The Safe Return of Face-to-Face Teaching in the Post-COVID-19 Era at a University in Southern Italy: Surface Monitoring as an Early Warning System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Environmental Sampling
2.2. Molecular Analysis
- -
- Reverse transcription phase (50 °C for 30 min)
- -
- Inactivation of the RT phase (+95 °C for 10 min)
- -
- 45 cycles of amplification (+95 °C for 15 s and +60 °C for 45 s).
2.3. Virus Isolation
2.4. Next-Generation Sequencing of Viral Genome
2.5. Sequence Data and Phylogenetic Analysis
2.6. Statistical Analysis
- Surface type (teacher and student desks, handrail, computer mouse, keyboard, door and window handles, other)
- Materials of surfaces sampled (plasticized wood, wood, metal, plastic)
- Number of students (classroom with <50 students, >50 students and environment <30 academic staff)
- Seasonality (spring–summer vs. autumn)
- Seasonality: the months from May to September 2022 were considered the spring–summer period and the months from October to December 2022 as the autumn period. In particular, the spring–summer period was given a value of “1” and the autumn period a value of “2”. The ordinal coding technique [28] was used to make this parameter comparable to the alphanumeric ones.
- Average number of COVID-19 cases among university students in the seven days before sampling.
- Average number of COVID-19 cases among university students in the seven days after sampling.
- Seasonality: as described in the analysis above.
- Average number of COVID-19 cases among university students in the seven days before sampling.
- % of swabs positive for SARS-CoV-2 on the day of sampling.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Status of Environmental Surveillance for SARS-CoV-2 Virus: Scientific Brief, 5 August 2020. Available online: www.who.int/newsroom/commentaries/detail/status-of-environmental-surveillance-for-sars-cov-2-virus (accessed on 24 August 2023).
- Sami, N.; Ahmad, R.; Afzal, B.; Naaz, H.; Fatma, T. SARS-CoV-2 in the Environment: Its Transmission, Mitigation, and Prospective Strategies of Safety and Sustainability. Rev. Environ. Contam. Toxicol. 2022, 260, 8. [Google Scholar] [CrossRef]
- Casabianca, A.; Orlandi, C.; Amagliani, G.; Magnani, M.; Brandi, G.; Schiavano, G.F. SARS-CoV-2 RNA Detection on Environmental Surfaces in a University Setting of Central Italy. Int. J. Environ. Res. Public Health 2022, 19, 5560. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Kasloff, S.B.; Leung, A.; Strong, J.E.; Funk, D.; Cutts, T.A. Stability of SARS-CoV-2 on Critical Personal Protective Equipment. Sci. Rep. 2021, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, G.; Triggiano, F.; Apollonio, F.; Diella, G.; Lopuzzo, M.; D’Ambrosio, M.; Fasano, F.; Stefanizzi, P.; Sorrenti, G.T.; Magarelli, P.; et al. SARS-CoV-2 RNA and Supermarket Surfaces: A Real or Presumed Threat? Int. J. Environ. Res. Public Health 2021, 18, 9404. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, S.; Carrà, G.; Bottino, P.; Zanotto, E.; De Santis, M.C.; Margaria, J.P.; Giorgio, A.; Mandili, G.; Martini, M.; Cavallo, R.; et al. A Novel Multiplex qRT-PCR Assay to Detect SARS-CoV-2 Infection: High Sensitivity and Increased Testing Capacity. Microorganisms 2020, 8, 1064. [Google Scholar] [CrossRef]
