Tannin Extraction from Chestnut Wood Waste: From Lab Scale to Semi-Industrial Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Chestnut Wood Matrix
2.3. Microwave-Assisted Subcritical Water Extraction (MASWE)
2.4. Semi-Industrial-Scale Subcritical Water Extraction (SI-SWE)
2.5. Membrane Filtration
2.6. Spray Drying
2.7. Colorimetric Assays
2.7.1. Total Polyphenol Content (TPC)—Folin–Ciocalteau Assay
2.7.2. Tannin Determination
2.7.3. Antioxidant Activity
DPPH Assay
ABTS Assay
2.8. LC-MS-DAD Characterization
3. Results and Discussions
3.1. MASWE
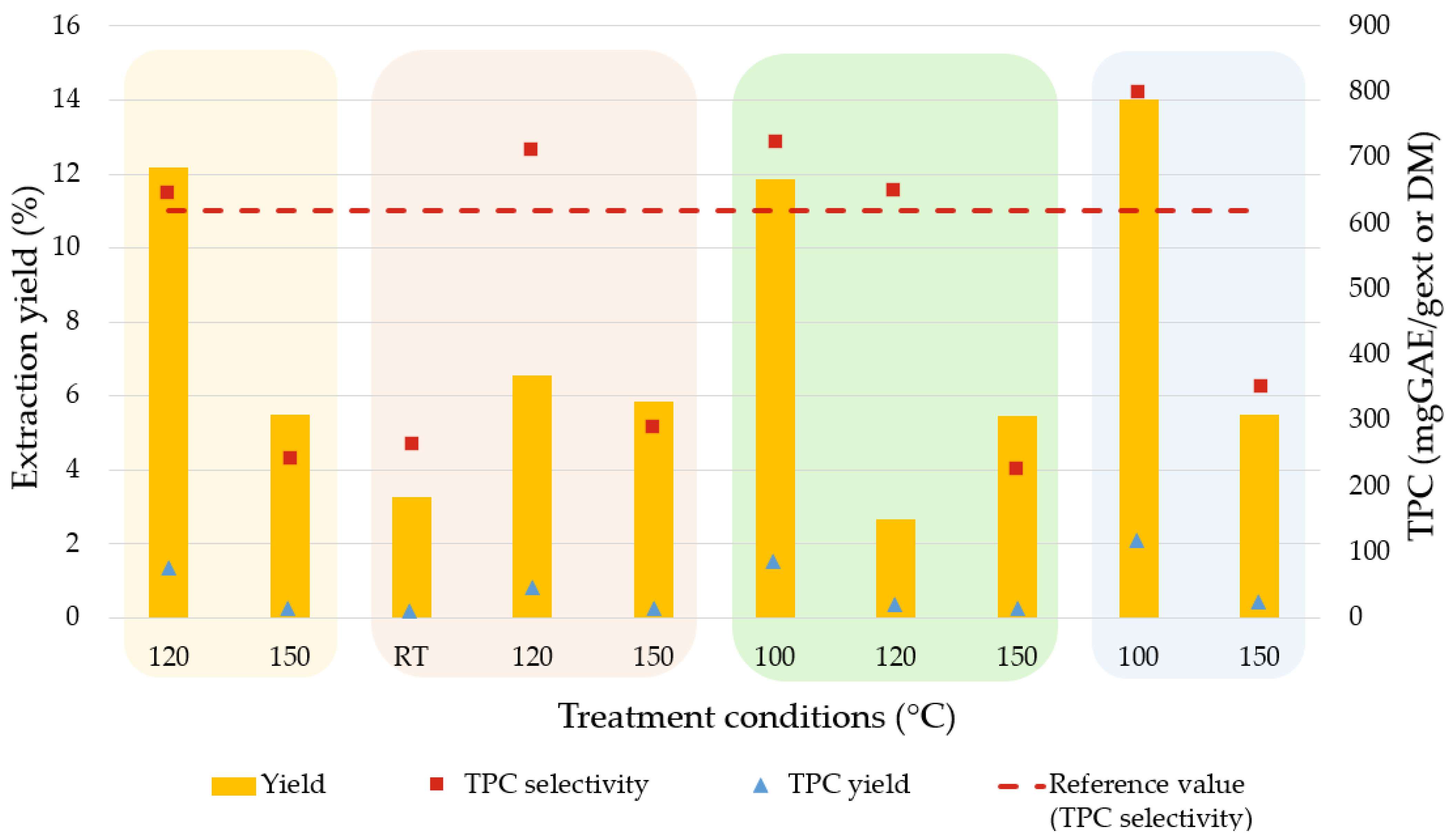
Sequential Extraction Screening
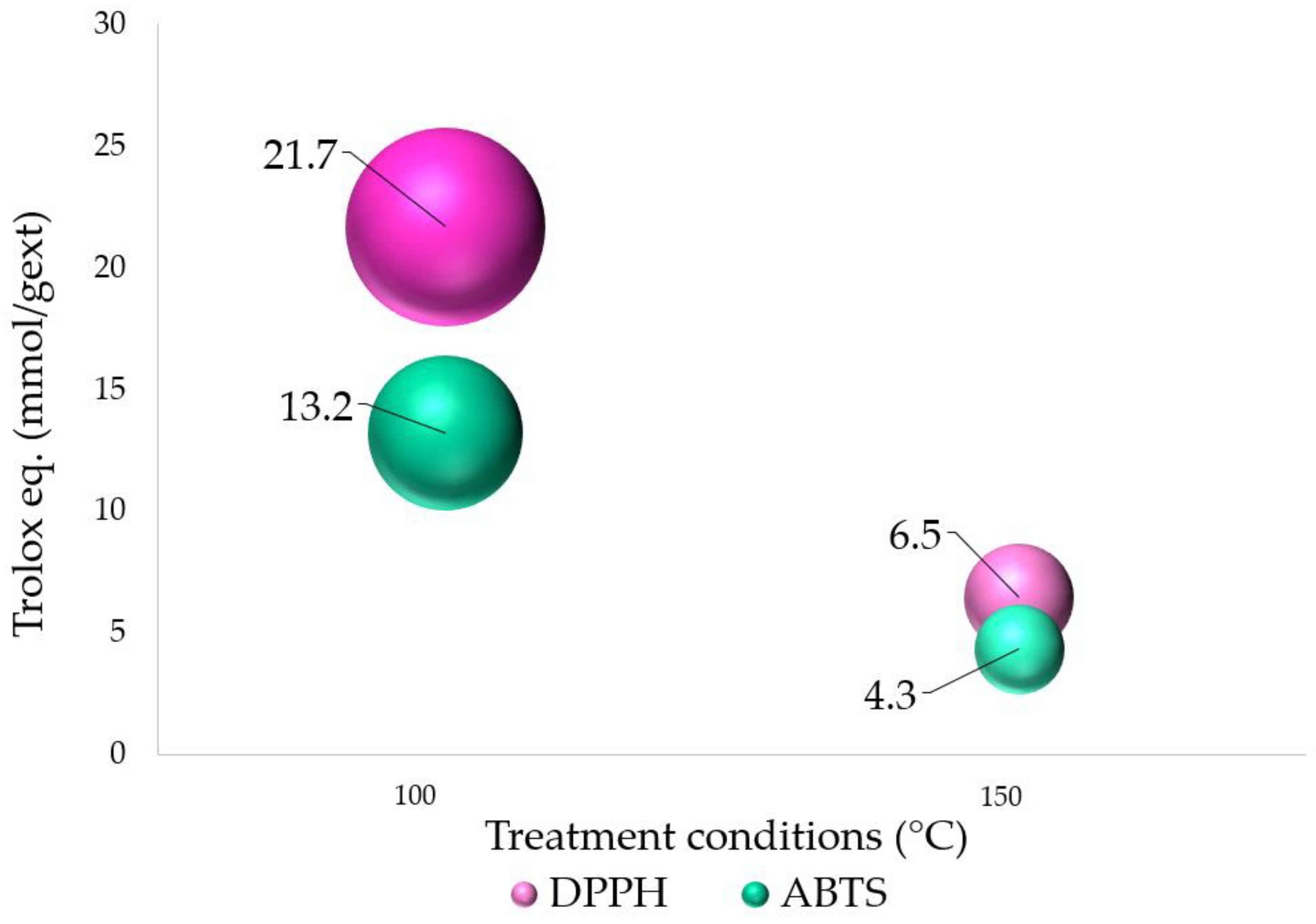
3.2. Antioxidant Activity, DPPH, and ABTS Assays
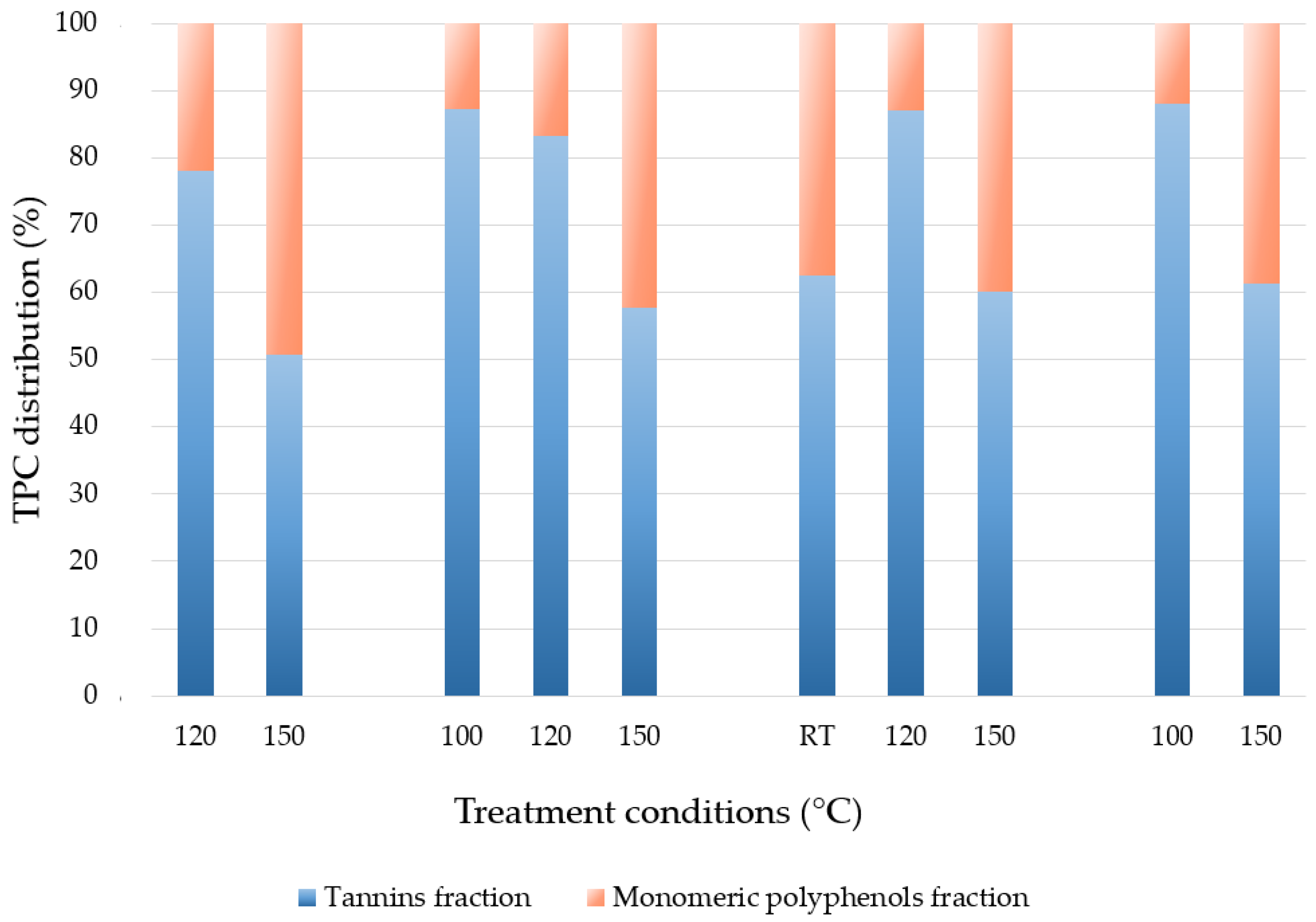
3.3. Tannin Analyses
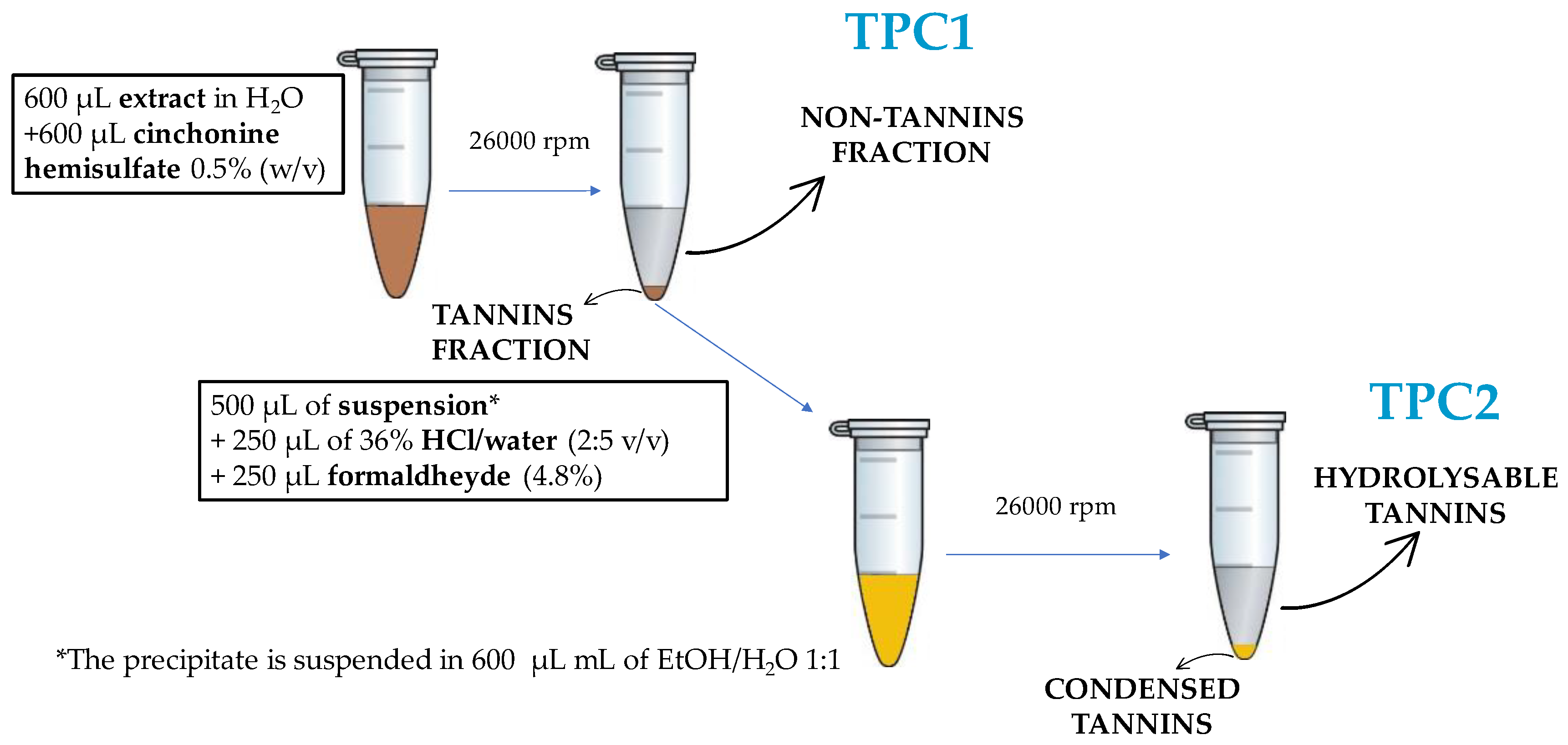
3.3.1. Tannins Precipitation—Cinchonine Hemisulfate Assay
3.3.2. Tannins—Formaldehyde and HCl Assay
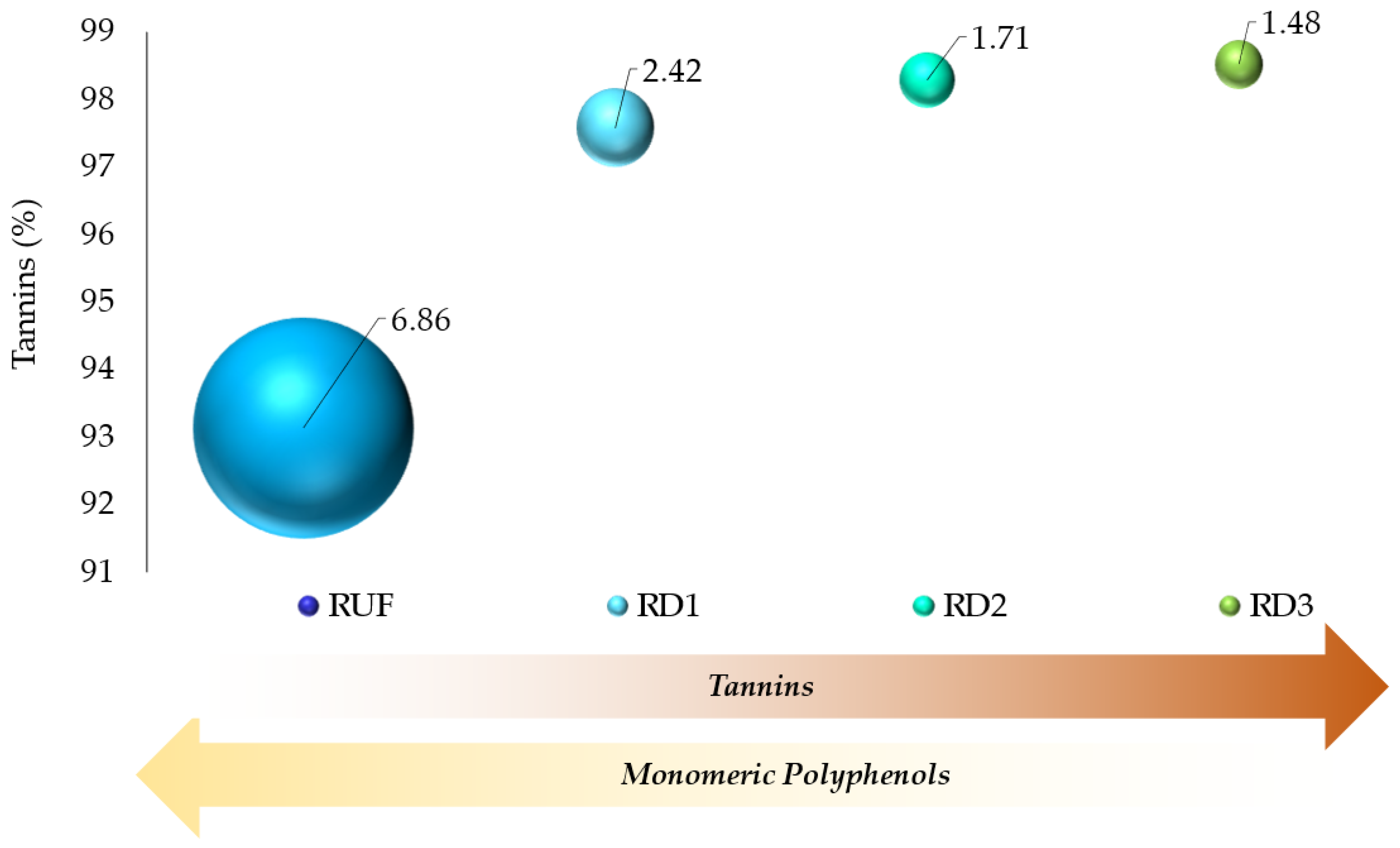
3.4. Lab-Scale Downstream—Membrane Filtration
3.5. Semi-Industrial-Scale Subcritical Water Extraction (SI-SWE)
3.6. Semi-Industrial Downstream
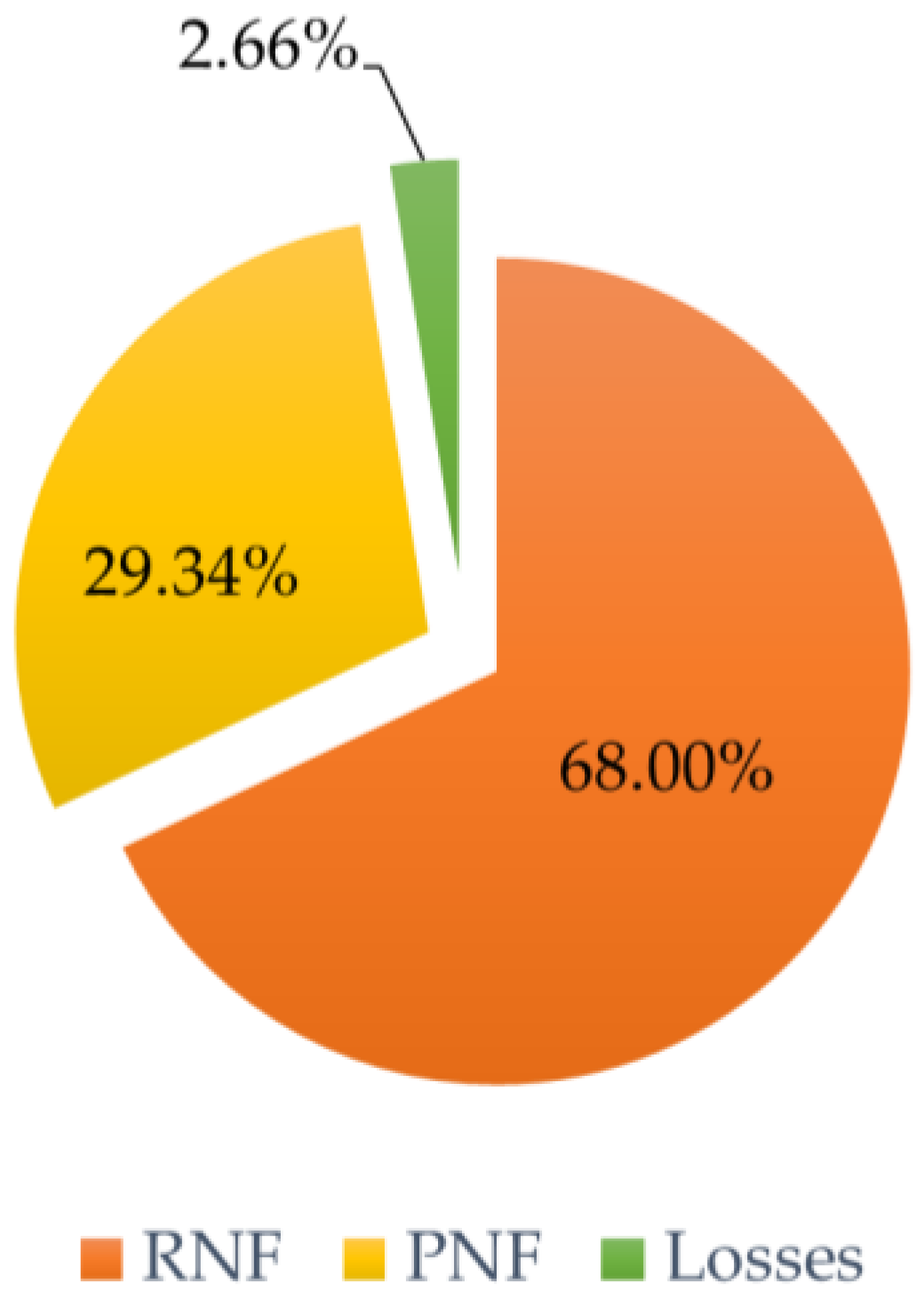
3.6.1. Membrane Filtration
3.6.2. Spray Drying
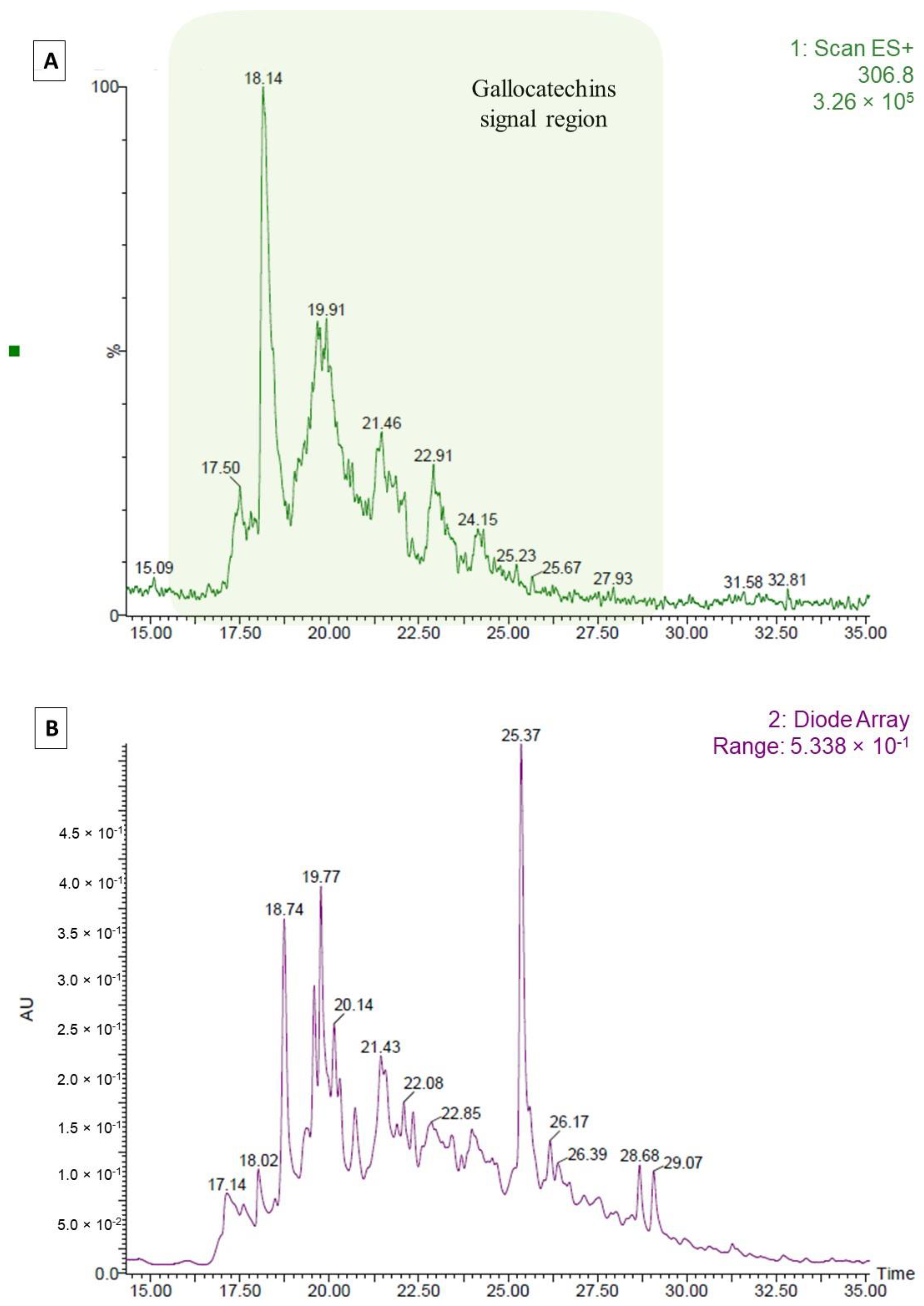
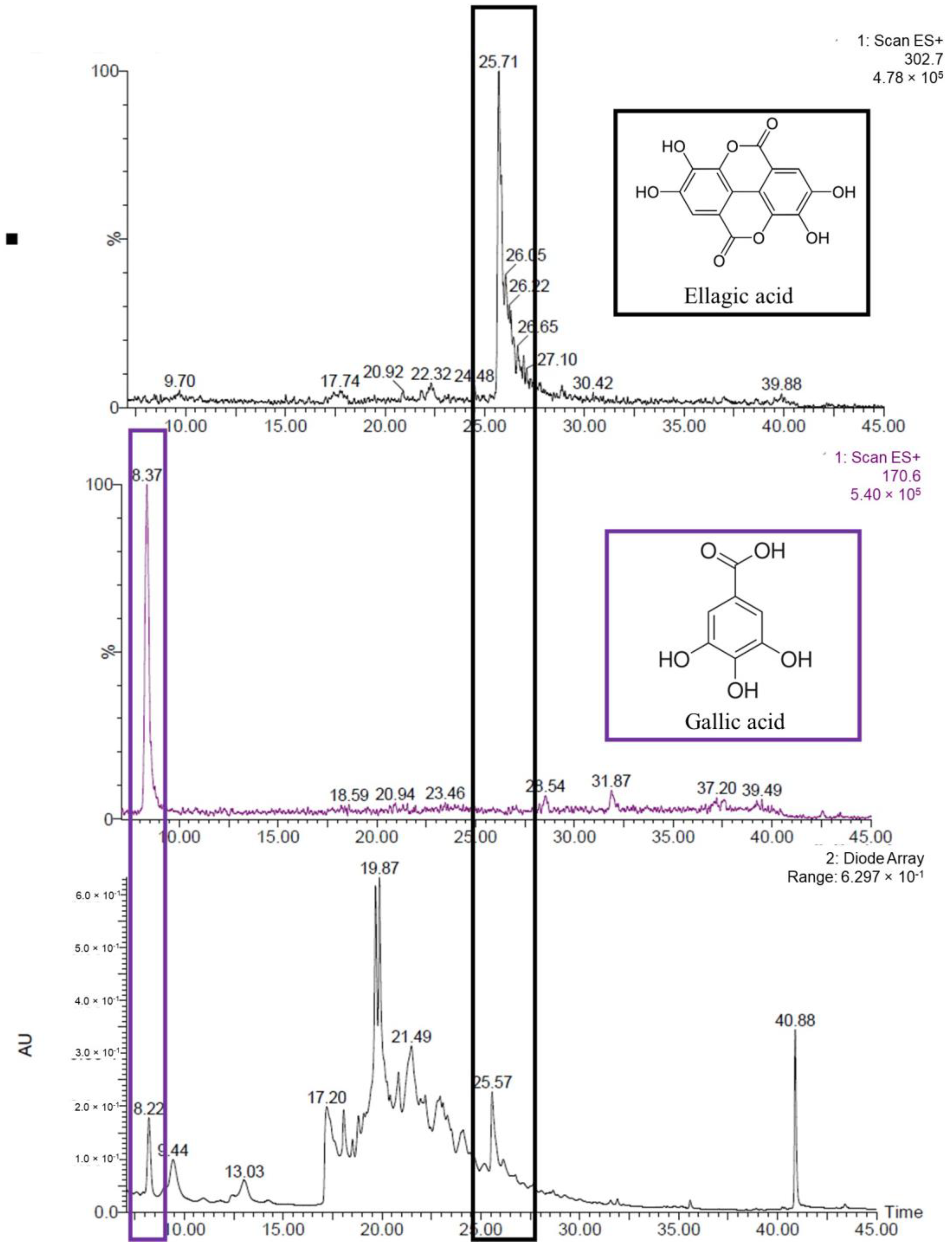
3.7. LC-MS-DAD Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Tannins: Precipitation and Hydrolysable/Condensed Discrimination
Appendix A.2. LC-MS-DAD Analysis of Tannin Fraction: Difference between Pre- and Post-Precipitation Samples

References
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Conedera, M.; Krebs, P.; Tinner, W.; Pradella, M.; Torriani, D. The cultivation of Castanea sativa (Mill.) in Europe, from its origin to its diffusion on a continental scale. Veg. Hist. Archaeobot. 2004, 13, 161–179. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Sharma, A.K.; Zhu, J.; Zheng, B.; Xiao, G.; Chen, L. Nutritional biology of chestnuts: A perspective review. Food Chem. 2022, 395, 133575. [Google Scholar] [CrossRef]
- Ozcan, T.; Yilmaz-Ersan, L.; Akpinar-Bayizit, A.; Delikanli, B. Antioxidant properties of probiotic fermented milk supplemented with chestnut flour (Castanea sativa Mill). J. Food Process. Preserv. 2017, 41, e13156. [Google Scholar] [CrossRef]
- Hu, M.; Yang, X.; Chang, X. Bioactive phenolic components and potential health effects of chestnut shell: A review. J. Food Biochem. 2021, 45, e13696. [Google Scholar] [CrossRef]
