Diversity of Bacterial Communities Associated with Solitary Bee Osmia excavata Alfken (Hymenoptera: Megachilidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and PCR Amplification of 16S rDNA
2.3. Bioinformatics Analysis
3. Results
3.1. Characteristics of the Sequencing Data
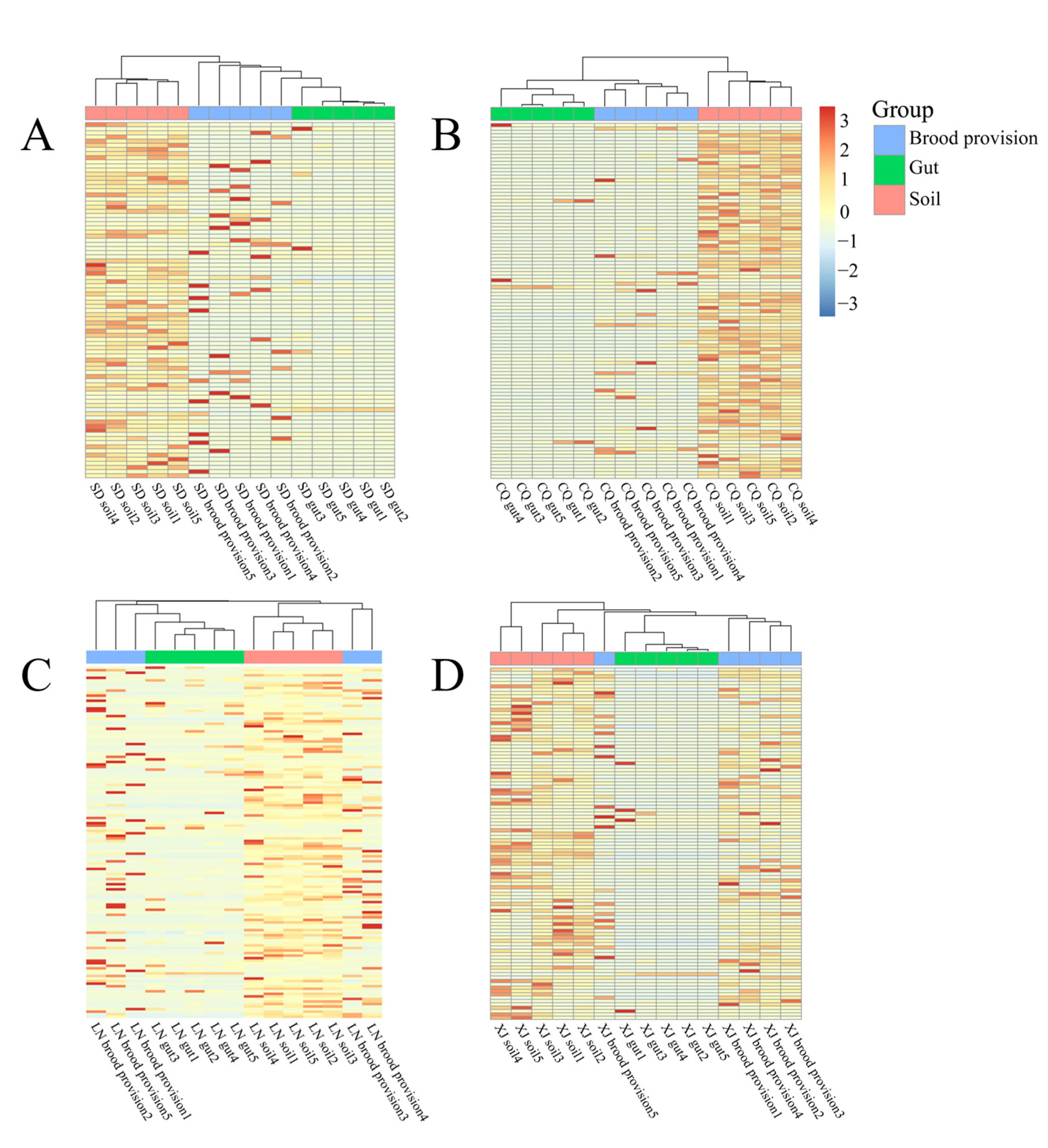
3.2. Homogeneity Analysis of Community Composition
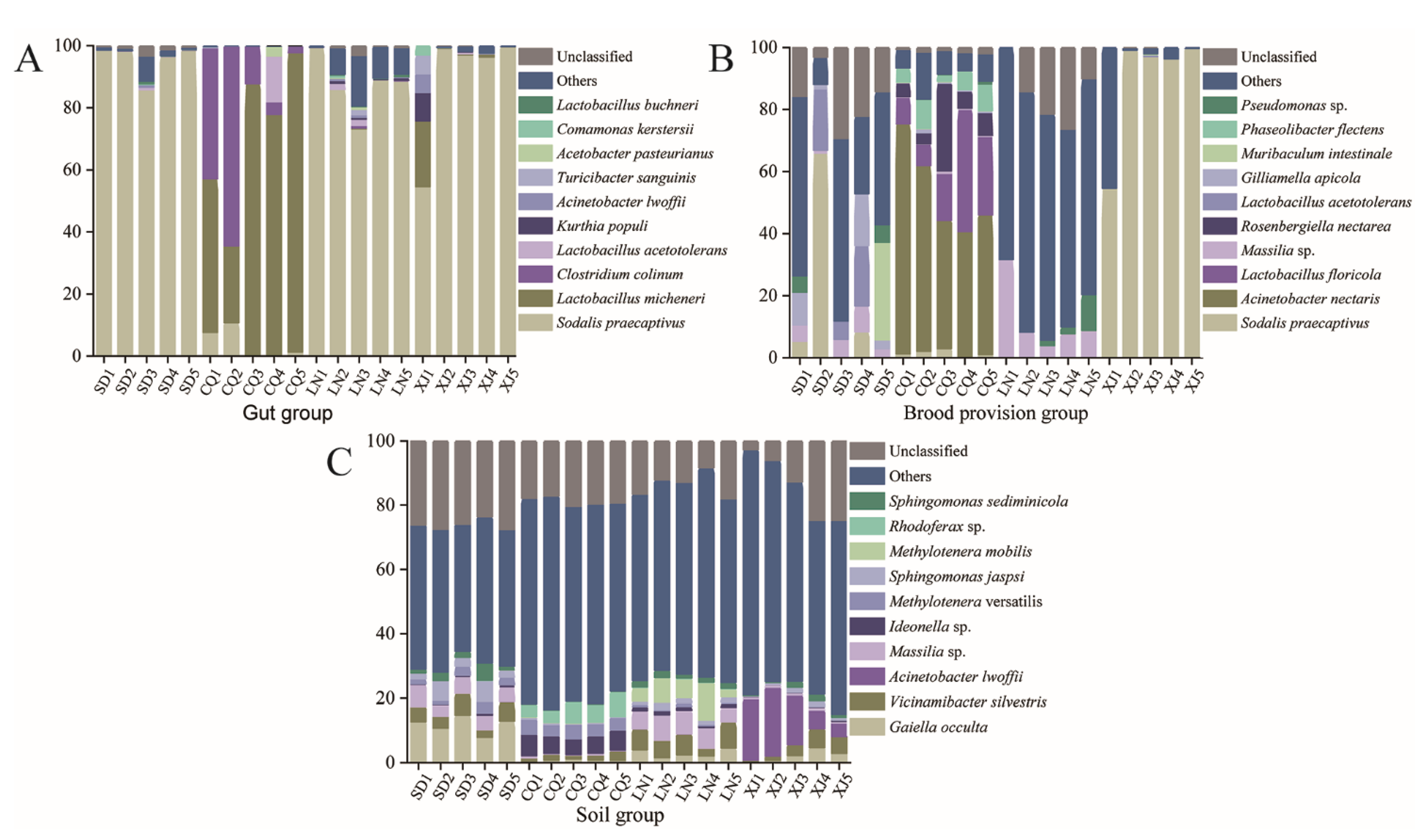
3.3. Analysis of Bacterial Communities Composition
3.3.1. Microbial Composition and Dominant Species
3.3.2. Differential Analysis of Bacterial Communities
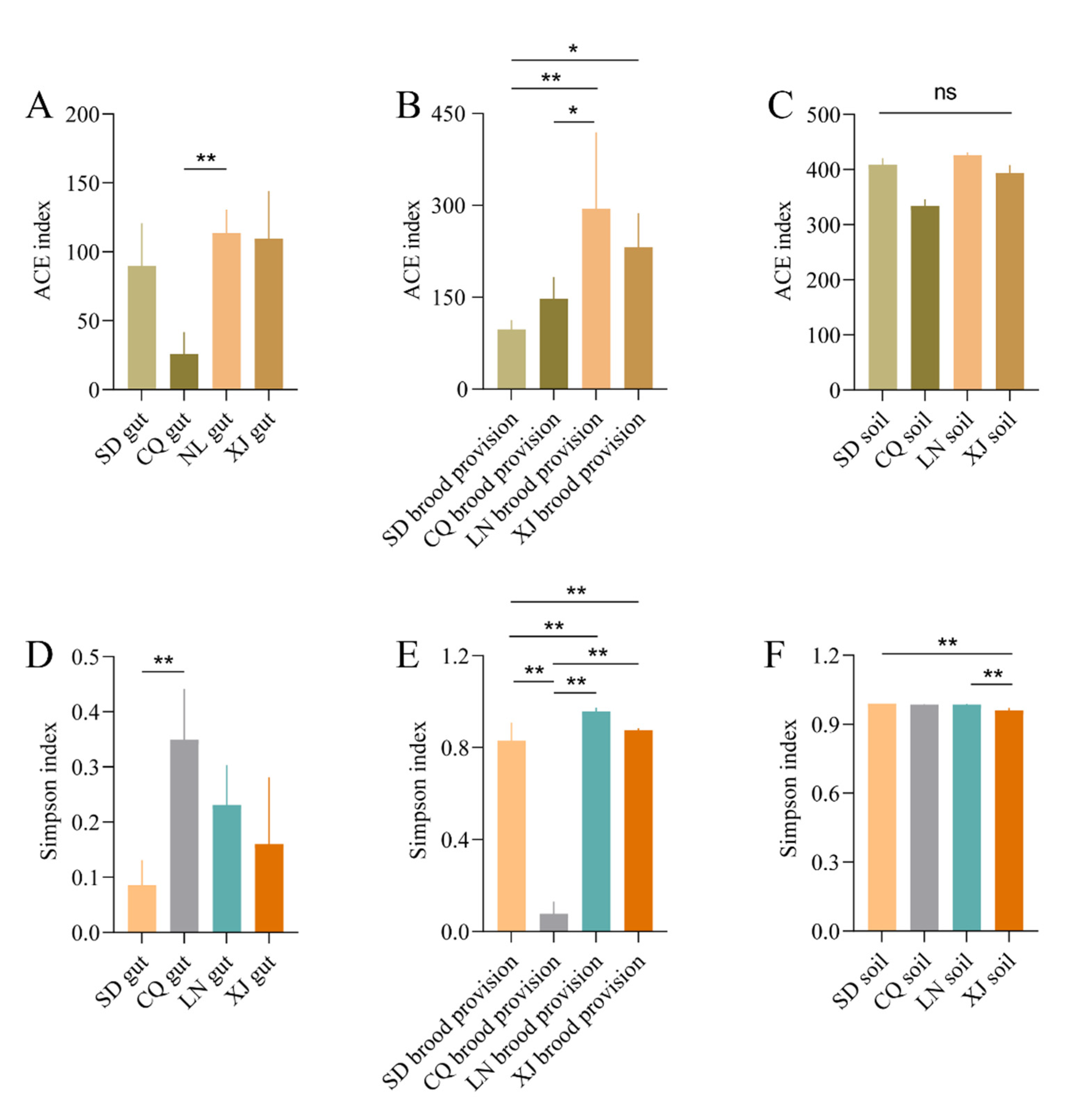
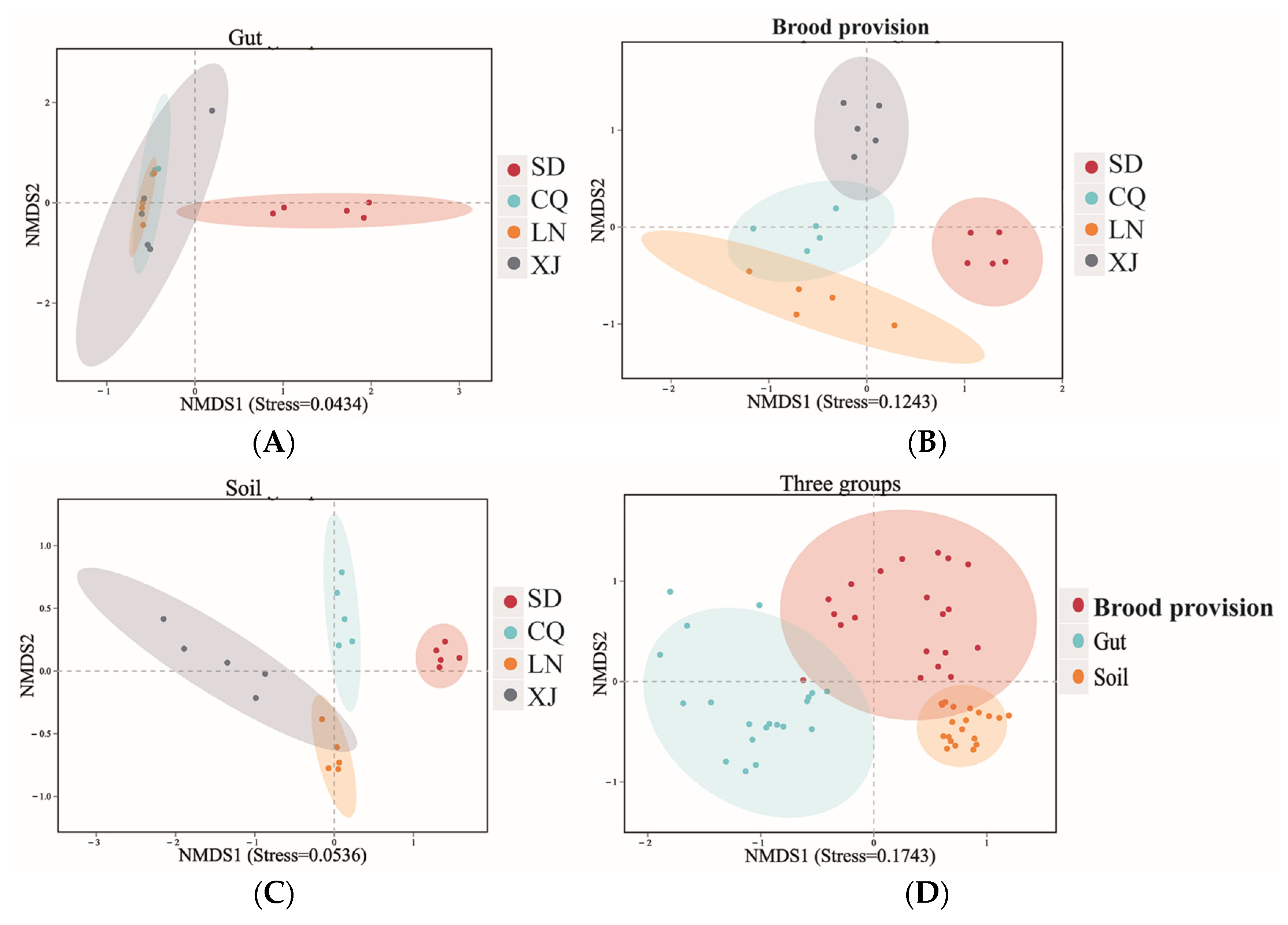
3.4. Diversity of Bacterial Communities in Different Groups
3.4.1. Alpha-Diversity Analysis
3.4.2. Beta-Diversity Analysis
3.5. Mantel Tests Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.; Vaissière, B.; Cane, J.; Steffan-Dewenter, I.; Cunningham, S.