Influence of Urea Content in Deep Eutectic Solvents on Thermoplastic Starch Films’ Properties
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Eutectic-Mixture Preparation
2.3. Differential Scanning Calorymetric Analysis of Eutectic Mixtures
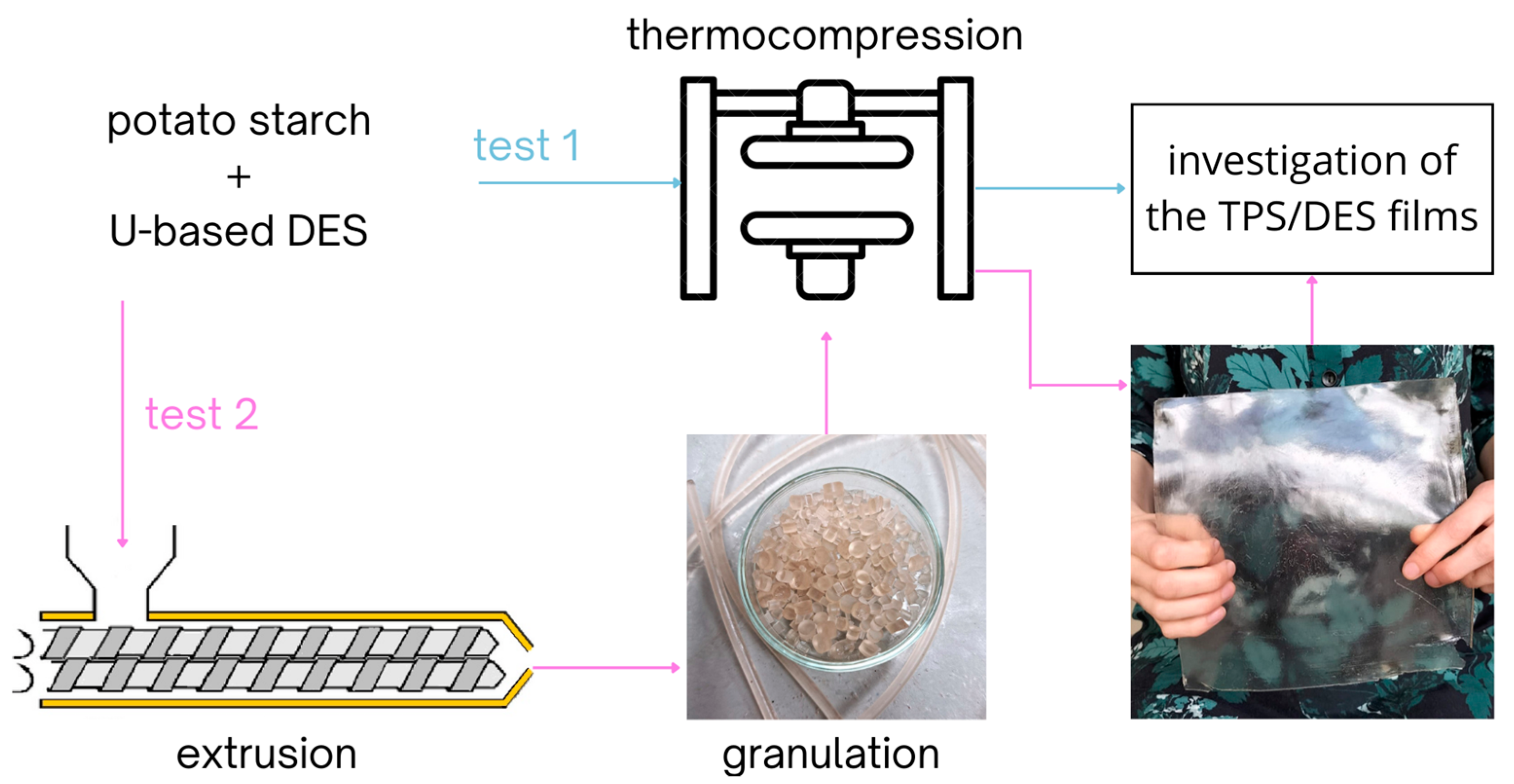
2.4. Preparation of TPS Films via Thermocompression (Test 1)
2.5. Preparation of TPS Films via Extrusion and Thermocompression (Test 2)
2.6. Mechanical Tests
2.7. DMTA of TPS Films
2.8. X-ray Diffraction Analysis of the Films
2.9. FTIR-ATR Spectroscopy of the Films
2.10. Moisture Sorption and Water Sorption Degrees
2.11. Surface Contact Angle Measurements of the Films
2.12. Statistical Analysis
3. Results and Discussion
3.1. Thermal Characteristic of Urea-Based Mixtures
3.2. Mechanical Test Results for the Films Obtained via Thermocompression as Well as via Extrusion and Thermcompression
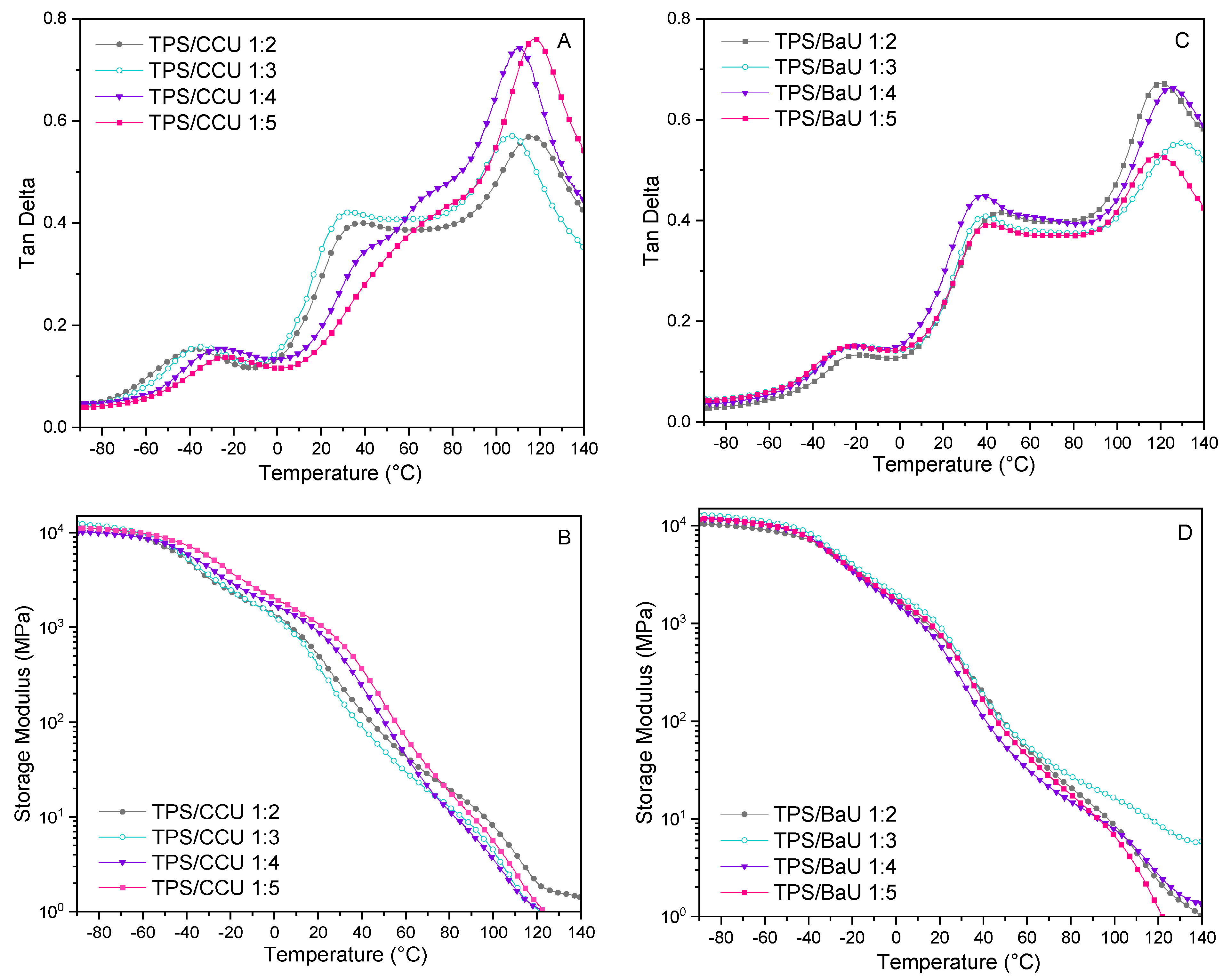
3.3. DMTA Results for TPS Films Obtained via Thermocompression (Test 1)
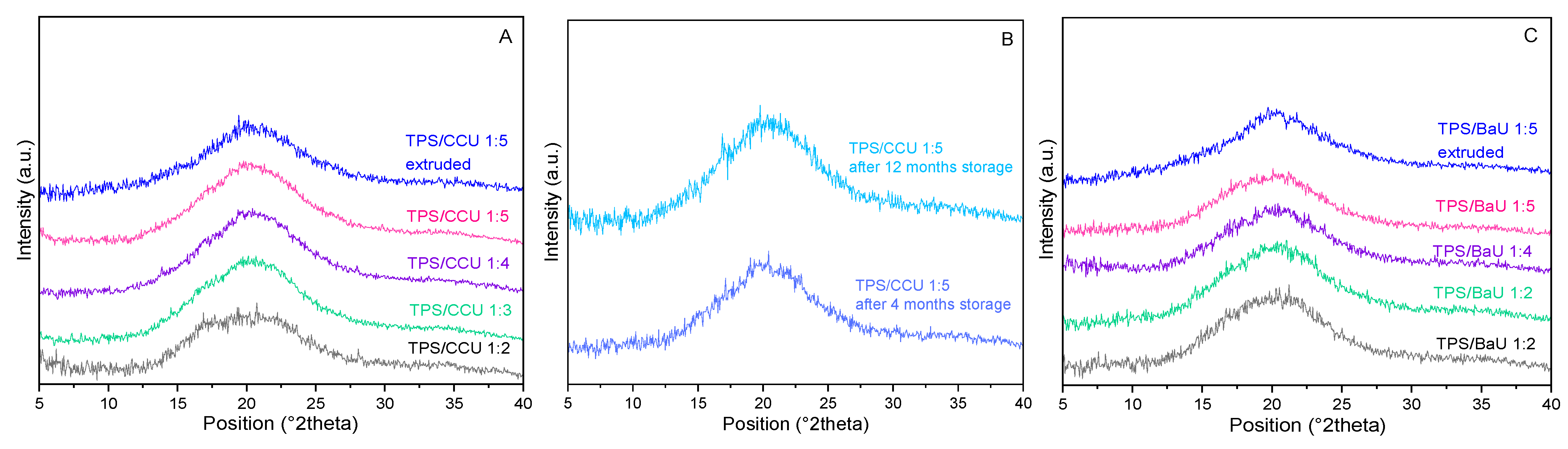
3.4. XRD Results for TPS/DES Films Obtained via Thermocompression and Extrusion and Thermocompression (Test 1 and 2)
3.5. FTIR-ATR Results for TPS/DES Films Obtained via Thermocompression (Test 1)
3.6. Behavior in Water and Contact Angle Determination Results for TPS Films Obtained via Thermocompression and Extrusion and Thermocompression (Test 1 and 2)
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellis, R.P.; Cochrane, M.P.; Dale, M.F.B.; Duffus, C.M.; Lynn, A.; Morrison, I.M.; Prentice, R.D.M.; Swanston, J.S.; Tiller, S.A. Starch Production and Industrial Use. J. Sci. Food Agric. 1998, 77, 289–311. [Google Scholar] [CrossRef]