- de Vasconcelos, M.d.C.B.M.; Bennett, R.N.; Quideau, S.; Jacquet, R.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Evaluating the potential of chestnut (Castanea sativa Mill.) fruit pericarp and integument as a source of tocopherols, pigments and polyphenols. Ind. Crops Prod. 2010, 31, 301–311. [Google Scholar] [CrossRef]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; De Lucia, A.; Colucci, G.; La Cara, F.; Morana, A. Chestnut (Castanea sativa Mill.) industrial wastes as a valued bioresource for the production of active ingredients. Process Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- Truong, V.-L.; Jeong, W.-S. Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci. Hum. Wellness 2022, 11, 502–511. [Google Scholar] [CrossRef]
- Macías-Cortés, E.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Moreno-Jiménez, M.R.; Cervantes-Cardoza, V.; Castillo-Herrera, G.A. Antioxidant and anti-inflammatory polyphenols in ultrasound-assisted extracts from salvilla (Buddleja scordioides Kunth). Ultrason. Sonochem. 2022, 83, 105917. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, A.; Alfarhan, A.; Rajagopal, R. Antimicrobial and anti-biofilm activities of polyphenols extracted from different Saudi Arabian date cultivars against human pathogens. J. Infect. Public Health 2021, 14, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Pinto, D.; Almeida, A.; López-Yerena, A.; Pinto, S.; Sarmento, B.; Lamuela-Raventós, R.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Appraisal of a new potential antioxidants-rich nutraceutical ingredient from chestnut shells through in-vivo assays—A targeted metabolomic approach in phenolic compounds. Food Chem. 2023, 404, 134546. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Yoshida, T.; Amakura, Y.; Yoshimura, M. Structural Features and Biological Properties of Ellagitannins in Some Plant Families of the Order Myrtales. Int. J. Mol. Sci. 2010, 11, 79–106. [Google Scholar] [CrossRef] [Green Version]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Fernández-Agulló, A.; Freire, M.S.; Antorrena, G.; Pereira, J.A.; González-Álvarez, J. Effect of the Extraction Technique and Operational Conditions on the Recovery of Bioactive Compounds from Chestnut (Castanea sativa Mill.) Bur and Shell. Sep. Sci. Technol. 2014, 49, 267–277. [Google Scholar] [CrossRef]
