Changes on Grape Aroma Composition as a Consequence of Foliar Application of Methyl Jasmonate and Nano-Sized Particles Doped with Methyl Jasmonate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vineyard Site, Grapevine Treatments and Samples
2.2. General Parameters Determination
2.3. Analysis of Grape Volatile Compounds by HS-SPME-GC-MS
2.4. Statistical Analyses
3. Results and Discussion
3.1. Effect of the Foliar MeJ and ACP-MeJ Treatments on the Must General Parameters
3.2. Influence of the Foliar MeJ and ACP-MeJ Treatments on Must Volatile Compounds
3.3. Factorial (Treatment, Season and Their Interaction) and Discriminant Analysis of the Aroma Compounds in Vitis vinifera L. cv. Tempranillo Grapes from 2019 and 2020 Seasons
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perestrelo, R.; Barros, A.S.; Rocha, S.M.; Câmara, J.S. Optimisation of solid-phase microextraction combined with gas chromatography–mass spectrometry based methodology to establish the global volatile signature in pulp and skin of Vitis vinifera L. grape varieties. Talanta 2011, 85, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre-Tudo, J.L.; Weightman, C.; Nieuwoudt, H.; Du Toit, W. Effect of skin contact before and during alcoholic fermentation on the chemical and sensory profile of south african chenin blanc white wines. S. Afr. J. Enol. Vitic. 2015, 36, 366–377. [Google Scholar] [CrossRef]
- Román, S.M.-S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Advancement in analytical techniques for the extraction of grape and wine volatile compounds. Food Res. Int. 2020, 137, 109712. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Floral scents and fruit aromas: Functions, compositions, biosynthesis, and regulation. Front. Plant Sci. 2022, 13, 860157. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Matarese, F.; Cuzzola, A. Effect of methyl jasmonate on the aroma of Sangiovese grapes and wines. Food Chem. 2018, 242, 352–361. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Gutiérrez-Gamboa, G.; López, R.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Influence of foliar application of phenylalanine and urea at two doses to vineyards on grape volatile composition and amino acids content. Vitis 2018, 141, 137–141. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Rubio-Bretón, P.; Román, S.M.-S.; de Urturi, I.S.; Pérez-Álvarez, E.P. Pre-fermentative maceration with SO2 enhanced the must aromatic composition. Food Chem. 2020, 345, 128870. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef]
- Darriet, P.; Thibon, C.; Dubourdieu, D. Aroma and Aroma Precursors in Grape Berry. In The Biochemistry of the Grape Berry; Bentham Science: Sharjah, United Arab Emirates, 2012; ISBN 9781608055401. [Google Scholar]
