Abstract
Previous study suggested that fixed dental prostheses (crowns) increase oral malodor production. There might be a role to temporary acrylic crowns contributing to oral malodor. In the current study, we analyzed the microbiome associated with malodorous temporary dental crowns. The study population comprised nineteen patients (mean age 45.8 ± 10.9, 8 females) who visited the Tel Aviv University dental clinic. Temporary crowns were scored by an odor judge using a 6-point malodor organoleptic scale (0–5) and temporary crowns that were scored 2 and above were assigned as malodor positive. Microbial DNA was extracted from the temporary dental crowns and analyzed using next generation 16S rDNA sequencing. Taxa identified could be classified into 11 phyla, 50 genera and 119 core species. Malodor positive samples demonstrated higher abundance of the phyla Proteobacteria and Actinobacteria and the genera Tannerella, Alloprevotella, Treponema, Olsenella and Bifidobacterium. Malodorous samples showed higher bacterial diversity and significant differences in microbial population. Taken together these results suggest a difference between the microbial populations of malodorous and non-malodorous temporary dental crowns both in composition and diversity.
1. Introduction
According to The Glossary of Prosthodontic Terms, the definition of prosthodontics suggests it “pertains to the diagnosis, treatment planning, rehabilitation and maintenance of the oral function, comfort, appearance, and health of patients…… by using biocompatible substitutes” []. One cannot ignore that oral malodor strongly impairs a patient’s comfort and self-confidence, hence hindering such aspects of oral rehabilitation.
The process of tooth restoration begins traditionally, and in most cases, with tooth preparation followed by temporary acrylic crown []. The acrylic material (PMMA) tends to exhibit substantial porosity as some acrylic monomer vaporizes away from the set acrylic dough. Another feature inherent within that material is water sorption. A volumetric shrinkage is also expected, yielding dimensional change of as much as 7% []. As a result, with time, the temporary restoration changes its dimensions and gaps will appear along the finish line of the prepared tooth [].
The temporary cement luting the temporary restoration to the tooth is not intended to hold for more than a period of several months []. Hence, coronal leakage is likely to appear, leading to bacterial colonization at the edges of the restoration. All of the above are at risk of becoming a reservoir for bacterial accumulation on the temporary restoration and its surroundings. No wonder that biofilm adhesion was significantly abundant on prepared teeth with provisional restorations compared to untreated reference teeth [,,,].
Oral malodor is considered a very common condition affecting according to several reports about 25–30% of the world’s adult population [,]. In more than 90% of the cases, this condition results from bacterial metabolism arising from the anaerobic activity of Gram-negative proteolytic oral bacteria at various niches within the oral cavity itself []. These bacteria utilize oral glycoproteins for energy and growth yielding malodorous metabolites comprising of volatile sulfide compounds (VSC), diamines, volatile fatty acids and indoles that are felt during speech and exhalation [,,].
According to data reported by several multidisciplinary breath odor clinics (involving various medical fields such as dentistry, E.N.T., internal medicine and psychology) in various medical and academic centers, faulty restorations (e.g., leaking crowns and bridges) are considered one of the causes for oral malodor [,,]. Furthermore, a recent study conducted on 96 subjects measured oral VSC levels in patients with and without permanent dental crowns and concluded that the presence of final fixed partial dentures significantly contributed to patients’ oral malodor [].
Since temporary dental crowns are faulty and leaky by definition, the aim of the current study was to characterize the microbial population (i.e., microbiome) associated with temporary dental crown malodor using next generation 16S microbial identification techniques. Given the microbial nature of this condition, we hypothesize that the microbial population will vary between malodorous and non-malodorous temporary acrylic crowns.
2. Materials and Methods
2.1. Study Population
Nineteen subjects (mean age 45.8 ± 10.9, 8 females) from the Tel Aviv University dental clinic participated in the study. Subjects scheduled to receive single fixed dental crowns on natural tooth preparation were included in the study. Subjects who were smokers or took antibiotics up to three weeks prior to the study were excluded. All subjects signed an informed consent form and the study protocol was approved by the Tel Aviv University institutional ethics committee (#0003165-2).
