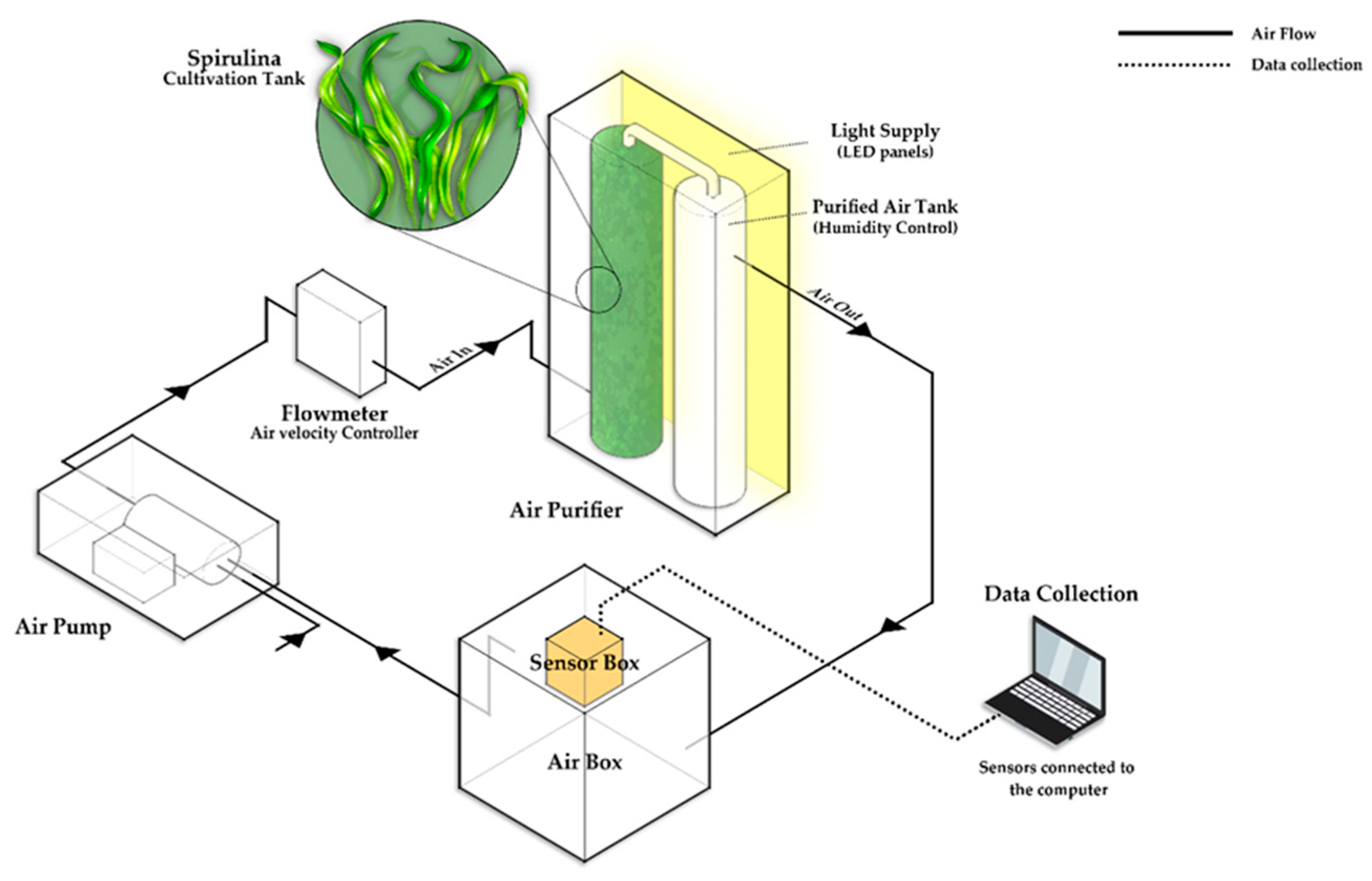
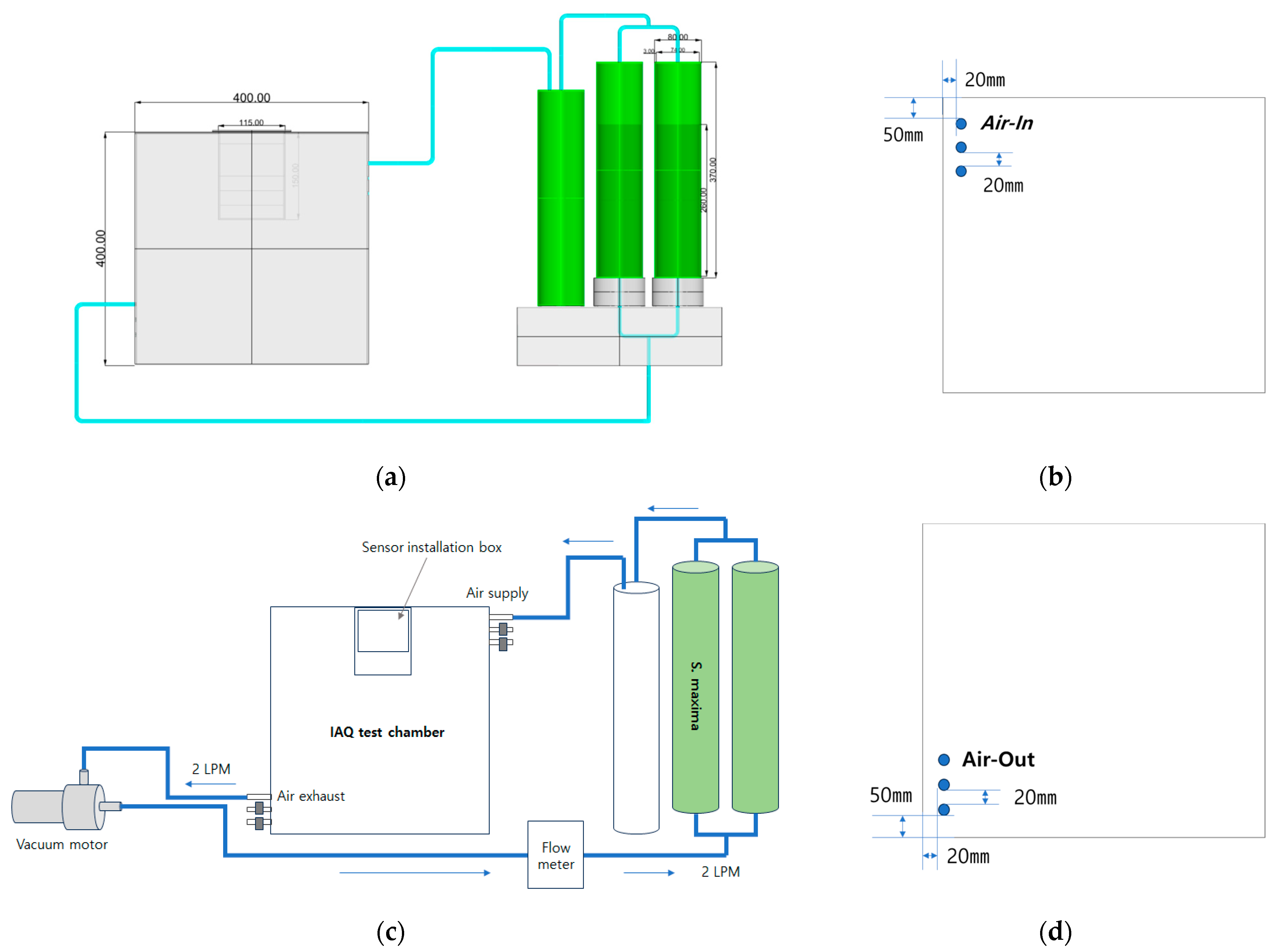
2.2. Equipment Development: Design and Fabrication of a Purification System
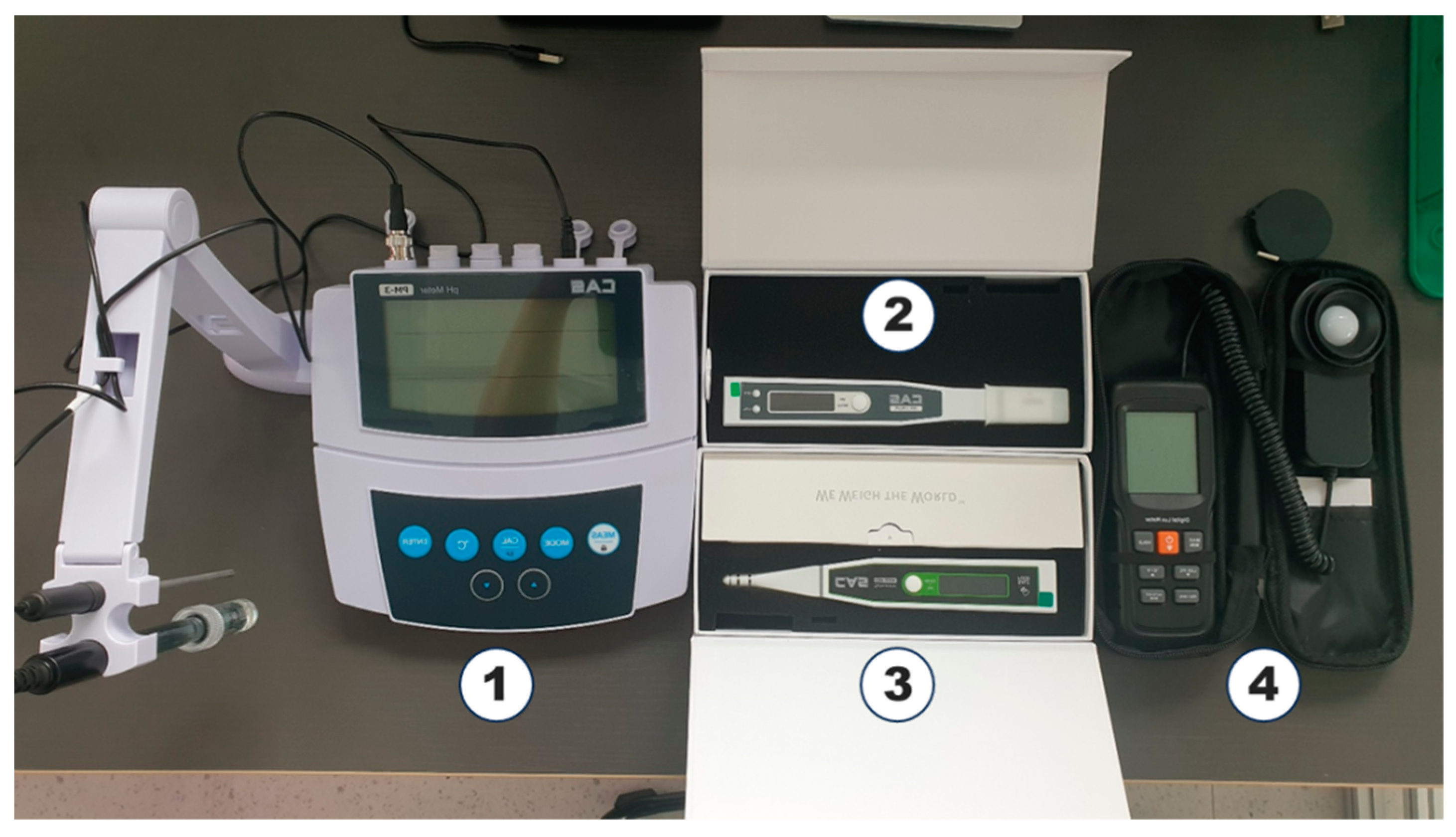
2.3. Experiment Procedure and Measurement Methods
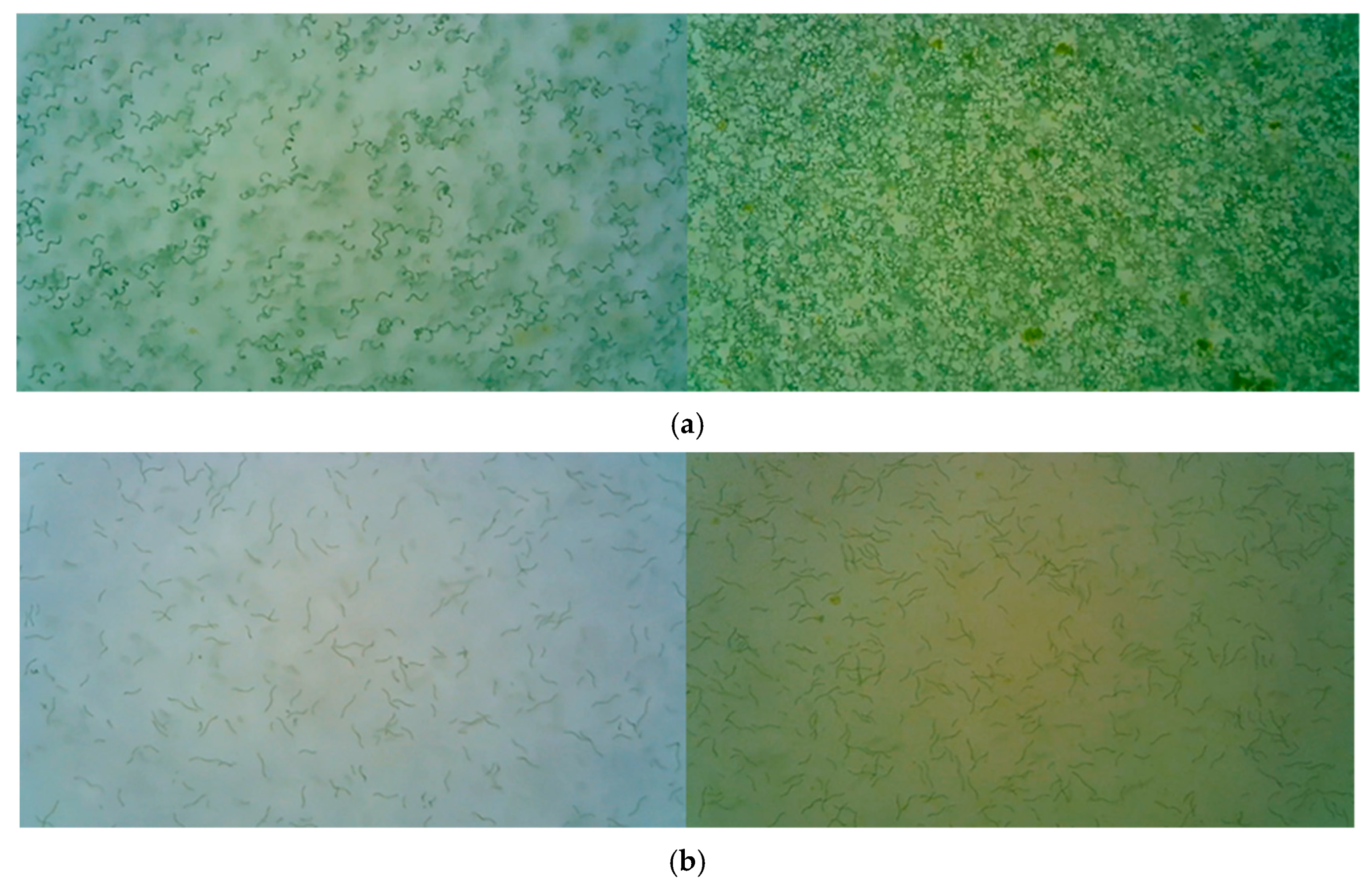
2.4. Culture Treatment, Observation, and Data Analysis
3. Results and Discussion
3.1. Examination of Culture Media Recipes
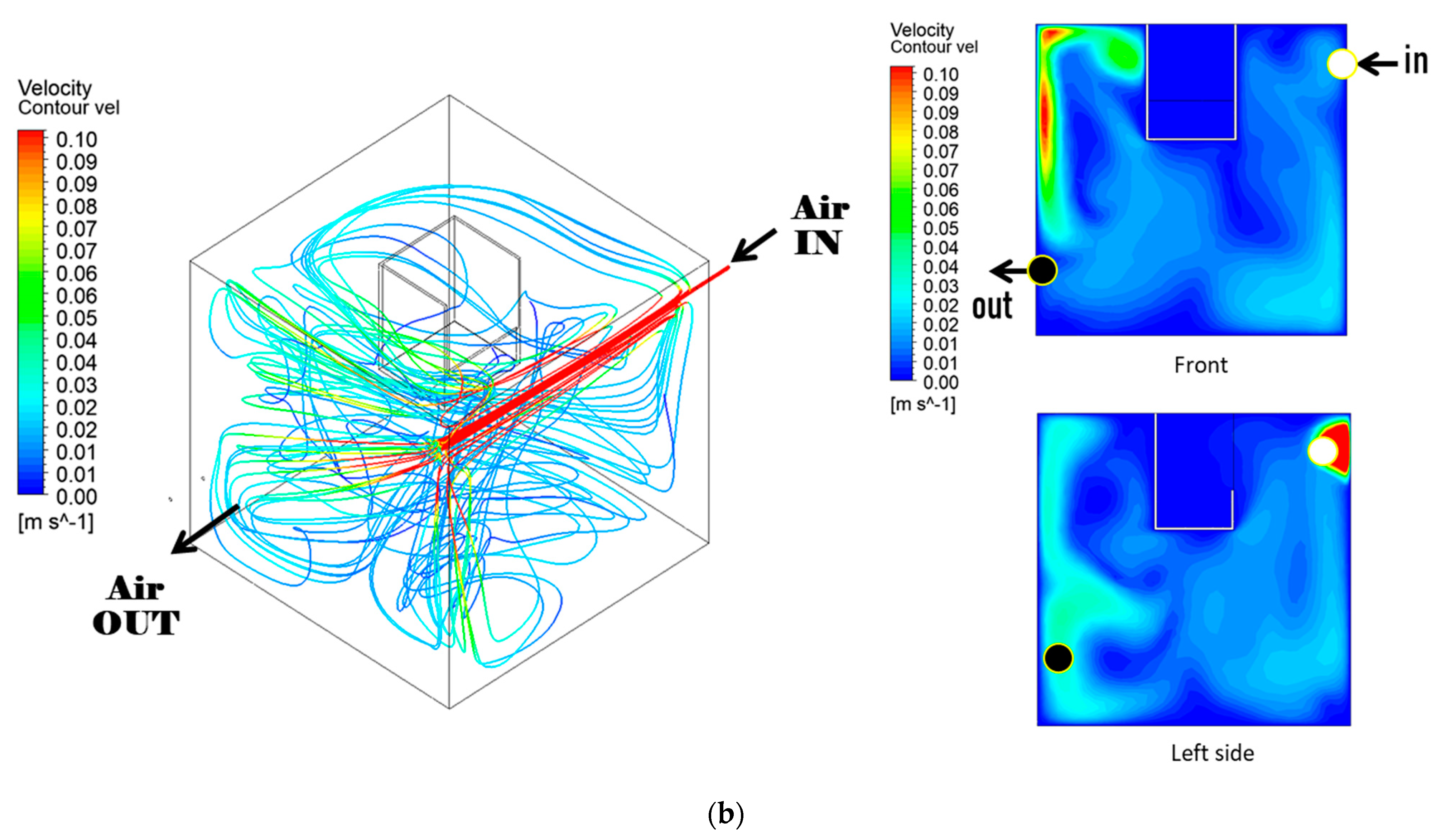
3.2. Air Dispersion in the Chamber
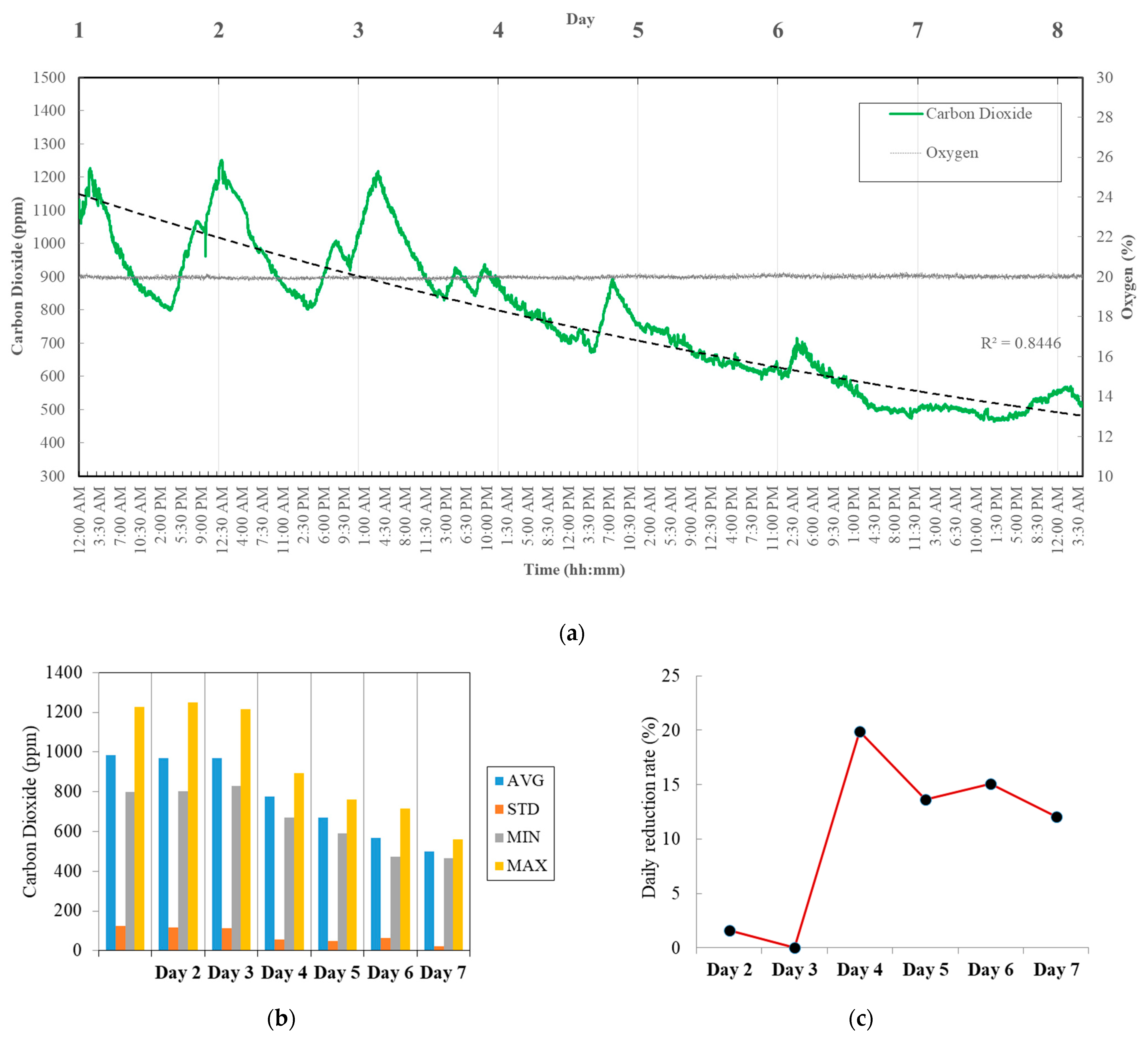
3.3. Air Purification Performance
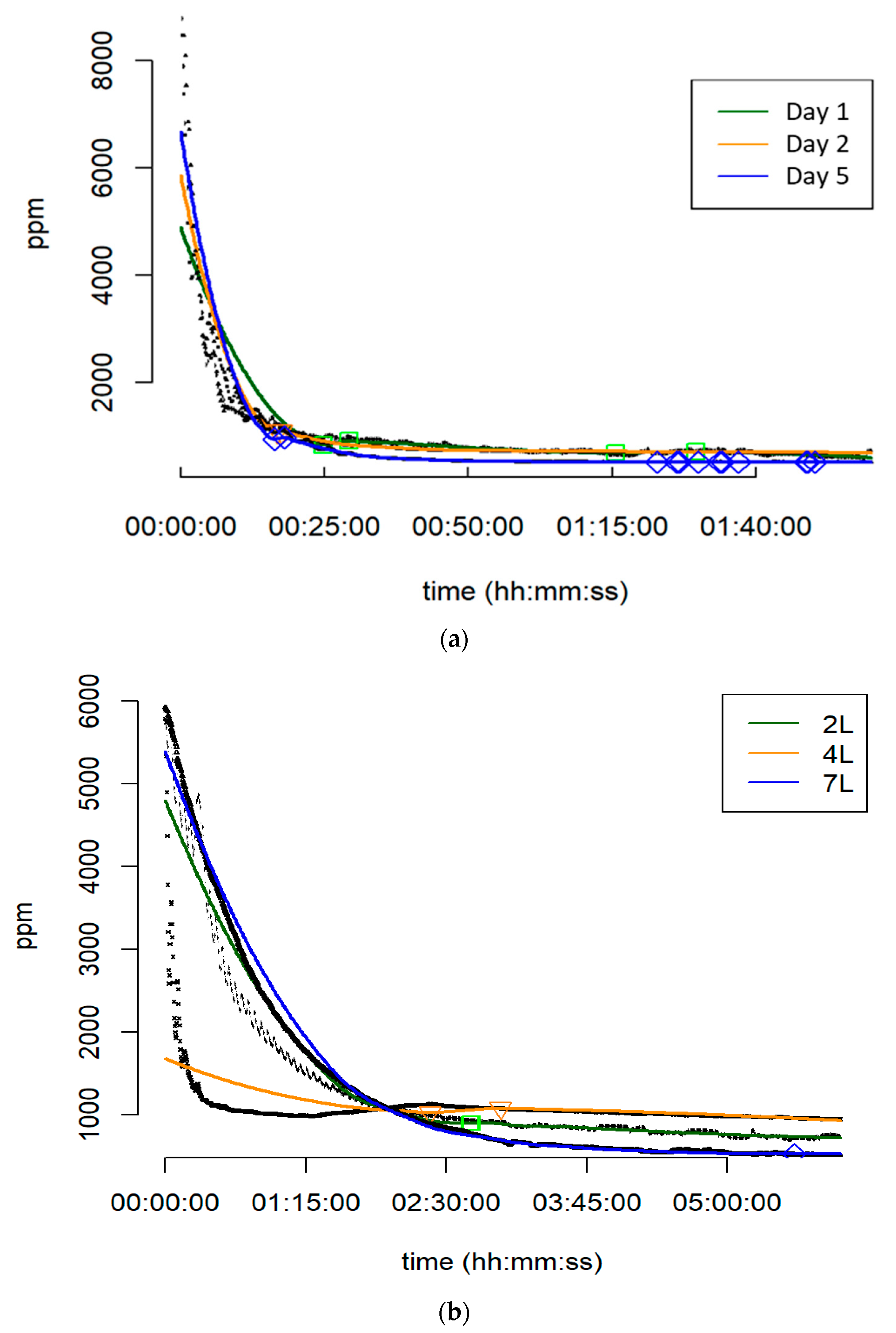
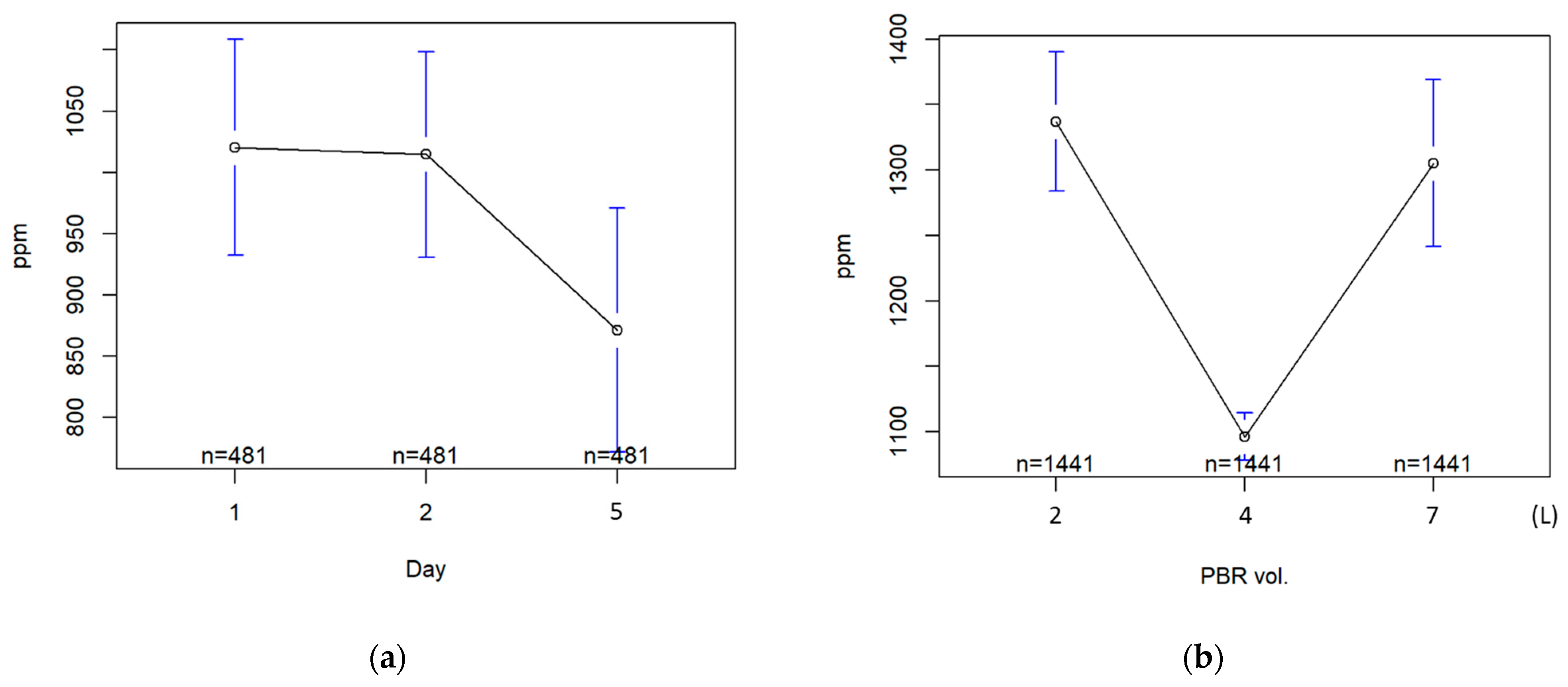
3.4. Performance of Individual PBRs under High-Grade CO2
3.5. Study Limitations and Further Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Fayad, M.A.; Al-Ogaidi, B.R.; Abood, M.K.; Al-Salihi, H.A. Influence of post-injection strategies and CeO2 nanoparticles additives in the C30D blends and diesel on engine performance, NOX emissions, and PM characteristics in diesel engine. Part. Sci. Technol. 2022, 40, 824–837. [Google Scholar] [CrossRef]
- Klepeis, N.; Nelson, W.; Ott, W.; Robinson, J.; Tsang, A.; Switzer, P.; Behar, J.; Hern, S.; Engelmann, W. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. Available online: https://escholarship.org/uc/item/1zg3q68x (accessed on 14 March 2023). [CrossRef] [PubMed]
- Persily, A. Development and application of an indoor carbon dioxide metric. Indoor Air 2022, 32, e13059. [Google Scholar] [CrossRef] [PubMed]