- Vella, F.M.; Laratta, B.; la Cara, F.; Morana, A. Recovery of bioactive molecules from chestnut (Castanea sativa Mill.) by-products through extraction by different solvents. Nat. Prod. Res. 2018, 32, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H. Ethanol-modified supercritical CO2 extraction of chestnut burs antioxidants. Chem. Eng. Process. Process Intensif. 2020, 156, 108092. [Google Scholar] [CrossRef]
- Zhu, T.; Shen, Q.; Xu, Y.; Li, C. Ionic liquid and ultrasound-assisted extraction of chestnut shell pigment with good hair dyeing capability. J. Clean. Prod. 2022, 335, 130195. [Google Scholar] [CrossRef]
- An, J.Y.; Wang, L.-T.; Lv, M.-J.; Wang, J.-D.; Cai, Z.-H.; Wang, Y.-Q.; Zhang, S.; Yang, Q.; Fu, Y.-J. An efficiency strategy for extraction and recovery of ellagic acid from waste chestnut shell and its biological activity evaluation. Microchem. J. 2021, 160, 105616. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem. Eng. Res. Des. 2011, 89, 857–862. [Google Scholar] [CrossRef]
- Ham, J.S.; Kim, H.Y.; Lim, S.T. Antioxidant and deodorizing activities of phenolic components in chestnut inner shell extracts. Ind. Crops Prod. 2015, 73, 99–105. [Google Scholar] [CrossRef]
- Shi, J.; Nawaz, H.; Pohorly, J.; Mittal, G.; Kakuda, Y.; Jiang, Y. Extraction of polyphenolics from plant material for functional foods—Engineering and technology. Food Rev. Int. 2005, 21, 139–166. [Google Scholar] [CrossRef]
- Grillo, G.; Tabasso, S.; Solarino, R.; Cravotto, G.; Toson, C.; Ghedini, E.; Menegazzo, F.; Signoretto, M. From Seaweeds to Cosmeceutics: A Multidisciplinar Approach. Sustainability 2021, 13, 13443. [Google Scholar] [CrossRef]
- Santagata, G.; Grillo, G.; Immirzi, B.; Tabasso, S.; Cravotto, G.; Malinconico, M. Non-conventional Ultrasound-Assisted Extraction of Alginates from Sargassum Seaweed: From Coastal Waste to a Novel Polysaccharide Source. In Springer Water, Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea (Capri, Italy); Cocca, M., Di Pace, E., Errico, M., Gentile, G., Montarsolo, A., Mossotti, R., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Lamberti, L.; Grillo, G.; Gallina, L.; Carnaroglio, D.; Chemat, F.; Cravotto, G. Microwave-Assisted Hydrodistillation of Hop (Humulus lupulus L.) Terpenes: A Pilot-Scale Study. Foods 2021, 10, 2726. [Google Scholar] [CrossRef]