- Garde-Cerdán, T.; Santamaría, P.; Rubio-Bretón, P.; González-Arenzana, L.; López-Alfaro, I.; López, R. Foliar application of proline, phenylalanine, and urea to Tempranillo vines: Effect on grape volatile composition and comparison with the use of commercial nitrogen fertilizers. LWT Food Sci. Technol. 2015, 60, 684–689. [Google Scholar] [CrossRef]
- Portu, J.; González-Arenzana, L.; Hermosín-Gutiérrez, I.; Santamaría, P.; Garde-Cerdán, T. Phenylalanine and urea foliar applications to grapevine: Effect on wine phenolic content. Food Chem. 2015, 180, 55–63. [Google Scholar] [CrossRef]
- Román, S.M.-S.; Garde-Cerdán, T.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Foliar application of phenylalanine plus methyl jasmonate as a tool to improve Grenache grape aromatic composition. Sci. Hortic. 2020, 272, 109515. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.; Moreno-Olivares, J.; Fernández-Fernández, J.; Bautista-Ortín, A.; Gil-Muñoz, R. Influence of methyl jasmonate and benzothiadiazole on the composition of grape skin cell walls and wines. Food Chem. 2019, 277, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; Santamaría, P.; López-Alfaro, I.; López, R.; Garde-Cerdán, T. Methyl jasmonate foliar application to tempranillo vineyard improved grape and wine phenolic content. J. Agric. Food Chem. 2015, 63, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Gil-Muñoz, R.; Fernández-Fernández, J.I.; Crespo-Villegas, O.; Garde-Cerdán, T. Elicitors used as a tool to increase stilbenes in grapes and wines. Food Res. Int. 2017, 98, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Elicitation with methyl jasmonate supported by precursor feeding with phenylalanine: Effect on Garnacha grape phenolic content. Food Chem. 2017, 237, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Román, S.M.-S.; Jofré, V.; Rubio-Bretón, P.; Pérez-Álvarez, E.; Garde-Cerdán, T. Effects on chlorophyll and carotenoid contents in different grape varieties (Vitis vinifera L.) after nitrogen and elicitor foliar applications to the vineyard. Food Chem. 2018, 269, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esplá, A.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Giménez, M.J.; García-Pastor, M.E.; Serrano, M.; Zapata, P.J. Preharvest application of methyl jasmonate as an elicitor improves the yield and phenolic content of artichoke. J. Agric. Food Chem. 2017, 65, 9247–9254. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Portu, J.; López, R.; Santamaría, P. Effect of methyl jasmonate application to grapevine leaves on grape amino acid content. Food Chem. 2016, 203, 536–539. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Bautista-Ortín, A.B.; Ruiz-García, Y.; I Fernández-Fernández, J.; Gil-Muñoz, R. Effect of elicitors on the evolution of grape phenolic compounds during the ripening period. J. Sci. Food Agric. 2016, 97, 977–983. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of benzothiadiazole and methyl jasmonate on the volatile compound composition of Vitis vinifera L. Monastrell grapes and wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]