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Fijen, T.; Scheper, J.; Boom, T.; Janssen, N.; Raemakers, I.; Kleijn, D. Insect pollination is at least as important for marketable crop yield as plant quality in a seed crop. Ecol. Lett. 2018, 21, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Orr, M.C.; Luo, A.; Dou, F.; Kou, R.; Hu, F.; Zhu, C.; Huang, D. Relationships between wild bee abundance and fruit set of Camellia oleifera Abel. J. Appl. Entomol. 2020, 145, 277–285. [Google Scholar] [CrossRef]
- Cox-Foster, D.; Conlan, S.; Holmes, E.; Palacios, G.; Evans, J.; Moran, N.; Quan, P.; Briese, T.; Hornig, M.; Geiser, D.; et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 2007, 318, 283–287. [Google Scholar] [CrossRef]
- Mattila, H.; Rios, D.; Walker-Sperling, V.; Roeselers, G.; Newton, I. Characterization of the active microbiotas associated with honey bees reveals healthier and broader communities when colonies are genetically diverse. PloS ONE 2012, 7, e32962. [Google Scholar] [CrossRef]
- Crotti, E.; Sansonno, L.; Prosdocimi, E.; Vacchini, V.; Hamdi, C.; Cherif, A.; Gonella, E.; Marzorati, M.; Balloi, A. Microbial symbionts of honeybees: A promising tool to improve honeybee health. New Biotechnol. 2013, 30, 716–722. [Google Scholar] [CrossRef]
- Engel, P.; Kwong, W.; McFrederick, Q.; Anderson, K.; Barribeau, S.; Chandler, J.; Cornman, R.; Dainat, J.; de Miranda, J.; Doublet, V.; et al. The Bee Microbiome: Impact on Bee Health and Model for Evolution and Ecology of Host-Microbe Interactions. mBio 2016, 7, e02164-15. [Google Scholar] [CrossRef]
- Anderson, K.; Ricigliano, V. Honey bee gut dysbiosis: A novel context of disease ecology. Curr. Opin. Insect Sci. 2017, 22, 125–132. [Google Scholar] [CrossRef]
- Martinson, V.; Danforth, B.; Minckley, R.; Rueppell, O.; Tingek, S.; Moran, N. A simple and distinctive microbiota associated with honey bees and bumble bees. Mol. Ecol. 2011, 20, 619–628. [Google Scholar] [CrossRef]
