Hydrodynamics and Musculature Actuation of Fish during a Fast Start
Abstract
:1. Introduction
2. Materials and Methods
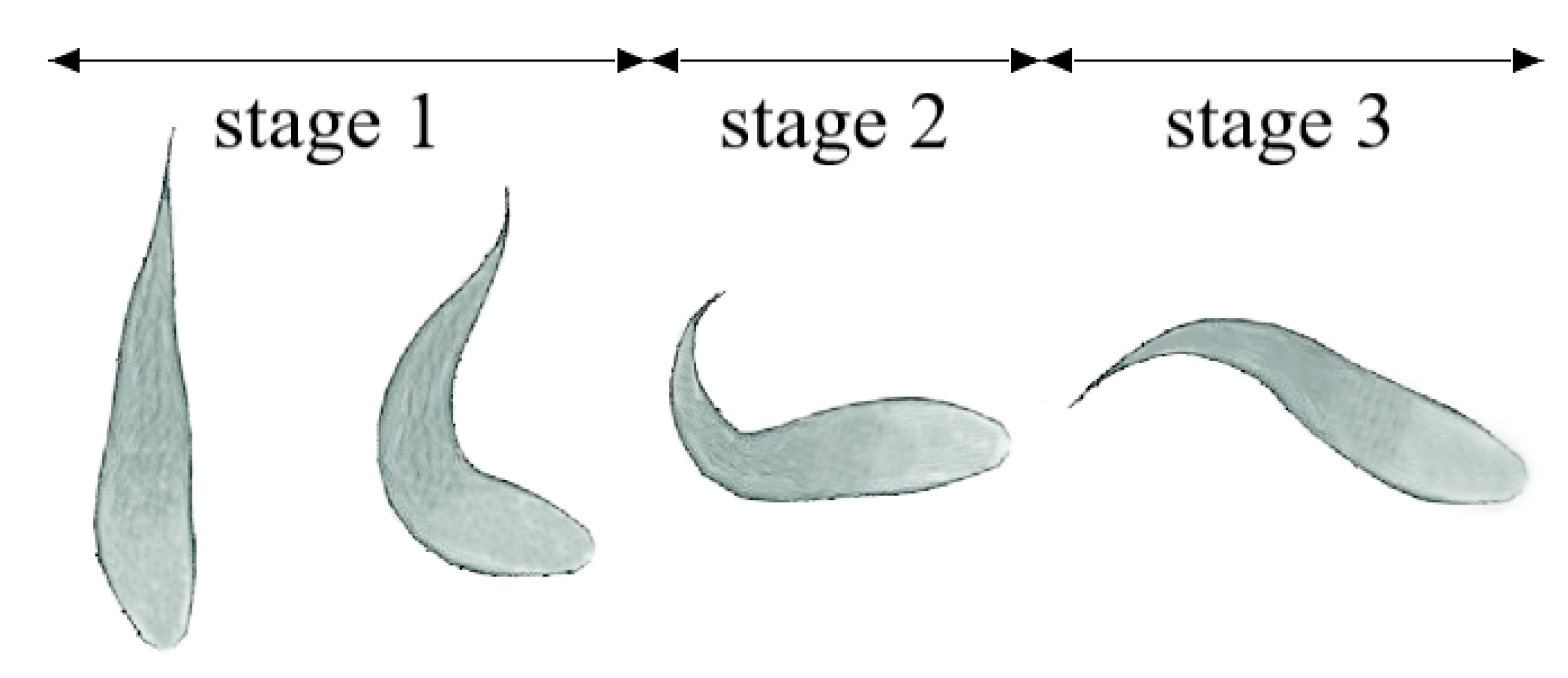
2.1. Geometric Modeling and Kinematics
2.2. Numerical Methods and Simulation Setup
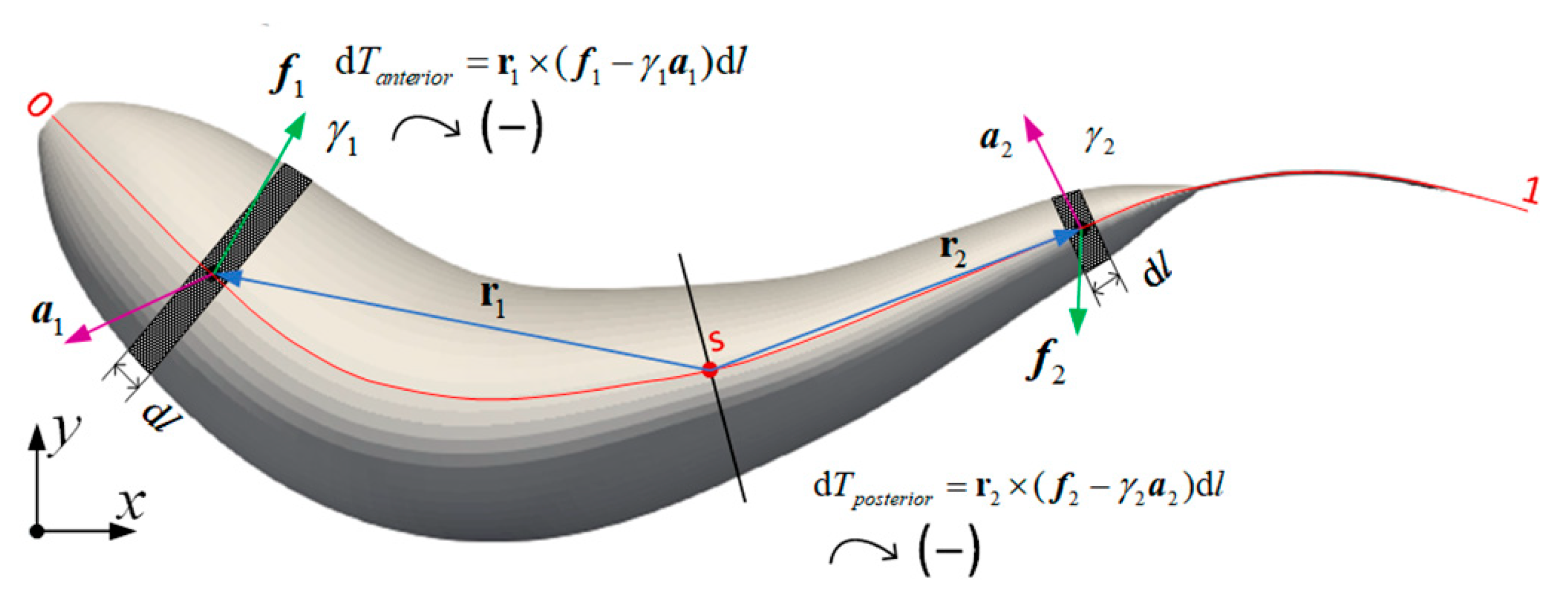
2.3. Internal Torque
3. Results
3.1. Swimming Performance
3.2. Force, Torque, and Power
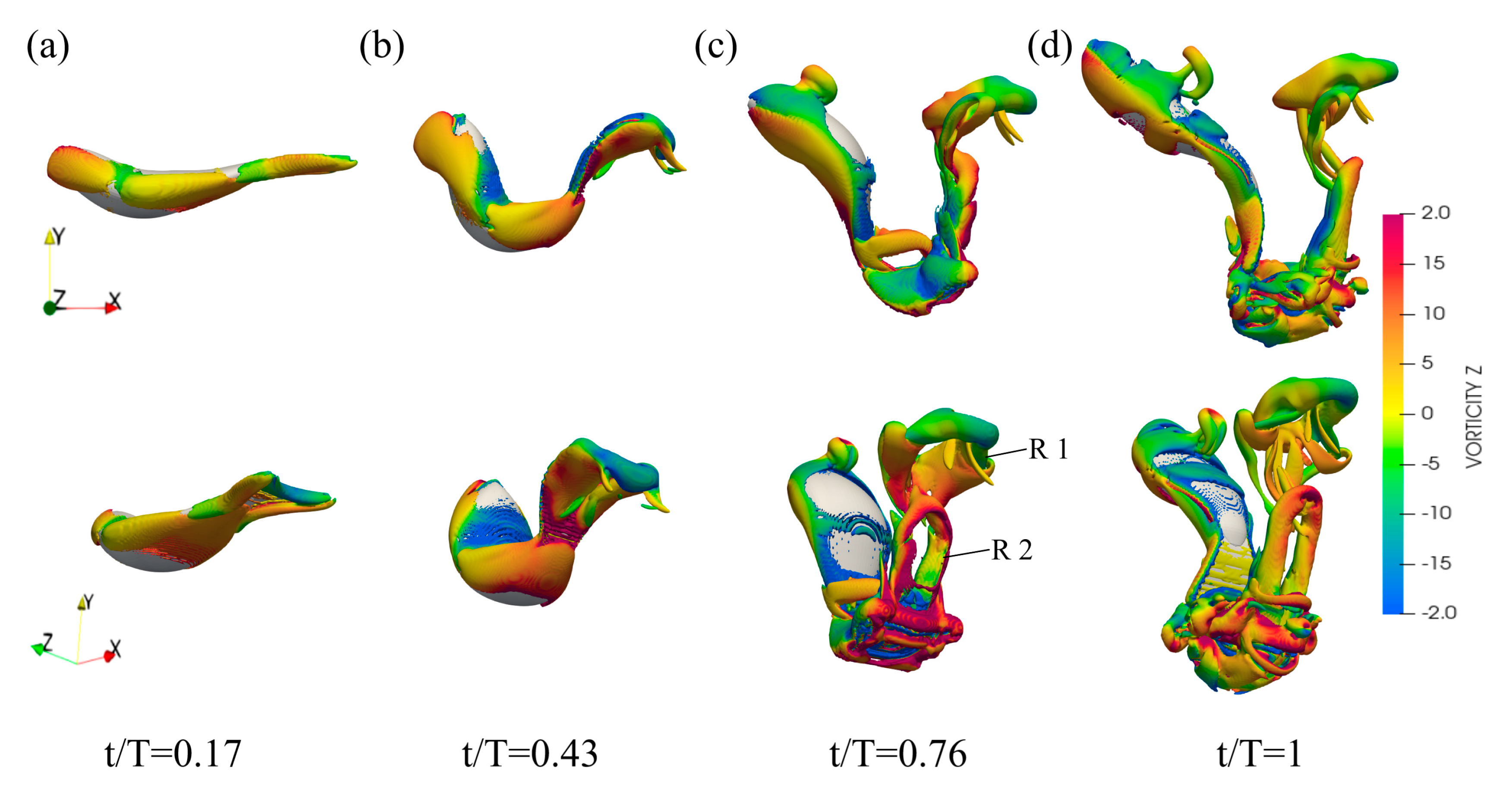
3.3. Flow Field
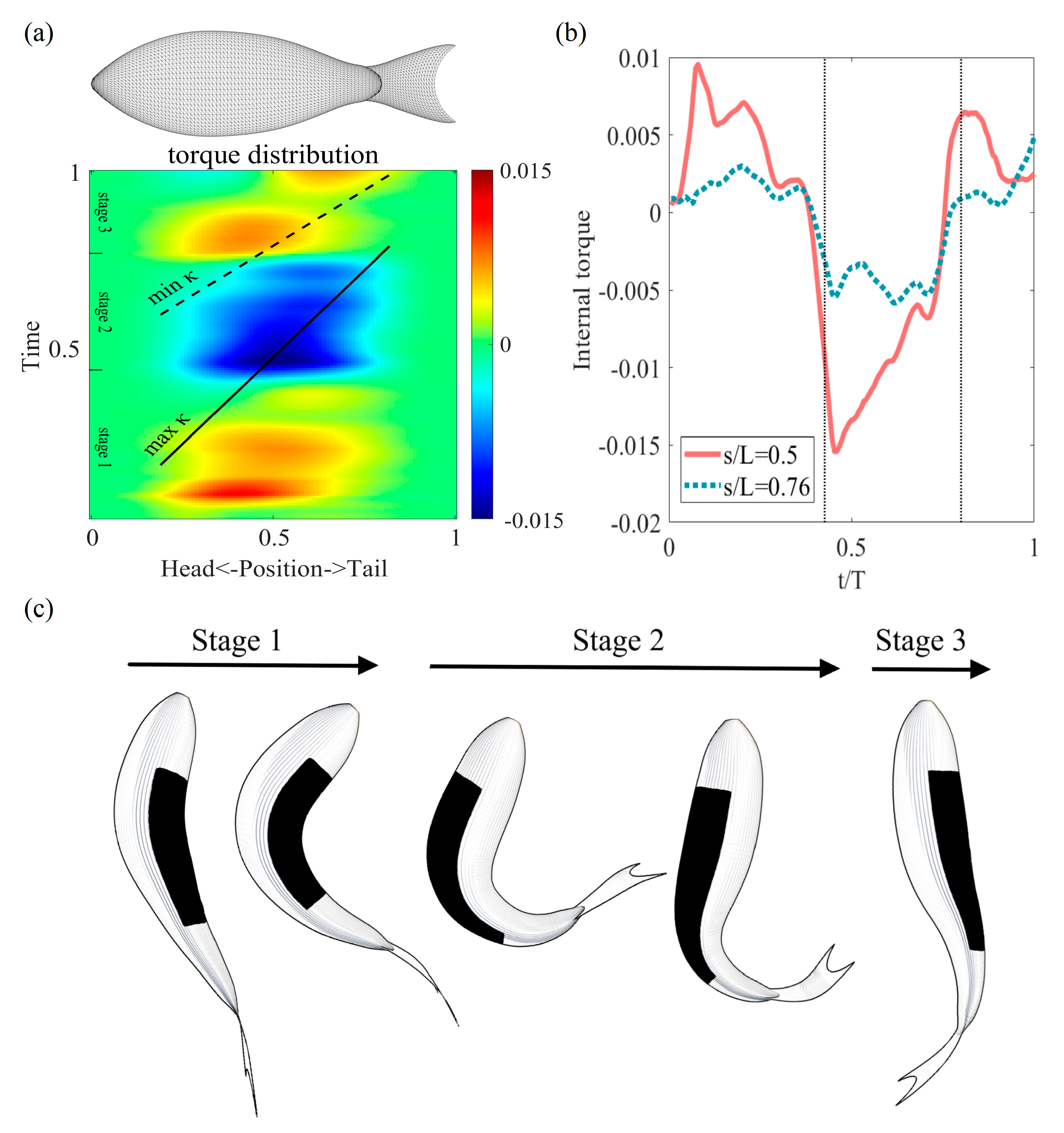
3.4. Internal Torque
4. Discussion
4.1. Roles of the Other Fins
4.2. Effects of the Reynolds Number
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fish, F.; Lauder, G.V. Passive and active flow control by swimming fishes and mammals. Annu. Rev. Fluid Mech. 2006, 38, 193–224. [Google Scholar] [CrossRef]
- Budick, S.A.; O’Malley, D.M. Locomotor repertoire of the larval zebrafish: Swimming, turning and prey capture. J. Exp. Biol. 2000, 203, 2565–2579. [Google Scholar] [CrossRef]
- Wakeling, J. Biomechanics of fast-start swimming in fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 131, 31–40. [Google Scholar] [CrossRef]
- Domenici, P.; Blake, R. The kinematics and performance of fish fast-start swimming. J. Exp. Biol. 1997, 200, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Webb, P. Fast-start performance and body form in seven species of teleost fish. J. Exp. Biol. 1978, 74, 211–226. [Google Scholar] [CrossRef]