2.2. Experimental Protocol
Protocol was adopted from previous work []. At the last restorative appointment, where final restorations were scheduled for installment, acrylic restorations were removed from the prepared teeth for the last time and kept in closed test tubes until analyzed. Subjects were asked to refrain from eating or drinking for three hours prior to the final restorative appointment. The temporary single crowns were evaluated organoleptically by a single dental practitioner (NF) using an odor judge malodor intensity scale of 0–5 []. Odor judge was trained and calibrated []. Temporary dental crowns that were given an odor judge score of 2 and above were defined as malodor positive []. Following organoleptic evaluation, the outer and inner surfaces of the temporary dental crowns were sampled using a swab (transfer buffer, −20 °C). To maintain standardization, all measurements were conducted in the late afternoon hours. Clinical information regarding health status, medication use and habits (e.g., smoking, denture hygiene) were recorded from patients’ files.
2.3. DNA Extraction
DNA was extracted from samples using 400 μL GT lysis buffer and 20 μL proteinase K (from Magcore Genomic DNA Tissue Kit, Taipei, Taiwan) in bead beating tubes type C (Geneaid, Taipei, Taiwan). Bead beating was performed for 2 min using a Biospec machine (RBC Bioscience, Taipei, Taiwan). Samples were then incubated at 60 degrees for 2 h and extracted on a Magcore machine (RBC Bioscience, Taipei, Taiwan) using Magcore Genomic DNA Tissue Kit cartridges and protocol.
2.4. PCR Amplification and Sequencing
DNA was quantified by nanodrop and 3 μL (~15 ng) was used as template for initial PCR. Amplification was performed using Kapa Hifi Hot start ready mix using custom primers covering the V4 region primers from the Earth Microbiome Project containing CS1/CS2 adaptors for 20 cycles in a volume of 15 μL:
CS1_515F (V4_F): ACACTGACGACATGGTTCTACAGTGCCAGCMGCCGCGGT;
CS2_806R (V4_R): TACGGTAGCAGAGACTTGGTCTGGACTACHVGGGTWTCT.
A 2 μL sample from the PCR-amplified sample containing CS1/CS2 adaptors was amplified for 10 cycles in 10 μL using Fluidigm Access Array Barcode library according to manufacturer’s protocol (2 μL barcode per reaction). DNA was purified using Kapa Pure Beads at a ratio of 0.8× and quantified with qubit using Denovix DsDNA high sensitivity assay. DNA size and integrity was quantified by Tapestation using Agilent DNA screen tape and reagents.
2.5. Sequencing Data Analysis
Samples were run on a dedicated Miseq (Illumina, San Diego, CA, USA) machine with 30% PhiX using MiSeq Reagent Kit v2 500PE. Demultiplexing was performed using bcl2fastq with default parameters allowing for 1 mismatch. Data was then mapped to PhiX using bwa and unmapped reads were quantified, collected and examined using fastQC.
Demultiplexed reads were uploaded into CLC genomics workbench (Qiagen, Hilden, Germany) and analyzed using their 16S microbiome pipeline. The analysis workflow consisting of quality filtration of the sequence data and operational taxonomic unit (OTU) clustering was performed with default parameter settings. The adaptor sequence was removed and the reads with a quality score lower than 25 or length < 150 were discarded. The maximum number of acceptable ambiguous nucleotides was set to 2 and the length of the reads was fixed at 200–500 bp. Chimeric sequences and singletons were detected and discarded. The remaining unique reads were used for OTU clustering, which was performed by alignment to the SILVA database at 97% sequence similarity.
2.6. Statistical Analysis
The data were described at the phylum, genus and species level in terms of abundance and prevalence. The differences between groups were analyzed using the Kruskal–Wallis test with the pairwise Wilcoxon test among subclasses. Diversity analysis was carried out for both groups using the Shannon diversity index and compared with the Mann–Whitney U test. Microbiome profile for both groups was assessed using principal coordinates analysis (PCoA) and compared with one way PERMANOVA with Bray–Curtis similarity distance. Correlations analyses were performed using the Spearman correlation coefficient. Heat map and hierarchical clustering were performed using UPGMA method (Heatmap 3 software). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Study Population
The study population demonstrated near normal distribution according to malodor scores and was statistically confirmed based on significant differences in variation and diversity between the tested groups (power 0.9). Clinical data comparison for both groups are shown in Table 1. Apart from the temporary dental crowns’ malodor scores, no differences were seen between the groups in any other parameter including gender, age, habits and dental crown parameters (e.g., location, service time).