- Blocken, B.; van Druenen, T.; Ricci, A.; Kang, L.; van Hooff, T.; Qin, P.; Xia, L.; Ruiz, C.A.; Arts, J.; Diepens, J.; et al. Ventilation and air cleaning to limit aerosol particle concentrations in a gym during the COVID-19 pandemic. Build. Environ. 2021, 193, 107659. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.; Poppendieck, D.; Dols, W.; Emmerich, S. Evaluating Indoor Air Quality and Energy Impacts of Ventilation in a Net-Zero Energy House Using a Coupled Model. Sci. Technol. Built Environ. 2018, 24, 124–134. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Mata, T.M.; Oliveira, G.M.; Monteiro, H.; Silva, G.V.; Caetano, N.S.; Martins, A.A. Indoor Air Quality Improvement Using Nature-Based Solutions: Design Proposals to Greener Cities. Int. J. Environ. Res. Public Health 2021, 18, 8472. [Google Scholar] [CrossRef]
- Hinkov, I.; Lamari, F.D.; Langlois, P.; Dicko, M.; Chilev, C.; Pentchev, I. Carbon Dioxide Capture by Absorption (Review). J. Chem. Technol. Metall. 2016, 51, 609–626. [Google Scholar]
- Fang, M.; Xiang, Q.; Zhou, X.; Ma, Q.; Luo, Z. Experimental Study on CO2 Absorption into Aqueous Ammonia-based Blended Absorbents. Energy Procedia 2014, 61, 2284–2288. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lin, H.-T. Removal of Carbon Dioxide from Indoor Air Using a Cross-Flow Rotating Packed Bed. Energy Procedia 2013, 37, 1187–1193. [Google Scholar] [CrossRef]
- Hu, S.-C.; Shiue, A.; Chang, S.-M.; Chang, Y.-T.; Tseng, C.-T.; Mao, C.-C.; Hsieh, A.; Chan, A. Removal of carbon dioxide in the indoor environment with sorption-type air filters. Int. J. Low-Carbon Technol. 2017, 12, 330–334. [Google Scholar] [CrossRef]
- Ito, T.; Otani, Y.; Namiki, N. Electrostatic Separation of Carbon Dioxide by Ionization in Bifurcation Flow. Aerosol Air Qual. Res. 2014, 4, 91–104. [Google Scholar] [CrossRef]