- Rahim, H.U.; Qaswar, M.; Uddin, M.; Giannini, C.; Herrera, M.L.; Rea, G. Nano-enable materials promoting sustainability and resilience in modern agriculture. Nanomaterials 2021, 11, 2068. [Google Scholar] [CrossRef] [PubMed]
- Fellet, G.; Pilotto, L.; Marchiol, L.; Braidot, E. Tools for nano-enabled agriculture: Fertilizers based on calcium phosphate, silicon, and chitosan nanostructures. Agronomy 2021, 11, 1239. [Google Scholar] [CrossRef]
- Fincheira, P.; Tortella, G.; Seabra, A.B.; Quiroz, A.; Diez, M.C.; Rubilar, O. Nanotechnology advances for sustainable agriculture: Current knowledge and prospects in plant growth modulation and nutrition. Planta 2021, 254, 1–25. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Costa, B.S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Nanotechnology: Recent advances in viticulture and enology. J. Sci. Food Agric. 2021, 101, 6156–6166. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Ramírez-Rodríguez, G.B.; Carmona, F.J.; Martínez-Vidaurre, J.M.; Masciocchi, N.; Guagliardi, A.; Garde-Cerdán, T.; Delgado-López, J.M. Towards a more sustainable viticulture: Foliar application of N-doped calcium phosphate nanoparticles on Tempranillo grapes. J. Sci. Food Agric. 2020, 101, 1307–1313. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.; Rubio-Bretón, P.; Intrigliolo, D.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.; Delgado-López, J.; Garde-Cerdán, T. Year, watering regime and foliar methyl jasmonate doped nanoparticles treatments: Effects on must nitrogen compounds in Monastrell grapes. Sci. Hortic. 2022, 297, 110944. [Google Scholar] [CrossRef]
- Gaiotti, F.; Lucchetta, M.; Rodegher, G.; Lorenzoni, D.; Longo, E.; Boselli, E.; Cesco, S.; Belfiore, N.; Lovat, L.; Delgado-López, J.; et al. Urea-doped calcium phosphate nanoparticles as sustainable nitrogen nanofertilizers for viticulture: Implications on yield and quality of pinot gris grapevines. Agronomy 2021, 11, 1026. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M. Effect of methyl jasmonate doped nanoparticles on nitrogen composition of Monastrell grapes and wines. Biomolecules 2021, 11, 1631. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Sáenz de Urturi, I.; Rubio-Bretón, P.; Marín-San Román, S.; Baroja, E.; Ramírez-Rodríguez, G.; Delgado-López, J.; Pérez-Álvarez, E. Foliar application of methyl jasmonate and methyl jasmonate supported on nanoparticles: Incidence on grape phenolic composition over two seasons. Food Chem. 2023, 402, 134244. [Google Scholar] [CrossRef]
- Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Gil-Muñoz, R.; Delgado-López, J.M. Nanoelicitors with prolonged retention and sustained release to produce beneficial compounds in wines. Environ. Sci. Nano 2021, 8, 3524–3535. [Google Scholar] [CrossRef]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Delgado-López, J.M.; Gil-Muñoz, R. Effects of methyl jasmonate and nano-methyl jasmonate treatments on monastrell wine volatile composition. Molecules 2022, 27, 2878. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Gamboa, G.G.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Influence of methyl jasmonate foliar application to vineyard on grape volatile composition over three consecutive vintages. Food Res. Int. 2018, 112, 274–283. [Google Scholar] [CrossRef] [PubMed]
- OIV. Compendium of Internationals Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Paris, France, 2009. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9780470010372. [Google Scholar]
- Li, W.; Li, W.; Yang, S.; Ma, Z.; Zhou, Q.; Mao, J.; Han, S.; Chen, B. Transcriptome and metabolite conjoint analysis reveals that exogenous methyl jasmonate regulates monoterpene synthesis in grape berry skin. J. Agric. Food Chem. 2020, 68, 5270–5281. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Pérez-Álvarez, E.; Rubio-Bretón, P.; Garde-Cerdán, T. Changes on grape volatile composition through elicitation with methyl jasmonate, chitosan, and a yeast extract in Tempranillo (Vitis vinifera L.) grapevines. Sci. Hortic. 2018, 244, 257–262. [Google Scholar] [CrossRef]
- Black, C.; Parker, M.; Siebert, T.; Capone, D.; Francis, I. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2018, 99, 975–985. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; González-Arenzana, L.; López, N.; López, R.; Santamaría, P.; López-Alfaro, I. Effect of different pulsed electric field treatments on the volatile composition of Graciano, Tempranillo and Grenache grape varieties. Innov. Food Sci. Emerg. Technol. 2013, 20, 91–99. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Dubery, I.A.; Teodorczuk, L.G.; Louw, A.E. Early responses in methyl jasmonate-preconditioned cells toward pathogen-derived elicitors. Mol. Cell Biol. Res. Commun. 2000, 3, 105–110. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M.; Ferreira, A.C.S.; Caris-Veyrat, C.; de Pinho, P.G. Carotenoid, chlorophyll, and chlorophyll-derived compounds in grapes and port wines. J. Agric. Food Chem. 2005, 53, 10034–10041. [Google Scholar] [CrossRef] [PubMed]
- Peña-Cortés, H.; Barrios, P.; Dorta, F.; Polanco, V.; Sánchez, C.; Sánchez, E.; Ramírez, I. Involvement of jasmonic acid and derivatives in plant responses to pathogens and insects and in fruit ripening. J. Plant Growth Regul. 2004, 23, 246–260. [Google Scholar] [CrossRef]