- Chia, P.Y.; Coleman, K.K.; Tan, Y.K.; Ong, S.W.X.; Gum, M.; Lau, S.K.; Lim, X.F.; Lim, A.S.; Sutjipto, S.; Lee, P.H.; et al. Singapore 2019 Novel Coronavirus Outbreak Research Team. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020, 11, 2800. [Google Scholar] [CrossRef]
- Marshall, D.L.; Bois, F.; Jensen, S.K.S.; Linde, S.A.; Higby, R.; Rémy-McCort, Y.; Murray, S.; Dieckelman, B.; Sudradjat, F.; Martin, G.G. Sentinel Coronavirus environmental monitoring can contribute to detecting asymptomatic SARS-CoV-2 virus spreaders and can verify effectiveness of workplace COVID-19 controls. Microb. Risk Anal. 2020, 16, 100137. [Google Scholar] [CrossRef]
- Vo, V.; Tillett, R.L.; Chang, C.L.; Gerrity, D.; Betancourt, W.Q.; Oh, E.C. SARS-CoV-2 variant detection at a university dormitory using wastewater genomic tools. Sci. Total Environ. 2021, 805, 149930. [Google Scholar] [CrossRef]
- Betancourt, W.Q.; Schmitz, B.W.; Innes, G.K.; Prasek, S.M.; Pogreba Brown, K.M.; Stark, E.R.; Foster, A.R.; Sprissler, R.S.; Harris, D.T.; Sherchanet, S.P.; et al. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci. Total Environ. 2021, 779, 146408. [Google Scholar] [CrossRef] [PubMed]
- Renninger, N.; Nastasi, N.; Bope, A.; Cochran, S.J.; Haines, S.R.; Balasubrahmaniam, N.; Stuart, K.; Bivins, A.; Bibby, K.; Hull, N.M.; et al. Indoor Dust as a Matrix for Surveillance of COVID-19. mSystems 2021, 6, e01350-20. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.P.; Fuhrmeister, E.R.; Cantrell, M.E.; Pitol, A.K.; Swarthout, J.M.; Powers, J.E.; Nadimpalli, M.L.; Julian, T.R.; Pickering, A.J. Longitudinal Monitoring of SARS-CoV-2 RNA on High-Touch Surfaces in a Community Setting. Environ. Sci. Technol. Lett. 2021, 8, 68–175. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.R.; Nancharaiah, Y.V.; Gu, X.; Lee, W.L.; Rajal, V.B.; Haines, M.B.; Girones, R.; Ng, L.C.; Alm, E.J.; Wuertz, S. Making waves: Wastewater surveillance of SARS-CoV-2 for population-based health management. Water Res. 2020, 184, 116181. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, O.; Martiny, D.; Rochas, O.; van Belkum, A.; Kozlakidis, Z. Considerations for diagnostic COVID-19 tests. Nat. Rev. Microbiol. 2021, 19, 171–183. [Google Scholar] [CrossRef] [PubMed]
- McElfish, P.A.; Purvis, R.; James, L.P.; Willis, D.E.; Andersen, J.A. Perceived Barriers to COVID-19 Testing. Int. J. Environ. Res. Public Health 2021, 18, 2278. [Google Scholar] [CrossRef] [PubMed]
- Presidency of the Italian Council of Ministries. Decreto del Presidente del Consiglio dei Ministri 8 marzo 2020. Ulteriori Disposizioni Attuative del Decreto-Legge 23 Febbraio 2020, n. 6, Recante Misure Urgenti in Materia di Contenimento e Gestione Dell’emergenza Epidemiologica da COVID-19 (20A01522). In Gazzetta Ufficiale della Repubblica Italiana Serie Generale n.59 del 8 Marzo 2020; Istituto Poligrafico e Zecca dello Stato: Rome, Italy, 2020. [Google Scholar]
- Coil, D.A.; Albertson, T.; Banerjee, S.; Brennan, G.; Campbell, A.J.; Cohen, S.H.; Dandekar, S.; Díaz-Muñoz, S.L.; Eisen, J.A.; Goldstein, T.; et al. SARSCoV-2 detection and genomic sequencing from hospital surface samples collected at UC Davis. PLoS ONE 2021, 16, e0253578. [Google Scholar] [CrossRef]
