Epoxy-Functionalized POSS and Glass Fiber for Improving Thermal and Mechanical Properties of Epoxy Resins
Abstract
:1. Introduction
2. Experiment
2.1. Materials
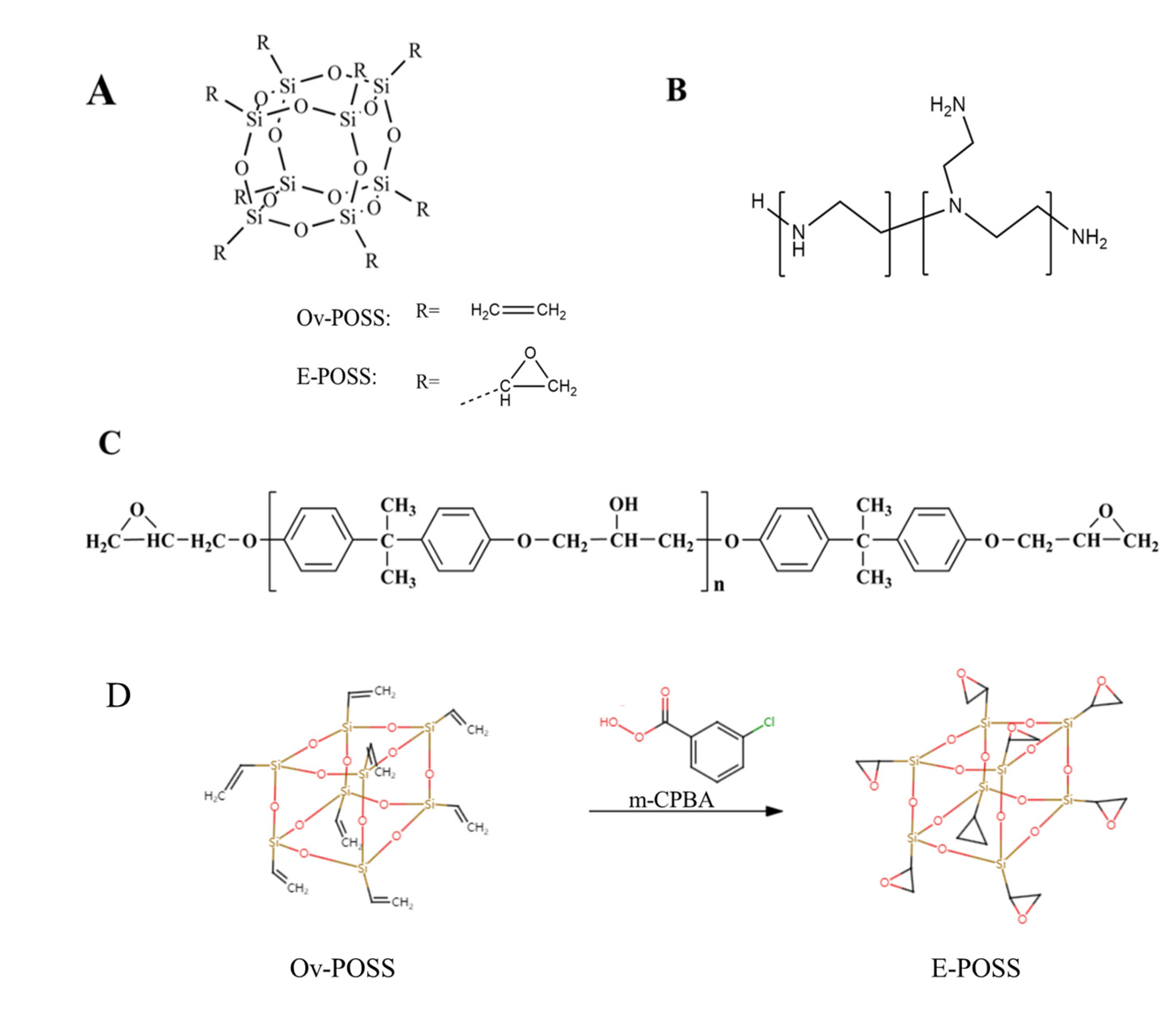
2.2. Synthesis of E-POSS
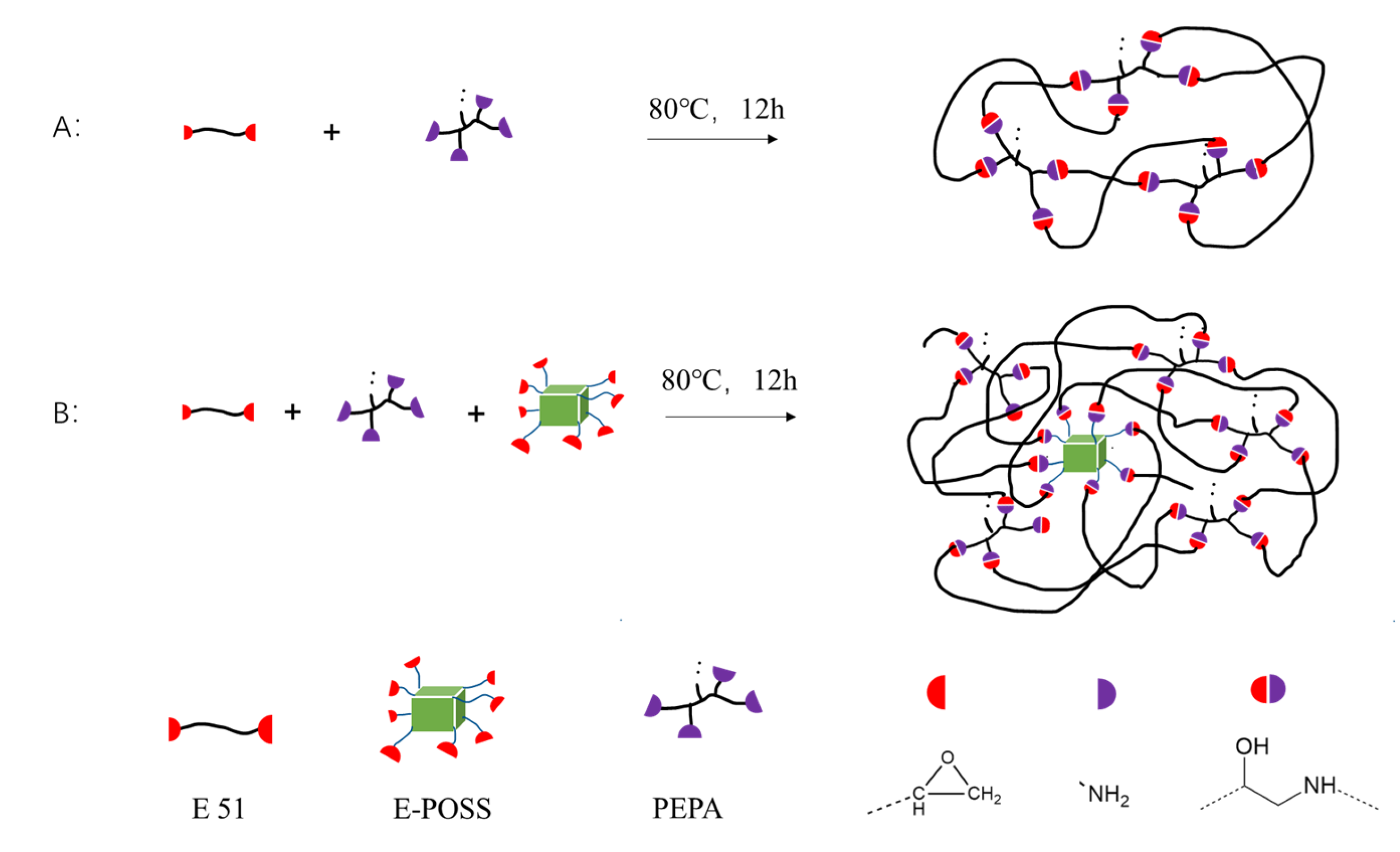
2.3. E51 Curing
2.4. Preparation of the E51 + E-POSS Composite
2.5. Preparation of the E51 + GF Composite
2.6. Preparation of the E51 + E-POSS + GF Composite
2.7. Measurements
3. Results and Discussion
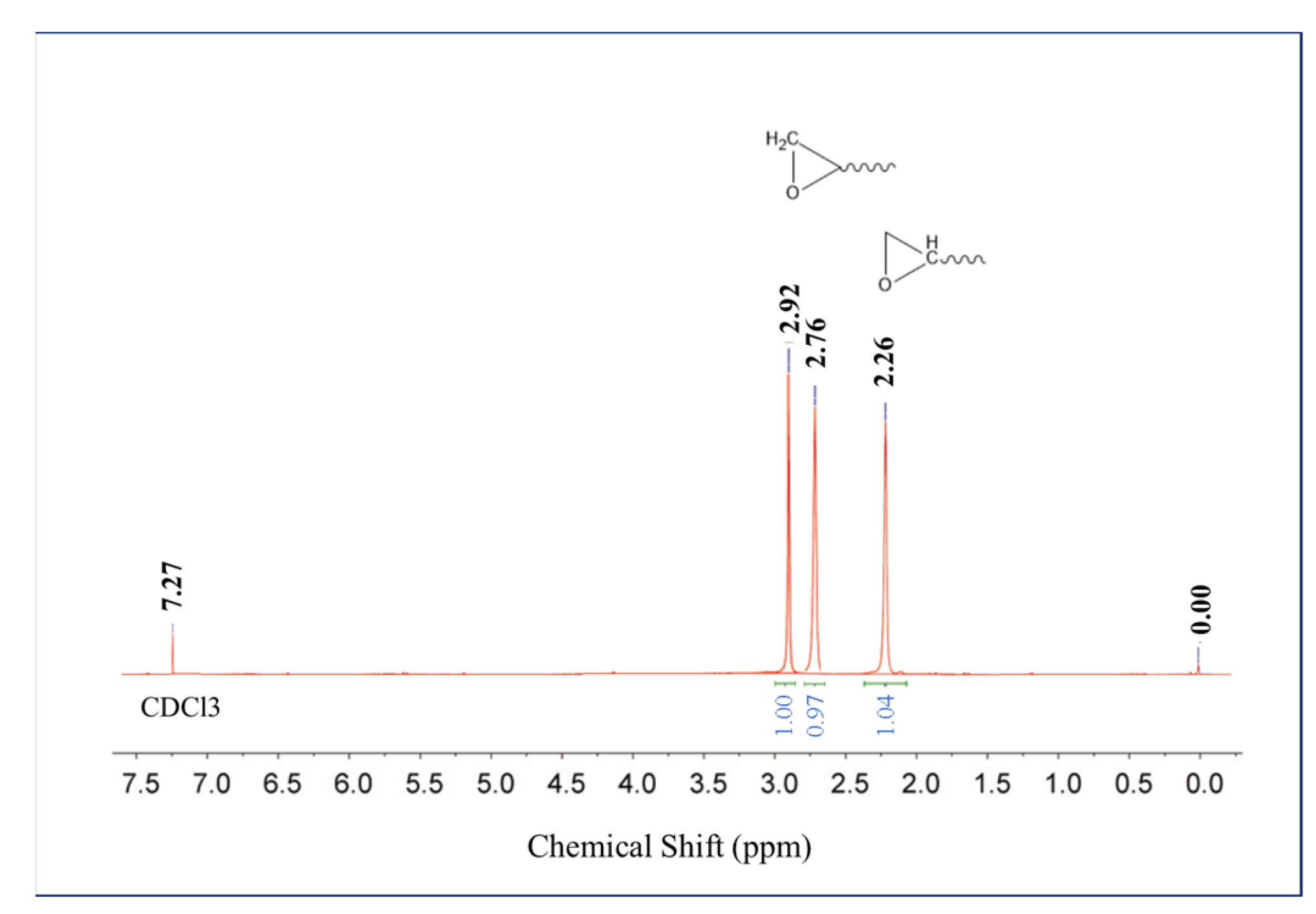
3.1. Characterization of E-POSS
3.2. Tensile Strength
3.3. Thermo-Mechanical Properties
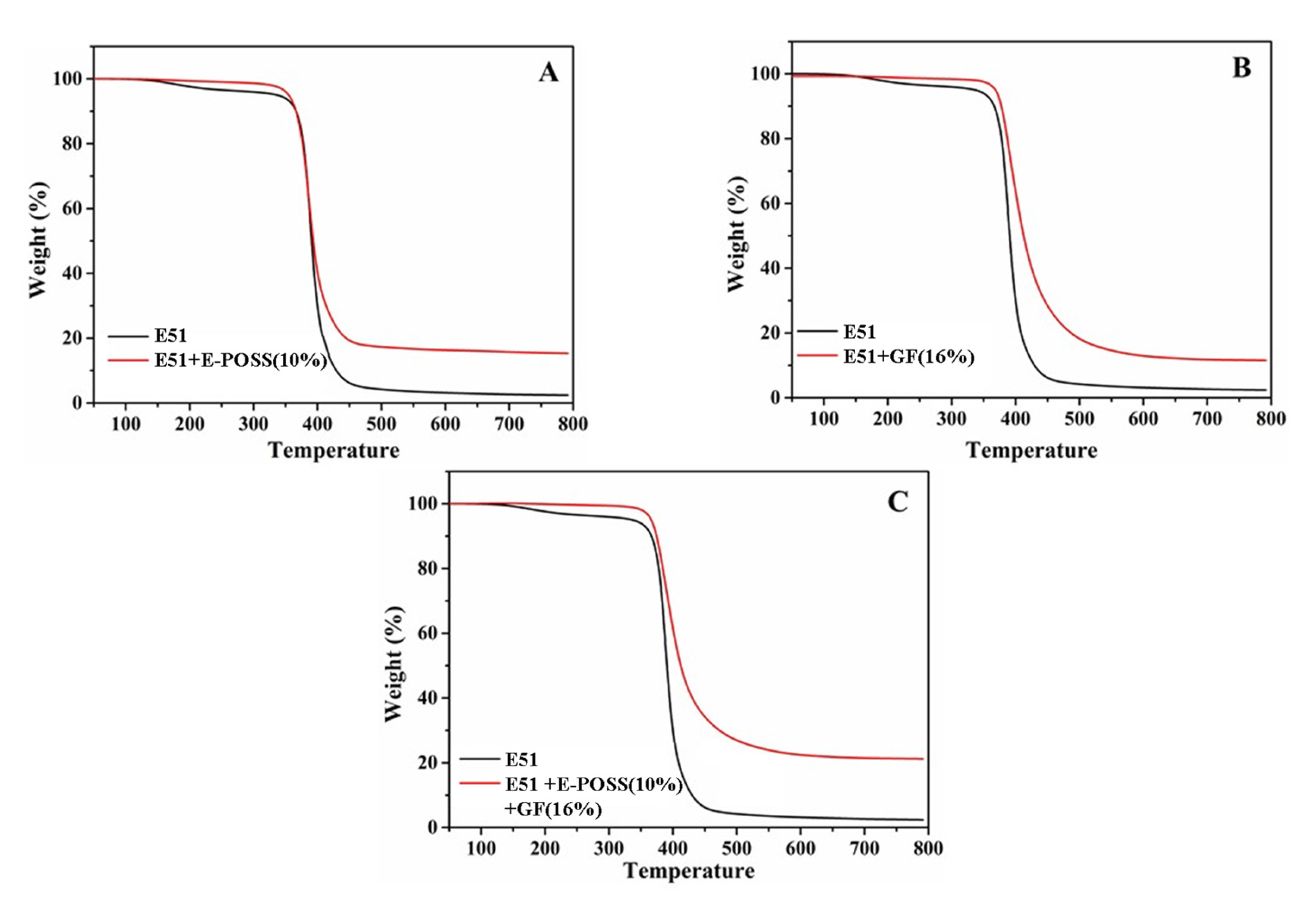
3.4. Thermal Stability
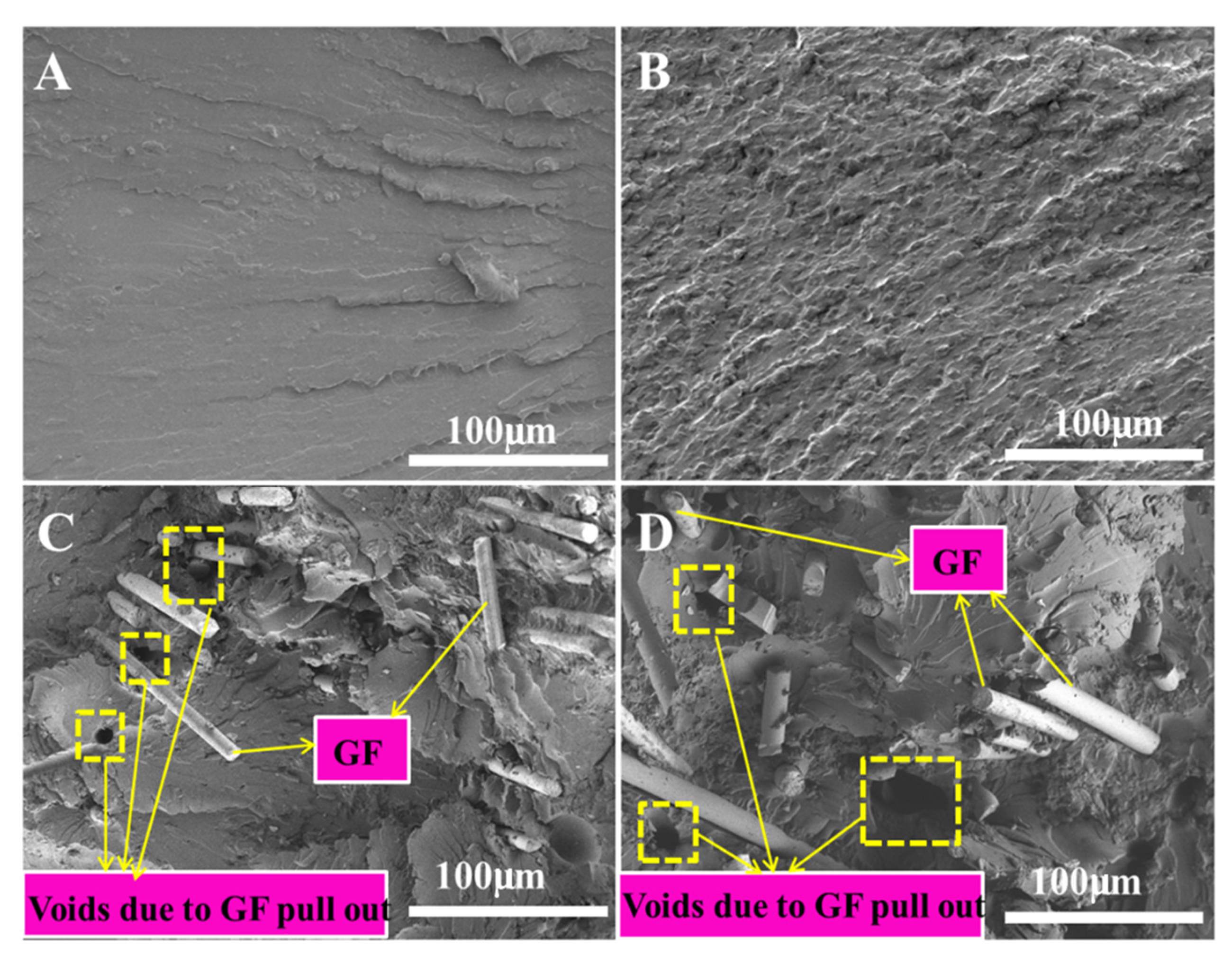
3.5. Fracture Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, W.; Wirasaputra, A.; Liu, S.; Yuan, Y.; Zhao, J. Highly effective flame retarded epoxy resin cured by DOPO-based co-curing agent. Polym. Degrad. Stabil. 2015, 122, 44–51. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Chruściel, J.J.; Leśniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Gu, J.W.; Liang, C.B.; Zhao, X.M.; Gan, B.; Qiu, H.; Guo, Y.; Yang, X.; Zhang, Q.; Wang, D.-Y. Highly thermally conductive flame-retardant epoxy nanocomposites with reduced ignitability and excellent electrical conductivities. Compos. Sci. Technol. 