- Sefton, M.A.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Occurrence, Sensory impact, formation, and fate of damascenone in grapes, wines, and other foods and beverages. J. Agric. Food Chem. 2011, 59, 9717–9746. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Traldi, P. Grape aroma compounds: Terpenes, C13-norisoprenoids, benzene compounds, and 3-Alkyl-2-methoxypyrazines. In Mass Spectrometry in Grape and Wine Chemistry; Jonh Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 97–116. [Google Scholar]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2013, 65, 1–24. [Google Scholar] [CrossRef] [Green Version]



| 2019 | 2020 | |||||
|---|---|---|---|---|---|---|
| Control | MeJ | ACP-MeJ | Control | MeJ | ACP-MeJ | |
| Weight of 100 berries (g) | 113.68 ± 11.07 a | 141.81 ± 27.18 a | 116.94 ± 4.62 a | 199.57 ± 7.27 a | 207.67 ± 40.39 a | 194.90 ± 20.65 a |
| °Brix | 24.70 ± 0.72 b | 22.23 ± 1.17 a | 23.37 ± 0.49 ab | 22.30 ± 0.92 a | 22.17 ± 2.31 a | 22.37 ± 0.38 a |
| Probable alcohol (% v/v) | 14.63 ± 0.49 b | 12.92 ± 0.80 a | 13.71 ± 0.35 ab | 12.97 ± 0.63 a | 12.89 ± 1.58 a | 13.01 ± 0.26 a |
| pH | 3.83 ± 0.05 a | 3.78 ± 0.10 a | 3.82 ± 0.09 a | 3.76 ± 0.01 a | 3.70 ± 0.07 a | 3.73 ± 0.06 a |
| Total acidity (g/L) * | 4.61 ± 0.11 a | 5.20 ± 0.36 b | 5.13 ± 0.26 ab | 4.12 ± 0.33 a | 4.54 ± 1.08 a | 4.03 ± 0.21 a |
| Glu + Fru (g/L) | 249.86 ± 9.97 b | 215.50 ± 12.29 a | 231.40 ± 10.82 ab | 216.42 ± 10.70 a | 218.62 ± 26.56 a | 223.84 ± 2.98 a |
| Glu (g/L) | 120.18 ± 5.13 b | 102.88 ± 6.89 a | 110.89 ± 4.94 ab | 107.31 ± 4.54 a | 106.08 ± 12.84 a | 108.61 ± 2.98 a |
| Fru (g/L) | 129.68 ± 4.84 b | 112.62 ± 5.43 a | 120.51 ± 6.26 ab | 109.11 ± 6.53 a | 112.54 ± 13.76 a | 114.72 ± 0.98 a |
| Malic acid (g/L) | 2.24 ± 0.24 a | 2.54 ± 0.32 a | 2.51 ± 0.56 a | 1.21 ± 0.08 a | 1.54 ± 0.22 a | 1.39 ± 0.18 a |
| Total phenols (mg/L) | 1185.33 ± 72.31 a | 1306.57 ± 61.35 b | 1351.40 ± 27.32 b | 541.60 ± 64.02 a | 603.07 ± 73.82 a | 582.70 ± 66.02 a |
| Ammonium nitrogen (mg N/L) | 78.00 ± 8.22 a | 106.34 ± 15.68 a | 101.40 ± 20.40 a | 121.16 ± 3.52 a | 101.66 ± 19.58 a | 114.66 ± 6.24 a |
| Amino nitrogen (mg N/L) | 118.51 ± 14.33 a | 202.11 ± 50.59 b | 175.71 ± 24.66 ab | 152.53 ± 14.33 a | 139.63 ± 35.64 a | 152.24 ± 5.50 a |
| YAN (mg N/L) | 196.51 ± 21.18 a | 308.45 ± 64.76 b | 277.11 ± 44.31 ab | 273.69 ± 17.69 a | 241.29 ± 55.05 a | 266.90 ± 11.62 a |
| 2019 | 2020 | |||||
|---|---|---|---|---|---|---|
| Control | MeJ | ACP-MeJ | Control | MeJ | ACP-MeJ | |
| Alcohols | ||||||
| n-Heptanol | 0.062 ± 0.010 c | 0.046 ± 0.008 b | 0.028 ± 0.005 a | 0.047 ± 0.002 a | 0.044 ± 0.009 a | 0.045 ± 0.009 a |
| n-Octanol | 0.191 ± 0.014 b | 0.174 ± 0.017 b | 0.107 ± 0.013 a | 0.326 ± 0.018 b | 0.234 ± 0.042 a | 0.238 ± 0.048 a |
| n-Nonanol | 0.064 ± 0.006 b | 0.059 ± 0.010 b | 0.031 ± 0.007 a | 0.197 ± 0.036 b | 0.245 ± 0.048 b | 0.093 ± 0.015 a |
| 1-Octen-3-ol | 0.595 ± 0.043 b | 0.296 ± 0.063 a | 0.243 ± 0.031 a | 0.174 ± 0.036 b | 0.074 ± 0.006 a | 0.147 ± 0.030 b |