- Gunjevic, V.; Grillo, G.; Carnaroglio, D.; Binello, A.; Barge, A.; Cravotto, G. Selective recovery of terpenes, polyphenols and cannabinoids from Cannabis sativa L. inflorescences under microwaves. Ind. Crops Prod. 2021, 162, 113247–113258. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Colletti, A.; Grillo, G.; Tabasso, S.; Cravotto, G. Emerging Processing Technologies for the Recovery of Valuable Bioactive Compounds from Potato Peels. Foods 2020, 9, 1598. [Google Scholar] [CrossRef] [PubMed]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep Eutectic Solvents and Nonconventional Technologies for Blueberry-Peel Extraction: Kinetics, Anthocyanin Stability, and Antiproliferative Activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Grillo, G.; Calcio Gaudino, E.; Rosa, R.; Leonelli, C.; Timonina, A.; Grygiškis, S.; Tabasso, S.; Cravotto, G. Green Deep Eutectic Solvents for Microwave-Assisted Biomass Delignification and Valorisation. Molecules 2021, 26, 798. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Grillo, G.; Tabasso, S.; Stevanato, L.; Cravotto, G.; Marjamaa, K.; Pihlajaniemi, V.; Koivula, A.; Aro, N.; Uusitalo, J.; et al. Optimization of ultrasound pretreatment and enzymatic hydrolysis of wheat straw: From lab to semi-industrial scale. J. Clean. Prod. 2022, 380, 134897. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Dominguez, J.M.; Sineiro, J.; Dominguez, H.; Nunez, M.J.; Parajo, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef]
- Gagić, T.; Knez, Ž.; Škerget, M. Subcritical water extraction of chestnut bark and optimization of process parameters. Molecules 2020, 25, 2774. [Google Scholar] [CrossRef]
- Alarcón, M.; Díaz-Maroto, M.C.; Pérez Coello, M.S.; Alañón, M.E. Isolation of natural flavoring compounds from cooperage woods by pressurized hot water extraction (PHWE). Holzforschung 2019, 73, 295–303. [Google Scholar] [CrossRef]
- Beaufils, N.; Boucher, J.; Peydecastaing, J.; Rigal, L.; Vilarem, G.; Villette, M.J.; Candy, L.; Pontaliera, P.Y. The effect of time and temperature on the extraction of xylose and total phenolic compounds with pressurized hot water from hardwood species used for pulp and paper production in the South of France. Bioresour. Technol. Rep. 2021, 16, 100832. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. The potential of subcritical water as a “green” method for the extraction and modification of pectin: A critical review. Food Res. Int. 2022, 161, 111849. [Google Scholar] [CrossRef]
- Song, E.J.; Ko, M.J. Extraction of monoterpenes from coriander (Coriandrum sativum L.) seeds using subcritical water extraction (SWE) technique. J. Supercrit. Fluids 2022, 188, 105668. [Google Scholar] [CrossRef]
- Gan, A.; Baroutian, S. Subcritical water extraction for recovery of phenolics and fucoidan from New Zealand Wakame (Undaria pinnatifida) seaweed. J. Supercrit. Fluids 2022, 190, 105732. [Google Scholar] [CrossRef]