- Montilla-Buitrago, C.E.; Gómez-López, R.A.; Solanilla-Duque, J.F.; Serna-Cock, L.; Villada-Castillo, H.S. Effect of Plasticizers on Properties, Retrogradation, and Processing of Extrusion-Obtained Thermoplastic Starch: A Review. Starch-Stärke 2021, 73, 2100060. [Google Scholar] [CrossRef]
- Ren, F.; Wang, J.; Xie, F.; Zan, K.; Wang, S.; Wang, S. Applications of Ionic Liquids in Starch Chemistry: A Review. Green Chem. 2020, 22, 2162–2183. [Google Scholar] [CrossRef]
- Zuo, Y.; Gu, J.; Tan, H.; Zhang, Y. Thermoplastic Starch Prepared with Different Plasticizers: Relation between Degree of Plasticization and Properties. J. Wuhan Univ. Technol.-Mat. Sci. Ed. 2015, 30, 423–428. [Google Scholar] [CrossRef]
- Zdanowicz, M. Starch Treatment with Deep Eutectic Solvents, Ionic Liquids and Glycerol. A Comparative Study. Carbohydr. Polym. 2020, 229, 115574. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M.; Staciwa, P.; Jędrzejewski, R.; Spychaj, T. Sugar Alcohol-Based Deep Eutectic Solvents as Potato Starch Plasticizers. Polymers 2019, 11, 1385. [Google Scholar] [CrossRef] [Green Version]
- Baran, A.; Vrábel, P.; Kovaľaková, M.; Hutníková, M.; Fričová, O.; Olčák, D. Effects of Sorbitol and Formamide Plasticizers on Molecular Motion in Corn Starch Studied Using NMR and DMTA. J. Appl. Polym. Sci. 2020, 137, 48964. [Google Scholar] [CrossRef]
- Lončarić, M.; Jakobek, L.; Molnar, M. Deep Eutectic Solvents in the Production of Biopolymer-Based Materials. Croat. Chem. Acta 2021, 94, 75–82. [Google Scholar] [CrossRef]
- Fričová, O.; Hutníková, M. Viscoelastic Behavior of Starch Plasticized with Urea and Glycero. AIP Conf. Proc. 2019, 2131, 020011. [Google Scholar]
- Dai, H.; Chang, P.R.; Yu, J.; Ma, X. N,N-Bis(2-Hydroxyethyl)Formamide as a New Plasticizer for Thermoplastic Starch. Starch-Stärke 2008, 60, 676–684. [Google Scholar] [CrossRef]
