Characterization of Natural Rubber, Styrene Butadiene Rubber, and Nitrile Butadiene Rubber Monomer Blend Composites Loaded with Zinc Stearate to Be Used in the Solid Tire Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Mastication and Compounding
2.4. Testing Method
3. Results
3.1. Characteristics of Maturation of Rubber Compound
3.2. Mechanical Properties of a Rubber Vulcanizate
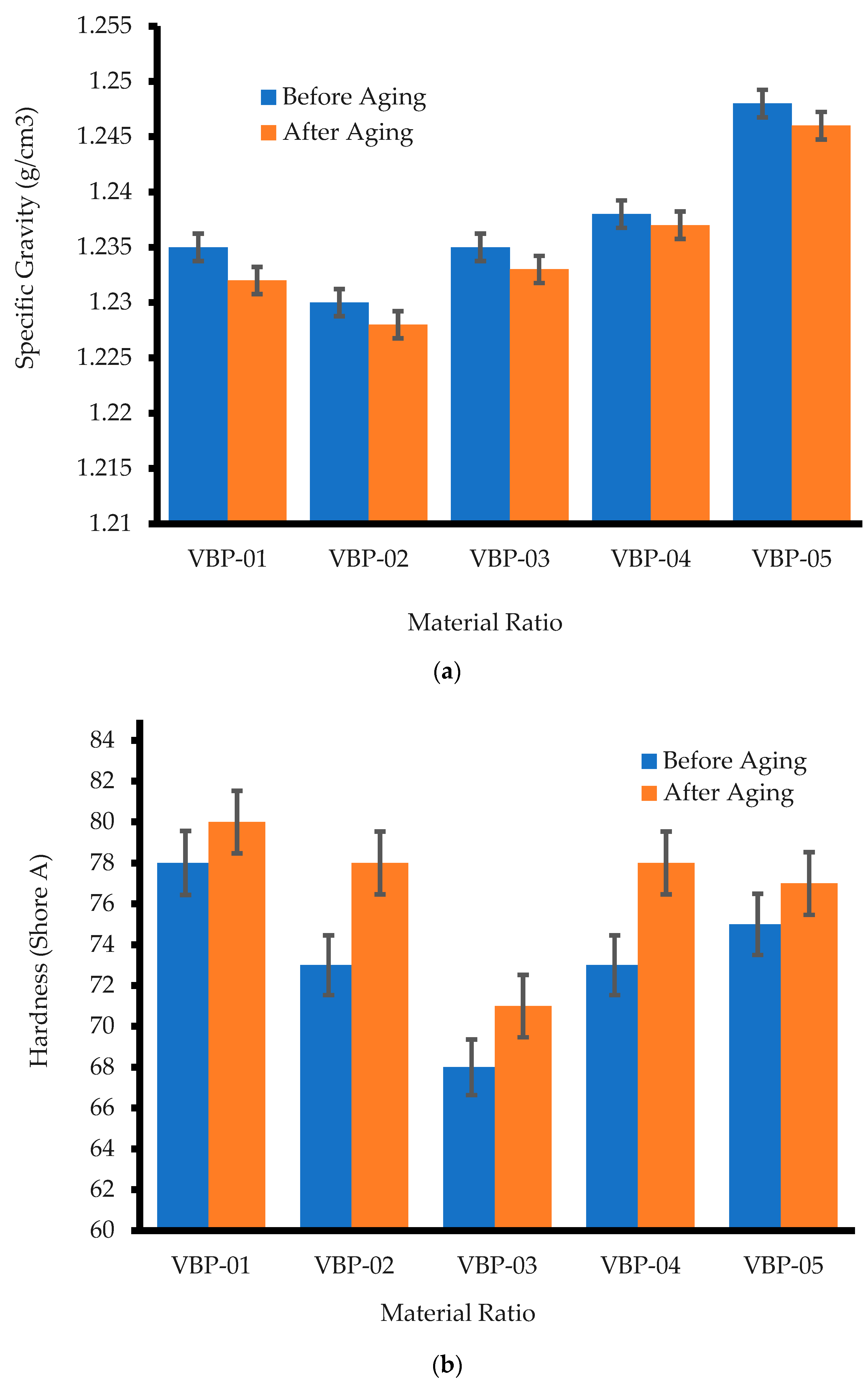
3.2.1. Specific Gravity
3.2.2. Hardness
3.2.3. Abrasion
3.2.4. Tensile Strength
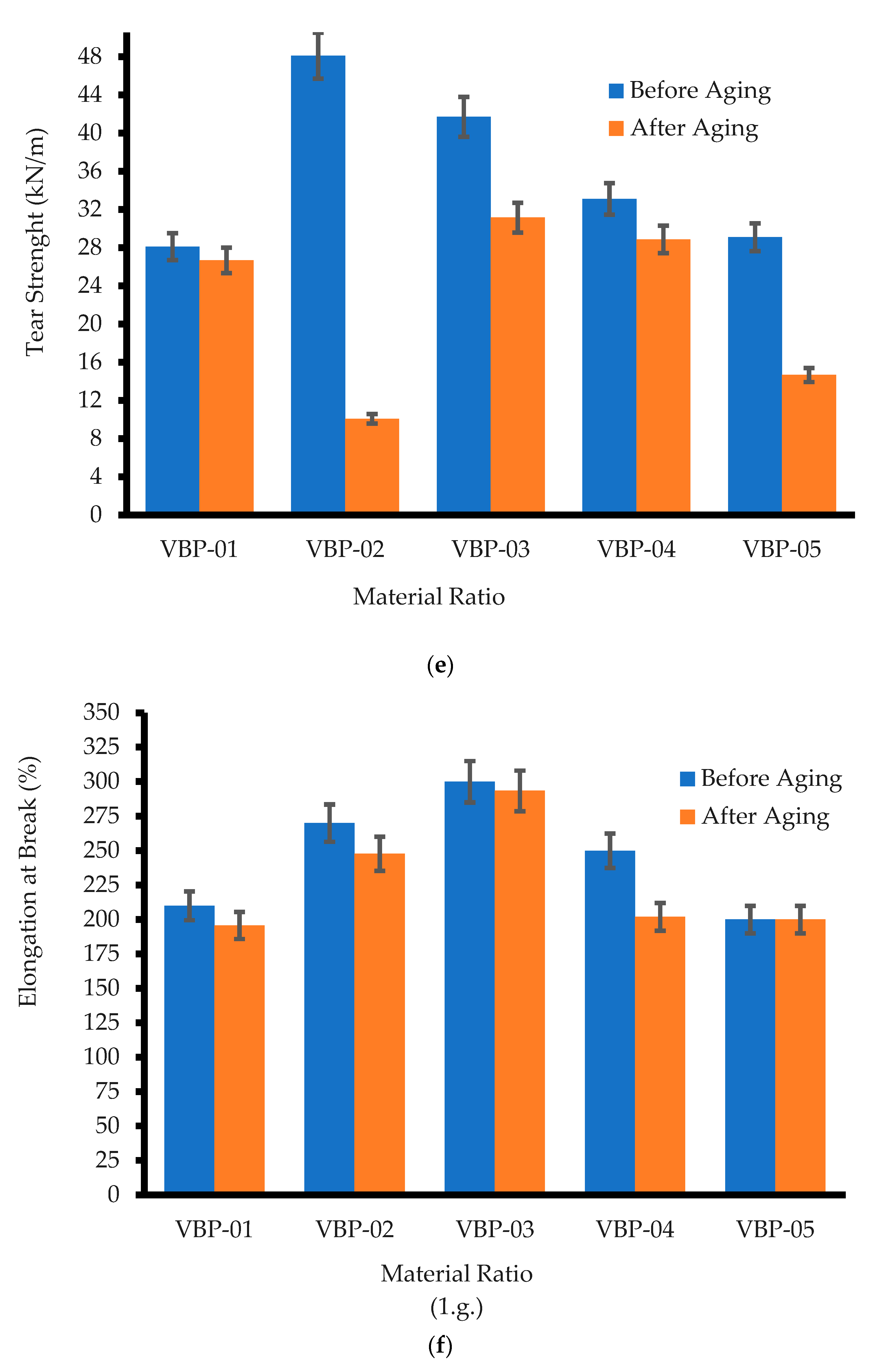
3.2.5. Tear Strength
3.2.6. Elongation at Break
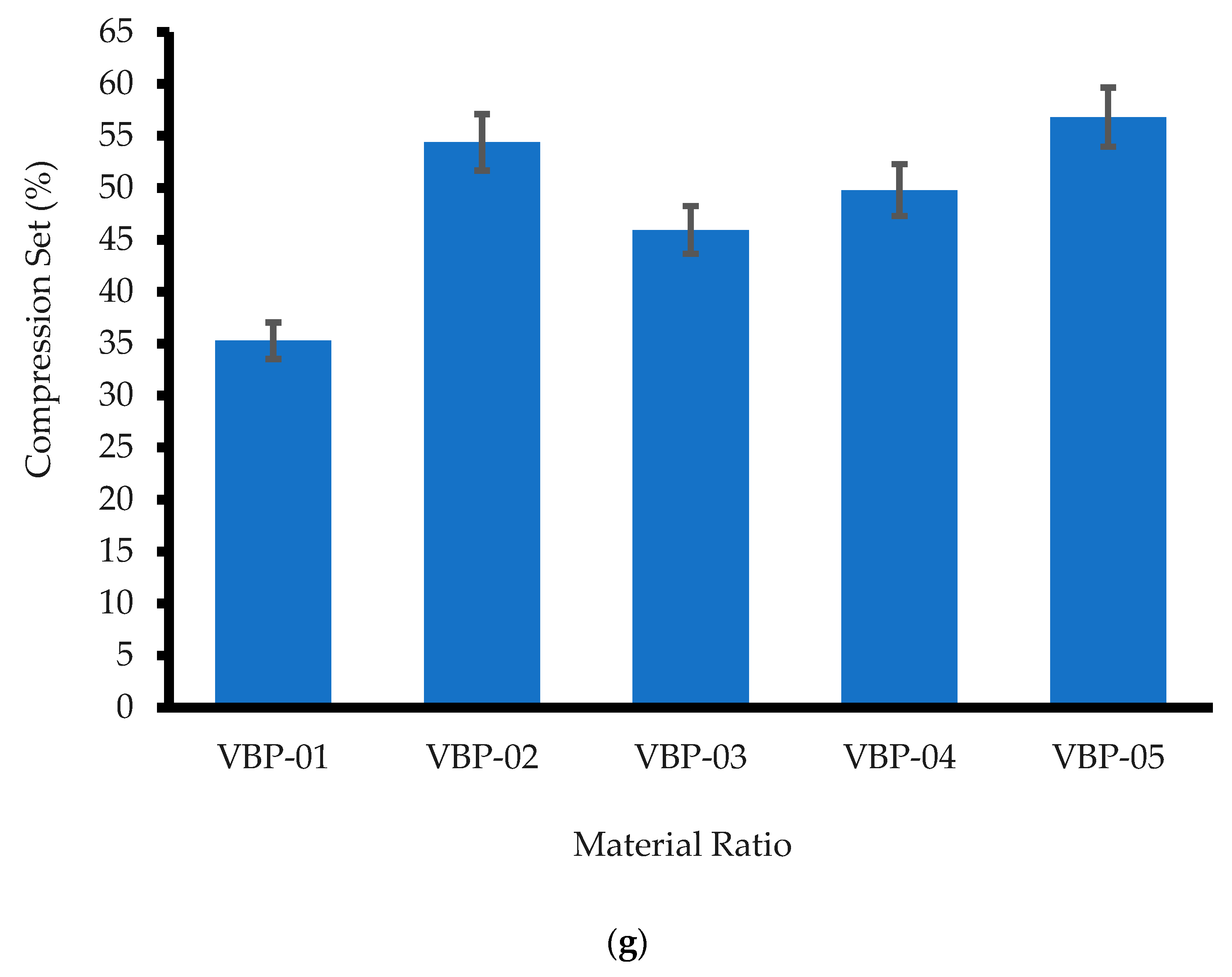
3.2.7. Compression Set
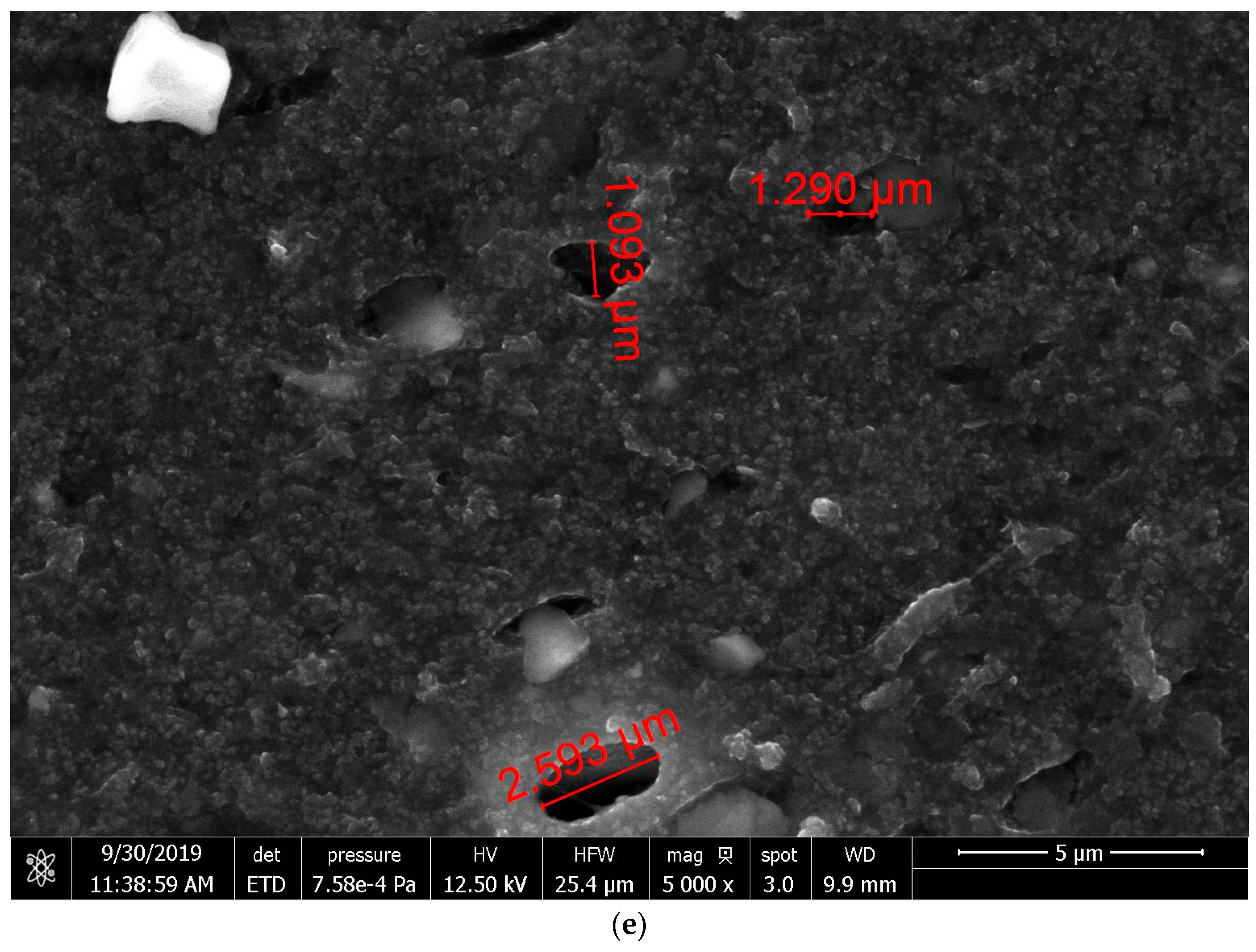
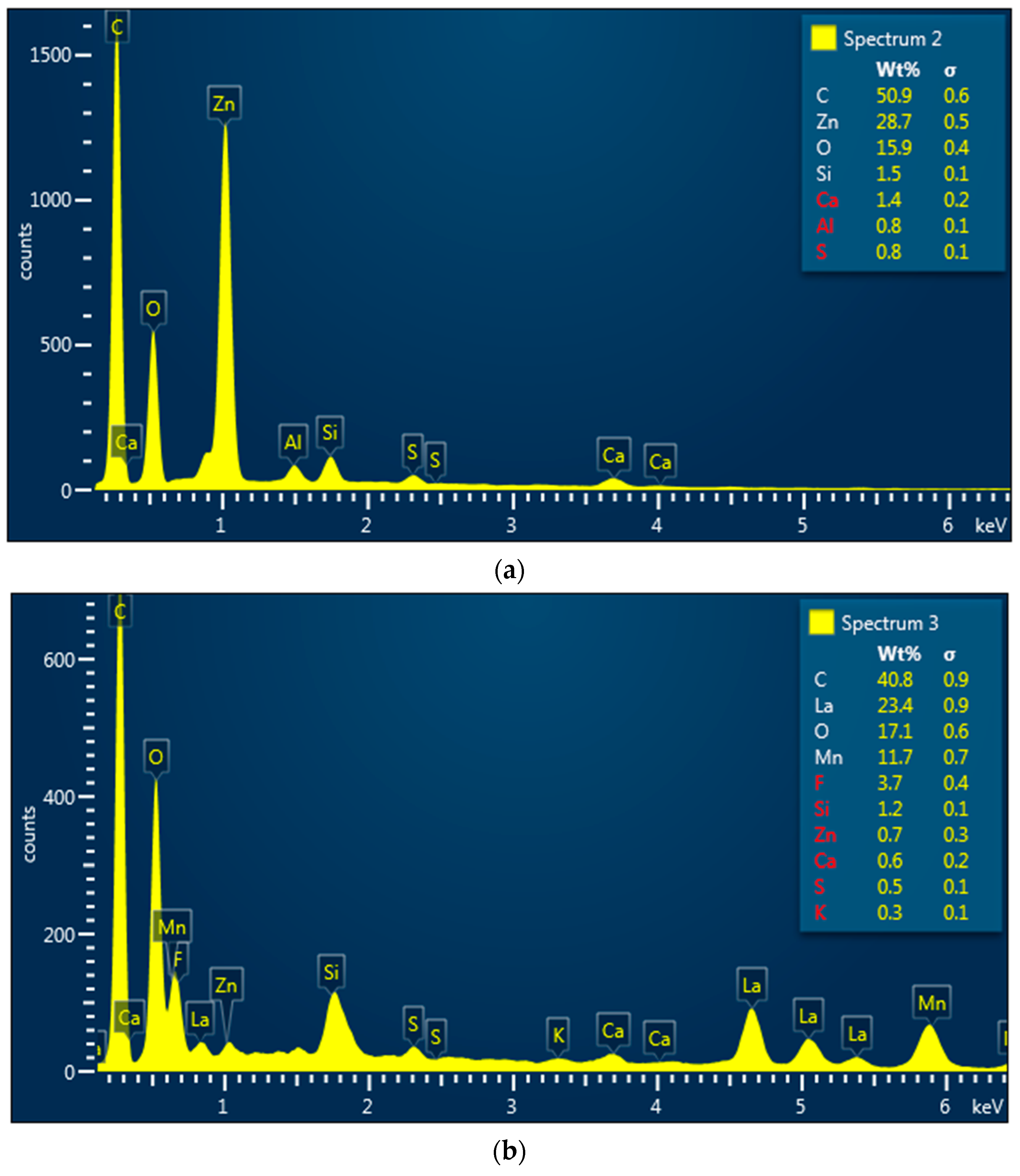
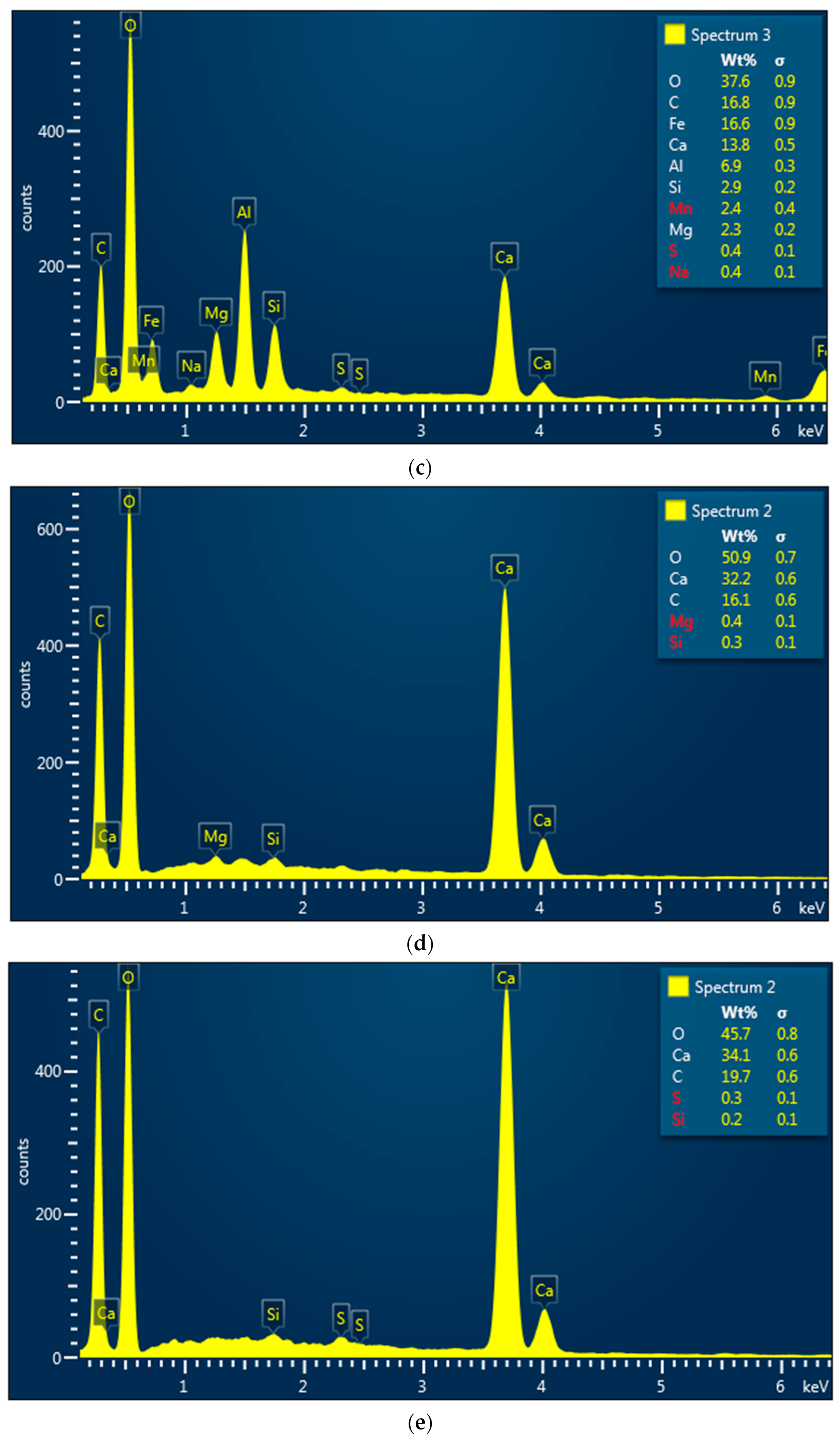
3.3. Morphological Properties of Vulcanized Rubber
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.Y.; Park, J.W.; Lee, D.Y.; Seo, K.H. Correlation between the Crosslink Characteristics and Mechanical Properties of Natural Rubber Compound via Accelerators and Reinforcement. Polymers 2020, 12, 2020. [Google Scholar] [CrossRef]
- Moolsin, S. Cure characteristics, thermal and mechanical properties of natural rubber/synthetic rubber blends with and without compatibilizer. Rangsit. J. Arts Sci. 2014, 4, 105–116. [Google Scholar] [CrossRef]