- da Rosa, G.M.; Moraes, L.; Cardias, B.B.; de Souza, M.R.A.Z.; Costa, J.A.V. Chemical absorption and CO2 biofixation via the cultivation of Spirulina in semicontinuous mode with nutrient recycle. Bioresour. Technol. 2015, 192, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Ghalkhani, M.; Dehkordi, Z.S.; Singh, J.; Wen, Y.; Baghayeri, M.; Rouhi, J.; Fu, L.; Rajendran, S. Mof-enabled pesticides as developing approach for sustainable agriculture and reducing environmental hazards. J. Ind. Eng. Chem. 2023, in press. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Teymourinia, H.; Ebrahimi, F.; Irannejad, N.; Karimi-Maleh, H.; Karaman, C.; Karimi, F.; Dragoi, E.N.; Lichtfouse, E.; Singh, J. Engineering and application of polysaccharides and proteins-based nanobiocatalysts in the recovery of toxic metals, phosphorous, and ammonia from wastewater: A review. Int. J. Biol. Macromol. 2023, 242, 124585. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Zaidi, S.J.; Ismail, A.F.; Goh, P.S. Advances of nanomaterials for air pollution remediation and their impacts on the environment. Chemosphere 2022, 287, 132083. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, G.; Igalavithana, A.D.; He, W.; Gao, B.; Tsang, D.C.; Ok, Y.S. Effects of elevated CO2 on the phytoremediation efficiency of Noccaea caerulescens. Environ. Pollut. 2019, 255, 113169. [Google Scholar] [CrossRef]
- Govindaraju, M.; Fowmitha Banu, J.; Senthamil Selvi, S.; Goel, M. CO2 Sequestration Through Phytoremediation Techniques with Special Emphasis on Urban Forestry to Mitigate Climate Change Impact. In Climate Change and Green Chemistry of CO2 Sequestration. Green Energy and Technology; Goel, M., Satyanarayana, T., Sudhakar, M., Agrawal, D.P., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Ravindra, K.; Mor, S. Phytoremediation potential of indoor plants in reducing air pollutants. Front. Sustain. Cities 2022, 4, 1039710. [Google Scholar] [CrossRef]