- Gazzola, M.; Van Rees, W.M.; Koumoutsakos, P. C-start: Optimal start of larval fish. J. Fluid Mech. 2012, 698, 5–18. [Google Scholar] [CrossRef]
- Li, G.; Müller, U.K.; van Leeuwen, J.L.; Liu, H. Body dynamics and hydrodynamics of swimming fish larvae: A computational study. J. Exp. Biol. 2012, 215, 4015–4033. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhong, Y.; Luo, H.; Ding, Y.; Du, R. Hydrodynamics of larval fish quick turning: A computational study. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2018, 232, 2515–2523. [Google Scholar] [CrossRef]
- Weihs, D. A hydrodynamical analysis of fish turning manoeuvres. Proc. R. Soc. London. Ser. B Biol. Sci. 1972, 182, 59–72. [Google Scholar]
- Weihs, D. The mechanism of rapid starting of slender fish. Biorheology 1973, 10, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Tytell, E.D.; Lauder, G.V. Hydrodynamics of the escape response in bluegill sunfish, Lepomis macrochirus. J. Exp. Biol. 2008, 211, 3359–3369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triantafyllou, M.S.; Triantafyllou, G.; Yue, D. Hydrodynamics of fishlike swimming. Annu. Rev. Fluid Mech. 2000, 32, 33–53. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Su, Y.; Su, Y. Numerical study on the hydrodynamics of C-turn maneuvering of a tuna-like fish body under self-propulsion. J. Fluids Struct. 2020, 94, 102954. [Google Scholar] [CrossRef]
- Ellerby, D.; Altringham, J. Spatial variation in fast muscle function of the rainbow trout Oncorhynchus mykiss during fast-starts and sprinting. J. Exp. Biol. 2001, 204, 2239–2250. [Google Scholar] [CrossRef]
