Recent Advances in the Targeting of Head and Neck Cancer Stem Cells
Abstract
:1. Introduction
2. Cancer Stem Cells
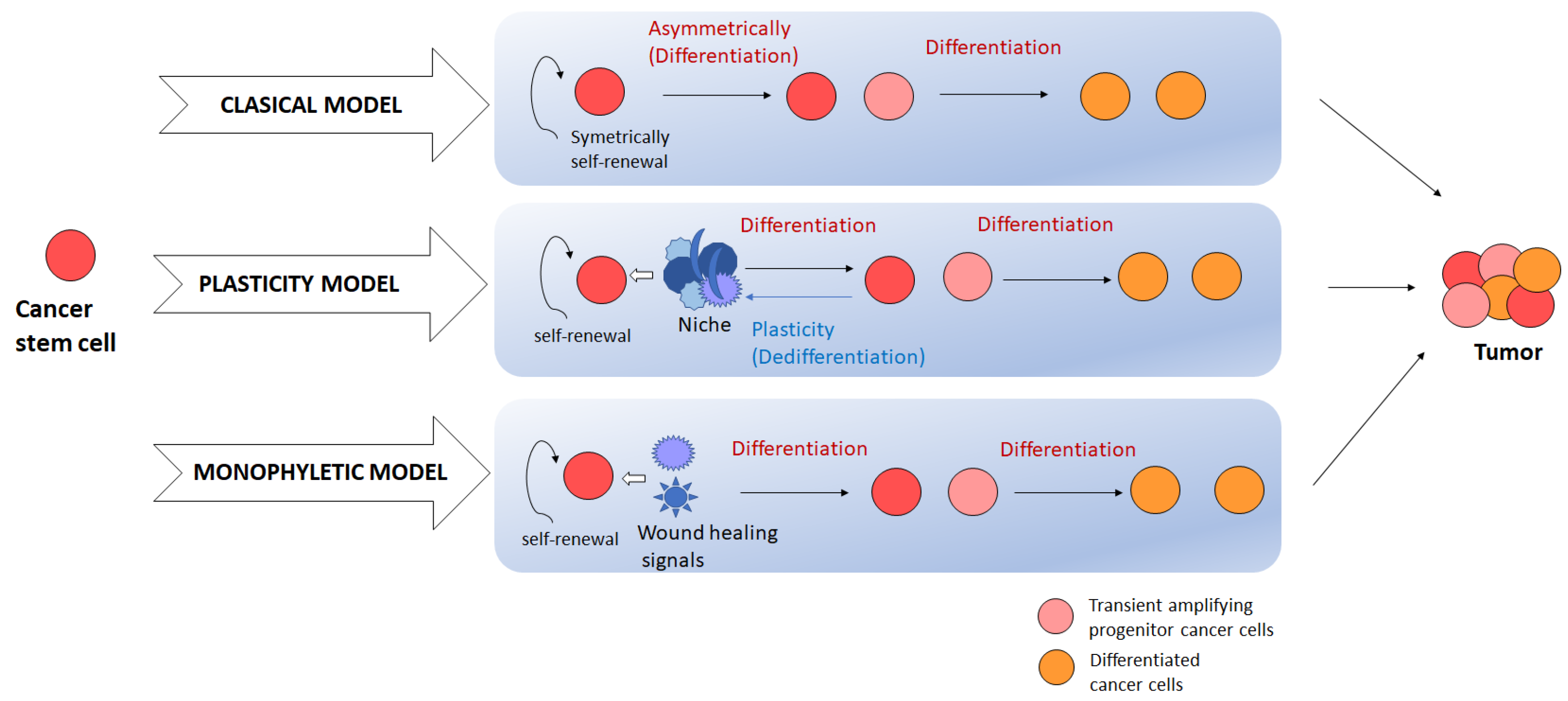
2.1. Evolution Model
2.2. Stemness-Associated Signaling Pathways
2.2.1. Wnt Signaling Pathway
2.2.2. Notch Signaling Pathway
2.2.3. Sonic-Hedgehog (SHh) Signaling Pathway
2.2.4. EGFR Signaling Pathway
2.2.5. PI3K/AKT/mTOR Signaling Pathway
2.2.6. Hippo Pathway
2.3. Stem Cell Factors
3. Identification of CSC
3.1. Marker-Based Isolation of CSCs
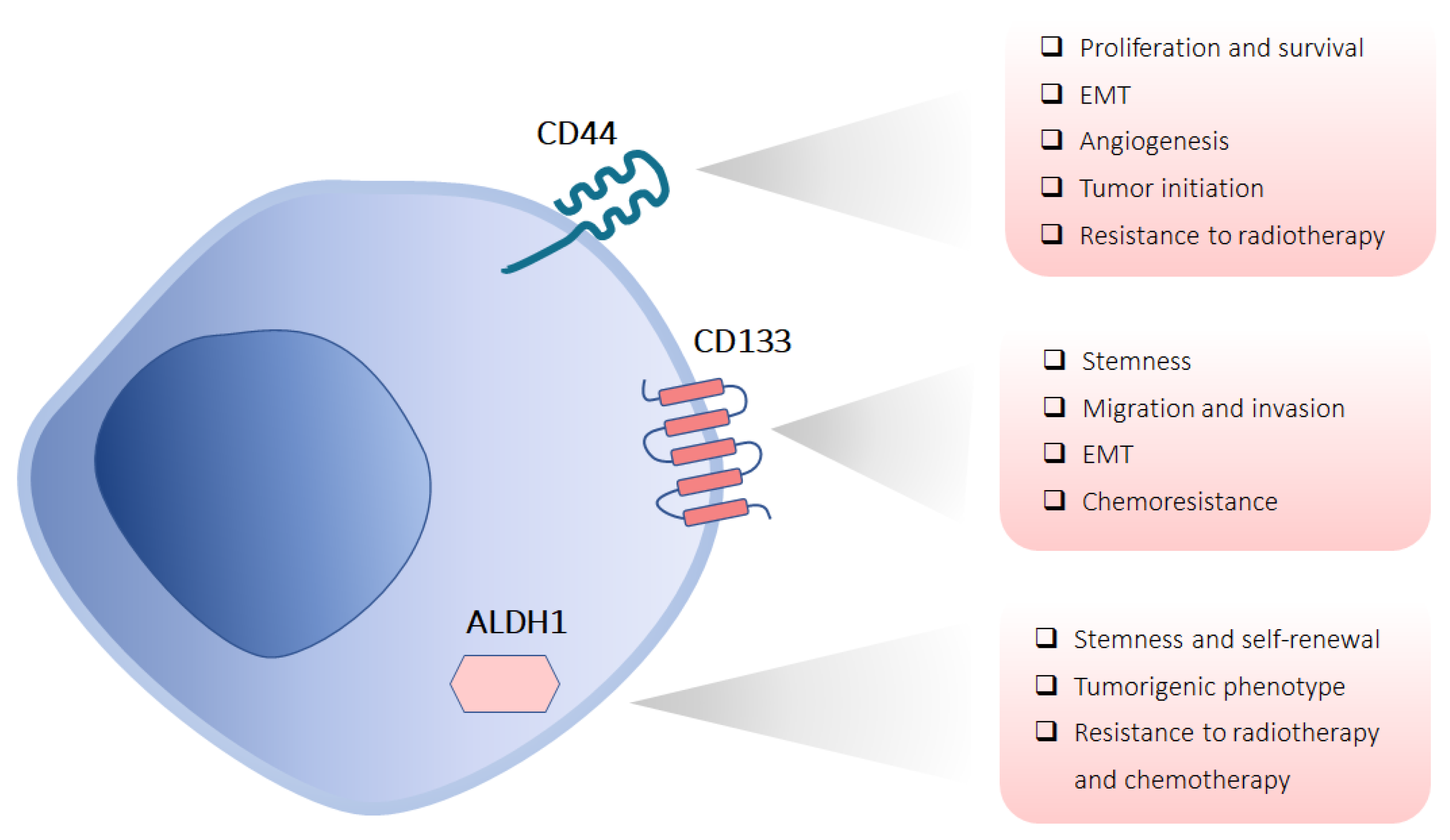
3.1.1. CD44
3.1.2. CD133
3.1.3. ALDH1
3.2. Side Population
3.3. Sphere-Forming Ability
4. Chemotherapy Resistance
5. Radiotherapy Resistance
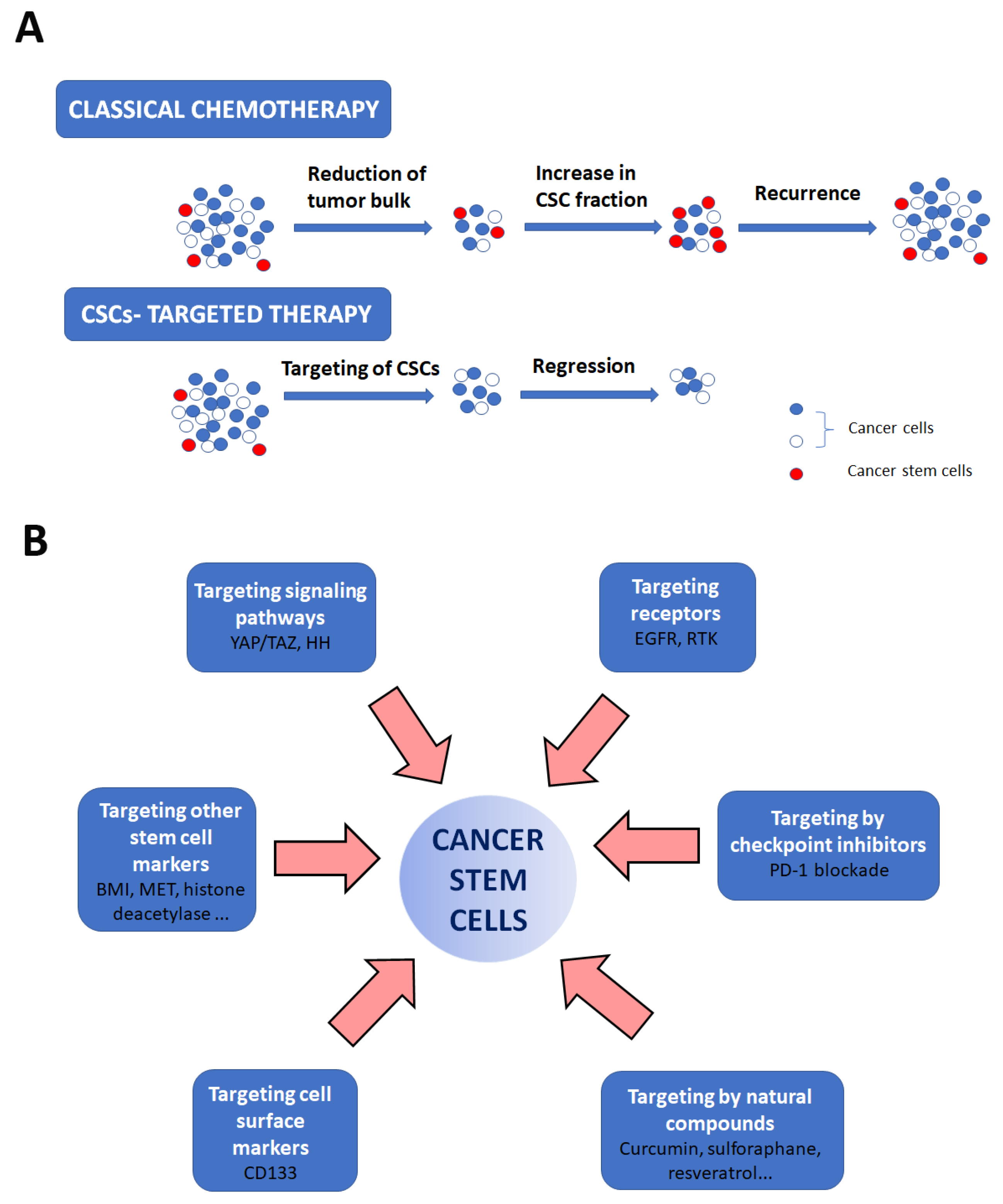
6. Therapeutic Targeting of CSCs
6.1. Targeting Cell Surface Markers
6.2. Targeting Other Stem Cell Markers
6.3. Targeting Signaling Pathways
6.4. Targeting Receptors
6.5. Targeting Metabolism
6.6. Targeting by Checkpoint Inhibitors
6.7. Targeting by Natural Compounds
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dang, S.; Zhang, S.; Zhao, J.; Li, X.; Li, W. Efficacy and safety of immune checkpoint inhibitors in recurrent or metastatic head and neck squamous cell carcinoma: A systematic review and meta-analysis of randomized clinical trials. Cancer Med. 2023, 12, 20277–20286. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wan, W.W.; Xiong, S.L.; Feng, H.; Wu, N. Cancer stem-like cells can be induced through dedifferentiation under hypoxic conditions in glioma, hepatoma and lung cancer. Cell Death Discov. 2017, 3, 16105. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhong, Z.; Huang, Y.; Deng, W.; Cao, J.; Tsao, G.; Liu, Q.; Pei, D.; Kang, T.; Zeng, Y.X. Stem-like cancer cells are inducible by increasing genomic instability in cancer cells. J. Biol. Chem. 2010, 285, 4931–4940. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, M.; Quintana, E.; Fearon, E.R.; Morrison, S.J. Heterogeneity in cancer: Cancer stem cells versus clonal evolution. Cell 2009, 138, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Altea-Manzano, P.; Cuadros, A.M.; Broadfield, L.A.; Fendt, S.M. Nutrient metabolism and cancer in the in vivo context: A metabolic game of give and take. EMBO Rep. 2020, 21, e50635. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. Evolutionary determinants of cancer. Cancer Discov. 2015, 5, 806–820. [Google Scholar] [CrossRef]
- Bolton, K.L.; Ptashkin, R.N.; Gao, T.; Braunstein, L.; Devlin, S.M.; Kelly, D.; Patel, M.; Berthon, A.; Syed, A.; Yabe, M.; et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat. Genet 2020, 52, 1219–1226. [Google Scholar] [CrossRef]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Nairuz, T.; Mahmud, Z.; Manik, R.K.; Kabir, Y. Cancer stem cells: An insight into the development of metastatic tumors and therapy resistance. Stem Cell Rev. Rep. 2023, 19, 1577–1595. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. How does multistep tumorigenesis really proceed? Cancer Discov. 2015, 5, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Liu, P.; Yu, P.; Qin, T. Cancer Stem Cells are Actually Stem Cells with Disordered Differentiation: The Monophyletic Origin of Cancer. Stem Cell Rev. Rep. 2023, 19, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Willert, K.; Jones, K.A. Wnt signaling: Is the party in the nucleus? Genes Dev. 2006, 20, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal. Transduct. Target Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Iwai, S.; Katagiri, W.; Kong, C.; Amekawa, S.; Nakazawa, M.; Yura, Y. Mutations of the APC, beta-catenin, and axin 1 genes and cytoplasmic accumulation of beta-catenin in oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2005, 131, 773–782. [Google Scholar] [CrossRef]
- Xie, J.; Huang, L.; Lu, Y.G.; Zheng, D.L. Roles of the Wnt Signaling Pathway in Head and Neck Squamous Cell Carcinoma. Front. Mol. Biosci. 2020, 7, 590912. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal. Transduct. Target Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Sakamoto, K. Notch signaling in oral squamous neoplasia. Pathol. Int. 2016, 66, 609–617. [Google Scholar] [CrossRef]
- Carballo, G.B.; Honorato, J.R.; de Lopes, G.P.F.; Spohr, T. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 2018, 16, 11. [Google Scholar] [CrossRef]
- Cierpikowski, P.; Leszczyszyn, A.; Bar, J. The Role of Hedgehog Signaling Pathway in Head and Neck Squamous Cell Carcinoma. Cells 2023, 12, 2083. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q.; Xu, L.; Chen, J.; Pu, Y.; Wang, L.; Sun, H.; Guo, Y.; Guo, C. Cancer stemness of CD10-positive cells regulated by Hedgehog pathway promotes the resistance to cisplatin in oral squamous cell carcinoma. Oral Dis. 2021, 27, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Z.; Huang, H.; Wang, H. Hedgehog signaling promotes multidrug resistance by regulation of ABC transporters in oral squamous cell carcinoma. J. Oral Pathol. Med. 2020, 49, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Gan, G.N.; Eagles, J.; Keysar, S.B.; Wang, G.; Glogowska, M.J.; Altunbas, C.; Anderson, R.T.; Le, P.N.; Morton, J.J.; Frederick, B.; et al. Hedgehog signaling drives radioresistance and stroma-driven tumor repopulation in head and neck squamous cancers. Cancer Res. 2014, 74, 7024–7036. [Google Scholar] [CrossRef] [PubMed]
- Patni, A.P.; Harishankar, M.K.; Joseph, J.P.; Sreeshma, B.; Jayaraj, R.; Devi, A. Comprehending the crosstalk between Notch, Wnt and Hedgehog signaling pathways in oral squamous cell carcinoma—Clinical implications. Cell Oncol. (Dordr.) 2021, 44, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tie, Y.; Alu, A.; Ma, X.; Shi, H. Targeted therapy for head and neck cancer: Signaling pathways and clinical studies. Signal. Transduct. Target Ther. 2023, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Bonner, J.A.; Bredel, M. EGFR Mutations in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 3818. [Google Scholar] [CrossRef]
- Marquard, F.E.; Jucker, M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem. Pharmacol. 2020, 172, 113729. [Google Scholar] [CrossRef]
- Tan, F.H.; Bai, Y.; Saintigny, P.; Darido, C. mTOR Signalling in Head and Neck Cancer: Heads Up. Cells 2019, 8, 333. [Google Scholar] [CrossRef]
