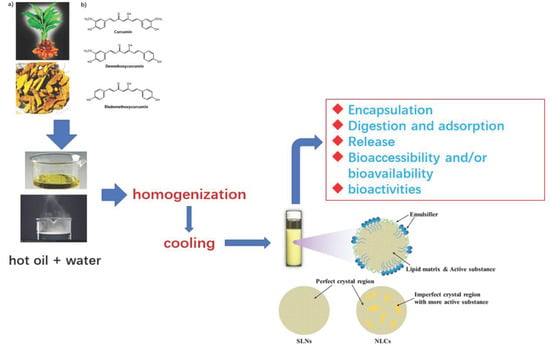
Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as Food-Grade Nanovehicles for Hydrophobic Nutraceuticals or Bioactives
Abstract
:1. Introduction
2. A General Summary of Food Biocompatible SLNs and NLCs: Composition and Preparation Methods
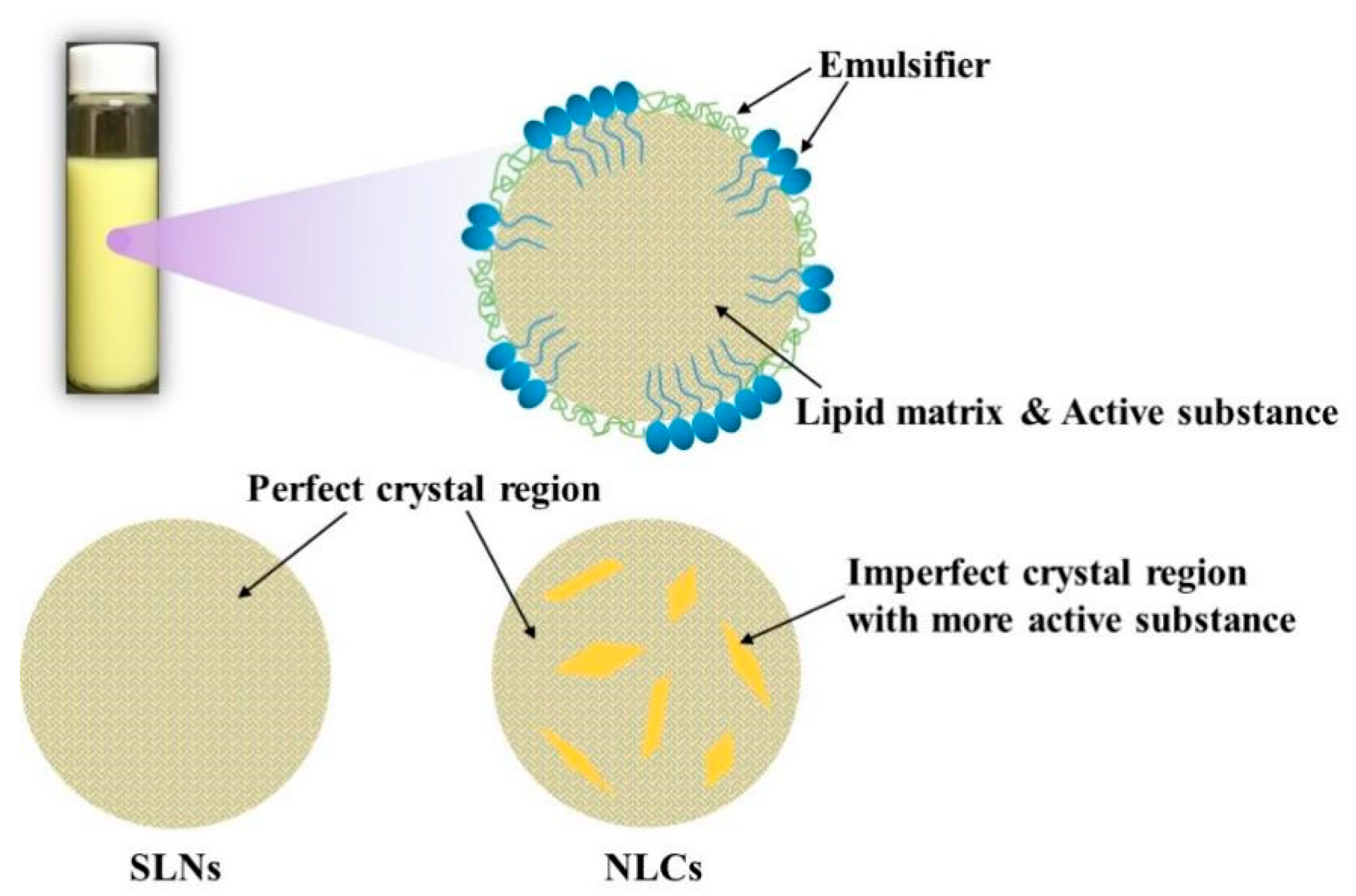
2.1. Structural Composition
- (i).
- the solubility of bioactive compounds in the lipid phase, and the efficiency of their incorporation;
- (ii).
- oxidative stability of the lipid phase, and storage stability of crystallized lipids in the nanoparticles;
- (iii).
- use of food biocompatible lipid components, from the viewpoint of acceptable toxicological evaluation.
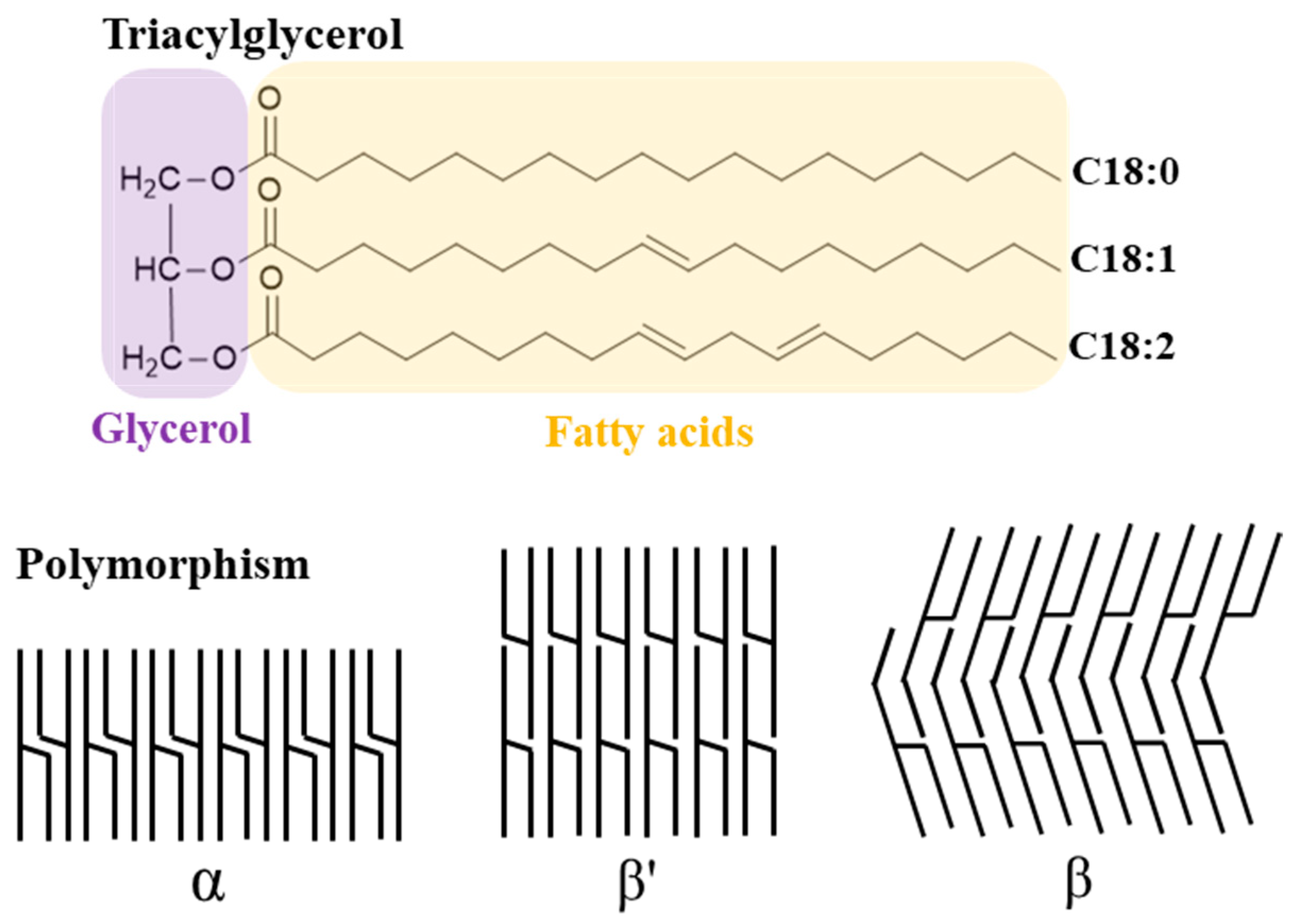
2.1.1. Lipids
2.1.2. Emulsifiers and Co-Emulsifiers
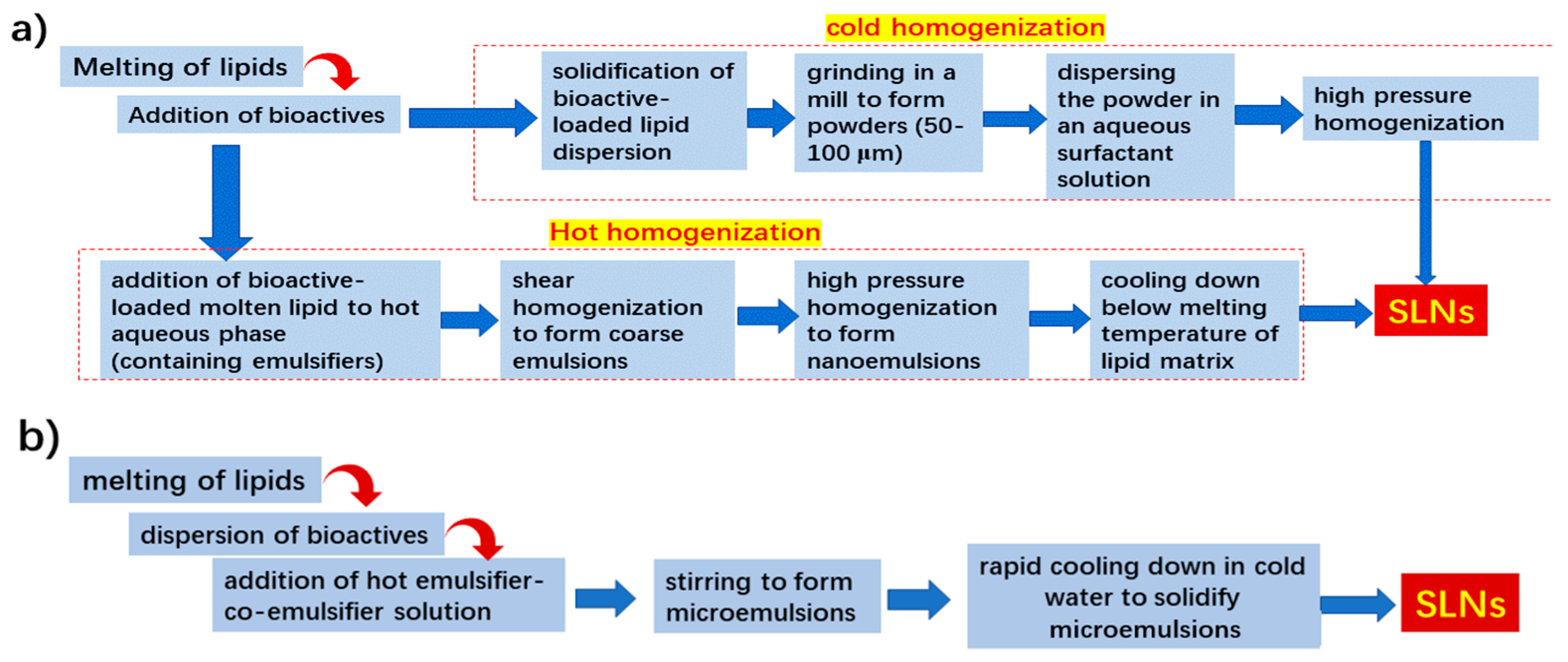
2.1.3. Preparation Methods
3. Some Key Issues on the Use of SLNs and NLCs as Oral Nanovehicles for Nutraceuticals or Bioactives
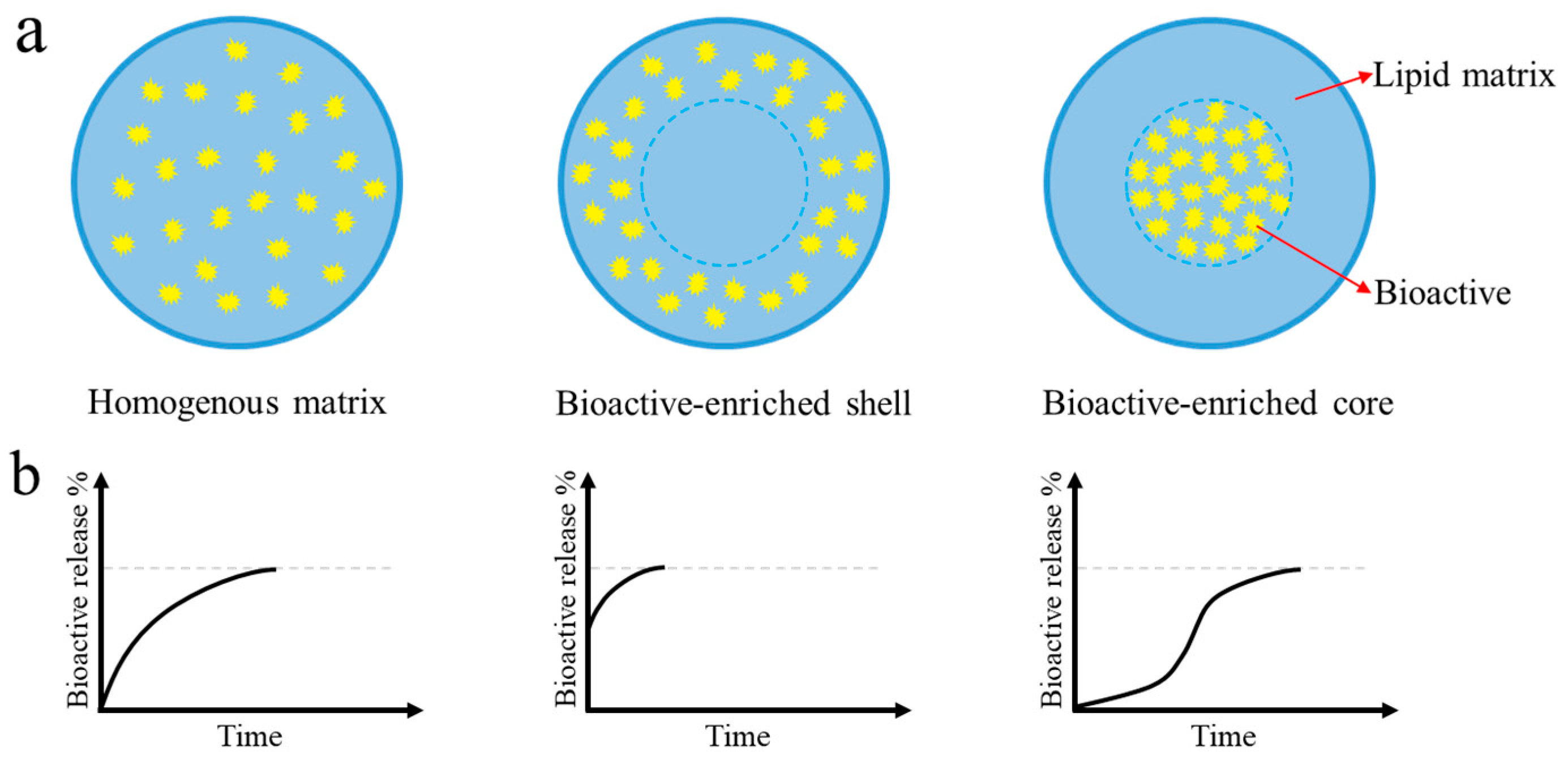
3.1. Incorporation and Release of Bioactives
3.2. Oxidative Stability
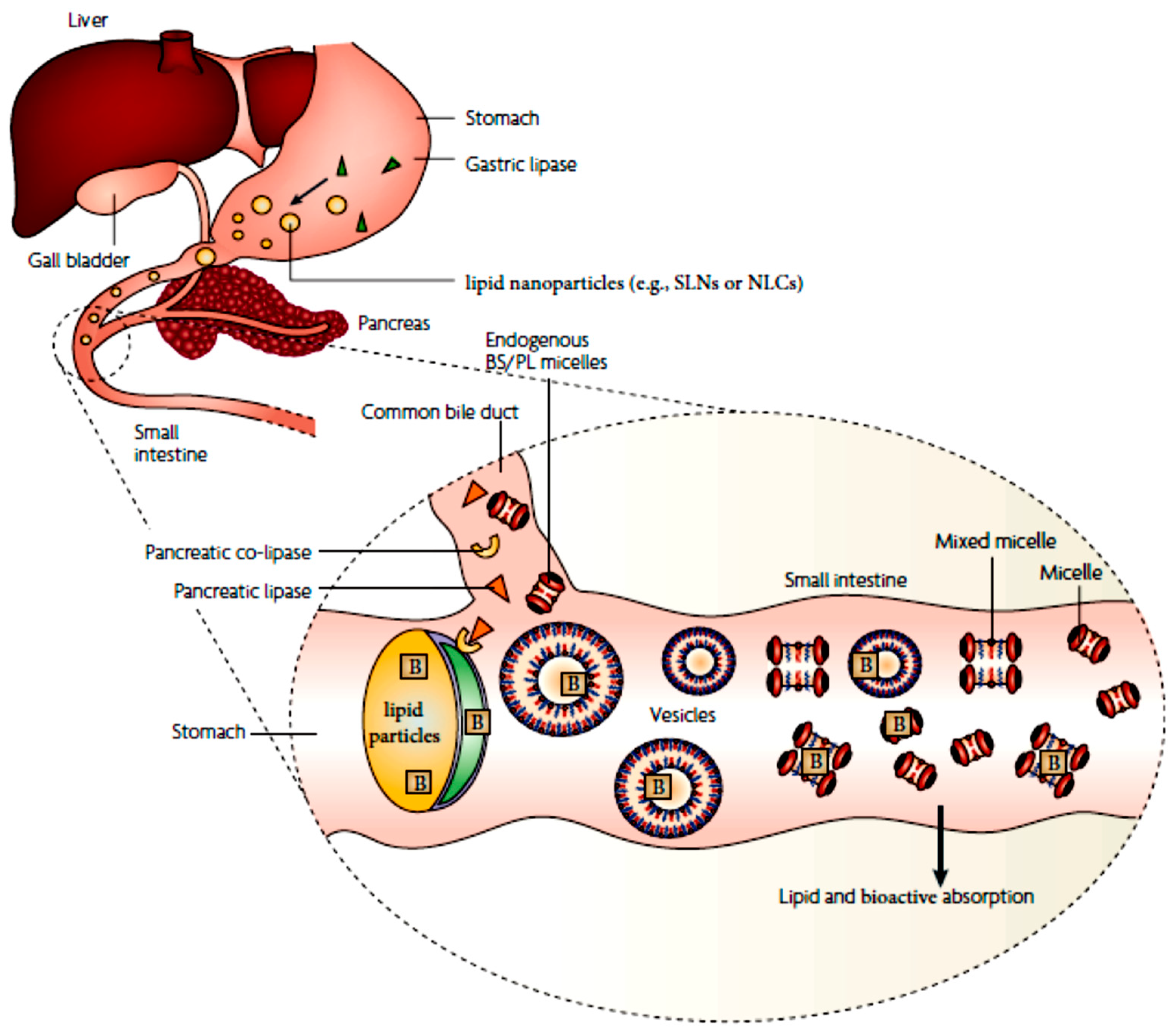
3.3. Lipid Digestion and Bioactive Solubilization
3.4. Bioactive Absorption and Intestinal Transport
4. Utilization of SLNs and NLCs as Nanovehicles for Encapsulation and Delivery of Hydrophobic Nutraceuticals or Bioactive Ingredients
| Liposoluble Nutraceuticals | Type of Lipid Nanoparticles a | Formulation Ingredients b | Processing Technique c | EE/DL d | Main Results | References |
|---|---|---|---|---|---|---|
| Carotenoids | ||||||
| β-carotene (10%; in sunflower oil) | NLCs | Propylene glycol monostearate; sunflower oil; Tween 80 | Hot HPH | -; 0.35% | The dispersions of β-carotene NLCs were stable at 4–8 °C after 30 weeks; the recrystallization of solid lipids occurred completely; the oxidative degradation of encapsulated β-carotene was enhanced by the dilution in water. | [49] |
| β-carotene | SLNs | Tripalmitin; lecithin or Tween 60 or 80 | Hot HPH | -; 0.1% | The crystallization of solid lipids was affected by the type of surfactants; the use of high melting surfactant (lecithin) in the SLNs provided a better protection against chemical degradation of encapsulated β-carotene. | [50] |
| β-carotene | NLCs | Palmitic acid; corn oil; Tween 20 | Solvent diffusion | 25–91%; - | The optimization of the formulation of β-carotene-NLCs was performed, with the aim to achieve the small particle size and high β-carotene retention; the NLCs were spherical in morphology, | [63] |
| β-carotene | NLCs | Milk fat; Tween 80 | Hot shear homogenization + phase-inversion-temperature | -;- | A kind of transparent β-carotene-NLCs dispersions were successfully fabricated; the NLCs showed stability against dilution, storage, and chemical degradation. | [64] |
| β-carotene | SLNs | Hydrogenated palm oil, cocoa butter; Tween 20 | Hot HPH | -; ~0.5% | The SLNs had better stability to droplet aggregation but lower β-carotene oxidation than liquid lipid nanoparticles; the impaired stability was attributed to the β-carotene exclusion from the crystallized lipids. | [51] |
| β-carotene | SLNs, NLCs | Eicosane, glyceryl trioctanoate (liquid); high-melting lecithin | Hot shear homogenization + ultrasonication | 16–100%; - | The exclusion of β-carotene occurred in the SLNs and NLCs, with the extent of exclusion decreasing with increasing the liquid lipid ratio; the oxidative stability of encapsulated β-carotene was much higher at 50–70% liquid lipid contents than that at 10–30% liquid lipid contents. | [46] |
| β-carotene | SLNs | Glyceryl stearate or hydrogenated palm oil (HPO); Tween 80 | Hot HPH | -;- | The solid lipid type affects the in vitro digestibility and β-carotene bioaccessiblity of SLNs; there was no close relationship between the digestibility and β-carotene bioaccessibiity. | [65] |
| lutein (20%; in corn oil) | NLCs | Glyceryl palmitostearate; Tween 80 and others | Hot ultrasonication | ~85%; 0.05–3.0% | The size of as-obtained NLCs depended on the treatment duration and lutein loading; the NLCs had a spherical morphology with an imperfect crystalline lattice structure, and showed a sustained-release delivery. | [66] |
| lutein (20%; in corn oil) | NLCs | cetyl palmitate, glyceryl tripalmitate, or wax; MCT; caprylyl/capryl glycoside | Hot HPH | 89–100%; ~2.0% | The nanoencapsulation in the NLCs greatly protected the UV-induced degradation of lutein; the encapsulated lutein released in a biphasic manner. | [67] |
| lutein (20%; in corn oil) | NLCs | Glycerol stearate, wax; fish oil; Tween 80+lecithin/poloxamer 407 | Hot shear homogenization | 50–88.5%; about 0.4–1.2% | The as-fabricated NLCs exhibited a high blocking effect against oxygen free radicals, and a good in vitro sustain release behavior. | [59] |
| lycopene | NLCs | orange wax; rice oil; sodium stearoyl glutamate | Hot HPH | 100%; 0.1–1.0 % | The fabricated lycopene-loaded NLCs showed a biphasic release profile, and exhibited an excellent colloidal stability upon storage of 120 days; the stability of lycopene was enhanced. | [68] |
| lycopene | SLNs | Glyceryl palmitostearate or glyceryl behenate; Tween 80 + Poloxamer 407 | Hot shear homogenization | 87–98%; 4.5–5.2 % | The size of SLNs was dependent on the type of applied solid lipids; the dispersions containing the lycopene-SLNs exhibited a good storage stability at 4 °C. | [69] |
