Template-Free Synthesis of One-Dimensional SnO2 Nanostructures Using Highly Efficient Hydrothermal Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
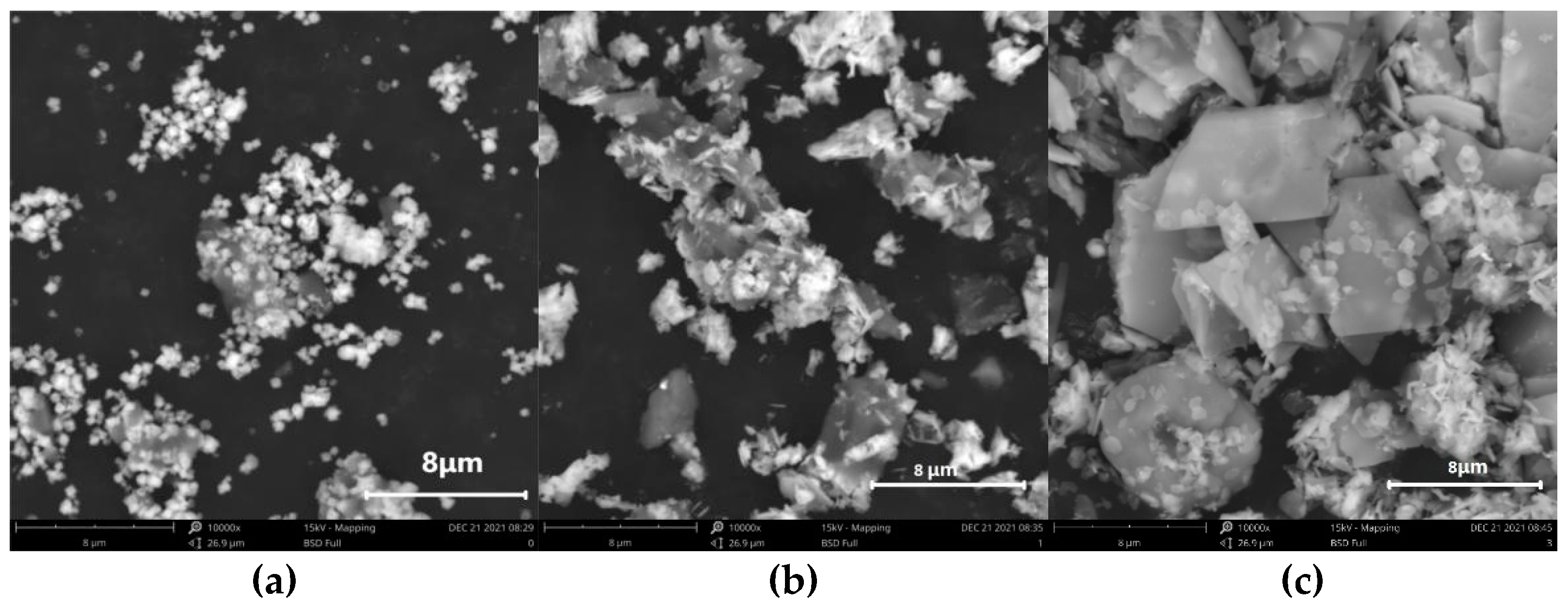
3.1. Effects of Varying Reaction Times in Absolute Alcohol on SnO2 Morphology
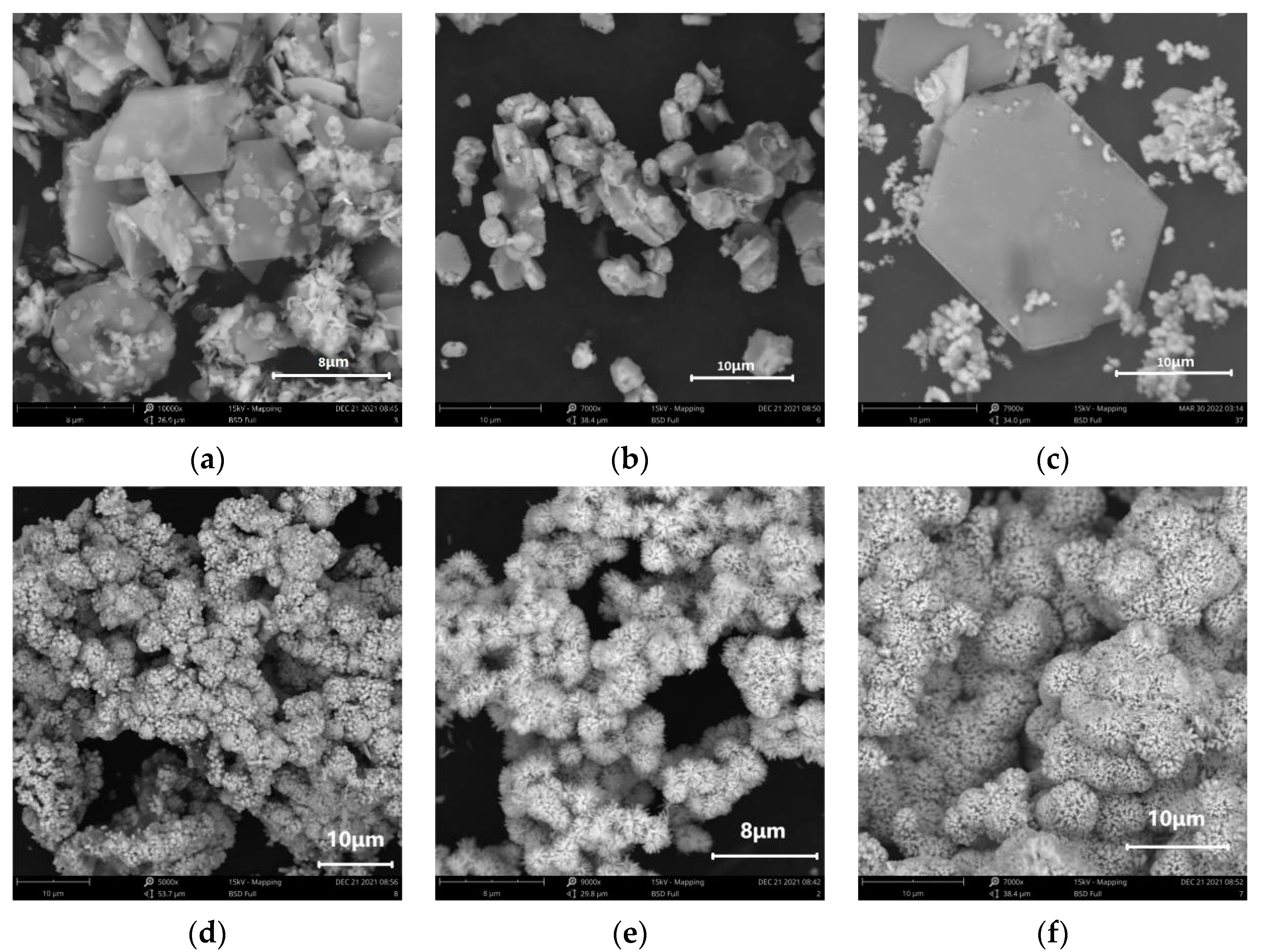
3.2. Effects of Varying Volume Ratios between Absolute Alcohol and Deionised Water on SnO2 Morphology
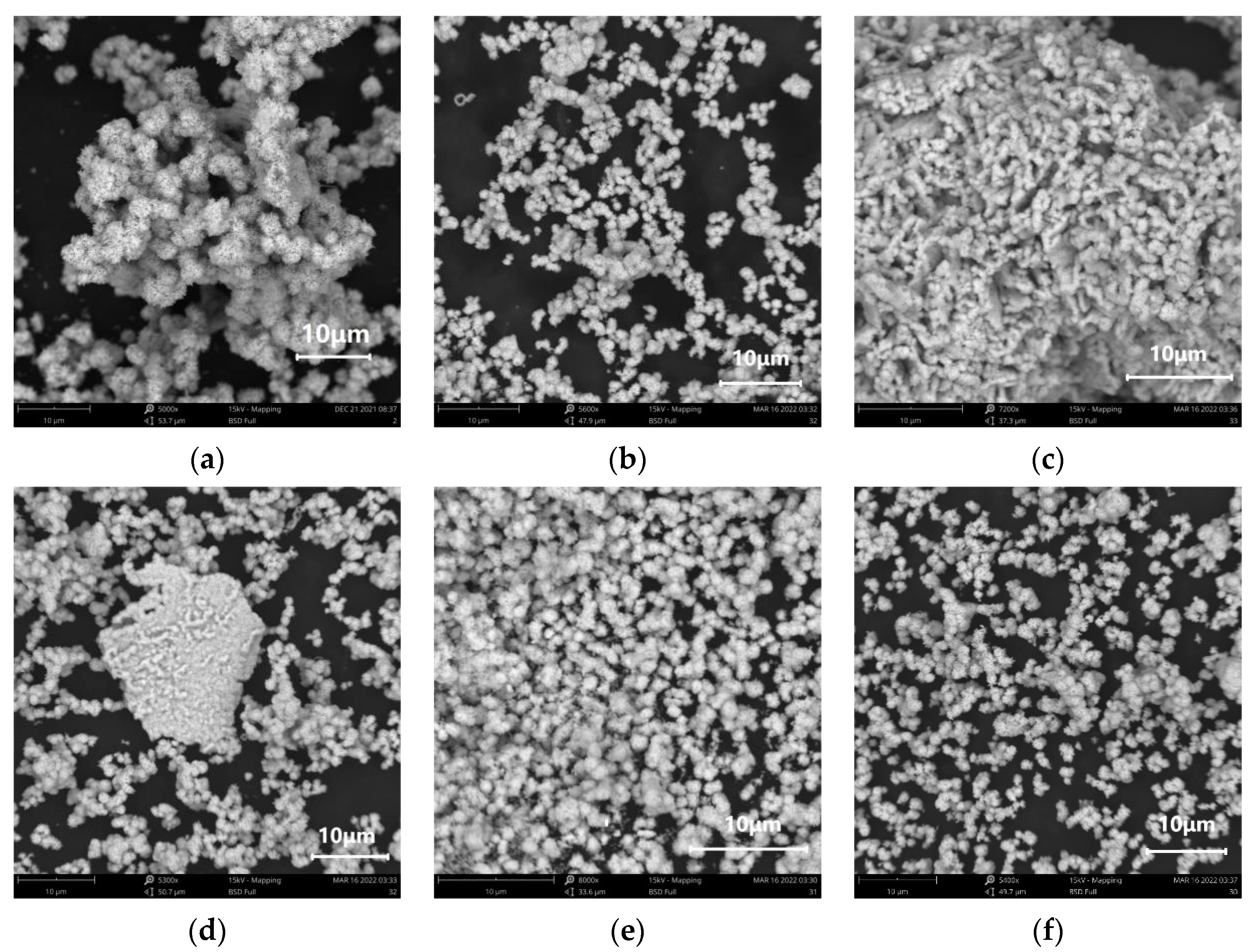
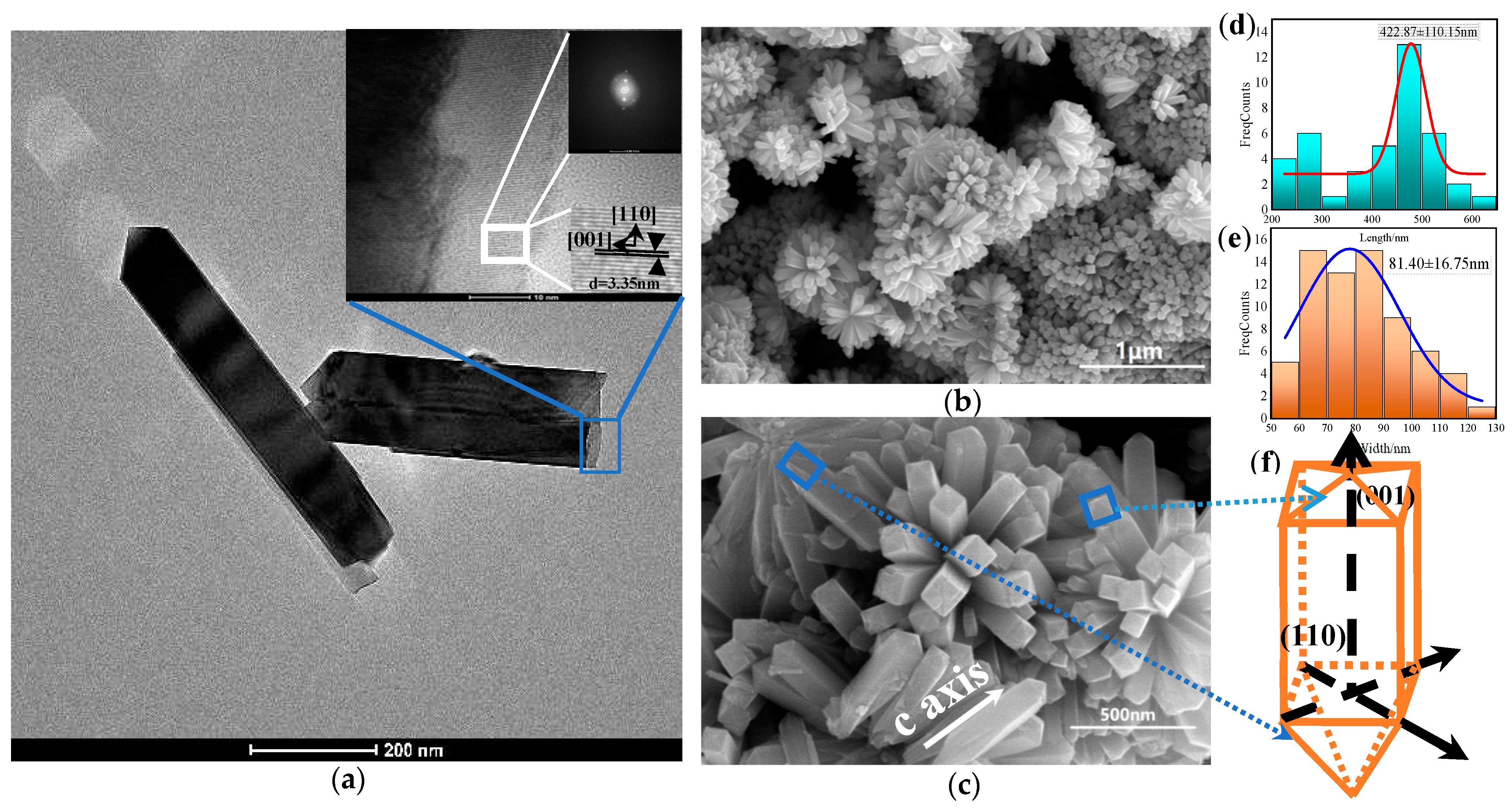
3.3. Effects of Reaction Time on SnO2 Morphology
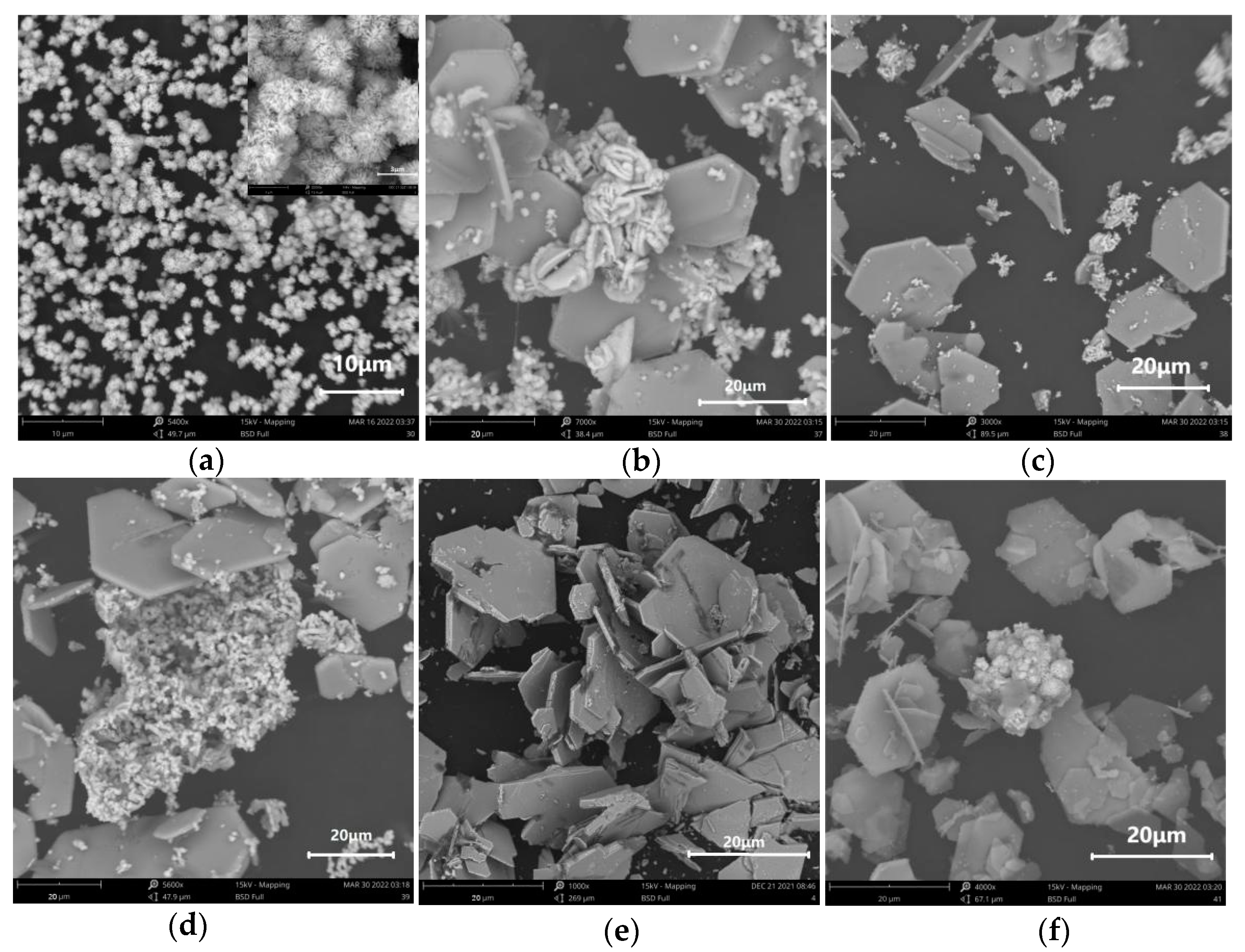
3.4. Effects of Reaction Temperature on SnO2 Morphology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Devan, R.S.; Patil, R.A.; Lin, J.H.; Ma, Y.R. One-dimensional metal-oxide nanostructures: Recent developments in synthesis, characterization, and applications. Adv. Funct. Mater. 2012, 22, 3326–3370. [Google Scholar] [CrossRef]
- Mathur, S.; Barth, S. One-dimensional semiconductor nanostructures: Growth, characterization and device applications. Z. Phys. Chem. 2008, 222, 307–317. [Google Scholar] [CrossRef]