- Caggiano, G.; Apollonio, F.; Triggiano, F.; Diella, G.; Stefanizzi, P.; Lopuzzo, M.; D’Ambrosio, M.; Bartolomeo, N.; Barbuti, G.; Sorrenti, G.T.; et al. SARS-CoV-2 and Public Transport in Italy. Int. J. Environ. Res. Public Health 2021, 18, 11415. [Google Scholar] [CrossRef]
- La Rosa, G.; Mancini, P.; Bonanno Ferraro, G.; Veneri, C.; Iaconelli, M.; Bonadonna, L.; Lucentini, L.; Suffredini, E. SARS-CoV-2 has been circulating in northern Italy since December 2019: Evidence from environmental monitoring. Sci. Total Environ. 2021, 750, 141711. [Google Scholar] [CrossRef]
- Montagna, M.T.; De Giglio, O.; Calia, C.; Pousis, C.; Apollonio, F.; Campanale, C.; Diella, G.; Lopuzzo, M.; Marzella, A.; Triggiano, F.; et al. First Detection of Severe Acute Respiratory Syndrome Coronavirus 2 on the Surfaces of Tourist-Recreational Facilities in Italy. Int. J. Environ. Res. Public Health 2021, 18, 3252. [Google Scholar] [CrossRef]
- Rondinone, V.; Pace, L.; Fasanella, A.; Manzulli, V.; Parisi, A.; Capobianchi, M.R.; Ostuni, A.; Chironna, M.; Caprioli, E.; Labonia, M.; et al. VOC 202012/01 Variant Is Effectively Neutralized by Antibodies Produced by Patients Infected before Its Diffusion in Italy. Viruses 2021, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Manzulli, V.; Scioscia, G.; Giganti, G.; Capobianchi, M.R.; Lacedonia, D.; Pace, L.; Cipolletta, D.; Tondo, P.; De Nittis, R.; Rondinone, V.; et al. Real Time PCR and Culture-Based Virus Isolation Test in Clinically Recovered Patients: Is the Subject Still Infectious for SARS-CoV2? J. Clin. Med. 2021, 10, 309. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, L.; Simone, D.; Bianco, A.; Del Sambro, L.; Rondinone, V.; Pace, L.; Manzulli, V.; Iacobellis, M.; Parisi, A. Emerging Mutations Potentially Related to SARS-CoV-2 Immune Escape: The Case of a Long-Term Patient. Life 2021, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Gurry, C.; Freitas, L.; Schultz, M.B.; Bach, G.; Diallo, A.; Akite, N.; Ho, J.; Lee, R.T.; Yeo, W.; et al. GISAID’s Role in Pandemic Response. China CDC Wkly. 2021, 3, 1049–1051. Available online: https://www.gisaid.org/ (accessed on 26 May 2023). [CrossRef] [PubMed]
- O’Toole, Á.; Scher, E.; Underwood, A.; Jackson, B.; Hill, V.; McCrone, J.T.; Colquhoun, R.; Ruis, C.; Abu-Dahab, K.; Taylor, B.; et al. Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus Evol. 2021, 7, veab064. Available online: https://github.com/cov-lineages/pangolin (accessed on 26 May 2023). [CrossRef]
- Potdar, K.; Pardawala, T.S.; Pai, C.D. A Comparative Study of Categorical Variable Encoding Techniques for Neural Network Classifiers. Int. J. Comput. Appl. 2017, 175, 7–9. [Google Scholar] [CrossRef]
- Wu, Y.; Kang, L.; Guo, Z.; Liu, J.; Liu, M.; Liang, W. Incubation Period of COVID-19 Caused by Unique SARS-CoV-2 Strains: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2228008, Erratum in: JAMA Netw. Open 2022, 5, e2235424. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Sorveglianza COVID-19. Sorveglianza Integrata Casi di Coronavirus COVID-19 in Italia. Available online: https://covid-19.iss.it/ (accessed on 22 October 2023).
- Apulia Statistical Office. Available online: https://www.regione.puglia.it/web/ufficio-statistico/andamento-dei-contagi (accessed on 13 August 2023).