Table 1.
Summarizes the clinical data on health, medications and habits.
3.2. Microbiome Analysis
Within the 19 tested specimens (8 malodorous and 11 non-malodorous), the microbial population identified could be taxonomically classified into 11 phyla, 50 genera and 119 species. Five of those phyla (Firmicutes, Proteobacteria, Actinobacteria, Bacteriodetes and Fusobacteria) were present in all the samples and accounted for 95% of the sequences of which 41% belonged to the phylum Firmicutes. At genus level, three genera (Streptococcus, Veillonella and Actinomyces) were present in all the samples of which Streptococcus and Actinomyces were most abundant (17% of the sequences each).
Microbiome analysis at phylum and genus levels in the malodorous and non-malodorous samples is shown in Figure 1 and Figure 2. At phylum level, malodor positive samples demonstrated higher prevalence of Actinobacteria (28%) and Proteobacteria (18%) as compared with malodor negative samples (22% and 12%, respectively). At genus level, the comparison between the two groups showed higher abundance of several genera including Tannerella, Alloprevotella, Treponema, Olsenella and Bifidobacterium in the malodor positive group. However, this difference was only statistically significant for the genus Tannerella (p = 0.01).
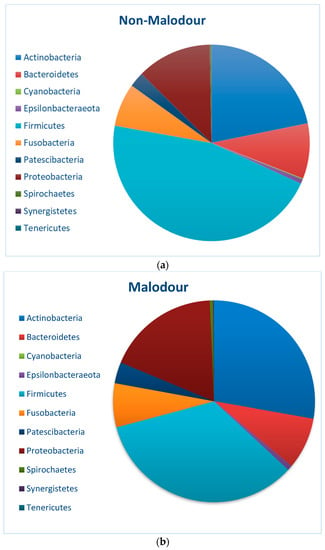
Figure 1.
Microbial abundance at phylum level for (a) non-malodor samples and (b) malodor samples.
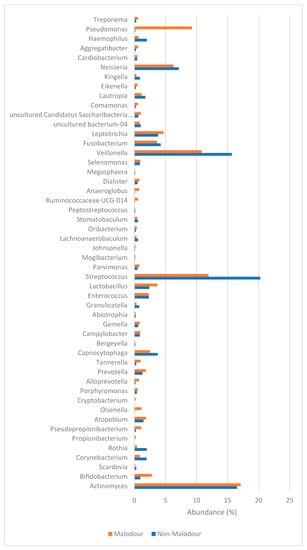
Figure 2.
Microbial abundance (%) at genus level according to (red) malodor and (blue) non-malodor.
3.3. Clustering and Diversity Analysis
3.3.1. Principal Coordinates Analysis (PCoA)
Principal coordinates analysis (PCoA) shown in Figure 3 demonstrates the level of similarity between the microbial population of the malodor and non-malodor samples. This analysis showed that the differences between these two groups were statistically significant (p = 0.038, F = 2.911, PERMANOVA).
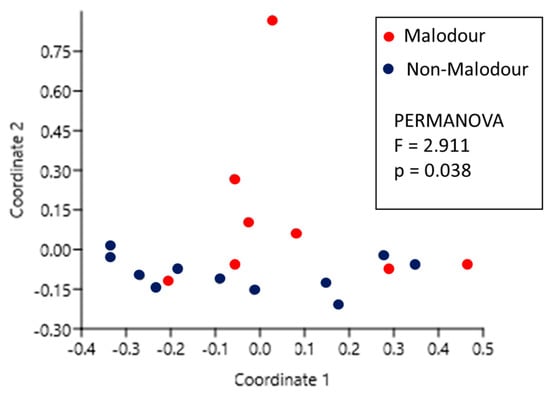
Figure 3.
Principal coordinates analysis (PCoA) comparison between the microbial population of malodor and non-malodor groups.
3.3.2. Diversity Analysis
Microbiome diversity analysis (Shannon diversity index) conducted on the tested samples showed significantly higher diversity (p < 0.001) in the malodor positive samples as compared with the malodor negative group (Figure 4).
