A New C2-Symmetric Atropisomeric Thiophene-Based Monomer for Inherently Chiral Electroactive Materials: Synthesis, HPLC Resolution, and Absolute Configuration Assignment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Chemicals and Reagents
2.1.2. Synthesis of 5-(trimethylstannyl)-2,2′:5′,2′’-terthiophene
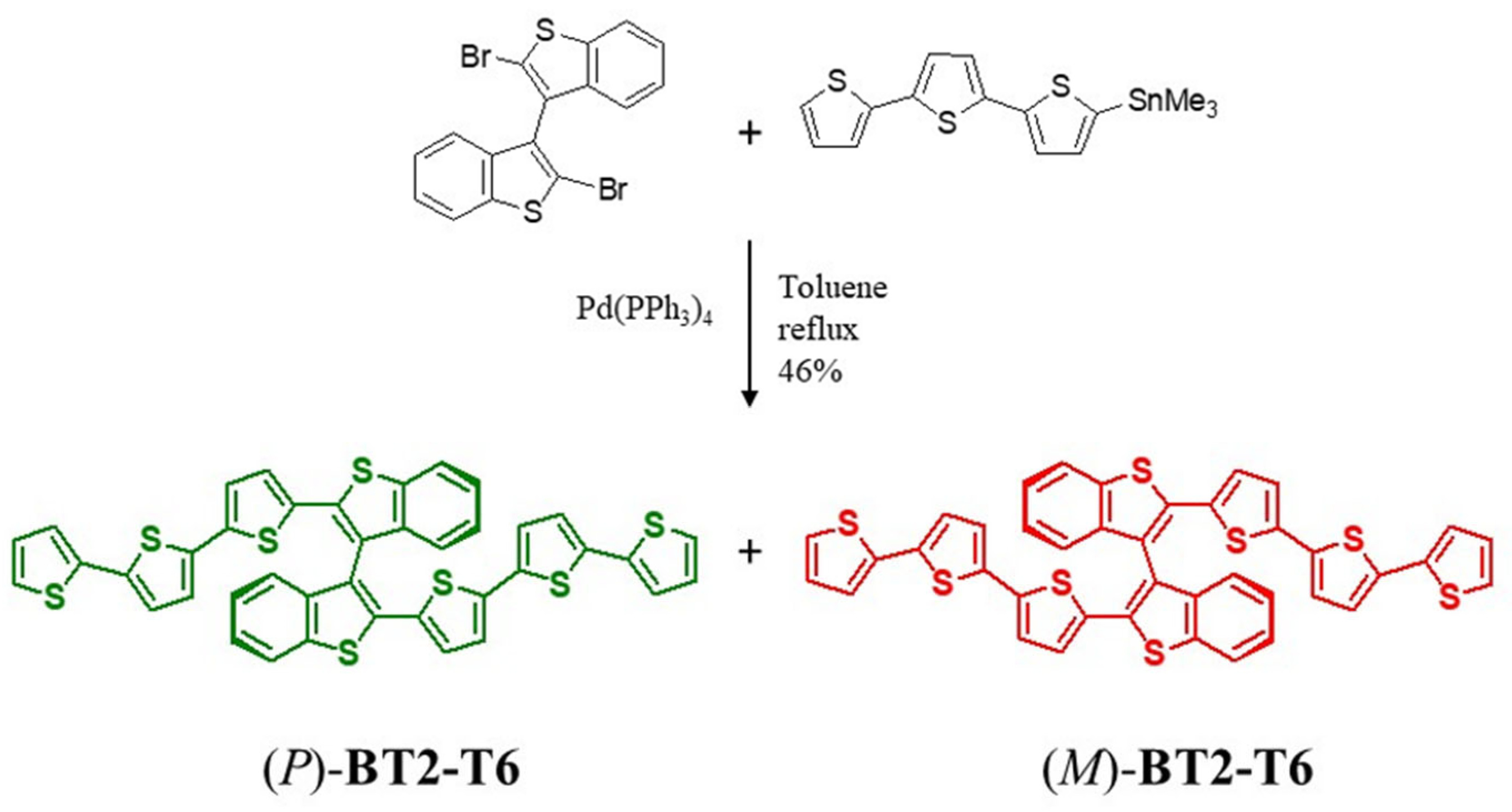
2.1.3. Synthesis of BT2-T6
2.2. Enantioselective HPLC
2.3. Chiroptical Measurements
3. Results
3.1. Synthesis
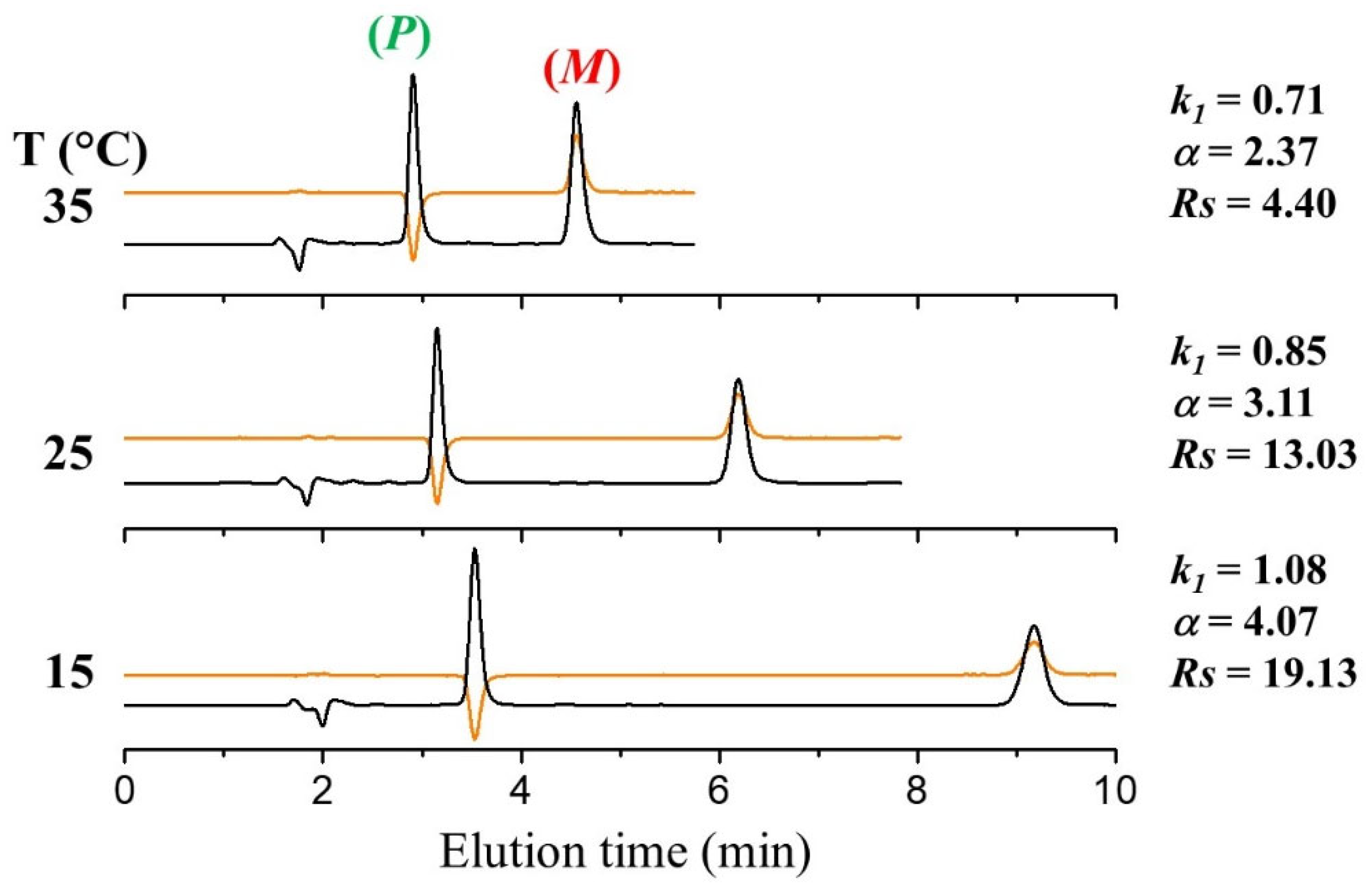
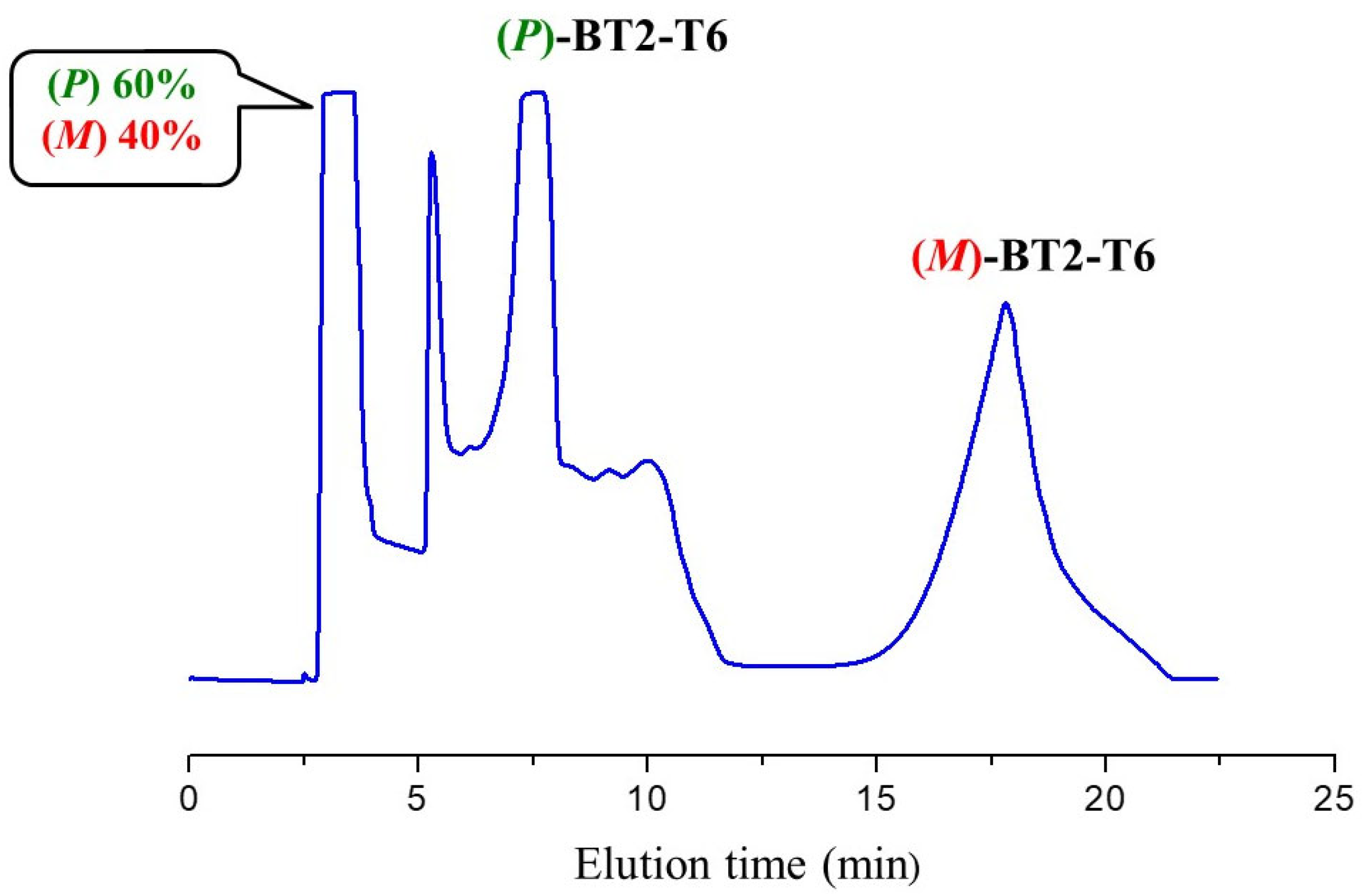
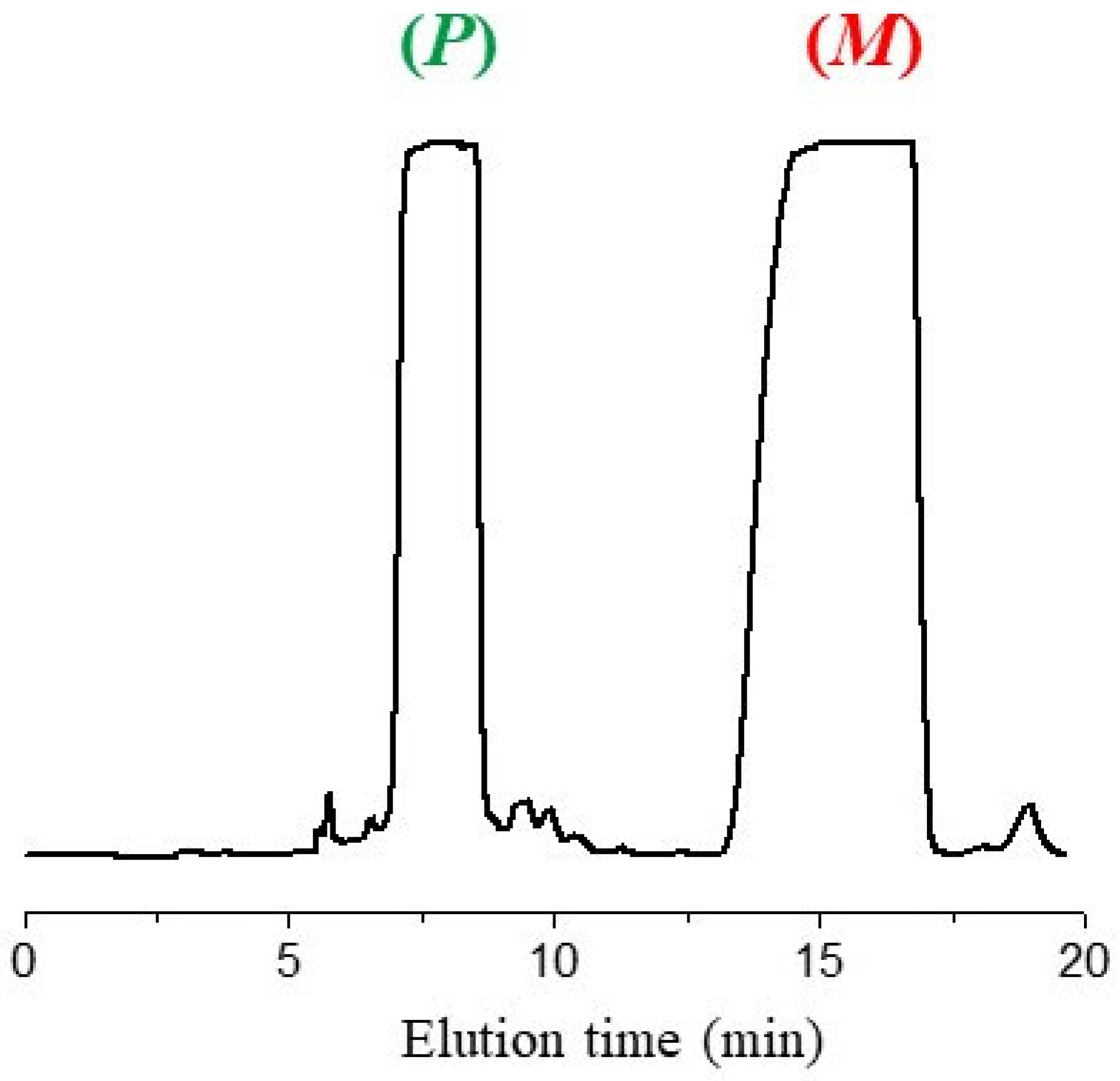
3.2. HPLC Resolution
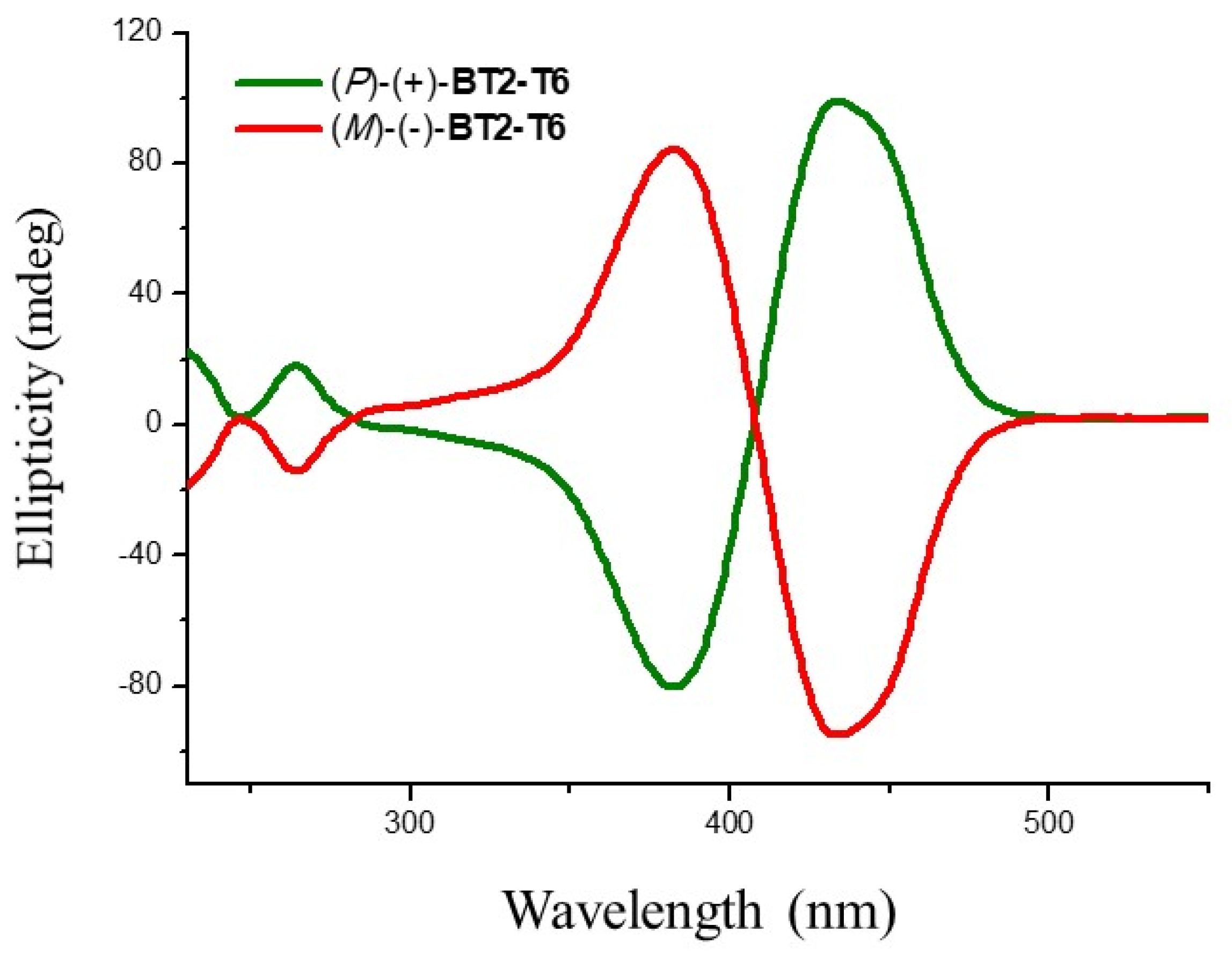
3.3. Absolute Configuration Assignment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnaboldi, S.; Magni, M.; Mussini, P.R. Enantioselective selectors for chiral electrochemistry and electroanalysis: Stereogenic elements and enantioselection performance. Curr. Opin. Electrochem. 2018, 8, 60–72. [Google Scholar] [CrossRef]
- Pop, F.; Zigon, N.; Avarvari, N. Main-group-based electro- and photoactive chiral materials. Chem. Rev. 2019, 19, 8435–84788. [Google Scholar] [CrossRef] [PubMed]
