Abstract
This study seeks to understand the effects of new hydrogels based on Oregano® essential oil, Frankincense® essential oil, and the Thieves® blend, which includes clove, lemon, cinnamon bark, eucalyptus radiata, rosemary extract, and Frankincense® essential oil through in vitro cytotoxicity and antimicrobial analysis. Mesenchymal stem cells (D1MSCs) generated from the dental papilla of human wisdom teeth germs were used to test the hydrogels’ cytotoxicity (D1MSCs). The chemical makeup of the tested essential oils (EO) was determined using GC-MS analysis, and their presence in the novel hydrogels was determined using UV-VIS analysis. The primary constituents of the essential oils identified as being present were eugenol, pinene, limonene, carvacrol, and cymene. The primary constituents from essential oils identified by the absorption spectra included eugenol, pinene, limonene, carvacrol, and cymene. The presence of essential oils in the hydrogel composition was also clearly discernible. All of the studied compounds had a reduced cytotoxic effect on cell cultures, proving the lack of in vitro toxicity of the gels. This study demonstrates that hydrogels enriched with pure natural extracts of essential oils have a tangible in vitro antimicrobial potential, especially for Staphylococcus aureus and Bacillus cereus.
1. Introduction
In human dentistry, the use of herbal extracts and essential oils as plaque-fighting and tooth-cleaning agents has traditionally been successful. These phytochemicals also provide a potential strategy in the prevention and treatment of dental caries, oral inflammation, and other oral infections [1] and could be a potent antibiotic substitute.
Essential oils are complex mixtures of many compounds, primarily produced from aromatic and medicinal plants, including terpenoids, aromatic components derived from phenol, and aliphatic components. From a chemical standpoint, their contents can change as a result of climate change, depending on the organ portion of the plant, age, species, and subspecies, and changes may occur as a result of the oil extraction mechanism [2]. As a result, their contents are unique to each of them. Certain essential oils have antimicrobial and antioxidant properties, and in combination with other substances, they can have bacteriostatic, bactericidal, and anti-inflammatory effects, so they are increasingly used in the treatment of certain conditions with a certain spectrum, especially since they are simple to include into hydrogel formulations, this opens up new possibilities for the administration of active chemicals at the level of mucosal membranes, particularly antibacterial, antioxidant, and anti-inflammatory drugs, which are all experiencing rapid growth [3].
According to the existing studies in the literature, there is still utility to using essential oils by incorporating and preserving the antimicrobial effect of the active constituents, these being integrated into wound dressings with the aim of preventing, limiting, or treating other infections that tend to occur at the level of wounds and at the same time they can facilitate the regeneration of soft tissues. These recent studies highlight the fact that more and more natural constituents extracted from plants can control the stages of healing [4].
The idea of various systems, which is anticipated to be in favor of the release of active substances as well as the avoidance of side effects and the reduction in dosing intervals, is the foundation of the current studies on drug delivery systems. Particularly, contemporary mucosal delivery systems for antibiotics, antioxidants, and anti-inflammatory drugs have improved in this area with regard to the avoidance of adverse effects, simple administration methods, and a tolerable concentration of the active ingredients in the mucosa [5].
In medicine and pharmacy, hydrogels are becoming increasingly important. They appear to be one of the most encouraging classes of biomaterials because of their favorable physicochemical characteristics, biocompatibility, and designed activity with living environments [6]. One of the most researched topics at the intersection of engineering and medicine is the development of hydrogels for biological applications [7]. Hydrogels have been created for and employed in a variety of medical practices due to their high-water content, chains of hydrophilic polymers, and strong biocompatibility [8]. Since the characteristics of hydrogels can be altered through various chemical studies, their rational design and engineering enabled new methods for the release of small molecules, proteins, and cells. Additionally, they served as tissue scaffolds for the management of cell lines, stem cell development, and tissue regeneration [7]. These are frequently enhanced with natural ingredients that set them apart and place them on a higher scale than traditional medications.
Dentistry is one of the domains of medicine where the most common pathologies have a bacterial and fungal origin. The most prevalent diseases, such as dental caries, periodontal disease, and endodontic lesions, appear because of well-known bacterial and fungal pathogens: Streptococcus mutans, Streptococcus salivarius, Streptococcus sanguinis, Porfiromonas gingivalis, Prevotella intermedia, Actinobacilus actinomycetemcomitans, Candidas facalibica, etc. [9]. The attention of many researchers has focused on the antimicrobial properties of traditional medical substances, such as essential oils [10].
Oregano-Origanum vulgare (family Lamiaceae) is a plant found in India, southern Iran, and the Mediterranean region, which has traditionally been used for antiseptic purposes. Scientific reports have shown that oregano oil possesses antimicrobial, antiseptic, and antispasmodic properties due to its bioactive compounds such as the phenolic isomers carvacrol and thymol; the monoterpenes p-cymene and γ terpinene, being effective antimicrobial agents against the pathogen Enterococcus faecalis [11]. Cinnamon, which has the scientific name Cinnamomum zeylanicum, and the use of the plant, due to its vast medicinal uses, has found a prominent position in traditional medicine, especially in Ayurveda, which is the traditional Indian medicinal system. Cinnamon is used in many cultures to treat a variety of health disorders, including diarrhea, arthritis, spasticity, yeast infections, mouth ulcers, colds, flu, and digestive problems [12]. Clove essential oil is extracted from the clove plant, Syzygium aromaticum. This is a good natural analgesic and antiseptic, mainly used in dentistry for its main ingredient, eugenol. The health benefits of the oil can be attributed to its antimicrobial, antifungal, antiseptic, antiviral, aphrodisiac, and stimulant properties. The germicidal properties of the oil make it very effective in relieving toothaches and gum and mouth ulcers. The most important and common use of clove oil is in dental care [13]. Rosmarinus officinalis, commonly known as rosemary, is a woody perennial plant native to the Mediterranean region. Rosemary essential oil is also used in oral inflammatory conditions. It is a rich source of antioxidants and anti-inflammatory compounds, which help strengthen the immune system and improve blood circulation. Laboratory studies have shown that rosemary is rich in antioxidants, which play an important role in neutralizing harmful compounds called free radicals [14]. Lemon essential oil has always been used to treat a variety of conditions, such as inflammation, cardiovascular disease, cancer, hepatobiliary dysfunction, etc. It is known to possess multiple biological activities, such as antibacterial and fungicidal properties against foodborne pathogens and spoilage bacteria, as well as considerable antioxidant properties. Studies have shown that lemon essential oil exhibited selective antibacterial activity with a greater effect on pathogenic bacteria (E. coli) than on beneficial bacteria (Lactobacillus). All of this points to the possibility of using this substance as a natural extract to prevent inflammation brought on by oxidative stress [15]. The Eucalyptus globules tree, which is endemic to China, is the source of eucalyptus. The primary active ingredient in eucalyptus essential oil is eucalyptol (1,8-cineole), which has been shown to have potent broad-spectrum antibacterial activity against a variety of microorganisms in clinical settings [16]. It also has potent antiseptic, astringent, diaphoretic, deodorant, inhalant, expectorant, and insect repellent.
Thus, natural phytochemicals incorporated into hydrogels could provide an effective alternative to the over-administration of antibiotics, antiseptics, and analgesics, and this represents a promising approach in therapeutic strategies seeking to combat dental problems and other oral infections in both humans and animals. Efforts to develop hydrogels enriched with natural extracts for biomedical applications represent one of the most studied fields at the interface of engineering and medicine at the present time [1].
In the beginning, our research team synthesized five gels used as photosensitizers containing oregano, methylene blue, Thieves®, Frankincense®, arnica oil, and curcuma extract. In our first study, these gels were used only as photosensitizers, whereas, in the current study, these compounds were combined as hydrogels in order to be tested as singular therapeutic agents, which have not been tested extensively in the literature, especially oregano and Thieves®. This was performed in order to compare the action of the agents as photosensitizers to their action as standalone therapeutic agents.
After an extensive search on the search engine database Pubmed, it was discovered that there are numerous reviews that propose essential oils as active constituents for periodontal treatment, but only a few of them were actually tested. We have found data regarding preclinical or clinical studies of periodontal treatment with Thieves® and Clove essential oil without them being embedded in hydrogels. In addition, we have found numerous hydrogel formulations with different therapeutic molecules such as hydroxyapatite, nanoparticles, metals, Metronidazole, Doxycyclin, honey incorporation, or other natural compounds, not specifically Oregano and Frankincense. Our research team has previously developed such materials containing essential oils, which were successfully published [9,10].
Previously, there were five essential oils tested embedded in hydrogels as photosensitizers, and the efficacy of these three (Oregano®, Thieves®, and Frankincense®) was highlighted, so the team decided to test further their therapeutical capacity in periodontitis used as single therapy molecules.
This study is unique in that it developed and described brand-new investigational dental drugs that can be used either alone or in conjunction with photodynamic therapy. These brand-new hydrogels are made of polymers and natural extracts (essential oils) that we want to use as photosensitizers in photodynamic therapy for periodontitis treatment.
The aim of this study was to characterize the proposed/studied hydrogels’ physicochemical properties and in vitro effects utilizing oxygen-enriched water and naturally derived essential oils.
2. Results and Discussion
GC-MS Analysis of Gels Containing Essential Oils
By using the gas chromatography-mass spectrometry (GC-MS) technology, the essential oils were examined. Due to the volatile and semi-volatile components (monoterpenes and sesquiterpenes) of essential oils being easily separated, recognized, and quantified, GC-MS is the most popular analytical approach for the examination of essential oils. The GC method is trustworthy because it can identify analytes in minute quantities (traces) recovered from gels and because its mass spectrometry (MS) coupling provides structural information about the detected compounds [17,18,19].
GC-MS chromatogram analysis shows the volatile compounds specific to experimental gels: Oregano® essential oil (O), Thieves® essential oil (T), and Frankincense® essential oil (F). The volatile compounds recovered from the composition are listed in Table 1.

Table 1.
The volatile constituents of essential oils.
The experimental gels contained, as shown in Table 1, a mixture of essential oils (Thieves®—T), Frankincense® essential oil (F), and Oregano® essential oil. The GC-MS analysis reveals the specific volatile components detected in the experimental gels (O).