- Patro, S.; Sahu, K.K. Normalization: A Preprocessing Stage. arXiv 2015, arXiv:1503.06462. [Google Scholar] [CrossRef]
- La Rosa, G.; Iaconelli, M.; Veneri, C.; Mancini, P.; Bonanno Ferraro, G.; Brandtner, D.; Lucentini, L.; Bonadonna, L.; Rossi, M.; Grigioni, M.; et al. The rapid spread of SARS-CoV-2 Omicron variant in Italy reflected early through wastewater surveillance. Sci. Total Environ. 2022, 837, 155767. [Google Scholar] [CrossRef]
- Wu, F.; Xiao, A.; Zhang, J.; Moniz, K.; Endo, N.; Armas, F.; Bushman, M.; Chai, P.R.; Duvallet, C.; Erickson, T.B.; et al. Wastewater surveillance of SARS-CoV-2 across 40 U.S. states from February to June 2020. Water Res. 2021, 202, 117400. [Google Scholar] [CrossRef]
- Gallè, F.; Sabella, E.A.; Ferracuti, S.; De Giglio, O.; Caggiano, G.; Protano, C.; Valeriani, F.; Parisi, E.A.; Valerio, G.; Liguori, G.; et al. Sedentary Behaviors and Physical Activity of Italian Undergraduate Students during Lockdown at the Time of COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 6171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, J.; Smith, L.M.; Li, X.; Yancey, O.; Franzblau, A.; Dvonch, J.T.; Xi, C.; Neitzel, R.L. Monitoring SARS-CoV-2 in air and on surfaces and estimating infection risk in buildings and buses on a university campus. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Yung, L.; Leung, L.Y.; Lee, K.H.; Morrell, S.; Fong, M.W.; Fung, N.H.Y.; Cheng, K.L.; Kaewpreedee, P.; Li, Y.; Cowling, B.J.; et al. A longitudinal environmental surveillance study for SARS-CoV-2 from the emergency department of a teaching hospital in Hong Kong. J. Hosp. Infect. 2023, 138, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Lessler, J.; Grabowski, M.K.; Grantz, K.H.; Badillo-Goicoechea, E.; Metcalf, C.J.E.; Lupton-Smith, C.; Azman, A.S.; Stuart, E.A. Household COVID-19 risk and in-person schooling. Science 2021, 372, 1092–1097. [Google Scholar] [CrossRef]
- La Scola, B.; Le Bideau, M.; Andreani, J.; Hoang, V.T.; Grimaldier, C.; Colson, P.; Gautret, P.; Raoult, D. Viral RNA load as determined by cell culture as a management tool for discharge of SARSCoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1059–1061. [Google Scholar] [CrossRef]
- La Rosa, G.; Brandtner, D.; Bonanno Ferraro, G.; Veneri, C.; Mancini, P.; Iaconelli, M.; Lucentini, L.; Del Giudice, C.; Orlandi, L.; The SARI Network. Wastewater surveillance of SARS-CoV-2 variants in October-November 2022 in Italy: Detection of XBB.1, BA.2.75 and rapid spread of the BQ.1 lineage. Sci. Total Environ. 2023, 873, 162339. [Google Scholar] [CrossRef] [PubMed]
- Crits-Christoph, A.; Kantor, R.S.; Olm, M.R.; Whitney, O.N.; Al-Shayeb, B.; Lou, Y.C.; Flamholz, A.; Kennedy, L.C.; Greenwald, H.; Hinkle, A.; et al. Genome Sequencing of Sewage Detects Regionally Prevalent SARS-CoV-2 Variants. MBio 2021, 12, e02703-20. [Google Scholar] [CrossRef] [PubMed]
- Nottmeyer, L.; Armstrong, B.; Lowe, R.; Abbott, S.; Meakin, S.; O’Reilly, K.; von Borries, R.; Schneider, R.; Royé, D.; Hashizume, M.; et al. The association of COVID-19 incidence with temperature, humidity, and UV radiation—A global multi-city analysis. Sci. Total Environ. 2022, 854, 158636. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, J.; Zhang, L.; Chen, S.; Gao, J.; Jiao, H. The global transmission of new coronavirus variants. Environ. Res. 2022, 206, 112240. [Google Scholar] [CrossRef]
