Abstract
In this study, the characterization of fatty acids and secondary metabolites in seeds of three cultivars of Opuntia ficus-indica L. (O. ficus-indica, yellow, orange, and green) harvested from the Souk Ahras area in Northeast Algeria was performed. The antioxidant activity of seed extracts was also assessed by using two methods, namely FRAP and DPPH tests. Results show that total phenolic content (TPC) ranged from 63.02 to 81.80 mg gallic acid equivalents (GAE)/100 g of seeds. The yellow cultivar had the highest TPC, followed by the orange and green cultivars. Concerning flavonoids and tannins, the orange cultivar showed higher concentrations, corresponding to 2.97 mg quercetin equivalent (QE)/100 g and 5.60 mg catechin equivalent (CE)/100 g, respectively. Analysis of the seed extracts by HPLC revealed the presence of phenolic compounds, including gallic acid and chlorogenic acid, while the GC analysis of oil showed that prickly pear oil is a major source of essential fatty acids (C18:2). The antioxidant activities of extracts from the three cultivars were comparable. The EC50 for the reduction of ferric iron was almost 0.05 g/mL for all extracts. Regarding the scavenging of DPPH, green and yellow cultivars showed the highest capacity (EC50 = 0.26 g/mL). Linear correlations between the content of some antioxidants (flavonoids, tannins) and different activities were observed, indicating their participation in the latter. Above all, a significant inverse correlation between the total flavonoid content and the EC50 calculated for the reducing activity of seed extracts was observed (r = −0.657; p ≤ 0.05). Overall, the results indicate that the seeds of O. ficus-indica growing in Algeria can be exploited as valuable sources of table oil, cooking oil, and antioxidants.
1. Introduction
Plants represent abundant sources of bioactive natural substances. Among these, secondary metabolites have the advantage of being widely variable in chemical structure, thus allowing potential applications in medical, pharmaceutical, cosmetic, and agro-food fields. Members of the Opuntia genus are widely used as food but have also been investigated for their potential usefulness in the prevention and treatment of several human diseases, ranging from atherosclerotic cardiovascular diseases to cancer and microbial infections [1]. Opuntia ficus-indica L., commonly called “prickly pear” or “prickly fig”, is native to Mexico, but it is also widely diffused in the Mediterranean basin thanks to its adaptation to the climate of this region [2]. Although this plant has long been marginalized, its culture is now growing and its socio-economic and environmental importance is increasing as a result [3]. Indeed, modern medical research is rediscovering this plant, and there is an increasing interest in its beneficial properties. In Algeria, despite its abundance, especially in the area of Souk Ahras, prickly pear generates little interest: it is not valued, and its consumption remains seasonal. The seeds of this plant are considered waste, although their importance continues to grow in other countries such as Mexico, Argentina, Spain, and even in neighboring countries like Morocco and Tunisia [4,5,6]. The content of their oil in unsaponifiable materials and essential fatty acids explains why they are widely used in cosmetology and as table oil or cooking oil [7,8]. The seeds also contain various phytochemicals such as secondary metabolites (polyphenols, flavonoids, and tannins), which could justify their industrial exploitation as a natural antioxidant [9,10]. Nevertheless, to the best of our knowledge, few studies have been reported in the literature on the phenolic composition and antioxidant properties of prickly pear seed oil [11]. Moreover, very little information exists on seeds from O. ficus-indica growing in Algeria. Hence, the present study aims to fill the gaps in the research by examining the composition and antioxidant activity of phenolic compounds from Algerian prickly pear seed oil; the larger goal is to valorize unexploited local natural sources. This is a comparative study carried out on three different cultivars (i.e., orange, green and yellow) collected from the Souk Ahras area located in the North-East of Algeria.
2. Materials and Methods
2.1. Plant Material Collection and Preparation
The study was performed on the seeds of three cultivars of O. ficus-indica L. (orange, green, and yellow) harvested in August 2020 from the Souk Ahras area in Northeast Algeria. Plant species were identified by an expert botanist from the Department of Plant Sciences and Microbiology, using the protocol developed by García-Rollán [12]. Plants were selected according to the presence or absence of thorns, color, and the shape of their fruits. The fruits, whose morphological characteristics are reported in Table 1, were manually picked at the same ripening stage and immediately transported to the laboratory. In this way, a total of 20 fruits per cultivar were collected. One voucher of each cultivar is kept at the Institute of Agriculture and Veterinary Sciences of the University of Souk Ahras, Algeria (8°1952751 E 36°1537121 N). Collected prickly pears were rinsed thoroughly with running water, then dried and peeled. The seeds were then separated from the pulp, rinsed, and dried at room temperature for 24 h. The dried seeds were crushed using the electric grinder A11 basic (IKA, Germany) until a fine powder was obtained. This latter was then stored in jars protected from light and moisture for further analyses.

Table 1.
Characteristics of the fruits of the different prickly pear cultivars collected from the Souk Ahras area, Northeast Algeria.
2.2. Extraction of Seed Oil by Using the Soxhlet Apparatus
Extraction of fat content in seeds was carried out in a Soxhlet-type apparatus using n-hexane (Merck) as solvent. This technique ensures a hot extraction of fat contained in a solid vegetable sample that is then placed in a cellulose cartridge and continuously soaked by the vapours of a solvent chosen according to the polarity of the lipids to be extracted. About 10 g of the ground sample was weighed in the cellulose tube, closed by carded cotton, and fed into a Soxhlet. The extraction was carried out with 300 mL of solvent at reflux for 8 h (distributed in 4 h + 2 h + 2 h). The procedure was performed in triplicate. Finally, the solvent was eliminated under reduced pressure at 45 °C, and the dried oil was kept at −20 °C in amber glass flasks until analysis.
2.3. Determination of Fatty Acid Content in Seed Oil
The fatty acid content of the seeds’ oil was determined by gas chromatography (GC). Before analysis, the oil underwent a trans-esterification in a methanol solution of potassium hydroxide (KOH; Merck), following a previously published protocol [13].