- Dai, H.; Chang, P.R.; Yu, J.; Geng, F.; Ma, X. N-(2-Hydroxypropyl)Formamide and N-(2-Hydroxyethyl)-N-Methylformamide as Two New Plasticizers for Thermoplastic Starch. Carbohydr. Polym. 2010, 80, 139–144. [Google Scholar] [CrossRef]
- Leroy, E.; Decaen, P.; Jaquet, P.; Coativy, G.; Pantoire, B.; Requerre, A.L.; Laurdin, D. Deep Eutectic Solvents as Functional Additives for Starch Based Plastics. Green Chem. 2012, 14, 3063–3066. [Google Scholar] [CrossRef]
- Ma, X.F.; Yu, J.G.; Wan, J.J. Urea and Ethanolamine as a Mixed Plasticizer for Thermoplastic Starch. Carbohydr. Polym. 2006, 64, 267–273. [Google Scholar] [CrossRef]
- Qiao, X.; Tang, Z.; Sun, K. Plasticization of Corn Starch by Polyol Mixtures. Carbohydr. Polym. 2011, 83, 659–664. [Google Scholar] [CrossRef]
- Adamus, J.; Spychaj, T.; Zdanowicz, M.; Jędrzejewski, R. Thermoplastic starch with deep eutectic solvents and montmorillonite as a base for composite materials. Ind. Crop. Prod. 2018, 123, 278–284. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Staciwa, P.; Spychaj, T. Low Transition Temperature Mixtures (LTTM) Containing Sugars as Potato Starch Plasticizers. Starch-Stärke 2019, 71, 1900004. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Johansson, C. Mechanical and Barrier Properties of Starch-Based Films Plasticized with Two- or Three Component Deep Eutectic Solvents. Carbohydr. Polym. 2016, 151, 103–112. [Google Scholar] [CrossRef]
- Paluch, M.; Ostrowska, J.; Tyński, P.; Sadurski, W.; Konkol, M. Structural and Thermal Properties of Starch Plasticized with Glycerol/Urea Mixture. J. Polym. Environ. 2022, 30, 728–740. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, F.; Zhu, P. Structure and Properties of Urea-Plasticized Starch Films with Different Urea Contents. Carbohydr. Polym. 2014, 101, 1109–1115. [Google Scholar] [CrossRef]
