Characterization of Pyrolysis Products of Forest Residues and Refuse-Derived Fuel and Evaluation of Their Suitability as Bioenergy Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Experimental Procedure of Pyrolysis Tests
2.3. Characterization of Pyrolysis Products
3. Results
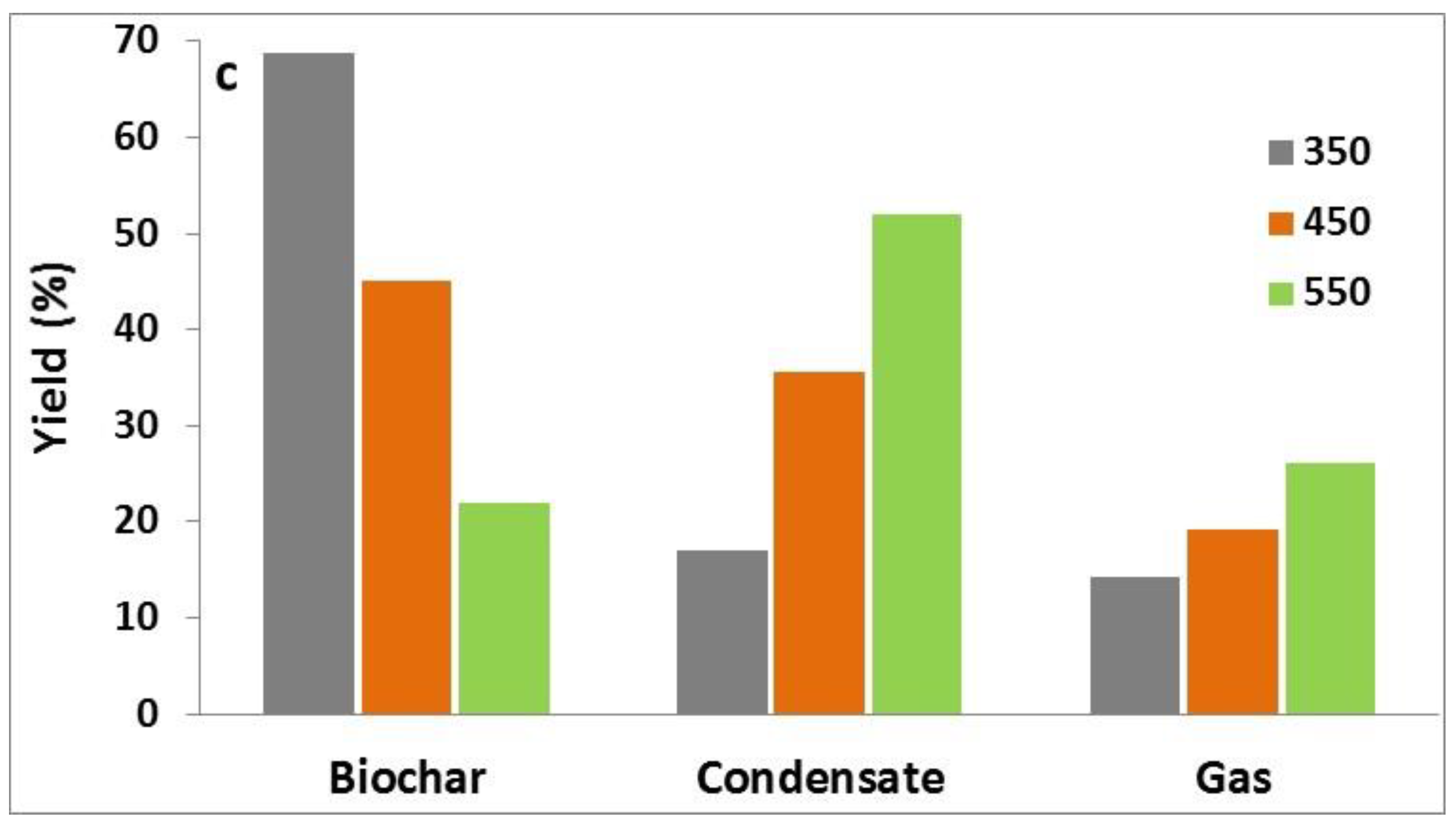
3.1. Characterization of Raw Materials and Product Yield
3.2. Solid Products—Biochars
3.3. Liquid Products–Bio-Oils
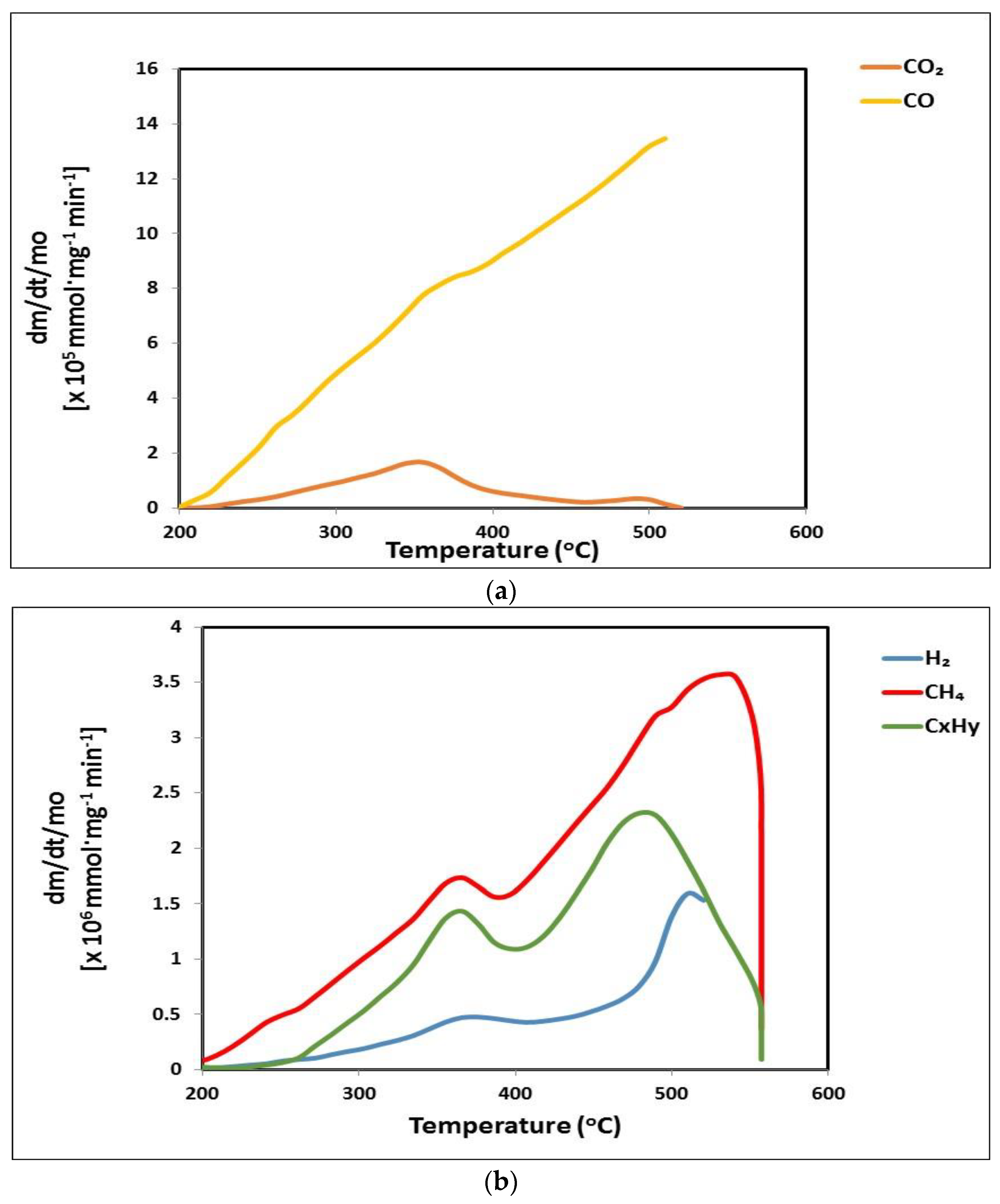
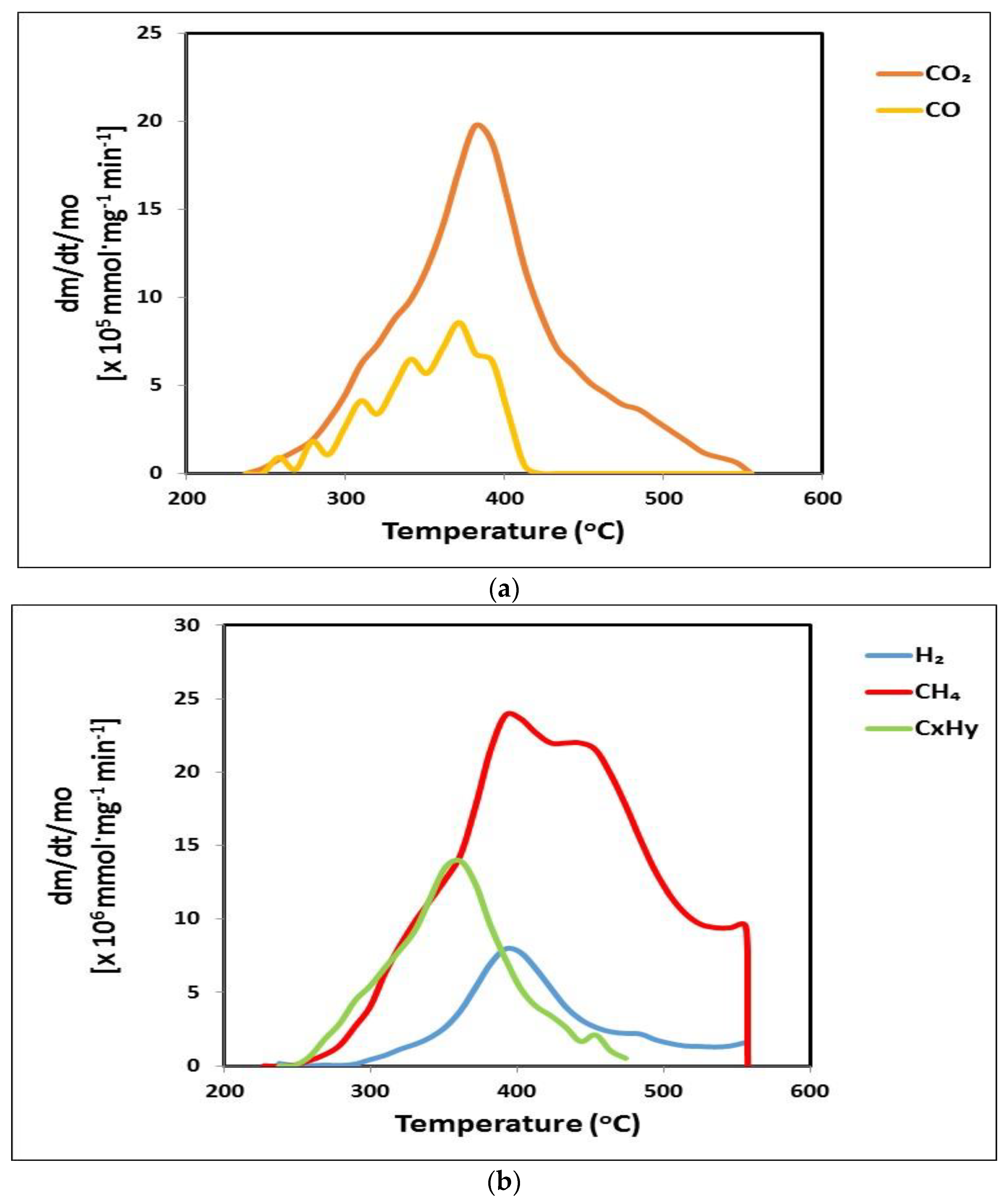
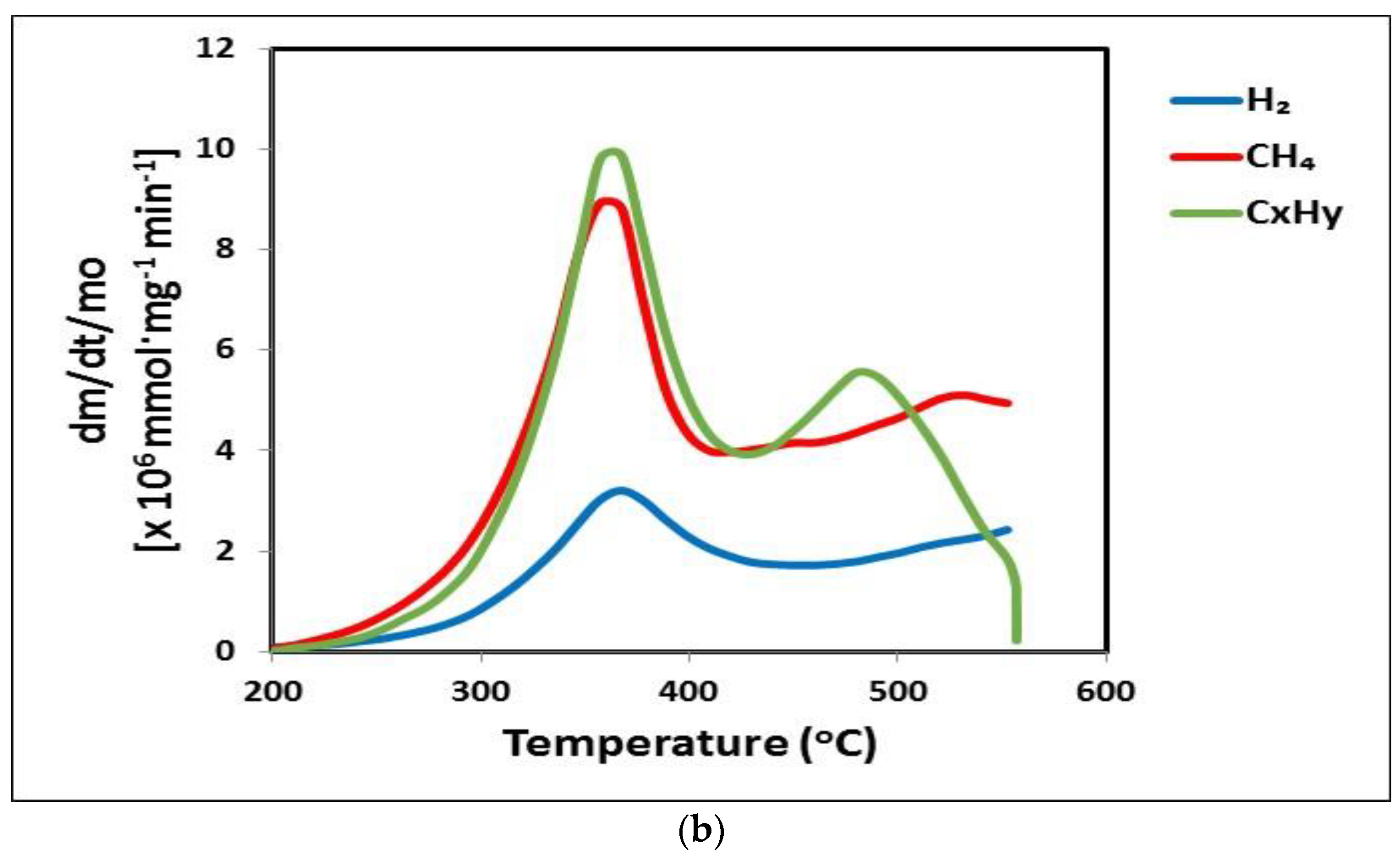
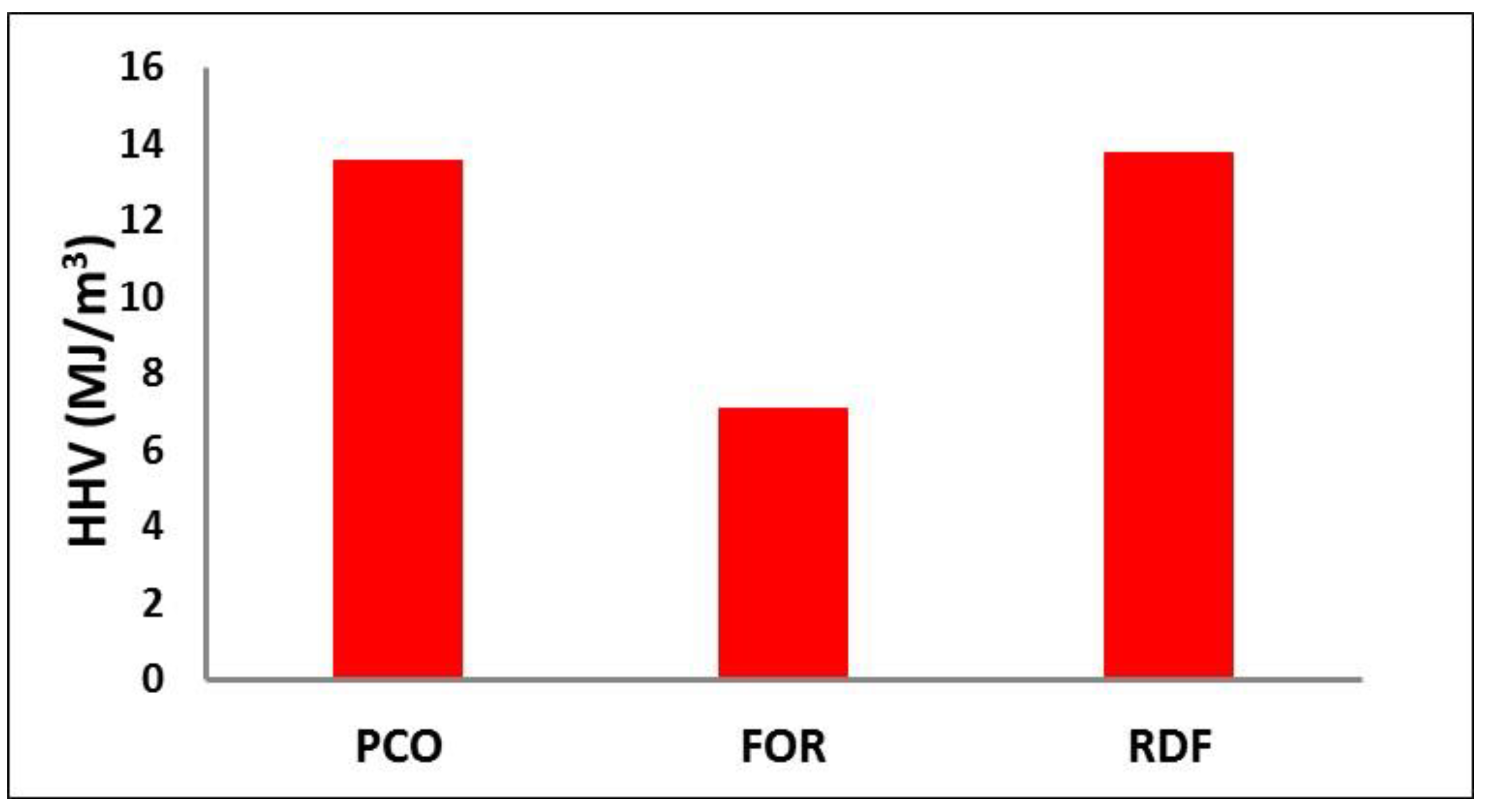
3.4. Gaseous Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mulvaney, D. Green New Deal. In Sol Power; University of California Press: Berkley, CA, USA, 2019; pp. 47–65. [Google Scholar]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrol. 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Pyrolysis of sugarcane bagasse in semi batch reactor: Effects of process parameters on product yields and characterization of products. Ind. Crops Prod. 2017, 95, 704–717. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S.; Pantelaki, O. Evaluation of gaseous and solid products from the pyrolysis of waste biomass blends for energetic and environmental applications. Fuel 2018, 236, 574–582. [Google Scholar] [CrossRef]