Briefly, 2 mL of the KOH solution (2 M) was added to 0.1 g of seed oil. The mixture was vigorously mixed, heated at 100 °C for 5 min in a water bath, and then cooled down at room temperature. 2 mL of a BF3 solution in methanol (14% w/w, diluted from a 50 w/w commercial solution; Sigma-Aldrich, St. Louis, MO, USA) was added and, after heating at 100 °C for 30 min, the mixture was diluted with 1 mL of hexane. The organic fraction containing the fatty acids methyl esters (FAMEs) was isolated by adding 5 mL of a saturated NaCl solution to facilitate the phase separation. The organic layer was finally withdrawn, concentrated under N2 flux, and used for chromatographic analysis. This was performed by using an Agilent 7820A GC equipped with a capillary column RTX-2330 (Restek; 105 m length, 0.25 mm i.d., 0.2 µm film thickness) and a flame ionization detector (FID). Injector and detector temperatures were 260 °C and 280 °C, respectively. Column temperature was set to 200 °C for 21 min, then increased to 250 °C at a rate of 10 °C/min; the final temperature was held for 6 min. Helium was used as a carrier gas at a linear flow rate of 35 cm/sec. Individual FAMEs were identified using the Supelco 37 Component FAME Mix (Sigma-Aldrich).
2.4. Extraction of Seeds for Phenol, Tannin, and Flavonoid Contents Determination
The method used was a solid-liquid extraction. The powder of prickly fig seeds was extracted with 30 mL of ethanol 40% (diluted from ethanol 96%; Merck). The mixture was stirred for 2 h in the dark and then filtered. A second extraction was carried out following the same protocol to obtain a better rate of extracted material. The recovered filtrate was concentrated by rotating evaporation at 40 °C until the solvent was completely removed and stored in the freezer at −20 °C until analysis.
2.5. Determination of Total Phenolic Content
Folin-Ciocalteu reagent, consisting of a mixture of phosphotungstic acid (H3PW12O40) and phosphomolybdic acid (H3PMo12O40), was reduced in the presence of phenolic compounds into a mixture of blue tungsten oxides and molybdenum. The coloring was proportional to the number of polyphenols present in plant extracts [14,15]. The total phenolic content (TPC) was estimated using the method previously described by other authors [16]. Briefly, it consisted of mixing 250 μL of a 1 mg/mL seed-extract solution in methanol and 1.5 mL of Folin-Ciocalteu’s phenol reagent (Merck). After 5 min, 1.5 mL of sodium carbonate (6%; Sigma-Aldrich) was added. Absorbance of the mixture was measured at 760 nm after 1 h of incubation in the dark. The concentration of phenolic compounds was determined using a calibration curve built with different concentrations (0.02, 0.04, 0.06, and 0.08 mg/mL) of gallic acid (monohydrate, ≥98%; Sigma-Aldrich). The results were expressed as mg equivalent of gallic acid/100 g of seeds (mg GAE/100 g). All tests were conducted on the three varieties of O. ficus-indica.
2.6. Determination of Total Flavonoid Content
The total flavonoid content (TFC) was determined based on the formation of a complex flavonoids-aluminum that absorbs at 430 nm [17]. According to the protocol described by other authors [17], the seed extract was added to 1.5 mL of aluminum trichloride (AlCl3: 2%; Sigma-Aldrich). After incubation for 15 min at room temperature, the absorbance of the mixture was read at 430 nm. The content of flavonoids in extracts was calculated by reference to a calibration curve obtained by analyzing quercetin solutions prepared in 30 mL of 70% ethanol in concentrations ranging from 0.5 to 4 µg/mL. The results were expressed in mg of quercetin equivalent/100 g of seeds (mg QE/100 g).
2.7. Determination of Tannin Content
Condensed tannins were measured using the butanol-HCl method, developed by Iqbal et al. [18], and based on a tannin depolymerisation reaction condensed in an acid medium. This reaction led to the release of anthocyanidins (colored molecules) corresponding to the cleaved monomers. The reaction medium, consisting of a known amount of each seed’s extract (50 mg) dissolved in 25 mL reagent [n-butanol: HCl, 3:2 (v/v) and 0.385 mg iron ammonium sulphate; solvent and reagents from Sigma-Aldrich], was placed in the oven at 95 °C for 15 min. Afterwards, the absorbance of the same solutions was measured at 530 nm, and the results were determined by the following formula [Equation (1)]:
where TC = tannin concentration in mg/L; A = absorbance recorded at 530 nm; DF = dilution factor; MC = molar mass of cyanidin (287.24 g/mol); and ε = molar extinction coefficient of cyanidin (34,700 L/mol).
TC = A × DF × MC × 1000/ε
2.8. HPLC-DAD Analysis of Secondary Metabolites
A raw assessment of the content of secondary metabolites in prickly pear seeds was performed by using an Agilent 1260 HPLC binary pump coupled to a 1260 diode array detector (DAD). As stationary phase, a Thermo Eclipse ODS Hypersil C18 column (150 × 4.6 mm, 5 µm) was used. The mobile phase consisted of a mixture of solvent A (water + 0.1% formic acid; Merck) and solvent B (acetonitrile + 0.1% formic acid; Merck). The elution gradient was 0–5 min: 95% A; 10 min; 90% A; 35 min, 50% A; 45 min, 95% A; 65 min, 95% A. The injection volume was 20 μL and the flow rate was 1 mL/min. DAD was operating at detection wavelengths = 280 nm and 292 nm. Prior to analysis, all the solutions of extracts and standards were filtered over 0.22 µm Millipore membranes to avoid damage to the column and to limit interference due to impurities.
Peak identification of phenolic compounds was performed by comparing the retention times (R.T.) of eluted compounds with those of reference standards (Merck). Quantification of secondary metabolites was performed by using calibration curves of gallic acid and quercetin, obtained by analyzing standard solutions in concentration range 0.02–0.08 mg/mL.