- Pornprasit, P.; Pechurai, W.; Chiangraeng, N.; Randorn, C.; Chandet, N.; Mungkornasawakul, P.; Nimmanpipug, P. Nanomodified ZnO in natural rubber and its effects on curing characteristics and mechanical proper-ties. Chiang Mai J. Sci. 2018, 45, 2195–2200. [Google Scholar]
- Mostoni, S.; Milana, P.; Di Credico, B.; D’Arienzo, M.; Scotti, R. Zinc-Based Curing Activators: New Trends for Reducing Zinc Content in Rubber Vulcanization Process. Catalysts 2019, 9, 664. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2012, 172, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, M.; Sowińska, A.; Kucharska, J. Organic Zinc Salts as Pro-Ecological Activators for Sulfur Vulcanization of Styrene–Butadiene Rubber. Polymers 2019, 11, 1723. [Google Scholar] [CrossRef] [Green Version]
- Tadiello, L.; Guerra, S.; Giannini, L. Sepiolite-Based Anisotropic Nanoparticles: A New Player in the Rubber Reinforcement Technology for Tire Application. Appl. Sci. 2022, 12, 2714. [Google Scholar] [CrossRef]
- Fathurrohman, M.I.; Soegijono, B.; Budianto, E. The Effect of Stearic Acid on Expanded Organoclay and Rheometric Properties of Natural Rubber/Expanded Organoclay Nanocomposites. J. Mater. Sci. Eng. B 2013, 3, 575–581. [Google Scholar] [CrossRef]
- Habeeb, S.A.; Alobad, Z.K.; Albozahid, M.A. Effect of zinc oxide loading levels on the cure characteristics, mechani-cal and aging properties of the EPDM rubber. Int. J. Mech. Eng. Technol. 2019, 10, 133–141. [Google Scholar]