- Sannicolò, F.; Arnaboldi, S.; Benincori, T.; Bonometti, V.; Cirilli, R.; Dunsch, L.; Kutner, W.; Longhi, G.; Mussini, P.R.; Panigati, M.; et al. Potential-driven chirality manifestations and impressive enantioselectivity of inherently chiral electroactive organic films. Angew. Chem. 2014, 126, 2661–2665. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ikai, T. Chiral HPLC for efficient resolution of enantiomers. Chem. Soc. Rev. 2008, 37, 2593–2608. [Google Scholar] [CrossRef] [PubMed]
- Peluso, P.; Mamane, V.; Dallocchio, R.; Dessì, A.; Cossu, S. Noncovalent interactions in high-performance liquid chromatography enantioseparations on polysaccharide-based chiral selectors. J. Chromatogr. A 2020, 1623, 461202. [Google Scholar] [CrossRef]
- Trapp, O.; Schoetz, G.; Schurig, V. Determination of enantiomerization barriers by dynamic and stopped-flow chromatographic methods. Chirality 2001, 13, 403–414. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Roussel, C.; Kitagawa, O.; Sorochinsky, A.E. Self-disproportionation of enantiomers via achiral chromatography: A warning and an extra dimension in optical purifications. Chem. Soc. Rev. 2012, 41, 4180–4188. [Google Scholar] [CrossRef]
- Fairchild, J.N.; Hill, J.F.; Iraneta, P.C. Influence of sample solvent composition for SFC separations. LCGC N. Am. 2013, 31, 326–333. [Google Scholar]
- Groeneveld, G.; Dunkle, M.N.; Pursch, M.; Mes, E.P.C.; Schoenmakers, P.J.; Gargano, A.F.G. Investigation of the effects of solvent-mismatch and immiscibility in normal-phase ×aqueous reversed-phase liquid chromatography. J. Chromatogr. A 2022, 1665, 462818. [Google Scholar] [CrossRef]
- Sannicolò, F.; Rizzo, S.; Benincori, T.; Kutner, W.; Noworyta, K.; Sobczak, J.W.; Bonometti, V.; Falciola, L.; Mussini, P.R.; Pierini, M. An effective multipurpose building block for 3D electropolymerisations: 2,2’-bis(2,2’-bithiophene-5-yl)-3,3’-bi-1-benzothiophene. Electrochim. Acta 2010, 55, 8352–8364. [Google Scholar] [CrossRef]
- Rosetti, A.; Bonetti, G.; Villani, C.; Benincori, T.; Cirilli, R. Multimilligram scale production implementation of atropisomers of 2,2’-bis(2,2’-bithiophene-5-yl)-3,3’-bithianaphthene. Chirality 2021, 33, 146–152. [Google Scholar] [CrossRef]
