Abstract
The thermal process of green amaranth leads to the partial or complete degradation of chlorophyll pigments and loss of green colour due to the formation of chlorophyll derivatives. This study aimed to evaluate a stabilisation process utilising metal ions to obtain a stable green colour of metal-chlorophyll derivative complexes. In this study, the effects of CuSO4 (0–240 ppm), ZnCl2 (0–1800 ppm) ions, pH (4–9), and temperature (60–100 °C) on green amaranth purees with a constant time of 15 min were investigated. In tapered leaf amaranths, the sample depicted higher contents of chlorophyll a (0.33 mg/g), chlorophyll b (0.34 mg/g), and total chlorophyll (0.68 mg/g) than round leaf amaranths (chlorophyll a = 0.28 mg/g, chlorophyll b = 0.29 mg/g, and total chlorophyll = 0.58 mg/g). A higher chlorophyll derivative content (0.62 mg/g), DPPH scavenging activity (93 mM TE/g), and FRAP value (54 mM TE/g) of Cu-amaranth purees were successfully achieved using 210 ppm of CuSO4 after heating at pH 6 and 80 °C. Zn-amaranth purees were produced using 1500 ppm of ZnCl2 at pH 8 and 90 °C for 15 min with chlorophyll derivative content of 0.39 mg/g, DPPH scavenging activity of 79 mM TE/g, and FRAP value of 57 mM TE/g. In HPLC chromatograms, two major peaks were identified as chlorophylls a and b in fresh amaranths. Nevertheless, these two peaks disappeared in Cu- and Zn-amaranth purees, presumably due to the formation of metallo-chlorophyll derivatives.
1. Introduction
The amaranth plant species belong to the genus Amaranthus and the family of Amaranthaceae [1]. Additionally, the genus Amaranthus comprises 60 to 70 species with over 400 varieties in temperate and tropical climates [2]. Three species are typically grown for their edible grains, while 17 species are planted for their edible leaves, such as A. blitum, A. cruentus, A. dubius, A. tricolor, and A. viridis [3]. Only three species of amaranths are discovered in Malaysia, which are “bayam itik” (A. blitum), “bayam putih” (A. paniculatus), and “bayam panjang” or “bayam hijau” (A. viridis). Nonetheless, these species are abundantly available in the market with round or tapered leaf characteristics [4].
The green amaranth (Amaranthus viridis L.) is an excellent source of phytochemicals, which include chlorophylls. Moreover, green amaranths are the least expensive vegetable compared to other local leafy vegetables, such as kale, mustard green, celery, pak choy, and cabbage. Amaranths have been reported to produce 1504 mg/kg of total chlorophyll as they are significantly higher in magnesium (Mg), calcium (Ca), potassium (K), copper (Cu), phosphorus (P), zinc (Zn), iron (Fe), and manganese (Mn) [5,6]. In addition, chlorophylls typically produce a natural green colour while exhibiting high antioxidant activity, and anti-inflammatory, anti-carcinogenic, anti-bacterial, and wound healing characteristics [7,8]. There are two primary forms of chlorophylls in plants consisting of chlorophyll a (blue-green) and chlorophyll b (yellow-green), which contain a methyl or formyl group, respectively.
Although green vegetables demonstrate excellent benefits, the chlorophyll pigments are unstable during extraction and thermal processing. The instability occurs in unfavourable temperatures and pH, contact with oxygen, and exposure to light [9,10]. In Malaysia, green amaranth is consumed directly, or its puree is incorporated into food products. However, this may involve some handling delay, which can be detrimental to its colour and chlorophyll content. Amaranth puree rapidly turns brown after thermal processing but remains green at chilling and frozen temperatures.
Nevertheless, its colour degrades on thawing. According to Schwartz et al., temperature and pH are the most critical factors affecting the stability of chlorophylls [11]. The authors reported that chlorophylls a and b could convert to magnesium ions (Mg2+)-free derivatives at pH less than 7 and 60 °C or higher temperatures. Furthermore, the converted products of the conversion process include pheophytin, pheophorbide, pyropheophytin, and pyropheophorbide [12]. Consequently, the conversion process reduced green colour intensity and antioxidant activity.
A study by Erge et al. indicated that the visual green colour loss in green peas occurred at a temperature range of approximately 70 to 100 °C [13]. Furthermore, the study observed that chlorophyll a degraded about 12 to 18 times more than chlorophyll b. Additionally, Edelenbos et al. reported that cooking green peas (Pisum sativum) in water for three minutes increased the amount of pheophytins a and b while decreasing the contents of chlorophylls a and b [14].
The formation of stable chlorophyll molecules in green amaranths can be produced to overcome these drawbacks by substituting divalent cations, such as Zn or Cu, in the porphyrin ring of Mg-free chlorophyll derivatives [15]. The stabilising process can form metallo-chlorophyll derivatives, similar in colour to chlorophylls. Furthermore, these metallo-chlorophyll derivatives are more stable and thermally resistant when compared to Mg-chlorophylls in low-pH foods [15,16,17]. The stabilisation process of chlorophyll derivatives using metal ions, such as Zn and Cu was also reported to improve the colour of pears, avocados, pandans, pennyworts, peas, and grapes [18,19,20,21,22,23,24].
A study by Senklang et al. reported the reaction process of fresh leaves with 300 mg/L of ZnCl2 at pH 5, 110 °C, and a reaction time of 15 min [25]. The study leads to the formation of Zn-chlorophyll derivatives in pandan leaves, such as Zn-pheophytin and Zn-pyropheophytin. Similar to native chlorophylls, these derivatives are green in colour but more stable to acid and heat while functioning as antioxidants [26]. Meanwhile, Guzmán et al. proved that the heating process of avocado purees added with Cu or Zn produced a higher puree colour than non-treated samples at approximately 87 to 89 °C [19]. The results agreed with those reported by Canjura et al. and Salama et al., in which the colour stability of Zn- and Cu-based chlorophylls in vegetables was improved by temperature values higher than 85 °C [22,27]. Rahayuningsih et al. [28] reported that the optimum stabilisation conditions of suji leaves (Pleomele Angustifolia Roxb.) were obtained using 700 mg/L of ZnCl2. The study was performed at pH 7, at a temperature of 85 °C, and with a total chlorophyll content of 47.29 mg/100 g fresh weight.
In Cu and Zn complexes, they are known not to be absorbed by the body and are removed as excretion products. Hence, these complexes are safe and permitted to be utilised in most countries as food additives. Nevertheless, the Food and Drug Administration (FDA) regulation limits that only 75 mg/L of Zn can remain in the final product, while the concentration of free ionisable Cu must be kept below 200 mg/L [17,29,30]. Furthermore, these metal ions, such as Cu, Zn, Mg, Ca, and Fe, play an important role in biological and biomedical processes [31]. A study by de Vogel et al. and Ferruzzi et al. discovered that in vitro anti-mutagenic activity of natural chlorophylls and commercial-grade derivatives, such as sodium copper chlorophyllin (SCC) was associated with a decreased risk of colon cancer and numerous dietary and environmental mutagens [12,32]. Gomes et al. [33] further proved that SCC has been utilised in several countries as a food colourant and nutritional supplement without adverse effects for over 50 years. Moreover, SCC is promoted for its anti-bacterial and anti-viral properties [34,35].
Another study by Ferruzzi et al. [16] discovered substantially higher antioxidant capacities of metallo-chlorophyll derivatives (such as Cu-pheophytin a, Cu-chlorophyllin, Zn-pheophytin, and Zn-pyropheophytin) than natural chlorophylls and Mg-free derivatives. Other researchers also reported similar results on the antioxidant activities of metal ions containing chlorophylls, such as Cu-pheophytin, Cu-chlorophyllin, Zn-pheophytin, Zn-pyropheophytin, and Mg-chlorophyll [36,37,38].
Although the method of stabilising chlorophylls has been successfully employed with several vegetables, there is still a lack of information on the use of Zn and Cu to stabilise the chlorophylls in green amaranth purees. Thus, this research aims to investigate the stabilisation process of chlorophylls in amaranth purees, which includes the effect of various metal concentrations, pH, and temperatures. Furthermore, the obtained results were further utilised to evaluate the colour intensities, chlorophylls and their derivative contents, and antioxidant activities of the metal-stabilised amaranth purees. The research suggests that stabilization of green amaranth with different metal ions and conditions has the potential to preserve the green colour and antioxidant activity of amaranth for further use in food processing.
2. Materials and Methods
2.1. Materials
Fresh green amaranths (tapered and round leaves) were purchased from a local market in Kuala Lumpur, Malaysia, and were processed immediately.
2.2. Chemicals
The 2,2-Diphenyl-1-picrylhydrazyl (DPPH), methanol, 2,4,6-Tripyridyl-s-Triazine (TPTZ), ZnCl2, CuSO4, and acetone chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Additionally, the chlorophyll a (MW = 893.5 g/mol) and chlorophyll b (MW = 907.5 g/mol) standards (HPLC grade) were purchased from Sigma Co. (St Louis, MO, USA). All other chemicals used in this study were either HPLC or analytical grades and purchased from Merck (Darmstadt, Germany).
2.3. Stabilisation of Amaranth Purees
The single-factor experiments were used for the stabilisation process of chlorophylls in green amaranths (A. viridis). A total of three factors were studied, which are different metal concentrations (CuSO4 and ZnCl2), pH, and temperature. Three responses were selected, which are colour value (priority), chlorophyll derivative content, and antioxidant activity (free radical DPPH) with ferric reducing antioxidant power (FRAP) assay. The steps involved in the stabilisation process of chlorophylls are further explained.
In Step 1, the stabilisation process of chlorophylls in green amaranths was performed according to Siti Faridah Mohd Amin et al. and Senklang et al. with slight modifications [25,39]. In brief, fresh amaranths were washed, drained, chopped, and the roots were discarded. The cleaned amaranth leaves with stalks were blended in a Waring blender for 5 min at high speed to obtain amaranth puree. The purees were then divided and mixed with varying concentrations of CuSO4 (0, 30, 60, 90, 120, 150, 180, 210, and 240 ppm) or ZnCl2 (0, 300, 600, 900, 1200, 1500, and 1800 ppm). Subsequently, the amaranth purees were kept constant at pH 6 (adjusted using citric acid or NaOH) before being incubated at a constant temperature of 80 °C for 15 min. The optimum values of CuSO4 and ZnCl2 concentrations were selected based on the purees with the highest green colour, chlorophyll derivative content, and antioxidant activity.
In Step 2, the chlorophylls in the green amaranth were stabilised using the optimum concentrations of CuSO4 and ZnCl2 obtained from Step 1. In addition, the chlorophyll stabilisation process was carried out at a pH range of approximately 4 to 9 at a constant temperature of 80 °C for a reaction time of 15 min. The optimum stabilisation pH value was selected based on the purees with the highest green colour, chlorophyll derivative content, and antioxidant activity.
In Step 3, the stabilisation process was performed at various temperatures (60 to 100 °C) using the optimum values of CuSO4 and ZnCl2 concentrations obtained from Step 1, and the optimum pH as determined in Step 2. Similarly, the optimum temperature was also chosen based on the purees with the highest green colour, chlorophyll derivative content, and antioxidant activity.
2.4. Physicochemical Properties
2.4.1. Determination of Colour
The colour parameters of the purees were determined using a Hunter Lab Ultra-Scan Colorimeter (Sphere Spectrocolorimeter, Hunter Association Inc., Reston, VA, USA) in the reflectance mode and with illuminant D65 to measure a* (+a* = red, −a* = green) and b* (+b* = yellow, −b* = blue). The −a*/b* ratio was used as an index of apparent changes in greenness, as described by Larrauri García et al. [40]. The high negative values of a*/b* ratios indicate that the amaranth puree is greener and less yellow.
2.4.2. Extraction and Determination of Total Chlorophylls
The measurement of chlorophylls and chlorophyll derivative contents in control (fresh amaranth puree) and amaranth purees stabilised with Cu or Zn were determined according to Senklang et al. with slight modifications [25]. In brief, the extraction process of chlorophylls from the purees was performed in dim light using stoppered tubes covered with aluminium foil to reduce the photo-destruction effect on the chlorophylls. Each lot of amaranths (1 g) was ground with 100% acetone using a mortar and pestle until the residue became colourless. The content of the mortar was then transferred into a centrifuge tube and washed several times with 100% acetone. The total amount of 100% acetone used in this extraction process was 50 mL. Subsequently, the mixture was centrifuged at 3500× g for 10 min. The absorbance values of the extracted chlorophylls in the supernatant were collected at 662 and 645 nm wavelengths for chlorophyll a and chlorophyll b, respectively. The absorbance measurements were performed using 100% acetone as the reference in the UV-Vis spectrophotometer (Shimadzu, Japan). The chlorophylls a and b and total chlorophyll (mg/g fresh weight) were then calculated according to the method of Costache et al. based on Equations (1)–(3), respectively [41]. Moreover, the absorbance spectra of the acetone extract of fresh amaranths and metal-treated amaranths were recorded using a wavelength range of approximately 300 to 700 nm.