| 2-Ethyl-1-hexanol | 3.088 ± 0.060 b | 1.798 ± 0.309 a | 1.625 ± 0.137 a | 1.870 ± 0.131 b | 0.863 ± 0.132 a | 2.140 ± 0.446 b |
| Total | 4.001 ± 0.108 b | 2.373 ± 0.387 a | 2.035 ± 0.167 a | 2.613 ± 0.048 b | 1.460 ± 0.156 a | 2.663 ± 0.502 b |
| Carbonyl compounds | ||||||
| Heptanal | 0.055 ± 0.009 b | 0.034 ± 0.007 a | 0.033 ± 0.004 a | 0.014 ± 0.002 b | 0.007 ± 0.001 a | 0.010 ± 0.001 a |
| (E)-2-Octenal | 0.059 ± 0.005 a | 0.051 ± 0.009 a | 0.051 ± 0.006 a | 0.043 ± 0.009 b | 0.024 ± 0.004 a | 0.042 ± 0.007 b |
| Nonanal | 0.204 ± 0.039 b | 0.115 ± 0.028 a | 0.083 ± 0.011 a | 0.381 ± 0.074 b | 0.143 ± 0.025 a | 0.236 ± 0.040 a |
| (E)-2-Nonenal | 0.065 ± 0.007 a | 0.068 ± 0.007 a | 0.065 ± 0.007 a | 0.047 ± 0.008 b | 0.031 ± 0.001 a | 0.024 ± 0.005 a |
| Decanal | 0.076 ± 0.013 b | 0.070 ± 0.011 b | 0.046 ± 0.009 a | 0.112 ± 0.023 b | 0.068 ± 0.014 a | 0.040 ± 0.005 a |
| (E,E)-2,4-Hexadienal | 1.177 ± 0.245 b | 1.567 ± 0.261 b | 0.691 ± 0.110 a | 0.711 ± 0.133 b | 0.208 ± 0.015 a | 0.836 ± 0.109 b |
| (E,E)-2,4-Nonadienal | 0.097 ± 0.011 b | 0.112 ± 0.026 b | 0.059 ± 0.001 a | 0.040 ± 0.005 b | 0.026 ± 0.005 a | 0.046 ± 0.007 b |
| γ-Decalactone | 0.125 ± 0.024 b | 0.157 ± 0.030 b | 0.054 ± 0.008 a | 0.146 ± 0.029 a | 0.141 ± 0.021 a | 0.274 ± 0.044 b |
| 6-Methyl-3,5-heptadien-2-one | 0.086 ± 0.017 b | 0.079 ± 0.015 b | 0.046 ± 0.009 a | 0.022 ± 0.005 a | 0.029 ± 0.004 a | 0.027 ± 0.005 a |
| Total | 1.942 ± 0.278 b | 2.254 ± 0.286 b | 1.128 ± 0.102 a | 1.515 ± 0.258 b | 0.676 ± 0.049 a | 1.535 ± 0.106 b |
| C6 compounds | ||||||
| n-Hexanol | 5.904 ± 1.031 b | 7.018 ± 1.447 b | 3.479 ± 0.575 a | 22.311 ± 3.544 a | 42.324 ± 4.178 b | 19.316 ± 4.032 a |
| n-Hexanal | 22.040 ± 2.145 b | 28.064 ± 5.929 b | 8.021 ± 1.150 a | 11.784 ± 1.942 b | 16.831 ± 2.431 c | 7.163 ± 1.427 a |
| (Z)-3-Hexen-1-ol +(E)-2-Hexen-1-ol | 1.027 ± 0.187 b | 0.340 ± 0.065 a | 0.361 ± 0.081 a | 0.669 ± 0.115 a | 1.080 ± 0.206 b | 0.553 ± 0.107 a |
| (E)-2-Hexenal | 5.474 ± 1.044 b | 10.305 ± 2.251 c | 1.346 ± 0.166 a | 9.629 ± 0.776 a | 19.002 ± 3.906 b | 8.177 ± 0.496 a |
| Total | 34.445 ± 3.815 b | 45.727 ± 8.718 c | 13.206 ± 1.925 a | 44.393 ± 4.949 a | 79.237 ± 5.398 b | 35.209 ± 5.113 a |
| Other compounds | ||||||
| Hexyl acetate | n.d. | n.d. | n.d. | 0.206 ± 0.043 a | 0.721 ± 0.159 b | 0.554 ± 0.115 b |
| Methyl jasmonate | 0.064 ± 0.006 a | 0.077 ± 0.009 a | 0.121 ± 0.016 b | 1.738 ± 0.381 b | 0.222 ± 0.038 a | 0.114 ± 0.022 a |
| Weight of 100 Berries (g) | °Brix | Probable Alcohol (% v/v) | pH | Total Acidity (g/L) | Glu + Fru (g/L) | Glu (g/L) | Fru (g/L) | Malic Acid (g/L) | Total Phenols (mg/L) | Ammonium Nitrogen (mg N/L) | Amino Nitrogen (mg N/L) | YAN (mg N/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T) | |||||||||||||
| Control | 156.63 a | 23.50 a | 13.80 a | 3.79 a | 4.37 a | 233.14 a | 113.74 a | 119.39 a | 1.73 a | 863.47 a | 99.58 a | 135.52 a | 235.10 a |