2017, 139, 83–89. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Lv, Z.; Li, N.; Liang, C.; Zhang, Q. Functionalized graphite nanoplatelets/epoxy resin nanocomposites with high thermal conductivity. Int. J. Heat. Mass. Tran. 2016, 92, 15–22. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Li, C.; Kou, K. Synthesis of Cyanate Ester Microcapsules via Solvent Evaporation Technique and Its Application in Epoxy Resins as a Healing Agent. Ind. Eng. Chem. Res. 2016, 55, 10941–10946. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohishi, T.; Goseki, R.; Otsuka, H. Degradable epoxy resins prepared from diepoxide monomer with dynamic covalent disulfide linkage. Polymer 2016, 82, 319–326. [Google Scholar] [CrossRef]
- Sprenger, S. Epoxy resins modified with elastomers and surface-modified silica nanoparticles. Polymer 2013, 54, 4790–4797. [Google Scholar] [CrossRef]
- Ma, L.; Meng, L.; Wu, G.; Wang, Y.; Zhao, M.; Zhang, C.; Huang, Y. Improving the interfacial properties of carbon fiber-reinforced epoxy composites by grafting of branched polyethyleneimine on carbon fiber surface in supercritical methanol. Compos. Sci. Technol. 2015, 114, 64–71. [Google Scholar] [CrossRef]