| lycopene | NLCs, SLNs | GDS and GMS; MCT oil; lecithin + Tween 80 | Hot shear homogenization + ultrasonication | 65–79%; 4.54–5.52% | The EE of NLCs was significantly higher than SLNs; the nanoencapsulation improved the solubility of the bioactive in aqueous drinks. | [70] |
| astaxanthin | SLNs | Stearic acid; lecithin + poloxamer | Double emulsion solvent displacement | -; 6.11% (max) | The astaxanthin-SLN exhibited a sustained release behavior at pH 7.4; the intranasal administration of the SLN achieved higher biodistribution in the brain (than the intravenous route); it had an antioxidant potential against oxidative stress in neurological disorders. | [71] |
| Astaxanthin (oleoresin; 40 wt%) | NLCs | Glyceryl behenate; oleic acid; Tween 80 + lecithin | Hot shear homogenization + ultrasonication | ~90%; 1.8% | NLCs containing astaxanthin have a potential to be used in beverage formulations. | [72] |
| Astaxanthin | NLCs | Glyceryl palmitostearate; sunflower oil; Poloxamer | Hot homogenization | ~90%; ~1.2% | The NLCs formulation enhanced the antioxidant capacity of astaxanthin. | [73] |
| bixin | Polymer-coated SLNs | Capric/caprylic triglycerides; sorbitan monostearate; Tween 80 | Spontaneous emulsification | 100%; - | Lipid core nanocapsules with high EE (100%) of bixin and good physical stability were fabricated; the nanoencapsulation increased the stability of bixin against photosensitization and oxidation. | [74,75] |
| bixin | SLNs | Trimyristin or glycerol monostearate; lecithin + poloxamer 188 | Hot shear homogenization + ultrasonication | >99%; 6~18% | The release of bixin from the SLNs at pH 7.7 was of non-Fick diffusion; the oral administration of the bixin-SLNs resulted in enhanced in vivo hepatoprotection in rats. | [76] |
| Liposoluble vitamins | ||||||
| vitamin A (retinol) | SLNs | Glyceryl behenate; Tween 80 | Hot HPH | ~100%; 3.3% | The encapsulated retinol in SLNs displayed a controlled release behavior; the increased retinol release was correlated with polymorphic transitions of the lipids. | [77] |
| vitamin D2 | SLNs | Tripalmitin; Tween 20 | Hot HPH | -; 15% (max) | Vitamin D-SLNs with a high LC (up to 15%) were successfully fabricated; increasing the vitamin proportion led to a progressive decrease in particle size of SLNs; the turbidity of the SLN dispersions reduced with increasing the loading. | [78] |
| vitamin D3 | SLNs | Stearic acid + beewax; SDS | Solvent emulsification/evaporation | 43–78%; 19–350% | SLNs with extraordinary LC of vitamin D were successfully fabricated; increasing beewax ratio in the lipid matrix improved the encapsulation and release performance of as-obtained SLNs as nanovehicles for vitamin D; the SLNs were not cytotoxic and immunocompatible. | [79] |
| vitamin D3 | NLCs | GMS; oleic acid; Tween 80 | Hot HPH | 68–86%; - | NLCs as nanovehicles for vitamin D were fabricated with widely available ingredients; the encapsulated vitamin D exhibited high stability upon storage or digestion and good controlled release behavior. | [80] |
| vitamin D3 | NLCs | Glyceryldistearate or glyceryldibehenate; caprylic/caprictriglycerides or octyloctanoat; Tween 80, Tween 20 or poloxamer | Hot shear homogenization | -; - | The formulation of NLCs as nanovehicles for vitamin D was optimized; the incorporation of the vitamin D into NLCs increased its oral absorption. | [81] |
| vitamin D3 | NLCs | GMS or polyglycerol polyricinoleate (PGPR); MCT or vegetable oils; poloxamer | Emulsification/evaporation | 85–92%; - | The NLCs with PGPR as the solid lipid exhibited higher liquid dispersion stability than those with GMS. | [82] |
| γ-tocotrienol | SLNs | Glyceryl behenate; poloxamer 188 | Hot shear homogenization + ultrasonication | -; 0.2% | The fabricated SLNs had much higher permeability and cell uptake than mixed micelles; the encapsulated γ-tocotrienol in the SLNs had threefold higher in vivo oral bioavailability. | [83] |
| α-tocotrienol | SLNs | Glyceryl behenate; lecithin + poloxamer | Hot HPH | 58.5~82%; - | The formulation of the SLNs was optimized; the preparation at the optimal conditions was stable after 21 days of storage at 6 °C; the solid lipids in the SLNs were mainly present in the α and β′ polymorphic forms. | [84] |
| α-tocotrienol | NLCs | Tristearin, MCT; poloxamer 188 | Hot shear homogenization + ultrasonication | 82.6%; 4.13% | The loading of the bioactive in the NLCs exhibited a lower toxicity on human cultured cells. | [85] |
| ω-3 fatty acids | ||||||
| ω-3 fatty acid-rich fish oil | SLNs, NLCs | Tripalmitin, fish oil; Tween 20 | Hot HPH | -; - | Increasing the fish oil in the lipid matrix (>10%) increased the stability of SLNs to aggregation, as well as the rate of α- to β-polymorphic transitions of the solid lipid. | [86] |
| Docosahexaenoic acid (DHA) and α-linolenic acid (ALA) | SLNs | Tripalmitin, tristearin, triolein; Tween 80 | Hot homogenization + microfluidization | -; - | The thermal behavior of the lipid matrix of SLNs could be modulated by changing the lipid components; the incorporation in the SLNs improved the oxidative stability and shelf life of DHA or ALA. | [87] |
| ω-3 fatty acid-rich fish oil | SLNs, NLCs | Tristearin; low-(LM) or high-melting (HM) lecithins, taurodeoxycholate | Hot shear homogenization + microfluidization | -; - | The use of HM lecithin led to significant inhibition of oxidation of ω-3 fatty acids in the NLCs, as a result of the formation of solidified surfactant shell layer. | [30] |