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. Room-temperature ultraviolet nanowire nanolasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef]
- Zhao, Q.X.; Willander, M.; Morjan, R.E.; Hu, Q.H.; Campbell, E.E.B. Optical recombination of ZnO nanowires grown on sapphire and Si substrates. Appl. Phys. Lett. 2003, 83, 165–167. [Google Scholar] [CrossRef]
- Xi, G.; Ye, J. Ultrathin SnO2 nanorods: Template-and surfactant-free solution phase synthesis, growth mechanism, optical, gas-sensing, and surface adsorption properties. Inorg. Chem. 2010, 49, 2302–2309. [Google Scholar] [CrossRef]
- Wang, Y.L.; Guo, M.; Zhang, M.; Wang, X.D. Hydrothermal preparation and photoelectrochemical performance of size-controlled SnO2 nanorod arrays. CrystEngComm 2010, 12, 4024–4027. [Google Scholar] [CrossRef]
- Chen, Y.J.; Xue, X.Y.; Wang, Y.G.; Wang, T.H. Synthesis and ethanol sensing characteristics of single crystalline SnO2 nanorods. Appl. Phys. Lett. 2005, 87, 233503. [Google Scholar] [CrossRef]
- Cheng, B.; Russell, J.M.; Shi, W.; Zhang, L.; Samulski, E.T. Large-scale, solution-phase growth of single-crystalline SnO2 nanorods. J. Am. Chem. Soc. 2004, 126, 5972–5973. [Google Scholar] [CrossRef]
- Chen, D.; Xu, J.; Xie, Z.; Shen, G. Nanowires assembled SnO2 nanopolyhedrons with enhanced gas sensing properties. ACS Appl. Mater. Interfaces 2011, 3, 2112–2117. [Google Scholar] [CrossRef]
- Qin, L.; Xu, J.; Dong, X.; Pan, Q.; Cheng, Z.; Xiang, Q.; Li, F. The template-free synthesis of square-shaped SnO2 nanowires: The temperature effect and acetone gas sensors. Nanotechnology 2008, 19, 185705. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Tian, J.; Cui, H.; Wang, X. Synthesis of 1D SnO2 nanorods/2D NiO porous nanosheets pn heterostructures for enhanced ethanol gas sensing performance. Vacuum 2022, 205, 111399. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Hong, S.Y.; Cho, I.S. All-solution-processed BiVO4/SnO2 nanorods-axial-heterostructure with improved charge collection properties for solar water-splitting. Ceram. Int. 2022, 48, 33101–33107. [Google Scholar] [CrossRef]