2.9. Evaluation of the Antioxidant Activity of Seed Extracts
2.9.1. Ferric Reducing Antioxidant Power Assay (FRAP)
The assay measures the reduction of ferric ion (Fe3+)-ligand complex to the intensely blue-colored ferrous (Fe2+) complex by antioxidants in an acidic medium [19,20]. The reducing power of prickly pear seed extracts was determined according to the method reported by Nagulendran et al. [21], with some modifications. Different concentrations of extracts in methanol were first prepared (ranging from 0.027 to 0.1 g/mL). 0.5 mL of each solution was mixed with 1.25 mL phosphate buffer (0.2 M and pH 6.6) and 1.25 mL potassium ferricyanide [K3Fe(CN)6] at 1%. Reagents were purchased from Sigma-Aldrich. After 20 min incubation at 50 °C, the reaction was stopped by adding 1.25 mL of trichloracetic acid (10% TCA; Sigma-Aldrich). 1.25 mL of the mixture was taken and added to 1.25 mL of distilled water and 0.25 mL of chloride iron (FeCl3; Sigma-Aldrich) at 0.1%. After incubation for 30 min, absorbance was measured at 700 nm against a calibration curve prepared by analyzing standard solutions of ascorbic acid (Sigma-Aldrich) at different concentrations (0.02; 0.04; 0.06 to 0.08 mg/mL). Results were expressed in mg ascorbic acid equivalent/100 mg of extract (mg AAE/100 mg).
2.9.2. DPPH Antiradical Activity
The method used DPPH (2,2-diphenyl 1-picrylhydrazyl) radical. The activity of the DPPH radical was estimated according to the protocol described by Lopes-Lutz et al. [22]. Solutions of seed extracts at different concentrations were first prepared (ranging from 0.08 to 0.33 g/mL). Each test solution was mixed with 2.44 mL of DPPH solution (6 × 10−5 M). Samples were placed in darkness and at room temperature for 1 h. The absorbance reading was made at 517 nm against a white. The anti-radical activity was estimated by the percentage inhibition of DPPH according to the following formula [Equation (2)] [23]:
I%: percentage of inhibition; AC: absorbance of the DPPH solution; AE: absorbance units of the sample.
I% = AC − AE/AC × 100
The percentage inhibition was used to calculate the EC50 values, defined as the effective concentration of the extract required to reduce the concentration of initial DPPH. The EC50 was determined graphically by linear regression of plots where the abscissa represented the concentration of the samples and the ordinate the antiradical activity in percentage. The antioxidant capacity of a sample was higher as its EC50 was small [24,25].
2.10. Statistical Analysis
The results were analyzed using the STATISTICA 5.5 software for Single Classification Criterion Variance Analysis (ANOVA). The degree of significance of the data was taken at the probability of p ≤ 0.05. Correlations between total phenolic, total flavonoid, and total tannin contents and antioxidant activities were also studied by performing a Spearman’s correlation analysis. The Spearman’s “r” coefficients were calculated: 0 < r < +1 indicated direct correlations, while −1 < r < 0 inverse correlations.
3. Results and Discussion
3.1. Determination of Fatty Acid Content in Seed Oil
The assessment of fatty acid composition of prickly pear seed oil was carried out by GC-FID after compound trans-esterification. The results are presented in Table 2. The results show that the different cultivars of prickly pear seed oil have a maximum content of linoleic acid (C18:2), followed by oleic acid (C18:1), palmitic acid (C16:0), stearic acid (C18:0), and finally palmitoleic acid (C16:1), with the highest relative concentration being around 1% in the oil from the yellow variety. Notably, the content of this latter in the yellow cultivar was almost ten times higher than the other two, and hence it can be considered as a characteristic factor of its seed oil. Nevertheless, further investigations are needed to elucidate if this variability is related to genetic factors or to other conditions.

Table 2.
Fatty acids composition of the seed oil from different cultivars (yellow, orange, and green) of prickly pear (g/100 g oil).
Prickly fig seed oil, like most vegetable oils, belongs to the category of polyunsaturated oils. Its chemical composition comprises mainly linoleic acid and oleic acid and is thus very similar in composition to corn oil. For this reason, it is therefore a good alternative source for essential fatty acids compared to other vegetable and seed oils. Regarding these main components, the amounts determined in the three prickly pear cultivars from the Souk Ahras area reflect those already reported in the same plant species growing in Tunisia (C18:1 = 19.96%; C18:2 = 67.60%) [26] and Algeria (specific region or area not specified; C18:1 = 12.92%; C18:2 = 55.81%) [27], for example. Conversely, variations in respect to other prickly pear seed oils were observed for the less abundant components. One of the particularities of the prickly fig oils studied in this work was the presence of traces of myristic acid. This data does not agree with the results of Ennouri et al. [5], Ramadan and Morsel [28], or Kadda et al. [29], for example, which showed the absence of this fatty acid in Tunisian prickly fig oil. However, small amounts of C14:0 (0.15%) have been reported in seeds from species growing in Algeria (specific region or area not specified) [27]. Hence, this could be a characteristic feature of Algerian prickly fig oil.
3.2. Determination of Antioxidants in Seeds
Prickly pear fruits have been largely characterized for their content in secondary metabolites [15,30,31], and several of these have been associated with antioxidant effects and anti-microbial activities in vitro [29,32,33,34]. Different antioxidant components have also been described in prickly pear seeds, such as flavonoids and tannins, which account for more than half of phenolic compounds [10]. However, interest has focused more on the oil content of these seeds, and very little work has been done on antioxidants or to assess their antioxidant activity, making it difficult to perform comparisons.
The results of the determinations of antioxidants (total phenolic compounds, flavonoids, tannins) in the seed extracts of the three varieties of prickly pears, determined spectrophotometrically according to different protocols, are summarized in Table 3.

Table 3.
Contents of total phenolic compounds, flavonoids, and tannins in the seeds of the three cultivars of prickly pear, namely orange, yellow, and green. Values in the same column followed by the same letter are not significantly different at p ≤ 0.05.