- Vamvuka, D.; Teftiki, A.; Sfakiotakis, S. Increasing the reactivity of waste biochars during their co-gasification with carbon dioxide using catalysts and bio-oils. Thermochim. Acta 2021, 704, 179015. [Google Scholar] [CrossRef]
- Policella, M.; Wang, Z.; Burra, K.G.; Gupta, A.K. Characteristics of syngas from pyrolysis and CO2-assisted gasification of waste tires. Appl. Energy 2019, 254, 113678. [Google Scholar] [CrossRef]
- Soni, B.; Karmee, S.K. Towards a continuous pilot scale pyrolysis based biorefinery for production of biooil and biochar from sawdust. Fuel 2020, 271, 117570. [Google Scholar] [CrossRef]
- Vamvuka, D.; Esser, K.; Komnitsas, K. Investigating the Suitability of Grape Husks Biochar, Municipal Solid Wastes Compost and Mixtures of Them for Agricultural Applications to Mediterranean Soils. Resources 2020, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhang, X.; Li, Y.; Han, L. Influence of pyrolysis temperature on chemical speciation, leaching ability and environmental risk of heavy metals in biochar derived from cow manure. Bioresour. Technol. 2020, 302, 122850. [Google Scholar] [CrossRef]
- Vamvuka, D.; Raftogianni, A. Evaluation of Pig Manure for Environmental or Agricultural Applications through Gasification and Soil Leaching Experiments. Appl. Sci. 2021, 11, 12011. [Google Scholar] [CrossRef]
- Yang, X.; Ng, W.; Wong, B.S.E.; Baeg, G.H.; Wang, C.-H.; Ok, Y.S. Characterization and ecotoxicological investigation of biochar produced via slow pyrolysis: Effect of feedstock composition and pyrolysis conditions. J. Hazard. Mater. 2019, 365, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Oliveira, B.; Pinheiro, W.; Correa, N.; França, L.; Ribeiro, N. Generation of biofuels by slow pyrolysis of palm empty fruit bunches: Optimization of process variables and characterization of physical-chemical products. Biomass Bioenergy 2020, 140, 105707. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Anand, A.; Aier, I.; Vijay, V.K.; Kaushal, P. Suitability of rice straw for biochar production through slow pyrolysis: Product characterization and thermodynamic analysis. Bioresour. Technol. Rep. 2021, 15, 100818. [Google Scholar] [CrossRef]
- Miranda, N.T.; Motta, I.L.; Filho, R.M.; Maciel, M.R.W. Sugarcane bagasse pyrolysis: A review of operating conditions and products properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Wang, L.; Olsen, M.N.; Moni, C.; Dieguez-Alonso, A.; de