- Xiaofei, M.; Jiugao, Y.; Jin, F. Urea and Formamide as a Mixed Plasticizer for Thermoplastic Starch. Polym. Int. 2004, 53, 1780–1785. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Spychaj, T. Ionic Liquids as Starch Plasticizers or Solvents. Polimery 2011, 56, 861–864. [Google Scholar] [CrossRef]
- Zdanowicz, M. Deep Eutectic Solvents Based on Urea, Polyols and Sugars for Starch Treatment. Int. J. Biol. Macromol. 2021, 176, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Shogren, R.L.; Swanson, C.L.; Thompson, A.R. Extrudates of Cornstarch with Urea and Glycols: Structure/Mechanical Property Relations. Starch-Stärke 1992, 44, 335–338. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Daik, R. Utilization of an Ionic Liquid/Urea Mixture as a Physical Coupling Agent for Agarose/Talc Composite Films. Materials 2013, 6, 682–698. [Google Scholar] [CrossRef] [Green Version]
- Ivanič, F.; Jochec-Mošková, D.; Janigová, I.; Chodák, I. Physical Properties of Starch Plasticized by a Mixture of Plasticizers. Euro. Polym. J. 2017, 93, 843–849. [Google Scholar] [CrossRef]
- de Souza Gamarano, D.; Pereira, I.M.; da Silva, M.C.; Mottin, A.C.; Ayres, E. Crystal Structure Transformations in Extruded Starch Plasticized with Glycerol and Urea. Polym. Bull. 2020, 77, 4971–4992. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.-X.; Qi, S.-J.; Xin, R.-P.; Yang, B.; Wang, Y.-H. Synergistic Behavior of Betaine–Urea Mixture: Formation of Deep Eutectic Solvent. J. Mol. Liq. 2016, 219, 74–78. [Google Scholar] [CrossRef]