3.2.1. Total Phenolic Compounds (TPC)
The TPC ranged from 63.02 ± 1.14 to 81.80 ± 1.01 mg GAE/100 g of seeds, in green and yellow cultivars, respectively, compared to 76.04 ± 1.59 mg GAE/100 g in the orange cultivar (Table 3). The highest value was recorded for the extract of the yellow cultivar, followed by the extracts of the orange and green cultivars. These results revealed a significant degree of variability in both yellow and orange cultivars as compared to the green cultivar. The statistical study revealed a variety effect at the threshold p ≤ 0.05. Our results are in consonance with those reported by other authors in seeds of the same plant species, as summarized by a recent review article [35]. However, other studies report higher TPC values, for example in the seeds of O. ficus-indica growing in Egypt, where TPC = 504.825 mg GAE/100 g [36]. This difference can be attributed to the method of extraction and analysis, the geographical origin of the sample, degree of maturity, or storage conditions. Indeed, the seeds used in this study derived from fruits that were harvested in August 2020 and it is likely that, during their storage, the degradation of some compounds occurred.
3.2.2. Total Flavonoid Content (TFC)
The colorimetric method used to estimate the TFC of O. ficus-indica seeds was based on the reaction between flavonoids and aluminum (Al3+) chloride. The C-4 keto group and either the C-3 or C-5 hydroxyl group of flavones and flavonols can chelate Al3+, and the products formed showed a maximum absorption of UV-vis light at 432 nm. The UV-vis absorption was proportional to the concentration of flavonoids present in the extracts, and could be determined spectrophotometrically [15,37].
Results of TFC determination in seeds of the three cultivars of O. ficus-indica from Algeria are reported in Table 3. In this case, these values are in line with those reported by other authors in seeds of the same plant species [35], although a significant variability is shown in the literature (e.g., [36]). It should be noted that the TFC is often expressed in equivalents of different standards (qurcetin, rutin, catechin) and the nature of the standard used could influence the final result, together with the factors mentioned above. Our results show a TFC range comprised between 1.15 ± 0.06 mg GAE/100 g of seeds (green cultivar) and 2.97 ± 0.11 mg GAE/100 g (orange cultivar).
3.2.3. Tannin Content (TC)
The astringency of fruits and drinks is often due to the interaction of tannins with salivary proteins [38]. The tannin content (TC) of seed samples of the cultivars studied ranged from 4.5 to 5.60 mg CE/100 g of seeds (Table 3). Seeds of the orange cultivar were, once again, the richest in tannins, followed by the yellow cultivar, with a value equal to 4.79 mg CE/100 g. In turn, this value was not statically different from that of the green one. These contents were also lower than those reported by other authors [36]. The type of cultivar and the abovementioned factors may be related to these differences.
3.3. Phytochemical Assessment by HPLC Analysis
Polyphenol identification was performed by HPLC-DAD, comparing R.T. of chromatographic peaks of unknown compounds with reference standards. In Figure S1 of Supplementary Material, a representative chromatogram of a standard mixture is reported, while a representative chromatogram obtained from the analysis of 40% ethanol extract of prickly pear seeds is reported in Figure S2. Results reported in Table 4 show that only six of the 12 phenols monitored were detected and quantified in the seed extracts, and the variability among the three cultivars was not significant.

Table 4.
Identification of different constituents of Opuntia ficus-indica seed extracts in different plant cultivars from the Souk Ahras area, Northeast Algeria.