la Rosa, J.M.; Stenrød, M.; Liu, X.; Mao, L. Comparison of properties of biochar produced from different types of lignocellulosic biomass by slow pyrolysis at 600 °C. Appl. Energy Combust. Sci. 2022, 12, 100090. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, T.-S.; Lee, S.-M.; Choi, D.; Yeo, H.; Choi, I.-G.; Choi, J.W. Comparison of physicochemical features of biooils and biochars produced from various woody biomasses by fast pyrolysis. Renew. Energy 2013, 50, 188–195. [Google Scholar] [CrossRef]
- Yu, S.; Park, J.; Kim, M.; Ryu, C.; Park, J. Characterization of biochar and byproducts from slow pyrolysis of hinoki cypress. Bioresour. Technol. Rep. 2019, 6, 217–222. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Alhayali, M.A.; Saeed, L.I. Date palm stones as a potential new feedstock for liquid bio-fuels production. Fuel 2017, 210, 165–176. [Google Scholar] [CrossRef]
- Struhs, E.; Sotoudehnia, F.; Mirkouei, A.; McDonald, A.G.; Ramirez-Corredores, M.M. Effect of feedstocks and free-fall pyrolysis on bio-oil and biochar attributes. J. Anal. Appl. Pyrol. 2022, 166, 105616. [Google Scholar] [CrossRef]