- Torani, D.; Crespo, J.D.S.; Brandalise, R.N. Influence of ZnO on the properties of elastomeric compositions and their leached extract. Polimeros 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Kruželák, J.; Ušaková, M.; Dosoudil, R.; Hudec, I.; Sýkora, R. Microstructure and Performance of Natural Rubber Based Magnetic Composites. Polym. Technol. Eng. 2014, 53, 1095–1104. [Google Scholar] [CrossRef]
- Poh, B.T.; Razai, M.J.B. Abrasion Property of Epoxidized Natural Rubber. Polym. Technol. Eng. 1999, 38, 341–350. [Google Scholar] [CrossRef]
- Ismail, H.; Chung, F.L. White Rice Husk Ash-Filled Natural Rubber Compounds: The Effect of Bonding Agents. Polym. Technol. Eng. 1999, 38, 1059–1079. [Google Scholar] [CrossRef]
- Nasruddin; Bondan, A.T. Natural rubber composites for solid tyre used for forklift tensile properties and morphological characteristics. J. Physics Conf. Ser. 2019, 1282, 012061. [Google Scholar] [CrossRef]
- Wilaiwong, W.; Smitthipong, W. Study of non-negligible chemical reduction of ZnO in the rubber industry. IOP Conf. Series: Mater. Sci. Eng. 2022, 1234, 01201. [Google Scholar] [CrossRef]
- Przybyszewska, M. Zinc chelates as new activators for sulphur vulcanization of acrylonitrile-butadiene elastomer. Express Polym. Lett. 2009, 3, 256–266. [Google Scholar] [CrossRef]
- Helaly, F.; El Sabbagh, S.; El Kinawy, O.; El Sawy, S. Effect of synthesized zinc stearate on the properties of natural rubber vulcanizates in the absence and presence of some fillers. Mater. Des. 2011, 32, 2835–2843. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Stöckelhuber, K.W.; Wagenknecht, U.; Heinrich, G. Advances in layered double hydroxide (LDH)-based elastomer composites. Prog. Polym. Sci. 2014, 39, 594–626. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Jia, Z.-X.; Liu, L.; Fu, W.-W.; Jia, D.-M.; Luo, Y.-F. Insight into vulcanization mechanism of novel binary accelerators for natural rubber. Chin. J. Polym. Sci. 2014, 32, 1077–1085. [Google Scholar] [CrossRef]
- Setianto, W.B.; Yohanes, H.; Astuti; Maisaroh; Atmaji, G. Synthesis of palm oil-base zinc stearate and its application on manufacture of rubber component. IOP Conf. Series: Earth Environ. Sci. 2022, 963, 012028. [Google Scholar] [CrossRef]
- Xiao, Y.; Hao, Y.; Yan, L.; Xu, Z.; Sui, Z.; Pan, Y.; Wang, C.; Bian, H.; Wang, X. Mechanism on surface hydrophobically modification of fibrous wollastonite and its reinforcement of natural rubber. J. Polym. Res. 2022, 29, 1–14. [Google Scholar] [CrossRef]
- Ikeda, Y.; Higashitani, N.; Hijikata, K.; Kokubo, Y.; Morita, Y.; Shibayama, M.; Osaka, N.; Suzuki, T.; Endo, H.; Kohjiya, S. Vulcanization: New Focus on a Traditional Technology by Small-Angle Neutron Scattering. Macromolecules 2009, 42, 2741–2748. [Google Scholar] [CrossRef]
- Kabakçi, G.C.; Aslan, O.; Bayraktar, E. A Review on Analysis of Reinforced Recycled Rubber Composites. J. Compos. Sci. 2022, 6, 225. [Google Scholar] [CrossRef]