Chlorophyll a = 11.75A662 − 2.350A645
Chlorophyll b = 18.61A645 − 3.960A662
Total chlorophylls = (16.26 A645 + 7.790 A642) × Dilution factor/1000
2.4.3. Preparation of Amaranth Puree Extracts for Antioxidant Activity Measurements
The stabilised amaranth purees were dried at 45 °C for 24 h in a convection oven (Venticell 111, MMM Group, Munich, Germany). Subsequently, the dried purees were ground to a fine powder using a Pulverisette 14-rotor mill (Fritsch, GmbH, Oberstein, Germany). The powders (0.25 g) were extracted with 10 mL of 80% methanol. The extraction process was performed in a test tube at 40 °C in a water bath with a continuous shaking process for 24 h. The tubes were then cooled to room temperature (27 ± 2 °C) and centrifuged at 3500× g for 15 min. Finally, the supernatants were placed in airtight glass vials and stored in a refrigerator until further use for antioxidant activity measurements [42].
2.4.4. Free Radical DPPH Assay Measurements
The antioxidant activity of the extracts from the stabilised purees was measured using the free radical DPPH assay through slight modifications of the spectrophotometric method by Brand-Williams [43]. In brief, a 3.9 mL aliquot of 0.1 mM methanolic DPPH solution (prepared using 80% v/v methanol) was mixed with 0.1 mL of amaranth puree extract in a test tube. The tube was vortexed for 15 s and incubated in the dark for 15 min at room temperature. Subsequently, the absorbance of the solution in the test tube was measured using a UV-Vis spectrophotometer (Shimadzu, Japan) using a wavelength of 515 nm with 80% methanol (v/v) as the reference. The Trolox solutions were used to construct the standard curves, and the results were expressed in mM of Trolox Equivalents (TE).
2.4.5. FRAP Assays for the Antioxidant Activity Study
The FRAP assays were performed according to Benzie et al. with minor modifications [44]. In brief, a FRAP reagent was prepared from sodium acetate buffer (300 mM, pH 3.6), 10 mM of 2,4,6-Tripyridyl-s-Triazine (TPTZ) solution in 40 mM HCl, and 20 mM FeCl3 solutions in proportions of 10:1:1 (v/v), respectively. Furthermore, the FRAP reagent was prepared daily and warmed to 37 °C with a water bath before further use. The FRAP reagent (2.85 mL) was combined with the sample extracts (150 L), and the mixture was left for 30 min at room temperature. Subsequently, the reaction mixture absorbances were measured at a wavelength of 593 nm, and the assay measurements were carried out in triplicate. The standard curves were constructed using the Trolox solutions, and the results were presented in mM of Trolox Equivalents (TE).
2.5. Identification of Chlorophylls and Their Derivatives in Fresh, Zn-, and Cu-Amaranth Purees Using Reversed-Phase High-Performance Liquid Chromatography (HPLC)
2.5.1. Extraction of Chlorophylls and Chlorophyll Derivatives
The extraction process of the chlorophylls from fresh, Cu- and Zn-amaranth purees was conducted by following the method of Canjura et al. [45]. In brief, 34 mL of acetone was added to 5 g of the amaranth puree. The mixture was homogenised with Ultra-Turrax homogeniser (IKA Werke, Labortechnik, Staufen, Germany) for 2 min. The homogenate was then filtered under vacuum through a Whatman 42 filter paper. Subsequently, the filtrate was brought to volume with acetone in a 25 mL volumetric flask.
2.5.2. Chromatographic Separation and Identification of Chlorophylls and Chlorophyll Derivatives
The chlorophylls a and b and metallo-chlorophyll derivatives in fresh and metal-stabilised amaranth purees were separated by HPLC using an Agilent system equipped with an autosampler, column thermostat, and diode array detector (DAD). The isocratic elution was performed with the mobile phase of ethyl acetate/methanol/water (40:54:10 v/v/v) as described by Ngo et al. [18]. In brief, the triplicate 20 μL of fresh and metal-amaranth extracts in acetone were injected into a Zorbax Eclipse Plus C18 reverse-phase column (5 μm particle size, 4.6 mm in internal diameter × 250 mm in length) (Agilent Technologies, Santa Clara, CA, USA) and maintained at 30 °C. All extracts were handled in the dark throughout the extraction, concentration, and injection processes. Furthermore, all extracts were filtered through a 13 mm diameter and 0.45 µm nylon filter (Captiva Econofilters, Agilent Technologies) before being injected into the HPLC system. All chlorophylls and their derivatives were detected at a wavelength of 658 nm. Finally, the identification of peaks was confirmed by comparing retention times (tret) obtained from chlorophyll a and b standards.
2.5.3. Preparation of Calibration Curves for Chlorophylls a and b Determined using HPLC
Chlorophyll a and b standards were each (1 g) dissolved in 20 mL of 100% acetone to obtain 0.05 mg/mL stock solutions. Eight concentrations from 0.002 to 0.010 mg/mL in 10 mL acetone were prepared from the stock solution of each standard. Each standard solution (20 µL) was injected into an Agilent 1200 series HPLC (Agilent, Santa Clara, CA, USA). The calibration curves were prepared by plotting the peak area against the concentration ratio. The concentrations were then calculated using the standard calibration curves of chlorophylls a and b. The chromatographic conditions employed in this measurement were as previously mentioned.
2.6. Statistical Analysis
MINITAB (version 16) statistical software was employed for one-way analysis of variance (ANOVA). Tukey’s test was performed to determine the significant differences among means at the 5% level. The data were expressed as means ± standard deviations of three replicate determinations.
3. Results and Discussion
3.1. Chlorophyll Content of Amaranth Leaves
The chlorophylls a and b and the total chlorophyll content in acetone extracts were estimated and tabulated in Table 1. The highest content of chlorophylls a and b and total chlorophyll was observed from amaranths with tapered leaves than round leaves. Therefore, the amaranths with tapered leaves were selected for further analysis.

Table 1.
The chlorophyll content (mg/100 g fresh weight) of green amaranth varieties.
3.2. Stabilisation Process of Chlorophylls
3.2.1. Effect of CuSO4 and ZnCl2 Concentrations
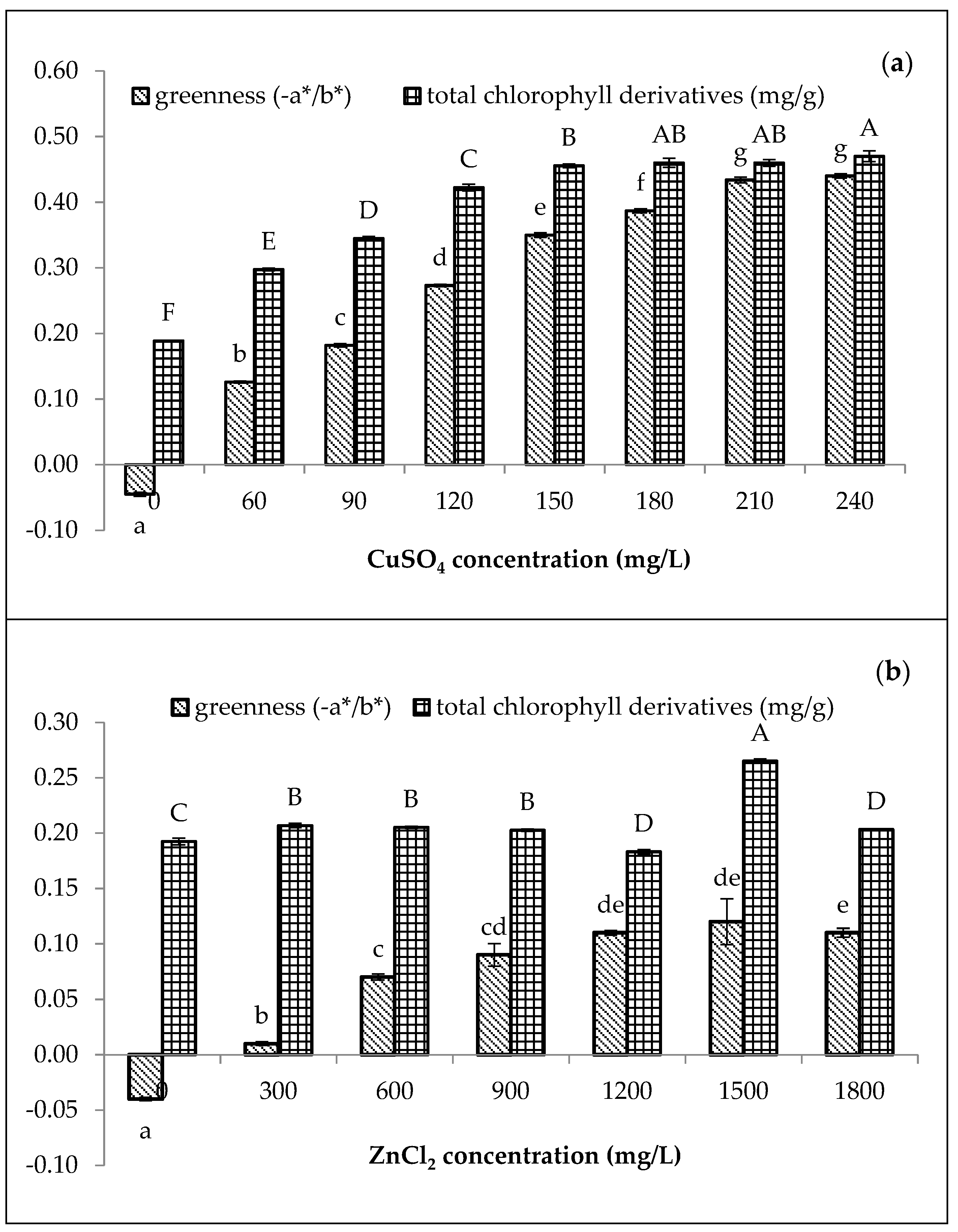
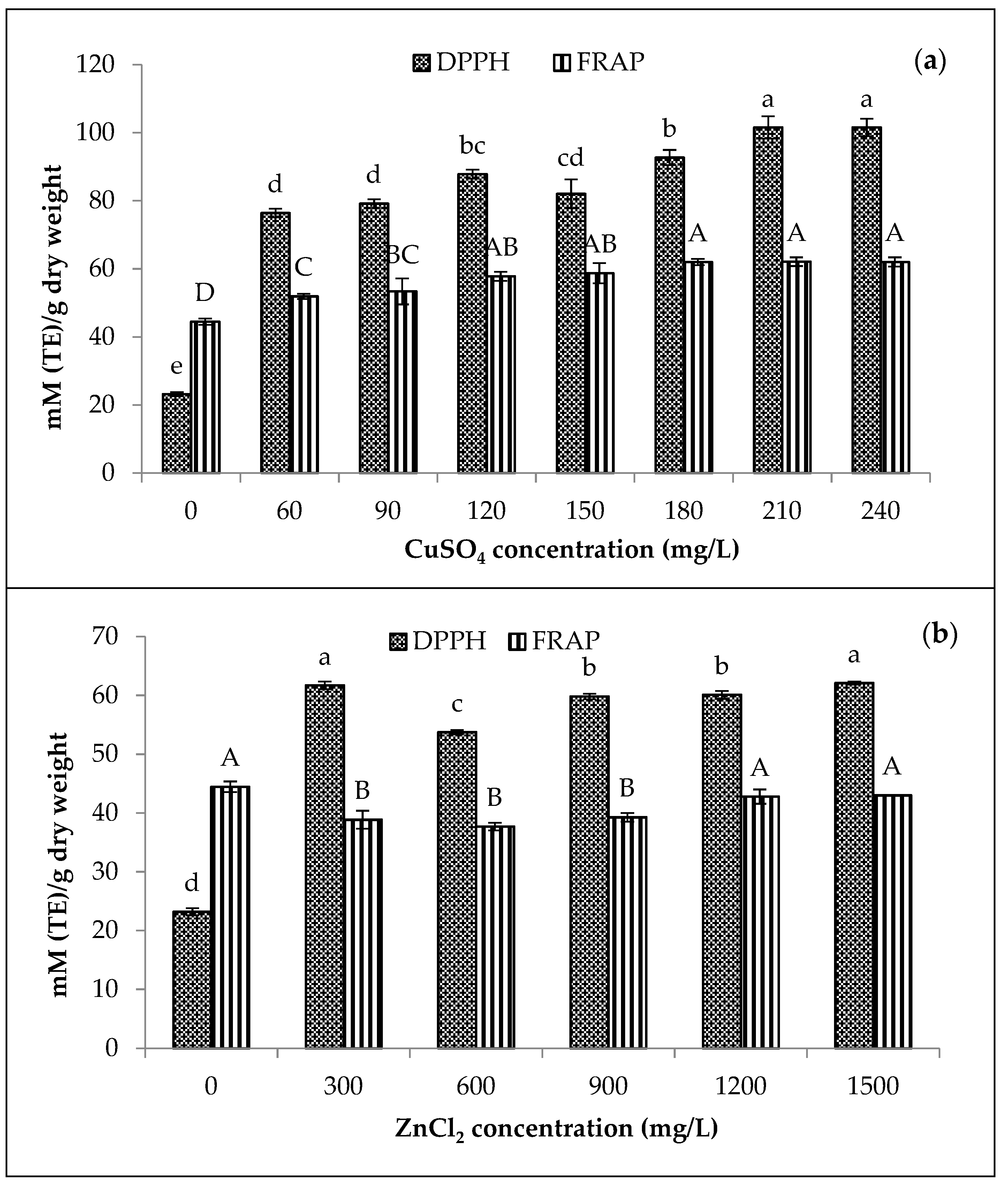
Compared to the chlorophyll content (0.30 mg/g) treated with 60 mg/L of CuSO4, only 0.21 mg/g of total chlorophyll content was achieved by the 300 mg/L of ZnCl2 (Figure 1). Moreover, the greenness (−a*/b*) of amaranth purees increased with increasing concentrations of both Cu and Zn. For Cu-amaranth purees (Figure 1a), the maximum level of greenness was reached when 210 mg/L of CuSO4 was added. Additionally, no significant (p > 0.05) differences were observed in the greenness values when 210 to 240 mg/L of CuSO4 was added. Thus, the results implied that 210 mg/L of CuSO4 resulted in the complete formation of Cu-chlorophyll derivatives in the amaranth puree.