- Faraji, F.; Ramirez, S.I.; Anguiano Quiroz, P.Y.; Mendez-Molina, A.N.; Gutkind, J.S. Genomic Hippo Pathway Alterations and Persistent YAP/TAZ Activation: New Hallmarks in Head and Neck Cancer. Cells 2022, 11, 1370. [Google Scholar] [CrossRef] [PubMed]
- Lian, I.; Kim, J.; Okazawa, H.; Zhao, J.; Zhao, B.; Yu, J.; Chinnaiyan, A.; Israel, M.A.; Goldstein, L.S.; Abujarour, R.; et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010, 24, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, X.; Maglic, D.; Dill, M.T.; Mojumdar, K.; Ng, P.K.; Jeong, K.J.; Tsang, Y.H.; Moreno, D.; Bhavana, V.H.; et al. Comprehensive Molecular Characterization of the Hippo Signaling Pathway in Cancer. Cell Rep. 2018, 25, 1304–1317.e5. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Noguchi, K.; Nakano, Y.; Yamamura, M.; Takaoka, K.; Hashimoto-Tamaoki, T.; Kishimoto, H. The Hippo pathway transcriptional co-activator, YAP, confers resistance to cisplatin in human oral squamous cell carcinoma. Int. J. Oncol. 2015, 46, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Zhu, Y.; Yuan, C.; Wang, D.; Zhang, W.; Qi, B.; Qiu, J.; Song, X.; Ye, J.; et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol. Oncol. 2015, 9, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Hiemer, S.E.; Zhang, L.; Kartha, V.K.; Packer, T.S.; Almershed, M.; Noonan, V.; Kukuruzinska, M.; Bais, M.V.; Monti, S.; Varelas, X. A YAP/TAZ-Regulated Molecular Signature Is Associated with Oral Squamous Cell Carcinoma. Mol. Cancer Res. 2015, 13, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Tsinias, G.; Nikou, S.; Mastronikolis, N.; Bravou, V.; Papadaki, H. Expression and prognostic significance of YAP, TAZ, TEAD4 and p73 in human laryngeal cancer. Histol. Histopathol. 2020, 35, 983–995. [Google Scholar]
- Liu, X.; Qiao, B.; Zhao, T.; Hu, F.; Lam, A.K.; Tao, Q. Sox2 promotes tumor aggressiveness and epithelial-mesenchymal transition in tongue squamous cell carcinoma. Int. J. Mol. Med. 2018, 42, 1418–1426. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, S.Y.; Do, S.I.; Lee, H.J.; Kang, H.J.; Rho, Y.S.; Bae, W.J.; Lim, Y.C. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br. J. Cancer 2014, 111, 2122–2130. [Google Scholar] [CrossRef]
- Boumahdi, S.; Driessens, G.; Lapouge, G.; Rorive, S.; Nassar, D.; Le Mercier, M.; Delatte, B.; Caauwe, A.; Lenglez, S.; Nkusi, E.; et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 2014, 511, 246–250. [Google Scholar] [CrossRef]
- Keysar, S.B.; Le, P.N.; Miller, B.; Jackson, B.C.; Eagles, J.R.; Nieto, C.; Kim, J.; Tang, B.; Glogowska, M.J.; Morton, J.J.; et al. Regulation of Head and Neck Squamous Cancer Stem Cells by PI3K and SOX2. J. Natl. Cancer Inst. 2017, 109, djw189. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.A.; Mannoor, K.; Cao, J.; Qadri, F.; Song, X. SOX2 in cancer stemness: Tumor malignancy and therapeutic potentials. J. Mol. Cell. Biol. 2020, 12, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Umemura, N.; Adachi, M.; Motoki, M.; Ohkoshi, E. ABCG2, CD44 and SOX9 are increased with the acquisition of drug resistance and involved in cancer stem cell activities in head and neck squamous cell carcinoma cells. Exp. Ther. Med. 2022, 24, 722. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ohnishi, Y.; Inoue, H.; Wato, M.; Tanaka, A.; Kakudo, K.; Nozaki, M. NANOG expression correlates with differentiation, metastasis and resistance to preoperative adjuvant therapy in oral squamous cell carcinoma. Oncol. Lett. 2014, 7, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, T.; Nath, N.; Mishra, P.; Jha, A.; Nagini, S.; Mishra, R. Pluripotency transcription factor Nanog and its association with overall oral squamous cell carcinoma progression, cisplatin-resistance, invasion and stemness acquisition. Head Neck 2020, 42, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yoon, C.; Zhou, X.H.; Zhou, Y.C.; Zhou, W.W.; Liu, H.; Yang, X.; Lu, J.; Lee, S.Y.; Huang, K. ERK1/2-Nanog signaling pathway enhances CD44(+) cancer stem-like cell phenotypes and epithelial-to-mesenchymal transition in head and neck squamous cell carcinomas. Cell Death Dis. 2020, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Major, A.G.; Pitty, L.P.; Farah, C.S. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int. 2013, 2013, 319489. [Google Scholar] [CrossRef]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Scholer, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef]
- Habu, N.; Imanishi, Y.; Kameyama, K.; Shimoda, M.; Tokumaru, Y.; Sakamoto, K.; Fujii, R.; Shigetomi, S.; Otsuka, K.; Sato, Y.; et al. Expression of Oct3/4 and Nanog in the head and neck squamous carcinoma cells and its clinical implications for delayed neck metastasis in stage I/II oral tongue squamous cell carcinoma. BMC Cancer 2015, 15, 730. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Dong, Z.; Vodopyanov, D.; Imai, A.; Helman, J.I.; Prince, M.E.; Wicha, M.S.; Nor, J.E. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010, 70, 9969–9978. [Google Scholar] [CrossRef]
- Nor, C.; Zhang, Z.; Warner, K.A.; Bernardi, L.; Visioli, F.; Helman, J.I.; Roesler, R.; Nor, J.E. Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia 2014, 16, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, M.; Li, Y.; Chang, I.; Yuan, Q.; Ekimyan-Salvo, M.; Deng, P.; Yu, B.; Yu, Y.; Dong, J.; et al. Targeting BMI1(+) Cancer Stem Cells Overcomes Chemoresistance and Inhibits Metastases in Squamous Cell Carcinoma. Cell Stem Cell 2017, 20, 621–634.e6. [Google Scholar] [CrossRef] [PubMed]
- Herzog, A.E.; Somayaji, R.; Nor, J.E. Bmi-1: A master regulator of head and neck cancer stemness. Front. Oral Health 2023, 4, 1080255. [Google Scholar] [CrossRef] [PubMed]
- Tsompana, M.; Gluck, C.; Sethi, I.; Joshi, I.; Bard, J.; Nowak, N.J.; Sinha, S.; Buck, M.J. Reactivation of super-enhancers by KLF4 in human Head and Neck Squamous Cell Carcinoma. Oncogene 2020, 39, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Paparella, M.L.; Abrigo, M.; Bal de Kier Joffe, E.; Raimondi, A.R. Oral-specific ablation of Klf4 disrupts epithelial terminal differentiation and increases premalignant lesions and carcinomas upon chemical carcinogenesis. J. Oral Pathol. Med. 2015, 44, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Rowland, B.D.; Bernards, R.; Peeper, D.S. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat. Cell Biol. 2005, 7, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.K.; Yang, M.H.; Chang, S.Y.; Chang, Y.C.; Li, W.Y.; Tsai, T.L.; Wang, Y.F.; Chu, P.Y.; Hsieh, S.L. Persistent Kruppel-like factor 4 expression predicts progression and poor prognosis of head and neck squamous cell carcinoma. Cancer Sci. 2011, 102, 895–902. [Google Scholar] [CrossRef]