- Kwong, W.; Medina, L.; Koch, H.; Sing, K.; Soh, E.; Ascher, J.; Jaffé, R.; Moran, N. Dynamic microbiome evolution in social bees. Sci. Adv. 2017, 3, e1600513. [Google Scholar] [CrossRef]
- McFrederick, Q.; Thomas, J.; Neff, J.; Vuong, H.; Russell, K.; Hale, A.; Mueller, U. Flowers and Wild Megachilid Bees Share Microbes. Microb. Ecol. 2017, 73, 188–200. [Google Scholar] [CrossRef]
- Voulgari-Kokota, A.; Ankenbrand, M.; Grimmer, G.; Steffan-Dewenter, I.; Keller, A. Linking pollen foraging of megachilid bees to their nest bacterial microbiota. Ecol. Evol. 2019, 9, 10788–10800. [Google Scholar] [CrossRef]
- Lozo, J.; Berić, T.; Terzić-Vidojević, A.; Stanković, S.; Fira, D.; Stanisavljević, L. Microbiota associated with pollen, bee bread, larvae and adults of solitary bee Osmia cornuta (Hymenoptera: Megachilidae). Bull. Entomol. Res. 2015, 105, 470–476. [Google Scholar] [CrossRef]
- Voulgari-Kokota, A.; McFrederick, Q.; Steffan-Dewenter, I.; Keller, A. Drivers, Diversity, and Functions of the Solitary-Bee Microbiota. Trends Microbiol. 2019, 27, 1034–1044. [Google Scholar] [CrossRef]
- Voulgari-Kokota, A.; Grimmer, G.; Steffan-Dewenter, I.; Keller, A. Bacterial community structure and succession in nests of two megachilid bee genera. FEMS Microbiol. Ecol. 2019, 95, fiy218. [Google Scholar] [CrossRef]
- Rothman, J.; Andrikopoulos, C.; Cox-Foster, D.; McFrederick, Q. Floral and Foliar Source Affect the Bee Nest Microbial Community. Microb. Ecol. 2019, 78, 506–516. [Google Scholar] [CrossRef]
- Yuan, F.; Wei, Y.P.; Zhang, Y.L.; He, Z.X.; Huang, S.Z.; Yuan, J.G. A faunistic survey of Osmia bees (Hymenoptera: Megachilidae) in Shaanxi and their application. Entomotaxonomia 1992, 2, 148–152. [Google Scholar]
- Zhang, F.L.; Yuan, F. Study on the Influence of Meteorological Factors on Daily Activity of Osmia Excavata Alfken. China Agric. Meteorol. 1998, 19, 52–54. [Google Scholar]
- Liu, L.; Li, L.L.; Ouyang, F.; Li, C.; Yu, Y.; Qu, C.H.; Qu, Z.L.; Men, X.Y.; Ye, B.H. Fruit-setting and yield increase for pear pollination by Osmia excavata Alfken and evaluation of economic value in Shandong Province. Bull. Agricul. Sci. Technol. 2019, 8, 233–236. [Google Scholar]
- Liu, L.; Li, L.L.; Ouyang, F.; LI, C.; Yu, Y.; Qu, C.H.; Qu, Z.L.; Ye, B.H.; Men, X.Y. Fruit-setting, yield increase and economic value evaluation for cherry pollination by Osmia excavata Alfken in Shandong Province. Shandong Agricul. Sci. 2019, 51, 125–128. [Google Scholar]
- Wang, G.P.; Lin, L.H.; Xue, X.M.; Wang, J.Z.; Tao, J.H. Research and application progress of Osmia pollination techniques on apple in China. Deciduous Fruits 2018, 50, 25–28. [Google Scholar]
- Lim, H.; Chu, C.; Seufferheld, M.; Cameron, S. Deep sequencing and ecological characterization of gut microbial communities of diverse bumble bee species. PloS ONE 2015, 10, e0118566. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 22 August 2021).