- Smejda-Krzewicka, A.; Słubik, A.; Strzelec, K.; Rybiński, P. Study on the Effect of Zinc on the Rheological, Mechanical and Thermal Properties and Fire Hazard of Unfilled and Filled CR/BR Vulcanizates. Polymers 2020, 12, 2904. [Google Scholar] [CrossRef] [PubMed]
- Indriasari; Noordermeer, J.; Dierkes, W. Incorporation of Oligomeric Hydrocarbon Resins for Improving the Properties of Aircraft Tire Retreads. Appl. Sci. 2021, 11, 9834. [Google Scholar] [CrossRef]
- Zhao, F.; Bi, W.; Zhao, S. Influence of Crosslink Density on Mechanical Properties of Natural Rubber Vulcanizates. J. Macromol. Sci. Part B 2011, 50, 1460–1469. [Google Scholar] [CrossRef]
- Ngamsurat, S.; Boonkerd, K.; Leela-Adisorn, U.; Potiyaraj, P. Curing Characteristics of Natural Rubber Filled with Gypsum. Energy Procedia 2011, 9, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K.; Nizami, S.S.; Riza, N.Z. Reinforcement of natural rubber hybrid composites based on marble sludge/Silica and marble sludge/rice husk derived silica. J. Adv. Res. 2013, 5, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Ojinmah, N.; To, U.; Vo, E.; Ogbobe, O. Studies on the Effect of Rice Husk Semi-Nano Filler on the Mechanical Proper-ties of Epoxidized Natural Rubber Composite. Eur. J. Adv. Eng. Technol. 2017, 4, 164–171. [Google Scholar]
- Bergmann, C.; Trimbach, J. Influence of plasticizers on the properties of natural rubber based compounds. KGK Kautschuk Gummi Kunststoffe 2014, 67, 40–49. [Google Scholar]