- Ingruber, J.; Savic, D.; Steinbichler, T.B.; Sprung, S.; Fleischer, F.; Glueckert, R.; Schweigl, G.; Skvortsova, I.I.; Riechelmann, H.; Dudas, J. KLF4, Slug and EMT in Head and Neck Squamous Cell Carcinoma. Cells 2021, 10, 539. [Google Scholar] [CrossRef]
- Lan, L.; Behrens, A. Are There Specific Cancer Stem Cell Markers? Cancer Res. 2023, 83, 170–172. [Google Scholar] [CrossRef]
- Kim, W.T.; Ryu, C.J. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017, 50, 285–298. [Google Scholar] [CrossRef]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef] [PubMed]
- Rajarajan, A.; Stokes, A.; Bloor, B.K.; Ceder, R.; Desai, H.; Grafstrom, R.C.; Odell, E.W. CD44 expression in oro-pharyngeal carcinoma tissues and cell lines. PLoS ONE 2012, 7, e28776. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Szczepanski, M.J.; Gluszko, A.; Szafarowski, T.; Azambuja, J.H.; Dolg, L.; Gellrich, N.C.; Kampmann, A.; Whiteside, T.L.; Zimmerer, R.M. CD44(+) tumor cells promote early angiogenesis in head and neck squamous cell carcinoma. Cancer Lett. 2019, 467, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Klement, J.D.; Paschall, A.V.; Redd, P.S.; Ibrahim, M.L.; Lu, C.; Yang, D.; Celis, E.; Abrams, S.I.; Ozato, K.; Liu, K. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J. Clin. Investig. 2018, 128, 5549–5560. [Google Scholar] [CrossRef] [PubMed]
- Spiegelberg, D.; Kuku, G.; Selvaraju, R.; Nestor, M. Characterization of CD44 variant expression in head and neck squamous cell carcinomas. Tumour Biol. 2014, 35, 2053–2062. [Google Scholar] [CrossRef]
- Mishra, A.; Sriram, H.; Chandarana, P.; Tanavde, V.; Kumar, R.V.; Gopinath, A.; Govindarajan, R.; Ramaswamy, S.; Sadasivam, S. Decreased expression of cell adhesion genes in cancer stem-like cells isolated from primary oral squamous cell carcinomas. Tumour Biol. 2018, 40, 1010428318780859. [Google Scholar] [CrossRef]
- Skandalis, S.S.; Karalis, T.T.; Chatzopoulos, A.; Karamanos, N.K. Hyaluronan-CD44 axis orchestrates cancer stem cell functions. Cell Signal. 2019, 63, 109377. [Google Scholar] [CrossRef]
- Biddle, A.; Liang, X.; Gammon, L.; Fazil, B.; Harper, L.J.; Emich, H.; Costea, D.E.; Mackenzie, I.C. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res. 2011, 71, 5317–5326. [Google Scholar] [CrossRef]
- Biddle, A.; Gammon, L.; Liang, X.; Costea, D.E.; Mackenzie, I.C. Phenotypic Plasticity Determines Cancer Stem Cell Therapeutic Resistance in Oral Squamous Cell Carcinoma. eBioMedicine 2016, 4, 138–145. [Google Scholar] [CrossRef]
- Tolg, C.; Hofmann, M.; Herrlich, P.; Ponta, H. Splicing choice from ten variant exons establishes CD44 variability. Nucleic Acids Res. 1993, 21, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Earle, C.; Shiina, M. Activation of Matrix Hyaluronan-Mediated CD44 Signaling, Epigenetic Regulation and Chemoresistance in Head and Neck Cancer Stem Cells. Int. J. Mol. Sci. 2017, 18, 1849. [Google Scholar] [CrossRef] [PubMed]
- Franzmann, E.J.; Weed, D.T.; Civantos, F.J.; Goodwin, W.J.; Bourguignon, L.Y. A novel CD44 v3 isoform is involved in head and neck squamous cell carcinoma progression. Otolaryngol. Head Neck Surg. 2001, 124, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Sheng, W.Q.; Du, X. CD133: A cancer stem cells marker, is used in colorectal cancers. World J. Gastroenterol. 2013, 19, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. CD133: A stem cell biomarker and beyond. Exp. Hematol. Oncol. 2013, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Gao, W.; Li, F.; Bo, Y.; Zhu, M.; Fu, R.; Liu, Q.; Wen, S.; Wang, B. Identification and characterization of CD133(+)CD44(+) cancer stem cells from human laryngeal squamous cell carcinoma cell lines. J. Cancer 2017, 8, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, S.; Yen, Y.; Brown, J.; Ta, J.Q.; Le, A.D. A subpopulation of CD133(+) cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett. 2010, 289, 151–160. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, C.; Liu, X.; Fang, F.; Liu, S.; Liao, X.; Tao, S.; Mai, H. Characterisation of a subpopulation of CD133(+) cancer stem cells from Chinese patients with oral squamous cell carcinoma. Sci. Rep. 2020, 10, 8875. [Google Scholar] [CrossRef]
- Chen, Y.S.; Wu, M.J.; Huang, C.Y.; Lin, S.C.; Chuang, T.H.; Yu, C.C.; Lo, J.F. CD133/Src axis mediates tumor initiating property and epithelial-mesenchymal transition of head and neck cancer. PLoS ONE 2011, 6, e28053. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, L.; Wu, S.; Gong, X.; Feng, Z.; Ma, L.; Zhu, B.; Yao, N.; Wang, D.; Dong, H. Clinicopathological significance of cancer stem cells marked by CD133 and KAI1/CD82 expression in laryngeal squamous cell carcinoma. World J. Surg. Oncol. 2014, 12, 118. [Google Scholar] [CrossRef]
- de Moraes, F.P.; Lourenco, S.V.; Ianez, R.C.; de Sousa, E.A.; Silva, M.M.; Damascena, A.S.; Kowalski, L.P.; Soares, F.A.; Coutinho-Camillo, C.M. Expression of stem cell markers in oral cavity and oropharynx squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Tanaka, K.; Tanaka, T.; Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018–11032. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, Y.W.; Hsu, H.S.; Tseng, L.M.; Huang, P.I.; Lu, K.H.; Chen, D.T.; Tai, L.K.; Yung, M.C.; Chang, S.C.; et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem. Biophys. Res. Commun. 2009, 385, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Xu, X.R.; Wu, T.F.; Sun, Z.J.; Zhang, W.F. Correlation of ALDH1, CD44, OCT4 and SOX2 in tongue squamous cell carcinoma and their association with disease progression and prognosis. J. Oral Pathol. Med. 2014, 43, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Lo, W.L.; Chen, Y.W.; Huang, P.I.; Hsu, H.S.; Tseng, L.M.; Hung, S.C.; Kao, S.Y.; Chang, C.J.; Chiou, S.H. Bmi-1 Regulates Snail Expression and Promotes Metastasis Ability in Head and Neck Squamous Cancer-Derived ALDH1 Positive Cells. J. Oncol. 2011, 2011, 609259. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; Tsakmaki, V.; Danielidis, V.; Sivridis, E. Cancer stem cell phenotype relates to radio-chemotherapy outcome in locally advanced squamous cell head-neck cancer. Br. J. Cancer 2012, 106, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ren, Y.; Yu, X.; Qian, F.; Bian, B.S.; Xiao, H.L.; Wang, W.G.; Xu, S.L.; Yang, J.; Cui, W.; et al. ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma. Mod. Pathol. 2014, 27, 775–783. [Google Scholar] [CrossRef]