To the best of our knowledge, only a small amount of information is available about the composition and concentration of phenolic compounds in prickly pear seeds, as opposed to other plant parts. Regarding the phenols in seeds, available information has been summarized in a recent review [35]. The studies here described show a high variability among different fruit varieties, for instance, high amounts of chlorogenic acid have been reported in O. ficus-indica seeds (885.31–1148.41 µg/g), but other compounds such as gallic acid and p-coumaric acid have not been found. It must be pointed out that the content of chlorogenic acid determined in our study is almost ten times lower than that reported in [35]. Nevertheless, as reported above for the TPC determination, and as suggested by other authors, all these variabilities can be attributed mainly to environmental factors, plant origin, species, developmental stage, and age [35].
Another point should be raised. In our study, only a limited number of phenolic constituents was monitored, and this contributed to the high discrepancy observed between the TPC, TFC, and TC, and the low amounts of derivatives detected in the seed extracts. Further studies will be performed for a deeper characterization of these constituents in seeds from O. ficus-indica growing in Algeria.
3.4. Antioxidant Activities
3.4.1. FRAP Assay
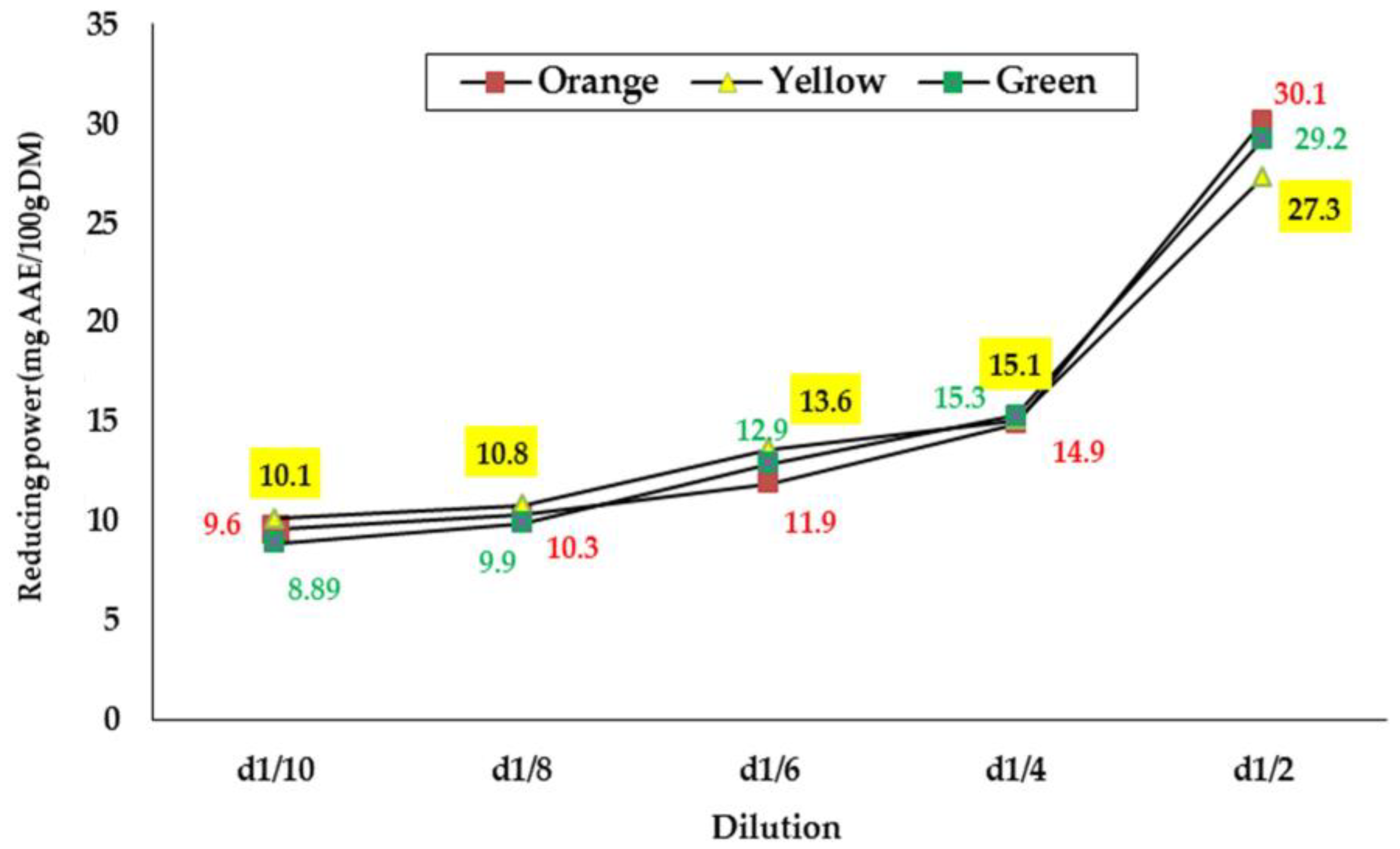
This test measured the ability of extracts to reduce metal ions, iron (Fe3+) potassium ferrocyanide to produce ferrous iron (Fe2+) [39]. Many authors consider the reductive capacity of a compound a significant indicator of antioxidant potency [19,25,39,40]. The results of the evaluation of the reducing power of seed extracts from the three cultivars of O. ficus-indica from Algeria are illustrated in Figure 1.
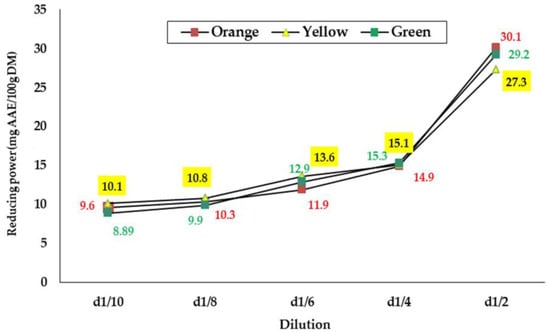
Figure 1.
Reducing power of the seed extracts from the three cultivars of prickly pear from the Souk Ahras area, Northeast Algeria.
According to the results obtained, the reducing power increases with the increase in concentration of seed extracts from all three varieties (Figure 1). This observation has been confirmed by several authors [21,41,42]. The plotted curves evolve in the same way and the values obtained oscillate between 8.89 and 30.1 mg AAE/100 g for all samples. Over all the concentrations tested, significant differences were noted only at dilutions D1/8 and D1/2, and the extracts of the seeds of the green and orange varieties are those with the best reducing potential. From the different measurements, the EC50 values were deduced for each sample and are presented in Table 5. As indicated, the EC50 was almost the same for all the seed extracts (0.05 g/mL), and no significant differences were detected.

Table 5.
EC50 values (g/mL) measured for the reducing power (RP; FRAP assay) and radical scavenging activity (DPPH assay) of seed extracts from the three cultivars of prickly pear. Values in the same column followed by the same letter are not significantly different at p ≤ 0.05.
3.4.2. DPPH Assay
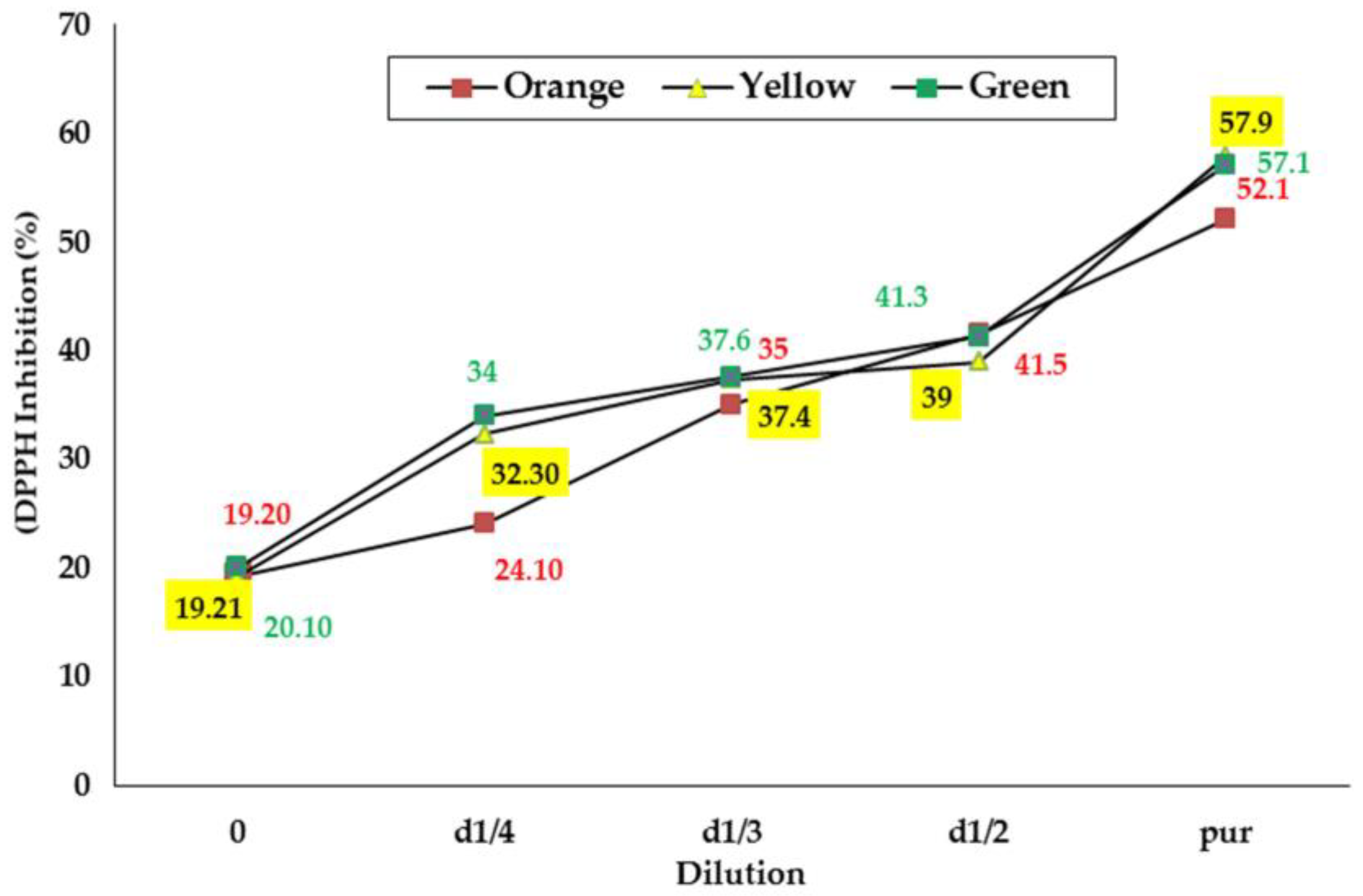
Results from the evaluation of the antiradical activity of the seed extracts from the three O. ficus indica cultivars are illustrated in Figure 2 Based on the results obtained, the anti-radical power of seed extracts from three cultivars increased dose-dependently [41]. The percentage of inhibition of the DPPH radical for the yellow, green, and orange cultivars increased from 19.20 to 52.10%, 20.10 to 57.10%, and 19.21 to 57.90%, respectively, for concentrations ranging from 0.064 to 0.4 g/mL. EC50 was also determined for each sample: the results, reported in Table 5, indicated that the amounts required to reduce the DPPH by 50% were significantly lower for seed extracts from green and yellow cultivars.
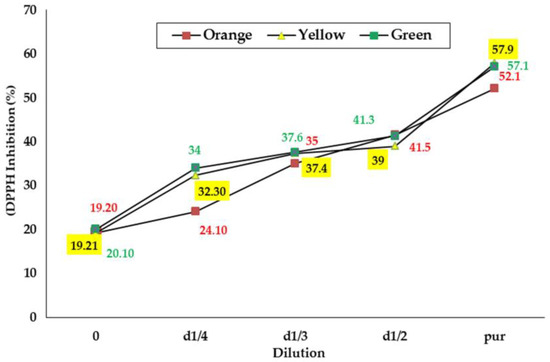