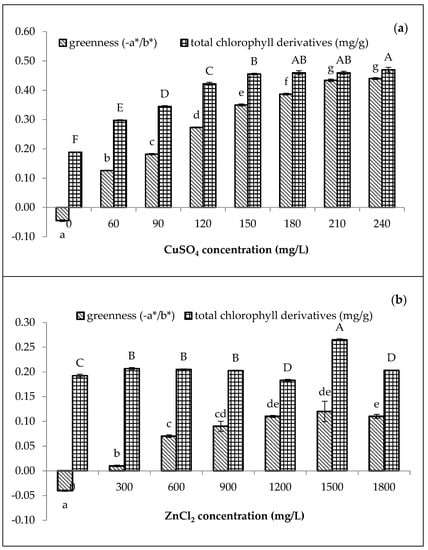
Figure 1.
Effect of metal (a) CuSO4 and (b) ZnCl2 concentrations on greenness (−a*/b*) and total chlorophyll derivative content (mg/g) of amaranth purees. The study was performed at pH 6 after heating at 80 °C for 15 min. Each value is expressed as mean ± standard deviation (n = 3) of triplicate analysis. The bars with different lowercase (a–g) and uppercase letters (A–F) indicate significant differences (p < 0.05) in Tukey’s HSD test.
In Figure 1a, the chlorophyll derivative contents of the Cu-amaranth purees increased with increasing metal concentrations. Furthermore, no significant (p > 0.05) differences were depicted at 180, 210, and 240 mg/L of CuSO4. Therefore, stabilization at 210 mg/L of CuSO4 was chosen due to its highest greenness value and chlorophyll derivative contents (0.46 mg/g FW). For Zn-amaranth purees (Figure 1b), no significant (p > 0.05) differences were observed when the a*/b* value reached its maximum from 1200 to 1800 mg/L of ZnCl2. Meanwhile, chlorophyll derivatives content was significantly highest (0.27 mg/g fresh weight) in Zn-amaranth puree treated with 1500 mg/L of ZnCl2. Hence, 1500 mg/L of ZnCl2 concentration was selected for subsequent experiments. The Zn concentration obtained in this study was close to the finding of Ngo et al., which retained the green colour of pears using 1300 mg/L of Zn ions [18]. In this study, the ZnCl2 concentrations were higher than CuSO4 concentrations. Hence, the higher ZnCl2 concentrations were due to Zn salts being less reactive than Cu salts [22,25]. In addition, the formation of Zn complexes with chlorophyll derivatives occurred much slower than Cu complexes [46]; therefore, more Zn ions are required to form metallo-chlorophyll complexes in amaranth purees.
Figure 1a,b, demonstrate that the green pigments of the control purees (0 mg/L of Cu or Zn and heated at 80 °C for 15 min) have mostly degraded. Both control purees turned brownish with a*/b* values of −0.05 and −0.04, respectively; therefore, the colour changes could be due to the formation of chlorophyll degradation compounds, which changed from bright green to brownish [47,48]. In addition, the chlorophyll degradation compounds are Mg-free derivatives, while the control puree had only 0.19 mg/g of total chlorophyll derivative content [49]. Previous studies reported that the formation of metallo-chlorophyll complexes depends on the metal and pigment concentrations, pH [50], temperature [51], and duration of contact between the metal solution and vegetable tissues [22]. Thus, Cu and Zn were used for subsequent studies with concentrations of 210 mg/L and 1500 mg/L, respectively.
3.2.2. Effect of pH
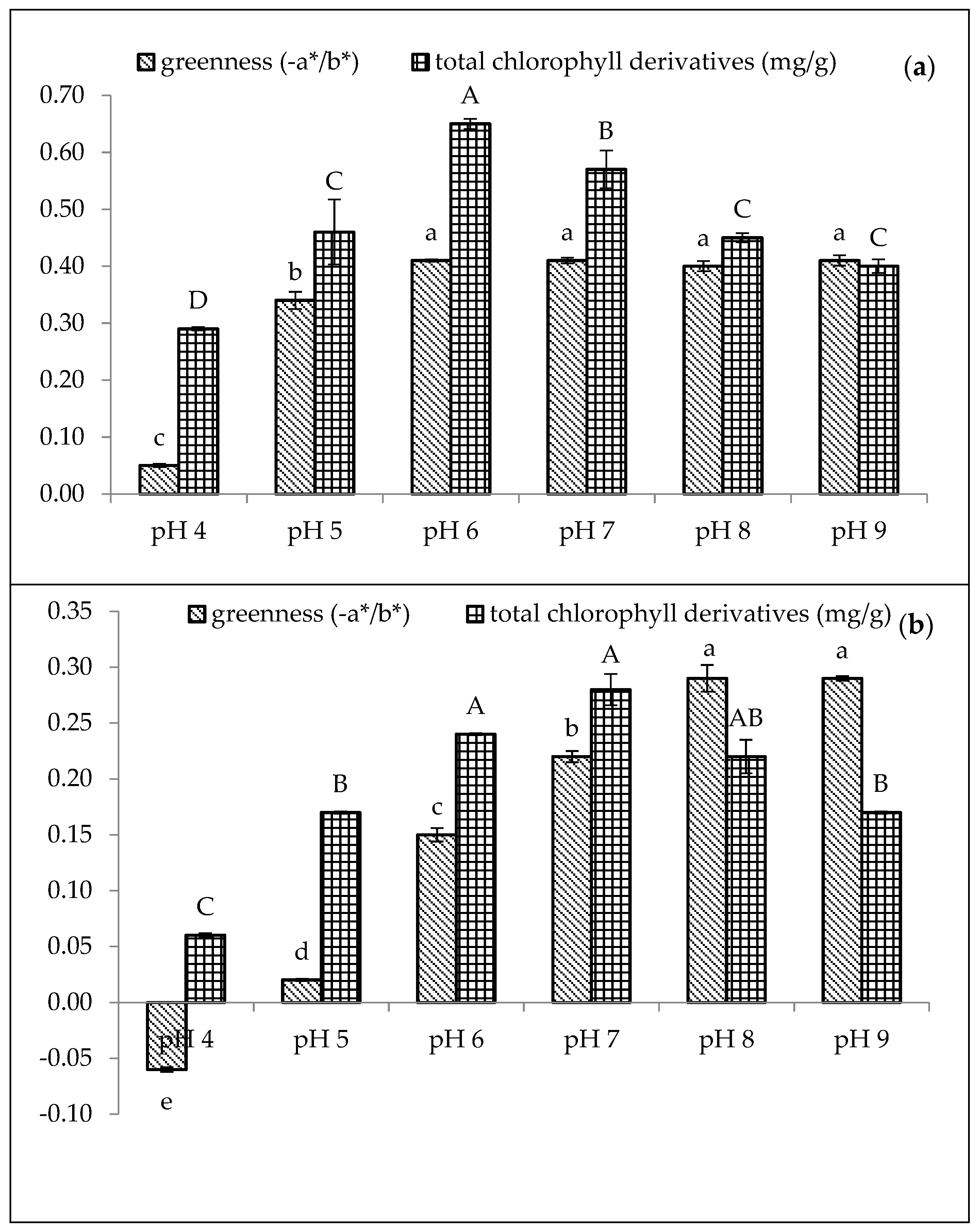
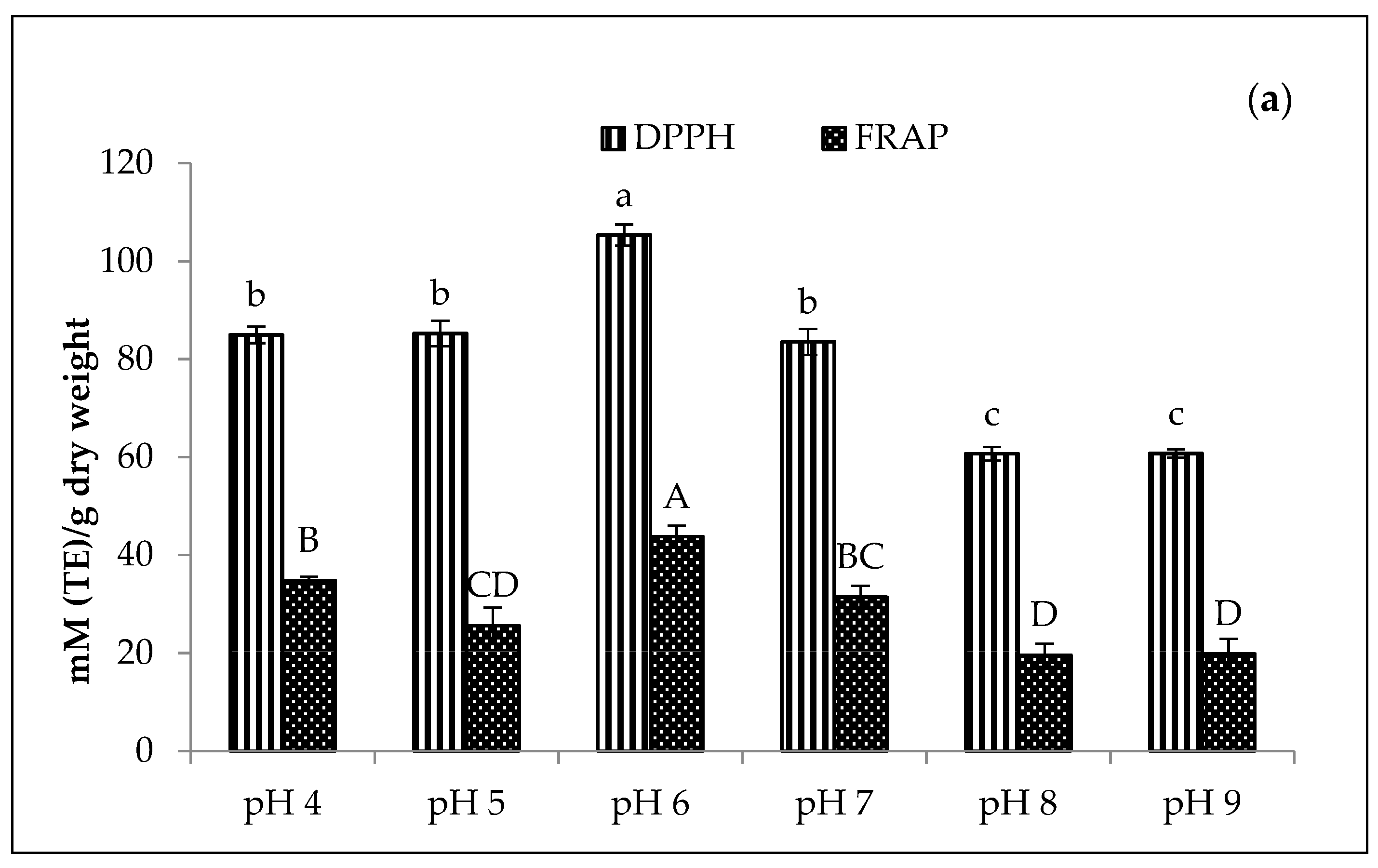
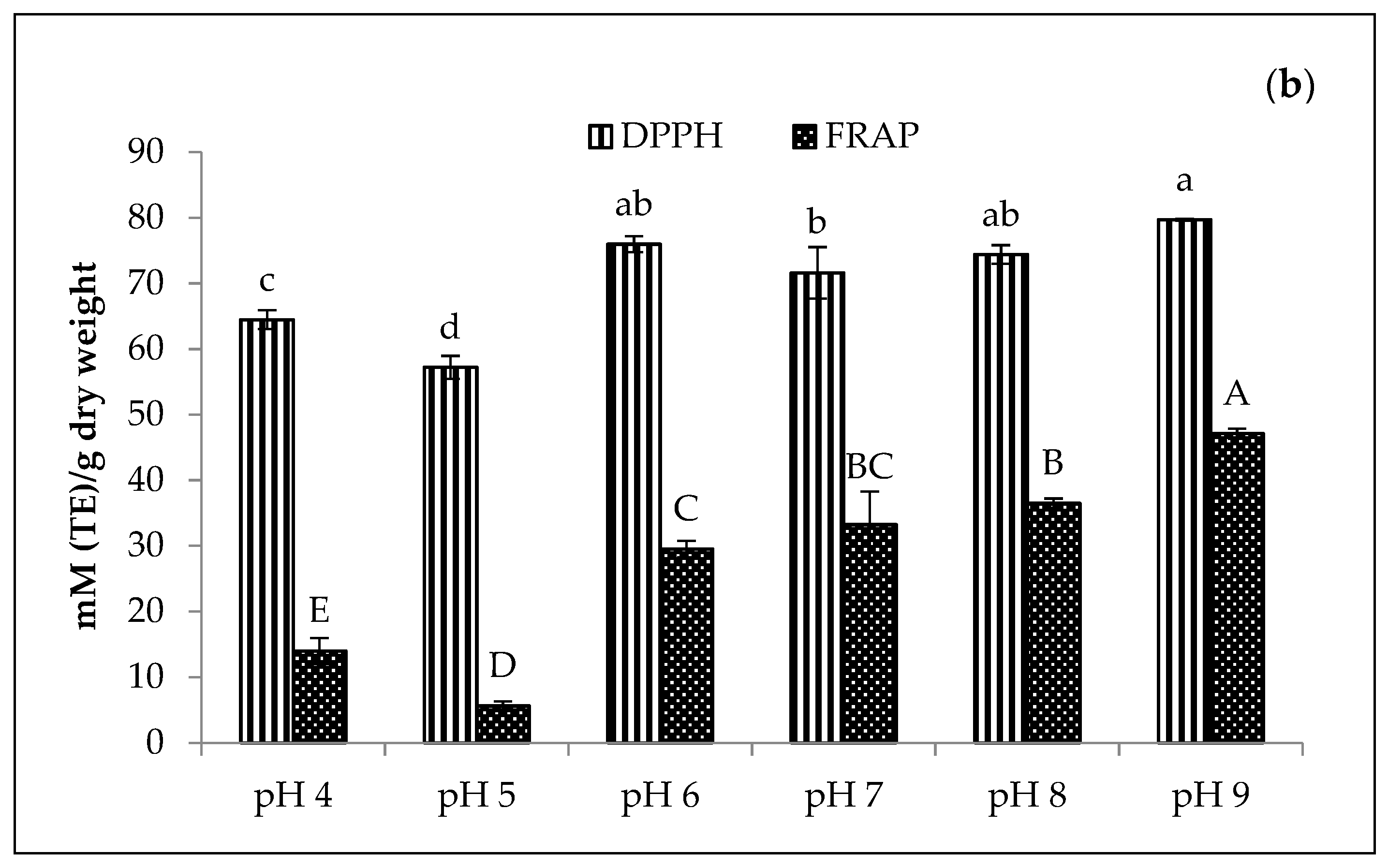