- Chukwu, M.; Ekhator, I.; Ekebafe, L. Effect of zinc oxide level as activator on the mechanical properties of natural rubber composite. Niger. J. Technol. 2019, 38, 675. [Google Scholar] [CrossRef] [Green Version]
- El-Sabbagh, S.A.; Yehia, A.A. Detection of Crosslink Density by Different Methods for Natural Rubber Blended with SBR and NBR. Egypt. J. Solids 2007, 30, 157–173. [Google Scholar] [CrossRef]
- Saeed, F.; Ansarifar, A.; Ellis, R.J.; Haile-Meskel, Y.; Farid, A.S. Effect of the blooming of chemical curatives on the cyclic fatigue life of natural rubber filled with a silanized silica nanofiller. J. Appl. Polym. Sci. 2010, 120, 2497–2507. [Google Scholar] [CrossRef]
- Frida, E.; Bukit, N.; Ginting, E.M.; Bukit, B.F. The effect of carbon black composition in natural rubber compound. Case Stud. Therm. Eng. 2019, 16, 100566. [Google Scholar] [CrossRef]
- Surya, I.; Ismail, H.; Azura, A. Alkanolamide as an accelerator, filler-dispersant and a plasticizer in silica-filled natural rubber compounds. Polym. Test. 2013, 32, 1313–1321. [Google Scholar] [CrossRef]
- Al Bodairy, O.H.; Kadhim, I.H. Effect of Zinc Oxide Percent on Transmission Properties for (X-ray) of Rubber Com-pound (SBR). Int. J. Enhanc. Res. Sci. Technol. Eng. 2013, 2, 1–5. Available online: https://www.researchgate.net/publication/271326730_Effect_of_Zinc_Oxide_Percent_on_Transmission_Properties_for_X-Ray_of_Rubber_Compound_SBR (accessed on 27 December 2022).