- Thomas, C.K.; Kim, K.J.; Kays, S.J. Phytoremediation of Indoor Air. HortScience 2015, 50, 765–768. [Google Scholar] [CrossRef]
- Thongsanit, P.; Ananpattarachai, J.; Sonklin, W.; Techowanich, A.; Jewyou, P.; Kooktaisong, A. Efficiency of Snake Plants Absorb Carbon Dioxide in Offices. In Proceedings of the International Conference on Chemical, Metallurgy and Material Science Engineering (CMMSE-2015), Pattaya, Thailand, 10–11 August 2015; pp. 16–20. [Google Scholar] [CrossRef]
- Suhaimi, M.M.; Leman, A.M.; Safii, H. Indoor Plants as Agents Deterioration of Gas Pollutants. ARPN J. Eng. Appl. Sci. 2016, 11, 10944–10949. [Google Scholar]
- Sharma, S.; Bakht, A.; Jahanzaib, M.; Lee, H.; Park, D. Evaluation of the Effectiveness of Common Indoor Plants in Improving the Indoor Air Quality of Studio Apartments. Atmosphere 2022, 13, 1863. [Google Scholar] [CrossRef]
- Irga, P.J.; Tropy, F.R.; Burchett, M.D. Can hydroculture be used to enhance the performance of indoor plants for the removal of air pollutants? Atmos. Environ. 2013, 77, 267–271. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Calheiros, C.S.C.; Villanueva, F.; Alonso-Cuevilla, N.P.; Gabriel, M.F.; Silva, G.V. Indoor Air Quality: A Review of Cleaning Technologies. Environments 2022, 9, 118. [Google Scholar] [CrossRef]
- Yewale, P.; Wagle, N.; Lenka, S.; Bannigol, P.; Junnarkar, M.; Prakash, D.; Mandal, A.; Stigh, C.; Sahasrabudhe, T.; Vannalwar, T.; et al. Studies on Biosmotrap: A multipurpose biological air purifier to minimize indoor and outdoor air pollution. J. Clean. Prod. 2022, 357, 132001. [Google Scholar] [CrossRef]