Figure 2 presents the effect of various pH values (4–9) on the amaranth purees stabilised with Cu and Zn concentrations of 210 mg/L and 1500 mg/L, respectively, at 80 °C for 15 min. Thus, the results indicated that the pH value significantly affected the greenness and chlorophyll derivative content of both Cu- and Zn-amaranth purees (p < 0.05). Moreover, these results are consistent with the findings of LaBorde et al., that the formation of metallo-chlorophyll derivatives in heated vegetables was a pH-dependent process [52].
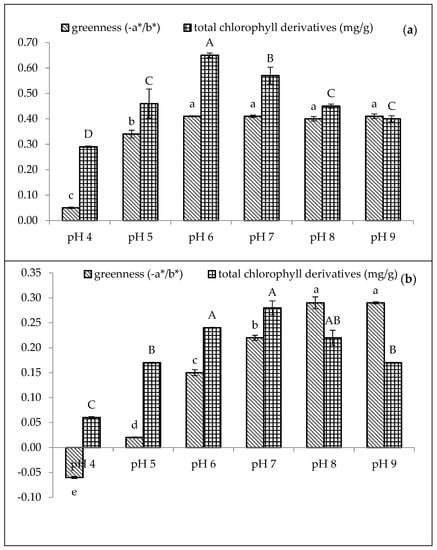
Figure 2.
Effect of pH on greenness (−a*/b*) and total chlorophyll derivative content (mg/g) in (a) 210 mg/L of CuSO4 and (b) 1500 mg/L of ZnCl2 stabilised amaranth purees after heating at 80 °C for 15 min. Each value is expressed as mean ± standard deviation (n = 3) of triplicate analysis. The bars with different lowercase (a–e) and uppercase (A–D) letters indicate significant differences (p < 0.05) in Tukey’s HSD test.
In Figure 2a, Cu-amaranth purees produced the highest greenness value (−0.41) with no significant difference (p > 0.05) between pH from 6 to 9. Comparatively, Zn-amaranth purees are significantly (p < 0.05) the highest greenness value (−0.29) at pH 8. Subsequently, the greenness value reached a plateau above pH 8. Guzmán et al. reported similar results that the avocado purees treated with copper chloride showed larger negative values of a*/b* (greener colour) than the avocado purees treated with ZnCl2 [19]. In contrast, Senklang et al. reported that the green colour of pandan leaf extracts was highest at pH 5 and lower at pH 7 and 8 [25]. Hence, the contradicting results of pH may be associated with variations in the plants’ raw materials and chlorophyll derivative contents.
The highest chlorophyll derivative content (0.65 mg/g FW) was observed in Cu-amaranth purees (prepared with 210 mg/L of CuSO4) at pH 6. Nevertheless, the value decreased at pH > 6 (Figure 2a). Based on these results, metallo-chlorophyll complexes rapidly formed when the pH was increased from 4 to 8.5. On the contrary, these formations decreased at pH 10 [49]. Therefore, the Cu-amaranth purees at pH 6 were used for subsequent experiments. Meanwhile, the chlorophyll derivative content of Zn-amaranth purees at pH 7 and 8 were 0.28 and 0.22 mg/g FW, respectively. However, the colour of the puree at pH 8 was brighter than the puree at pH 7 (Figure 2b). Based on these observations, the Zn-amaranth puree treated at pH 8 was selected for subsequent experiments.
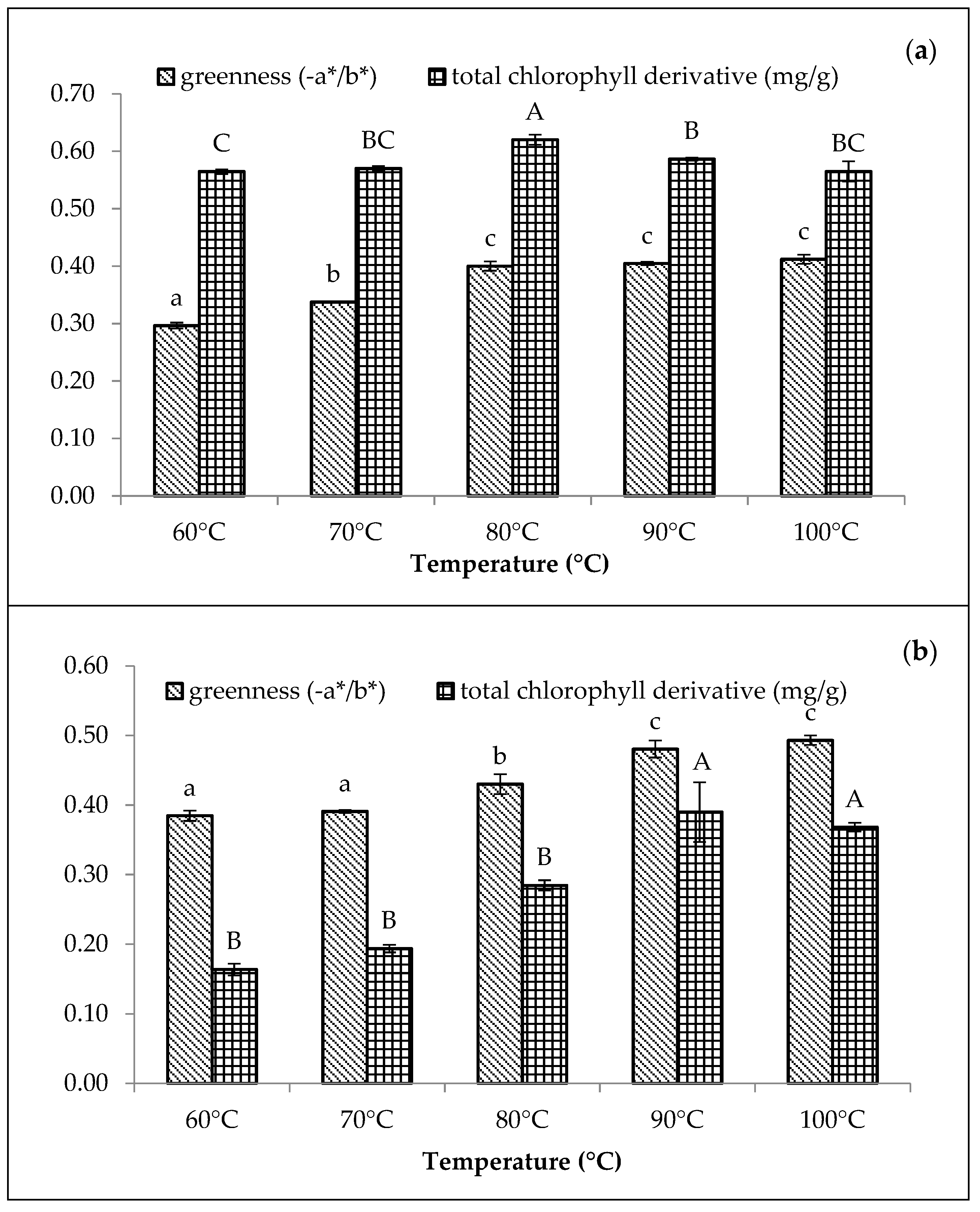
3.2.3. Effect of Temperature
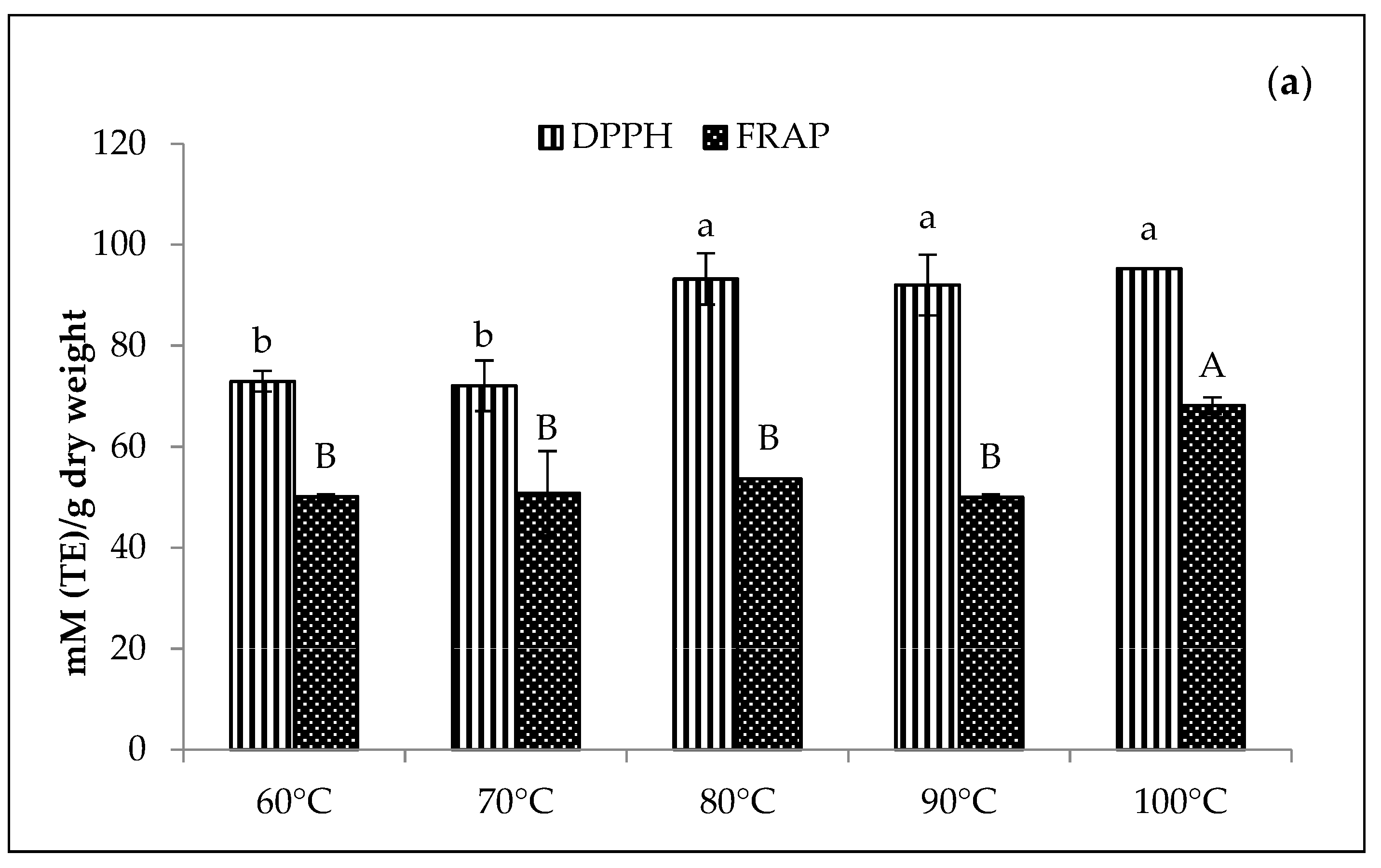
The greenness and chlorophyll derivative content of the Cu-amaranth and Zn-amaranth purees increased with the increasing temperature in Figure 3. Based on the results, the Cu-amaranth puree produced the highest chlorophyll derivative content (0.62 mg/g FW) at 80 °C and greenness value (−0.40) at 80 to 100 °C (Figure 3a). The thermal treatment process was also reported as essential when using metals to retain green pigments. The heat is important in removing carbomethoxyl at