- Imperato, G.; Eibler, E.; Niedermaier, J.; Konig, B. Low-Melting Sugar-Urea-Salt Mixtures as Solvents for Diels-Alder Reactions. Chem. Commun. 2005, 7, 1170–1172. [Google Scholar] [CrossRef]
- Otey, F.H.; Trimnell, D.; Westhoff, R.P.; Shasha, B.S. Starch Matrix for Controlled Release of Urea Fertilizer. J. Agric. Food Chem. 1984, 32, 1095–1098. [Google Scholar] [CrossRef]
- Versino, F.; Urriza, M.; García, M.A. Eco-Compatible Cassava Starch Films for Fertilizer Controlled-Release. Int. J. Biol. Macromol. 2019, 134, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Rychter, P.; Kot, M.; Bajer, K.; Rogacz, D.; Šišková, A.; Kapuśniak, J. Utilization of Starch Films Plasticized with Urea as Fertilizer for Improvement of Plant Growth. Carbohydr. Polym. 2016, 137, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Giroto, A.S.; Guimarães, G.G.; Colnago, L.A.; Klamczynski, A.; Glenn, G.; Ribeiro, C. Controlled Release of Nitrogen Using Urea-Melamine-Starch Composites. J. Clean. Prod. 2019, 217, 448–455. [Google Scholar] [CrossRef]
- Mnasri, A.; Khiari, R.; Dhaouadi, H.; Halila, S.; Mauret, E. Acidic and Alkaline Deep Eutectic Solvents Pre-Treatment to Produce High Aspect Ratio Microfibrillated Cellulose. Biores. Technol. 2023, 368, 128312. [Google Scholar] [CrossRef] [PubMed]
- Willberg-Keyriläinen, P.; Hiltunen, J.; Ropponen, J. Production of Cellulose Carbamate Using Urea-Based Deep Eutectic Solvents. Cellulose 2018, 25, 195–204. [Google Scholar] [CrossRef]
- Sirviö, J.A. Cationization of Lignocellulosic Fibers with Betaine in Deep Eutectic Solvent: Facile route to Charge Stabilized Cellulose and Wood Nanofibers. Carbohydr. Polym. 2018, 198, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Zdanowicz, M.; Jędrzejewski, R.; Pilawka, R. Deep eutectic solvents as simultaneous plasticizing and crosslinking agents for starch. Int. J. Biol. Macromol. 2019, 129, 1040–1046. [Google Scholar] [CrossRef]