- Nazir, A.; Khan, K.; Maan, A.; Zia, R.; Giorno, L.; Schroën, K. Membrane separation technology for the recovery of nutraceuticals from food industrial streams. Trends Food Sci. Technol. 2019, 86, 426–438. [Google Scholar] [CrossRef]
- Kumar, A. Chapter 8, Membrane Separation Technology in Processing Bioactive Components. In Functional Food Ingredients and Nutraceuticals; Shi, J., Ed.; CRC Press: Boca Raton, FL, USA, 2006; p. 16. [Google Scholar]
- Conidi, C.; Drioli, E.; Cassano, A. Membrane-based agro-food production processes for polyphenol separation, purification and concentration. Curr. Opin. Food Sci. 2018, 23, 149–164. [Google Scholar] [CrossRef]
- Cravotto, C.; Grillo, G.; Binello, A.; Gallina, L.; Olivares-Vicente, M.; Herranz-López, M.; Micol, V.; Barrajón-Catalán, E.; Cravotto, G. Bioactive Antioxidant Compounds from Chestnut Peels through Semi-Industrial Subcritical Water Extraction. Antioxidants 2022, 11, 988. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Talarico, S.; Solarino, R.; Binello, A.; Cavaglià, G.; Bensaid, S.; Telysheva, G.; Cravotto, G. Batch and Flow Ultrasound-Assisted Extraction of Grape Stalks: Process Intensification Design up to a Multi-Kilo Scale. Antioxidants 2020, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; De Luca, G.; Conidi, C.; Drioli, E. Effect of polyphenols-membrane interactions on the performance of membrane-based processes. A review. Coord. Chem. Rev. 2017, 351, 45–75. [Google Scholar] [CrossRef]
- Conidi, C.; Rodriguez-Lopez, A.D.; Garcia-Castello, E.M.; Cassano, A. Purification of artichoke polyphenols by using membrane filtration and polymeric resins. Sep. Purif. Technol. 2015, 144, 153–161. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Separation of sugarcane bagasse mild alkaline extract components by ultrafiltration–Membrane screening and effect of filtration parameters. Process Biochem. 2019, 78, 91–99. [Google Scholar] [CrossRef] [Green Version]
- de Santana Magalhães, F.; de Souza Martins Sá, M.; Cardoso, V.L.; Miranda Reis, M.H. Recovery of phenolic compounds from pequi (Caryocar brasiliense Camb.) fruit extract by membrane filtrations: Comparison of direct and sequential processes. J. Food Eng. 2019, 257, 26–33. [Google Scholar] [CrossRef]