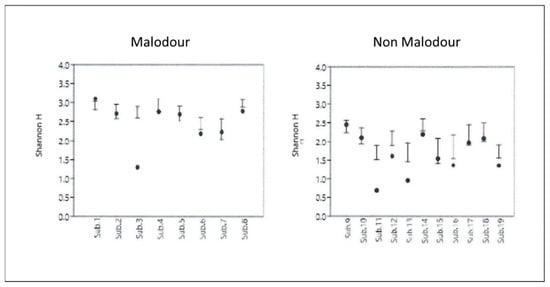
Figure 4.
Diversity analysis (Shannon diversity index) conducted on the microbial populations sampled from the malodor and non-malodor groups.
3.4. Heat Map
The genus-based clustering analysis shown in Figure 5 clearly shows the presence of two distinct clusters: one mostly dominated by malodor positive subjects (7/8) and the other that is mostly dominated by the presence of non-malodorous subjects (10/11).
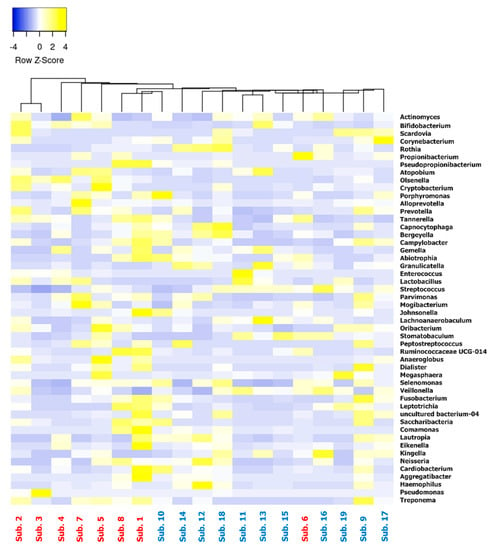
Figure 5.
Heat map clustered according to genera found within the samples and ordered according to their relative abundance. Subjects (malodor—red; non-malodor—blue) are clustered according to genera found in each sample.
3.5. Correlations Analysis
Within the malodor group, two parameters were significantly correlated with temporary dental crown malodor levels: service time (r = 0.734, p = 0.021) and abundance of the genera Treponema (r = 0.730, p = 0.024).
4. Discussion
Recent advancements in molecular microbiology including the “Human Oral Microbiome Database (HOMD)” project [] and the application of deep sequencing technology has opened up new ways for examining the vast microbial population of the oral cavity (i.e., oral microbiome) in health and disease. In the current study, we implemented these new tools in order to investigate the microbial population of temporary dental crowns, specifically regarding oral malodor production.
Results of the current study demonstrated several characteristic differences between the microbiome associated with malodorous temporary dental crowns as compared with non-malodorous ones both regarding composition as well as diversity. At phyla level, the phylum Firmicutes demonstrated higher abundance in the malodor negative group. This observation could be partially explained by the increased abundance of the genera Veillonella and Streptococcus within this group. Although some studies reported Veillonella spp. as an important hydrogen sulfide producer [], it is not traditionally considered a malodor producing genus since it does not normally produce any malodors when cultivated in growth media.
Genus level analysis on the other hand provided a clearer explanation for the differences between the groups. Some genera that are well recognized for their malodor producing properties such as Prevotella, Tannerella, Treponema and Porphyromonas [] showed higher abundance in the malodor-positive samples. Despite the fact that these bacteria are associated with periodontal disease, they can often be found in patients without (or at risk of developing) periodontal disease since their initial colonization is on the tongue []. These Gram-negative, anaerobic, proteolytic bacteria are known to putrefy proteins and produce malodorous metabolites such as volatile sulfides, diamines (e.g., cadaverine and putrecine), volatile fatty acids and indoles.
The oral cavity hosts hundreds of different bacterial species and shows high variability in microbial composition between different individuals. This, in addition to the relatively high prevalence of halitosis, suggests that oral malodor is not produced by a specific bacterium but is most likely the result of a biochemical “joint effort” of several bacterial species. The wide view given by the 16S deep sequencing microbiome database analysis used in the current study provided some support for this assumption by demonstrating the differences between malodor positive and negative samples with an emphasis on two key features: diversity and clustering. The observation that a more richly diverse bacterial population shows higher levels of malodor production has been previously reported over 75 years ago []. Furthermore, there is ample evidence for the co-dependence of various oral bacterial species that rely on one another for metabolism and growth as well as malodor production []. In the current study, results of the microbial population analysis demonstrated a higher diversity in samples extracted from malodorous temporary dental crowns. Furthermore, clustering analysis using PCoA and heat map centroid clustering demonstrated two definite clusters: one dominantly representing the non-malodor group and another more scattered, comprised mainly by malodor samples.