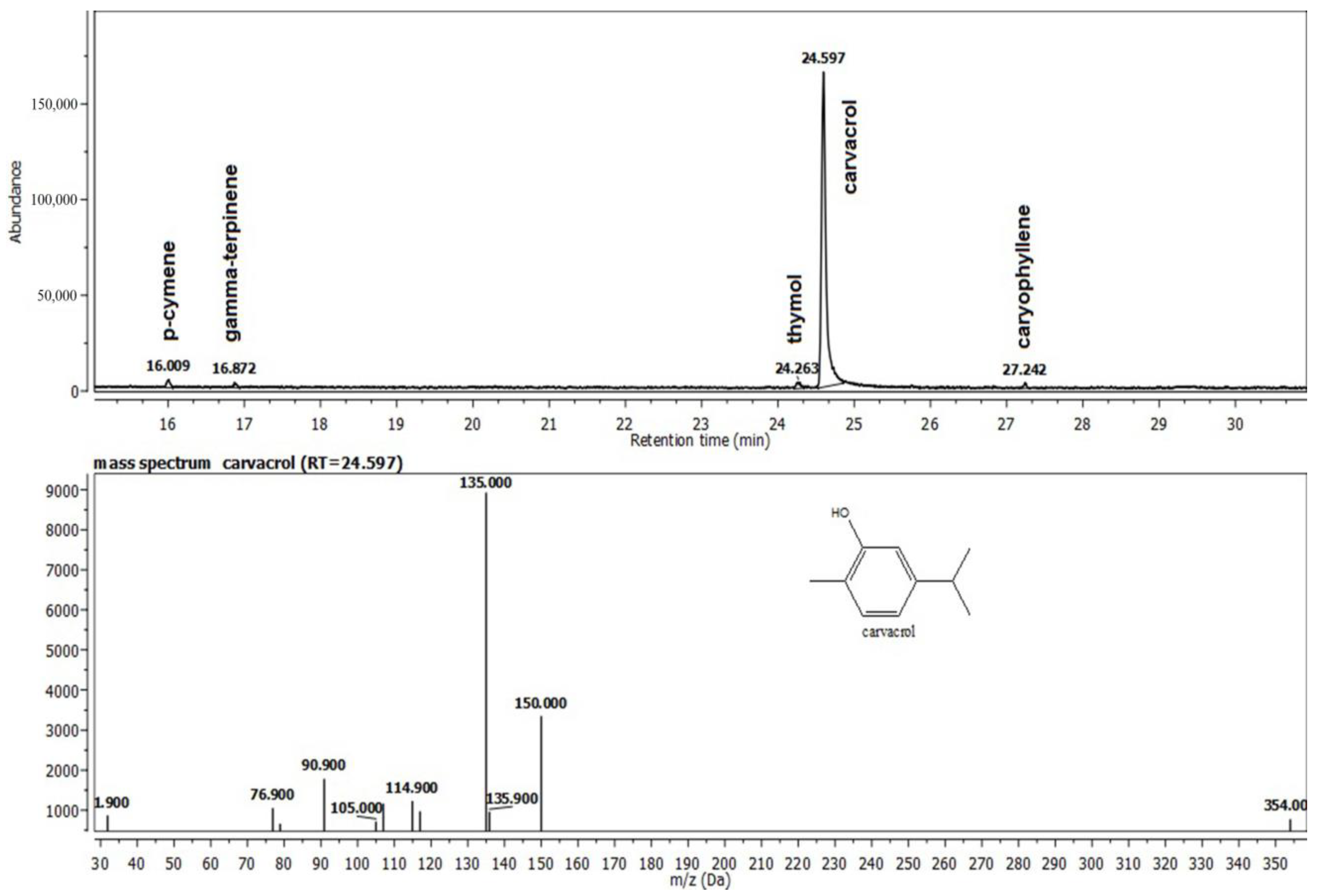
Oregano essential oil has a complex composition, with the main compounds being carvacrol, p-cymene, and γ-terpinene (Figure 1).
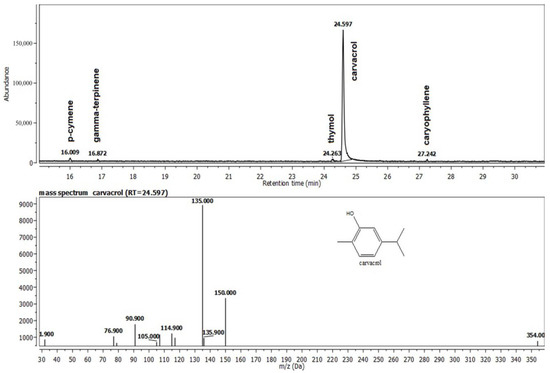
Figure 1.
The composition of the Oregano® oil (O) recovered from the gel G1O.
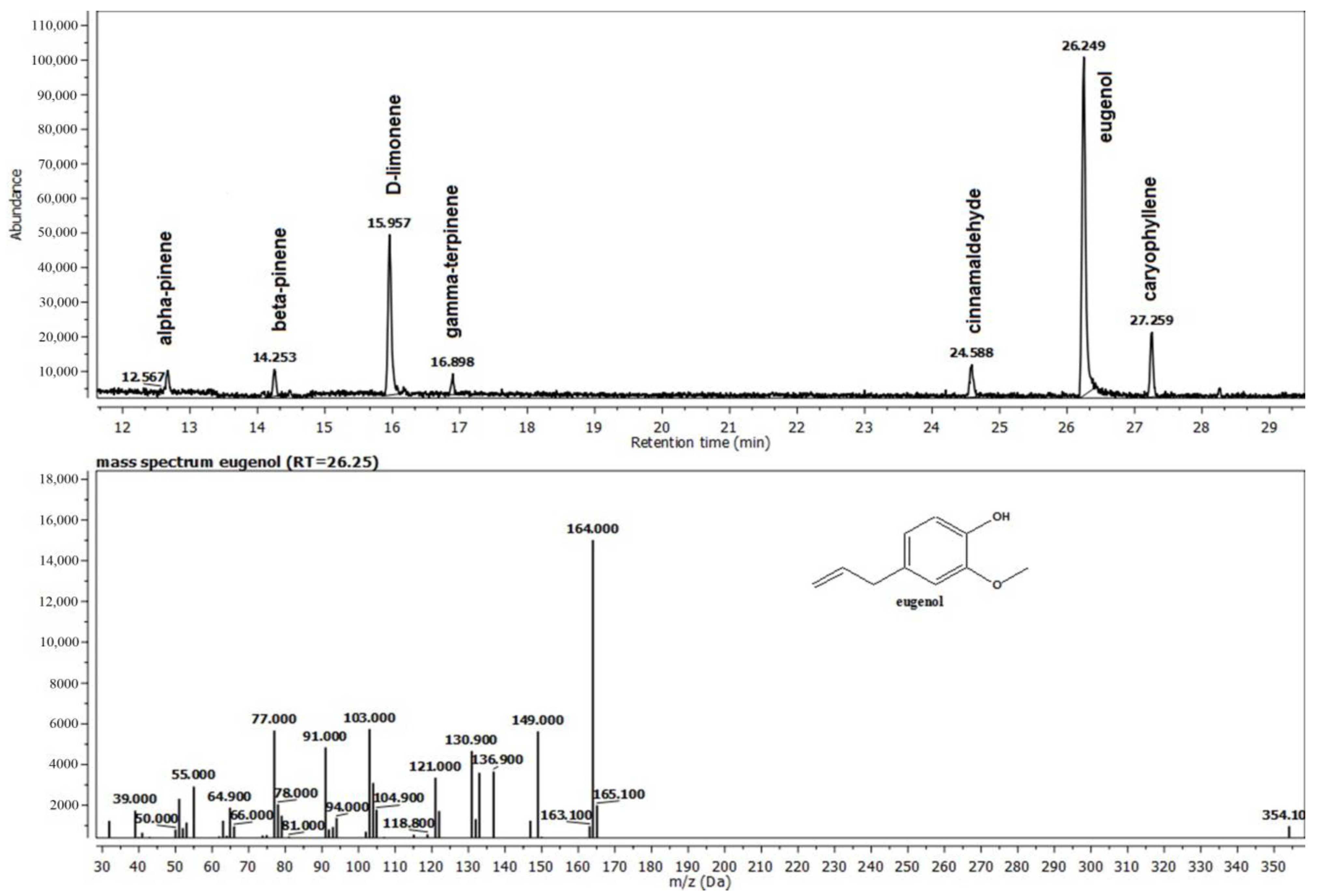
Eugenol (42.3 percent), monoterpene (22.52%), D-limonene, and eucalyptol are the main active elements in the blend of Thieves essential oils, which is made up of a variety of distinct compounds (Figure 2). They support the essential oil’s antioxidant and antibacterial capabilities.
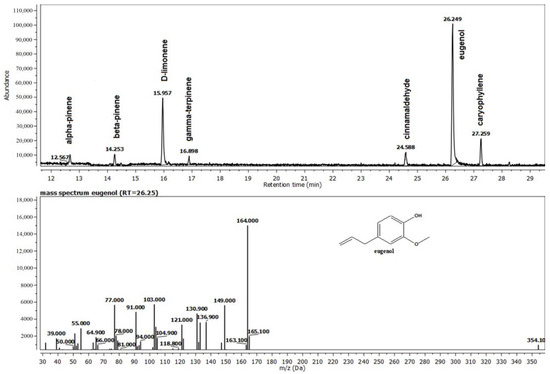
Figure 2.
The composition of the Thieves® oil (T) recovered from the gel G2T.