- Heideman, G.; Noordermeer, J.W.; Datta, R.N.; van Baarle, B. Various Ways to Reduce Zinc Oxide Levels in S-SBR Rubber Compounds. Macromol. Symp. 2006, 245–246, 657–667. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. ASTM D412-15 Standard Test Methods for Vulcanized Rubber and Thermo-plastic Elastomers–Tension. Annu. B. ASTM Stand. 2002, 2002, 1–14. [Google Scholar]
- Yalcin, D. Tensile Testing Concepts & Definitions. 2021. Available online: https://www.researchgate.net/publication/351392014_Tensile_Testing_Concepts_Definitions (accessed on 27 December 2022).
- Introduction to Tensile Testing; ASM International: Almere, The Netherlands, 2004; pp. 1–12. [CrossRef]
- ASTM E8/E8M-22; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2009; Volume I, pp. 1–27. [CrossRef]
- Roth, F.; Holt, W. Tensile properties of rubber compounds at high rates of stretch. J. Res. Natl. Bur. Stand. 1939, 23, 603. [Google Scholar] [CrossRef]
- Imanah, J.E.; Okieimen, F.E. Rheological and mechanical properties of natural rubber reinforced with agricultural byproduct. J. Appl. Polym. Sci. 2003, 90, 3718–3722. [Google Scholar] [CrossRef]
- Tang, H.; Dai, W.; Chen, B. A New Method for Producing High Melt Strength Polypropylene With Reactive Extrusion. Polym. Eng. Sci. 2008, 48, 1339–1344. [Google Scholar] [CrossRef]
- Heideman, G.; Noordermeer, J.W.M.; Datta, R.N.; Van Baarle, B. Effect of Zinc Complexes as Activator for Sulfur Vulcanization in Various Rubbers. Rubber Chem. Technol. 2005, 78, 245–257. [Google Scholar] [CrossRef]
- Al-Hartomy, O.A.; Al-Ghamdi, A.A.; Al-Said, S.A.F.; Dishovsky, N.; Mihaylov, M.; Ivanov, M. Investigation on the Influence of Various Kinds of Soaps on the Mechanical Properties of Silica Filled Composites Based on Natural Rubber. Polym. Polym. Compos. 2018, 26, 325–334. [Google Scholar] [CrossRef]
- Neuhaus, C.; Lion, A.; Johlitz, M.; Heuler, P.; Barkhoff, M.; Duisen, F. Fatigue behaviour of an elastomer under consideration of ageing effects. Int. J. Fatigue 2017, 104, 72–80. [Google Scholar] [CrossRef]
- Somers, A.; Bastow, T.; Burgar, M.; Forsyth, M.; Hill, A. Quantifying rubber degradation using NMR. Polym. Degrad. Stab. 2000, 70, 31–37. [Google Scholar] [CrossRef]
- Poikelispää, M.; Shakun, A.; Sarlin, E. Nanodiamond—Carbon Black Hybrid Filler System for Demanding Applications of Natural Rubber—Butadiene Rubber Composite. Appl. Sci. 2021, 11, 10085. [Google Scholar] [CrossRef]
- Ibrahim, M.; Sapuan, S.; Faieza, A. Mechanical and Thermal Properties of Composites from Unsaturated Polyester Filled with Oil Palm Ash. J. Mech. Eng. Sci. 2012, 2, 133–147. [Google Scholar] [CrossRef]
- Rahmah, M.; Norazira, W.Z.; Mohd, A.F.; Norizan, M.N. Effect of Epoxidized Oil on Tensile and Tear Strength of NR Vulcanizate and its Comparison with Aromatic Oil NR Vulcanizates. Adv. Mater. Res. 2013, 812, 204–209. [Google Scholar] [CrossRef]
- Ahmed, K.; Nizami, S.S.; Raza, N.Z.; Habib, F. The effect of silica on the properties of marble sludge filled hybrid natural rubber composites. J. King Saud Univ. Sci. 2013, 25, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Antony, P.; De, S. The effect of zinc stearate on melt-processable ionomeric blends based on zinc salts of maleated high-density polyethylene and maleated EPDM rubber. Polymer 1999, 40, 1487–1493. [Google Scholar] [CrossRef]
- Rattanasom, N.; Prasertsri, S.; Ruangritnumchai, T. Comparison of the mechanical properties at similar hardness level of natural rubber filled with various reinforcing-fillers. Polym. Test. 2009, 28, 8–12. [Google Scholar] [CrossRef]
- Pal, K.; Rajasekar, R.; Kang, D.J.; Zhang, Z.X.; Pal, S.K.; Das, C.K.; Kim, J.K. Effect of fillers on natural rubber/high styrene rubber blends with nano silica: Morphology and wear. Mater. Des. 2010, 31, 677–686. [Google Scholar] [CrossRef]
- Hamzah, M.; Alibadi, A.A. Effect of Carbon Black Type on The Mechanical Behaviour of Elastomeric Material. AL-Qadisiya J. Eng. Sci. 2016, 6, 268–296. [Google Scholar]
- Candau, N.; Oguz, O.; Federico, C.E.; Stoclet, G.; Tahon, J.-F.; Maspoch, M.L. Strain induced crystallization in vulcanized natural rubber containing ground tire rubber particles with reinforcement and nucleation abilities. Polym. Test. 2021, 101, 107313. [Google Scholar] [CrossRef]
- Tamási, K.; Kollár, M.S. Effect of different sulfur content in Natural Rubber mixtures on their thermo-mechanical and surface properties. Int. J. Eng. Res. Sci. 2018, 4, 28–37. [Google Scholar]
- Kim, J.W.; Kim, H.G.; Gil Lee, D. Compaction of thick carbon/phenolic fabric composites with autoclave method. Compos. Struct. 2004, 66, 467–477. [Google Scholar] [CrossRef]