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Boutros, P. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef]
- Coche, S.; Sprangers, B.; Van Laecke, S.; Weekers, L.; De Meyer, V.; Hellemans, R.; Castanares, D.; Ameye, H.; Goffin, E.; Demoulin, N.; et al. Recurrence and Outcome of Anti-Glomerular Basement Membrane Glomerulonephritis after Kidney Transplantation. Kidney Int. Rep. 2021, 6, 1888–1894. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2011, 26, 32–46. [Google Scholar]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- He, W.Z.; Zhou, R.W. Effects of apple pollination on Osmia excavata Alfken, Apis mellifera L. and artificical pollination. Apicult. China 2009, 60, 9–11. [Google Scholar]
- Keller, A.; Grimmer, G.; Steffan-Dewenter, I. Diverse microbiota identified in whole intact nest chambers of the red mason bee Osmia bicornis (Linnaeus 1758). PLoS ONE 2013, 8, e78296. [Google Scholar] [CrossRef]
- McFrederick, Q.S.; Rehan, S.M. Characterization of pollen and bacterial community composition in brood provisions of a small carpenter bee. Mol. Ecol. 2016, 25, 2302–2311. [Google Scholar] [CrossRef]
- Elbein, A.; Pan, Y.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Chari, A.; Oakeson, K.; Enomoto, S.; Jackson, D.; Fisher, M.; Dale, C. Phenotypic characterization of Sodalis praecaptivus sp. nov., a close non-insect-associated member of the Sodalis-allied lineage of insect endosymbionts. Int. J. Syst. Evol. Microbiol. 2015, 65, 1400–1405. [Google Scholar] [CrossRef]
- McFrederick, Q.; Rehan, S. Wild Bee Pollen Usage and Microbial Communities Co-vary across Landscapes. Microb. Ecol. 2019, 77, 513–522. [Google Scholar] [CrossRef]
- Diao, Y.X.; Li, J.Q.; Chen, Q.P.; Wng, H.B. Isolation and identification of Clostridum Colinum. Chin. J. Prev. Vet. Med. 2000, 4, 5–6. [Google Scholar]
- Yang, E.D.; Cui, D.X.; Wang, W.Y. Research progress on the genus Massilia. Microbiol. China 2019, 46, 1537–1548. [Google Scholar]
- Halpern, M.; Fridman, S.; Atamna-Ismaeel, N.; Izhaki, I. Rosenbergiella nectarea gen. nov., sp. nov., in the family Enterobacteriaceae, isolated from floral nectar. Int. J. Syst. Evol. Microbiol. 2013, 63, 4259–4265. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Huber, K.; Geppert, A.; Wanner, G.; Fösel, B.; Wüst, P.; Overmann, J. The first representative of the globally widespread subdivision 6 Acidobacteria, Vicinamibacter silvestris gen. nov., sp. nov., isolated from subtropical savannah soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 2971–2979. [Google Scholar] [CrossRef]
- Hosokawa, T.; Kikuchi, Y.; Fukatsu, T. How many symbionts are provided by mothers, acquired by offspring, and needed for successful vertical transmission in an obligate insect-bacterium mutualism? Mol. Ecol. 2007, 16, 5316–5325. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.P.; Yuan, F.; Zhang, Y.L. The resistance and reproductive potential of Osima excavata Alfken. Chin. J. Appl. Entomol. 2001, 2, 122–124. [Google Scholar]
- Gilliam, M.; Buchmann, S.L.; Lorenz, B.J.; Schmalzel, R.J. Bacteria belonging to the genus Bacillus associated with three species of solitary bees. Apidologie 1990, 21, 99–105. [Google Scholar] [CrossRef]