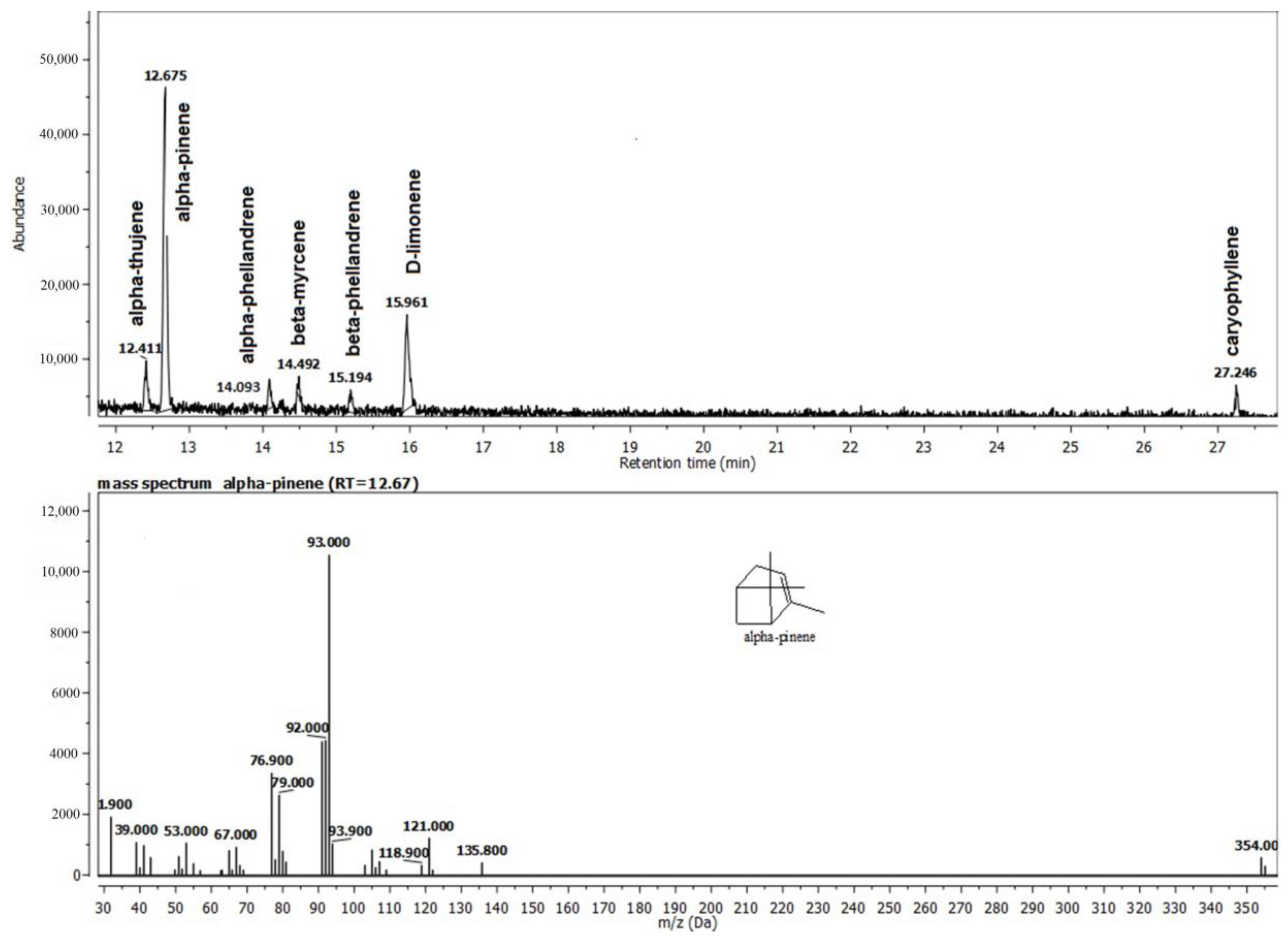
The frankincense essential oil was characterized by the high content of monoterpenes, which constituted 84.86% of the total and among which α-pinene and D-limonene were the major constituents (Figure 3). The remaining 14.53% was accounted for by sesquiterpenes, among which caryophyllene was the major constituent.
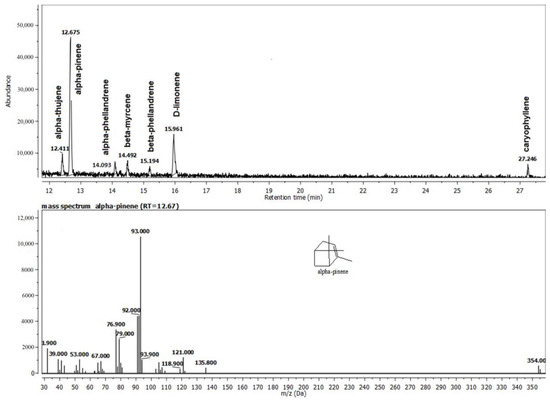
Figure 3.
The composition of the Frankincense® oil (F) recovered from the gel G2F.
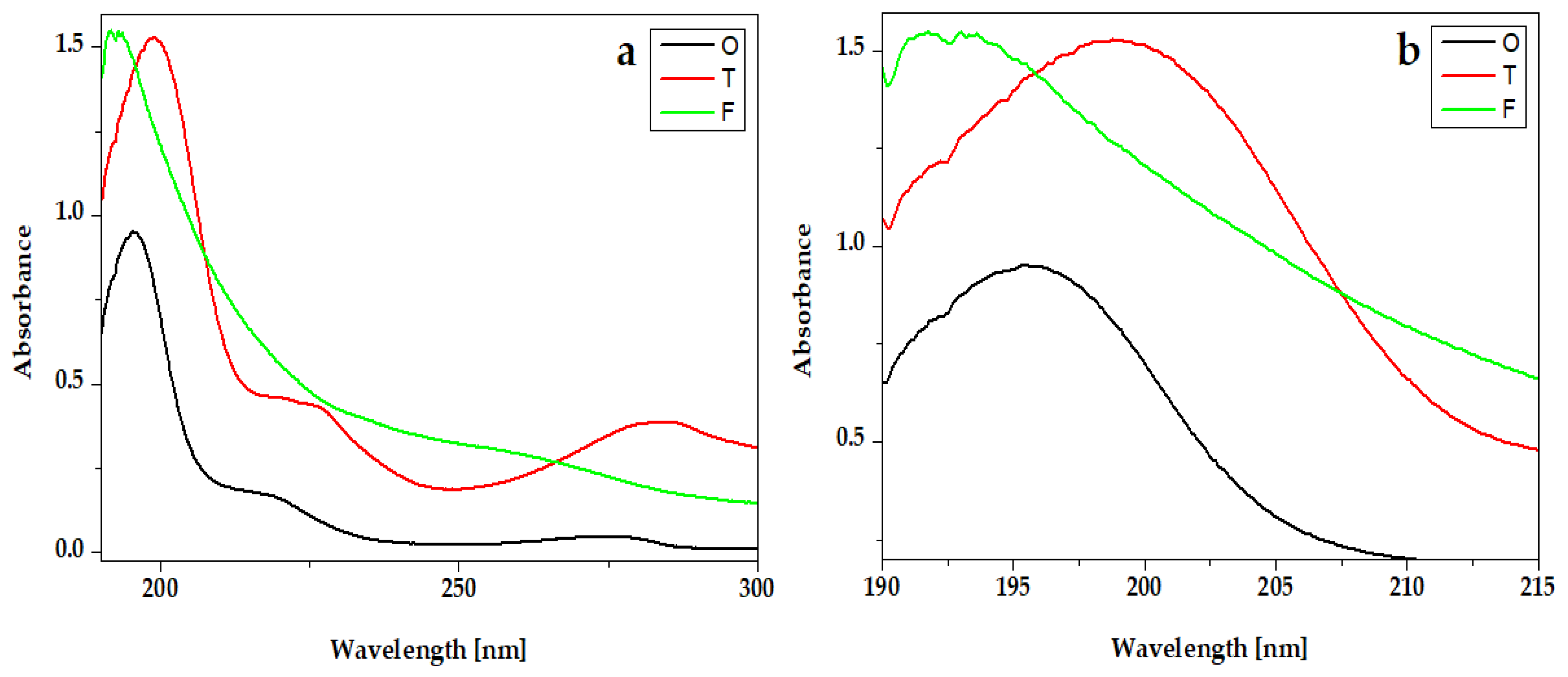
3. UV-Vis Absorption Spectroscopy of the Hydrogels
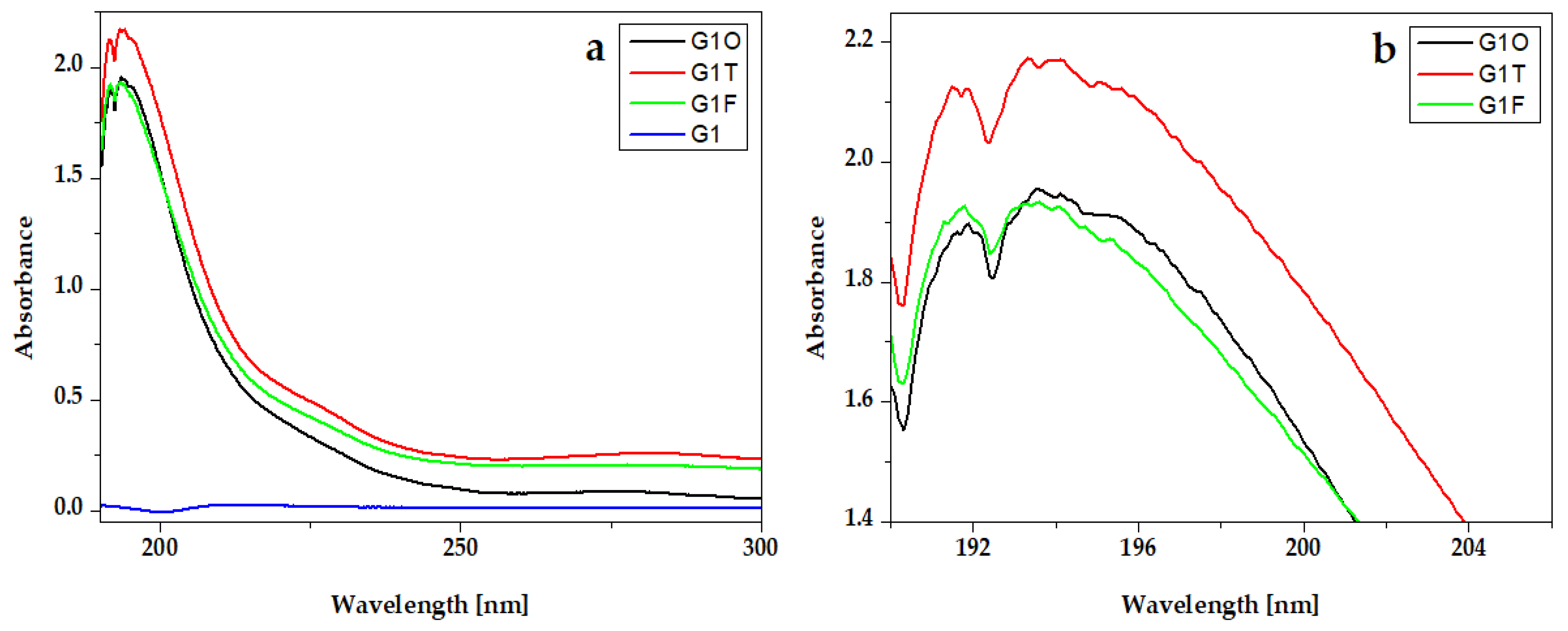
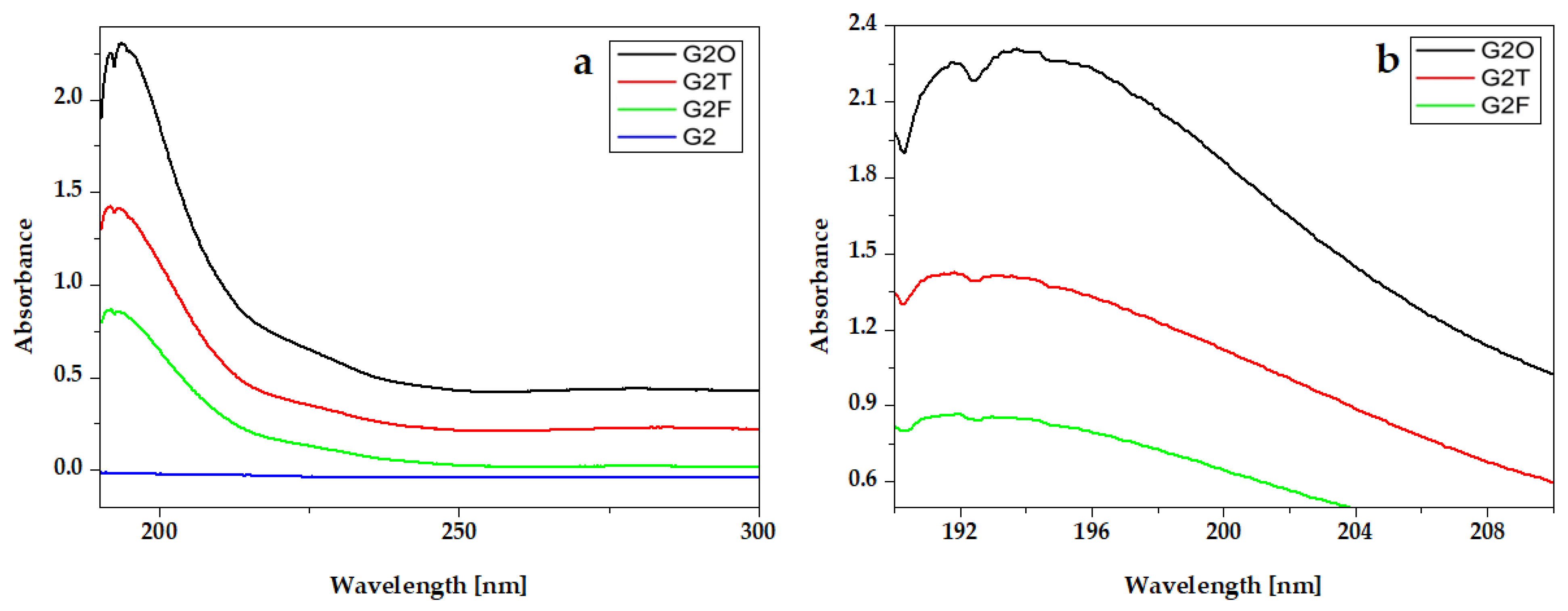
The UV-Vis absorption spectra of essential oils from hydrogel compositions are presented in Figure 4. All spectra present an intense peak around 200 nm. For Oregano, the peak is around 192 nm and for Frankincense oil is around 191 nm. The absorption spectra of Thieves present one intense peak at 208 nm and two absorption bands around 225 nm and 282 nm.