| ω-3 fatty acid-rich krill oil | NLCs | Palm stearin; lecithin | Hot shear homogenization + ultrasonication | >96%; - | NLCs with small sizes and high loading efficiency were successfully fabricated using krill oil as the liquid lipid; the NLCs provided a good protection to the encapsulated bioactives against UV, and showed good physical and chemical stabilities upon long-term storage. | [88] |
| Linseed oil + quercetin | NLCs | GMS; linseed oil (liquid lipid); Tween 80 | Hot HPH | -; - | Both quercetin and linseed oil were successfully co-loaded in NLCs with better lipid oxidation or storage stability; the addition of linseed oil increased the antioxidant capacities of quercetin. | [89] |
| Conjugated linoleic acid | NLCs | Cocoa butter; conjugated linoleic acid; Poloxamer | Hot high-shear homogenization | -; 98.2% | The formation of NLCs greatly protected conjugated linoleic acid against oxidation and heating. | [90] |
| Essential oils | ||||||
| citral | NLCs | Glyceryl palmitostearate; MCT; poloxamer | Hot shear homogenization | 99.8%; 12.5% | The encapsulation in NLCs provided prolonged preservative effect and storage stability. | [91] |
| citral | SLNs | GMS; Tween 80 + Span 80 | Hot HPH | 48–73%; 22.8% | The incorporation of citral in the SLNs reduced the ordered crystallinity of GMS; the nanoencapsulation significantly improved the retention of citral upon 12 days of storage. | [92] |
| eugenol | NLCs | Tristearin, MCT; poloxamer 188 | Hot shear homogenization + ultrasonication | 81.4%; 3.92% | Better antimicrobial activity and a lower toxicity on human cultured cells were demonstrated for encapsulated eugenol in the NLCs | [85] |
| carvacrol | SLNs | Propylene glycol monopalmitate, glyceryl monostearate; Tween 80 | Microemulsion template method | >98%; up to 30% | Carvacrol was homogenously distributed within the SLNs; the encapsulated carvacrol exhibited more effective anti-microbial activites. | [93] |
| carvacrol | SLNs | Beeswax; Tween 80 + lecithin | Hot shear homogenization + ultrasonication | 88.5%; - | Both carvacrol and astaxanthin were successfully co-loaded in the SLNs with high EE; encapsulated bioactives were stable under oxidative, acidic and alkaline conditions, and showed better anti-microbial activities. | [94] |
| Frankincense and myrrh essential oil | SLNs | Glyceryl dibehenate/behenate; lecithin + Tween 80 | Hot HPH | 80.6%; ~53.7% | The encapsulation of the oil decreased the ordered crystallization of solid lipids in the SLNs, and significantly improved its antitumor efficacy in mice. | [95] |
| Other liposoluble bioactives | ||||||
| CoQ10 | SLNs | Cetyl palmitate; Tego Care 450 | Hot HPH | -; 2.4% | The majority of CoQ10 was homogenously mixed with the solid lipid matrix, while the others formed separate domains. | [47] |
4.1. Liposoluble Nutraceuticals
4.1.1. Carotenoids
4.1.2. Fat-Soluble Vitamins
4.1.3. Omega-3 Polyunsaturated Fatty Acids (ω-3 PUFAs)
4.1.4. Essential Oils
4.2. Poorly Soluble Bioactive Compounds
4.2.1. Curcuminoids (and Curcumin in Particular)
| Curcuminoids | Type of Lipid Nanoparticles a | Formulation Ingredients (Lipids and Emulsifiers) b | Processing Technique c | Mode of Drug Incorporation d | EE/DL e | Main Results | References |
|---|---|---|---|---|---|---|---|
| curcumin | SLNs | Compritol®888 ATO; Tween 80 + soy lecithin | Microemulsion template method | Dissolution (in hot emulsifier mix) | 82%; 10% | The in vitro release of encapsulated curcumin followed the diffusion pattern; the encapsulated curcumin in the SLNs exhibited good long-term stability, and high bioavailability (32–155 times as free curcumin) in rats. | [130] |
| curcuminoids | NLCs | MCT, refined castor oil, soybean oil, TM or TS; polomaxer | Hot HPH | Dissolution | ~97%; max. 0.1% | The encapsulated curcuminoids exhibited good long-term storage stability; their release from the nanoparticles was dependent on the applied medium and physical state of the lipid carrier, which was much more affected by the degradation of the lipid matrix. | [52] |
| curcumin | SLNS | Trimyristin; different emulsifiers | Hot HPH | dissolution | ~66%; ~0.05 wt% | The encapsulation of curcumin in SLNs showed enhanced delivery (compared to unencapsulated or emulsified curcumin); the transport route of the SLNs was simple diffusion; most of encapsulated curcumin was absorbed and metabolized by the cells. | [122] |
| Curcumin (+ genistein) | NLCs | Oleic acid (liquid), GMS (solid); Tween 80 | Hot shear homogenization + ultrasonication | Dissolution | >75%; 1.2% (alone) or 0.7% (in combination) | The co-loading with genistein increased the loading efficiency, and the inhibition against prostate cancer cells. | [131] |
| curcumin | SLNs | Compritol®888 ATO; sodium caseinate (NaCas) | Solvent-diffusion+ hot shear homogenization + ultrasonication | Mixing (in ethanol) | 40–80%; - | Novel SLNs with biopolymeric double layer coating (using NaCas and pectin) were successfully fabricated as nanovehicles for curcumin; the cross-linking of the layer coating improved the EE, DL, stability and release behavior, as well as the antioxidant activity of encapsulated curcumin in aqueous phase; the cross-linking further facilitated the spray drying of SLNs to form homogenous powders. | [129] |