- Sudhakar, K.; Suresh, S.; Premalatha, M. An Overview of CO2 Mitigation Using Algae Cultivation Technology. Int. J. Chem. Res. 2011, 3, 110–117. Available online: http://www.bioinfo.in/contents.php?id=23 (accessed on 3 April 2023).
- Iglina, T.; Iglin, P.; Pashchenko, D. Industrial CO2 Capture by Algae: A Review and Recent Advances. Sustainability 2022, 14, 3801. [Google Scholar] [CrossRef]
- Miranda, A.M.; Hernandez-Tenorio, F.; Ocampo, D.; Vargas, G.J.; Sáez, A.A. Trends on CO2 Capture with Microalgae: A Bibliometric Analysis. Molecules 2022, 27, 4669. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of CO2 concentration on algal growth: A review. Renew. Sustain. Energy Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Suali, E.; Sarbatly, R. Conversion of Microalgae to Biofuel. Renew. Sustain. Energy Rev. 2012, 16, 4316–4342. [Google Scholar] [CrossRef]
- German Power Plant Testing CO2-Scrubbing Algae, 2010, Space Daily. Available online: https://www.spacedaily.com/reports/German_power_plant_testing_CO2-scrubbing_algae_999.html (accessed on 28 March 2023).
- Clarens, A.F.; Resurrection, E.P.; White, M.A.; Colosi, L.M. Environmental Life Cycle Comparison of Algae to Other Bioenergy Feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef]
- The Aerium 3.0, AlgenAir. Available online: https://algenair.com (accessed on 15 March 2023).
- LIQUID3: Urban Photo-Bioreactor. Available online: https://liquid3.rs (accessed on 15 March 2023).
- de Leon, S.P. Hypergiant Is Using AI And Algae To Take on Climate Change, 23 January 2020, Forbes. Available online: https://www.forbes.com/sites/cognitiveworld/2020/01/23/hypergiant-ai-algae-climate-change/?sh=1a5a8bb728c3 (accessed on 15 March 2023).