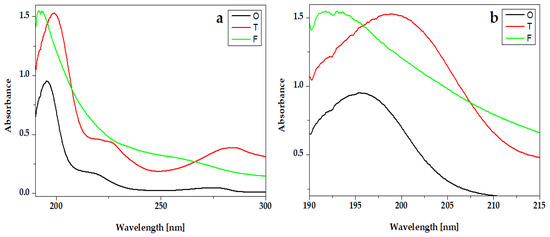
Figure 4.
UV-VIS absorption spectra of essential oils Oregano® (O), Thieves® (T), and Frankincense® (F): (a) full-length spectra and (b) peaks details.
The absorption peaks of essential oils are presented in the UV-Vis spectra of hydrogels that contain them (Figure 5 and Figure 6).
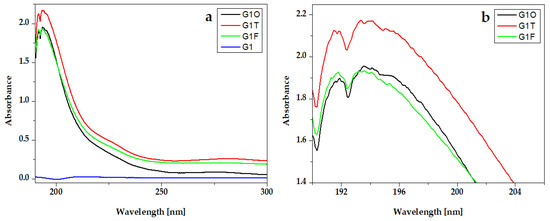
Figure 5.
UV-VIS absorption spectra of experimental gels G1 with Oregano® (G1O), Thieves® (G2O), and Frankincense® (G2F) essential oils: (a) full-length spectra and (b) peaks details.
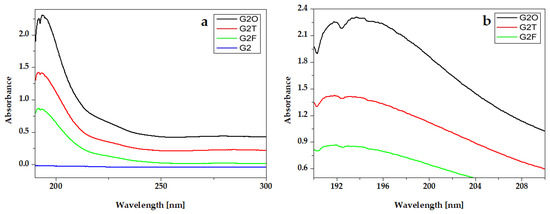
Figure 6.
UV-VIS absorption spectra of experimental gels G2 with Oregano® (G2O), Thieves® (G2T), and Frankincense® (G2F) essential oils: (a) full-length spectra and (b) peaks details.
In the experimental hydrogels, G1T and G2T containing the Thieves® blend had an absorption peak at 194 nm. An intense peak at 191 nm is registered for hydrogels G1O, G2O, G1F, and G2F, which present similar absorption spectra. Gels whose composition contains Kaqun water (Figure 6) have a less obvious band in the 250–300 nm range.
3.1. Viability and Cytotoxicity Assessment of the Experimental Gels
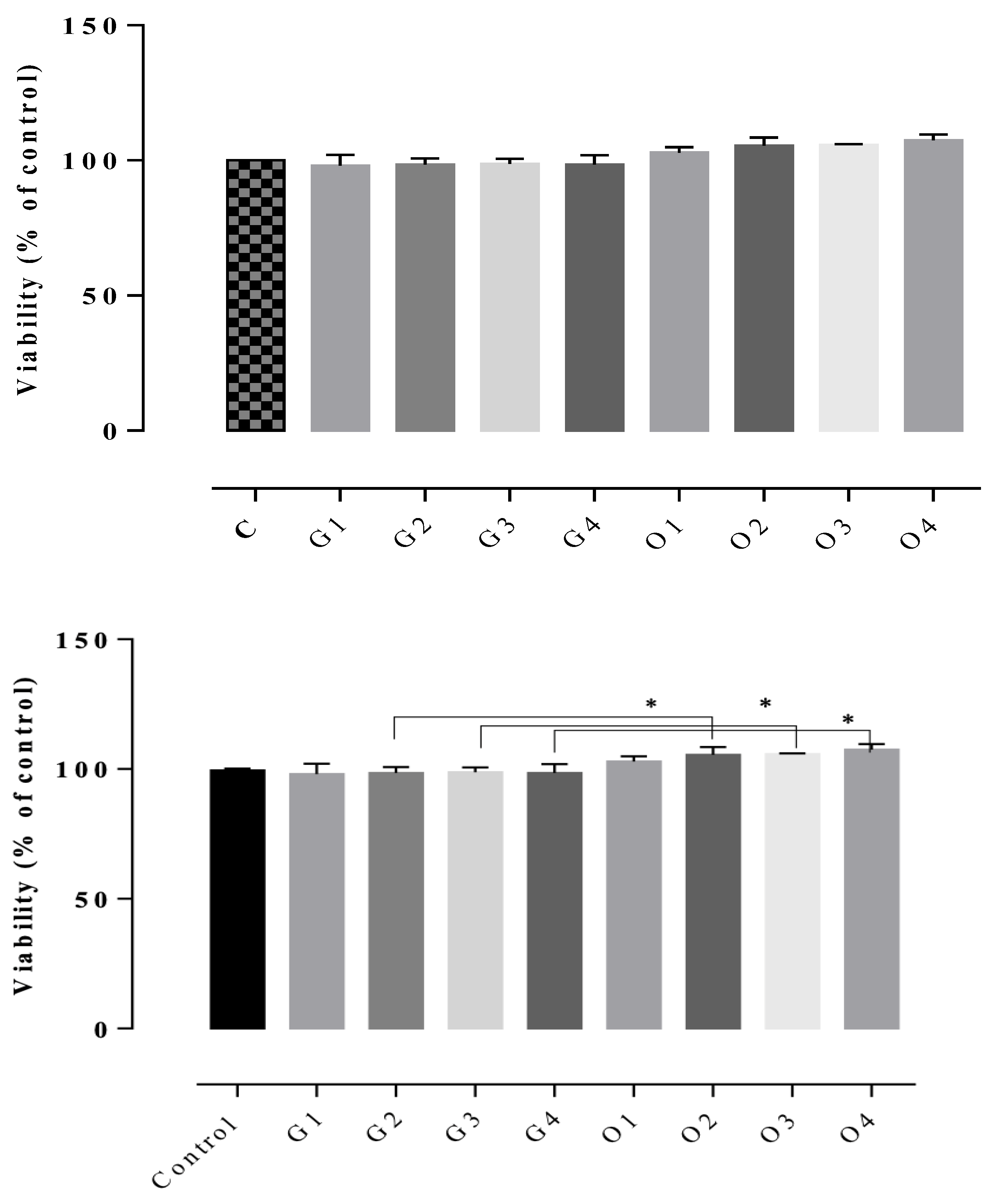
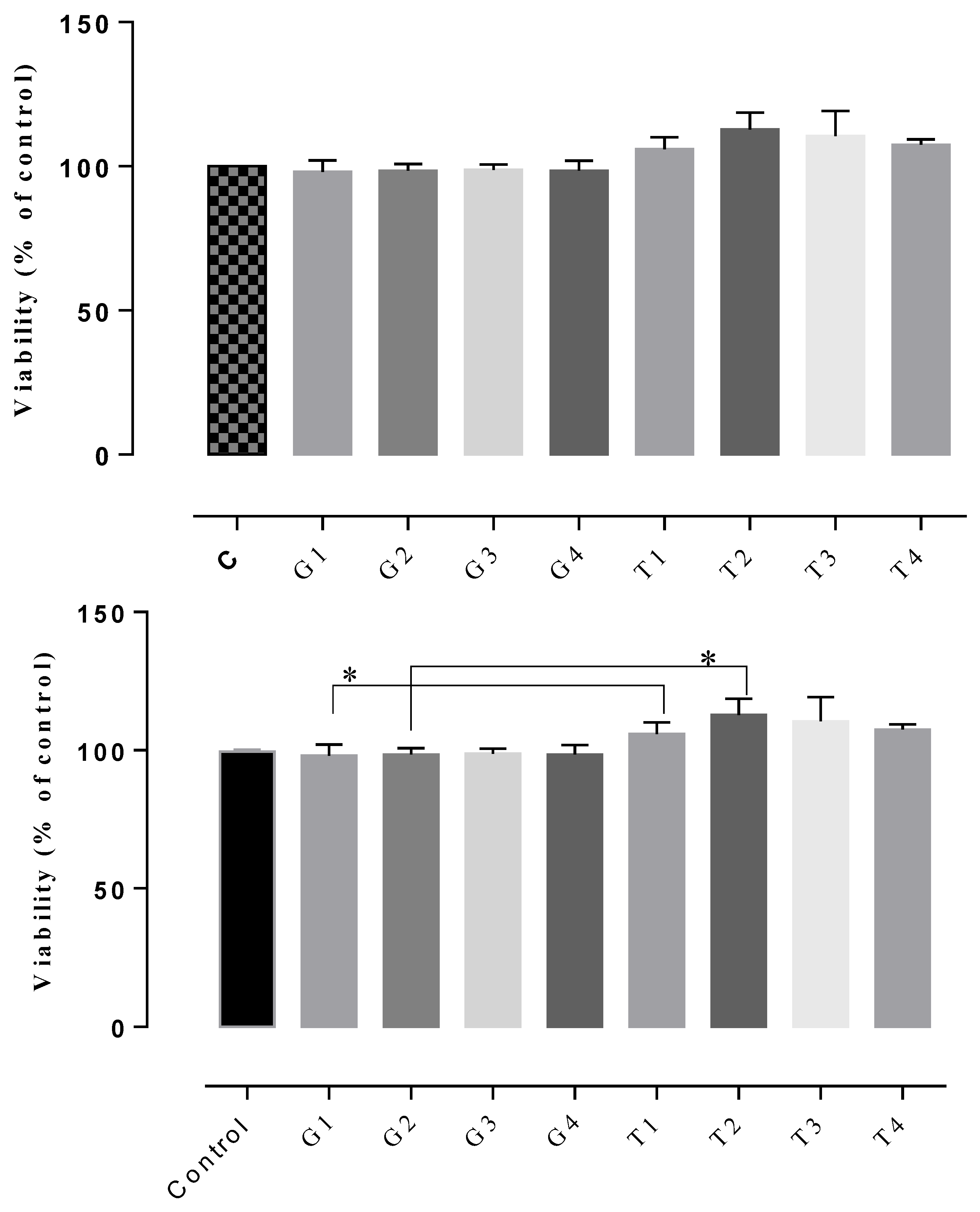
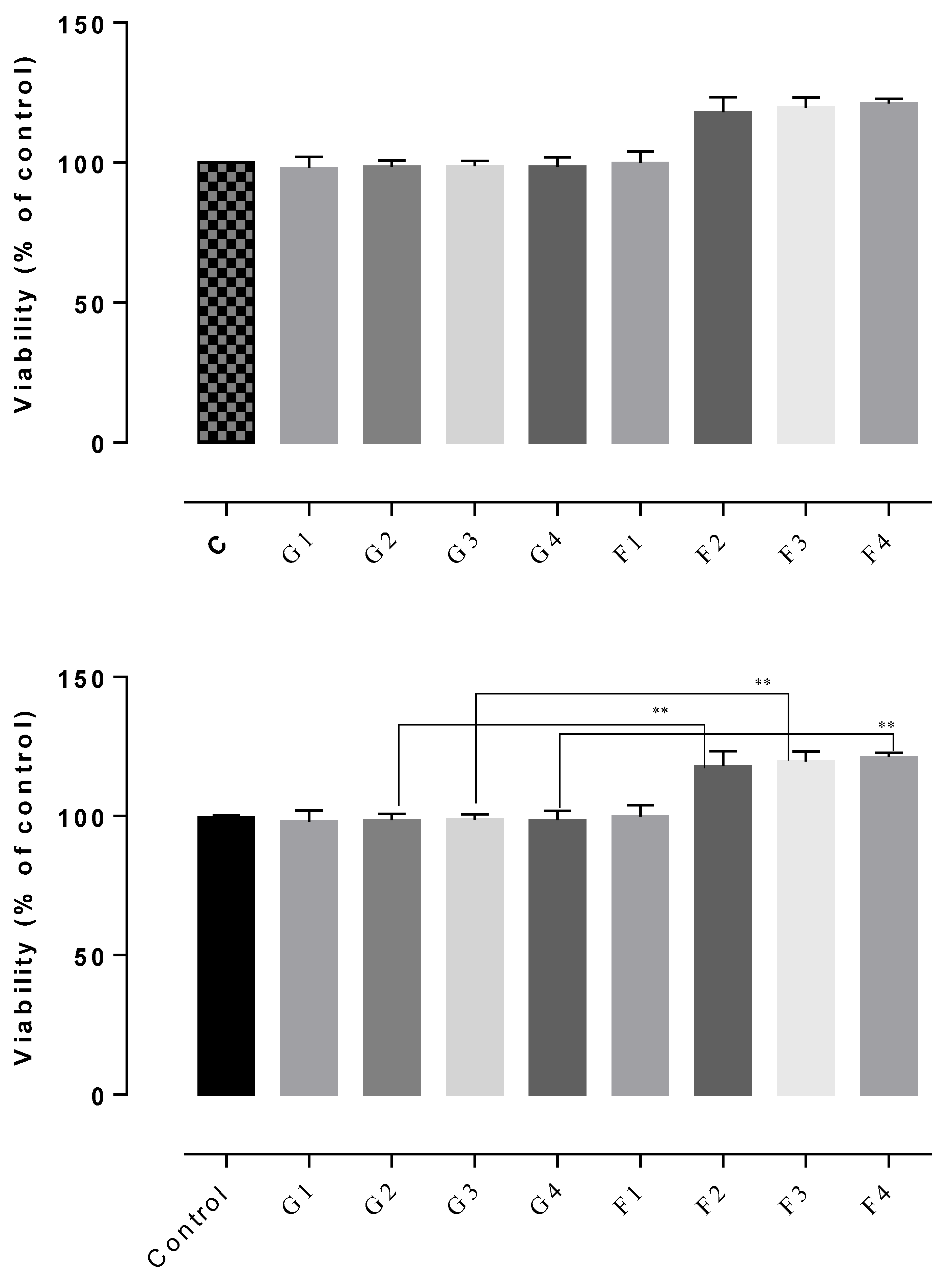
The results of the cytotoxicity assay indicated non-cytotoxic potential in all of the tested gels, the cell viability rates being between 102–122% (Figure 7, Figure 8 and Figure 9). The highest cell survival rate was recorded for the gel supplemented with Frankincense® essential oil (99–121%), suggesting a healing potential for this compound (Figure 8) for each tested concentration, followed