- Sannicolò, F.; Arnaboldi, S.; Benincori, T.; Cirilli, R.; Kutner, W.; Longhi, G.; Martinazzo, R.; Mussini, P.R.; Panigati, M.; Pappini, M.; et al. Inherently chiral macrocyclic oligothiophenes. Easily accessible electrosensitive cavities with outstanding enantioselection performances. Chem. Eur. J. 2014, 20, 15298–15302. [Google Scholar] [CrossRef] [Green Version]
- Arnaboldi, S.; Benincori, T.; Cirilli, R.; Kutner, W.; Magni, M.; Mussini, P.R.; Noworyta, K.; Sannicolò, F. Inherently chiral electrodes: The tool for chiral voltammetry. Chem. Sci. 2015, 6, 1706–1711. [Google Scholar] [CrossRef] [Green Version]
- Arnaboldi, S.; Benincori, T.; Cirilli, R.; Grecchi, S.; Santagostini, L.; Sannicolò, F.; Mussini, P.R. Inherent chiral thiophene-based electrodes at work: A screening of enantioselection ability toward a series of pharmaceutically relevant phenolic or catecholic amino acids, amino esters and amine. Anal. Bioanal. Chem. 2016, 408, 7243–7254. [Google Scholar] [CrossRef]
- Grecchi, S.; Arnaboldi, S.; Korb, M.; Cirilli, R.; Araneo, S.; Guglielmi, V.; Tomboni, G.; Magni, M.; Benincori, T.; Lang, H.; et al. Widening the scope of “inherently chiral” electrodes: Enantiodiscrimination of chiral electroactive probes with planar stereogenicity. ChemElectroChem 2020, 7, 3429–3438. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Gupta, B.; Benincori, T.; Bonetti, G.; Cirilli, R.; Kuhn, A. Absolute chiral recognition with hybrid wireless electrochemical actuators. Anal. Chem. 2020, 92, 10042–10047. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Gupta, B.; Benincori, T.; Bonetti, G.; Cirilli, R.; Kuhn, A. Large scale chirality transduction with functional molecular materials. Chem. Mat. 2020, 32, 10663–10669. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Salinas, G.; Karajić, A.; Garrigue, P.; Benincori, T.; Bonetti, G.; Cirilli, R.; Bichon, S.; Gounel, S.; Mano, N.; et al. Direct dynamic read-out of molecular chirality with autonomous enzyme-driven swimmers. Nat. Chem. 2021, 13, 241–1247. [Google Scholar] [CrossRef]