| curcumin | SLNs | Glycerol stearate, propylene glycol esters of fatty acids, palmitic acid; Tween 80 | Hot shear homogenization + ultrasonication | dissolution | 100%; 10% | The oral bioavailability of encapsulated curcumin in the SLNs was significantly improved by the coating with chitosan. | [124] |
| curcumin | SLNs, NLCs | Compritol®888 ATO (solid), oleic acid (liquid); sodium caseinate (NaCas) + Tween 80 (emulsifiers) | Solvent-diffusion + hot ultrasonication | Mixing (in an acetone and ethanol mixture) | 33–66%; max. 4.95% | SLNs and NLCs with high loading capacity and exceptional gastrointestinal stability were fabricated using NaCas (together with a minimal concentration of Tween 80) as the emulsifier and pectin as the coating, especially when the layer coating was crosslinked. | [132] |
| curcumin | NLCs | MCT (liquid), Compritol®888 ATO (solid); Tween 80 | Hot HPH | Mixing (in acetone) | -; - | The release of encapsulated curcumin from the NLCs was consistent with the release of free fatty acids, which could be modulated by altering the lipid type and composition, and the use of lipase inhibitors. | [54] |
| curcumin | SLNs | Stearic acid; NaCas (emulsifier) | Solvent-diffusion + hot ultrasonication | Mixing (in ethanol) or dissolution | -; - | The influence of loading processes on the efficacy of encapsulation of curcumin in the SLNs was investigated; the strategy of adding curcumin into deprotonated NaCas followed by addition of melted lipid and pectin at pH 12 was more effective in fabrication of uniform and small SLNs (with gastrointestinal-stable), than that of introducing curcumin in ethanol. | [35] |
| Turmeric powder | NLCs | MCT (liquid), GMS (solid); Tween 80 | Hot shear homogenization +ultrasonication | dissolution | 78–93.3%; 40–46.6% | More than 95% of the encapsulated curcuminoids were mainly released during the simulated intestinal digestion; and their bioaccessibility was around 75% (4-fold increase compared to that of free turmeric). | [126] |
| Turmeric extract | NLCs | MCT (liquid), Campritol (solid); poloxamer (emulsifier) | Hot shear homogenization | Dissolution | ~99%; - | The encapsulated turmeric extract in NLCs exhibited good physical stability, higher antioxidant and antimicrobial activities than the free extract. | [125] |
| curcumin | SLNs | Chinese white wax; Tween 20 + lecithin (emulsifiers) | Hot shear homogenization + ultrasonication | dissolution | Max. 84.6%; 10% | Wax SLNs were confirmed to be effective carriers for loading curcumin; the as-fabricated SLNs exhibited a sustained drug release behavior, and better inhibition of the biofilm formation (than free curcumin). | [123] |
| curcumin | SLNs | Tristearin; PEG-modified stearyl ether | Hot shear homogenization +ultrasonication | Mixing (in ethanol) | 91–95%; max 5% | The lipolysis of SLNs was modulated by altering types and concentrations of emulsifiers; high bioaccessibility (>91%) and fast epithelium permeation of encapsulated curcumin in the SLNs were confirmed, resulting in a > 12-fold increase in bioavailability (in rats). | [127] |
| curcumin | NLCs | MCT (liquid), GTS (solid); denatured ovalbumin | Hot shear homogenization +ultrasonication | Dissolution | -; max. 1.0% | It was demonstrated that the oil composition of NLCs produced an influence on transformation, bioaccessibility and intestinal absorption of encapsulated curcumin; NLC containing 20% MCT in the lipid matrix exhibited highest curcumin bioavailability. | [55] |
| curcumin | SLNs | Compritol®888 ATO + GMS; tween 80 + phospholipon 90G | Hot HPH | Mixing (in PEG 600) | ~80%; 15% (w/v) | A SLN with high LC (15%) of curcumin was successfully fabricated; the nanoencapsulation enhanced the stability (against photo and chemical degradation) and bioavailability (~70 times higher than free curcumin) of curcumin. | [128] |
| curcumin | SLNs | Propylene glycol monopalmitate, GMS; NaCas-lactose covalent conjugate (emulsifier) | Hot shear homogenization + ultrasonication | Dissolution | >90%; - | The SLNs using NaCas-lactose conjugate as the emulsifier exhibited much better physicochemical properties than those using NaCas alone; the nanoencapsulation improved the antioxidant activity and storage stability of curcumin. | [133] |
| curcumin | SLNs | Compritol 888 ATO; Tween 80 | Modified microemulsion method | Dissolution | 100%; 1.8% | The curcumin in the SLNs exhibited higher antimicrobial effect against Staphylococcus aureaus and Escherichia coli. | [121] |
| curcumin | SLNs | Murumuru butter; a mixture of Span 20 and Tween 80 | phase-inversion temperature method | dissolution | 98.9%; 1.0% | The curcumin loaded in SLNs was more toxic to colon adenocarcinoma cells. | [132] |
4.2.2. Polyphenols
4.2.3. Phytosterols and Phytostanols
5. Strategies to Improve the Performance of SLNs and NLCs as Nanovehicles for Nutraceuticals
5.1. Improving Oral Bioavailability of Loaded Nutraceuticals in SLNs (or NLCs) by Coating with Chitosan or Its Derivatives
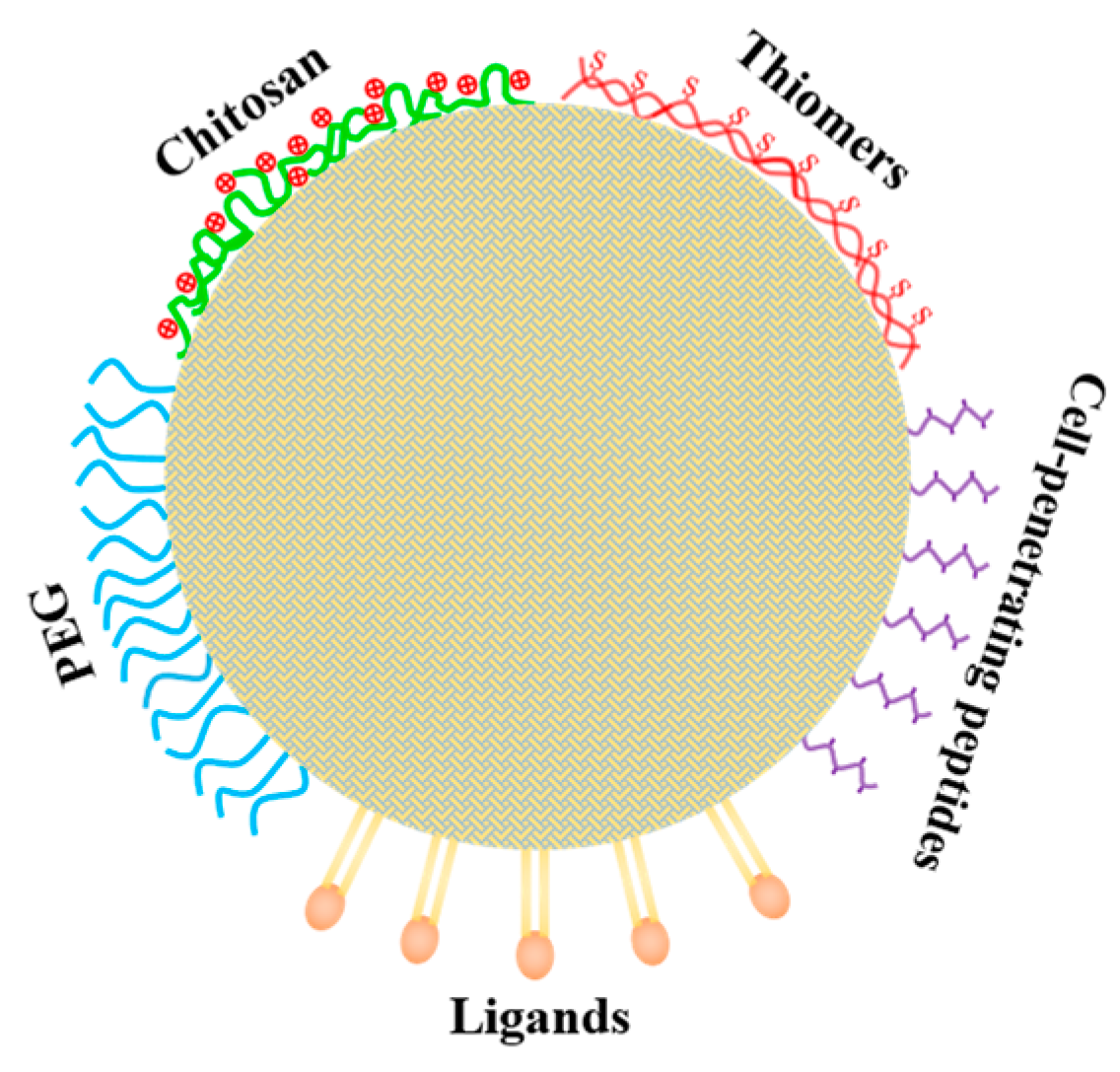
5.2. Improving the Transport across Intestinal Barrier and Target-Delivery of Loaded Nutraceuticals by Surface Modifications with Functional Molecules
6. Concluding Remarks and Future Research Prospects
- (i).