- Hu, L.; Luo, J.; Lu, D.; Tang, Q. Urea Decomposition: Efficient Synthesis of Pyrroles Using the Deep Eutectic Solvent Choline Chloride/Urea. Tetrahedron Lett. 2018, 59, 1698–1701. [Google Scholar] [CrossRef]
- Simeonov, S.P.; Afonso, C.A.M. Basicity and Stability of Urea Deep Eutectic Solvents. ACS Adv. 2016, 6, 5485–5490. [Google Scholar]
- Liu, W.-C.; Halley, P.J.; Gilbert, R.G. Mechanism of Degradation of Starch, a Highly Branched Polymer, during Extrusion. Macromolecules 2010, 43, 2855–2864. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Sałasińska, K.; Lewandowski, K.; Skórczewska, K. Thermoplastic Starch/Ternary Deep Eutectic Solvent/Lignin Materials: Study of Physicochemical Properties and Fire Behavior. ACS Sustain. Chem. Eng. 2022, 10, 4579–4587. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Flanagan, B.M.; Gilbert, E.P.; Gidley, M.J. A Novel Approach for Calculating Starch Crystallinity and Its Correlation with Double Helix Content: A Combined XRD and NMR Study. Biopolymers 2008, 89, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I.; Farag, S.; Mostafa, K.M.; Hebeish, A. Some Studies on Starch Carbamate. Starch-Stärke 1994, 46, 312–316. [Google Scholar] [CrossRef]
- Menzel, C.; Seisenbaeva, G.; Agback, P.; Gällstedt, M.; Boldizar, A.; Koch, K. Wheat Starch Carbamate: Production, Molecular Characterization, and Film Forming Properties. Carbohydr. Polym. 2017, 172, 365–373. [Google Scholar] [CrossRef] [PubMed]
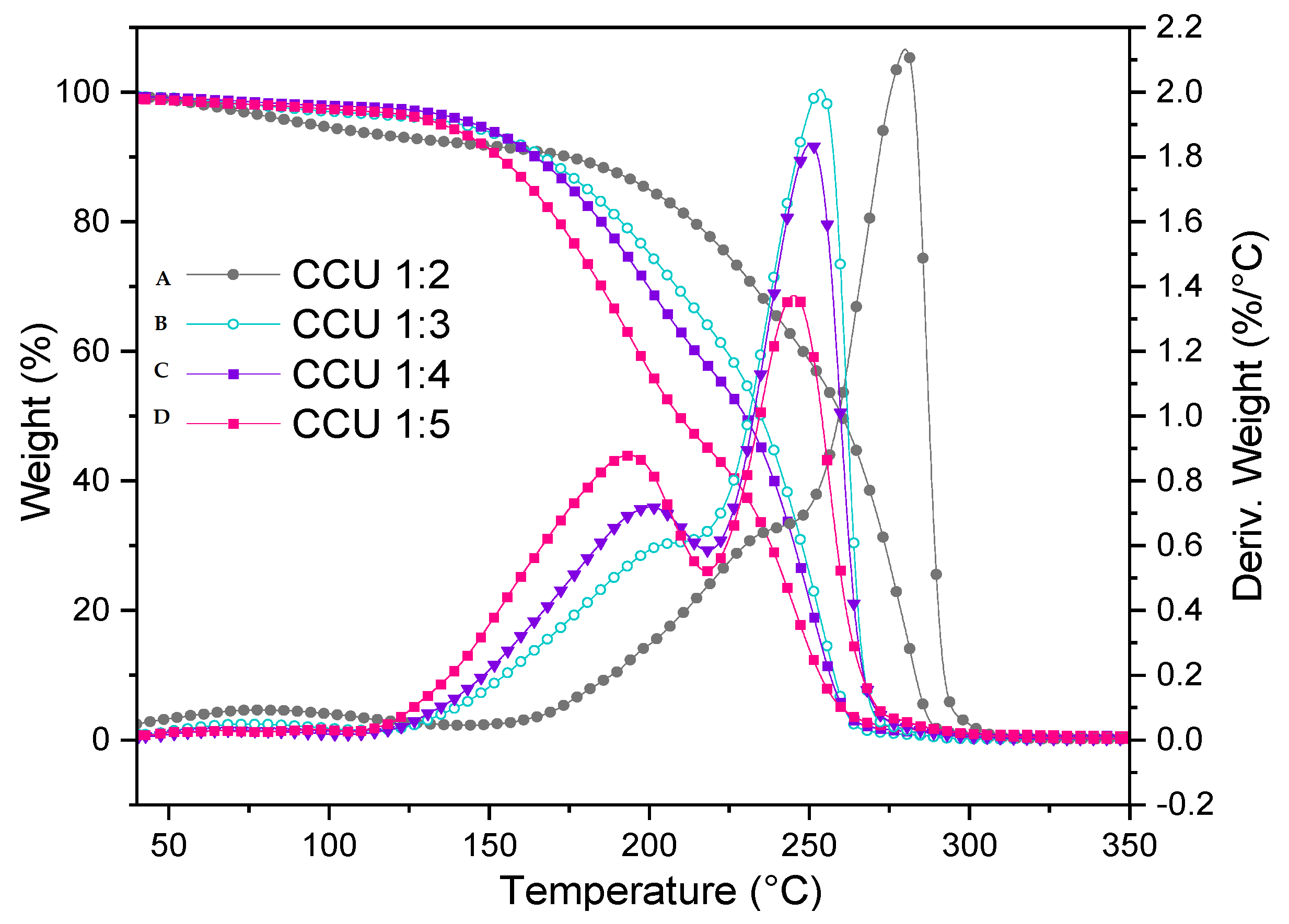