- Farina, V. New perspectives in the cross-coupling reaction of organostannanes. Pure Appl. Chem. 1996, 68, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Meilstein, D.; Stille, J.K. Palladium catalysed coupling of organotin compounds with aryl and benzylhalides. Synthetic utility and mechanism. J. Am. Chem. Soc. 1979, 101, 73–78. [Google Scholar]
- Panella, C.; Ferretti, R.; Casulli, A.; Cirilli, R. Temperature and eluent composition effects on enantiomer separation of carvedilol by high-performance liquid chromatography on immobilized amylose-based chiral stationary phases. J. Pharm. Anal. 2019, 9, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B. Recent developments on polysaccharide-based chiral stationary phases for liquid-phase separation of enantiomers. J. Chromatogr. A 2012, 1269, 26–51. [Google Scholar] [CrossRef] [PubMed]
- El Montassir, D.; Aamouche, A.; Vanthuyne, N.; Jean, M.; Vanloot, P.; Taourirte, M.; Dupuy, N.; Roussel, C. Attempts to separate (-)-α-thujone, (+)-β-thujone epimers from camphor enantiomers by enantioselective HPLC with polarimetric detection. J. Sep. Sci. 2013, 36, 832–839. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosetti, A.; Apolloni, G.; Villani, C.; Benincori, T.; Cirilli, R. A New C2-Symmetric Atropisomeric Thiophene-Based Monomer for Inherently Chiral Electroactive Materials: Synthesis, HPLC Resolution, and Absolute Configuration Assignment. Appl. Sci. 2023, 13, 1407. https://doi.org/10.3390/app13031407
Rosetti A, Apolloni G, Villani C, Benincori T, Cirilli R. A New C2-Symmetric Atropisomeric Thiophene-Based Monomer for Inherently Chiral Electroactive Materials: Synthesis, HPLC Resolution, and Absolute Configuration Assignment. Applied Sciences. 2023; 13(3):1407. https://doi.org/10.3390/app13031407
Chicago/Turabian StyleRosetti, Alessia, Giulio Apolloni, Claudio Villani, Tiziana Benincori, and Roberto Cirilli. 2023. "A New C2-Symmetric Atropisomeric Thiophene-Based Monomer for Inherently Chiral Electroactive Materials: Synthesis, HPLC Resolution, and Absolute Configuration Assignment" Applied Sciences 13, no. 3: 1407. https://doi.org/10.3390/app13031407