- Prodanov, M.; Garrido, I.; Vacas, V.; Lebron-Aguilar, R.; Dueñas, M.; Gómez-Cordovés, C.; Bartolomé, B. Ultrafiltration as alternative purification procedure for the characterization of low and high molecular-mass phenolics from almond skins. Anal. Chim. Acta 2008, 609, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.C.R.; Mota, I.F.; Louriero, J.M.; Rodrigues, A.E. Membrane performance and application of ultrafiltration and nanofiltration to ethanol/water extract of Eucalyptus bark. Sep. Purif. Technol. 2014, 132, 234–243. [Google Scholar] [CrossRef]
- Conidi, C.; Donato, L.; Algieri, C.; Cassano, A. Valorization of chestnut processing by-products: A membrane-assisted green strategy for purifying valuable compounds from shells. J. Clean. Prod. 2022, 378, 134564. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Moure, A.; Franco, D.; Sineiro, J.; Domínguez, H.; Núñez, M.J. Simulation of multistage extraction of antioxidants from Chilean hazelnut (Gevuina avellana) hulls. J. Am. Oil Chem. Soc. 2003, 80, 389–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, H.; Lv, X.; Zheng, C.; Wu, Z.; Wang, N.; Wang, J.; Chen, H.; Wei, F. Profiling and spatial distribution of phenolic compounds in rapeseed by two-step extraction strategy and targeted metabolomics combined with chemometrics. Food Chem. 2023, 401, 134151. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Cör, D.; Verboten, M.T.; Knez, Z. Application of supercritical and subcritical fluids in food processing. Food Qual. Saf. 2018, 2, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Both, S.; Chemat, F.; Strube, J. Extraction of polyphenols from black tea—Conventional and ultrasound assisted extraction. Ultrason. Sonochem. 2014, 21, 1030–1034. [Google Scholar] [CrossRef]
- Binello, A.; Grillo, G.; Barge, A.; Allegrini, P.; Ciceri, D.; Cravotto, G. A Cross-Flow Ultrasound-Assisted Extraction of Curcuminoids from Curcuma longa L.: Process Design to Avoid Degradation. Foods 2020, 9, 743. [Google Scholar] [CrossRef]
- Cardoso Ugarte, G.A.; Sosa Morales, M.E.; Ballard, T.; Liceaga, A.; San Martín González, M.F. Microwave-assisted extraction of betalains from red beet (Beta vulgaris). Food Sci. Technol. 2014, 59, 276–282. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Peri, C.; Pompei, C. Estimation of Different Phenolic Groups in Vegetable Extracts. Phytochemistry 1971, 10, 2187–2189. [Google Scholar] [CrossRef]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in wood: Comparison of different estimation methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Locatelli, M.; Gindro, R.; Travaglia, F.; Coïsson, J.D.; Rinaldi, M.; Arlorio, M. Study of the DPPH-scavenging activity: Development of a free software for the correct interpretation of data. Food Chem. 2009, 114, 889–897. [Google Scholar] [CrossRef]