- elucidating the potential and effectiveness of SLNs and/or NLCs as nanovehicles for nutraceuticals with specific properties, e.g., heat-labile or lipid-insoluble bioactives;
- (ii).
- characterizing the stability and absorption of SLNs and/or NLCs during in vitro simulated digestion and unravelling the relationships between the bioaccessibility and bioavailability of encapsulated nutraceuticals;
- (iii).
- developing surfactant-free, food-grade SLNs (or NLCs) as oral nanovehicles for the delivery of nutraceuticals or bioactives;
- (iv).
- elucidating the health and function-related effectiveness of encapsulated nutraceuticals, e.g., using in vitro cell models or in vivo animal models;
- (v).
- determining the suitability of incorporating SLNs and NLCs into different types of food formulations, e.g., beverages and milks, and investigating effective drying techniques for obtaining powdered products.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bazana, M.T.; Codevilla, C.F.L.; de Menezes, C.R. Nanoencapsulation of bioactive compounds: Challenges and perspectives. Curr. Opin. Food Sci. 2019, 26, 47–56. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation techniques for food bioactive components: A review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Shende, P.; Mallick, C. Nanonutraceuticals: A way towards modern therapeutics in healthcare. J. Drug Deliv. Sci. Technol. 2020, 58, 101838. [Google Scholar] [CrossRef]
- Yao, M.; McClements, D.J.; Xiao, H. Improving oral bioavailability of nutraceuticals by engineered nanoparticle-based delivery systems. Curr. Opin. Food Sci. 2015, 2, 14–19. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso, L.; Viegas, C.; Ferreira-Pégo, C.; Costa, J.; Fonte, P. Lipid-based carriers for food ingredients delivery. J. Food Eng. 2021, 295, 110451. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control. Release 2019, 298, 38–67. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtk, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. 1), S131–S155. [Google Scholar] [CrossRef]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mat. Sci. Eng. C 2016, 68, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Randhawa, J.K. High melting lipid based approach for drug delivery: Solid lipid nanoparticles. Mat. Sci. Eng. C 2013, 33, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Bansal, K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceuticals 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Salah, E.; Abouelfetouh, M.M.; Pan, Y.; Chen, D.; Xie, S. Solid lipid nanoparticles for enhanced oral absorption: A review. Colloids Surf B 2020, 196, 111305. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef] [PubMed]
- Aditya, N.P.; Ko, S. Solid lipid nanoparticles (SLNs): Delivery vehicles for food bioactives. RSC Adv. 2015, 5, 30902–30911. [Google Scholar] [CrossRef]
- de Silva Santos, V.; Ribeiro, A.P.B.; Santana, M.H.A. Solid lipid nanoparticles as carriers for lipophilic compounds for applications in foods. Food Res. Int. 2019, 122, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Decker, E.A.; McClements, D.J.; Kristbergsson, K.; Helgason, T.; Adwad, T. Solid lipid nanoparticles as delivery systems for bioactive food components. Food Biophy. 2008, 3, 146–154. [Google Scholar] [CrossRef]
- Yousefi, M.; Ehsani, A.; Jafari, S.M. Lipid-based nano delivery of antimicrobials to control food-borne bacteria. Adv. Colloid Interface Sci. 2019, 270, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Edible lipid nanoparticles: Digestion, absorption, and potential toxicity. Progress Lipid Res. 2013, 52, 409–423. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discovery 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Katopodi, A.; Detsi, A. Solid lipid nanoparticles and nanostructured lipid carriers of natural products as promising systems for their bioactivity enhancement: The case of essential oils and flavonoids. Colloids Surf. A 2021, 630, 127529. [Google Scholar] [CrossRef]
- Azar, F.A.N.; Pezeshki, A.; Ghanbarzadeh, B.; Hamishehkar, H.; Mohammadi, M. Nanostructured lipid carriers: Promising delivery systems for encapsulation of food ingredients. J. Agric. Food Res. 2020, 2, 100084. [Google Scholar] [CrossRef]
- Gomaa, E.; Fathi, H.A.; Eissa, N.G.; Elsabahy, M. Methods for preparation of nanostructured lipid carriers. Methods 2022, 199, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bunjes, H.; Steiniger, F.; Richter, W. Visualizing the structure of triglyceride nanoparticle in different crystal modifications. Langmuir 2007, 23, 4005–4011. [Google Scholar] [CrossRef] [PubMed]
- Salminen, H.; Helgason, T.; Kristinsson, B.; Kristbergsson, K.; Weiss, J. Formation of solid shell nanoparticles with liquid ω-3 fatty acid core. Food Chem. 2013, 141, 2934–2943. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Rühl, D.; Runge, S.; Schulze-Forster, K.; Mehnert, W. Cytotoxicity of solid lipid nanoparticles as a function of the lipid matrix and the surfactant. Pharm. Res. 1997, 14, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xue, J.; Hu, Q.; Zhou, M.; Luo, Y. Preparation of lipid nanoparticles with high loading capacity and exceptional gastrointestinal stability for potential oral delivery applications. J. Colloid Interface Sci. 2017, 507, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, Q.; Zhou, M.; Xia, Y.; Nieh, M.P.; Luo, Y. Development of “all natural” layer-by-layer redispersible solid lipid nanoparticles by nano spray drying technology. Eur. J. Pharm. Biopharm. 2016, 107, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, Q.; Zhou, M.; Xue, J.; Luo, Y. Preparation of ultra-fine powders from polysaccharide-coated solid lipid nanoparticles and nanostructured lipid carriers by innovative nano spray drying technology. Int. J. Pharm. 2016, 511, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wang, T.; Hu, Q.; Zhou, M.; Luo, Y. Insight into natural biopolymer-emulsified solid lipid nanoparticles for encapsulation of curcumin: Effect of loading methods. Food Hydrocolloid 2018, 79, 110–116. [Google Scholar] [CrossRef]
- Gasco, M.R. Method for Producing Solid Lipid Microspheres Having a Narrow Size Distribution. U.S. Patent Application No. 5250236, 5 October 1993. [Google Scholar]
- Kakkar, V.; Kumar Muppu, S.; Chopra, K.; Pal Kaur, I. Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur. J. Pharm. Biopharm. 2013, 85, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Mishra, A.K.; Chuttani, K.; Pal Kaur, I. Proof of concept studies to confirm the delivery of curcumin loaded solid lipid nanoparticles (C-SLNs) to brain. Int. J. Pharm. 2013, 448, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Pal Kaur, I. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol. 2011, 49, 2906–2913. [Google Scholar] [CrossRef]
- Nayak, A.P.; Tiyaboonchai, W.; Patankar, S.; Madhusudhan, B.; Souto, E.B. Curcuminoids-loaded lipid nanoparticles: Novel approach towards malaria treatment. Colloids Surf. B 2010, 81, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.M.; Eldridge, D.S.; Palombo, E.S.; Harding, I.H. Stability mechanisms for microwave-produced solid lipid nanoparticles. Colloids Surf. A 2022, 643, 128774. [Google Scholar] [CrossRef]
- Manea, A.M.; Vasile, B.S.; Meghea, A. Antioxidant and antimicrobial activities of green tea extract loaded into nanostructured carriers. Comptes. Rendus. Chim. 2014, 17, 331–341. [Google Scholar] [CrossRef]
- Prombutara, P.; Kulwatthanasal, Y.; Supaka, N.; Sramala, I.; Chareonpornwattana, S. Production of nisin-loaded solid lipid nanoparticles for sustained antimicrobial activity. Food Control. 2012, 24, 184–190. [Google Scholar] [CrossRef]
- Salminen, H.; Helgason, T.; Aulbach, S.; Kristinsson, B.; Kristbergsson, K.; Weiss, J. Influence of co-surfactants on crystallization and stability of solid lipid nanoparticles. J. Colloid Interface Sci. 2014, 426, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Anantachaisilp, S.; Smith, S.M.; Treetong, A.; Pratontep, S.; Puttipitatkhachorn, S.; Ruktanonchai, U.R. Chemical and structural investigation of lipid nanoparticles: Drug-lipid interaction and molecular distribution. Nanotechnology 2010, 21, 125102. [Google Scholar] [CrossRef]
- Pan, Y.; Tikekar, R.V.; Nitin, N. Distribution of a model bioactive within solid lipid nanoparticles and nanostructured lipid carriers influences its loading efficiency and oxidative stability. Int. J. Pharm. 2016, 511, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Wissing, S.A.; Müller, R.H.; Manthei, L.; Mayer, C. Structural characterization of Q10-loaded solid lipid nanoparticles by NMR spectroscopy. Pharm. Res. 2004, 21, 400–405. [Google Scholar] [CrossRef]
- Radomska-Soukharev, A. Stability of lipid excipients in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 411–418. [Google Scholar] [CrossRef]
- Hentschel, A.; Gramdorf, S.; Müller, R.H.; Kurz, T. β-Carotene-loaded nanostructured lipid carriers. J. Food Sci. 2008, 73, N1–N6. [Google Scholar] [CrossRef]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; Decker, E.A.; McClements, D.J.; Weiss, J. Impact of surfactant properties on oxidative stability of β-carotene encapsulated within solid lipid nanoparticles. J. Agric. Food Chem. 2009, 57, 8033–8040. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Impact of lipid nanoparticle physical state on particle aggregation and β-carotene degradation: Potential limitations of solid lipid nanoparticles. Food Res. Int. 2013, 52, 342–349. [Google Scholar] [CrossRef]
- Noack, A.; Oidtmann, J.; Kutza, J.; Mäder, K. In vitro digestion of curcuminoid-loaded lipid nanoparticles. J. Nanoparticle Res. 2012, 14, 1113. [Google Scholar] [CrossRef]
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Nanotechnology for increased micronutrient bioavailability. Trend Food Sci. Technol. 2014, 40, 168–182. [Google Scholar] [CrossRef]