- Wakeling, J.M.; Johnston, I.A. Muscle power output limits fast-start performance in fish. J. Exp. Biol. 1998, 201, 1505–1526. [Google Scholar] [CrossRef] [PubMed]
- Jayne, B.; Lauder, G. Red and white muscle activity and kinematics of the escape response of the bluegill sunfish during swimming. J. Comp. Physiol. A 1993, 173, 495–508. [Google Scholar] [CrossRef]
- Westneat, M.W.; Hale, M.E.; McHenry, M.J.; Long, J.H. Mechanics of the fast-start: Muscle function and the role of intramuscular pressure in the escape behavior of Amia calva and Polypterus palmas. J. Exp. Biol. 1998, 201, 3041–3055. [Google Scholar] [CrossRef]
- Tytell, E.D.; Lauder, G.V. The C-start escape response of Polypterus senegalus: Bilateral muscle activity and variation during stage 1 and 2. J. Exp. Biol. 2002, 205, 2591–2603. [Google Scholar] [CrossRef]
- Wakeling, J.M.; Johnston, I.A. Predicting muscle force generation during fast-starts for the common carp Cyprinus carpio. J. Comp. Physiol. B 1999, 169, 391–401. [Google Scholar] [CrossRef]
- Borazjani, I. The functional role of caudal and anal/dorsal fins during the C-start of a bluegill sunfish. J. Exp. Biol. 2013, 216, 1658–1669. [Google Scholar] [CrossRef]