Figure 2.
SPercentage of inhibition of the DPPH radical calculated for the seed extracts from the three cultivars of prickly pear.
3.4.3. Correlations between Phenolic Constituents of Seeds and Antioxidant Activities
To get an overview of the possible involvement of the phenolic constituents of prickly pear seeds in their antioxidant activities, correlations between these variables were assessed. The correlation coefficients “r” are shown in Table 6. The results indicate a lack of significant correlation between the TPC of seeds and their reducing power (r = 0.261). It should be recalled that, in this case, the EC50 found for all three samples tested was the same, and this could indicate that the type of effective molecules against ferric iron are identical. This would explain the lack of a linear relationship between these two parameters. In contrast, a strong positive correlation (r = 0.890; p ≤ 0.05) was observed between the content of these compounds and the EC50 of seed extracts for antiradical activity. This result represents an interesting aspect, since usually phenols are reported as effective radical-quenching agents. Nevertheless, it must be considered that these compounds can respond differently in antiradical assays depending on their chemical structures, and mainly on the number of phenolic groups [43,44]. The correlations assessed between the antioxidant activities of seed extracts and their TFC and TC further support this observation. In fact, considering the DPPH assay, both TFC and TC were inversely correlated to the measured EC50 values. Nevertheless, the results were not significant, although the correlation coefficient for the TFC (r = −0.520) could suggest a partial involvement of flavonoids in the activity. More importantly, a significant inverse correlation was assessed between the TFC and the EC50 measured for the reducing power of seed extracts (r = −0.657; p ≤ 0.05), indicating in this case that flavonoids are among those responsible for the observed effects.

Table 6.
Results of the correlation analysis between measured antioxidant activities of prickly pear seeds and the classes of antioxidants monitored.
Finally, the study of the correlation between the two assays performed (reducing power and scavenging effect on the DPPH) did not reveal any linear effect, which might suggest that reducing agents do not necessarily exert a significant antiradical activity and vice versa [45].
4. Conclusions
Overall, the results of this study show that the seeds of three different cultivars of O. ficus-indica from the Souk Ahras area of Algeria represent a valuable source of nutrients and bioactive compounds. An oil rich in essential fatty acids can be extracted that can be potentially used as table or cooking oil. Moreover, the results show that these seeds contain significant levels of antioxidants. The concentration of total phenolic compounds was higher in the yellow cultivar, while flavonoid and tannin contents were higher in the orange one. The antioxidant activity of seed extracts is significant, but a variability that depends on the cultivar was observed in the different assays performed. The green and orange cultivars had a higher reducing power, while yellow and green cultivars had higher anti-radical activity. Positive and negative correlations were observed between the different activities and the concentrations of antioxidants measured, indicating that these compounds participate, according to the concentration, in antioxidant and antiradical effects. Also, these observations indicate that the concentration factor alone does not explain the results obtained. Therefore, it would be desirable to purify and identify the compounds present in seed extracts, apply more experimental protocols, and carry out the test on other varieties of prickly pear.