- Shin, P.-S.; Wang, Z.-J.; Kwon, D.-J.; Choi, J.-Y.; Sung, I.; Jin, D.-S.; Kang, S.-W.; Kim, J.-C.; DeVries, K.L.; Park, J.-M. Optimum mixing ratio of epoxy for glass fiber reinforced composites with high thermal stability. Compos. Part B 2015, 79, 132–137. [Google Scholar] [CrossRef]
- Sprenger, S. Epoxy resin composites with surface-modified silicon dioxide nanoparticles: A review. J. Appl. Polym. Sci. 2013, 130, 1421–1428. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Braga, R.A.; Magalhaes, P.A.A. Analysis of the mechanical and thermal properties of jute and glass fiber as reinforcement epoxy hybrid composites. Mat. Sci. Eng C 2015, 56, 269–273. [Google Scholar] [CrossRef]
- Naresh, K.; Shankar, K.; Rao, B.S.; Velmurugan, R. Effect of high strain rate on glass/carbon/hybrid fiber reinforced epoxy laminated composites. Compos. Part B 2016, 100, 125–135. [Google Scholar] [CrossRef]
- Kwon, S.C.; Adachi, T.; Araki, W.; Yamaji, A. Effect of composing particles of two sizes on mechanical properties of spherical silica-particulate-reinforced epoxy composites. Compos. Part B 2008, 39, 740–746. [Google Scholar] [CrossRef]
- Jumahat, A.; Soutis, C.; Jones, F.R.; Hodzic, A. Effect of silica nanoparticles on compressive properties of an epoxy polymer. J. Mater. Sci. 2010, 45, 5973–5983. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H. Thermal degradation behaviors of epoxy resin/POSS hybrids and phosphorus–silicon synergism of flame retardancy. J. Polym. Sci. Pol. Phys. 2010, 48, 693–705. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Yang, R.J. Novel flame retardancy effects of DOPO-POSS on epoxy resins. Polym Degrad. Stabil. 2011, 96, 2167–2173. [Google Scholar] [CrossRef]
- Hernandez, C.P.; Guo, L.J.; Fu, P.F. High-Resolution Functional Epoxysilsesquioxane-Based Patterning Layers for Large-Area Nanoimprinting. ACS Nano. 2010, 4, 4776–4784. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.G.; Laine, R.M. Organic/Inorganic Hybrid Epoxy Nanocomposites from Aminophenylsilsesquioxanes. Macromolecules 2004, 37, 99–109. [Google Scholar] [CrossRef]
- Hou, G.X.; Gao, J.G.; Tian, C. Hybrid free radical-cationic thermal polymerization of methylacryloylpropyl-POSS/epoxy resins nanocomposites. J. Polym Res. 2013, 20, 221–231. [Google Scholar] [CrossRef]
- Xin, C.; Ma, X.; Chen, F.; Song, C.; Qu, X. Synthesis of EP-POSS Mixture and the Properties of EP-POSS/Epoxy, SiO2/Epoxy, and SiO2/EP-POSS/Epoxy Nanocomposite. J. Appl. Polym. Sci. 2013, 130, 810–819. [Google Scholar] [CrossRef]
- Wu, K.; Kandola, B.K.; Kandare, E.; Hu, Y. Flame retardant effect of polyhedral oligomeric silsesquioxane and triglycidyl isocyanurate on glass fibre-reinforced epoxy composites. Polym. Compos. 2011, 32, 378–389. [Google Scholar] [CrossRef]