- Li, H.L.; Yang, B.Y.; Wang, L.J.; Liao, K.; Sun, N.; Liu, Y.C.; Ma, R.F.; Yang, X.D. A meta-analysis result: Uneven influences of season, geo-spatial scale and latitude on relationship between meteorological factors and the COVID-19 transmission. Environ. Res. 2022, 212, 113297. [Google Scholar] [CrossRef]
- Mecenas, P.; Bastos, R.T.R.M.; Vallinoto, A.C.R.; Normando, D. Effects of temperature and humidity on the spread of COVID-19: A systematic review. PLoS ONE 2020, 15, e0238339. [Google Scholar] [CrossRef] [PubMed]
- Briz-Redón, Á.; Serrano-Aroca, Á. The effect of climate on the spread of the COVID-19 pandemic: A review of findings, and statistical and modelling techniques. Prog. Phys. Geogr. Earth Environ. 2020, 44, 591–604. [Google Scholar] [CrossRef]
- Majumder, P.; Ray, P.P. A systematic review and meta-analysis on correlation of weather with COVID-19. Sci. Rep. 2021, 11, 10746. [Google Scholar] [CrossRef] [PubMed]
- Triggiano, F.; De Giglio, O.; Apollonio, F.; Brigida, S.; Fasano, F.; Mancini, P.; Bonanno Ferraro, G.; Veneri, C.; La Rosa, G.; Suffredini, E.; et al. Wastewater-based Epidemiology and SARS-CoV-2: Variant Trends in the Apulia Region (Southern Italy) and Effect of Some Environmental Parameters. Food Environ. Virol. 2023, 15, 331–341. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Y.; Zhang, Y.; Yan, R. Structural Basis for the Enhanced Infectivity and Immune Evasion of Omicron Subvariants. Viruses 2023, 15, 1398. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of respiratory viral infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Contreras, S.; Villavicencio, H.A.; Medina-Ortiz, D.; Saavedra, C.P.; Olivera-Nappa, Á. Real-Time Estimation of Rt for Supporting Public-Health Policies Against COVID-19. Front. Public Health 2020, 8, 556689. [Google Scholar] [CrossRef]
- Report Esteso iss COVID-19: Sorveglianza, Impatto Delle Infezioni ed Efficacia Vaccinale. Aggiornamento Nazionale 27/07/2022. Istituto Superiore di Sanità, 29 luglio 2022. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_27-luglio-2022.pdf (accessed on 22 October 2023).
- Report Esteso iss COVID-19: Sorveglianza, Impatto Delle Infezioni ed Efficacia Vaccinale. Aggiornamento Nazionale 21/09/2022. Istituto Superiore di Sanità, 23 settembre 2022. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_21-settembre-2022.pdf (accessed on 22 October 2023).
- Draisci, R.; Attias, L.; Baldassarri, L.; Catone, T.; Cresti, R.; Fidente, R.M.; Marcello, I.; Buonanno, G.; Bertinato, L. Interim Recommendations on Cleaning and Disinfection of Non-Healthcare Settings during COVID-19 Health Emergency: Indoor Environments/surfaces. Updating Rapporto ISS COVID-19 n. 25/2020. Version of 20 May 2021. Available online: https://www.iss.it/rapporti-covid-19/-/asset_publisher/btw1J82wtYzH/content/prossima-pubblicazione.-rapporto-iss-covid-19-n.-25-2020.-raccomandazioni-ad-interim-sulla-sanificazione-di-strutture-non-sanitarie-nell-attuale-emergenza-covid-19-superfici-ambienti-interni-e-abbigliamento (accessed on 19 September 2023).
- Circolare 17644 del 22 maggio 2020. Indicazioni per L’attuazione di Misure Contenitive del Contagio da SARS-CoV-2 Attraverso Procedure di Sanificazione di Strutture non Sanitarie (Superfici, Ambienti Interni) e Abbigliamento. Available online: https://www.sicp.it/normative/2020/05/ministero-della-salute-circolare-n-17644-del-22-mag/ (accessed on 22 October 2023).