- Hernández, M.; Bernal, M.D.M.; Verdejo, R.; Ezquerra, T.A.; López-Manchado, M.A. Overall performance of natural rubber/graphene nanocomposites. Compos. Sci. Technol. 2012, 73, 40–46. [Google Scholar] [CrossRef]
- González, N.; Custal, M.D.; Riba, J.-R.; Armelin, E.; Rodríguez, D. Influence of ZnO and TiO2 Particle Sizes in the Mechanical and Dielectric Properties of Vulcanized Rubber. Mater. Res. 2017, 20, 1082–1091. [Google Scholar] [CrossRef] [Green Version]
- Arayapranee, W.; Rempel, G.L. Effects of Polarity on the Filler-Rubber Interaction and Properties of Silica Filled Grafted Natural Rubber Composites. J. Polym. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Girão, A.V.; Caputo, G.; Ferro, M.C. Application of Scanning Electron Microscopy–Energy Dispersive X-Ray Spectroscopy (SEM-EDS). Compr. Anal. Chem. 2017, 75, 153–168. [Google Scholar] [CrossRef]
- Modi, S. Green Synthesis of Zinc Oxide Nanoparticles Using Garlic Skin Extract and Its Green Synthesis of Zinc Oxide Na-noparticles Using Garlic Skin Extract and Its Characterization. J. Nanostruct. 2021, 10, 20–27. [Google Scholar] [CrossRef]
- Joseph, A.; George, B. The Current Status of Sulphur Vulcanization and Devulcanization Chemistry: Devulcanization The Current Status of Sulphur Vulcanization and Devulcanization Chemistry: Devulcanization. Rubber Sci. 2016, 29, 62–100. [Google Scholar]



| Material | Ratio Formula * | ||||
|---|---|---|---|---|---|
| VBP-01 | VBP-02 | VBP-03 | VBP-04 | VBP-05 | |
| NR | 60.00 | 60.00 | 60.00 | 60.00 | 60.00 |
| SBR | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| NBR | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| ZnO | 5.00 | - | - | - | - |
| SA | 2.50 | - | - | - | - |
| ZS | 0.00 | 6.00 | 7.00 | 8.00 | 9.00 |
| PEG-4000 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Black carbon | 60.00 | 60.00 | 60.00 | 60.00 | 60.00 |
| CaCO3 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Kaolin | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Silica | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Coumarone resin | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 |
| Paraffin wax | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 |
| DOP | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| BHT | 1.75 | 1.75 | 1.75 | 1.75 | 1.75 |
| IPPD | 1.75 | 1.75 | 1.75 | 1.75 | 1.75 |
| TMTD | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| DPG | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| CBS | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Sulfur | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Curing Characteristic Rheometer, 150 °C | VBP-01 | VBP-02 | VBP-03 | VBP-04 | VBP-05 |
|---|---|---|---|---|---|
| S* maximum, kg-cm | 30.21 | 22.03 | 23.19 | 18.85 | 26.16 |
| S* minimum, kg-cm | 3.79 | 1.69 | 1.69 | 1.18 | 1.95 |
| Delta torsion (Δs), kg-cm | 26.45 | 20.34 | 21.50 | 17.67 | 24.21 |
| Opt cure time (t90), min;sec | 9;12 | 2;53 | 2;56 | 2;51 | 3;50 |
| Scorch time (ts2), min;sec | 1;07 | 0;05 | 0;07 | 0;52 | 0;46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasruddin; Setianto, W.B.; Yohanes, H.; Atmaji, G.; Lanjar; Yanto, D.H.Y.; Wulandari, E.P.; Wiranata, A.; Ibrahim, B. Characterization of Natural Rubber, Styrene Butadiene Rubber, and Nitrile Butadiene Rubber Monomer Blend Composites Loaded with Zinc Stearate to Be Used in the Solid Tire Industry. Appl. Sci. 2023, 13, 1277. https://doi.org/10.3390/app13031277
Nasruddin, Setianto WB, Yohanes H, Atmaji G, Lanjar, Yanto DHY, Wulandari EP, Wiranata A, Ibrahim B. Characterization of Natural Rubber, Styrene Butadiene Rubber, and Nitrile Butadiene Rubber Monomer Blend Composites Loaded with Zinc Stearate to Be Used in the Solid Tire Industry. Applied Sciences. 2023; 13(3):1277. https://doi.org/10.3390/app13031277
Chicago/Turabian StyleNasruddin, Wahyu Bahari Setianto, Heryoki Yohanes, Gigih Atmaji, Lanjar, Dede Heri Yuli Yanto, Enasty Pratiwi Wulandari, Arya Wiranata, and Bahruddin Ibrahim. 2023. "Characterization of Natural Rubber, Styrene Butadiene Rubber, and Nitrile Butadiene Rubber Monomer Blend Composites Loaded with Zinc Stearate to Be Used in the Solid Tire Industry" Applied Sciences 13, no. 3: 1277. https://doi.org/10.3390/app13031277
APA StyleNasruddin, Setianto, W. B., Yohanes, H., Atmaji, G., Lanjar, Yanto, D. H. Y., Wulandari, E. P., Wiranata, A., & Ibrahim, B. (2023). Characterization of Natural Rubber, Styrene Butadiene Rubber, and Nitrile Butadiene Rubber Monomer Blend Composites Loaded with Zinc Stearate to Be Used in the Solid Tire Industry. Applied Sciences, 13(3), 1277. https://doi.org/10.3390/app13031277