- Ming, T.; Jin, B.; Song, J.; Luo, H.; Du, R.; Ding, Y. 3D computational models explain muscle activation patterns and energetic functions of internal structures in fish swimming. PLoS Comput. Biol. 2019, 15, e1006883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Kan, J. A second-order accurate pressure-correction scheme for viscous incompressible flow. SIAM J. Sci. Stat. Comput. 1986, 7, 870–891. [Google Scholar] [CrossRef]
- Dong, H.; Mittal, R.; Najjar, F. Wake topology and hydrodynamic performance of low-aspect-ratio flapping foils. J. Fluid Mech. 2006, 566, 309–343. [Google Scholar] [CrossRef]
- Song, J.; Zhong, Y.; Du, R.; Yin, L.; Ding, Y. Tail shapes lead to different propulsive mechanisms in the body/caudal fin undulation of fish. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2021, 235, 351–364. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, J.; Wang, C.; Li, Y.; Song, J. Hydrodynamic effects of the caudal fin shape of fish in carangiform undulatory swimming. Proc. Inst. Mech. Eng. Part C: J. Mech. Eng. Sci. 2022, 236, 6385–6394. [Google Scholar] [CrossRef]
- Song, J.; Luo, H.; Hedrick, T.L. Three-dimensional flow and lift characteristics of a hovering ruby-throated hummingbird. J. R. Soc. Interface 2014, 11, 20140541. [Google Scholar] [CrossRef]
- Song, J.; Tobalske, B.W.; Powers, D.R.; Hedrick, T.L.; Luo, H. Three-dimensional simulation for fast forward flight of a calliope hummingbird. R. Soc. Open Sci. 2016, 3, 160230. [Google Scholar] [CrossRef]
- Borazjani, I.; Sotiropoulos, F.; Tytell, E.D.; Lauder, G.V. Hydrodynamics of the bluegill sunfish C-start escape response: Three-dimensional simulations and comparison with experimental data. J. Exp. Biol. 2012, 215, 671–684. [Google Scholar] [CrossRef]