the C10 carbon position on the isocyclic ring of chlorophyll, transforming the pyrrole nucleus into a cation [44]. Thus, the transformation improves the diffusion of divalent metal ions (Cu2+ or Zn2+) in dislodging Mg2+ ions to form metal complexes with chlorophylls [17]. The highest greenness value was depicted with the Zn-amaranth purees at 90 °C. Meanwhile, the highest chlorophyll derivative content was observed at 90 °C (0.39 mg/g FW) and 100 °C (0.37 mg/g FW).
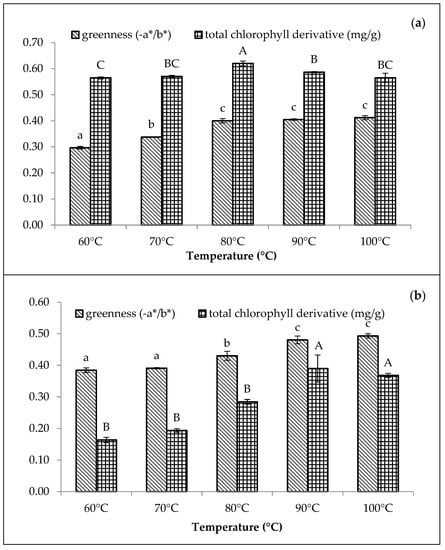
Figure 3.
Effect of temperatures (reaction time of 15 min) on colour (−a*/b*) and total chlorophyll derivative content (mg/g) of (a) 210 mg/L of CuSO4 stabilised amaranth purees at pH 6 and (b) 1500 mg/L of ZnCl2 stabilised amaranth purees at pH 8. Each value is expressed as mean ± standard deviation (n = 3) of triplicate analysis. The bars with different lowercase (a–c) and uppercase (A–C) letters indicate significant differences (p < 0.05) in Tukey’s HSD test.
In this study, the findings were in line with Guzmán et al., which reported that avocado purees treated with Cu or Zn at 87 to 89 °C produced greener colour than untreated samples [19]. Similarly, Canjura et al. and Salama et al. reported that treating green vegetables with Cu and Zn and heating them at higher temperatures (>85 °C) would result in colour improvements [22,27]. Canjura et al. also reported that peas blanched in Zn solution (50–500 mg/L) showed increased complex formation during the heat process (121–125 °C) [22]. The heat treatment process causes chlorophyll derivatives, such as pheophytin, to react with metal ions to form metal-pheophytin complexes with a stable green colour [22,44]. Therefore, heat treatments at 80 °C (Cu-amaranth puree) and 90 °C (Zn-amaranth puree) were selected as the optimum stabilisation temperatures.
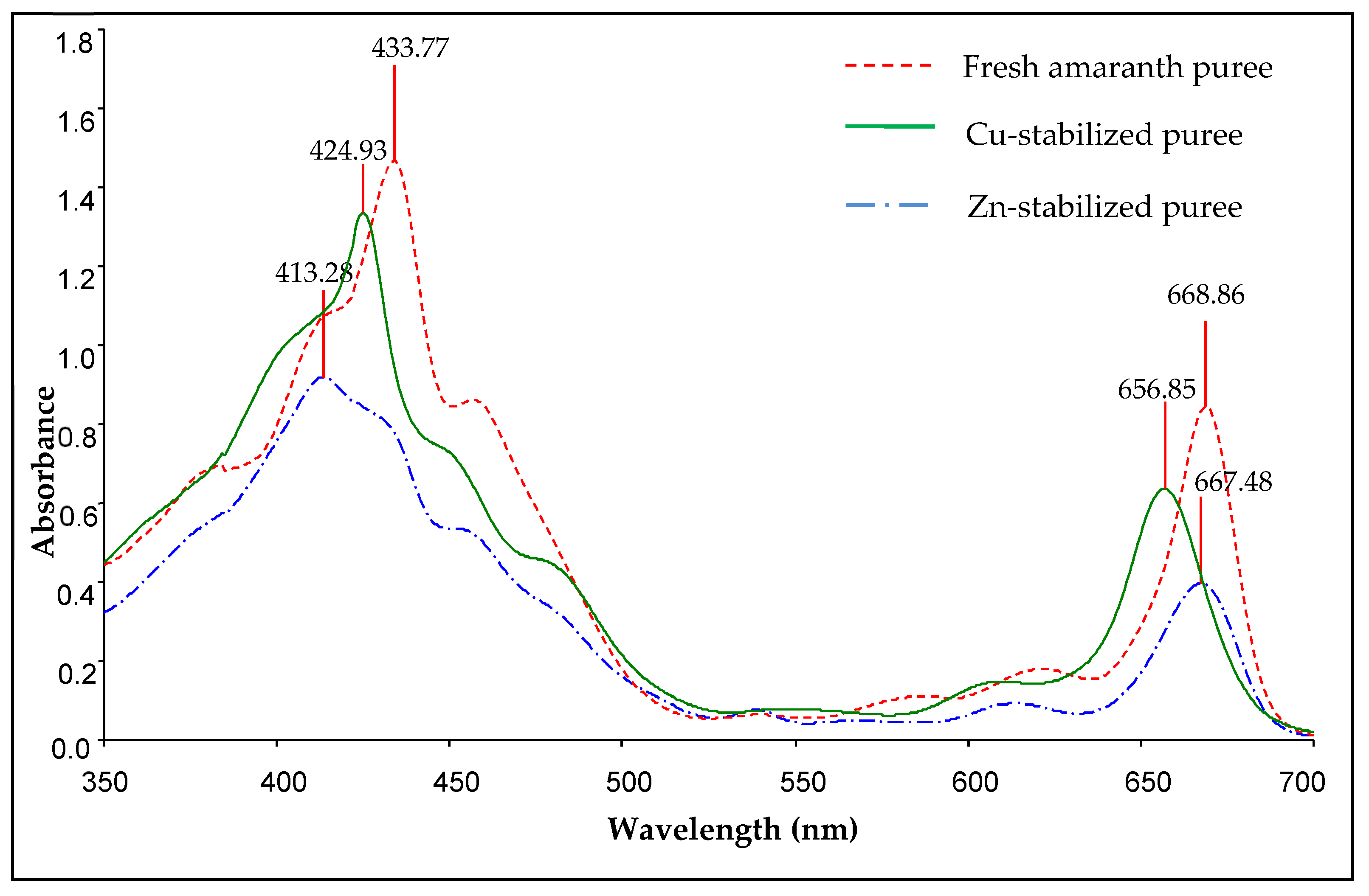
Figure 4 displays the three absorbance spectra of acetone extracts of fresh and metal-stabilised amaranth purees. The amaranth purees are mostly absorbed in the blue and red regions of visible light due to the presence of chlorophylls and their derivative forms [53]. All acetone extracts of fresh and metal-stabilised amaranth purees have two major absorption bands in the visible range, which are “red” (Q-) and “blue” (Soret- or B-) bands [53]. The “red” and “blue” bands of chlorophyll a (Q- and B-bands) were located at wavelengths of 662 and 430 nm for acetone solutions, respectively [54]. A difference in the maximum absorption of the amaranth extracts at wavelengths from 350 to 500 nm was displayed in Figure 5 due to the colour differences between fresh amaranth and metal-stabilised purees [18].
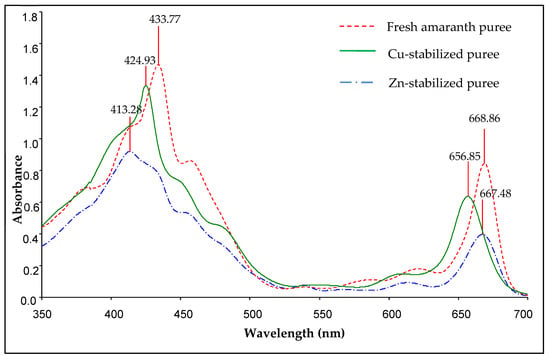
Figure 4.
Absorbance spectra of acetone extracts of fresh and metal-stabilised amaranth purees (Cu-amaranth puree: 120 mg/L of CuSO4 at 80 °C for 15 min, Zn-amaranth puree: 1500 mg/L of ZnCl2 at 90 °C for 15 min.
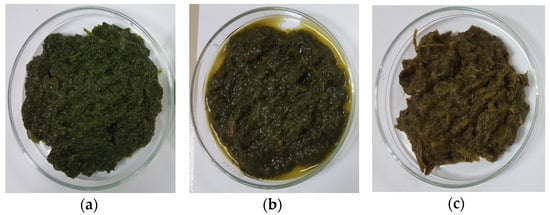
Figure 5.