- Wang, W.N.; Soulis, J.; Yang, Y.J.; Biswas, P. Comparison of CO2 Photoreduction Systems: A Review. Aerosol Air Qual. Res. 2014, 14, 533–549. [Google Scholar] [CrossRef]
- Klinthong, W.; Yang, Y.-H.; Huang, C.-H.; Tan, C.-S. A Review: Microalgae and Their Applications in CO2 Capture and Renewable Energy. Aerosol Air Qual. Res. 2015, 15, 712–742. [Google Scholar] [CrossRef]
- Zhu, B.; Shen, H.; Li, Y.; Liu, Q.; Jin, G.; Han, J.; Zhao, Y.; Pan, K. Large-Scale Cultivation of Spirulina for Biological CO2 Mitigation in Open Raceway Ponds Using Purified CO2 from a Coal Chemical Flue Gas. Front. Bioeng. Biotechnol. 2020, 7, 441. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Lim, J.S.M.; Chong, C.C.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Foo, H.C.W.; Show, P.L.; Lim, S. Unravelling CO2 capture performance of microalgae cultivation and other technologies via comparative carbon balance analysis. J. Environ. Chem. Eng. 2021, 9, 106519. [Google Scholar] [CrossRef]
- de Morais, M.G.; Costa, J.A.V. Carbon Dioxide Fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. Cultivated in Flasks and Vertical Tubular Photobioreactors. Biotechnol. Lett. 2007, 29, 1349–1352. [Google Scholar] [CrossRef]
- Cheng, I.; Zhang, I.; Chen, H.; Gao, C. Carbon Dioxide Removal from Air by Microalgae Cultured in a Membrane-Photobioreactor. Sep. Purif. Technol. 2006, 50, 324–329. [Google Scholar] [CrossRef]
- Voskian, S.; Hatton, T.A. Faradaic electro-swing reactive adsorption for CO2 capture. Energy Environ. Sci. 2019, 12, 3530–3547. [Google Scholar] [CrossRef]
- Sydney, E.B.; Sturm, W.; de Carvalho, J.C.; Thomaz-Soccol, V.; Larroche, C.; Pandey, A.; Soccol, C.R. Potential carbon dioxide fixation by industrially important microalgae. Bioresour. Technol. 2010, 101, 5892–5896. [Google Scholar] [CrossRef]
- Margarites, A.C.; Volpato, N.; Araújo, E.; Cardoso, L.G.; Bertolin, T.E.; Colla, L.M.; Costa, J.A.V. Spirulina platensis is more efficient than Chlorella homosphaera in carbohydrate productivity. Environ. Technol. 2017, 38, 2209–2216. [Google Scholar] [CrossRef]
- Shabani, M.; Sayadi, M.H.; Rezaei, M.R. CO2 bio-sequestration by Chlorella vulgaris and Spirulina platensis in response to different levels of salinity and CO2. Proc. Int. Acad. Ecol. Environ. Sci. 2016, 6, 53–61. [Google Scholar]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Comparative study on the growth performance of Spirulina platensis on modifying culture media. Energy Rep. 2019, 5, 327–336. [Google Scholar] [CrossRef]
- Jung, C.H.G.; Braune, S.; Waldeck, P.; Küpper, J.-H.; Petrick, I.; Jung, F. Morphology and Growth of Arthrospira platensis during Cultivation in a Flat-Type Bioreactor. Life 2021, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Lamtham, S.; Sornchai, P. Biofixation of CO2 from a power plant through large-scale cultivation of Spirulina maxima. South Afr. J. Bot. 2022, 147, 840–851. [Google Scholar] [CrossRef]
- Ogawa, T.; Terui, G. Studies on the growth of Spirulina platensis. (I) On the pure culture of Spirulina platensis. J. Ferment. Technol. 1970, 48, 361–367. [Google Scholar]
- Kantarci, N.; Borak, F.; Ulgen, K.O. Bubble column reactors. Process Biochem. 2005, 40, 2263–2283. [Google Scholar] [CrossRef]