by the gel supplemented with Thieves® essential oil (105–112%), (Figure 7). In general, all tested gels promoted cell proliferation being non-toxic, compared to the control gel without essential oil. The absolute control gel (no essential oil) is also non-cytotoxic, having a viability rate of 98% but not indicating healing potential (Figure 7, Figure 8 and Figure 9).
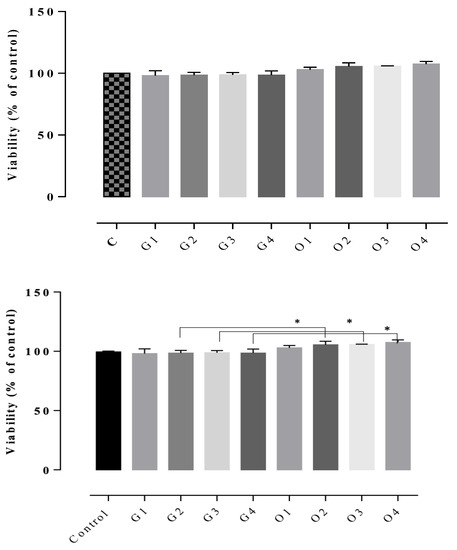
Figure 7.
D1MSCs cells viability assessment after treatment with control gel and gel with Oregano® essential oil, where Control = D1MSCs cells in normal propagation medium; G1, 2, 3, 4 = the gel without essential oil in 4 different concentrations (2.4; 4.7; 7 and 9% (v/v)); O = the gel with Oregano® essential oil in 4 different concentrations of gels (2.4; 4.7; 7 and 9% (v/v)), * p < 0.05.
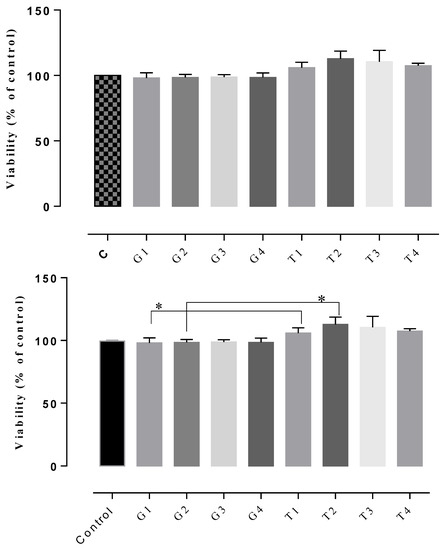
Figure 8.
D1MSCs cells viability assessment after treatment with control gel and gel with Thieves® essential oil, where Control = D1MSCs cells in normal propagation medium; G1, 2, 3, 4 = the gel without essential oil in 4 different concentrations (2.4; 4.7; 7 and 9% (v/v)); T = the gel with Thieves® essential oil in 4 different concentrations of gels (2.4; 4.7; 7 and 9% (v/v)), * p < 0.05.
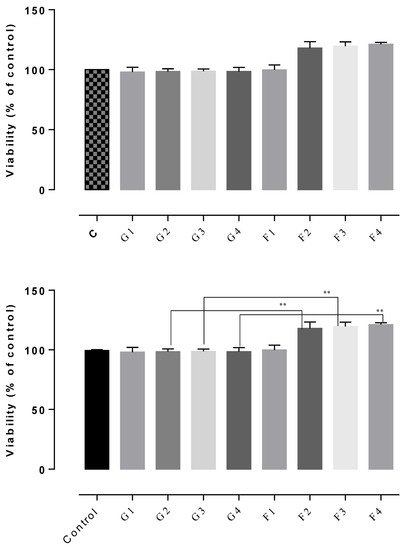
Figure 9.
D1MSCs cells viability assessment after treatment with control gel and gel with Frankincense® essential oil, where Control = D1MSCs cells in normal propagation medium; G1, 2, 3, 4 = the gel without essential oil in 4 different concentrations (2.4; 4.7; 7 and 9% (v/v)); F = the gel with Frankincense® essential oil in 4 different concentrations of gels (2.4; 4.7; 7 and 9% (v/v)), ** p < 0.001.