Results of the present study showed that whereas no significant differences were noted in clinical parameters, medication or patient habits between the malodor positive and negative groups, only in the malodor positive group there was a significant correlation between malodor scores and service time. This observation might suggest that there is a pre-inclination for this condition and once it exists it may worsen over time. That pre-inclination may be attributed to the aforementioned surface characteristics within the acrylic rough surface which attracts both saliva proteins [] and oral bacteria []. None of the tested temporary restorations undergo high polish procedure or liquid polish coating and this allowed quick bacterial accumulation on the PMMA provisionals [], which increased with time. However, a previous in vitro study suggested that pre-immersion of PMMA in an antiseptic solution before exposing it to salivary flora may inhibit malodor production []. Within the limited focus of the present study on the V4 NGS microbiome analysis of malodorous acrylic temporary crowns, it is clear that there is a marked difference in microbial profiles between malodor positive and negative groups. However, the underlying reason for these differences warrants further investigation.
Author Contributions
Conceptualization, N.S. and O.R.; methodology, G.M.; validation, N.F., U.J. and N.D.; formal analysis, N.D.; investigation, G.M. and N.F.; resources, N.S. and O.R.; data curation, U.J. and N.D.; writing—original draft preparation, N.S.; writing—review and editing, O.R.; visualization, N.D.; supervision, N.S.; project administration, U.J.; funding acquisition, N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by The Ernst and Tova Turnheim (Née Alexandrovitz) Clinical Research Fund in Dentistry.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and research protocol was approved by the Tel Aviv University IRB (#0003165-2).
Informed Consent Statement
Informed Parental consent was obtained for all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patients’ privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferro, K.; Morgano, S.M. The glossary of prosthodontic terms ninth edition. J. Prosthet. Dent. 2017, 117, e1–e106. [Google Scholar]
- Rosensteel, S.F.; Land, M.F.; Fujimoto, J. (Eds.) Interim Fixed Restorations. In Contemporary Fixed Prosthodontics, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 401–429. [Google Scholar]
- Anusavich, K.J.; Shen, C.; Rawls, R.H. (Eds.) Prosthetic Polymers and Resins. In Phillips Science of Dental Materials, 12th ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 471–479. [Google Scholar]
- Luthardt, R.G.; Stossel, M.; Hinz, M.; Vollandt, R. Clinical performance and periodontal outcome of temporary crowns and fixed partial dentures. A randomized clinical trial. J. Prosthet Dent. 2000, 83, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Anusavich, K.J.; Shen, C.; Rawls, R.H. (Eds.) Overview of Preventive and Restorative Materials. In Phillips Science of Dental Materials, 12th ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 5–6. [Google Scholar]
- Steinberg, D.; Shahar, E. Early formation of Streptococcus Sobrinus biofilm on various dental restorative materials. J. Dent. 2002, 30, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Perez Davidi, M.; Beyth, N.; Sterer, N.; Feuerstein, O.; Weiss, E.I. Effect of liquid—polish coating on in vivo biofilm formation on provisional restorations. Part 1. Quintessence Int. 2007, 38, 591–596. [Google Scholar] [PubMed]
- Perez Davidi, M.; Beyth, N.; Sterer, N.; Feuerstein, O.; Weiss, E.I. Effect of liquid—polish coating on in vivo biofilm formation on provisional restorations. Part 2. Quintessence Int. 2008, 39, 45–49. [Google Scholar]