- Yang, T.S.; Liu, T.T.; Liu, H.I. Effects of aroma compounds and lipid composition on release of functional substances encapsulated in nanostructured lipid carriers lipolyzed by lipase. Food Hydrocolloid 2017, 62, 280–287. [Google Scholar] [CrossRef]
- Feng, J.; Huang, M.; Chai, Z.; Li, C.; Huang, W.; Cui, L.; Li, Y. The influence of oil composition on the transformation, bioaccessibility, and intestinal absorption of curcumin in nanostructured lipid carriers. Food Funct. 2020, 11, 5223–5239. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, N.L.; Charman, W.N.; Porter, C.J.H. Lipid-based delivery systems and intestinal lymphatic drug transport: A mechanistic update. Adv. Drug Deliv. Rev. 2008, 60, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Lucio, M.; Martins, S.; Lima, J.L.; Reis, S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar]
- Roger, E.; Lagarce, F.; Garcio, E.; Benoit, J.P. Lipid nanocarriers improve paclitaxel transport throughout human intestinal epithelial cells by using vesicle-mediated transcytosis. J. Control. Release 2009, 140, 174–181. [Google Scholar] [CrossRef]
- Lacatusa, I.; Mitrea, E.; Badea, N.; Stan, R.; Oprea, O.; Meghea, A. Lipid nanoparticles based on omega-3 fatty acids as effective carriers for lutein delivery. Preparation and in vitro characterization studies. J. Funct. Food 2013, 5, 1260–1269. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—From molecule to biological function. Angew. Chim. 2012, 51, 5308–5332. [Google Scholar] [CrossRef]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Controlled Release 2008, 127, 97–109. [Google Scholar] [CrossRef]
- Hejri, A.; Khosra, V.I.A.; Gharanjig, K.; Hejazi, M. Optimisation of the formulation of β-carotene loaded nanostructured lipid carriers prepared by solvent diffusion method. Food Chem. 2013, 141, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hayes, D.G.; Chen, G.; Zhong, Q. Transparent dispersions of milk-fat-based nanostructured lipid carriers for delivery of β-carotene. J. Agric. Food Chem. 2013, 61, 9435–9443. [Google Scholar] [CrossRef] [PubMed]
- de Abreu-Martins, H.H.; Artiga-Artigas, M.; Piccoli, R.H.; Martín-Belloso, O.; Salvia-Trujillo, L. The lipid type affects the in vitro digestibility and β-carotene bioaccessibility of liquid or solid lipid nanoparticles. Food Chem. 2020, 311, 126024. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Wu, C.T. Optimization of nanostructured lipid carriers for lutein delivery. Colloid Surf. A 2010, 353, 149–156. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, G.; Müller, R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability and performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef]
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Nazemiyeh, E.; Eskandani, M.; Sheikhloie, H.; Nazemiyeh, H. Formulation and physicochemical characterization of lycopene-loaded solid lipid nanoparticles. Adv. Pharm. Bull. 2016, 6, 235. [Google Scholar] [CrossRef] [Green Version]
- Zardini, A.A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. J. Food Sci. Technol. 2018, 55, 287–298. [Google Scholar] [CrossRef]
- Bhatt, P.C.; Srivastava, P.; Pandey, P.; Khan, W.; Panda, B.P. Nose to brain delivery of astaxanthin-loaded solid lipid nanoparticles: Fabrication, radio labeling, optimization and biological studies. RSC Adv. 2016, 6, 10001–10010. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Stability of astaxanthin-loaded nanostructured lipid carriers in beverage systems. J. Sci. Food Agric. 2018, 98, 511–518. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, V.; Salatti-Dorado, J.Á.; Barzegari, A.; Nicolas-Boluda, A.; Houaoui, A.; Caballo, C. Astaxanthin-loaded nanostructured lipid carriers for preservation of antioxidant activity. Molecules 2018, 23, 2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Sousa Lobato, K.B.; Paese, K.; Forgearini, J.C.; Guterres, S.S.; Jablonski, A.; de Oliveira Rios, A. Characterization and stability evaluation of bixin nanocapsules. Food Chem. 2013, 141, 3906–3912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Sousa Lobato, K.B.; Paese, K.; Forgearini, J.C.; Guterres, S.S.; Jablonski, A.; de Oliveira Rios, A. Evaluation of stability of bixin in nanocapsules in model systems of photosensitization and heating. LWT 2015, 60, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.P.; Manjunath, K.; Bhagawati, S.T.; Thippeswamy, B.S. Bixin loaded solid lipid nanoparticles for enhanced hepatoprotection—Preparation, characterisation and in vivo evaluation. Int. J. Pharm. 2014, 473, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Jenning, V.; Schäfer-Korting, M.; Gohla, S. Vitamin A-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Controlled Release 2000, 66, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; San Martin-Gonzalez, M.F. Characterization of ergocalciferol loaded solid lipid nanoparticles. J. Food Sci. 2012, 71, N8–N13. [Google Scholar] [CrossRef]
- Demirbilek, M.; Türkoglu, N.L.; Aktürk, S.; Akça, C. Vit D3-loaded solid lipid nanoparticles: Stability, cytotoxicity and cytokine levels. J. Microencapsul. 2017, 34, 454–462. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3. Food Chem. 2017, 225, 213–219. [Google Scholar] [CrossRef]
- Mohammadi, M.; Pezeshki, A.; Abbasi, M.M.; Ghanbarzadeh, B.; Hamishehkar, H. Vitamin D3-loaded nanostructured lipid carriers as a potential approach for fortifying food beverages; in vitro and in vivo evaluation. Adv. Pharm. Bull. 2017, 7, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, T.R.; Lee, I.; Chun, Y.G.; Park, D.J.; Lee, S.H.; Kim, B.K. Improved stability of polyglycerol polyricinoleate-substituted nanostructured lipid carrier cholecalciferol emulsions with different carrier oils. J. Food Sci. 2019, 84, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Abuasal, B.S.; Lucas, C.; Peyton, B.; Alayoubi, A.; Nazzal, S.; Sylvester, P.W.; Kaddoumi, A. Enhancement of intestinal permeability utilizing solid lipid nanoparticles increases γ-tocotrienol oral bioavailability. Lipids 2012, 47, 461–469. [Google Scholar] [CrossRef]
- de Carvalho, S.M.; Noronha, C.M.; Floriani, C.L.; Lino, R.C.; Rocha, G.; Bellettini, I.C.; Ogliari, P.J.; Barreto, P.L.M. Optimization of α-tocopherol loaded solid lipid nanoparticles by central composite design. Ind. Crops Products 2013, 49, 278–285. [Google Scholar] [CrossRef]
- Cortesi, R.; Valacchi, G.; Muresan, X.M.; Drechsler, M.; Contado, C.; Esposito, E.; Grandini, A.; Alessandra, G.; Forlani, G.; Sacchetti, G. Nanostructured lipid carriers (NLC) for the delivery of natural molecules with antimicrobial activity: Production, characterization and in vitro studies. J. Microencapsul. 2017, 34, 63–72. [Google Scholar] [CrossRef]
- Awad, T.; Helgason, T.; Weiss, J.; Decker, E.A.; McClements, D.J. Effect of omega-3 fatty acids on crystallization, polymorphic transformation and stability of tripalmitin solid lipid nanoparticle suspensions. Cry Growth Design 2009, 9, 3405–3411. [Google Scholar] [CrossRef]
- Holser, R. Encapsulation of polyunsaturated fatty acid esters with solid lipid particles. Lipid Insights 2012, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zhuang, P.; Luan, L.; Sun, Q.; Cao, F. Preparation and characterization of novel nanocarriers containing krill oil for food application. J. Funct. Foods 2015, 19, 902–912. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Li, T.; Xia, N.; Xia, Q. Nanostructured lipid carrier (NLC) as a strategy for encapsulation of quercetin and linseed oil: Preparation and in vitro characterization studies. J. Food Eng. 2017, 215, 1–12. [Google Scholar] [CrossRef]
- Hashemi, F.S.; Farzadnia, F.; Ahajani, A.; NovariAzar, F.A.; Pezeshki, A. Conjugated linoleic acid loaded nanostructured lipid carrier as a potential antioxidant nanocarrier for food applications. Food Sci. Nutr. 2020, 8, 4185–4195. [Google Scholar] [CrossRef]
- Mokarizadeh, M.; Kafil, H.S.; Ghanbarzadeh, S.; Alizadeh, A.; Hamishehkar, H. Improvement of citral antimicrobial activity by incorporation into nanostructured lipid carriers: A potential application in food stuffs as a natural preservative. Res. Pharm. Sci. 2017, 12, 409–415. [Google Scholar]
- Tian, H.; Lu, Z.; Li, D.; Hu, J. Preparation and characterization of citral-loaded solid lipid nanoparticles. Food Chem. 2018, 248, 78–85. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, S.; Sun, X.; Han, L.; Chang, C.; Zhang, W.; Zhong, Q. Carvacrol loaded solid lipid nanoparticles of propylene glycol monopalmitate and glyceryl monostearate: Preparation, characterization, and synergistic antimicrobial activity. Nanomaterials 2019, 9, 1162. [Google Scholar] [CrossRef]
- Shakeri, M.; Razavi, S.H.; Shakeri, S. Carvacrol and astaxanthin co-entrapment in beeswax solid lipid nanoparticles as an efficient nano-system with dual antioxidant and anti-biofilm activities. LWT 2019, 107, 280–290. [Google Scholar] [CrossRef]
- Shi, F.; Zhao, J.H.; Liu, Y.; Wang, Z.; Zhang, Y.T.; Feng, N.P. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012, 7, 2033–2043. [Google Scholar]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Brit. J. Pharm. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [Green Version]
- Seigio, S.A.R.; Russell, R.M. β-Carotene and other carotenoids as antioxidants. J. Am. College Nutr. 1999, 18, 426–433. [Google Scholar]
- Boonlao, N.; Ruktanonchai, U.R.; Anal, A.K. Enhancing bioaccessibility and bioavailability of carotenoids using emulsion-based delivery systems. Colloid Surf. B 2022, 209, 112211. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Rostamabadi, H.; Babazadeh, A.; Tarhan, ö.; Rashidinejad, A.; Boostani, S. Lycopene nanodevliery systems; recent advances. Trend Food Sci. Technol. 2022, 119, 378–399. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Wang, P.W.; Alalaiwe, A.; Lin, Z.C.; Fang, J.Y. Use of lipid nanocarriers to improve oral delivery of vitamins. Nutrients 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rejinold, N.S.; Kim, H.K.; Isakovic, A.F.; Gater, D.L.; Kim, Y.C. Therapeutic vitamin delivery: Chemical and physical methods with future directions. J. Controlled Release 2019, 298, 83–98. [Google Scholar] [CrossRef]