- Pimolrat, P.; Direkbusarakom, S.; Chinajariyawong, C.; Powtongsook, S. The effect of sodium bicarbonate concentrations on growth and biochemical composition of Chaetoceros gracilis Schutt. Kasetsart Univ. Fish. Res. Bull. 2010, 34, 40–47. [Google Scholar]
- Korea Research Institute of Bioscience and Biotechnology. The Medium Composition for Optimum Growth and Maximum Biomass of Spirulina genus. Korean Patent KR1020040065536A, 2006. Available online: https://patents.google.com/patent/KR100622025B1/ko (accessed on 29 August 2022).
- Peng, X.; Liu, S.; Zhang, W.; Zhao, Y.; Chen, L.; Wang, H.; Liu, T. Triacylglycerol accumulation of Phaeodactylum tricornutum with different supply of inorganic carbon. J. Appl. Phycol. 2014, 26, 131–139. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Ferreira, L.S.; Converti, A.; Sato, S.; Carvalho, J.C.M. Fed-batch cultivation of Arthrospira (Spirulina) platensis: Potassium nitrate and ammonium chloride as simultaneous nitrogen sources. Bioresour. Technol. 2010, 101, 4491–4498. [Google Scholar] [CrossRef]
- Aishvarya, V.; Pradhan, N.; Nayak, R.R.; Sukla, L.B.; Mishra, B.K. Enhanced inorganic carbon uptake by Chlorella sp. IMMTCC-2 under autotrophic conditions for lipid production and CO2 sequestration. J. Appl. Phycol. 2012, 24, 1455–1463. Available online: https://grow-organic-spirulina.com/product-category/live-spirulina-culture/ (accessed on 9 March 2023). [CrossRef]
- Monteir, M.P. da C.; Luchese, R.H.; Absher, T.M. Effect of Three Different Types of Culture Conditions. Arch. Biol. Technol. 2010, 53, 369–373. [Google Scholar] [CrossRef]
- Mokashi, K.; Shetty, V.; George, S.A.; Sibi, G. Sodium Bicarbonate as Inorganic Carbon Source for Higher Biomass and Lipid Production Integrated Carbon Capture in Chlorella vulgaris. Achiev. Life Sci. 2016, 10, 111–117. [Google Scholar] [CrossRef]
- Soletto, D.; Binaghi, L.; Ferrari, L.; Lodi, A.; Carvalho, J.C.M.; Zilli, M.; Converti, A. Effects of carbon dioxide feeding rate and light intensity on the fed-batch pulse-feeding cultivation of Spirulina platensis in helical photobioreactor. Biochem. Eng. J. 2008, 39, 369–375. [Google Scholar] [CrossRef]
- Wati, A.; Rusva, R.; Umar, L. Effect of LED Wavelengths and Light-Dark Cycle on Photosynthetic Production of Chlorella Kessleri for AlgaeBased Biosensor Optimization. J. Phys. Conf. Ser. 2019, 1351, 012003. [Google Scholar] [CrossRef]
- Bialevich, V.; Zachleder, V.; Bišová, K. The Effect of Variable Light Source and Light Intensity on the Growth of Three Algal Species. Cells 2022, 11, 1293. [Google Scholar] [CrossRef] [PubMed]
- Pe’rez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; Mata, T.M.; Martins, A.A.; Freitas, M.A.; Caetano, N.S. Economic analysis of microalgae biodiesel production in a small-scale facility. Energy Rep. 2020, 6, 325–332. [Google Scholar] [CrossRef]
- Llamas, B.; Suárez-Rodríguez, M.C.; González-López, C.V.; Mora, P.; Gabriel Acién, F. Techno-economic analysis of microalgae related processes for CO2 bio-fixation. Algal Res. 2021, 57, 102339. [Google Scholar] [CrossRef]




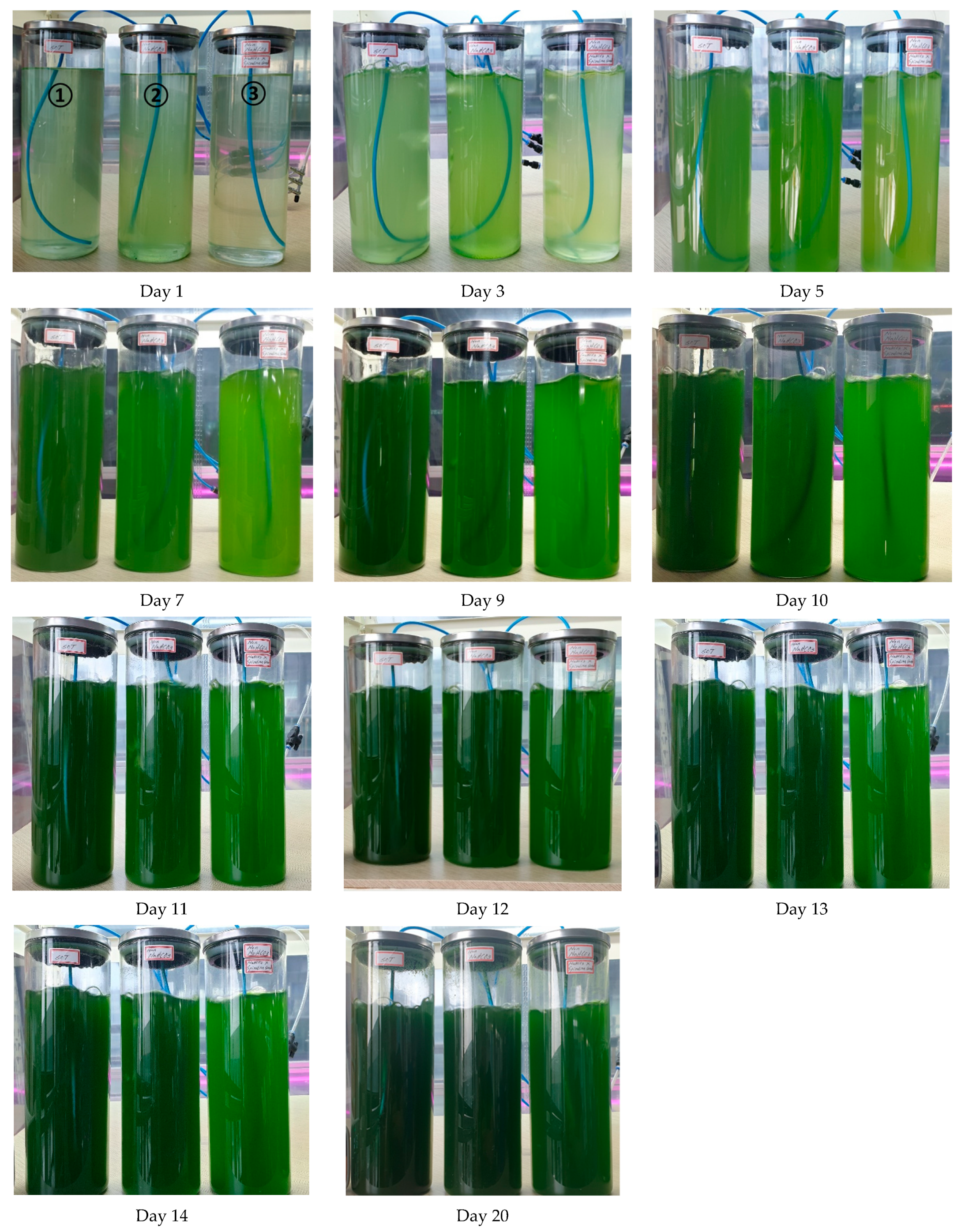
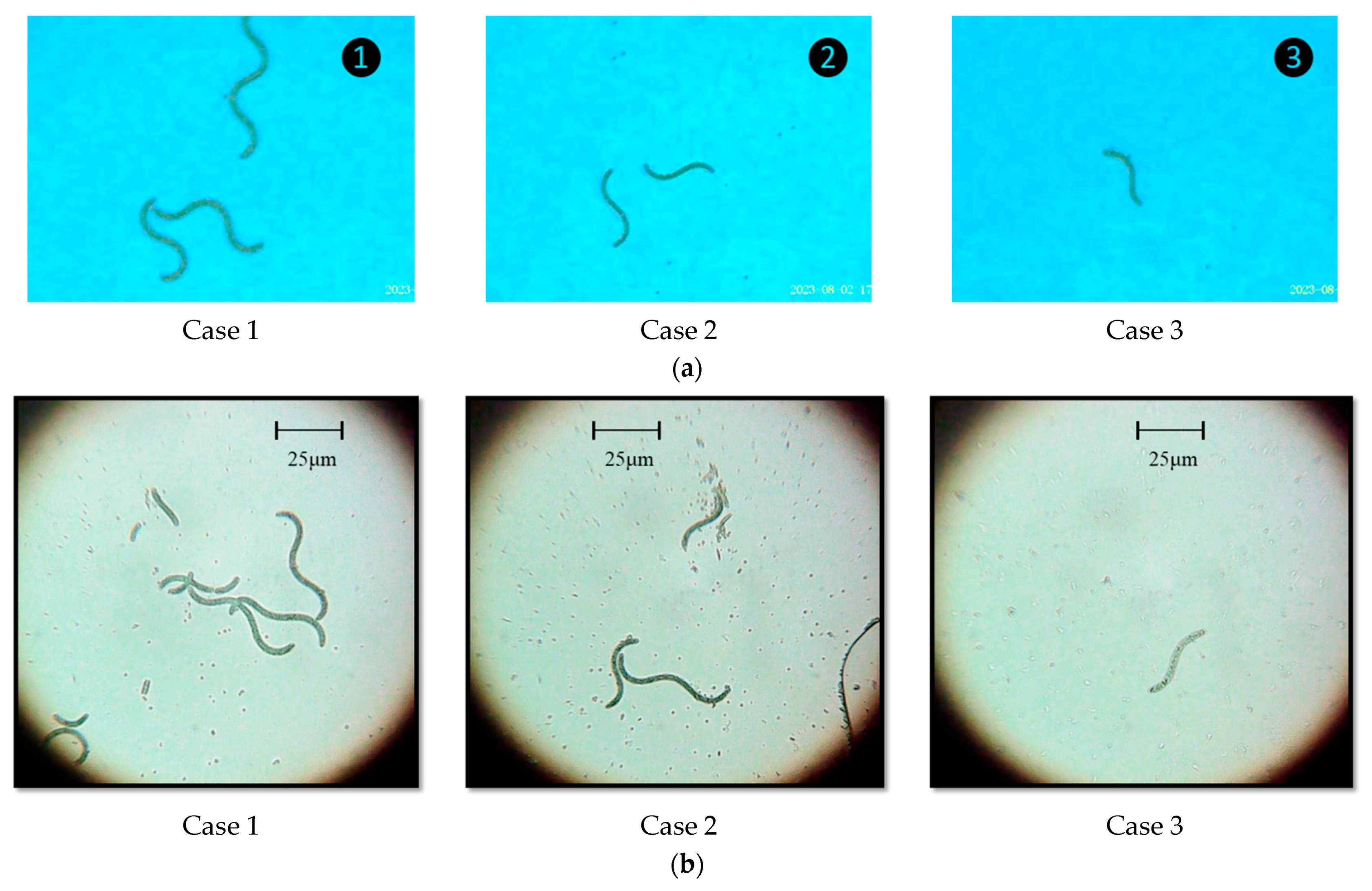