- Qian, X.; Wagner, S.; Ma, C.; Coordes, A.; Gekeler, J.; Klussmann, J.P.; Hummel, M.; Kaufmann, A.M.; Albers, A.E. Prognostic significance of ALDH1A1-positive cancer stem cells in patients with locally advanced, metastasized head and neck squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2014, 140, 1151–1158. [Google Scholar] [CrossRef]
- Sterz, C.M.; Kulle, C.; Dakic, B.; Makarova, G.; Bottcher, M.C.; Bette, M.; Werner, J.A.; Mandic, R. A basal-cell-like compartment in head and neck squamous cell carcinomas represents the invasive front of the tumor and is expressing MMP-9. Oral Oncol. 2010, 46, 116–122. [Google Scholar] [CrossRef]
- Hirschmann-Jax, C.; Foster, A.E.; Wulf, G.G.; Nuchtern, J.G.; Jax, T.W.; Gobel, U.; Goodell, M.A.; Brenner, M.K. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 14228–14233. [Google Scholar] [CrossRef]
- Wu, C.; Alman, B.A. Side population cells in human cancers. Cancer Lett. 2008, 268, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chang, I.; Chen, Z.; Kang, M.; Wang, C.Y. Characterization of side populations in HNSCC: Highly invasive, chemoresistant and abnormal Wnt signaling. PLoS ONE 2010, 5, e11456. [Google Scholar] [CrossRef] [PubMed]
- Loebinger, M.R.; Giangreco, A.; Groot, K.R.; Prichard, L.; Allen, K.; Simpson, C.; Bazley, L.; Navani, N.; Tibrewal, S.; Davies, D.; et al. Squamous cell cancers contain a side population of stem-like cells that are made chemosensitive by ABC transporter blockade. Br. J. Cancer 2008, 98, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Tabor, M.H.; Clay, M.R.; Owen, J.H.; Bradford, C.R.; Carey, T.E.; Wolf, G.T.; Prince, M.E. Head and neck cancer stem cells: The side population. Laryngoscope 2011, 121, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Dong, P.; Li, D.; Gao, S. Expression and function of ABCG2 in head and neck squamous cell carcinoma and cell lines. Exp. Ther. Med. 2011, 2, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Nor, J.E. Orosphere assay: A method for propagation of head and neck cancer stem cells. Head Neck 2013, 35, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Oh, S.Y.; Cha, Y.Y.; Kim, S.H.; Jin, X.; Kim, H. Cancer stem cell traits in squamospheres derived from primary head and neck squamous cell carcinomas. Oral Oncol. 2011, 47, 83–91. [Google Scholar] [CrossRef]
- Pozzi, V.; Sartini, D.; Rocchetti, R.; Santarelli, A.; Rubini, C.; Morganti, S.; Giuliante, R.; Calabrese, S.; Di Ruscio, G.; Orlando, F.; et al. Identification and characterization of cancer stem cells from head and neck squamous cell carcinoma cell lines. Cell Physiol. Biochem. 2015, 36, 784–798. [Google Scholar] [CrossRef]
- Kaseb, H.O.; Fohrer-Ting, H.; Lewis, D.W.; Lagasse, E.; Gollin, S.M. Identification, expansion and characterization of cancer cells with stem cell properties from head and neck squamous cell carcinomas. Exp. Cell Res. 2016, 348, 75–86. [Google Scholar] [CrossRef]
- Kuch, V.; Schreiber, C.; Thiele, W.; Umansky, V.; Sleeman, J.P. Tumor-initiating properties of breast cancer and melanoma cells in vivo are not invariably reflected by spheroid formation in vitro, but can be increased by long-term culturing as adherent monolayers. Int. J. Cancer 2013, 132, E94–E105. [Google Scholar] [CrossRef]
- Lee, J.; Park, M.; Ko, Y.; Kim, B.; Kim, O.; Hyun, H.; Kim, D.; Sohn, H.; Moon, Y.L.; Lim, W. Ectopic overexpression of CD133 in HNSCC makes it resistant to commonly used chemotherapeutics. Tumour Biol. 2017, 39, 1010428317695534. [Google Scholar] [CrossRef] [PubMed]
- Kulsum, S.; Sudheendra, H.V.; Pandian, R.; Ravindra, D.R.; Siddappa, G.; Nisheena, R.; Chevour, P.; Ramachandran, B.; Sagar, M.; Jayaprakash, A.; et al. Cancer stem cell mediated acquired chemoresistance in head and neck cancer can be abrogated by aldehyde dehydrogenase 1 A1 inhibition. Mol. Carcinog. 2017, 56, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Melissaridou, S.; Wiechec, E.; Magan, M.; Jain, M.V.; Chung, M.K.; Farnebo, L.; Roberg, K. The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int. 2019, 19, 16. [Google Scholar] [CrossRef]
- Lu, B.C.; Li, J.; Yu, W.F.; Zhang, G.Z.; Wang, H.M.; Ma, H.M. Elevated expression of Nrf2 mediates multidrug resistance in CD133(+) head and neck squamous cell carcinoma stem cells. Oncol. Lett. 2016, 12, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- McDermott, S.C.; Rodriguez-Ramirez, C.; McDermott, S.P.; Wicha, M.S.; Nor, J.E. FGFR signaling regulates resistance of head and neck cancer stem cells to cisplatin. Oncotarget 2018, 9, 25148–25165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, Z.; Gao, N.; Xiong, G.; Chen, P.; Li, H.; Chen, D.; He, Q.; Peng, L. SOX18 meditates the resistance of Bmi1-expressing cells to cetuximab in HNSCC. Oral Dis. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Magan, M.; Wiechec, E.; Roberg, K. CAFs affect the proliferation and treatment response of head and neck cancer spheroids during co-culturing in a unique in vitro model. Cancer Cell Int. 2020, 20, 599. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.F.; Tang, X.X.; Zhang, D.J.; Zheng, Y.; Yu, D.J.; Zhao, Y.; Lu, Y.Q.; Zhu, L. Constitutive secretion of Interleukin-4 dictates CD133+ side population cells to resist drug treatment and cell death. J. BUON 2015, 20, 1350–1359. [Google Scholar]
- Reid, P.A.; Wilson, P.; Li, Y.; Marcu, L.G.; Bezak, E. Current understanding of cancer stem cells: Review of their radiobiology and role in head and neck cancers. Head Neck 2017, 39, 1920–1932. [Google Scholar] [CrossRef]