- Bhatt, M.; Wagh, S.; Chakinala, A.G.; Pant, K.K.; Sharma, T.; Joshi, J.B.; Shah, K.; Sharma, A. Conversion of refuse derived fuel from municipal solid waste into valuable chemicals using advanced thermo-chemical process. J. Clean. Prod. 2021, 329, 129653. [Google Scholar] [CrossRef]
- Krutof, A.; Hawboldt, K.A. Thermodynamic model of fast pyrolysis bio-oil advanced distillation curves. Fuel 2020, 261, 116446. [Google Scholar] [CrossRef]
- Chireshe, F.; Collard, F.-X.; Görgens, J.F. Production of an upgraded bio-oil with minimal water content by catalytic pyrolysis: Optimisation and comparison of CaO and MgO performances. J. Anal. Appl. Pyrol. 2020, 146, 104751. [Google Scholar] [CrossRef]
- Rijo, B.; Dias, A.P.S.; Ramos, M.; Ameixa, M. Valorization of forest waste biomass by catalyzed pyrolysis. Energy 2022, 243, 122766. [Google Scholar] [CrossRef]
- Radojevic, M.; Jankovic, B.; Stojiljkovic, D.; Jovanovic, V.; Cekovic, I.; Manic, N. Improved TGA-MS measurements for evolved gas analysis during pyrolysis process of various biomass feedstocks. Syngas energy balance determination. Thermochim. Acta 2021, 699, 178912. [Google Scholar] [CrossRef]
- Fermoso, J.; Mašek, O. Thermochemical decomposition of coffee ground residues by TG-MS: A kinetic study. J. Anal. Appl. Pyrol. 