- Kiani, A.; Fathi, M.; Ghasemi, S.M. Production of novel vitamin D3 loaded lipid nanocapsules for milk fortification. Int. J. Food Prop. 2017, 20, 2466–2476. [Google Scholar] [CrossRef] [Green Version]
- Rabelo, R.S.; Oliveira, I.F.; da Silva, V.M.; Prata, A.S.; Hubinger, M.D. Chitosan coated nanostructured lipid carriers (NLCs) for loading vitamin D: A physical stability study. Int. J. Biol. Macromol. 2018, 119, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Mauya, V.K.; Bashir, K.; Aggarwal, M. Vitamin D microencapsulation and fortification: Trends and technologies. J. Steroid. Biochem. Mol. Biol. 2020, 196, 105489. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Aggarwal, M. Fabrication of nano-structured lipid carrier for encapsulation of vitamin D3 for fortification of ‘Lassi’; A milk based beverage. J. Steroid. Biochem. Mol. Biol. 2019, 193, 105429. [Google Scholar] [CrossRef] [PubMed]
- Zai, K.; Hirota, M.; Yamada, T.; Ishihara, N.; Mori, T.; Kishimura, A.; Suzuki, K.; Hase, K.; Katayama, Y. Therapeutic effect of vitamin D3-containing nanostructured lipid carriers on inflammatory bowel disease. J. Controlled Release 2018, 286, 94–102. [Google Scholar] [CrossRef]
- Mu, Y.; Li, J.; Kang, J.H.; Eto, H.; Zai, K.; Kishimura, A.; Hyodo, F.; Mori, T.; Katayama, Y. A lipid-based nanocarrier containing active vitamin D3 ameliorates NASH in mice via direct and intestine-mediated effects on liver inflammation. Biol. Pharm. Bull. 2020, 43, 1413–1420. [Google Scholar] [CrossRef]
- Sabzichi, M.; Mohammadian, J.; Mohammadi, M.; Jahanfar, F.; Pour, A.A.M.; Hamishehkar, H.; Ostad-Rahimi, A.O. Vitamin D-loaded nanostructured lipid carrier (NLC): A new strategy for enhancing efficacy of doxorubicin in breast cancer treatment. Nutr. Cancer 2017, 69, 1–9. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Bramley, P.M.; Elmadfa, I.; Kafatos, A.; Kelly, F.J.; Manios, Y.; Roxborough, H.E.; Schuch, W.; Sheehy, P.J.A.; Wagner, K.-H. Vitamin E. J. Sci. Food Agric. 2000, 80, 913–938. [Google Scholar] [CrossRef]
- Schneider, C. Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 2005, 49, 7–30. [Google Scholar] [CrossRef]
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 21, 781–792. [Google Scholar] [CrossRef]
- Gogus, U.; Smith, C. n-3 Omega fatty acids: A review of current knowledge. Int. J. Food Sci. Technol. 2010, 45, 417–436. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Omega-3 polyunsaturated fatty acids and human health outcomes. BioFactors 2009, 35, 266–272. [Google Scholar] [CrossRef]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essential Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Fathi, M.; Vinceković, M.; Jurić, S.; Viskić, M.; Jambrak, A.R.; Donsì, F. Food-grade colloidal systems for the delivery of essential oils. Food Rev. Int. 2021, 37, 1–45. [Google Scholar] [CrossRef]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herbal. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective properties of the golden spice curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Alanchari, M.; Mohammadi, M.; Yazdian, F.; Ahangari, H.; Ahmadi, N.; Emam-Djomeh, Z.; Homayouni-Rad, A.; Ehsani, A. Optimization and antimicrobial efficacy of curcumin loaded solid lipid nanoparticles against foodborne bacteria in hamburger patty. J. Food Sci. 2021, 86, 2242–2254. [Google Scholar] [CrossRef]
- Guri, A.; Gülseren, I.; Corredig, M. Utilization of solid lipid nanoparticles for enhanced delivery of curcumin in cocultures of HT29-MTX and Caco-2 cells. Food Funct. 2013, 4, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Chi, Z.; Liu, C. Chinese white wax solid lipid nanoparticles as a novel nanocarrier of curcumin for inhibiting the formation of Staphylococcus aureus biofilms. Nanomaterials 2019, 9, 763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalingam, P.; Yoo, S.W.; Ko, Y.T. Nanodelivery systems based on mucoadhesive polymer coated solid lipid nanoparticles to improve the oral intake of food curcumin. Food Res. Int. 2016, 84, 113–119. [Google Scholar] [CrossRef]
- Karimi, N.; Ghanbarzadeh, B.; Hamishehkar, H.; Mehramuz, B.; Kafil, H.S. Antioxidant, antimicrobial and physicochemical properties of turmeric extract-loaded nanostructured lipid carrier (NLC). Colloid Interface Sci. Comm. 2018, 22, 16–24. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Improvement of curcuminoid bioaccessibility from turmeric by a nanostructured lipid carrier system. Food Chem. 2018, 251, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.-H.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing bioavailability and stability of curcumin using solid lipid nanoparticles (CLEN): A covenant for its effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 879. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, X.; Lei, Y.; Luo, Y. Solid lipid nanoparticles coated with cross-linked polymeric double layer for oral delivery of curcumin. Colloid Surf. B 2016, 148, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Singh, S.; Singla, D.; Pal Kaur, I. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011, 55, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Aditya, N.P.; Shim, M.; Lee, I.; Lee, Y.; Im, M.H.; Ko, S. Curcumin and genistein coloaded nanostructured lipid carriers: In vitro digestion and antiprostate cancer activity. J. Agric. Food Chem. 2013, 61, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Ganassin, R.; de Silva, V.C.M.; Araujo, V.H.S.; Tavares, G.R.; da Silva, P.B.; Cáceres-Vélez, P.R. Solid lipid nanoparticles loaded with curcumin: Development and in vitro toxicity against CT26 cells. Nanomedicine 2022, 17, 167–179. [Google Scholar] [CrossRef]
- Huang, S.; He, J.; Cao, L.; Lin, H.; Zhang, W.; Zhong, Q. Improved physicochemical properties of curcumin-loaded solid lipid nanoparticles stabilized by sodium caseinate-lactose Maillard conjugate. J. Agric. Food Chem. 2020, 68, 7072–7081. [Google Scholar] [CrossRef]
- Malvajerd, S.S.; Azadi, A.; Izadi, Z.; Kurd, M.; Dara, T.; Dibaei, M.; Zadeh, M.S.; Javar, H.A.; Hamidi, M. Brain delivery of curcumin using solid lipid nanoparticles and nanostructured lipid carriers: Preparation, optimization, and pharmacokinetic evaluation. ACS Chem. Neurosci. 2019, 10, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Madane, R.G.; Mahajan, H.S. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: Design, characterization, and in vivo study. Drug Deliv. 2016, 23, 1326–1334. [Google Scholar] [CrossRef]
- Fang, M.; Jin, Y.; Bao, W.; Gao, H.; Xu, M.; Wang, D.; Wang, X.; Yao, P.; Liu, L. In vitro characterization and in vivo evaluation of nanostructured lipid curcumin carriers for intragastric administration. Int. J. Nanomed. 2012, 7, 5395–5404. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Pan, L.; Jiang, M.; Li, D.; Jin, L. Nanostructured lipid carriers enhance the bioavailability and brain cancer inhibitory efficacy of curcumin both in vitro and in vivo. Drug Deliv. 2016, 23, 1383–1392. [Google Scholar] [CrossRef]
- Meng, F.; Asghar, S.; Xu, Y.; Wang, J.; Jin, X.; Wang, Z.; Wang, J.; Ping, Q.; Zhou, J.; Xiao, Y. Design and evaluation of lipoprotein resembling curcumin-encapsulated protein-free nanostructured lipid carriers for brain targeting. Int. J. Pharm. 2016, 506, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bi, C.; Chan, H.M.; Sun, S.; Zhang, Q.; Zheng, Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumor activity, cellular uptake and improved in vivo bioavailability. Colloid Surf. B 2013, 111, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Malvajerd, S.S.; Izadi, Z.; Azadi, A.; Kurd, M.; Derakhshankhah, H.; Zadeh, M.S.; Javar, H.A.; Hamidi, M. Neuroprotective potential of curcumin-loaded nanostructured lipid carrier in an animal model of Alzheimer’s disease: Behavioral and biochemical evidence. J. Alzheimer Dis. 2019, 69, 671–686. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhu, R.; Liu, Q.; Fei, J.; Wang, S. Anti-inflammatory activity of curcumin-loaded solid lipid nanoparticles in IL-1β transgenic mice subjected to the lipopolysaccharide-induced sepsis. Biomaterials 2015, 53, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Vandita, K.; Shashi, B.; Santosh, K.G.; Pal, K.I. Enhanced apoptotic effect of curcumin loaded solid lipid nanoparticles. Mol. Pharm. 2012, 9, 3411–3421. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, J.; Dai, W.; He, Z.; Zhai, D.; Chen, W. Pharmacokinetic studies and anticancer activity of curcumin-loaded nanostructured lipid carriers. Acta Pharm. 2017, 67, 357–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanotto-Filho, A.; Coradini, K.; Braganhol, E.; Schröder, R.; de Oliveira, C.M.; Simóes-Pires, A. Curcumin-loaded lipid-core nanocapsules as a strategy to improve pharmacological efficacy of curcumin in glioma treatment. Eur. J. Pharm. Biopharm. 2013, 83, 156–167. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, L.; Wang, M.; Zhuang, X.; Huang, R.; Zhu, R.; Wang, S. Targeting the endocannabinoid/CB1 receptor system for treating major depression through antidepressant activities of curcumin and dexanabinol-loaded solid lipid nanoparticles. Cell Physiol. Biochem. 2017, 42, 2281–2294. [Google Scholar] [CrossRef]
- He, X.; Yang, L.; Wang, Z.; Huang, R.; Zhu, R.; Cheng, L. Solid lipid nanoparticles loading with curcumin and dexanabinol to treat major depressive disorder. Neural. Regen. Res. 2021, 16, 537–542. [Google Scholar]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef]
- Aditya, N.P.; Macedo, A.S.; Doktorovova, S.; Souto, E.B.; Kim, S.; Chang, P.S.; Ko, S. Development and evaluation of lipid nanocarriers for quercetin delivery: A comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE). LWT 2014, 59, 115–121. [Google Scholar] [CrossRef]