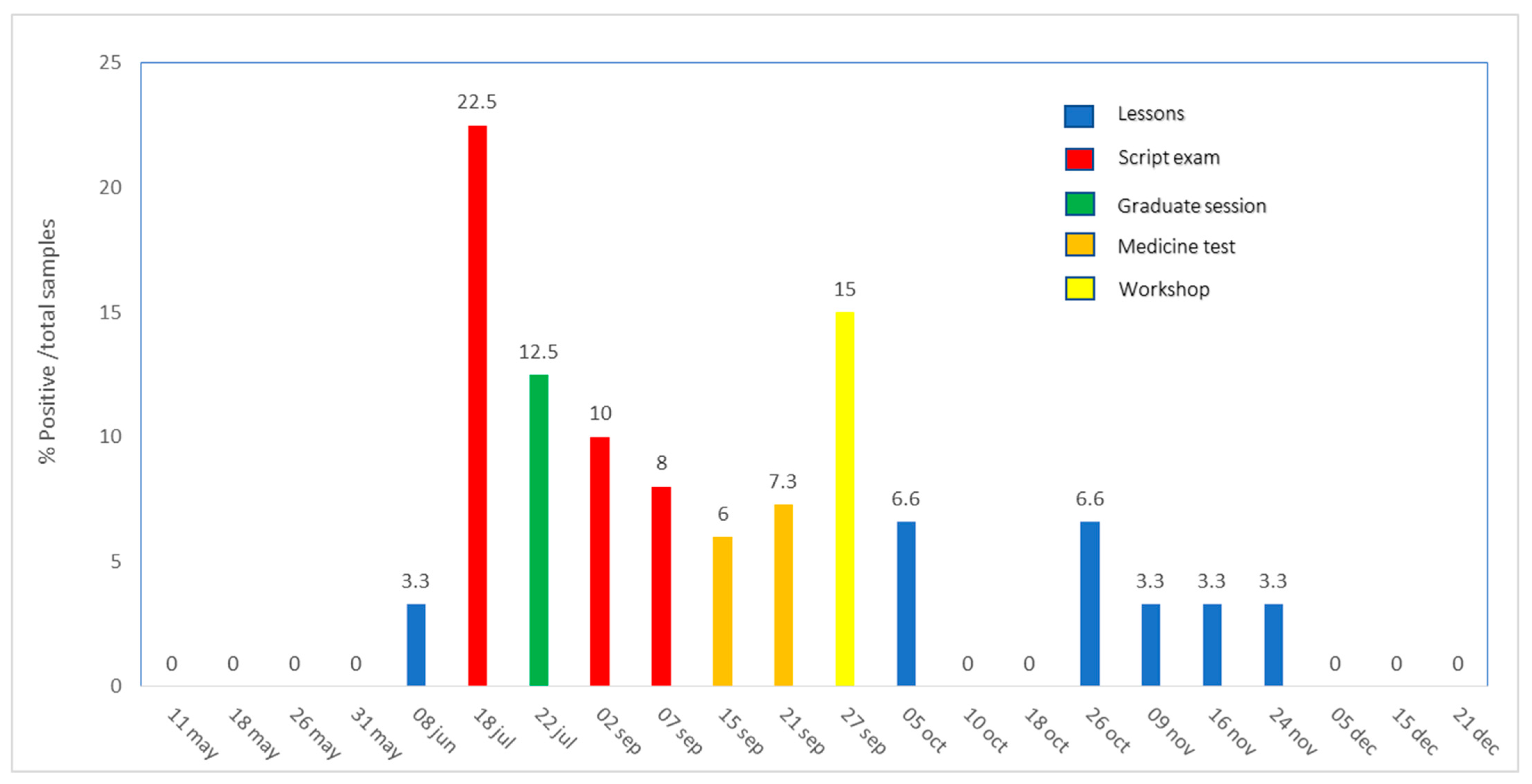
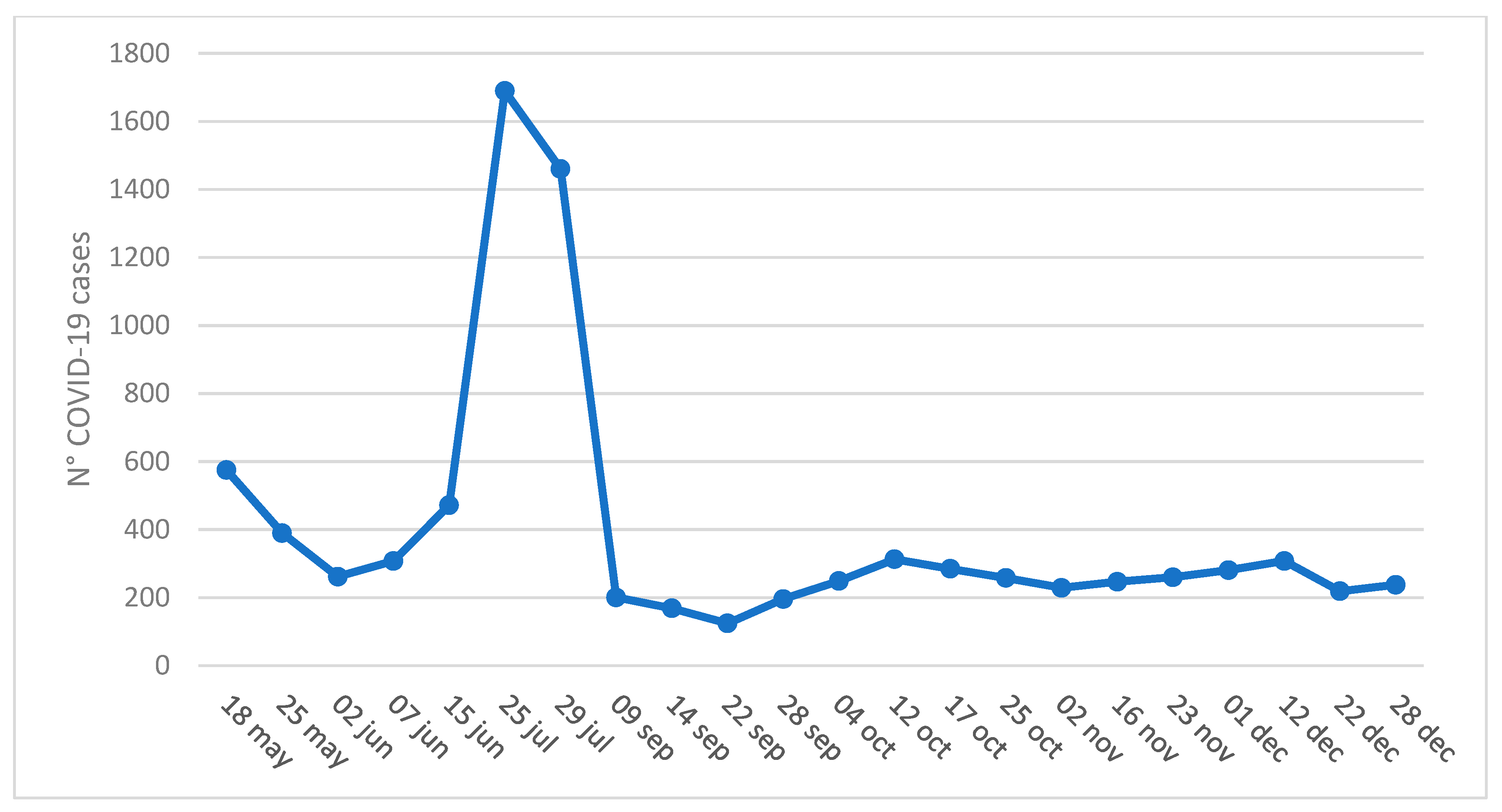
| Tested Surface (No.) | Positive (No.) | Negative (No.) | % |
|---|---|---|---|
| Students’ desks (686) | 41 | 645 | 6 |
| Handles (20) | 0 | 20 | 0 |
| Handrail (11) | 0 | 11 | 0 |
| Mouse (7) | 0 | 7 | 0 |
| Keyboard (7) | 0 | 7 | 0 |
| Teachers’ desks (6) | 0 | 6 | 0 |
| Others (26) | 0 | 26 | 0 |
| Total (763) | 41 | 722 | 5.4 |
| Material of Surface | Positive/Total Samples No. (%) |
|---|---|
| Plasticized-wood | 32/448 (7.1) |
| Non-plasticized wood | 9/265 (3.4) |
| Metal | 0/36 (0) |
| Plastic | 0/14 (0) |
| Total | 41/763 (5.4) |
| Environmental Crowding | Positive/Total Samples No. (%) |
|---|---|
| classrooms with >50 students | 38/534 (7.1) |
| classrooms with <50 students | 3/191 (1.5) |
| other with <30 academic staff | 0/35 (0) |
| Total | 41/763 (5.4) |
| Βeta | (eβ − 1) = RR (%) | p-Value | |
|---|---|---|---|
| Preliminary model | |||
| Intercept | 5.622603 | <0.001 | |
| Seasonality | −3.188502 | −95.8 | 0.04 |
| Average COVID-19 cases among university students in the seven days before sampling | −0.009186 | −0.9 | 0.41 |
| Average COVID-19 cases among university students in the seven days post sampling | 0.020769 | 2.1 | 0.05 |