2018, 130, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.H.; Zhao, Z.; Ren, J.; Rasool, T.; Naqvi, S.R. Thermo-kinetics and gaseous product analysis of banana peel pyrolysis for its bioenergy potential. Biomass Bioenergy 2019, 122, 193–201. [Google Scholar] [CrossRef]
- Tian, B.; Wang, X.; Zhao, W.; Xu, L.; Bai, L. Pyrolysis behaviors, kinetics and gaseous product evolutions of two typical biomass wastes. Catal. Today 2021, 374, 77–85. [Google Scholar] [CrossRef]
- Hai, A.; Bharath, G.; Ali, I.; Daud, M.; Othman, I.; Rambabu, K.; Haija, M.A.; Hasan, S.W.; Banat, F. Pyrolysis of date seeds loaded with layered double hydroxide: Kinetics, thermodynamics and pyrolytic gas properties. Energy Convers. Manag. 2022, 252, 115127. [Google Scholar] [CrossRef]
- Li, J.; Burra, K.G.; Wang, Z.; Liu, X.; Gupta, A.K. Effect of alkali and alkaline metals on gas formation behavior and kinetics during pyrolysis of pine wood. Fuel 2021, 290, 120081. [Google Scholar] [CrossRef]
- Vamvuka, D. Biomass, Bioenergy and the Environment; Tziolas Publications: Salonica, Greece, 2009. [Google Scholar]
- Vamvuka, D.; Loulashi, C. Catalytic Co-gasification of Lignites Blended with a Forest Residue under the Carbon Dioxide Stream. Catal. Res. 2022, 2, 2203031. [Google Scholar] [CrossRef]