| Sample | Ammonium Compund: U Molar Ratio | DSC | TGA | ||
|---|---|---|---|---|---|
| Tf [°C] | Tdeg [°C] | Tdeg | DTAmax | ||
| TPS/CCU 1:2 | 1:2 | Tg -67; Tm 5; | 169 | 182 | 260 |
| TPS/CCU 1:3 | 1:3 | Tg -48; Tm 12 | 168 | 144 | 254 |
| TPS/CCU 1:4 | 1:4 | Tg Tm 62; Tcc -14 | 186 | 138 | 250 |
| TPS/CCU 1:5 | 1:5 | Tg -11; 86; Tc 31 | 171 | 125 | 244 |
| TPS/BaU 1:2 | 1:2 | Tg -19; Tm 106, Tcc 37 | 167 | 180 | 254 |
| TPS/BaU 1:3 | 1:3 | Tg -22; Tm 100; Tcc 51 | 166 | 150 | 245 |
| TPS/BaU 1:4 | 1:4 | Tg -30; Tm 88 Tcc 55 | 166 | 148 | 241 |
| TPS/BaU 1:5 | 1:5 | Tg -20; Tm 107; Tcc 57 | 168 | 131 | 237 |
| TPS/BhU 1:2 | 1:2 | Tm 91 | 147 | 177 | 246 |
| TPS/BhU 1:3 | 1:3 | Tg -22; Tm 76 | 130 | 161 | 245 |
| TPS/BhU 1:4 | 1:4 | Tg -24; Tm 88 | 136 | 155 | 245 |
| TPS/BhU 1:5 | 1:5 | Tg -18; Tm 101 | 139 | 144 | 247 |
| Sample | YM [MPa] | TS [MPa] | EB [%] | Thickness [mm] |
|---|---|---|---|---|
| TPS/CCU 1:2 | 32 (±1.6) | 2.2 (±0.1) | 119 (±2.2) | 0.62 (±0.01) |
| TPS/CCU 1:3 | 12 (±0.6) | 1.7 (±0.3) | 131 (±9.2) | 0.58 (±0.02) |
| TPS/CCU 1:4 | 15 (±4.0) | 1.6 (±0.2) | 125 (±8.4) | 0.60 (±0.02) |
| TPS/CCU 1:5 | 22 (±6.9) | 1.8 (±0.3) | 190 (±27.0) | 0.63 (±0.05) |
| TPS/BhU 1:2 | 156 (±22.7) | 3.8 (±0.09) | 74 (±11.6) | 0.60 (±0.02) |
| TPS/BhU 1:3 | 80 (±22.3) | 3.2 (±0.29) | 80 (±9.8) | 0.57 (±0.01) |
| TPS/BhU 1:4 | 77 (±7.8) | 2.3 (±0.30) | 64 (±17.6) | 0.55 (±0.03) |
| TPS/BhU 1:5 | 74 (±22.6) | 2.0 (±0.28) | 49 (±9.3) | 0.57 (±0.00) |
| TPS/BaU 1:2 | 70 (±15.8) | 3.3 (±0.20) | 117 (±2.8) | 0.56 (±0.06) |
| TPS/BaU 1:3 | 30 (±8.9) | 2.2 (±0.17) | 141 (±24.1) | 0.56 (±0.04) |
| TPS/BaU 1:4 | 40 (±9.8) | 2.5 (±0.21) | 159 (±5.0) | 0.54 (±0.02) |
| TPS/BaU 1:5 | 62 (±9.4) | 3.0 (±0.38) | 181 (±8.8) | 0.60 (±0.06) |