3.2. Antimicrobial Activity of the Experimental Gels Containing Natural Essential Oils
The increased bacteriostatic potential was recorded for the gel supplemented with Thieves® essential oil and Oregano® essential oil at dilution no. 3 = 16.6 µL containing 0.036 µL of essential oil against the Staphylococcus aureus strain, while the bacteriostatic potency of the gel supplemented with Frankincense® essential oil was observed only at ½ of the dilution involving the use of a volume of 25 µL of gel containing an amount of 0.055 µL of essential oil. The same results were also obtained against the Bacillus cereus strain. A higher bactericidal potential indicated the gel supplemented with Oregano® essential oil for all studied bacterial strains, especially against Bacillus cereus strain, having potency at the 4th dilution (10 µL) of the gel. A similar biological effect was observed for a gel with Frankincense oil, noting a lower efficacy against the Bacillus cereus strain.
Thieves essential oil supplemented gel did not appear to have a bactericidal effect against Escherichia coli and Enterococcus faecalis strains compared to the other two essential oils, but results are similar against Staphylococcus aureus and Bacillus cereus strains, respectively (Table 2).

Table 2.
Bacteriostatic effect: MIC (minimum inhibitory concentration).
Bactericidal efficacy was demonstrated against all tested bacterial strains for gels supplemented with Oregano® and Frankincense® essential oil, but gels supplemented with Thieves® essential oil expressed bactericidal potential only against S. aureus and B. cereus strains (MBC/MIC ≤ 4—this means the composites have a bactericidal effect and if MBC/MIC = 4, the composites have only bacteriostatic effect) (Table 3).

Table 3.
Antibacterial activity (MIC/MBC index).
Thieves® essential oil-supplemented gel did not appear to have a bactericidal effect against Escherichia coli and Enterococcus faecalis strains compared to the other two essential oils, but results are similar against Staphylococcus aureus and Bacillus cereus strains, respectively (Table 3).
Dental hydrogels should be biocompatible and offer a non-toxic delivery mechanism with the regulated release of substances containing natural extracts. These extracts are primarily effective at local therapy, either by themselves or in combination with more traditional techniques such as mechanical cleaning or laser-assisted photodynamic therapy. These kinds of hydrogels with essential oils embedded could be used in a single treatment to reduce patient expenditures and possibly treat chronic mouth diseases. Nevertheless, the biological properties these gels possess make them perfect candidates for oral procedures, which contain natural products which are safe for oral cavity tissues and exhibit not only protective effects but also regenerative potential for soft and hard tissues. Research on innovative drug delivery devices focuses on designing multiple delivery systems for active substances, preventing drug side effects, and reducing dosing intervals [20]. Essential oils are great antibacterial, antifungal, antioxidant, and anti-inflammatory effects that can be augmented by combining them with nanotechnological designs [21]. Herbal materials, which have antibacterial, anti-inflammatory, and antioxidant properties, are widely and frequently utilized in the treatment of numerous oral illnesses that affect the periodontium, including periodontitis. Numerous studies support the positive effects of phytotherapy in treating periodontitis [22]. Natural materials are made up of a variety of chemical structures with a range of biological functions. They are hence the focus of discussion in this decade. Due to their overall efficacy and safety, herbal medications have been utilized to treat periodontal disease [23].
The first studied compound was the Thieves® blend gel, in which four main constituents have different biological roles. Eugenol is said to have positive effects on human health, which is supported by a substantial body of contemporary scientific research. It is one of the major constituents of Clove (Syzygium aromaticum) oil. These effects are mostly linked to anti-inflammatory and antioxidant properties. In investigations, eugenol has also demonstrated exceptional antibacterial action, being effective against fungi and a variety of gram-positive and gram-negative bacteria [24]. In general, monoterpenes alter the characteristics of the plant they are found based on their natural habitat. For instance, citral is a key ingredient in lemon’s aroma. Mandarin orange flavor is influenced by thymol. Other monoterpenes that are components of flower smells and draw pollinators to plants include limonene and geranyl. Menthol, camphor, carvone, thymol, fenchone, and a-pinene are a few examples of monoterpenes that are significant commercially. Cineole, citral, geraniol, linalool, and menthol are other examples of monoterpenes that were evaluated against several bacterial strains, including gram-positive cocci and rods, gram-negative rods, and yeast-like and filamentous fungi [25]. Salmonella typhimurium and Staphylococcus aureus viable counts have been demonstrated to be significantly reduced by EO of thyme or its component thymol, which is another constituent of our experimental gel. The chemically similar terpenes p-cymene and y-terpinene displayed no antagonistic effects against S. typhimurium, in contrast to the phenolic components of the oil, thymol, and carvacrol [25,26].
The monoterpenes, which made up 84.86% of the total and were primarily composed of α-pinene and D-limonene, were also a defining characteristic of the experimental Frankincense® essential oil gel. The majority of the monoterpenoids found in medicinal plants’ essential oils, such as limonene, myrcene, α-terpineol, linalool, β-pinene, p-cymene, and endo-Borneol, are present. All essential oils with a high D-limonene content not only have a potent anti-inflammatory action but also significantly reduce the generation of reactive oxygen species by scavenging free radicals [27]. Several studies indicated the antioxidant potential of D-limonene inhibits lipid peroxidation and fights cell damage induced by free radicals [28]. The monoterpenes include the well-known compound—α-pinene, which is present in the essential oils of numerous plants. Antibiotic resistance modulation, anticoagulant, anti-cancer, antibacterial, antimalarial, antioxidant, anti-inflammatory, anti-Leishmania, and analgesic properties are only a few of the many pharmacological activities that have been identified [29]. The most important effects of α and β -pinene are outlined, including their cytogenetic, gastroprotective, anxiolytic, cytoprotective, anticonvulsant, and neuroprotective effects, as well as their effects on pancreatitis, stress-induced hyperthermia, pulpal pain, and H2O2-stimulated oxidative stress [29].
The three primary constituents of the last tested oregano essential oil are carvacrol, p-cymene, and γ–terpinene. The monoterpenes carvacrol and cymene are frequently present in Origanum essential oils. These show antibacterial and antifungal effects, as well as anti-inflammatory and analgesic properties [30]. These characteristics make these products excellent candidates for the creation of tissue-healing solutions.
All the experimental hydrogels present an important absorption peak around 200 nm and a large band between 260 and 300 nm due to the presence of carvacrol and eugenol, which are the majority constituents of the essential oils extracted from oregano and clove plants. In order to evaluate the potential of the main constituents of the essential oils regarding specific effects in the oral cavity, further in vitro tests were performed, whereas the cytotoxic effect of hydrogels was tested on mesenchymal stem cells (D1MSCs) derived from the dental papilla of human wisdom teeth germs (D1MSCs). No cytotoxic effects were observed in any of our experimental gels. In contradiction with earlier findings [31], where a drop of viability (75%) was recorded in similarly tested gels filled with natural essential oils, our findings prove a lack of in vitro toxicity in the new gel formulations. Even though these results differ from some published studies [21], where the tested gels with essential oils having the same compounds identified by us (carvacrol, thymol) but also cinnamaldehyde were cytotoxic, reducing fibroblast viability below 70%, they are consistent with [32], where hydrogels having Clove essential incorporated showed good cell viability rates to the same human gingival fibroblast cells.
Even if our major interest is to have non-cytotoxic gels, the antibacterial or even bacteriostatic effect is truly desired because the basic etiology of periodontal diseases is infectious and originates from the bacteria found in dental plaque, which forms a specific microbial biofilm made up of a complex population of microorganisms. Approximately 500 bacterial species have been identified in these biofilms, both in healthy and diseased oral cavities, from dogs and cats [33]. Therefore, the best performance was seen in Oregano® essential oil gel. This highlights the usefulness of carvacrol as an antibacterial agent. The antibacterial action of carvacrol, which is stronger against Gram-positive than Gram-negative bacteria, principally relies on bacterial membrane damage; it results in the dissolution of the proton motive force and subsequent reduction in ATP synthesis that leads to a reduction in other energy-dependent cell processes, including synthesis of enzymes and toxins [34]. In particular, carvacrol has extensively been tested as an antimicrobial agent in food to control gram-positive and gram-negative pathogens, including Bacillus cereus, Enterococcus faecalis, Listeria monocytogenes, Staphylococcus aureus, Escherichia coli, Pseudomonas fluorescens, Salmonella typhimurium, Vibrio cholerae, and V. vulnificus [35,36]. The most striking fact to emerge from these data is that all of the pathogens are the main etiologies for oral pathologies concerning the mouth cavity and also gastrointestinal diseases.
These results further widen our knowledge about the importance of phytotherapy and specific natural mini constituents with potent biological activity. Our formula captures the results of the chemical analysis of Oregano® essential oil, which reveals the presence of several ingredients, most of which possess important antioxidant and antimicrobial properties [37]. Carvacrol and thymol, two phenols with antibacterial properties, make up the majority of oregano essential oil (78–85%) [38]. Additionally, small components, including the monoterpene hydrocarbons p-cymene and -terpinene, contributed to the oil’s antimicrobial action [39]. There are several references on the chemical makeup, antibacterial properties, and use as antimicrobials and antioxidants of some oregano species’ essential oils [40].
With the completion of these two test procedures, further data were produced on the effectiveness of the gels, demonstrating that the Thieves®’ hydrogel had the least effective effect and oregano-based gels had the most optimal effect. However, a PubMed search revealed only three other study teams utilizing Oregano® essential oil and none employing Frankincense® or Thieves® for the treatment of periodontitis or other oral disorders, emphasizing the uniqueness of the current work.
Our work has some limitations due to the lack of antibacterial testing on all of the specific bacteria which are considered the etiological causes of periodontitis. After thoroughly testing all three essential oils on the above-mentioned spectrum of bacteria, the best should be chosen, and further specific tests should be carried out to demonstrate the biological effect of the new hydrogels prior to in vivo testing. Other specific tests could include immunofluorescence assays and spectrophotometry analyses allowing us to determine the best essential oil that could be used in the synthesis of the hydrogel, being able to stand out as a singular therapy in periodontitis. Despite all of this, we believe our work could be a starting point in establishing new treatments for oral pathologies.
4. Materials and Methods
4.1. Chemical Composition of the Newly Developed Experimental Gels
Preparation of Hydrogels
In this work, new hydrogels based on essential oils were created and employed as photo-sensitizers in an experimental treatment for periodontitis. Eight new hydrogels were synthesized, six with essential oils and two without (G1, G2).