- Mohammadi, H.; Salehzadeh, H.; Khezri, S.M.; Zanganeh, M.; Soori, M.M.; Keykhosravi, S.S.; Kamarehie, B. Improving Nitrification and Denitrification Processes in Urban Wastewater Treatment Using Optimized MBBR Reactor Design Considerations. J. Biochem. Technol. 2020, 11, 85–100. [Google Scholar]
- Vamvuka, D.; Chatzifotiadis, I. Energy Recovery from Solid Waste Materials via a Two-step Gasification Process by Steam. Eur. J. Energy Res. 2022, 2, 20–24. [Google Scholar] [CrossRef]
- European Standard CEN/TC335; Directive 2000/76/EC of the European Parliament and of the Council of 4 December 2000 on the Incineration of Waste. Technical Committee of European Standards: Brussels, Belgium, 2000.
- Vamvuka, D.; Trikouvertis, M.; Pentari, D.; Alevizos, G. Characterization and evaluation of fly and bottom ashes from combustion of residues from vineyards and processing industry. J. Energy Inst. 2017, 90, 574–587. [Google Scholar] [CrossRef]
- Garcia-Maraver, A.; Mata-Sanchez, J.; Carpio, M.; Perez-Jimenez, J.A. Critical review of predictive coefficients for biomass ash deposition tendency. J. Energy Inst. 2017, 90, 214–228. [Google Scholar] [CrossRef]
- ASTM 1298; Refractories, activated carbon, advanced ceramics. In ASTM Book of Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2007.