- Parveen, A.; Surumbarkuzhali, N.; Shkir, M.; Massoud, E.E.S.; Manjunath, V.; Ahn, C.H.; Park, S.H. Design of SnO2 nanorods/polypyrrole nanocomposite photocatalysts for photocatalytic activity towards various organic pollutants under the visible light irradiation. Inorg. Chem. Commun. 2022, 142, 109685. [Google Scholar] [CrossRef]
- Yadav, V.; Singh, N.; Meena, D. Investigation of structural and optical properties of pure SnO2, ZnO and SnO2/ZnO composite nanorods. Mater. Today Proc. 2022, 62, 3368–3375. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, W.; Xu, H.; Shi, J. Fabrication of ordered SnO2 nanotube arrays via a template route. Mater. Chem. Phys. 2006, 99, 127–130. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Y.; Sun, S.; Li, R.; Sun, X. Three growth modes and mechanisms for highly structure-tunable SnO2 nanotube arrays of template-directed atomic layer deposition. J. Mater. Chem. 2011, 21, 12321–12330. [Google Scholar] [CrossRef]
- Lai, M.; Martinez, J.A.G.; Grätzel, M.; Riley, D.J. Preparation of tin dioxide nanotubes via electrosynthesis in a template. J. Mater. Chem. 2006, 16, 2843–2845. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, W.; Yang, H.; Ma, D.; Cao, J.; Leng, Y.; Guo, J.; Zhang, Y.; Sui, Y.; Zhao, W.; et al. Synthesis and ethanol-sensing properties of flowerlike SnO2 nanorods bundles by poly (ethylene glycol)-assisted hydrothermal process. Mater. Chem. Phys. 2010, 124, 614–618. [Google Scholar] [CrossRef]
- Chen, D.; Gao, L. Facile synthesis of single-crystal tin oxide nanorods with tunable dimensions via hydrothermal process. Chem. Phy. Lett. 2004, 398, 201–206. [Google Scholar] [CrossRef]
- Shen, X.S. Shape-Controlled Synthesis, Self-Assembly and Sers Properties of Noble Metal Nanoparticles. Ph.D. Thesis, University of Science and Technology of China, Hefei, China, 2010. [Google Scholar]
- Lin, Z.J. Microstructure Control and Properties of Ag-SnO2 and Ag-Ni Electrical Contact Materials. Ph.D. Thesis, Northeastern University, Shenyang, China, 2016. [Google Scholar]
- Wang, W.S.; Zhen, L.; Xu, C.; Shao, W. Hollow inorganic micro/nanostructures synthesized by solution-phase method. Prog. Chem. 2008, 20, 679–689. [Google Scholar]