Hydrogel G1 was obtained as a mix of two gels separately obtained (gel A and gel B) and then mixed together. The ingredients and their proportion are presented in Table 4. Next, we will describe the steps and conditions of mixing them. Gel A was obtained by mixing xanthan gum (Mayam® Elements, Oradea, Romania) and glycerine (Sigma-Aldrich Inc., St. Louis, MO, USA) at room temperature for 30 min until a homogeneous composition. Gel B was obtained by mixing lyophilized whey with polyvinylpyrrolidone, in which a mix of PEG and distilled water were gradually added, and mixing continued for 30 min until a homogeneous composition was obtained. After gel A and B were obtained, they were mixed together to obtain hydrogel G1. We added 2-Hydroxyethyl salicylate (Tokyo Chemical Industry CO., LTD, Tokyo, Japan) to stabilize the system. The composition was divided into four equal amounts, and into three were added different essential oils (Oregano®—G1O, Thieves®—G1T, and Frankincense®—G1F); one composition remained as control material (G1). The obtained hydrogels were mixed for 30 min with a magnetic stirrer AREX Heating Magnetic stirrer (VELP SCIENTIFICA, Usmate, Italy) at 800 rpm without heating only at room temperature (22 °C) for better homogenization.

Table 4.
The chemical composition and the pH of the experimental hydrogels based on essential oils.
Hydrogel G2 was obtained using the same procedure, but the difference is water. We used Kaqun® water.
As a final step, after mixing all the ingredients and obtaining 8 compositions of hydrogels, these were subjected to ultrasound for 15 min in an Ultrasonic bath P 70 H (ELMA, Germany) in a degas mode for the elimination of air bubbles.
4.2. Gas Chromatography-Mass Spectrometry (GC-MS)
One of the goals of the current work was to use GS-MS to demonstrate the presence of essential oils (Oregano®, Thieves®, and Frankincense®) in the gel composition. A volume of 1 µL was fed into the apparatus after the essential oils (3 µL) were diluted in hexane (1 mL). The gel (1 g) was dispersed for two hours in a mixture of hexane and acetone (6 mL each, at a ratio of 2:1), then centrifuged at 4400 rpm for 15 min before being used as a sample for GS-MS. The solvent phase was dried over anhydrous sodium sulfate after centrifugation. By using GC-MS, the extracted essential oils from the gel were evaluated.
Agilent GC-MS Gas Chromatograph-7890A/5975/2008 was used for the analyses (Agilent Technologies, Inc., Europe, Waldbronn, Germany). The studies were carried out in scan mode on an Agilent DB-5MS capillary column (30 m × 0.25 mm × 0.25 m) using high-purity helium as the carrier gas at a flow rate of 1 mL/min. Programming of the temperature included the following: the GS oven temperature for the column set was 40 °C, 5 °C/min to 220 °C (10 min hold), then with 20 °C up to 280 °C (5 min hold), injector temperature 250 °C, injection volume of 1 µL, 100:1 split ratio, MS 70 eV, and mass range u.a.m. 30–400. The NIST library was utilized to locate and confirm the existence of components from essential oils. For the calculation of the linear retention index (LRI), the C8–C20 standard alkanes solution (Alkane Standard Solution C8–C20, Sigma Aldrich, St. Louis, MO, USA) was used. The experimental values matched those reported in the literature for similar chromatographic columns and identical conditions. The compounds identified in the oil samples are presented in Table 2. Each value in Table 2 represents the mean percentage value ± standard deviation (SD) of triple analyses. The percentage peak area method was used for the qualitative analysis of the investigated compounds.
4.3. UV-Vis Absorption Spectroscopy
To evidence the presence of absorption bands of the essential oils in the experimental hydrogels, we employed a twin beam Jasco V-750 UV/Vis Spectro-photometer (JASCO Corporation, Tokyo, Japan). The spectral range of the visible and near-infrared allowed for the measurement of the optical characteristics (between 400 and 1000 nm). A syringe is used to transfer about 3 mL of gel into a quartz cuvette, rectangular in shape, with an internal width of 1 cm through which light crosses. To check the repeatability of the measurements, each experiment was run three times.
4.4. Cytotoxicity Assay
Human mesenchymal stem cells (D1MSCs) generated from the dental papilla of human wisdom teeth germs were used to evaluate the hydrogels’ cytotoxicity (D1MSCs). The mixed technique was used to obtain D1MSCs (tissue explants and enzymatic treatment). Dental pulp was removed, diced (1–2 mm2), and then subjected to a 30-min enzymatic treatment using a 0.1% IV collagenase and trypsin-EDTA solution from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA).
The remaining tissue explants and the cell solution were put into culture plates that had already been coated with 4% porcine skin gelatin (Sigma-Aldrich, St. Louis, MO, USA). The propagation medium was composed of 10% fetal calf serum (FCS) (Gibco Life Technologies, Paisley, UK), 100 mg/mL streptomycin (Gibco Life Technologies, Paisley, UK), 100 U/mL penicillin (Gibco Life Technologies, Paisley, UK), and 50 mg/mL gentamicin (Gibco Life Technologies, Paisley, UK) (Gibco Life Technologies, Paisley, UK). The evaluation was carried out daily while the plates were incubated at 37 °C in a microenvironment enhanced with 5% CO2 and 90% humidity. After 72 h, the culture media was replaced. The passages were carried out at a confluence of 60–70% after the explants were removed after 5 days. A concentration of 5 × 103 cells/plate was used for subculturing. After 6 passages, the immunophenotypic (CD 44, CD79a, HLA-DR, CD105, CD29, CD34/45, CD49f, CD14, and CD90) and functional (trilinear differentiation, clonal capacity, migration capacity, and adhesion) characterization were assessed.
The cytotoxic potential of the essential oils supplemented hydrogels was assessed through MTT assay (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). In order to obtain cell suspensions, the D1MSCs cultures were treated with 0.25% trypsin-EDTA, and after centrifugation (1500 rpm for 5 min), 1 × 104 cells/well were seeded on 96 wells plates in 200 µL complete culture medium. After 24 h, the hydrogels were added at the predetermined concentrations (4 concentrations: 2.4, 4.7, 7, and 9% (v/v) of gel). The concentration of 100% oil extracted by the cold press method implies the quantity of 0.01 µL, 0.02 µL, 0.03 µL and 0.04 µL of pure oil for each type of tested composite.
As a control, cells kept in the typical propagation medium were employed. Three duplicates of each experimental condition were carried out. After 24 h, the degree of cell proliferation was evaluated. The culture media was taken out, 100 L of MTT solution (1 mg/mL) was added, and the mixture was incubated at 37 °C for three hours. The formazan was dissolved in DMSO (dimethyl sulfoxide; Honeywell, Fluka, Seelze, Germany) solution, and the chromogenic reaction was assessed at 450 nm using a BioTek Synergy 2 microplate reader (Winooski, VT, USA). By reporting the optical density in comparison to the control, the results were calculated.
4.5. The Antimicrobial Effect
Using agar well-diffusion experiments, the hydrogels’ antibacterial efficacy was assessed. Four bacterial reference strains (n = 4 each of Staphylococcus aureus ATCC 25923, Bacillus cereus ATCC 14579, Enterococcus faecalis ATCC 29219, and Escherichia coli ATCC 25922) and two clinical Staphylococcus aureus isolates (n = 2) were used. A concentration of 1 × 107 colony forming units (CFU)/mL, determined using the McFarland scale, was employed for the 24-h bacterial culture. Flooding was used to inoculate the Muller Hinton agar plates, and then 6 mm diameter wells were cut (in triplicate). As a control, amoxicillin/clavulanic acid (20/10 mg, Oxoid) was used. The widths of the growth inhibition zones were measured following a 24-h incubation period at 37 °C. In addition, the minimum inhibitory concentrations (MIC) and minimum bactericidal (MBC) concentrations of the hydrogels were established using a broth microdilution method. Serial dilutions were made in 100 µL broths for the selected hydrogels; 5.0 µL of 1 × 107 CFU/mL bacterial inoculum was added in each well, followed by cultivation at 37 °C for 24 h.
The most crucial “one-number characterization” of how effective an antimicrobial drug is against a target organism is the minimum inhibitory concentration (MIC).
The minimum inhibitory concentration assay is one of the main techniques for evaluating the emergence of antibiotic resistance in bacteria. These assays result in censoring, which, as opposed to a precise value, indicates a minimum inhibitory value from an interval of concentrations. The MIC is described as the lowest concentration (in mg L−1) of the antibacterial element that impedes the visible growth of a microorganism under defined circumstances noticed with the unaided eye. In clinical application, this in vitro parameter is used to classify the tested microorganism as clinically susceptible, intermediate, or resistant to the tested drug. For new drug candidates, MIC determination is one of the first steps to evaluate the antimicrobial potential [41,42,43].
The quantities of hydrogels were 33.6 mg (50 µL), 16.8 mg (25 µL), 11.15 mg (16.6 µL), 8.4 mg (12.5 µL) and 6.72 mg (10 µL). The amount of pure essential oils was 0.11 µL, 0.055 µL, 0.036 µL, 0.0275 µL, and 0.022 µL. The lowest concentration of hydrogels with growth inhibition potential (the absence of turbidity) of bacteria is considered the MIC.
For MBC evaluation, 10.0 µL of bacterial culture from each well was added to Muller-Hinton agar plates. After 24 h, the lowest hydrogel concentration that induces bacterial growth inhibition in the agar plate is considered the MBC.
Three duplicates of each experiment were carried out. The MBC/MIC ratio (bactericidal—MBC/MIC 4 and bacteriostatic—MBC/MIC > 4) was used to express the bactericidal and bacteriostatic potential of the studied hydrogels.
4.6. Statistical Analysis
The one-way ANOVA and t-test were used for the statistical analysis. The results were expressed as the mean ± standard deviation (SD), and p ≤ 0.05 was considered statistically significant.
5. Conclusions
The specific active compounds from essential oils (eugenol, pinene, limonene, carvacrol, and cymene) were identified by GC-MS analysis, and the absorption bands of the essential oils from the hydrogel’s composition were evidenced by UV-Vis. The lack of in vitro toxicity of the gels was demonstrated by the reduced cytotoxic effect all of the investigated chemicals had on cell cultures.
This study demonstrates that hydrogels enriched with pure natural extracts of essential oils have a tangible in vitro antimicrobial potential, especially for Staphylococcus aureus and Bacillus cereus, two principal bacteria found in oral-digestive diseases.
The findings of this study indicate that the best essential oil with the highest biological antimicrobial and the non-toxic effect was Oregano® essential oil embedded in our experimental hydrogel.
We have found an innovative solution for the treatment of several strains of oral-specific bacteria by using natural compounds with antibacterial properties in order to avoid local mouth antimicrobial resistance, and also, we have developed a strategy for periodontitis treatment. These strategies will be discussed in future works concerning in vivo action of these hydrogels.