- Zhang, M.; Atkinson, R.L.; Rosen, J.M. Selective targeting of radiation-resistant tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2010, 107, 3522–3527. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, S.L.; Finniss, S.; Xiang, C.; Decarvalho, A.; Umansky, F.; Kalkanis, S.N.; Mikkelsen, T.; Brodie, C. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int. J. Cancer 2009, 125, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Rovida, E.; Peppicelli, S.; Bono, S.; Bianchini, F.; Tusa, I.; Cheloni, G.; Marzi, I.; Cipolleschi, M.G.; Calorini, L.; Sbarba, P.D. The metabolically-modulated stem cell niche: A dynamic scenario regulating cancer cell phenotype and resistance to therapy. Cell Cycle 2014, 13, 3169–3175. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Dubrovska, A.; Linge, A.; Baumann, M. Cancer stem cells: Radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv. Drug Deliv. Rev. 2017, 109, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Gupta, R.; Mishra, A.; Kumar, V.; Bhadauria, S.; Bhatt, M.L.B. Evaluation of correlation between CD44, radiotherapy response, and survival rate in patients with advanced stage of head and neck squamous cell carcinoma (HNSCC). Cancer Med. 2022, 11, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.C.; La Fleur, L.; Melissaridou, S.; Roberg, K. The relationship between EMT, CD44(high)/EGFR(low) phenotype, and treatment response in head and neck cancer cell lines. J. Oral Pathol. Med. 2016, 45, 640–646. [Google Scholar] [CrossRef] [PubMed]
- La Fleur, L.; Johansson, A.C.; Roberg, K. A CD44high/EGFRlow subpopulation within head and neck cancer cell lines shows an epithelial-mesenchymal transition phenotype and resistance to treatment. PLoS ONE 2012, 7, e44071. [Google Scholar] [CrossRef]
- Park, S.J.; Min, H.J.; Yoon, C.; Kim, S.H.; Kim, J.H.; Lee, S.Y. Integrin beta1 regulates the perineural invasion and radioresistance of oral squamous carcinoma cells by modulating cancer cell stemness. Cell Signal. 2023, 110, 110808. [Google Scholar] [CrossRef]
- Nathansen, J.; Lukiyanchuk, V.; Hein, L.; Stolte, M.I.; Borgmann, K.; Lock, S.; Kurth, I.; Baumann, M.; Krause, M.; Linge, A.; et al. Oct4 confers stemness and radioresistance to head and neck squamous cell carcinoma by regulating the homologous recombination factors PSMC3IP and RAD54L. Oncogene 2021, 40, 4214–4228. [Google Scholar] [CrossRef]
- Kurth, I.; Hein, L.; Mabert, K.; Peitzsch, C.; Koi, L.; Cojoc, M.; Kunz-Schughart, L.; Baumann, M.; Dubrovska, A. Cancer stem cell related markers of radioresistance in head and neck squamous cell carcinoma. Oncotarget 2015, 6, 34494–34509. [Google Scholar] [CrossRef]
- Suzuki, H.; Kawasaki, Y.; Suzuki, S.; Yamada, T.; Ito, A.; Suzuki, M.; Miura, M.; Hatakeyama, H.; Omori, Y. CD98 expression can be a predictive factor of resistance to radiotherapy in head and neck squamous cell carcinoma. Pol. J. Pathol. 2023, 74, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Digomann, D.; Kurth, I.; Tyutyunnykova, A.; Chen, O.; Lock, S.; Gorodetska, I.; Peitzsch, C.; Skvortsova, I.I.; Negro, G.; Aschenbrenner, B.; et al. The CD98 Heavy Chain Is a Marker and Regulator of Head and Neck Squamous Cell Carcinoma Radiosensitivity. Clin. Cancer Res. 2019, 25, 3152–3163. [Google Scholar] [CrossRef] [PubMed]
- Moncharmont, C.; Guy, J.B.; Wozny, A.S.; Gilormini, M.; Battiston-Montagne, P.; Ardail, D.; Beuve, M.; Alphonse, G.; Simoens, X.; Rancoule, C.; et al. Carbon ion irradiation withstands cancer stem cells’ migration/invasion process in Head and Neck Squamous Cell Carcinoma (HNSCC). Oncotarget 2016, 7, 47738–47749. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.B.; Espenel, S.; Louati, S.; Gauthier, A.; Garcia, M.A.; Vial, N.; Malesys, C.; Ardail, D.; Alphonse, G.; Wozny, A.S.; et al. Combining radiation to EGFR and Bcl-2 blockade: A new approach to target cancer stem cells in head and neck squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2021, 147, 1905–1916. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Suzuki, H.; Suzuki, S.; Yamada, T.; Suzuki, M.; Ito, A.; Hatakeyama, H.; Miura, M.; Omori, Y. GPNMB-Positive Cells in Head and Neck Squamous Cell Carcinoma-Their Roles in Cancer Stemness, Therapy Resistance, and Metastasis. Pathol. Oncol. Res. 2022, 28, 1610450. [Google Scholar] [CrossRef] [PubMed]
- Elkashty, O.A.; Abu Elghanam, G.; Su, X.; Liu, Y.; Chauvin, P.J.; Tran, S.D. Cancer stem cells enrichment with surface markers CD271 and CD44 in human head and neck squamous cell carcinomas. Carcinogenesis 2020, 41, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, C.Y. Targeting cancer stem cells in squamous cell carcinoma. Precis. Clin. Med. 2019, 2, 152–165. [Google Scholar] [CrossRef]
- Mai, Y.; Su, J.; Yang, C.; Xia, C.; Fu, L. The strategies to cure cancer patients by eradicating cancer stem-like cells. Mol. Cancer 2023, 22, 171. [Google Scholar] [CrossRef]
- Waldron, N.N.; Barsky, S.H.; Dougherty, P.R.; Vallera, D.A. A bispecific EpCAM/CD133-targeted toxin is effective against carcinoma. Target Oncol. 2014, 9, 239–249. [Google Scholar] [CrossRef]
- Lai, Y.J.; Yu, W.N.; Kuo, S.C.; Ho, C.T.; Hung, C.M.; Way, T.D.; Chen, C.T. CSC-3436 inhibits TWIST-induced epithelial-mesenchymal transition via the suppression of Twist/Bmi1/Akt pathway in head and neck squamous cell carcinoma. J. Cell Physiol. 2019, 234, 9118–9129. [Google Scholar] [CrossRef]
- Yu, C.C.; Hu, F.W.; Yu, C.H.; Chou, M.Y. Targeting CD133 in the enhancement of chemosensitivity in oral squamous cell carcinoma-derived side population cancer stem cells. Head Neck 2016, 38 (Suppl. S1), E231–E238. [Google Scholar] [CrossRef]