- Inglis, G.D.; Yanke, L.J.; Goettel, M.S. Anaerobic bacteria isolated from the alimentary canals of alfalfa leafcutting bee larvae. Apidologie 1998, 29, 327–332. [Google Scholar] [CrossRef][Green Version]

| Location | Experimental Plot | Latitude/Longitude | Elevation (m) | Sampling Environment |
|---|---|---|---|---|
| Tangwang Town, Jinan, Shandong | SD | N36.8193/E117.2758 | 26 | pear orchard |
| Huxi Street, Shapingba District, Chongqing | CQ | N29.6145/E106.2994 | 292 | semi-natural habitat |
| Sanrunbao Street, Dalian, Liaoning | LN | N38.9400/E121.1704 | 30 | cherry orchard |
| Shayidong Horticulture Farm, Bayinguoleng, Xinjiang | XJ | N41.7271/E86.0232 | 921 | pear orchard |
| Category | SD | CQ | LN | XJ |
|---|---|---|---|---|
| ACE index | ||||
| Gut | 89.75 ± 61.84 AB b | 28.39 ± 20.76 B c | 113.56 ± 34.06 A c | 109.64 ± 68.79 AB c |
| Brood provision | 97.71 ± 30.38 C b | 147.73 ± 71.23 BC b | 294.54 ± 44.62 A b | 231.67 ± 48.94 AB b |
| Soil | 408.55 ± 24.01 A a | 334.01 ± 24.46 A a | 425.92 ± 10.47 A a | 393.64 ± 28.61 A a |
| Simpson index | ||||
| Gut | 0.09 ± 0.03 B c | 0.35 ± 0.19 A c | 0.23 ± 0.14 AB c | 0.16 ± 0.24 AB c |
| Brood provision | 0.83 ± 0.15 C b | 0.64 ± 0.11 B b | 0.96 ± 0.03 A b | 0.88 ± 0.01 A b |
| Soil | 0.99 ± 0.00 A a | 0.99 ± 0.00 AB a | 0.99 ± 0.01 A a | 0.96 ± 0.02 B a |
| Plot | Sample 1 | Sample 2 | Mantel Statistics R * | p-Value |
|---|---|---|---|---|
| SD | Gut | Brood provision | −0.01818 | 0.49167 |
| Gut | Soil | −0.5273 | 0.96667 | |
| Brood provision | Soil | 0.5515 | 0.14167 | |
| CQ | Gut | Brood provision | 0.3939 | 0.1 |
| Gut | Soil | 0.2606 | 0.21667 | |
| Brood provision | Soil | 0.6485 | 0.05 | |
| LN | Gut | Brood provision | 0.1879 | 0.31667 |
| Gut | Soil | −0.4545 | 0.96667 | |
| Brood provision | Soil | −0.05455 | 0.55833 | |
| XJ | Gut | Brood provision | −0.2606 | 0.79167 |
| Gut | Soil | 0.0303 | 0.5 | |
| Brood provision | Soil | −0.2242 | 0.70833 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Li, Y.; Lu, H.; Hao, Y.; Zhang, K.; Dang, X.; Fan, X.; Zhang, H.; Zhou, Z.; Zhu, C.; et al. Diversity of Bacterial Communities Associated with Solitary Bee Osmia excavata Alfken (Hymenoptera: Megachilidae). Appl. Sci. 2023, 13, 1524. https://doi.org/10.3390/app13031524
Liu W, Li Y, Lu H, Hao Y, Zhang K, Dang X, Fan X, Zhang H, Zhou Z, Zhu C, et al. Diversity of Bacterial Communities Associated with Solitary Bee Osmia excavata Alfken (Hymenoptera: Megachilidae). Applied Sciences. 2023; 13(3):1524. https://doi.org/10.3390/app13031524
Chicago/Turabian StyleLiu, Wenping, Yue Li, Huanhuan Lu, Youjin Hao, Ke Zhang, Xiaoqun Dang, Xiaodong Fan, Huan Zhang, Zeyang Zhou, Chaodong Zhu, and et al. 2023. "Diversity of Bacterial Communities Associated with Solitary Bee Osmia excavata Alfken (Hymenoptera: Megachilidae)" Applied Sciences 13, no. 3: 1524. https://doi.org/10.3390/app13031524
APA StyleLiu, W., Li, Y., Lu, H., Hao, Y., Zhang, K., Dang, X., Fan, X., Zhang, H., Zhou, Z., Zhu, C., Luo, A., & Huang, D. (2023). Diversity of Bacterial Communities Associated with Solitary Bee Osmia excavata Alfken (Hymenoptera: Megachilidae). Applied Sciences, 13(3), 1524. https://doi.org/10.3390/app13031524