Finally, for a complete valorization of this plant species in Algeria, the current study should be integrated with analyses of other parts of the fig tree that are considered as waste in the future, and with further bioactivity tests on whole extracts and isolated chemical constituents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13031444/s1, Figure S1: Chromatogram of the standard phenols mixture; Figure S2: Representative HPLC chromatograms of O. ficus-indica seed extract at 280 nm.
Author Contributions
Conceptualization, A.B. and F.B.; methodology, A.B., F.B., H.B., H.M. and G.P.; software, A.B.; validation, G.P., H.B. and A.B.; formal analysis, A.B.; investigation, G.P.; resources, A.B.; data curation, G.P.; writing—original draft preparation, A.B.; writing—review and editing, G.P.; visualization, A.B.; supervision, H.B.; project administration, A.B.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to Barbaros Balabanlı (Ankara Gazi University, Department of Biology, Developmental Biology and Biotechnology, Turkey) for the plant identification. They want also to acknowledge the Ministry of Higher Education and Scientific Research (MHESR, Algeria) for the financial support. Finally, thanks also to the Laboratory of Science and Techniques for Living, Department of Agricultural Sciences, Institute of Agriculture and Veterinary Sciences, University of Souk Ahras, and NOPALTEC Algeria Cooperative, Sidi-Fredj, Souk Ahras region, Algeria.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Faedda, R.; Pane, A.; Cacciola, S.O.; Granata, G.; Salafia, L.; Sinatra, F. Penicillium polonicum causing a postharvest soft rot of cactus pear fruits. Acta Hortic. 2015, 1067, 193–197. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of Sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [PubMed]
- Maataoui, B.; Hmyene, A.; Hilali, S. Anti-radical activities of fruit juice extracts from Opuntia ficus-indica. Leban. Sci. J. 2006, 1, 3–8. [Google Scholar]
- Sepúlveda, E.; Sáenz, C. Industrialización de la tuna (Opuntia ficus-indica). I. Aceite de la semilla. Alimentos 1988, 13, 35–38. [Google Scholar]
- Ennouri, M.; Evelyne, B.; Laurence, M.; Hamadi, A. Fatty acid composition and rheological behavior of prickly pear seed oils. Food. Chem. 2005, 93, 431–437. [Google Scholar] [CrossRef]
- Ghazi, Z.; Ramdani, M.; Tahri, M.; Rmili, R.; Elmsellem, H.; El Mahi, B.; Fauconnier, M.L. Chemical composition and antioxidant activity of seeds oils and fruit juice of Opuntia Ficus Indica and Opuntia Dillenii from Morocco. J. Mater. Environ. Sci. 2015, 6, 2338–2345. [Google Scholar]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Arabinan-rich polysaccharides isolated and characterized from the endosperm of the seed of Opuntia ficus-indica prickly pear fruits. Carbohydr. Polym. 2005, 60, 319–329. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Morphological and structural study of seed pericarp of Opuntia ficus-indica prickly pear fruits. Carbohydr. Polym. 2008, 72, 102–112. [Google Scholar] [CrossRef]
- El Kossori, R.L.; Villaume, C.; El Boustani, E.; Sauvaire, Y.; Méjean, L. Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus indica sp.). Plant Foods Hum. Nutr. 1998, 52, 263–270. [Google Scholar] [CrossRef]
- Cardador-Martínez, A.; Jiménez-Martínez, C.; Sandoval, G. Revalorization of cactus pear (Opuntia spp.) wastes as a source of antioxidants. Ciênciae Tecnol. Aliment. 2011, 31, 782–788. [Google Scholar] [CrossRef]
- Sumaya Martìnez, M.T.; Cruz Jaime, S.; Marigal Santillàn, E.; Garcìa Paredes, J.D.; Cariño Cortés, R.; Cruz Cansino, N.; Valadez Vega, C.; Martinez Cardenas, L.; Alanìs Carcìa, E. Betalain, acid ascorbic, phenolic contents and antioxidant properties of purple, red, yellow and white cactus pears. Int. J. Mol. Sci. 2011, 12, 6452–6468. [Google Scholar] [CrossRef] [PubMed]
- García-Rollán, M. Claves de la Flora de España (Península y Baleares), Pteridofitas, Gimnospermas, Dicotiledoneas (A–J); Ediciones Mundi-Prensa: Madrid, Spain, 1981; Volume 1. [Google Scholar]
- Labuschagne, M.T.; Hugo, A. Oil content and fatty acid composition of cactus pear seed compared with cotton and grape seed. J. Food Biochem. 2010, 34, 93–100. [Google Scholar] [CrossRef]
- Boizot, N.; Charpentier, J.P. Rapid method for evaluating the content of phenolic compounds in the organs of a foustier tree. In INRA’s Book of Techniques; INRAE: Montpellier, France, 2006; pp. 79–82. [Google Scholar]
- Romero-Orejon, F.L.; Muñoz, A.M.; Luciana de la Fuente-Carmelino, L.; Jimenez-Champi, D.; Contreras-López, E.; Best, I.; Aguilar, L.; Ramos-Escudero, F. Secondary Metabolites of Edible Cacti (Cactaceae) from the South American Andes. In Secondary Metabolites—Trends and Reviews; Vijayakumar, R., Raja, S.S.S., Eds.; IntechOpen: London, UK, 2022. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sajid, M.S.; Abbas, R.Z.; Sindhu, Z. Determination of Condensed Tannin Contents from Different Plants of Kherimurat Rangeland (Attock, Pakistan). J. Agric. Soc. Sci. 2011, 7, 114–116. [Google Scholar]
- Gülçin, I.; Alici, H.A.; Cesur, M. Determination of in Vitro Antioxidant and Radical Scavenging Activities of Propofol. Chem. Pharm. Bull. 2005, 53, 281–285. [Google Scholar] [CrossRef]