| Sample | Extrusion Parameters [°C/rpm] | YM [MPa] | TS [MPa] | EB [%] |
|---|---|---|---|---|
| eTPS/CCU 1:5 | 110/100 | 22 (±2.3) | 1.8 (±0.10) | 170 (±12.2) |
| eTPS/CCU 1:5 | 110/200 | 24 (±2.1) | 2.0 (±0.10) | 188 (±9.3) |
| eTPS/BaU 1:5 | 110/100 | 62 (±13.1) | 2.5 (±0.24) | 96 (±23.9) |
| eTPS/BaU 1:5 | 110/200 | 91 (±14.5) | 2.9 (±0.17) | 166 (±17.6) |
| Sample | Swelling Degree [%] | Solubility Degree [%] | Contact Angle [°] |
|---|---|---|---|
| TPS/CCU 1:2 | 253 (±30.6) | 23.7 (±0.4) | 69 (±2.9) |
| TPS/CCU 1:3 | 154 (±11.7) | 22.3 (±1.2) | 66 (±2.7) |
| TPS/CCU 1:4 | 165 (±8.5) | 22.6 (±1.2) | 72 (±3.6) |
| TPS/CCU 1:5 | 209 (±30.1) | 21.4 (±1.1) | 76 (±4.3) |
| TPS/BaU 1:2 | 371 (±34.4) | 24.7 (±5.8) | 60 (±4.3) |
| TPS/BaU 1:3 | 436 (±30.9) | 27.2 (±1.3) | 90 (±6.7) |
| TPS/BaU 1:4 | 589 (±30.9) | 32.0 (±4.2) | 82 (±7.8) |
| TPS/BaU 1:5 | 426 (±33.8) | 32.5 (±5.0) | 68 (±7.4) |
| eTPS/CCU 1:5 110/100 | gelled/defragemented | 95 (±4.0) | |
| eTPS/CCU 1:5 110/200 | 97 (±8.5) | ||
| eTPS/BaU 1:5 110/100 | 93 (±4.2) | ||
| eTPS/BaU 1:5 110/200 | 72 (±8.2) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdanowicz, M. Influence of Urea Content in Deep Eutectic Solvents on Thermoplastic Starch Films’ Properties. Appl. Sci. 2023, 13, 1383. https://doi.org/10.3390/app13031383
Zdanowicz M. Influence of Urea Content in Deep Eutectic Solvents on Thermoplastic Starch Films’ Properties. Applied Sciences. 2023; 13(3):1383. https://doi.org/10.3390/app13031383
Chicago/Turabian StyleZdanowicz, Magdalena. 2023. "Influence of Urea Content in Deep Eutectic Solvents on Thermoplastic Starch Films’ Properties" Applied Sciences 13, no. 3: 1383. https://doi.org/10.3390/app13031383