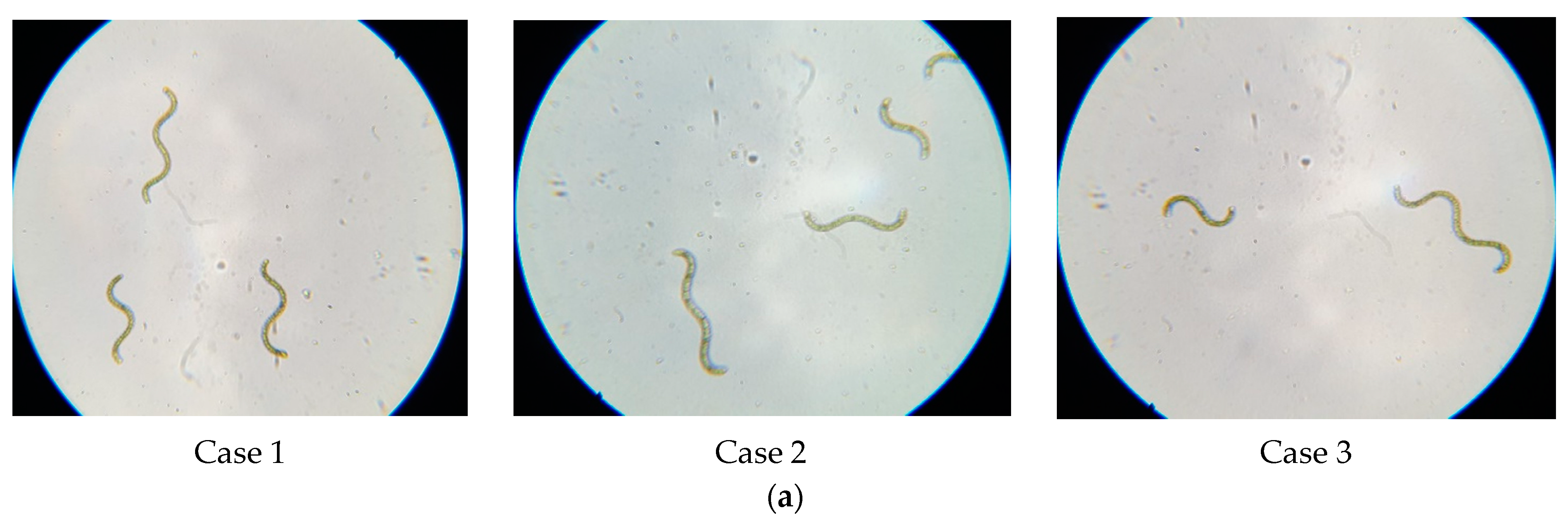

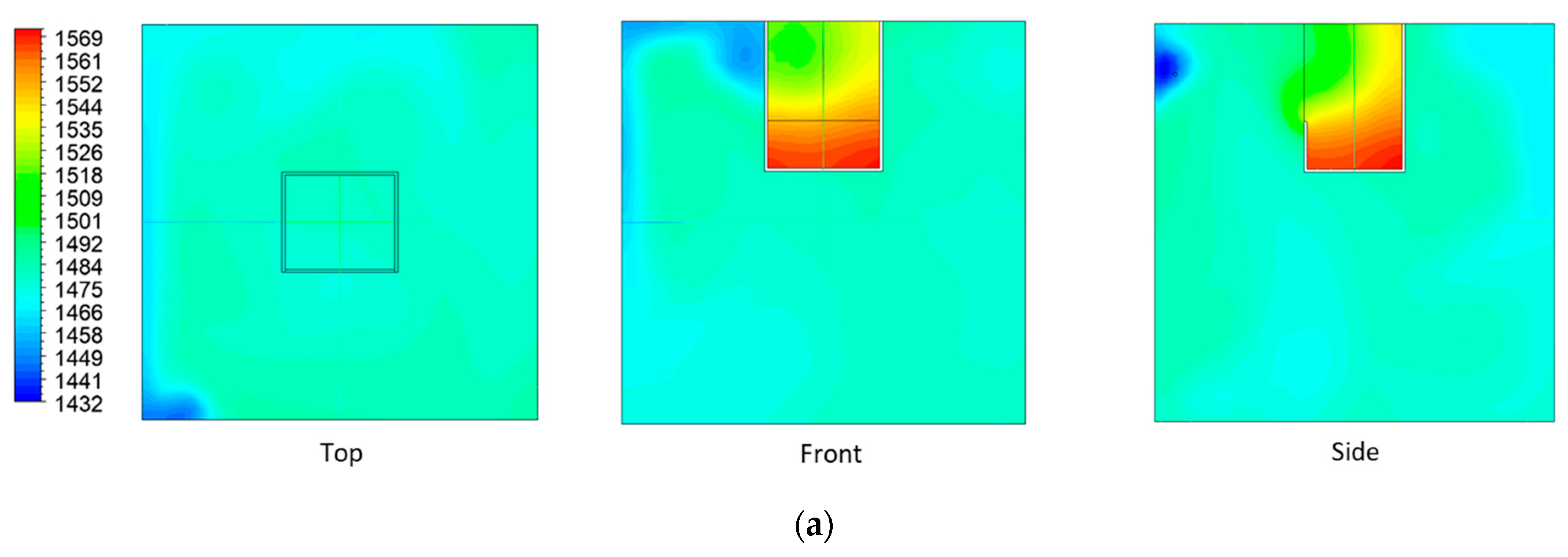

| Factor | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| Date | A | B | A | B | A | B |
| pH | 10.01 | 9.41 | 8.69 | 8.83 | 7.92 | 8.16 |
| Medium temperature (°C) | 30.1 | 29.2 | 30.2 | 29.0 | 30.1 | 29.1 |
| Lighting intensity (lux) | 6842.5 ± 869.0 (Min: 5948, Max: 7629) | |||||
| CO2 supply (ppm) | 732.5 ± 44.5 | |||||
| (Lab) Air temperature (°C) | 32.0 ± 0.3 | |||||
| (Lab) Air humidity (%) | 98 ± 0.7 | |||||
| O.D. | 0.52 | 0.81 | 0.41 | 0.68 | 0.37 | 0.49 |
| No. | Day 9 | Day 14 | Day 30 |
|---|---|---|---|
| 1 |  | 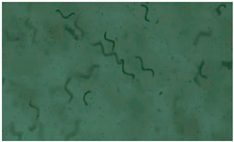 | 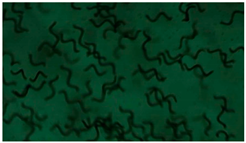 |
| 2 |  |  |  |
| 3 |  |  | 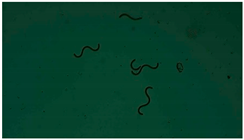 |
| VOCs (ppb) | CO (ppm) | O2 (%) | CH4 | NO2 | NH3 | |
|---|---|---|---|---|---|---|
| μ | 129 | 98.6 | 19.98 | 621.8 | 100.4 | 96.7 |
| σ | 93 | 36.5 | 0.07 | 31.9 | 14.0 | 12.7 |
| Min. | 0 | 6 | 19.74 | 519 | 82 | 76 |
| Max. | 474 | 242 | 20.25 | 704 | 175 | 165 |
| Medium | Day 1 | Day 2 | Day 3 | Day 5 | Day 7 | |
|---|---|---|---|---|---|---|
| SOT excl. NaHCO3 | μ | 817.9 | 885.5 | 709.6 | 497.6 | 521.0 |
| σ | 6.1 | 23.1 | 17.9 | 5.8 | 16.6 | |
| p-Value | ** | *** | *** | *** | *** | |
| Max. | 828 | 928 | 743 | 510 | 547 | |
| Min. | 807 | 842 | 671 | 479 | 483 | |
| Standard SOT | μ | 533.0 | 525.6 | 413.9 | 442.6 | 532.2 |
| σ | 10.2 | 23.6 | 12.8 | 16.9 | 17.1 | |
| p-Value | *** | *** | *** | *** | *** | |
| Max. | 542 | 580 | 476 | 465 | 588 | |
| Min. | 486 | 452 | 365 | 373 | 489 | |
| Mann–Whitney U | p-Value | *** | *** | *** | *** | *** |
| Brown–Forsythe | p-Value | 0.767 | *** | *** | *** | 0.805 |
| Test | Parameter | Day 1 | Day 2 | Day 5 | 2 L | 4 L | 7 L |
|---|---|---|---|---|---|---|---|
| μ | 1020.4 | 1014.5 | 871.2 | 1337.0 | 1096.3 | 1305.3 | |
| σ | 982.9 | 940.1 | 1112.6 | 1028.5 | 349.2 | 1231.0 | |
| Min. | 584 | 686 | 504 | 695 | 949 | 515 | |
| Kruskal | p-Value | *** | *** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Park, J.; Kim, I.; Yi, H. A Microalgae Photobioreactor System for Indoor Air Remediation: Empirical Examination of the CO2 Absorption Performance of Spirulina maxima in a NaHCO3-Reduced Medium. Appl. Sci. 2023, 13, 12991. https://doi.org/10.3390/app132412991
Han M, Park J, Kim I, Yi H. A Microalgae Photobioreactor System for Indoor Air Remediation: Empirical Examination of the CO2 Absorption Performance of Spirulina maxima in a NaHCO3-Reduced Medium. Applied Sciences. 2023; 13(24):12991. https://doi.org/10.3390/app132412991
Chicago/Turabian StyleHan, Myungho, Jinsuck Park, Inhan Kim, and Hwang Yi. 2023. "A Microalgae Photobioreactor System for Indoor Air Remediation: Empirical Examination of the CO2 Absorption Performance of Spirulina maxima in a NaHCO3-Reduced Medium" Applied Sciences 13, no. 24: 12991. https://doi.org/10.3390/app132412991
APA StyleHan, M., Park, J., Kim, I., & Yi, H. (2023). A Microalgae Photobioreactor System for Indoor Air Remediation: Empirical Examination of the CO2 Absorption Performance of Spirulina maxima in a NaHCO3-Reduced Medium. Applied Sciences, 13(24), 12991. https://doi.org/10.3390/app132412991