- Dhanapal, D.; Srinivasan, A.K.; Ramalingam, N. Role of POSS as coupling agent for DGEBA/GF reinforced nanocomposites. Silicon 2018, 10, 537–546. [Google Scholar] [CrossRef]
- Chen, S.; Gao, J.; Han, H.; Wang, C. Mechanical and thermal properties of epoxy-POSS reinforced-(biphenyl diol formaldehyde/epoxy hybrid resin) composites. Iranian. Polym. J. 2014, 23, 609–617. [Google Scholar] [CrossRef]
- Zhang, C.X.; Laine, R.M. Silsesquioxanes as synthetic platforms. II. Epoxy-functionalized inorganic-organic hybrid species. J. Organomet. Chem. 1996, 521, 199–201. [Google Scholar] [CrossRef]
- Metroke, T.L.; Kachurina, O.; TKnobbe, E. Spectroscopic and corrosion resistance characterization of GLYMO–TEOS Ormosil coatings for aluminum alloy corrosion inhibition. Prog. Org. Coat. 2002, 44, 295–305. [Google Scholar] [CrossRef]
- Huang, J.-C.; He, C.-B.; Xiao, Y.; Mya, K.Y.; Dai, J.; Siow, Y.P. Polyimide/POSS nanocomposites: Interfacial interaction, thermal properties and mechanical properties. Polymer 2003, 44, 4491–4499. [Google Scholar] [CrossRef]
- Ananthakrishnan, D.; Venkatesvaran, H.; Kannan, A.; Gandhi, S. Simplistic one-pot synthesis of an inorganic-organic cubic caged material: A new interface for detecting toxic bisphenol-A electrochemically. New J. Chem. 2020, 44, 20192–20202. [Google Scholar] [CrossRef]