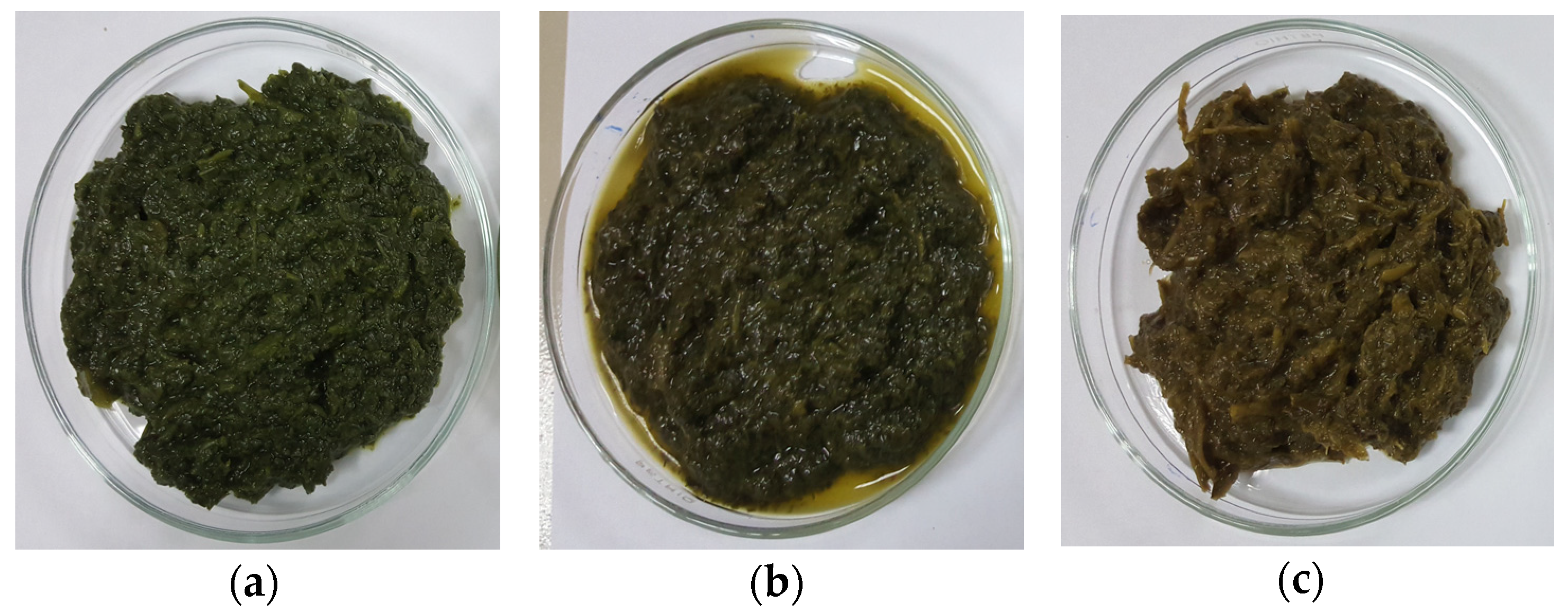
Amaranth purees of (a) Cu-amaranth puree stabilised at pH 6 and 80 °C for 15 min with 210 mg/L of CuSO4, (b) Zn-amaranth puree stabilised at pH 8 and 90 °C for 15 min with 1500 mg/L ZnCl2, and (c) control amaranth puree (0 mg/L of Zn or Cu and heated at 80 °C for 15 min).
The maximum absorption peaks of fresh amaranth puree were located at 433.77 and 668.86 nm wavelengths. Hence, the results were closely related to Von Elbe et al. [52], which concluded that the absorption maxima of chlorophyll a were at 428.5 and 660.5 nm wavelengths. Furthermore, Ngo et al. also reported that the fresh pear peel absorption peaks occurred at wavelengths of 430 and 660.5 nm [18]. Thus, these results indicated that the green colour of fresh amaranth puree was dominated by chlorophyll a [18].
For Cu-stabilised purees, the absorption maxima peaks were at wavelengths of 424.93 and 656.85 nm, respectively. The results were consistent with the findings of Scotter et al., in which the absorption maxima at the Soret (S-) and Q-bands of Cu chlorophyll a were at wavelengths of 422 and 652 nm [55], respectively. Based on the amaranth stabilised with Zn, the extracts revealed absorption maxima at 413.28 and 667.48 nm wavelengths.
In addition, Zheng et al. elucidated that the absorption maxima of Thomson seedless grape purees occurred at 664 to 670 nm wavelengths for the complexes with ZnCl2 [23]. Nonetheless, these data contradict the data of Von Elbe et al., who reported that the absorption maxima for the Zn stabilization process were at wavelengths of 655 and 423 nm [52]. The contradicting results were probably due to the different concentrations of Zn and the heat treatment processes used.
3.3. Antioxidant Activities of Cu- and Zn-Amaranth Purees
3.3.1. Effect of Metal Concentration
Figure 6 displays the significant effects of metal concentrations on the antioxidant activity of methanolic extracts of Cu- and Zn-amaranth purees. Based on the Cu-amaranth purees, the DPPH antioxidant activities reached their maximum levels when the puree was stabilised with 210 and 240 mg/L of CuSO4. Furthermore, the FRAP values were highest when treated with CuSO4 above 120 mg/L. The Zn-amaranth purees depicted no significant differences at the highest value of the DPPH antioxidant activity (300 and 1500 mg/L of ZnCl2). Nevertheless, the FRAP values increased when ZnCl2 was above 1200 mg/L.
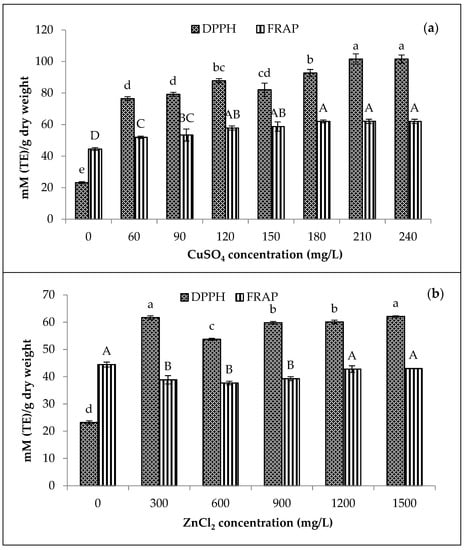
Figure 6.
Effect of metal (a) CuSO4 and (b) ZnCl2 concentrations on antioxidant activity of amaranth puree at pH 6 after heating at 80 °C for 15 min. Each value is expressed as mean ± standard deviation (n = 3) of triplicate analysis. The bars with different lowercase (a–e) and uppercase (A–D) letters indicate significant differences (p < 0.05) in Tukey’s HSD test.
Figure 6 shows lower antioxidant activity in amaranth puree with metal-free derivatives (at 0 mg/L of CuSO4 and ZnCl2) than in amaranth puree containing metallo-chlorophyll derivatives. Interestingly, the FRAP values of amaranth purees were significantly (p < 0.05) higher than Zn-amaranth purees in the range of approximately 300 to 900 mg/L of ZnCl2. The higher FRAP values could be related to the other components in amaranth purees, which contributed to their higher antioxidant activity. According to Hoshina et al. and Ferruzzi et al., the antioxidant content was related to metal-treated samples [16,56].
In another study, Wrolstad et al. elucidated that metallo-chlorophyll derivatives, such as Cu-pyropheophytin and Zn-pheophytin produced higher antioxidant activities than metal-free derivatives (pheophytin and pyropheophytin) [57]. The findings in Figure 6 supported the higher antioxidant activity. Furthermore, the CuSO4 (210 mg/L) and ZnCl2 (1500 mg/L) stabilisation process on amaranth purees revealed the highest chlorophyll derivative contents. Thus, the relationship between antioxidant activity and chlorophyll derivative content of metal-treated amaranth purees was observed. Furthermore, metallo-chlorophyll derivatives, such as Cu- and Zn- pheophytin or phyropheophytin were reported to exhibit significantly higher antioxidant capacity than metal-free chlorophyll derivatives [57].
3.3.2. Effect of pH
The DPPH and FRAP values of stabilised amaranth purees affected by pH are presented in Figure 7. The results depicted that the Cu-amaranth puree at pH 6 had the highest DPPH (105.33 mM TE/dry weight) and FRAP (43.75 mM TE/dry weight) values. Additionally, the antioxidant activity of Zn-amaranth puree increased when treated at above pH 6. The DPPH and FRAP values of Zn-amaranth purees ranged from 76 to 80 mM TE/dry weight and 30 to 47 mM TE/dry weight, respectively. Hence, these values were lower than the values of Cu-amaranth purees.
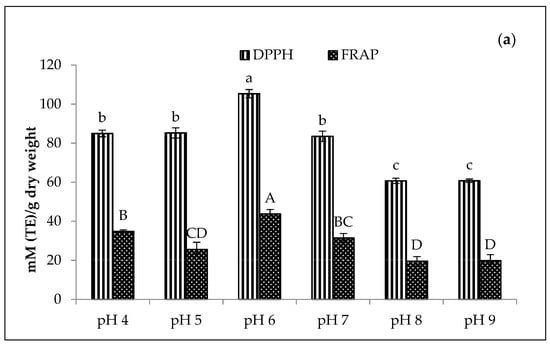
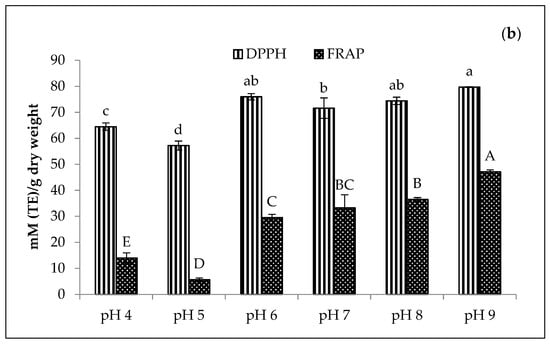
Figure 7.
Effect of pH on antioxidant activities of (a) 210 mg/L of CuSO4 and (b) 1500 mg/L of ZnCl2 stabilised amaranth purees after heating at 80 °C for 15 min. Each value was expressed as mean ± standard deviation (n = 3) of triplicate analysis. The bars with different lowercase (a–d) and uppercase (A–E) letters indicate significant differences (p < 0.05) in Tukey’s HSD test.
3.3.3. Effect of Temperature
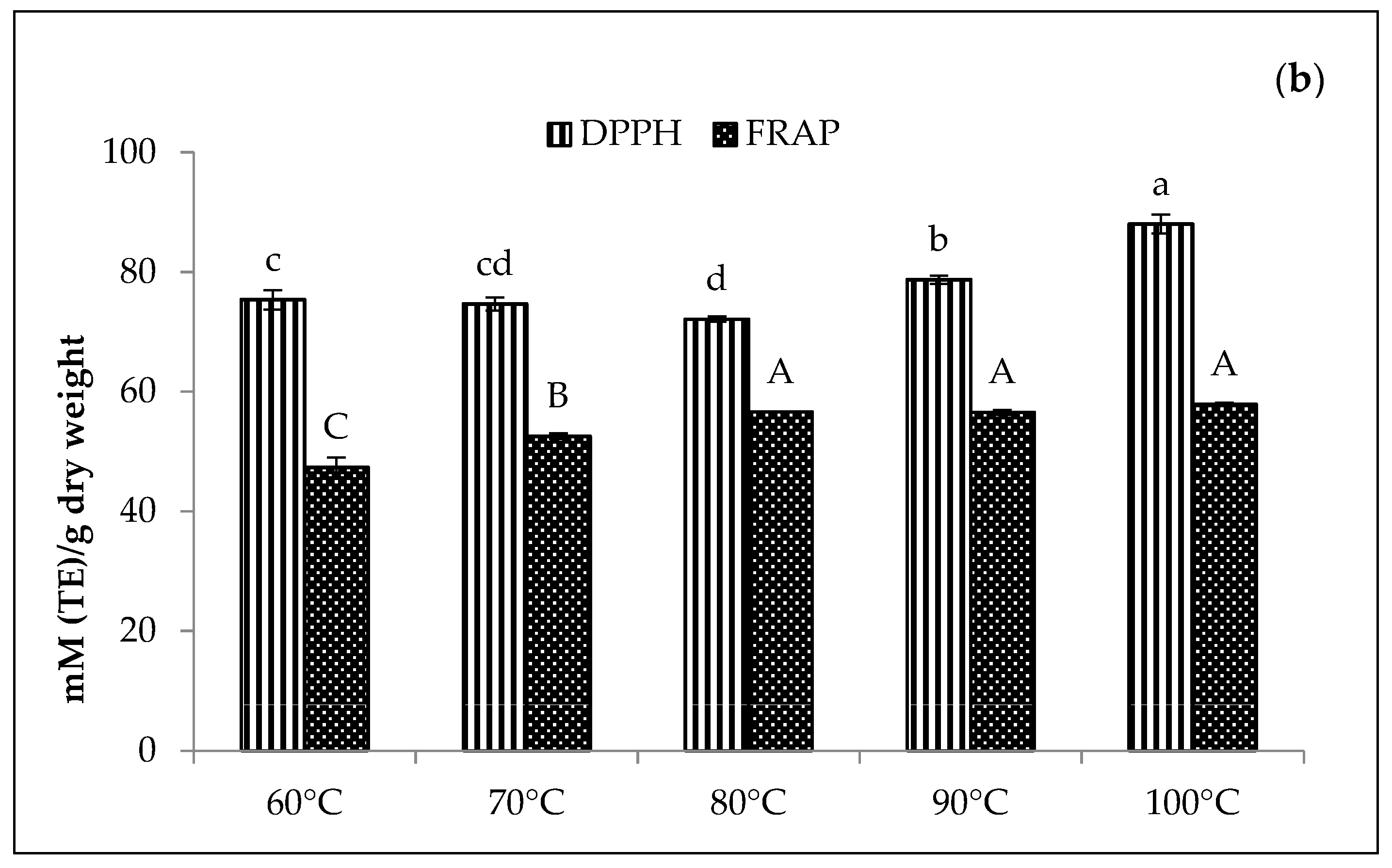
In Figure 8, the Zn-amaranth purees significantly (p < 0.05) displayed the highest DPPH values at 88 mM TE/dry weight at 100 °C. Conversely, the highest FRAP values range from 57 to 58 mM TE/dry weight at 80 to 100 °C. A similar trend was also observed for Cu-amaranth purees with the highest DPPH values ranging from 93 to 95 mM TE/dry weight at 80 to 100 °C. Moreover, the DPPH values were higher than Zn-amaranth purees. Thus, these findings confirmed that the antioxidant activities increased with increasing temperatures, probably due to the formation of firm bonds between Zn or Cu with chlorophyll derivatives. In addition, the metallo-chlorophyll derivatives complexes are more resistant to acid and heat treatments than natural Mg2+-chlorophyll complexes [58].