Author Contributions
Conceptualization, S.M.C.M., M.M. and A.D.; methodology, C.D. and O.R.; software, S.P.; validation, R.A.P. and C.R.; formal analysis, C.D., R.A.P. and S.P.; investigation, S.M.C.M., E.P., C.R., I.C., A.G.M., C.S. and O.R.; data curation, L.O.; writing—original draft preparation, S.M.C.M., A.D. and E.P.; writing—review and editing, A.D. and C.S.; visualization, M.M.; supervision, L.O. All authors have read and agreed to the published version of the manuscript.
Funding
National Research Development Projects to finance excellence (PFE)—14/2022-2024 granted by the Romanian Ministry of Research and Innovation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data may be provided on request from the main author.
Acknowledgments
This work was supported by the project “The Development of Advanced and Applicative Research Competencies in the Logic of STEAM + Health”/POCU/993/6/13/153310, project co-financed by the European Social Fund through The Romanian Operational Programme Human Capital 2014–2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chandrasekaran, S. Antibacterial Activity of the Three Essential Oils on Oral Pathogens-An In-Vitro Study. Res. J. Pharm. Technol. 2014, 7, 1128. [Google Scholar]
- Kumari, S.; Pundhir, S.; Priya, P.; Jeena, G.; Punetha, A.; Chawla, K.; Jafaree, Z.F.; Mondal, S.; Yadav, G. EssOilDB: A Database of Essential Oils Reflecting Terpene Composition and Variability in the Plant Kingdom. Database 2014, 2014, bau120. [Google Scholar] [CrossRef]
- Tavares Bekner Correa, G.; Alves Costa Veranio, G.; Esmaeraldo Silva, L.; Hirata Junior, R.; Coil, J.M.; Zaccaro Scelza, M.F. Cytotoxicity Evaluation of Two Root Canal Sealers and a Commercial Calcium Hydroxide Paste on Thp1 Cell Line By Trypan Blue Assay. J. Appl. Oral Sci. 2009, 17, 457–461. [Google Scholar] [CrossRef] [PubMed]
- De Luca, I.; Pedram, P.; Moeini, A.; Cerruti, P.; Peluso, G.; di Salle, A.; Germann, N. Nanotechnology Development for Formulating Essential Oils in Wound Dressing Materials to Promote the Wound-Healing Process: A Review. Appl. Sci. 2021, 11, 1713. [Google Scholar] [CrossRef]
- Alvarez Echazú, M.I.; Olivetti, C.E.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development and Evaluation of Thymol-Chitosan Hydrogels with Antimicrobial-Antioxidant Activity for Oral Local Delivery. Mater. Sci. Eng. C 2017, 81, 588–596. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Sobczak, M. Pharmaceutics Hydrogel-Based Active Substance Release Systems for Cosmetology and Dermatology Application: A Review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the Clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Kuang, X.; Chen, V.; Xu, X. Novel Approaches to the Control of Oral Microbial Biofilms. BioMed Res. Int. 2018, 2018, 6498932. [Google Scholar] [CrossRef]
- Ashrafi, B.; Rashidipour, M.; Marzban, A.; Soroush, S.; Azadpour, M.; Delfani, S.; Ramak, P. Mentha Piperita Essential Oils Loaded in a Chitosan Nanogel with Inhibitory Effect on Biofilm Formation against S. Mutans on the Dental Surface. Carbohydr. Polym. 2019, 212, 142–149. [Google Scholar] [CrossRef]
- Johari, S.; Pushpalatha, C.; Deveswaran, R. Formulation and Antimicrobial Assessment of Intracanal Medicament Containing Oregano Essential Oil. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2020; Volume 2274. [Google Scholar]
- Yanakiev, S. Effects of Cinnamon (Cinnamomum spp.) in Dentistry: A Review. Molecules 2020, 25, 4184. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (Rosemary) as Therapeutic and Prophylactic Agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, W.; Canada, A.-F.; Zhang, G.; Li, Y.; Zhao, C.; Zhang, Z.; Nie, D. Protective Effect of Lemon Essential Oil and Its Major Active Component, D-Limonene, on Intestinal Injury and Inflammation of E. Coli-Challenged Mice. Front. Nutr. 2022, 1, 843096. [Google Scholar] [CrossRef]
- Elaissi, A.; Rouis, Z.; Abid Ben Salem, N.; Mabrouk, S.; ben Salem, Y.; Bel Haj Salah, K.; Aouni, M.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F.; et al. Chemical Composition of 8 Eucalyptus Species’ Essential Oils and the Evaluation of Their Antibacterial, Antifungal and Antiviral Activities. BMC Complement. Altern. Med. 2012, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Kodadová, A.; Vitková, Z.; Herdová, P.; Ťažký, A.; Oremusová, J.; Grančai, D.; Mikuš, P. Formulation of Sage Essential Oil (Salvia officinalis, L.) Monoterpenes into Chitosan Hydrogels and Permeation Study with GC-MS Analysis. Drug Dev. Ind. Pharm. 2015, 41, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Rusu, L.C.; Kaya, D.A.; Ghica, M.V.; Albu, M.G.; Popa, L.; Butu, A.; Dinu-Pirvu, C.E. Eucalyptus-collagen composite gels for dentistry applications. Biology 2014, 9, 317–323. [Google Scholar] [CrossRef]
- Horváth, B.; Balázs, V.L.; Varga, A.; Böszörményi, A.; Kocsis, B.; Horváth, G.; Széchenyi, A. Preparation, Characterisation and Microbiological Examination of Pickering Nano-Emulsions Containing Essential Oils, and Their Effect on Streptococcus mutans Biofilm Treatment. Sci. Rep. 2019, 9, 16611. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J.; Paulson, J.A. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef]
- Gościniak, A.; Paczkowska-Walendowska, M.; Skotnicka, A.; Ruchała, M.A.; Cielecka-Piontek, J. Can Plant Materials Be Valuable in the Treatment of Periodontal Diseases? Practical Review. Pharmaceutics 2021, 13, 2185. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, P.E.F.; Ranjbar, R.; Yazdanian, M.; Tahmasebi, E.; Alam, M.; Abbasi, K.; Tebyaniyan, H. The Current Natural/Chemical Materials and Innovative Technologies in Periodontal Diseases Therapy and Regeneration: A Narrative Review. Mater. Today Commun. 2022, 32, 104099. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial Activity of Eugenol and Essential Oils Containing Eugenol: A Mechanistic Viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Loza-Tavera, H. Monoterpenes in Essential Oils. Biosynthesis and Properties. Adv. Exp. Med. Biol. 1999, 464, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Juven, B.J.; Kanner, J.; Schved, F.; Weisslowicz, H.; Schved, A.N.F.; Weisslowicz, D.H. Factors That Interact with the Antibacterial Action of Thyme Essential Oil and Its Active Constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- Banjari, I.; Balkić, J.; Yashasvi Waisundara, V. Analgesic Potential of Monoterpenes from Citrus Essential Oils. In Pain Management—Practices, Novel Therapies and Bioactives; IntechOpen: London, UK, 2021. [Google Scholar]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-Limonene: A Multifunctional Compound with Potent Therapeutic Effects. J. Food Biochem. 2020, 45, e13566. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Kumar, N.V.A.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Costa, M.F.; Durço, A.O.; Rabelo, T.K.; Barreto, R.d.S.S.; Guimarães, A.G. Effects of Carvacrol, Thymol and Essential Oils Containing Such Monoterpenes on Wound Healing: A Systematic Review. J. Pharm. Pharmacol. 2019, 71, 141–155. [Google Scholar] [CrossRef]
- Dascalu, L.M.; Moldovan, M.; Prodan, D.; Ciotlaus, I.; Popescu, V.; Baldea, I.; Carpa, R.; Sava, S.; Chifor, R.; Badea, M.E. Materials Assessment and Characterization of Some New Photosensitizers for Antimicrobial Photodynamic Therapy (APDT). Materials 2020, 13, 3012. [Google Scholar] [CrossRef]
- Huerta, R.R.; Silva, E.K.; El-Bialy, T.; Saldaña, M.D.A. Clove Essential Oil Emulsion-Filled Cellulose Nanofiber Hydrogel Produced by High-Intensity Ultrasound Technology for Tissue Engineering Applications. Ultrason. Sonochem. 2019, 64, 104845. [Google Scholar] [CrossRef]
- Melo, R.S.; Azevedo, M.A.; Pereira, A.M.G.; Rocha, R.R.; Cavalcante, R.M.B.; Matos, M.N.C.; Lopes, P.H.R.; Gomes, G.A.; Rodrigues, T.H.S.; dos Santos, H.S.; et al. Molecules Chemical Composition and Antimicrobial Effectiveness of Ocimum gratissimum L. Essential Oil Against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli. Molecules 2019, 24, 3864. [Google Scholar] [CrossRef]
- Nostro, A.; Papalia, T. Antimicrobial Activity of Carvacrol: Current Progress and Future Prospectives. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L.; Hayashi, M.A.F.; Knapp, C. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between Essential Oil Components and Antibiotics: A Review. Crit. Rev. Microbiol. 2013, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Özkan, G.; Saǧdiç, O.; Özcan, M. Note: Inhibition of Pathogenic Bacteria by Essential Oils at Different Concentrations. Food Sci. Technol. Int. 2003, 9, 85–88. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Dardioti, A.; Krigas, N.; Lanaras, T. Autumn Essential Oils of Greek Oregano. Phytochemistry 1997, 44, 883–886. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Theodoridou, I.; Papaemmanouil, V.; Kapsiotis, I.; Panopoulou, M.; Stavropoulou, E.; Bezirtzoglou, E.E.; et al. Antimicrobial Activity of Essential Oils of Cultivated Oregano (Origanum vulgare), Sage (Salvia officinalis), and Thyme (Thymus vulgaris) against Clinical Isolates of Escherichia Coli, Klebsiella Oxytoca and Klebsiella Pneumoniae. Microb. Ecol. Health Dis. 2015, 26, 23289. [Google Scholar] [CrossRef]
- Jepson, A.K.; Schwarz-Linek, J.; Ryan, L.; Ryadnov, M.G.; Poon, W.C.K. What Is the ‘Minimum Inhibitory Concentration’ (MIC) of Pexiganan Acting on Escherichia coli?—A Cautionary Case Study. Adv. Exp. Med. Biol. 2016, 915, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Kelman, T.; Pitesky, M. Overview of Quantitative Methodologies to Understand Antimicrobial Resistance via Minimum Inhibitory Concentration. Animals 2020, 10, 1405. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).