- Bornstein, M.M.; Kislig, K.; Hoti, B.B.; Seemann, R.; Lussi, A. Prevalence of halitosis in the population of the city of Bern, Switzerland: A study comparing self-reported and clinical data. Eur. J. Oral. Sci. 2009, 117, 261–267. [Google Scholar] [CrossRef]
- Liu, X.N.; Shinada, K.; Chen, X.C.; Zhang, B.X.; Yaegaki, K.; Kawaguchi, Y. Oral malodor-related parameters in the Chinese general population. J. Clin. Periodont. 2006, 33, 31–36. [Google Scholar] [CrossRef]
- Quirynen, M.; Van Eldere, J.; Pauwels, M.; Bollen, C.M.; Van Steenberghe, D. In vitro volatile sulfur compound production of oral bacteria in different culture media. Quintessence Int. 1999, 30, 351–356. [Google Scholar]
- Berg, M.; Fosdick, L.S. Studies in periodontal disease. II. Putative organisms in the mouth. J. Dent. Res. 1946, 25, 73–81. [Google Scholar] [CrossRef]
- McNamara, T.F.; Alexander, J.F.; Lee, M. The role of microorganisms in the production of oral malodor. Oral Surg. 1972, 34, 41–48. [Google Scholar] [CrossRef]
- De Boever, E.H.; Loesche, W.J. Assessing the contribution of anaerobic microflora of the tongue to oral malodor. J. American. Dent. Ass. 1995, 126, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Dadamio, J.; Van den Velde, S.; De Smit, M.; Dekeyser, C.; Van Tornout, M.; Vandekerckhove, B. Characteristics of 2000 patients who visited a halitosis clinic. J. Clin. Periodontol. 2009, 36, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Leib, E. Experiences of an Israeli malodor clinic. In Bad Breath Research Perspectives; Rosenberg, M., Ed.; Ramot Publishing: Tel Aviv, Israel, 1997; pp. 137–148. [Google Scholar]
- Seemann, R.; Bizhang, M.; Djamchidi, C.; Kage, A.; Nachnani, S. The proportion of pseudo-halitosis patients in a multidisciplinary breath malodor consultation. Int. Dent. J. 2006, 56, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Alzoman, H.; Rashid Habib, S.; Alghamdi, S.; Al-Juhani, H.; Daabash, R.; Al-Khalid, W.; Al-Askar, M.; Al-Johany, S. Relationship between Fixed Dental Crowns and Volatile Sulphur Compounds. Int. J. Environ. Res. Pub. Health 2021, 18, 1283. [Google Scholar] [CrossRef] [PubMed]
- Yitzhaki, S.; Reshef, L.; Gophna, U.; Rosenberg, M.; Sterer, N. Microbiome associated with denture malodour. J. Breath Res. 2018, 12, 027103. [Google Scholar] [CrossRef]
- Rosenberg, M.; Kulkarni, G.V.; Bosy, A.; McCulloch, C.A. Reproducibility and sensitivity of oral malodor measurements with a portable sulphide monitor. J. Dent.Res. 1991, 70, 1436–1440. [Google Scholar] [CrossRef]
- Nachnani, S.; Majerus, G.; Lanton, P.; Hodges, J.; Magallanes, E. Effect of training on odor judges scoring intensity. Oral Dis. 2005, 11, 40–44. [Google Scholar] [CrossRef]
- Sterer, N.; Hendler, A.; Davidi, M.P.; Rosenberg, M. A novel microscopic assay for oral malodor-related microorganisms. J. Breath Res. 2008, 2, 026003. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Washio, J.; Shimada, Y.; Yamada, M.; Sakamaki, R.; Takahashi, N. Effects of pH and lactate on hydrogen sulfide production by oral Veillonella spp. Appl. Environ. Microbiol. 2014, 80, 4184–4188. [Google Scholar] [CrossRef]
- Faveri, M.; Feres, M.; Shibli, J.A.; Hayacibara, R.F.; Hayacibara, M.M.; De Figueiredo, L.C. Microbiota of the dorsum of the tongue after plaque accumulation: An experimental study in humans. J. Periodontol. 2006, 77, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Sterer, N.; Rosenberg, M. Streptococcus salivarius promotes mucin putrefaction and malodor production by Porphyromonas gingivalis. J. Dent. Res. 2006, 85, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Rosner, O.; Melamed, G.; Livne, S.; Jeffet, U.; Dolev, E.; Ben Izhack, G.; Heller, H.; Sterer, N. Pre-disinfection of Poly-Methyl-Methacrylate (PMMA) reduces volatile sulfides compounds (VSC) production in experimental biofilm in vitro. Appl. Sci. 2020, 12, 1947. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).