- Kalish, J.M.; Tang, X.H.; Scognamiglio, T.; Zhang, T.; Gudas, L.J. Doxycycline-induced exogenous Bmi-1 expression enhances tumor formation in a murine model of oral squamous cell carcinoma. Cancer Biol. Ther. 2020, 21, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Mirshahidi, S.; Simental, A.; Lee, S.C.; De Andrade Filho, P.A.; Peterson, N.R.; Duerksen-Hughes, P.; Yuan, X. Cancer stem cell self-renewal as a therapeutic target in human oral cancer. Oncogene 2019, 38, 5440–5456. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Schober, M. Joining Forces: Bmi1 Inhibition and Cisplatin Curb Squamous Carcinogenesis. Cell Stem Cell 2017, 20, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, W.; Wang, C.Y. BMI1 Inhibition Eliminates Residual Cancer Stem Cells after PD1 Blockade and Activates Antitumor Immunity to Prevent Metastasis and Relapse. Cell Stem Cell 2020, 27, 238–253.e6. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Qian, X.; Ochsenreither, S.; Soldano, F.; DeLeo, A.B.; Sudhoff, H.; Oppel, F.; Kuppig, A.; Klinghammer, K.; Kaufmann, A.M.; et al. Disulfiram Acts as a Potent Radio-Chemo Sensitizer in Head and Neck Squamous Cell Carcinoma Cell Lines and Transplanted Xenografts. Cells 2021, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Kerk, S.A.; Finkel, K.A.; Pearson, A.T.; Warner, K.A.; Zhang, Z.; Nor, F.; Wagner, V.P.; Vargas, P.A.; Wicha, M.S.; Hurt, E.M.; et al. 5T4-Targeted Therapy Ablates Cancer Stem Cells and Prevents Recurrence of Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2017, 23, 2516–2527. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, H.; Luo, W.; Zhang, Q.; Liu, J.; Yao, K. 5T4-specific chimeric antigen receptor modification promotes the immune efficacy of cytokine-induced killer cells against nasopharyngeal carcinoma stem cell-like cells. Sci. Rep. 2017, 7, 4859. [Google Scholar] [CrossRef]
- Tavares, M.O.; Milan, T.M.; Bighetti-Trevisan, R.L.; Leopoldino, A.M.; de Almeida, L.O. Pharmacological inhibition of HDAC6 overcomes cisplatin chemoresistance by targeting cancer stem cells in oral squamous cell carcinoma. J. Oral Pathol. Med. 2022, 51, 529–537. [Google Scholar] [CrossRef]
- Marques, A.E.M.; do Nascimento Filho, C.H.V.; Marinho Bezerra, T.M.; Guerra, E.N.S.; Castilho, R.M.; Squarize, C.H. Entinostat is a novel therapeutic agent to treat oral squamous cell carcinoma. J. Oral Pathol. Med. 2020, 49, 771–779. [Google Scholar] [CrossRef]
- Khedkar, H.N.; Chen, L.C.; Kuo, Y.C.; Wu, A.T.H.; Huang, H.S. Multi-Omics Identification of Genetic Alterations in Head and Neck Squamous Cell Carcinoma and Therapeutic Efficacy of HNC018 as a Novel Multi-Target Agent for c-MET/STAT3/AKT Signaling Axis. Int. J. Mol. Sci. 2023, 24, 10247. [Google Scholar] [CrossRef] [PubMed]
- Luttich, L.; Besso, M.J.; Heiden, S.; Koi, L.; Baumann, M.; Krause, M.; Dubrovska, A.; Linge, A.; Kurth, I.; Peitzsch, C. Tyrosine Kinase c-MET as Therapeutic Target for Radiosensitization of Head and Neck Squamous Cell Carcinomas. Cancers 2021, 13, 1865. [Google Scholar] [CrossRef] [PubMed]
- Milan, T.M.; Eskenazi, A.P.E.; Oliveira, L.D.; Silva, G.D.; Bighetti-Trevisan, R.L.; Freitas, G.P.; Almeida, L.O. Interplay between EZH2/beta-catenin in stemness of cisplatin-resistant HNSCC and their role as therapeutic targets. Cell Signal. 2023, 109, 110773. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, X.; Li, J.; Yu, S.; Ke, X.; Yan, T.; Zhu, Y.; Cheng, J.; Yang, J. HMGA2-Snai2 axis regulates tumorigenicity and stemness of head and neck squamous cell carcinoma. Exp. Cell Res. 2022, 418, 113271. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Lee, S.H.; Koo, B.S.; Kim, J.M.; Huang, S.; Cho, J.H.; Eun, Y.G.; Shin, H.A.; Lim, Y.C. Slug is a novel molecular target for head and neck squamous cell carcinoma stem-like cells. Oral Oncol. 2020, 111, 104948. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, J.; Li, Y.; Ma, Z.; Yu, Y.; Wang, C.Y. Transcriptional super-enhancers control cancer stemness and metastasis genes in squamous cell carcinoma. Nat. Commun. 2021, 12, 3974. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, W.; Liu, S.; Wang, Y.; Peng, X.; Jia, L. PVT1 inhibition stimulates anti-tumor immunity, prevents metastasis, and depletes cancer stem cells in squamous cell carcinoma. Cell Death Dis. 2023, 14, 187. [Google Scholar] [CrossRef]
- Garcia-Mayea, Y.; Mir, C.; Carballo, L.; Castellvi, J.; Temprana-Salvador, J.; Lorente, J.; Benavente, S.; Garcia-Pedrero, J.M.; Allonca, E.; Rodrigo, J.P.; et al. TSPAN1: A Novel Protein Involved in Head and Neck Squamous Cell Carcinoma Chemoresistance. Cancers 2020, 12, 3269. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.C.; Wu, L.; Yu, G.T.; Zhang, W.F.; Huang, C.F.; Sun, Z.J. TRAF6 regulates tumour metastasis through EMT and CSC phenotypes in head and neck squamous cell carcinoma. J. Cell Mol. Med. 2018, 22, 1337–1349. [Google Scholar] [CrossRef]
- Subramanian, C.; Kovatch, K.J.; Sim, M.W.; Wang, G.; Prince, M.E.; Carey, T.E.; Davis, R.; Blagg, B.S.J.; Cohen, M.S. Novel C-Terminal Heat Shock Protein 90 Inhibitors (KU711 and Ku757) Are Effective in Targeting Head and Neck Squamous Cell Carcinoma Cancer Stem cells. Neoplasia 2017, 19, 1003–1011. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wu, Y.; Wang, Y.; Wang, D.; Zhang, W.; Yuan, H.; Ye, J.; Song, X.; Yang, J.; et al. The Hippo effector TAZ promotes cancer stemness by transcriptional activation of SOX2 in head neck squamous cell carcinoma. Cell Death Dis. 2019, 10, 603. [Google Scholar] [CrossRef]
- Omori, H.; Sato, K.; Nakano, T.; Wakasaki, T.; Toh, S.; Taguchi, K.; Nakagawa, T.; Masuda, M. Stress-triggered YAP1/SOX2 activation transcriptionally reprograms head and neck squamous cell carcinoma for the acquisition of stemness. J. Cancer Res. Clin. Oncol. 2019, 145, 2433–2444. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Zhang, L.; Huang, C.F.; Ma, S.R.; Bu, L.L.; Liu, J.F.; Yu, G.T.; Liu, B.; Gutkind, J.S.; Kulkarni, A.B.; et al. NOTCH1 inhibition enhances the efficacy of conventional chemotherapeutic agents by targeting head neck cancer stem cell. Sci. Rep. 2016, 6, 24704. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Do, S.I.; Lee, H.J.; Kang, H.J.; Koo, B.S.; Lim, Y.C. Notch1 signaling contributes to stemness in head and neck squamous cell carcinoma. Lab. Investig. 2016, 96, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Kalafut, J.; Czerwonka, A.; Anameric, A.; Przybyszewska-Podstawka, A.; Misiorek, J.O.; Rivero-Muller, A.; Nees, M. Shooting at Moving and Hidden Targets-Tumour Cell Plasticity and the Notch Signalling Pathway in Head and Neck Squamous Cell Carcinomas. Cancers 2021, 13, 6219. [Google Scholar] [CrossRef]
- Rodrigues, M.; Miguita, L.; De Andrade, N.P.; Heguedusch, D.; Rodini, C.O.; Moyses, R.A.; Toporcov, T.N.; Gama, R.R.; Tajara, E.E.; Nunes, F.D. GLI3 knockdown decreases stemness, cell proliferation and invasion in oral squamous cell carcinoma. Int. J. Oncol. 2018, 53, 2458–2472. [Google Scholar] [CrossRef] [PubMed]
- Kleszcz, R.; Frackowiak, M.; Dorna, D.; Paluszczak, J. Combinations of PRI-724 Wnt/beta-Catenin Pathway Inhibitor with Vismodegib, Erlotinib, or HS-173 Synergistically Inhibit Head and Neck Squamous Cancer Cells. Int. J. Mol. Sci. 2023, 24, 10448. [Google Scholar] [CrossRef]