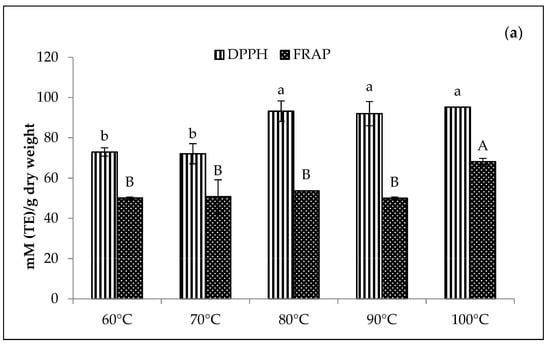
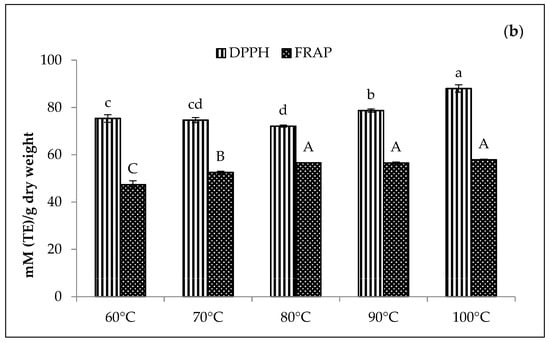
Figure 8.
Effect of temperatures for the reaction time of 15 min on antioxidant activities of (a) 210 mg/L of CuSO4 stabilised amaranth purees at pH 6 and (b) 1500 mg/L of ZnCl2 stabilised amaranth purees at pH 8. Each value was expressed as mean ± standard deviation (n = 3) of triplicate analysis. The bars with different lowercase (a–d) and uppercase (A–C) letters indicate significant differences (p < 0.05) in Tukey’s HSD test.
3.4. Identification of Chlorophylls and Their Derivatives in Fresh, Cu-, and Zn-Amaranth Purees Using Reversed-Phase HPLC
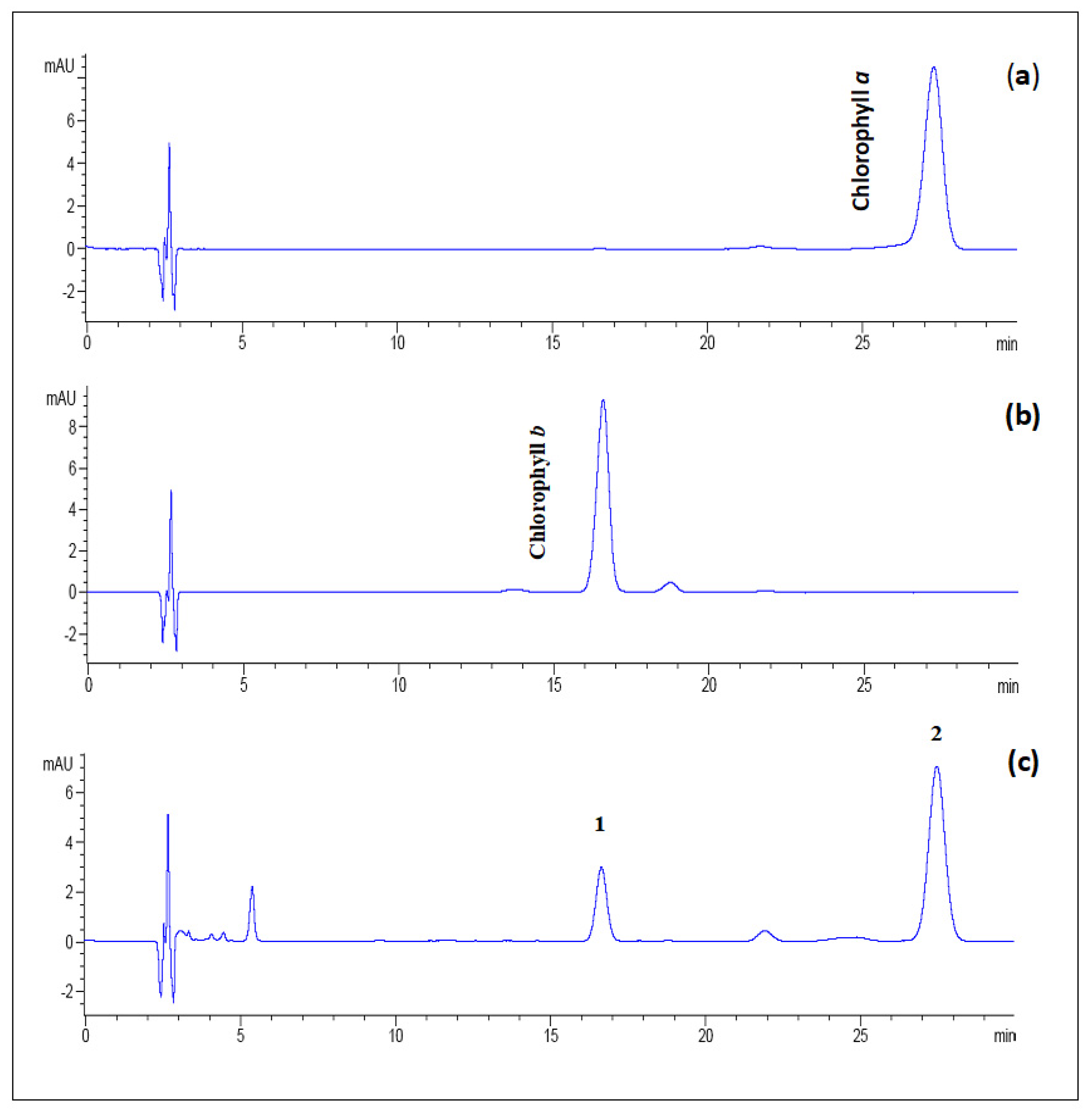
The identification of peaks was confirmed by comparing retention times (tret) of the peaks in the sample with standard chlorophylls a and b (Figure 9a,b). As shown in Figure 9c, the HPLC chromatogram of the chlorophyll fraction from the fresh amaranth extract depicted two major peaks at retention times (tret) of 16.73 (peak 1) and 27.65 min (peak 2). Hence, these peaks corresponded to chlorophyll b and chlorophyll a, respectively.
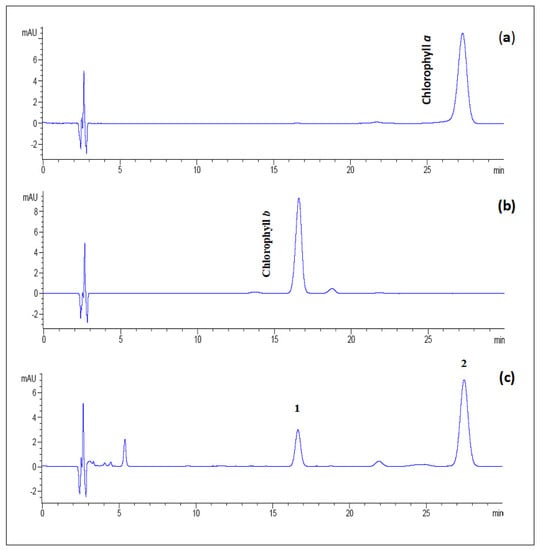
Figure 9.
The reversed-phase HPLC chromatograms of (a) standard chlorophyll a, (b) standard chlorophyll b, and (c) fresh amaranth puree extracts (peaks identification: 1 = chlorophyll b and 2 = chlorophyll a) were detected at a wavelength of 658 nm. The separation process was carried out using a reversed-phase C18 column with an isocratic mobile phase of ethyl acetate/methanol/water (40:54:10 v/v/v).
Chlorophyll b appeared to have a shorter retention time in the non-polar C18 type of column, which is more polar and eluting than chlorophyll a [59,60]. The elution order of chlorophylls a and b of fresh amaranth extracts coincided with green bean and spinach extracts [51]. Additionally, chlorophylls a and b in fresh amaranths were 5.01 mg/g and 1.26 mg/g, respectively. In this study, the chlorophyll content of fresh amaranth puree recorded was higher than green beans (chlorophyll a: 1.11 mg/g, b: 0.74 mg/g) and peas (chlorophyll a: 0.8 mg/g, b: 0.51 mg/g) [61]. Nevertheless, the chlorophyll content of chlorophylls a and b in spinach leaves (chlorophyll a: 14.1 mg/g, b: 6.2 mg/g DW) was higher than the amaranth purees reported in this study [62].
4. Conclusions
In conclusion, the findings of this study established that metal concentration, pH, and temperature can influence the stabilisation process of the green colour, chlorophyll derivative content, and antioxidant activity of amaranth purees. The optimal stabilisation process conditions for the formation of Cu-amaranth purees were successfully achieved at pH 6 and a temperature of 80 °C for a reaction time of 15 min using 210 mg/L of CuSO4. Alternatively, Zn-amaranth occurred more readily at pH 8 and a temperature of 90 °C for a reaction time of 15 min using 1500 mg/L of ZnCl2. The chromatograms in both Cu- and Zn-amaranth extracts showed no peaks, which corresponded to the complete degradation of chlorophylls a and b due to the formation of metallo-chlorophyll derivatives. The colour of Cu-amaranth puree was olive-green, while Zn-amaranth puree was bluish-green. Based on the results obtained in this study, the outcome will be useful in developing stable green vegetables with antioxidant activity to be applied in the food processing industry.