- Re, R.; Pellegrino, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical Cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Caprarulo, V.; Giromini, C.; Rossi, L. Review: Chestnut and quebracho tannins in pig nutrition: The effects on performance and intestinal health. Animal 2021, 15, 100064. [Google Scholar] [CrossRef]
- Auad, P.; Spier, F.; Gutterres, M. Vegetable tannin composition and its association with leather tanning effect. Chem. Eng. Commun. 2020, 207, 722–732. [Google Scholar] [CrossRef]
- Spina, S.; Zhou, X.; Segovia, C.; Pizzi, A.; Romagnoli, M.; Giovando, S.; Pasch, H.; Rode, K.; Delmotte, L. Phenolic resin adhesives based on chestnut (Castanea sativa) hydrolysable tannins. J. Adhes. Sci. Technol. 2013, 27, 2103–2111. [Google Scholar] [CrossRef]




| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) | PNF | NF permeate |
| CTs | Condensed tannins | PUF | UF permeate |
| CW | Chestnut wood | RD | Diafiltration retentate |
| CWC | Chestnut wood chips | RNF | NF retentate |
| CWS | Chestnut wood sawdust | ROS | Reactive oxygen species |
| DAD | Diode array detector | RT | Room temperature |
| DM | Dry matrix | RUF | UF retentate |
| DPPH | 2,2-diphenyl-1-picrilidraziyl | S/L | Solid/Liquid ratio |
| GAE | Gallic acid equivalents | SI-SWE | Semi-industrial SWE |
| HTs | Hydrolysable tannins | SWE | Subcritical water extraction |
| IC50 | Half-maximal inhibitory concentration | TPC | Total polyphenol content |
| MASWE | Microwave-assisted subcritical water extraction | UF | Ultrafiltration |
| NF | Nanofiltration | US | Ultrasound |
| T (°C) | t (min) | Yield (%) | TPC | |
|---|---|---|---|---|
| Selectivity (mg GAE/gext) | Yield (mg GAE/gDM) | |||
| 120 | 5 | 12.15 | 646.36 ± 10.45 | 78.53 ± 1.27 |
| 150 | 15 | 5.48 | 242.77 ± 5.72 | 13.30 ± 0.31 |
| T (°C) | t (min) | Yield (%) | TPC | |
|---|---|---|---|---|
| Selectivity (mg GAE/gext) | Yield (mg GAE/gDM) | |||
| RT | 120 | 3.25 | 266.16 ± 24.43 | 8.65 ± 0.79 |
| 120 | 5 | 6.52 | 713.35 ± 2.30 | 46.51 ± 0.15 |
| 150 | 15 | 5.82 | 288.13 ± 3.57 | 16.77 ± 0.21 |
| T (°C) | t (min) | Yield (%) | TPC | |
|---|---|---|---|---|
| Selectivity (mg GAE/gext) | Yield (mg GAE/gDM) | |||
| 100 | 5 | 11.83 | 723.93 ± 27.27 | 85.64 ± 3.23 |
| 120 | 5 | 2.64 | 653.89 ± 3.62 | 17.26 ± 0.10 |
| 150 | 15 | 5.43 | 226.26 ± 3.95 | 12.29 ± 0.21 |