- Mateus, M.M.; Gaspar, D.; Matos, S.; Reigo, A.; Motta, C.; Castenheira, I. Converting a residue from an edible source into a bio-oil. J. Environ. Chem. Eng. 2019, 7, 103004. [Google Scholar] [CrossRef]
- Alburquerque, J.; Sanchez, M.; Mora, M.; Barron, M. Slow pyrolysis of relevant biomasses in the Mediterranean basin. Part 2. Char characterization for carbon sequestration and agricultural uses. J. Clean. Prod. 2014, 120, 191–197. [Google Scholar] [CrossRef]
- Vamvuka, D. Bio-oil, solid and gaseous biofuels from biomass pyrolysis processes-An overview. Int. J. Energy Res. 2011, 35, 835–862. [Google Scholar] [CrossRef]
- Cai, W.; Liu, R.; He, Y.; Chai, M.; Cai, J. Bio-oil production from fast pyrolysis of rice husk in a commercial-scale plant with a downdraft circulating fluidized bed reactor. Fuel Process. Technol. 2018, 171, 308–317. [Google Scholar] [CrossRef]
- Qiang, L.; Wen-Zhi, L.; Xi-Feng, Z. Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers. Manag. 2009, 50, 1376–1383. [Google Scholar]




| Sample | Volatile Matter | Fixed Carbon | Ash | C | H | N | O | S | HHV (MJ/kg) |
|---|---|---|---|---|---|---|---|---|---|
| PCO | 76.9 | 17.5 | 5.6 | 47.8 | 6.7 | 0.2 | 39.6 | 0.05 | 19.9 |
| FOR | 84.5 | 15.0 | 0.5 | 46.0 | 6.5 | - | 47.0 | - | 17.3 |
| RDF | 84.8 | 0.3 | 14.9 | 52.8 | 8.2 | 0.3 | 23.8 | - | 26.3 |
| Sample | Temperature (°C) | Organic Matter | Ash | C | H | N | O | HHV (MJ/kg) |
|---|---|---|---|---|---|---|---|---|
| PCO | 350 | 87.9 | 12.1 | 62.7 | 4.9 | 1.1 | 19.2 | 24.8 |
| 450 | 84.9 | 15.1 | 62.9 | 3.2 | 1.0 | 17.8 | 22.7 | |
| 550 | 79.5 | 20.5 | 63.4 | 1.9 | 1.0 | 13.2 | 21.8 | |
| FOR | 350 | 98.6 | 1.4 | 68.4 | 4.0 | - | 26.2 | 24.2 |
| 450 | 98.4 | 1.6 | 62.7 | 2.9 | - | 32.8 | 19.5 | |
| 550 | 97.7 | 2.3 | 56.5 | 2.1 | - | 39.1 | 15.2 | |
| RDF | 350 | 80.2 | 19.8 | 69.2 | 10.8 | 0.1 | 0.1 | 38.7 |
| 450 | 66.7 | 33.3 | 52.0 | 6.0 | 0.1 | 8.6 | 24.6 | |
| 550 | 46.1 | 53.9 | 34.2 | 1.0 | 0.2 | 10.7 | 11.1 |
| Sample | B/A | Sv | AI | Deposition Tendency |
|---|---|---|---|---|
| PCO | 0.93 | 51.9 | 0.14 | medium/high/low |
| FOR | 4.3 | 18.2 | 0.02 | high/high/low |
| RDF | 1.5 | 28.8 | 0.19 | high/high/low |
| Sample | PCO | FOR | RDF | Heavy Fuel Oil [31] | Flash Pyrolysis Oil [31] |
|---|---|---|---|---|---|
| Density (g/cm3) | 0.94 | 0.93 | 1.30 | 0.94 | 1.23 |
| Dynamic viscosity (cP) | 7.0 | 1.5 | 110.6 | 180.0 | 55–180 |
| pH | 3.0 | 2.1 | 3.1 | - | 2.4 |
| C (%) | 59.0 | 52.4 | 43.5 | 85.2 | 44–46 |
| H (%) | 8.9 | 7.9 | 5.0 | 11.1 | 6.6–7.1 |
| N (%) | 1.2 | 0.1 | 3.9 | 0.3 | - |
| S (%) | - | - | - | 2.3 | - |
| O (%) | 30.9 | 39.6 | 47.6 | 1.0 | 47–49 |
| HHV (MJ/kg) | 27.1 | 21.9 | 13.4 | 40.0 | 15.0–17.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamvuka, D.; Esser, K.; Marinakis, D. Characterization of Pyrolysis Products of Forest Residues and Refuse-Derived Fuel and Evaluation of Their Suitability as Bioenergy Sources. Appl. Sci. 2023, 13, 1482. https://doi.org/10.3390/app13031482
Vamvuka D, Esser K, Marinakis D. Characterization of Pyrolysis Products of Forest Residues and Refuse-Derived Fuel and Evaluation of Their Suitability as Bioenergy Sources. Applied Sciences. 2023; 13(3):1482. https://doi.org/10.3390/app13031482
Chicago/Turabian StyleVamvuka, Despina, Katerina Esser, and Dimitrios Marinakis. 2023. "Characterization of Pyrolysis Products of Forest Residues and Refuse-Derived Fuel and Evaluation of Their Suitability as Bioenergy Sources" Applied Sciences 13, no. 3: 1482. https://doi.org/10.3390/app13031482