- Slater, B.; Catlow, C.R.A.; Gay, D.H.; Williams, D.E.; Dusastre, V. Study of surface segregation of antimony on SnO2 surfaces by computer simulation techniques. J. Phys. Chem. B 1999, 103, 10644–10650. [Google Scholar] [CrossRef]
- Oviedo, J.; Gillan, M.J. Energetics and structure of stoichiometric SnO2 surfaces studied by first-principles calculations. Surf. Sci. 2000, 463, 93–101. [Google Scholar] [CrossRef]
- Peng, Q.; Li, Y.D. Controlled synthesis, assembly of functional nanomaterials and their properties exploration based on structures. Sci. China Chem. 2009, 39, 1028–1052. [Google Scholar]


| Name | Grade | Manufacturer |
|---|---|---|
| Stannic chloride pentahydrate (SnCl4˙5H2O) | Analytically pure | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) |
| Sodium hydroxide (NaOH) | Analytically pure | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) |
| Absolute alcohol (C2H5OH) | Analytically pure | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) |
| Deionised water (H2O) | Self-manufactured | |
| Polyvinylpyrrolidone (PVP-k30) | Analytically pure | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Li, H.; Chen, Y.; Xie, M.; Bi, Y. Template-Free Synthesis of One-Dimensional SnO2 Nanostructures Using Highly Efficient Hydrothermal Method. Appl. Sci. 2023, 13, 2048. https://doi.org/10.3390/app13042048
Zhao J, Li H, Chen Y, Xie M, Bi Y. Template-Free Synthesis of One-Dimensional SnO2 Nanostructures Using Highly Efficient Hydrothermal Method. Applied Sciences. 2023; 13(4):2048. https://doi.org/10.3390/app13042048
Chicago/Turabian StyleZhao, Jingchen, Hongmei Li, Yongtai Chen, Ming Xie, and Yanan Bi. 2023. "Template-Free Synthesis of One-Dimensional SnO2 Nanostructures Using Highly Efficient Hydrothermal Method" Applied Sciences 13, no. 4: 2048. https://doi.org/10.3390/app13042048
APA StyleZhao, J., Li, H., Chen, Y., Xie, M., & Bi, Y. (2023). Template-Free Synthesis of One-Dimensional SnO2 Nanostructures Using Highly Efficient Hydrothermal Method. Applied Sciences, 13(4), 2048. https://doi.org/10.3390/app13042048