- Goldbogen, J.A.; Shadwick, R.E.; Fudge, D.S.; Gosline, J.M. Fast-start muscle dynamics in the rainbow trout Oncorhynchus mykiss: Phase relationship of white muscle shortening and body curvature. J. Exp. Biol. 2005, 208, 929–938. [Google Scholar] [CrossRef]
- Eaton, R.C.; Bombardieri, R.A.; Meyer, D.L. The Mauthner-initiated startle response in teleost fish. J. Exp. Biol. 1977, 66, 65–81. [Google Scholar] [CrossRef]
- Webb, P. Effects of median-fin amputation on fast-start performance of rainbow trout (Salmo gairdneri). J. Exp. Biol. 1977, 68, 123–135. [Google Scholar] [CrossRef]





| Parameter | Value |
|---|---|
| Mass | 0.0192 |
| Width | 0.166 |
| Height | 0.3406 |
| Area of tail | 0.0382 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Song, J.; Yin, L.; Jin, B.; Yin, B.; Zhong, Y. Hydrodynamics and Musculature Actuation of Fish during a Fast Start. Appl. Sci. 2023, 13, 2365. https://doi.org/10.3390/app13042365
Li Y, Song J, Yin L, Jin B, Yin B, Zhong Y. Hydrodynamics and Musculature Actuation of Fish during a Fast Start. Applied Sciences. 2023; 13(4):2365. https://doi.org/10.3390/app13042365
Chicago/Turabian StyleLi, Yuhan, Jialei Song, Ling Yin, Bowen Jin, Bo Yin, and Yong Zhong. 2023. "Hydrodynamics and Musculature Actuation of Fish during a Fast Start" Applied Sciences 13, no. 4: 2365. https://doi.org/10.3390/app13042365