- Lv, X.X.; Zheng, X.Y.; Yu, J.J.; Ma, H.R.; Hua, C.; Gao, R.T. EGFR enhances the stemness and progression of oral cancer through inhibiting autophagic degradation of SOX2. Cancer Med. 2020, 9, 1131–1140. [Google Scholar] [CrossRef]
- Sato, F.; Kubota, Y.; Natsuizaka, M.; Maehara, O.; Hatanaka, Y.; Marukawa, K.; Terashita, K.; Suda, G.; Ohnishi, S.; Shimizu, Y.; et al. EGFR inhibitors prevent induction of cancer stem-like cells in esophageal squamous cell carcinoma by suppressing epithelial-mesenchymal transition. Cancer Biol. Ther. 2015, 16, 933–940. [Google Scholar] [CrossRef]
- Setubal Destro Rodrigues, M.F.; Gammon, L.; Rahman, M.M.; Biddle, A.; Nunes, F.D.; Mackenzie, I.C. Effects of Cetuximab and Erlotinib on the behaviour of cancer stem cells in head and neck squamous cell carcinoma. Oncotarget 2018, 9, 13488–13500. [Google Scholar] [CrossRef]
- Tanei, T.; Choi, D.S.; Rodriguez, A.A.; Liang, D.H.; Dobrolecki, L.; Ghosh, M.; Landis, M.D.; Chang, J.C. Antitumor activity of Cetuximab in combination with Ixabepilone on triple negative breast cancer stem cells. Breast Cancer Res. 2016, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Li, G.; Lei, C.; Qian, K.; Zhang, S.; Zhao, J.; Hu, S. Bispecific antibodies targeting EGFR/Notch enhance the response to talazoparib by decreasing tumour-initiating cell frequency. Theranostics 2023, 13, 3641–3654. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, F.; Zotti, A.I.; Roca, M.S.; Grumetti, L.; Lombardi, R.; Moccia, T.; Vitagliano, C.; Milone, M.R.; Ciardiello, C.; Bruzzese, F.; et al. Valproic Acid Synergizes With Cisplatin and Cetuximab in vitro and in vivo in Head and Neck Cancer by Targeting the Mechanisms of Resistance. Front. Cell Dev. Biol. 2020, 8, 732. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.B.; Mery, B.; Ollier, E.; Espenel, S.; Vallard, A.; Wozny, A.S.; Simonet, S.; Lauret, A.; Battiston-Montagne, P.; Ardail, D.; et al. Dual “mAb” HER family blockade in head and neck cancer human cell lines combined with photon therapy. Sci. Rep. 2017, 7, 12207. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, Y.; Sedhom, W.; Lu, L.; Liu, Y.; Wang, H.; Oka, M.; Bornstein, S.; Said, S.; Song, J.; et al. Distinct roles of PIK3CA in the enrichment and maintenance of cancer stem cells in head and neck squamous cell carcinoma. Mol. Oncol. 2020, 14, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Teijeiro, S.; Garcia-Inclan, C.; Villaronga, M.A.; Casado, P.; Hermida-Prado, F.; Granda-Diaz, R.; Rodrigo, J.P.; Calvo, F.; Del-Rio-Ibisate, N.; Gandarillas, A.; et al. Factors Secreted by Cancer-Associated Fibroblasts that Sustain Cancer Stem Properties in Head and Neck Squamous Carcinoma Cells as Potential Therapeutic Targets. Cancers 2018, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Macha, M.A.; Rachagani, S.; Qazi, A.K.; Jahan, R.; Gupta, S.; Patel, A.; Seshacharyulu, P.; Lin, C.; Li, S.; Wang, S.; et al. Afatinib radiosensitizes head and neck squamous cell carcinoma cells by targeting cancer stem cells. Oncotarget 2017, 8, 20961–20973. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Peiris-Pages, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 113. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, J.; Qiao, X. Research Progress of Metformin in the Treatment of Oral Squamous Cell Carcinoma. Endocrinology 2023, 164, bqad139. [Google Scholar] [CrossRef]
- Patil, S. Metformin treatment decreases the expression of cancer stem cell marker CD44 and stemness related gene expression in primary oral cancer cells. Arch. Oral Biol. 2020, 113, 104710. [Google Scholar] [CrossRef]
- Marles, H.; Biddle, A. Cancer stem cell plasticity and its implications in the development of new clinical approaches for oral squamous cell carcinoma. Biochem. Pharmacol. 2022, 204, 115212. [Google Scholar] [CrossRef]
- Han, Y.; Xu, S.; Ye, W.; Wang, Y.; Zhang, X.; Deng, J.; Zhang, Z.; Liu, L.; Liu, S. Targeting LSD1 suppresses stem cell-like properties and sensitizes head and neck squamous cell carcinoma to PD-1 blockade. Cell Death Dis. 2021, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Q.C.; Li, Y.C.; Yang, L.L.; Liu, J.F.; Li, H.; Xiao, Y.; Bu, L.L.; Zhang, W.F.; Sun, Z.J. Targeting CMTM6 Suppresses Stem Cell-Like Properties and Enhances Antitumor Immunity in Head and Neck Squamous Cell Carcinoma. Cancer Immunol. Res. 2020, 8, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, L.L.; Junior, T.C.T.; Rangel, M.C. Sulforaphane: An emergent anti-cancer stem cell agent. Front. Oncol. 2023, 13, 1089115. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, C.; McKee, D.L. The “Big Five” Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol and Genistein. Curr. Med. Chem. 2021, 28, 4321–4342. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Chatterjee, S.; Hembram, K.C.; Sethy, C.; Mandal, M.; Kundu, C.N. Nano formulated Resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. J. Nutr. Biochem. 2021, 92, 108624. [Google Scholar] [CrossRef] [PubMed]
- Amorntaveechai, A.; Osathanon, T.; Pavasant, P.; Sooampon, S. Effect of resveratrol and oxyresveratrol on deferoxamine-induced cancer stem cell marker expression in human head and neck squamous cell carcinoma. J. Oral Biol. Craniofac. Res. 2022, 12, 253–257. [Google Scholar] [CrossRef]
- Lin, C.S.; Bamodu, O.A.; Kuo, K.T.; Huang, C.M.; Liu, S.C.; Wang, C.H.; Tzeng, Y.M.; Chao, T.Y.; Yeh, C.T. Investigation of ovatodiolide, a macrocyclic diterpenoid, as a potential inhibitor of oral cancer stem-like cells properties via the inhibition of the JAK2/STAT3/JARID1B signal circuit. Phytomedicine 2018, 46, 93–103. [Google Scholar] [CrossRef]
- de Camargo, M.R.; Frazon, T.F.; Inacio, K.K.; Smiderle, F.R.; Amor, N.G.; Dionisio, T.J.; Santos, C.F.; Rodini, C.O.; Lara, V.S. Ganoderma lucidum polysaccharides inhibit in vitro tumorigenesis, cancer stem cell properties and epithelial-mesenchymal transition in oral squamous cell carcinoma. J. Ethnopharmacol. 2022, 286, 114891. [Google Scholar] [CrossRef]
- Chen, P.Y.; Chao, S.C.; Hsieh, P.L.; Liao, Y.W.; Chu, P.M.; Harn, H.J.; Yu, C.C. Butylidenephthalide Abrogates the Snail-Induced Cancer Stemness in Oral Carcinomas. Int. J. Mol. Sci. 2022, 23, 6157. [Google Scholar] [CrossRef]