| T (°C) | t (min) | Yield (%) | TPC | |
|---|---|---|---|---|
| Selectivity (mg GAE/gext) | Yield (mg GAE/gDM) | |||
| 100 | 10 | 14.50 | 802.84 ± 20.52 | 116.37 ± 2.97 |
| 150 | 15 | 5.47 | 350.74 ± 1.04 | 22.87 ± 0.07 |
| Sample | IC50 (μg/mL) | Trolox Eq. (mmol/gext) | ABTS/DPPH· Ratio | ||
|---|---|---|---|---|---|
| DPPH· | ABTS· | DPPH· | ABTS· | ||
| 100 °C | 1.4 | 2.4 | 21.7 | 13.2 | 0.61 |
| 150 °C | 4.7 | 7.3 | 6.5 | 4.3 | 0.67 |
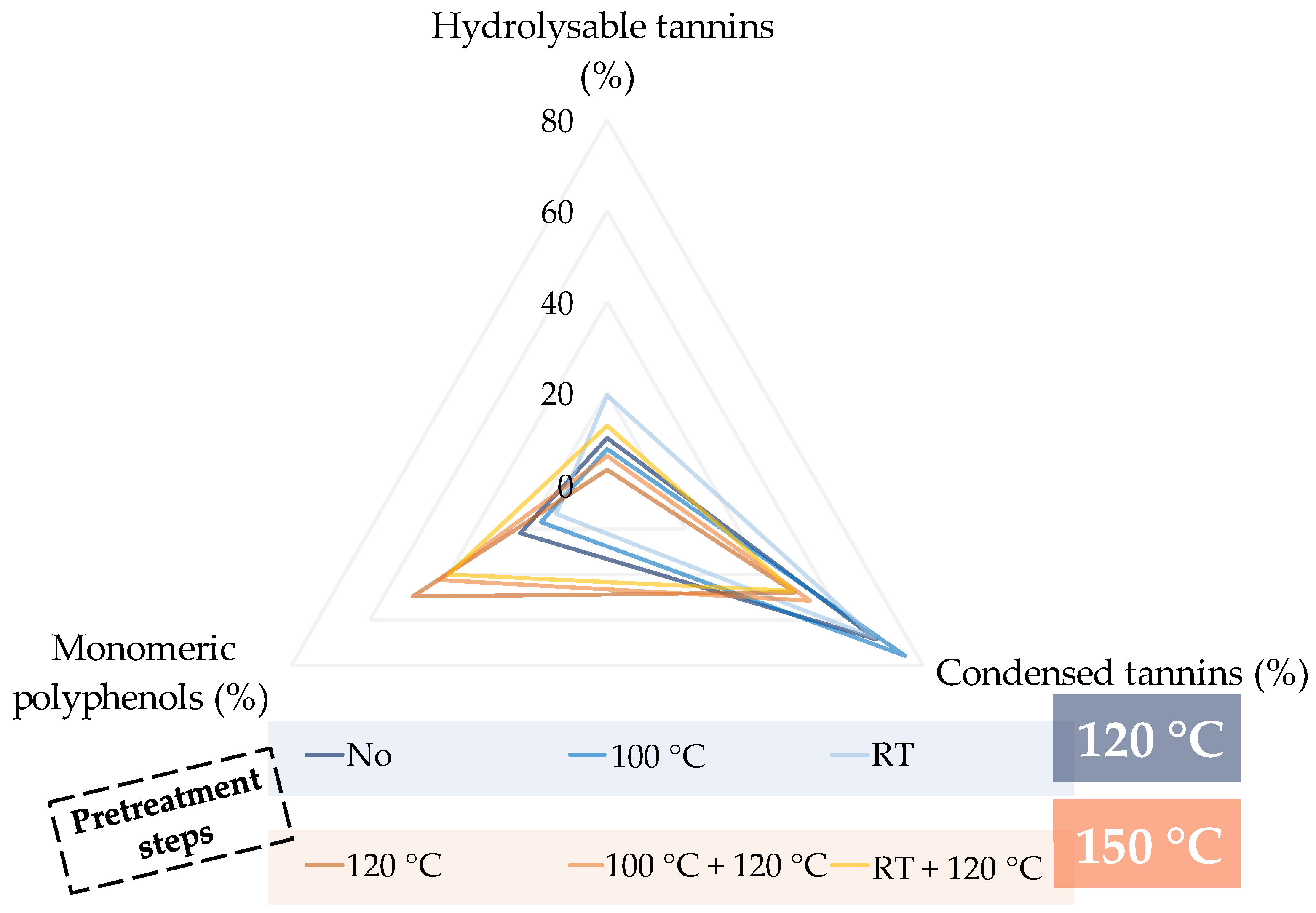
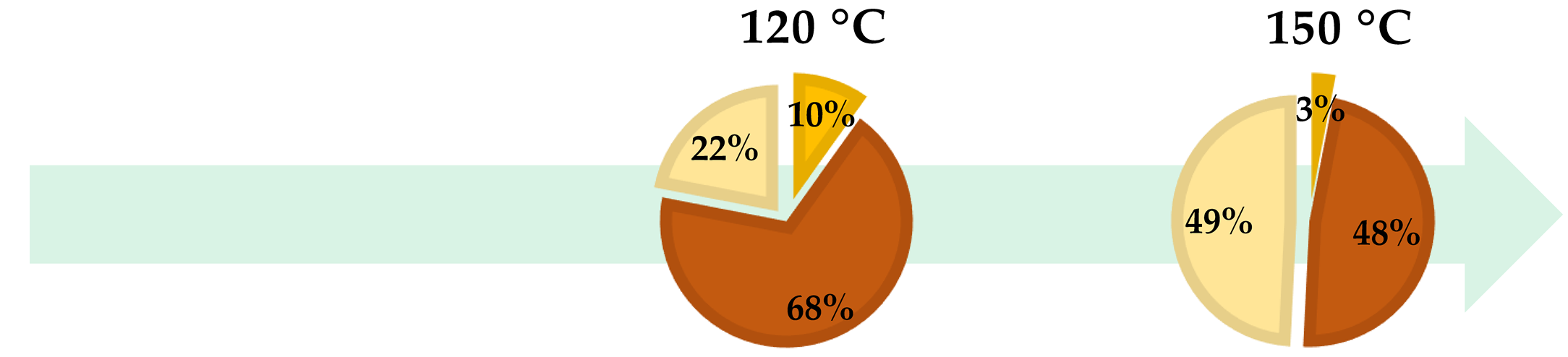
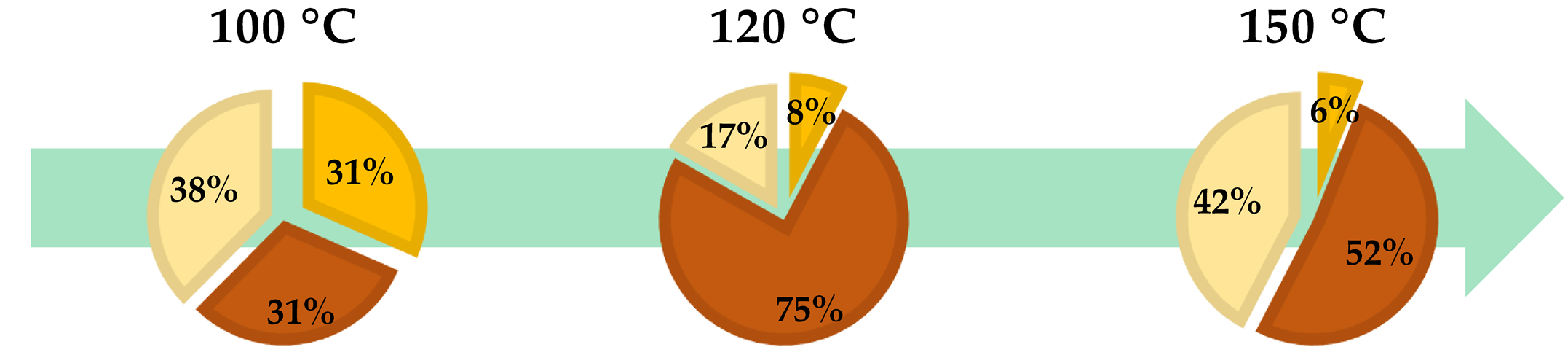
| ● Hydrolysable Tannins | ● Condensed Tannins | ● Monomeric Polyphenols |
|---|---|---|
 | ||
 | ||
 | ||
 | ||
| Temperature (°C) | Time (min) | Yield (%) | TPC Selectivity (mgGAE/gext) | TPC Yield (mgGAE/gDM) |
|---|---|---|---|---|
| 120 | 5 | 2.89 | 516.33 ± 9.65 | 14.92 ± 0.28 |
| 150 | 15 | 3.47 | 666.94 ± 18.12 | 23.12 ± 0.63 |
| Global Process | 6.26 | - | 37.36 | |
| Temperature (°C) | Time (min) | Yield (%) | TPC Selectivity (mgGAE/gext) | TPC Yield (mgGAE/gDM) |
|---|---|---|---|---|
| 100 | 60 | 3.84 | 678.10 ± 6.63 | 26.04 ± 0.25 |
| 120 | 30 | 6.21 | 714.13 ± 13.93 | 44.36 ± 0.87 |
| 150 | 30 | 4.64 | 651.98 ± 5.29 | 30.23 ± 0.24 |
| Global Process | 13.99 | - | 95.96 | |
| Temperature (°C) | Time (min) | Tannins | Polyphenols (%) | ||
|---|---|---|---|---|---|
| Total (%) | Hydrolysable (%) | Condensed (%) | |||
| 100 | 60 | 83.96 | 9.34 | 74.62 | 16.04 |
| 120 | 30 | 77.65 | 8.34 | 69.31 | 22.35 |
| 150 | 30 | 75.34 | 7.12 | 68.22 | 24.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aimone, C.; Grillo, G.; Boffa, L.; Giovando, S.; Cravotto, G. Tannin Extraction from Chestnut Wood Waste: From Lab Scale to Semi-Industrial Plant. Appl. Sci. 2023, 13, 2494. https://doi.org/10.3390/app13042494
Aimone C, Grillo G, Boffa L, Giovando S, Cravotto G. Tannin Extraction from Chestnut Wood Waste: From Lab Scale to Semi-Industrial Plant. Applied Sciences. 2023; 13(4):2494. https://doi.org/10.3390/app13042494
Chicago/Turabian StyleAimone, Clelia, Giorgio Grillo, Luisa Boffa, Samuele Giovando, and Giancarlo Cravotto. 2023. "Tannin Extraction from Chestnut Wood Waste: From Lab Scale to Semi-Industrial Plant" Applied Sciences 13, no. 4: 2494. https://doi.org/10.3390/app13042494
APA StyleAimone, C., Grillo, G., Boffa, L., Giovando, S., & Cravotto, G. (2023). Tannin Extraction from Chestnut Wood Waste: From Lab Scale to Semi-Industrial Plant. Applied Sciences, 13(4), 2494. https://doi.org/10.3390/app13042494