- Florea, N.; Lungu, A.; Badica, P.; Craciun, L.; Enculescu, M.; Ghita, D.; Ionescu, C.; Zgirian, R.; Iovu, H. Novel nanocomposites based on epoxy resin/epoxy-functionalized polydimethylsiloxane reinforced with POSS. Compos. Part B 2015, 75, 226–234. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Fu, F.; Liu, X. Copolymerization and Phase Separation Behaviors of Benzoxazine Amine Thermoset. RSC Adv. 2016, 6, 100590–100597. [Google Scholar] [CrossRef]
- Tang, L.-C.; Wan, Y.-J.; Yan, D.; Pei, Y.-B.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Gong, L.-X.; Zhao, L.; Tang, L.-C.; Liu, H.-Y.; Mai, Y.-W. Balanced electrical, thermal and mechanical properties of epoxy composites filled with chemically reduced graphene oxide and rubber nanoparticles. Compos. Sci. Technol. 2015, 121, 104–114. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, G.; Ren, P.; Wang, J. Thermodegradation kinetics of epoxy/DDS/POSS system. Polym. Compos. 2007, 28, 755–761. [Google Scholar] [CrossRef]
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Q.; Zhao, L.; Li, S.-N.; Wu, L.-B.; Jiang, J.-X.; Tang, L.-C. A novel and facile strategy for highly flame retardant polymer foam composite materials: Transforming silicone resin coating into silica self-extinguishing layer. J. Hazard. Mater. 2017, 336, 222–231. [Google Scholar] [CrossRef]
- Yu, Z.-R.; Li, S.-N.; Zang, J.; Zhang, M.; Gong, L.-X.; Song, P.; Zhao, L.; Zhang, G.-D.; Tang, L.-C. Enhanced mechanical property and flame resistance of graphene oxide nanocomposite paper modified with functionalized silica nanoparticles. Compos. Part B 2019, 177, 107347. [Google Scholar] [CrossRef]
- Gong, L.-X.; Hu, L.-L.; Zang, J.; Pei, Y.-B.; Zhao, L.; Tang, L.-C. Improved interfacial properties between glass fibers and tetra-functional epoxy resins modified with silica nanoparticles. Fiber. Polym. 2015, 16, 2056–2065. [Google Scholar] [CrossRef]

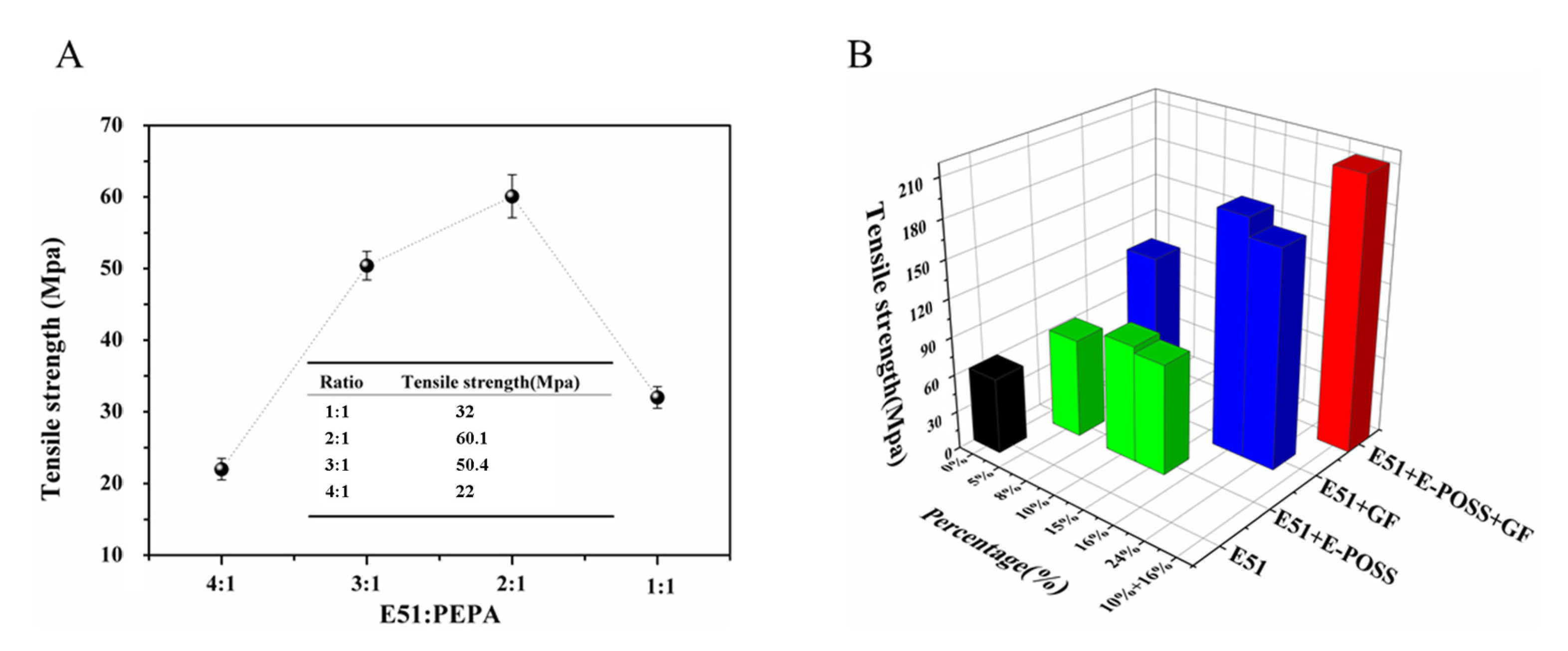

| Sample ID | E-POSS (wt%) | GF (wt%) | Modulus (GPa) | Tensile Strength (MPa) | |
|---|---|---|---|---|---|
| E51 | - | - | 3.7 ± 0.1 | 60.1 ± 6.5 | 100% |
| E51 + E-POSS (5%) | 5 | - | 4.5 ± 0.2 | 78.3 ± 7.4 | 130.3% |
| E51 + E-POSS (10%) | 10 | - | 4.9 ± 02 | 92.5 ± 7.8 | 153.9% |
| E51 + E-POSS (15%) | 15 | - | 4.7 ± 0.2 | 87.6 ± 8.3 | 145.8% |
| E51 + GF (8%) | - | 8 | 5.2 ± 0.2 | 132.1 ± 10.4 | 219.8% |
| E51 + GF (16%) | - | 16 | 6.6 ± 0.2 | 186.7 ± 11.5 | 310.6% |
| E51 + GF (24%) | - | 24 | 5.8 ± 0.2 | 172.1 ± 15.3 | 286.4% |