| Final model | |||
| Intercept | 5.622603 | <0.001 | |
| Seasonality | −2.737769 | −93.5 | 0.04 |
| Average COVID-19 cases among university students in the seven days post sampling | 0.08199 | 8.5 | 0.008 |
| Βeta | (eβ − 1) = RR (%) | p-Value | |
|---|---|---|---|
| Preliminary model | |||
| Intercept | 5.342 | <0.001 | |
| Seasonality | −0.01092 | −1.1 | 0.695 |
| Average COVID-19 cases among university students in the seven days before sampling | 0.0011 | 0.001 | <0.001 |
| Positive swabs for SARS-CoV-2 (%) | 0.01853 | 0.02 | <0.001 |
| Final model | |||
| Intercept | 5.32498 | <0.001 | |
| Average COVID-19 cases among university students in the seven days before sampling | 0.00111 | 0.11 | <0.001 |
| Positive swabs for SARS-CoV-2 (%) | 0.01847 | 1.9 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Giglio, O.; Triggiano, F.; Apollonio, F.; Buonavoglia, C.; Capozzi, L.; Camero, M.; Colafemmina, G.; Del Prete, R.; Fasano, F.; Lanave, G.; et al. The Safe Return of Face-to-Face Teaching in the Post-COVID-19 Era at a University in Southern Italy: Surface Monitoring as an Early Warning System. Appl. Sci. 2023, 13, 13214. https://doi.org/10.3390/app132413214