| MeJ | 174.74 a | 22.20 a | 12.91 a | 3.74 a | 4.87 a | 217.06 a | 104.48 a | 112.58 a | 2.04 a | 954.82 b | 104.00 a | 170.87 a | 274.87 a |
| ACP-MeJ | 155.92 a | 22.87 a | 13.36 a | 3.77 a | 4.58 a | 227.37 a | 109.75 a | 117.62 a | 1.95 a | 967.05 b | 108.03 a | 163.97 a | 272.00 a |
| Season (S) | |||||||||||||
| 2019 | 124.14 a | 23.43 a | 13.75 a | 3.81 a | 4.98 b | 232.25 a | 111.32 a | 120.94 b | 2.43 b | 1281.10 b | 95.25 a | 165.44 a | 260.68 a |
| 2020 | 200.71 b | 22.28 a | 12.96 a | 3.73 a | 4.23 a | 219.46 a | 107.33 a | 112.12 a | 1.38 a | 575.79 a | 112.49 b | 148.13 a | 260.63 a |
| Interaction | |||||||||||||
| T × S | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | * | * | * |
| Treatment (T) | Season (S) | |||||
|---|---|---|---|---|---|---|
| Control | MeJ | ACP-MeJ | 2019 | 2020 | Interaction (T × S) | |
| Terpenoids | ||||||
| Limonene | 0.101 b | 0.129 c | 0.078 a | 0.092 a | 0.113 b | * |
| p-Cymene | 0.154 a | 0.319 b | 0.141 a | 0.156 a | 0.254 b | * |
| Linalool | 0.058 a | 0.140 b | 0.052 a | 0.089 a | 0.078 a | *** |
| α-Terpineol | 0.045 a | 0.107 b | 0.056 a | 0.073 a | 0.066 a | *** |
| Geraniol | 0.026 a | 0.025 a | 0.024 a | 0.029 b | 0.021 a | *** |
| Geranic acid | 0.098 a | 0.104 a | 0.090 a | 0.111 b | 0.084 a | *** |
| Geranyl acetone | 0.019 ab | 0.021 b | 0.016 a | 0.021 b | 0.016 a | *** |
| Total | 0.501 a | 0.846 b | 0.457 a | 0.570 a | 0.632 b | *** |
| C13 norisoprenoids | ||||||
| (E)-β-Damascenone | 4.093 a | 4.964 a | 4.080 a | 1.815 a | 6.944 b | * |
| (Z)-β-Damascenone | 0.275 a | 0.353 a | 0.327 a | 0.129 a | 0.508 b | * |
| β-Ionone | 0.105 b | 0.186 c | 0.076 a | 0.170 b | 0.061 a | N.S. |
| β-Cyclocitral | 0.112 b | 0.113 b | 0.074 a | 0.148 b | 0.051 a | *** |
| TDN | 0.210 a | 0.346 c | 0.262 b | 0.197 a | 0.347 b | *** |
| Total | 4.795 a | 5.942 a | 4.936 a | 2.500 a | 7.949 b | * |
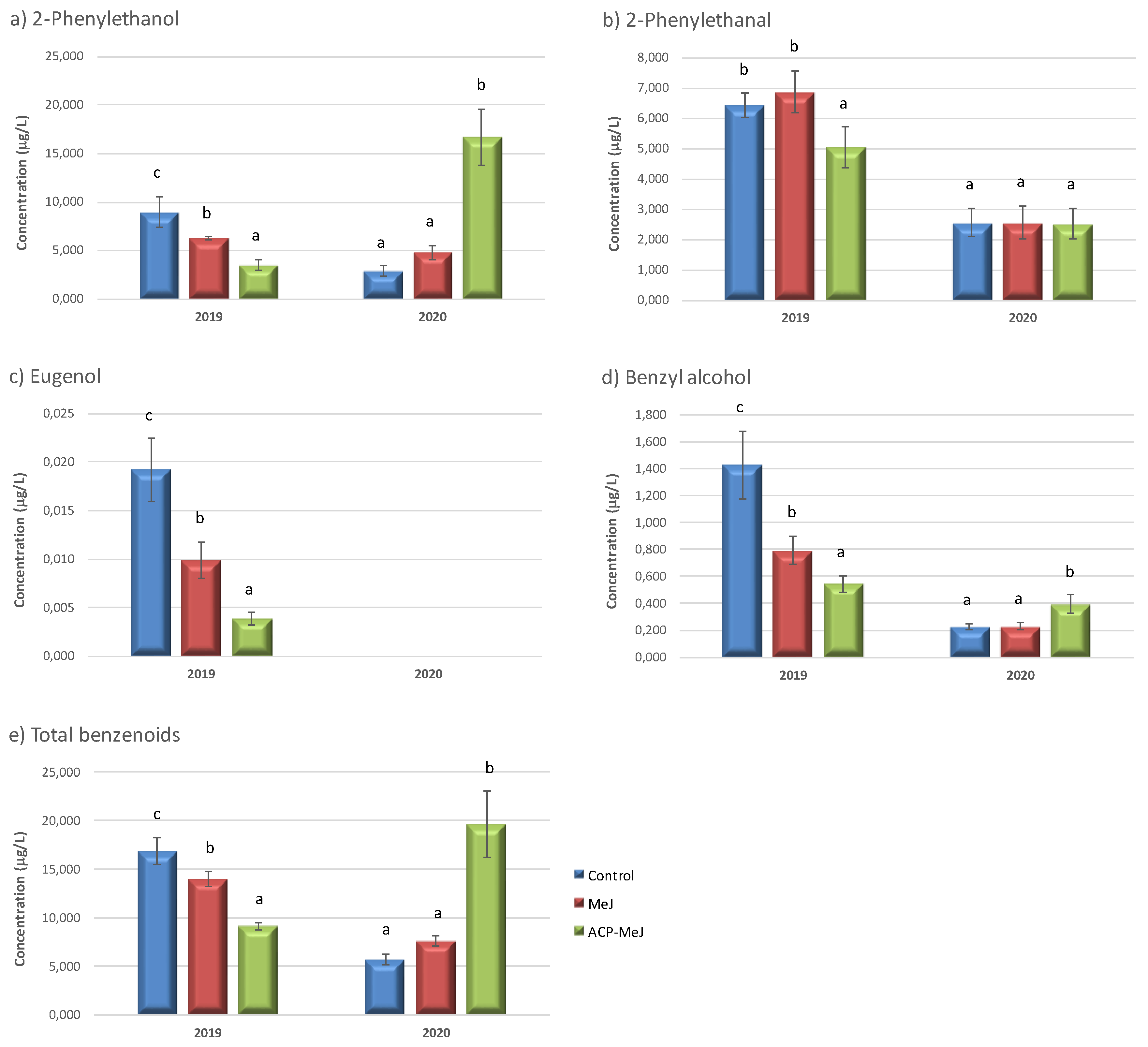
| Benzenoid compounds | ||||||
| 2-Phenylethanol | 5.917 a | 5.528 a | 10.099 b | 6.254 a | 8.109 b | *** |
| 2-Phenylethanal | 4.492 b | 4.722 b | 3.794 a | 6.128 b | 2.544 a | * |
| Eugenol | 0.010 c | 0.005 b | 0.002 a | 0.011 b | n.d. a | *** |
| Benzyl alcohol | 0.826 b | 0.511 a | 0.468 a | 0.921 b | 0.282 a | *** |
| Total | 11.245 a | 10.766 a | 14.362 b | 13.314 b | 10.935 a | *** |
| Alcohols | ||||||
| n-Heptanol | 0.055 b | 0.045 ab | 0.037 a | 0.045 a | 0.045 a | ** |
| n-Octanol | 0.258 b | 0.204 a | 0.173 a | 0.157 a | 0.266 b | N.S. |
| n-Nonanol | 0.130 b | 0.152 b | 0.062 a | 0.051 a | 0.178 b | ** |
| 1-Octen-3-ol | 0.384 b | 0.185 a | 0.195 a | 0.378 b | 0.131 a | *** |
| 2-Ethyl-1-hexanol | 2.479 c | 1.330 a | 1.883 b | 2.170 b | 1.624 a | *** |
| Total | 3.307 c | 1.916 a | 2.349 b | 2.803 b | 2.245 a | *** |
| Carbonyl compounds | ||||||