- Bose, S.; Michniak-Kohn, B. Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur. J. Pharm. Sci. 2013, 48, 442–452. [Google Scholar] [CrossRef]
- Dhawa, S.; Kapil, R.; Singh, B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J. Pharm. Pharmacol. 2011, 63, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Controlled Release 2009, 133, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Pandita, D.; Kumar, S.; Poonia, N.; Lather, V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res. Int. 2014, 62, 1165–1174. [Google Scholar] [CrossRef]
- Jose, S.; Anju, S.S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int. J. Pharm. 2014, 474, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Karamchedu, S.; Tunki, L.; Julhari, H.; Pooja, D. Morin hydrate loaded solid lipid nanoparticles: Characterization, stability, anticancer activity, and bioavailability. Chem. Phys. Lipid. 2020, 233, 104988. [Google Scholar] [CrossRef] [PubMed]
- Sim, G.S.; Lee, B.C.; Cho, H.S.; Lee, J.W.; Kim, J.H.; Lee, D.H.; Kim, J.H.; Pyo, H.B.; Moon, D.C.; Oh, K.W.; et al. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch. Pharm. Res. 2007, 30, 290–298. [Google Scholar] [CrossRef]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Rad. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.R.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef]
- Teskac, K.; Kristl, J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 2010, 390, 61–69. [Google Scholar] [CrossRef]
- Carlotti, M.; Sapino, S.; Ugazio, E.; Gallarate, M.; Morel, S. Resveratrol in solid lipid nanoparticles. J. Dispers. Sci. Technol. 2012, 33, 465–471. [Google Scholar] [CrossRef]
- Berger, A.; Jones, P.J.H.; Abumweis, S.S. Plant sterols: Factors affecting their efficacy and safety as functional food ingredients. Lipid Health Dis. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKay, D.S.; Jones, P.J. Phytosterols in human nutrition: Type, formulation, delivery, and physiological function. Eur. J. Lipid. Sci. Technol. 2011, 113, 1427–1432. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Progress Lipid. Res. 2002, 41, 457–500. [Google Scholar] [CrossRef] [PubMed]
- Lacatusa, I.; Badea, N.; Stan, R.; Meghea, A. Novel bio-active lipid nanocarriers for the stabilization and sustained release of sitosterol. Nanotechnology 2012, 23, 455–702. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.S.; Gupta, R.; Smith, K.W.; van Malssen, K.F.; Popp, A.K.; Velikov, K.P. Super-cooled and amorphous lipid-based colloidal dispersions for the delivery of phytosterols. Soft Mat. 2016, 12, 5835–5846. [Google Scholar] [CrossRef]
- Soleimanian, Y.; Goli, S.A.H.; Varshosaz, J.; Maestrelli, F. Propolis wax nanostructured lipid carrier for delivery of β sitosterol: Effect of formulation variables on physicochemical properties. Food Chem. 2018, 260, 97–105. [Google Scholar] [CrossRef]
- Soleimanian, Y.; Goli, S.A.H.; Varshosaz, J.; Varshosaz, J.; Mannelli, L.D.C.; Ghelardini, C.; Cirri, M.; Maestrelli, F. β-Sitosterol loaded nanostructured lipid carrier: Physical and oxidative stability, in vitro simulated digestion and hypocholesterolemic activity. Pharmaceutics 2020, 12, 386. [Google Scholar] [CrossRef]
- da Silva, M.G.; de Godoi, K.R.R.; Gigante, M.L.; Cardoso, L.P.; Ribeiro, A.P.B. Nanostructured lipid carriers for delivery of free phytosterols: Effect of lipid composition and chemical interesterification on physical stability. Colloid Surf. A 2022, 640, 128425. [Google Scholar] [CrossRef]
- Harde, H.; Das, M.; Jain, S. Solid lipid nanoparticles: An oral bioavailability enhancer vehicle. Expert Opinion 2011, 8, 1407–1424. [Google Scholar] [CrossRef]
- Talegaonkar, S.; Bhattacharyya, A. Potential of lipid nanoparticles (SLNs and NLCs) in enhancing oral bioavailability of drugs with poor intestinal permeability. AAPS PharmSciTech 2019, 20, 121. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Controlled Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloid Surf. B 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Souto, E.B.; Pinho, S.C.; Santana, M.H. Hydrophilic coating of mitotane-loaded lipid nanoparticles: Preliminary studies for mucosal adhesion. Pharm. Develop. Technol. 2013, 18, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Günzel, D.; Finger, C.; Krug, S.M.; Richter, J.F.; Schulzke, J.-D.; Fromm, M.; Amasheh, S. The effect of chitosan on transcellular and paramcellular mechanisms in the intestinal epithelial barrier. Biomaterials 2012, 33, 2791–2800. [Google Scholar] [CrossRef]
- Piazzini, V.; Cinci, L.; D’Ambrosio, M.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Solid lipid nanoparticles and chitosan-coated solid lipid nanoparticles as promising tool for silybin delivery: Formulation, characterization, and in vitro evaluation. Curr. Drug Deliv. 2019, 16, 142–152. [Google Scholar] [CrossRef]
- Shi, L.L.; Lu, J.; Cao, Y.; Liu, J.Y.; Zhang, X.X.; Zhang, H.; Cui, J.H.; Cao, Q.R. Gastrointestinal stability, physicochemical characterization and oral bioavailability of chitosan or its derivatives-modified solid lipid nanoparticles loading docetaxel. Drug Develop. Ind. Pharm. 2017, 43, 839–846. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; Gökçe, E.H.; Ferrari, F.; Rossi, S.; Patrini, M.; Caramella, C. Chitosan-associated SLN: In vitro and ex vivo characterization of cyclosporine A loaded ophthalmic systems. J. Microencapsul. 2010, 27, 735–746. [Google Scholar] [CrossRef]
- Baek, J.S.; Cho, C.W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef]
- Perteghella, S.; Mandracchia, D.; Torre, M.L.; Tamma, R.; Ribatti, D.; Trapani, A.; Tripodo, G. Anti-angiogenic activity of uncoated- and N,O-carboxymethyl-chitosan surface modified-Gelucire® 50/13 based solid lipid nanoparticles for oral delivery of curcumin. J. Drug Deliv. Sci. Technol. 2020, 56, 101494. [Google Scholar] [CrossRef]
- Ramalingam, P.; Ko, Y.T. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: Pharmacokinetic and brain distribution evaluations. Pharm. Res. 2015, 32, 389–402. [Google Scholar] [CrossRef]
- Santonocito, D.; Sarpietro, M.G.; Carbone, C.; Panico, A.; Campisi, A.; Siciliano, E.A.; Sposito, G.; Castelli, F.C.; Puglia, C. Curcumin containing PEGylated solid lipid nanoparticles for systemic administration: A preliminary study. Molecules 2020, 25, 2991. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Chen, C.; Guo, H.; Xu, J.; Zhang, J.; Zhu, X.; Yang, Y.; Zhou, Z.; Li, L.; Huang, Y. Design and evaluation of solid lipid nanoparticles modified with peptide ligand for oral delivery of protein drugs. Eur. J. Pharm. Biopharm. 2014, 88, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Asghar, S.; Gao, S.; Su, Z.; Song, J.; Huo, M.; Meng, W.; Ping, Q.; Xiao, Y. A novel LDL-mimic nanocarrier for the targeted delivery of curcumin into the brain to treat Alzheimer’s disease. Colloid Surf. B 2015, 134, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Tang, B.; Chao, Y.; Xu, H.; Gou, J.; Zhang, Y.; Xu, H.; Tang, X. Cysterine-functionalized nanostructured lipid carriers for oral delivery of docetaxel: A permeability and pharmacokinetic study. Mol. Pharm. 2015, 12, 2384–2395. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, K.; Bernkop-Schnurch, A. Thiomers: Forms, functions and applications to nanomedicine. Nanomedicine 2007, 2, 41–50. [Google Scholar] [CrossRef]
- Gradauer, K.; Dunnhaupt, S.; Vonach, C.; Szollosi, H.; Pali-Scholl, I.; Mangge, H.; Jensen-Jarolim, E.; Bernkop-Schnurch, A.; Prassl, R. Thiomer-coated liposomes harbor permeation enhancing and efflux pump inhibitory properties. J. Control. Release 2013, 165, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, C.; Asghar, S.; Wu, Y.; Chen, Z.; Jin, X.; Yin, L.; Huang, L.; Ping, Q.; Xiao, Y. Improving intestinal absorption and oral bioavailability of curcumin via taurocholic acid-modified nanostructured lipid carriers. Int. J. Nanomed. 2017, 12, 7897–7911. [Google Scholar] [CrossRef] [Green Version]
- Madani, F.; Lindberg, S.; Langel, U.; Futaki, S.; Graslund, A. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 1, 414729. [Google Scholar] [CrossRef] [Green Version]
- Bolhassani, A. Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. BBA 2011, 1816, 232–246. [Google Scholar] [CrossRef]
- Tian, C.; Asghar, S.; Wu, Y.; Amerigos, D.K.; Chen, Z.; Zhang, M.; Yin, L.; Huang, L.; Ping, Q.; Xiao, Y. N-acetyl-L-cysteine functionalized nanostructured lipid carrier for improving oral bioavailability of curcumin: Preparation, in vitro and in vivo evaluations. Drug Deliv. 2017, 24, 1605–1616. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Low, P.S. Folate-targeted therapies for cancer. J. Med. Chem. 2010, 53, 6811–6824. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Teng, L.; Wang, Y.; Zhang, J.; Sun, X. Curcumin-guided nanotherapy: A lipid-based nanomedicine for targeted drug delivery in breast cancer therapy. Drug Deliv. 2016, 23, 1420–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawar, H.; Surapaneni, S.K.; Tikoo, K.; Singh, C.; Burman, R.; Gill, M.S.; Suresh, S. Folic acid functionalized long-circulating co-encapsulated docetaxel and curcumin solid lipid nanoparticles: In vitro evaluation, pharmacokinetic and biodistribution in rats. Drug Deliv. 2016, 23, 1453–1468. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.-H.; Chen, H.-L.; Dong, J.-R. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as Food-Grade Nanovehicles for Hydrophobic Nutraceuticals or Bioactives. Appl. Sci. 2023, 13, 1726. https://doi.org/10.3390/app13031726
Tang C-H, Chen H-L, Dong J-R. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as Food-Grade Nanovehicles for Hydrophobic Nutraceuticals or Bioactives. Applied Sciences. 2023; 13(3):1726. https://doi.org/10.3390/app13031726
Chicago/Turabian StyleTang, Chuan-He, Huan-Le Chen, and Jin-Ru Dong. 2023. "Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as Food-Grade Nanovehicles for Hydrophobic Nutraceuticals or Bioactives" Applied Sciences 13, no. 3: 1726. https://doi.org/10.3390/app13031726