De Giglio O, Triggiano F, Apollonio F, Buonavoglia C, Capozzi L, Camero M, Colafemmina G, Del Prete R, Fasano F, Lanave G, et al. The Safe Return of Face-to-Face Teaching in the Post-COVID-19 Era at a University in Southern Italy: Surface Monitoring as an Early Warning System. Applied Sciences. 2023; 13(24):13214. https://doi.org/10.3390/app132413214
Chicago/Turabian StyleDe Giglio, Osvalda, Francesco Triggiano, Francesca Apollonio, Canio Buonavoglia, Loredana Capozzi, Michele Camero, Giuseppe Colafemmina, Raffaele Del Prete, Fabrizio Fasano, Gianvito Lanave, and et al. 2023. "The Safe Return of Face-to-Face Teaching in the Post-COVID-19 Era at a University in Southern Italy: Surface Monitoring as an Early Warning System" Applied Sciences 13, no. 24: 13214. https://doi.org/10.3390/app132413214
APA StyleDe Giglio, O., Triggiano, F., Apollonio, F., Buonavoglia, C., Capozzi, L., Camero, M., Colafemmina, G., Del Prete, R., Fasano, F., Lanave, G., Mateos, H., Pace, L., Mosca, A., Palazzo, G., Parisi, A., Stefanizzi, P., Terio, V., Tafuri, S., & Montagna, M. T. (2023). The Safe Return of Face-to-Face Teaching in the Post-COVID-19 Era at a University in Southern Italy: Surface Monitoring as an Early Warning System. Applied Sciences, 13(24), 13214. https://doi.org/10.3390/app132413214