Author Contributions
Supervision, project administration, funding acquisition, and writing—review and editing, K.M.; conceptualization, methodology, and resources, K.M., R.K., and Y.A.Y.; formal analysis, data curation, writing—original draft preparation, and writing—review and editing, S.F.M.A. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank Universiti Putra Malaysia (Vot. 6360600) for providing financial support for this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sarker, U.; Hossain, M.M.; Oba, S. Nutritional and antioxidant components and antioxidant capacity in green morph Amaranthus leafy vegetable. Sci. Rep. 2020, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Alegbejo, J.O. Nutritional value and utilization of Amaranthus (Amaranthus spp.)—A Review. Bayero J. Pure Appl. Sci. 2013, 6, 136–143. [Google Scholar] [CrossRef]
- Achigan-Dako, E.G.; Sogbohossou, O.E.D.; Maundu, P. Current knowledge on Amaranthus spp.: Research avenues for improved nutritional value and yield in leafy amaranths in sub-Saharan Africa. Euphytica 2014, 197, 303–317. [Google Scholar] [CrossRef]
- Amin, I.; Norazaidah, Y.; Hainida, K.I.E. Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem. 2006, 94, 47–52. [Google Scholar] [CrossRef]
- Vivek, P.; Prabhakaran, S.; Shankar, S.R. Assessment of nutritional value in selected edible greens based on the chlorophyll content in leaves. Res. Plant Biol. 2013, 3, 45–49. [Google Scholar]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Genotypic variability for nutrient, antioxidant, yield and yield contributing traits in vegetable amaranth. J. Food Agric. Environ. 2014, 12, 168–174. [Google Scholar]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Phenotypic divergence in vegetable amaranth for total antioxidant capacity, antioxidant profile, dietary fiber, nutritional and agronomic traits. Acta Agric. Scand. B Soil Plant Sci. 2018, 68, 67–76. [Google Scholar] [CrossRef]
- Hosikian, A.; Lim, S.; Halim, R.; Danquah, M.K. Chlorophyll extraction from microalgae: A review on the process engineering aspects. Int. J. Chem. Eng. 2010, 2010, 1–11. [Google Scholar] [CrossRef]
- Schoefs, B. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci. Technol. 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Sinnecker, P. Chlorophylls: Properties, biosynthesis, degradation and functions. In Food Colorants: Chemical and Functional Properties; Socaciu, C., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 25–45. [Google Scholar]
- Schwartz, S.J.; Lorenzo, T.V. Chlorophylls in foods. Crit. Rev. Food Sci. Nutr. 1990, 29, 1–17. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Erge, H.S.; Karadeniz, F.; Koca, N.; Soyer, Y. Effect of heat treatment on chlorophyll degradation and color loss in green peas. Gida Derg. 2008, 33, 225–233. [Google Scholar]
- Edelenbos, M.; Christensen, L.P.; Grevsen, K. HPLC determination of chlorophyll and carotenoid pigments in processed green pea cultivars (Pisum sativum L.). J. Agric. Food Chem. 2001, 49, 4768–4774. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, characterization, and functional properties of chlorophylls, pheophytins, and Zn-pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Böhm, V.; Courtney, P.D.; Schwartz, S.J. Antioxidant and antimutagenic activity of dietary chlorophyll derivatives determined by radical scavenging and bacterial reverse mutagenesis assays. J. Food Sci. 2002, 67, 2589–2595. [Google Scholar] [CrossRef]
- Ngo, T.; Zhao, Y. Retaining Green Pigments on Thermally Processed Peels-on Green Pears. J. Food Sci. 2005, 70, C568–C574. [Google Scholar] [CrossRef]
- Ngo, T.; Zhao, Y. Formation of Zinc-Chlorophyll-Derivative Complexes in Thermally Processed Green Pears (Pyrus communis L.). J. Food Sci. 2007, 72, C397–C404. [Google Scholar] [CrossRef]
- Guzmán, G.R.; Dorantes, A.L.; Hernández, U.H.; Hernández, S.H.; Ortiz, A.; Mora, E.R. Effect of zinc and copper chloride on the color of avocado puree heated with microwaves. Innov. Food Sci. Emerg. Technol. 2002, 3, 47–53. [Google Scholar] [CrossRef]
- Porrarud, S.; Pranee, A. Microencapsulation of Zn-chlorophyll pigment from Pandan leaf by spray drying and its characteristic. Int. Food Res. J. 2010, 17, 1031–1042. [Google Scholar]
- Chaiwanichsiri, S.; Dharmsuriya, N.; Sonthornvit, N.; Janjarasskul, T. Process improvement to preserve the color of instant pennywort Centella asiatica (Linn. ) Urban. J. Sci. Res. Chula. Univ. 2000, 25, 233–243. [Google Scholar]
- Canjura, F.L.; Watkins, R.H.; Schwartz, S.J. Color Improvement and Metallo-chlorophyll Complexes in Continuous Flow Aseptically Processed Peas. J. Food Sci. 1999, 64, 987–990. [Google Scholar] [CrossRef]
- Zheng, Y.; Shi, J.; Pan, Z.; Cheng, Y.; Zhang, Y.; Li, N. Effect of heat treatment, pH, sugar concentration, and metal ion addition on green color retention in homogenized puree of Thompson seedless grape. LWT-Food Sci. Technol. 2014, 55, 595–603. [Google Scholar] [CrossRef]
- Ngo, T.X. Understanding the Principles and Procedures to Retain Green and Red Pigments in Thermally Processed Peels-on Pears (Pyrus Communis L.). Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2008. [Google Scholar]
- Senklang, P.; Anprung, P. Optimizing Enzymatic Extraction of Zn–Chlorophyll Derivatives from Pandan Leaf Using Response Surface Methodology. J. Food Process. Preserv. 2010, 34, 759–776. [Google Scholar] [CrossRef]
- Tonucci, L.H.; Von Elbe, J.H. Kinetics of the formation of zinc complexes of chlorophyll derivatives. J. Agric. Food Chem. 1992, 40, 2341–2344. [Google Scholar] [CrossRef]
- Salama, M.F.; Moharram, H.A. Relationship between colour improvement and metallo-chlorophyll complexes during blanching of peas and broccoli. Alex. J. Food Sci. Technol. 2007, 4, 11–18. [Google Scholar]
- Rahayuningsih, E.; Pamungkas, M.S.; Olvianas, M.; Putera, A.D.P. Chlorophyll extraction from suji leaf (Pleomele angustifolia Roxb.) with ZnCl2 stabilizer. J. Food Sci. Technol. 2018, 55, 1028–1036. [Google Scholar] [CrossRef]
- FDA. Listing of Color Additives Exempt from Certification; Sodium Copper Chlorophyllin; Food and Drug Administration: Silver Spring, MD, USA, 2002; pp. 35429–35431. [Google Scholar]
- EFSA. Scientific Opinion on re-evaluation of copper complexes of chlorophylls (E 141(i)) and chlorophyllins (E 141(ii)) as food additives. EFSA J. 2015, 13, 4151. [Google Scholar]
- Soldatović, T. Mechanism of interactions of Zinc(II) and Copper(II) complexes with small biomolecules. In Basic Concepts Viewed from Frontier in Inorganic Coordination Chemistry; Intechopen: London, UK, 2018. [Google Scholar]
- de Vogel, J. Green Vegetables and Colon Cancer: The Mechanism of a Protective Effect by Chlorophyll. Ph.D. Thesis, Wageningen University and Research Centre, Wageningen, The Netherlands, 2006; p. 151. [Google Scholar]
- Gomes, B.B.; Barros, S.B.M.; Andrade-Wartha, E.R.S.; Silva, A.M.O.; Silva, V.V.; Lanfer-Marquez, U.M. Bioavailability of dietary sodium copper chlorophyllin and its effect on antioxidant defence parameters of Wistar rats. J. Sci. Food Agric. 2009, 89, 2003–2010. [Google Scholar] [CrossRef]
- Ulbricht, C.; Bramwell, R.; Catapang, M.; Giese, N.; Isaac, R.; Le, T.D. An evidence-based systematic review of chlorophyll by the Natural Standard Research Collaboration. J. Diet. Suppl. 2014, 11, 198–239. [Google Scholar] [CrossRef]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their derivatives used in food industry and medicine. Mini-Rev. Med. Chem. 2017, 17, 1194–1222. [Google Scholar] [CrossRef]
- Zhan, R.; Wu, J.; Ouyang, J. In vitro antioxidant activities of sodium zinc and sodium iron chlorophyllins from pine needles. Food Technol. Biotechnol. 2014, 52, 505. [Google Scholar] [CrossRef] [PubMed]
- Praveena, B.; Murthy, S. Role of photosynthetic pigments in protection against oxidative damage. Int. J. Plant Anim. Environ. Sci. 2013, 4, 167–171. [Google Scholar]
- Hsu, C.Y.; Chao, P.Y.; Hu, S.P.; Yang, C.M. The antioxidant and free radical scavenging activities of chlorophylls and pheophytins. Nutr. Food Sci. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Amin, S.F.M.; Karim, R.; Yusof, Y.A.; Muhammad, K. Effects of enzymatic liquefaction, drying techniques, and wall materials on the physicochemical properties, bioactivities, and morphologies of Zinc-amaranth (Amaranthus viridis L.) powders. Int. J. Food Sci 2021, 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Larrauri García, J.A.; Saura Calixto, F. Evaluation of CIE-lab colour parameters during the clarification of a sugar syrup from Mesquite pods (Prosopis Pallida L.). Int. J. Food Sci. Technol. 2000, 35, 385–389. [Google Scholar] [CrossRef]
- Costache, M.A.; Campeanu, G.; Neata, G. Studies concerning the extraction of chlorophyll and total carotenoids from vegetables. Rom. Biotechnol. Lett. 2012, 17, 7703–7708. [Google Scholar]
- Li, H.B.; Wong, C.C.; Cheng, K.W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT-Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Canjura, F.L.; Schwartz, S.J. Separation of chlorophyll compounds and their polar derivatives by high-performance liquid chromatography. J. Agric. Food Chem. 1991, 39, 1102–1105. [Google Scholar] [CrossRef]
- Gaur, S.; Shivhare, U.; Ahmed, J. Degradation of chlorophyll during processing of green vegetables: A review. Stewart Postharvest Rev. 2006, 2, 1–8. [Google Scholar]
- Weemaes, C.A.; Ooms, V.; Loey, A.M.V.; Hendrickx, M.E. Kinetics of chlorophyll degradation and colour loss in heated broccoli juice. J. Agric. Food Chem. 1999, 47, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Östbring, K.; Rayner, M.; Sjöholm, I.; Otterström, J.; Albertsson, P.Å.; Emek, S.C.; Erlanson-Albertsson, C. The effect of heat treatment of thylakoids on their ability to inhibit in vitro lipase/co-lipase activity. Food Funct. 2014, 5, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Heaton, J.W.; Marangoni, A.G. Chlorophyll degradation in processed foods and senescent plant tissues. Trends Food Sci. Technol. 1996, 7, 8–15. [Google Scholar] [CrossRef]
- LaBorde, L.F.; Von Elbe, J.H. Zinc complex formation in heated vegetable purees. J. Agric. Food Chem. 1990, 38, 484–487. [Google Scholar] [CrossRef]
- Lin, Z.; Schyvens, E. Influence of blanching treatments on the texture and colour of some processed vegetables and fruits. J. Food Process. Preserv. 1995, 19, 451–465. [Google Scholar] [CrossRef]
- LaBorde, L.F.; Von Elbe, J.H. Chlorophyll degradation and zinc complex formation with chlorophyll derivatives in heated green vegetables. J. Agric. Food Chem. 1994, 42, 1100–1103. [Google Scholar] [CrossRef]
- Von Elbe, J.; Schwartz, S. Colourants. Food Chem. 1996, 3, 651–723. [Google Scholar]
- Hoff, A.J.; Amesz, J. Chlorophylls; Scheer, H., Ed.; CRC Press: Boca Raton, FL, USA, 1991; p. 723. [Google Scholar]
- Scotter, M.J.; Castle, L.; Roberts, D. Method development and HPLC analysis of retail foods and beverages for copper chlorophyll (E141 [i]) and chlorophyllin (E141 [ii]) food colouring materials. Food Addit. Contam. 2005, 22, 1163–1175. [Google Scholar] [CrossRef]
- Hoshina, C.; Tomita, K.; Shioi, Y. Antioxidant activity of chlorophylls: Its structure-activity relationship. In Photosynthesis: Mechanisms Effects; Springer: Dordrecht, The Netherlands, 1998; Volume 4, pp. 3281–3284. [Google Scholar]
- Wrolstad, R.E.; Acree, T.E.; Decker, E.A.; Penner, M.; Reid, D.; Schwartz, S.; Shoemaker, C.; Smith, D.; Sporns, P. Pigments, Colorants, Flavors, Texture, and Bioactive Food Components. In Handbook of Food Analytical Chemistry; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Leunda, M.; Guerrero, S.; Alzamora, S. Color and chlorophyll content changes of minimally processed kiwifruit. J. Food Process. Preserv. 2000, 24, 17–38. [Google Scholar] [CrossRef]
- Lim, C.K. High-Performance Liquid Chromatography and Mass Spectrometry of Porphyrins, Chlorophylls and Bilins; World Scientific Publishing Co., Pte Ltd.: Singapore, 2009. [Google Scholar]
- Gökmen, V.; Savaş Bahçeci, K.; Serpen, A.; Acar, J. Study of lipoxygenase and peroxidase as blanching indicator enzymes in peas: Change of enzyme activity, ascorbic acid and chlorophylls during frozen storage. LWT-Food Sci. Technol. 2005, 38, 903–908. [Google Scholar] [CrossRef]
- Turkmen, N.; Poyrazoglu, E.S.; Sari, F.; Sedat Velioglu, Y. Effects of cooking methods on chlorophylls, pheophytins and colour of selected green vegetables. Int. J. Food Sci. Technol. 2005, 41, 281–288. [Google Scholar] [CrossRef]
- Teng, S.S.; Chen, B.H. Formation of pyrochlorophylls and their derivatives in spinach leaves during heating. Food Chem. 1999, 65, 367–373. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).