- Patel, H.; Joshi, J.; Raval, A.; Shah, F. Identification of Natural Compounds to Inhibit Sonic Hedgehog Pathway in Oral Cancer. Anticancer Agents Med. Chem. 2022, 22, 905–913. [Google Scholar] [PubMed]
- Chang, M.T.; Lee, S.P.; Fang, C.Y.; Hsieh, P.L.; Liao, Y.W.; Lu, M.Y.; Tsai, L.L.; Yu, C.C.; Liu, C.M. Chemosensitizing effect of honokiol in oral carcinoma stem cells via regulation of IL-6/Stat3 signaling. Environ. Toxicol. 2018, 33, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.Y.; Yu, C.C.; Huang, C.C.; Liao, Y.W.; Hsieh, P.L.; Chu, P.M.; Yu, C.H.; Lin, S.S. Magnolol inhibits cancer stemness and IL-6/Stat3 signaling in oral carcinomas. J. Formos. Med. Assoc. 2022, 121 Pt 1, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.Y.; Hsieh, P.L.; Wang, T.H.; Yu, C.C.; Lu, M.Y.; Liao, Y.W.; Lee, T.H.; Peng, C.Y. Andrographolide impedes cancer stemness and enhances radio-sensitivity in oral carcinomas via miR-218 activation. Oncotarget 2017, 8, 4196–4207. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.K.; Shih, P.H.; Lee, W.H.; Bamodu, O.A.; Wu, A.T.H.; Huang, C.C.; Tzeng, Y.M.; Hsiao, M.; Yeh, C.T.; Lin, C.M. Antrodia cinnamomea sensitizes radio-/chemo-therapy of cancer stem-like cells by modulating microRNA expression. J. Ethnopharmacol. 2017, 207, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kantapan, J.; Dechsupa, N.; Tippanya, D.; Nobnop, W.; Chitapanarux, I. Gallotannin from Bouea macrophylla Seed Extract Suppresses Cancer Stem-like Cells and Radiosensitizes Head and Neck Cancer. Int. J. Mol. Sci. 2021, 22, 9253. [Google Scholar] [CrossRef] [PubMed]
- Nor, F.; Nor, C.; Bento, L.W.; Zhang, Z.; Bretz, W.A.; Nor, J.E. Propolis reduces the stemness of head and neck squamous cell carcinoma. Arch. Oral Biol. 2021, 125, 105087. [Google Scholar] [CrossRef]
- Qi, W.; Zhu, F.; Wang, M.; Teng, Z.; Xu, R.; Xi, Y.; Meng, Q.; Wu, X.; Zhao, H.; Ma, M.; et al. The Antitumoral Effect of Paris Saponin II on Head and Neck Squamous Cell Carcinomas Mediated via the Nitric Oxide Metabolic Pathway. Front. Cell Dev. Biol. 2021, 9, 803981. [Google Scholar] [CrossRef]
- Ahn, K.; Ji, H.; Kim, H.E.; Cho, H.; Sun, Q.; Shi, S.; He, Y.; Kim, B.G.; Kim, O. Raphanus sativus L. seed extracts induce apoptosis and reduce migration of oral squamous cell carcinoma KB and KB(CD133+)cells by downregulation of beta-catenin. Nutr. Cancer 2020, 72, 1378–1389. [Google Scholar] [CrossRef]
- Elkashty, O.A.; Tran, S.D. Broccoli extract increases drug-mediated cytotoxicity towards cancer stem cells of head and neck squamous cell carcinoma. Br. J. Cancer 2020, 123, 1395–1403. [Google Scholar] [CrossRef]
- Farag, A.F.; Hassabou, N.F. CD24-gold nanocomposite as promising and sensitive biomarker for cancer stem cells in salivary gland tumors. Nanomedicine 2022, 46, 102598. [Google Scholar] [CrossRef] [PubMed]
- Ning, N.; Pan, Q.; Zheng, F.; Teitz-Tennenbaum, S.; Egenti, M.; Yet, J.; Li, M.; Ginestier, C.; Wicha, M.S.; Moyer, J.S.; et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 2012, 72, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Bowles, D.W.; Keysar, S.B.; Eagles, J.R.; Wang, G.; Glogowska, M.J.; McDermott, J.D.; Le, P.N.; Gao, D.; Ray, C.E.; Rochon, P.J.; et al. A pilot study of cetuximab and the hedgehog inhibitor IPI-926 in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2016, 53, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Riechelmann, H.; Sauter, A.; Golze, W.; Hanft, G.; Schroen, C.; Hoermann, K.; Erhardt, T.; Gronau, S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol. 2008, 44, 823–829. [Google Scholar] [CrossRef]
| Name of the Study | Type of Tumor | Status | Brief Summary | Clinical Trial ID | References |
|---|---|---|---|---|---|
| Gold Nanoparticles as Novel Biomarkers for Cancer Stem Cells in Salivary Gland Tumors: A Diagnostic and Prognostic Accuracy Study | Salivary glands | Completed in 2021. | The current work aimed to introduce a novel diagnostic and prognostic approach in early detection of cancer stem cells in salivary gland tumors using gold nanoparticles conjugated to CD24 (CD24-Gold Nanocomposite). | NCT04907422 | [191] |
| The Immunotherapy of Nasopharyngeal Cancer Using Cancer Stem Cells Vaccine | Nasopharyngeal Cancer | Completed in 2015. | To assess the feasibility of generating CSC-loaded DC vaccines for clinical use, the investigators will harvest peripheral blood and tumor specimen from patients with Nasopharyngeal Cancer. | NCT02115958 | [192] |
| Biopsy of Human Tumors for Cancer Stem Cell Characterization: a Feasibility Study | Head and Neck | Completed in 2012. | To see if a limited sampling of tumor tissue from human subjects is a feasible way to gather adequate tissue for cancer stem cell quantification. | NCT00610415 | No publications found. |
| Pilot Study of Cetuximab and the Hedgehog Inhibitor IPI-926 in Recurrent Head and Neck Cancer | Recurrent Head and Neck Cancer | Completed in 2013. | This study will evaluate the clinical activity of ipilimumab (IPI)-926 in combination with cetuximab in patients with advanced head and neck cancer. | NCT01255800 | [193] |
| Single Dose Escalation Study of Bivatuzumab Mertansine in Patients With Advanced Squamous Cell Carcinoma of the Head and Neck | Head and Neck Neoplasms | Completed in 2005. | To determine maximum tolerated dose (MTD), safety, and efficacy of bivatuzumab mertansine in patients with HNSCC (immunoconjugate bivatuzumab mertansine (BIWI 1) consists of a highly potent antimicrotubule agent coupled to a monoclonal antibody against CD44v6). | NCT02254018 | [194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukovic Đerfi, K.; Vasiljevic, T.; Matijevic Glavan, T. Recent Advances in the Targeting of Head and Neck Cancer Stem Cells. Appl. Sci. 2023, 13, 13293. https://doi.org/10.3390/app132413293
Vukovic Đerfi K, Vasiljevic T, Matijevic Glavan T. Recent Advances in the Targeting of Head and Neck Cancer Stem Cells. Applied Sciences. 2023; 13(24):13293. https://doi.org/10.3390/app132413293
Chicago/Turabian StyleVukovic Đerfi, Kristina, Tea Vasiljevic, and Tanja Matijevic Glavan. 2023. "Recent Advances in the Targeting of Head and Neck Cancer Stem Cells" Applied Sciences 13, no. 24: 13293. https://doi.org/10.3390/app132413293