- Koksal, E.; Bursal, E.; Dikici, E.; Tozoglu, F.; Gulcin, I. Antioxidant activity of Melissa officinalis leaves. J. Med. Plants Res. 2011, 5, 217–222. [Google Scholar]
- Nagulendran, K.R.; Velavan, S.; Mahesh, R.; Begum, V.H. In vitro antioxidant activity and total polyphenolic content of Cyperus rotundus rhizomes. E. J. Chem. 2007, 4, 440–449. [Google Scholar] [CrossRef]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef]
- Wallace, P.A.; Marfo, E.K.; Plahar, W.A. Nutritional quality and anti-nutritional composition of four non-conventional leafy vegetables. Food Chem. 1998, 61, 287–291. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity Songklanakarin. J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Huang, D.; Ou, B.; Ronald, L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Masmoudi, M.; Baccouche, A.; Borchani, M.; Besbes, S.; Blecker, C.; Attia, H. Physico-chemical and antioxidant properties of oils and by-products obtained by cold press-extraction of Tunisian Opuntia spp. Seeds. Appl. Food Res. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Brahmi, F.; Haddad, S.; Bouamara, K.; Yalaoui-Guellal, D.; Prost-Camus, E.; Pais de Barros, J.P.; Prost, M.; Atanasov, A.G.; Madani, K.; Boulekbache-Makhlouf, L.; et al. Comparison of chemical composition and biological activities of Algerian seed oils of Pistacia lentiscus L., Opuntia ficus indica (L.) mill. and Argania spinosa L. Skeels. Ind. Crop. Prod. 2020, 151, 112456. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mörsel, J.-T. Oil cactus pear (Opuntia-indica L.). Food Chem. 2003, 82, 339–345. [Google Scholar] [CrossRef]
- Kadda, S.; Belabed, A.; Conte, R.; Ouahhoud, S.; Hamdaoui, H.; Mechchate, H.; Elarbi, Z. Phytochemical analysis for the residues of Opuntia ficus indica L. seed oil of eastern region of Morocco. Mater. Today Proc. 2023, 72, 3362–3668. [Google Scholar] [CrossRef]
- Hernández, F.; Andreu-Coll, L.; Bento-Silva, A.; Serra, A.T.; Mena, P.; Legua, P.; Bronze, M.R. Phytochemical Profile of Opuntia ficus-indica (L.) Mill Fruits (cv. ‘Orito’) Stored at Different Conditions. Foods 2022, 11, 160. [Google Scholar] [CrossRef]
- Ghanemi, F.Z.; Abdelhafid, N.; Patoli, D.; Khaldi, D.; Zoubida, M.; Belarbi, M. Evaluation of the nutritional value and antioxidant activity of Opuntia ficus indica seeds in the western region of Algeria. J. Nat. Prod. Res. Applic. 2022, 2, 54–66. [Google Scholar]
- Ali, S.K.; Mahmoud, S.M.; El-Masry, S.S.; Alkhalifah, D.H.M.; Hozzein, W.N.; Aboel-Ainin, M.A. Phytochemical screening and characterization of the antioxidant, anti-proliferative and antibacterial effects of different extracts of Opuntia ficus-indica peel. J. King Saud Univ. Sci. 2022, 34, 102216. [Google Scholar] [CrossRef]
- Armas Diaz, Y.; Machì, M.; Salinari, A.; Mazas Pérez-Oleaga, C.; Martínez López, N.M.; Briones Urbano, M.; Cianciosi, D. Prickly pear fruits from Opuntia ficus-indica varieties as a source of potential bioactive compounds in the Mediterranean diet. Med. J. Nutr. Metab. 2022, 15, 581–592. [Google Scholar] [CrossRef]
- Attanzio, A.; Restivo, I.; Tutone, M.; Tesoriere, L.; Allegra, M.; Livrea, M.A. Redox Properties, Bioactivity and Health Effects of Indicaxanthin, a Bioavailable Phytochemical from Opuntia ficus indica, L.: A Critical Review of Accumulated Evidence and Perspectives. Antioxidants 2022, 11, 2364. [Google Scholar] [CrossRef] [PubMed]
- Zeghbib, W.; Boudjouan, F.; Vasconcelos, V.; Lopes, G. Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review. Molecules 2022, 27, 4763. [Google Scholar] [CrossRef] [PubMed]
- AbdelFattah, M.S.; Badr, S.E.A.; Elsaid, A.S. Nutritional composition, prickly pear (Opunti aficus-indica (L.) seeds, fatty acids, fiber, minerals, protein, amino acids, antimicrobial activity. Int. J. Agric. Pol. Res. 2022, 8, 1–10. [Google Scholar]
- Ying, C.; Wan, D. Quantitative determination of total and individual flavonoids in stems and leaves of Buddleja davidii and Buddleja albiflora. Pharmacogn. Mag. 2012, 8, 273–279. [Google Scholar] [PubMed]
- Pascal, C.; Bigey, F.; Ratomahenina, R.; Boze, H.; Moulin, G.; Sarni-Manchado, P. Overexpression and characterization of two human salivary proline rich proteins. Protein Expr. Purif. 2006, 47, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Campestrini, L.H.; Vieira, D.L.; Pritsch, I.; Yamassaki, F.T.; Zawadzki-Baggio, S.F.; Maurer, J.B.B.; Molento, M.B. Chemical characterization of Opuntia ficus-indica (L.) Mill. hydroalcoholic extract and its efficiency against gastrointestinal nematodes of sheep. Vet. Sci. 2018, 5, 80. [Google Scholar] [CrossRef]
- Allai, L.; Karym, E.M.; El Amiri, B.; Nasser, B.; Essamad, A.; Terzioğlu, P.; Ertas, A.; Öztürk, M. Evaluation of antioxidant activity and phenolic composition of Opuntia ficus-indica cladodes collected from Moroccan Settat region. Eurasian J. Anal. Chem. 2017, 12, 105–117. [Google Scholar] [CrossRef]
- Ozsoy, N.; Can, A.; Yanardag, R.; Akev, N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem. 2008, 110, 571–583. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, H.R.; Kim, J.; Jang, Y.S. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J. Agric. Food Chem. 2002, 50, 6490–6496. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH-assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).