| E51 + E-POSS (10%) + GF (16%) | 10 | 16 | 7.5 ± 0.2 | 214.9 ± 18.8 | 357.6% |
| Sample ID | Td5% (°C) | Residues (wt%) |
|---|---|---|
| E51 | 336 | 2.4 |
| E51 + E-POSS (10%) | 354 | 11.5 |
| E51 + GF (16%) | 366 | 15.3 |
| E51 + E-POSS (10%) + GF (16%) | 378 | 21.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Shen, J.; Yang, X.; Zhao, D.; Feng, Y. Epoxy-Functionalized POSS and Glass Fiber for Improving Thermal and Mechanical Properties of Epoxy Resins. Appl. Sci. 2023, 13, 2461. https://doi.org/10.3390/app13042461
Jiang J, Shen J, Yang X, Zhao D, Feng Y. Epoxy-Functionalized POSS and Glass Fiber for Improving Thermal and Mechanical Properties of Epoxy Resins. Applied Sciences. 2023; 13(4):2461. https://doi.org/10.3390/app13042461
Chicago/Turabian StyleJiang, Jianliang, Jingbo Shen, Xiao Yang, Dongqi Zhao, and Yakai Feng. 2023. "Epoxy-Functionalized POSS and Glass Fiber for Improving Thermal and Mechanical Properties of Epoxy Resins" Applied Sciences 13, no. 4: 2461. https://doi.org/10.3390/app13042461
APA StyleJiang, J., Shen, J., Yang, X., Zhao, D., & Feng, Y. (2023). Epoxy-Functionalized POSS and Glass Fiber for Improving Thermal and Mechanical Properties of Epoxy Resins. Applied Sciences, 13(4), 2461. https://doi.org/10.3390/app13042461