| Heptanal | 0.034 b | 0.020 a | 0.021 a | 0.041 b | 0.010 a | * |
| (E)-2-Octenal | 0.051 b | 0.038 a | 0.047 b | 0.054 b | 0.037 a | N.S. |
| Nonanal | 0.292 b | 0.129 a | 0.159 a | 0.134 a | 0.253 b | * |
| (E)-2-Nonenal | 0.056 b | 0.049 ab | 0.045 a | 0.066 b | 0.034 a | * |
| Decanal | 0.094 c | 0.069 b | 0.043 a | 0.064 a | 0.073 a | * |
| (E,E)-2,4-Hexadienal | 0.944 a | 0.888 a | 0.763 a | 1.145 b | 0.585 a | *** |
| (E,E)-2,4-Nonadienal | 0.069 a | 0.069 a | 0.053 a | 0.090 b | 0.037 a | *** |
| γ-Decalactone | 0.135 a | 0.149 a | 0.164 a | 0.112 a | 0.0187 b | *** |
| 6-Methyl-3,5-heptadien-2-one | 0.054 b | 0.054 b | 0.036 a | 0.070 b | 0.026 a | * |
| Total | 1.729 b | 1.465 a | 1.331 a | 1.775 b | 1.242 a | *** |
| C6 compounds | ||||||
| n-Hexanol | 14.107 a | 24.671 b | 11.398 a | 5.467 a | 27.984 b | *** |
| n-Hexanal | 16.912 b | 22.448 c | 7.592 a | 19.375 b | 11.926 a | * |
| (Z)-3-Hexen-1-ol + (E)-2-Hexen-1-ol | 0.848 b | 0.710 b | 0.457 a | 0.576 a | 0.767 b | *** |
| (E)-2-Hexenal | 7.552 b | 14.653 c | 4.761 a | 5.708 a | 12.269 b | N.S. |
| Total | 39.419 b | 62.482 c | 24.207 a | 31.126 a | 52.946 b | ** |
| Other compounds | ||||||
| Hexyl acetate | 0.103 a | 0.361 b | 0.277 b | n.d. a | 0.494 b | *** |
| Methyl jasmonate | 0.901 b | 0.149 a | 0.117 a | 0.087 a | 0.691 b | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-San Román, S.; Pérez-Álvarez, E.P.; Sáenz de Urturi, I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Garde-Cerdán, T. Changes on Grape Aroma Composition as a Consequence of Foliar Application of Methyl Jasmonate and Nano-Sized Particles Doped with Methyl Jasmonate. Appl. Sci. 2023, 13, 2487. https://doi.org/10.3390/app13042487
Marín-San Román S, Pérez-Álvarez EP, Sáenz de Urturi I, Parra-Torrejón B, Ramírez-Rodríguez GB, Delgado-López JM, Garde-Cerdán T. Changes on Grape Aroma Composition as a Consequence of Foliar Application of Methyl Jasmonate and Nano-Sized Particles Doped with Methyl Jasmonate. Applied Sciences. 2023; 13(4):2487. https://doi.org/10.3390/app13042487
Chicago/Turabian StyleMarín-San Román, Sandra, Eva Pilar Pérez-Álvarez, Itziar Sáenz de Urturi, Belén Parra-Torrejón, Gloria B. Ramírez-Rodríguez, José Manuel Delgado-López, and Teresa Garde-Cerdán. 2023. "Changes on Grape Aroma Composition as a Consequence of Foliar Application of Methyl Jasmonate and Nano-Sized Particles Doped with Methyl Jasmonate" Applied Sciences 13, no. 4: 2487. https://doi.org/10.3390/app13042487
APA StyleMarín-San Román, S., Pérez-Álvarez, E. P., Sáenz de Urturi, I., Parra-Torrejón, B., Ramírez-Rodríguez, G. B., Delgado-López, J. M., & Garde-Cerdán, T. (2023). Changes on Grape Aroma Composition as a Consequence of Foliar Application of Methyl Jasmonate and Nano-Sized Particles Doped with Methyl Jasmonate. Applied Sciences, 13(4), 2487. https://doi.org/10.3390/app13042487







