Influence of Sample Preparation/Extraction Method on the Phytochemical Profile and Antimicrobial Activities of 12 Commonly Consumed Medicinal Plants in Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. The Preparation of Plant Material
2.3. The Obtaining of Plant Extracts
2.3.1. Conventional Extraction with Solvents (CES)
2.3.2. Ultrasound-Assisted Extraction (UAE)
2.3.3. Microwave Extraction (MWE)
2.4. Chemical Analysis
2.4.1. Proximate Composition of Medicinal Plants
2.4.2. Elemental Composition of Medicinal Plants
2.4.3. Total Phenolic Content (TPC) of Medicinal Plant Extracts
2.4.4. Total Flavonoids Content (TFC) of Medicinal Plant Extracts
2.4.5. Antioxidant Activity (AA) of Medicinal Plant Extracts
2.4.6. Individual Polyphenols Content Detected by LC-MS
2.5. Microbiological Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of Medicinal Plants
3.2. Elemental Composition of Medicinal Plants
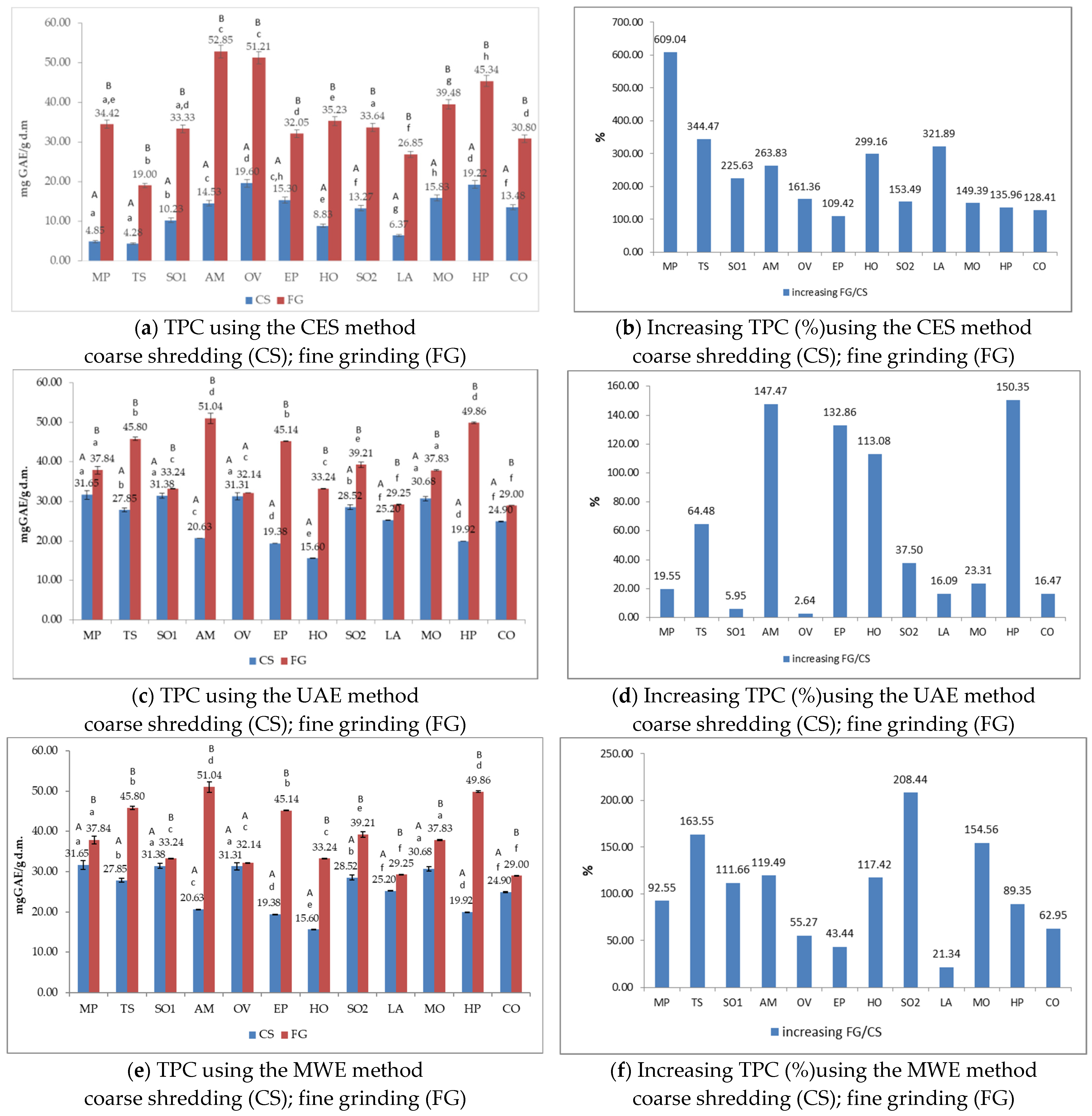
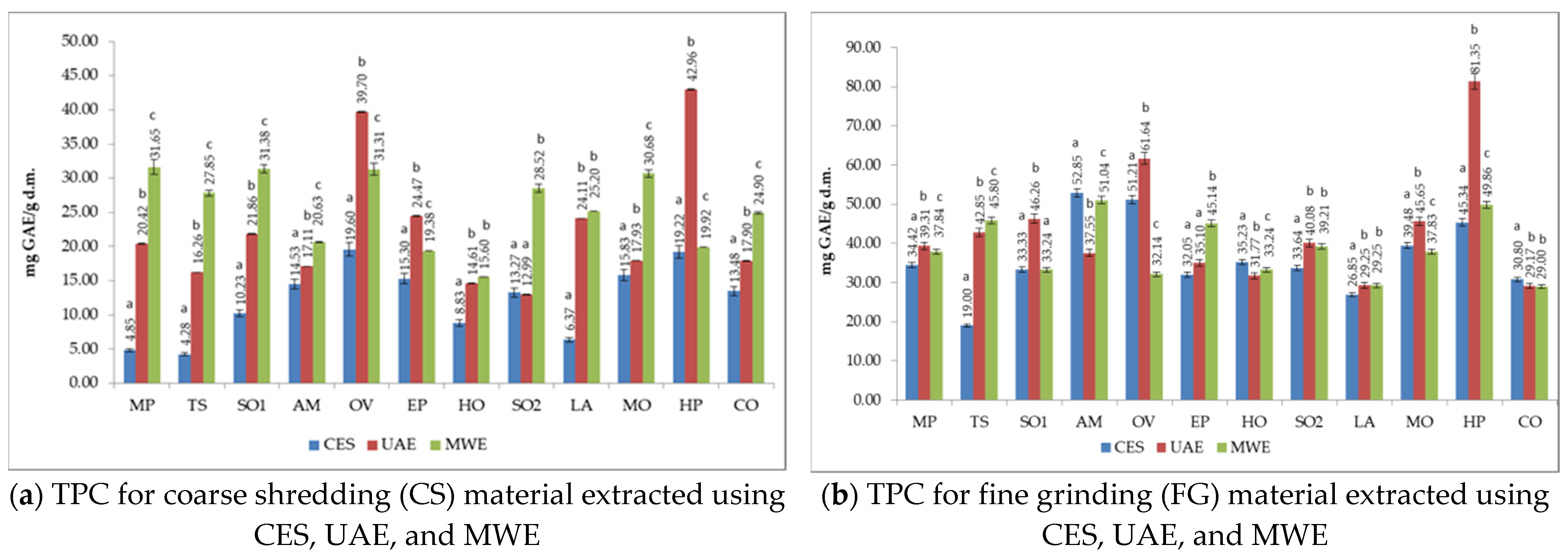
3.3. Total Phenols Content (TPC) of Medicinal Plant Extracts
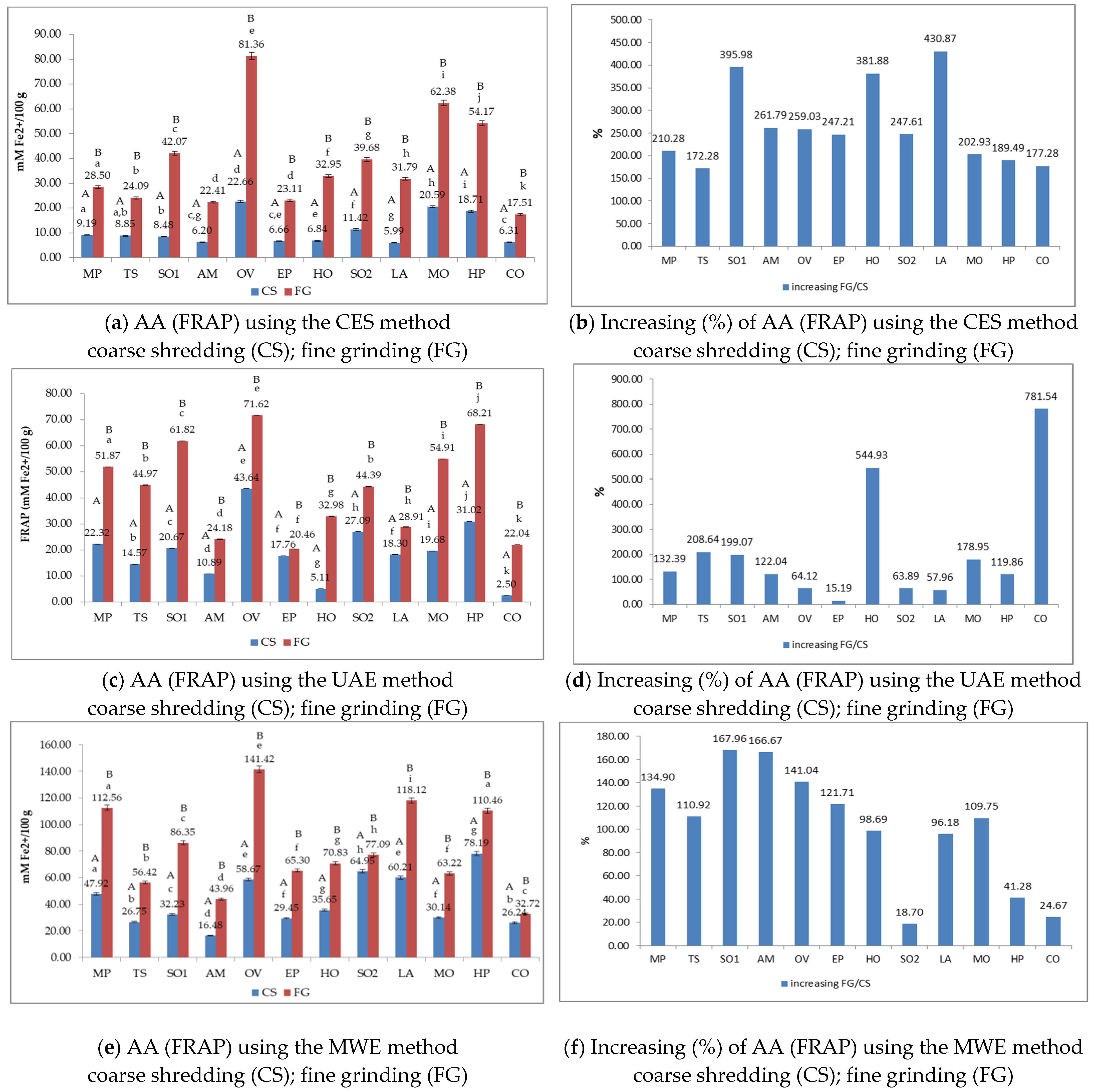
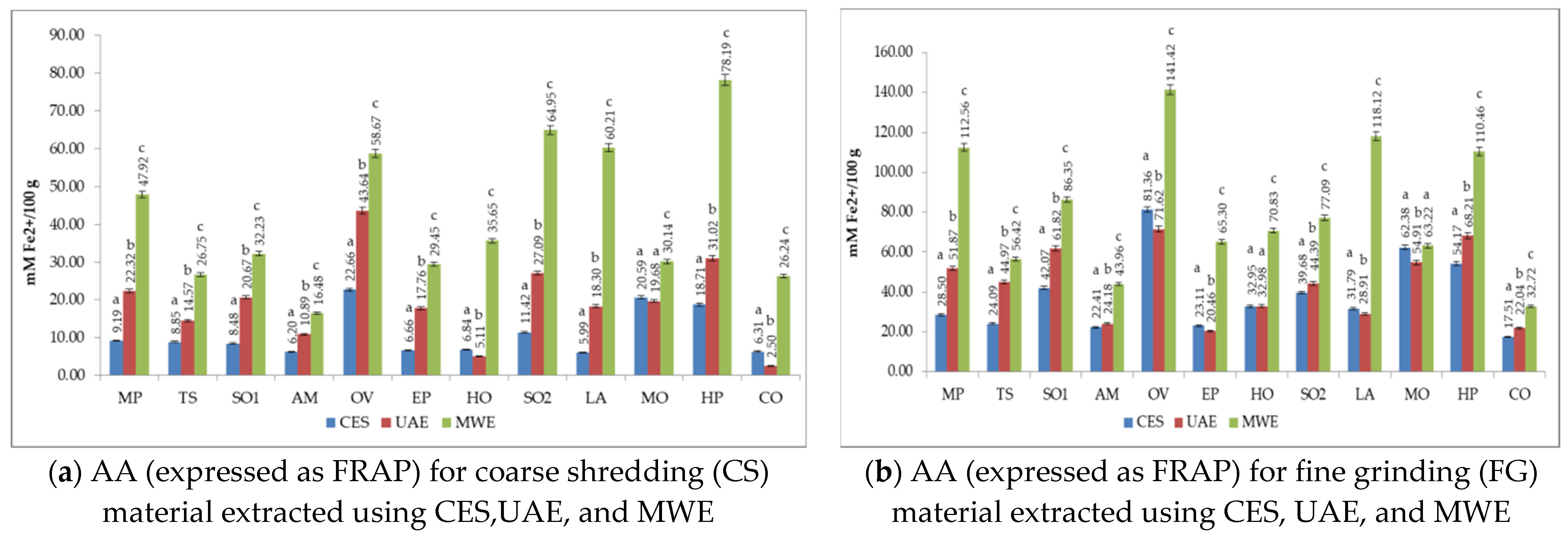
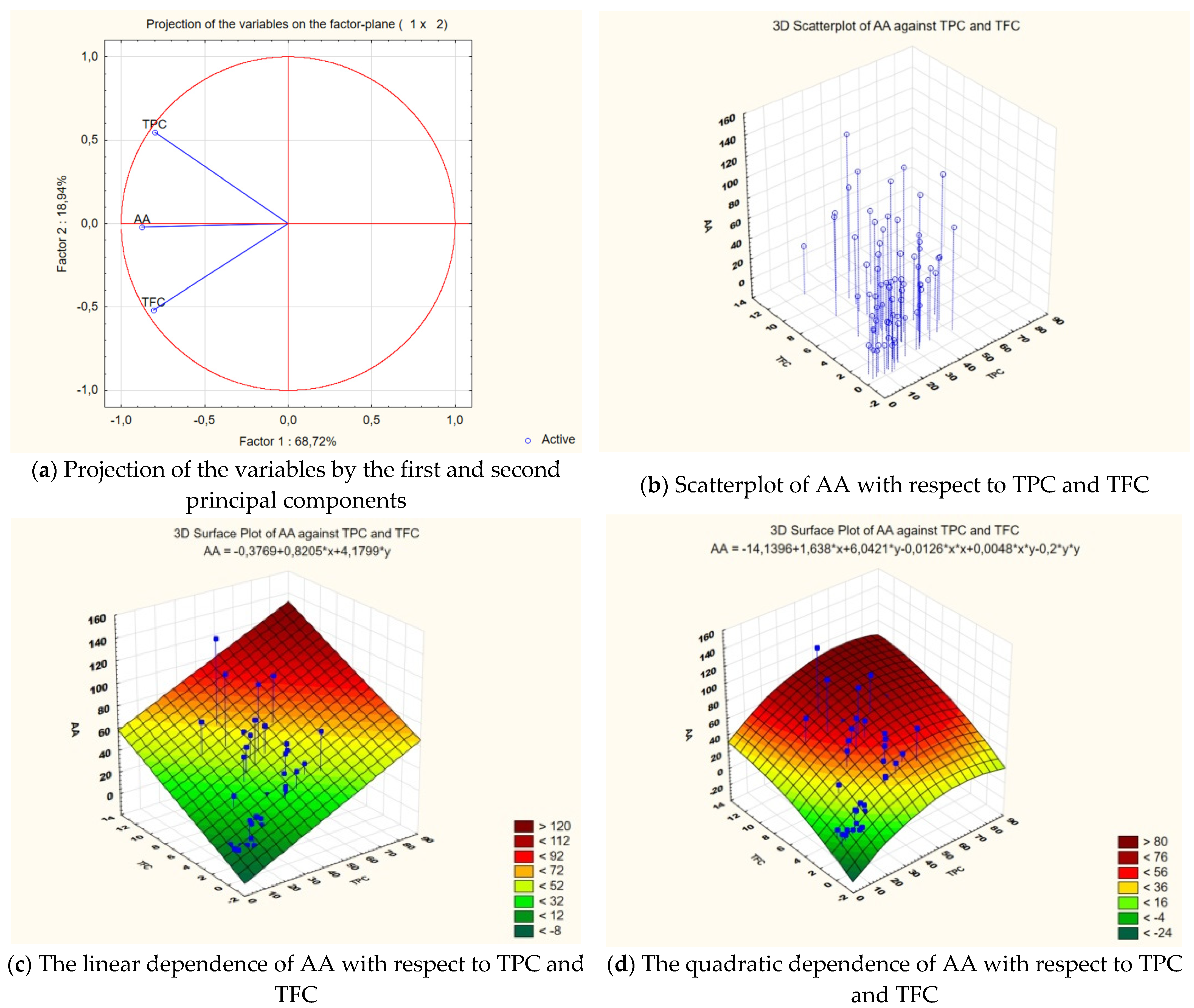
3.4. The Antioxidant Activity (AA) of Medicinal Plant Extracts
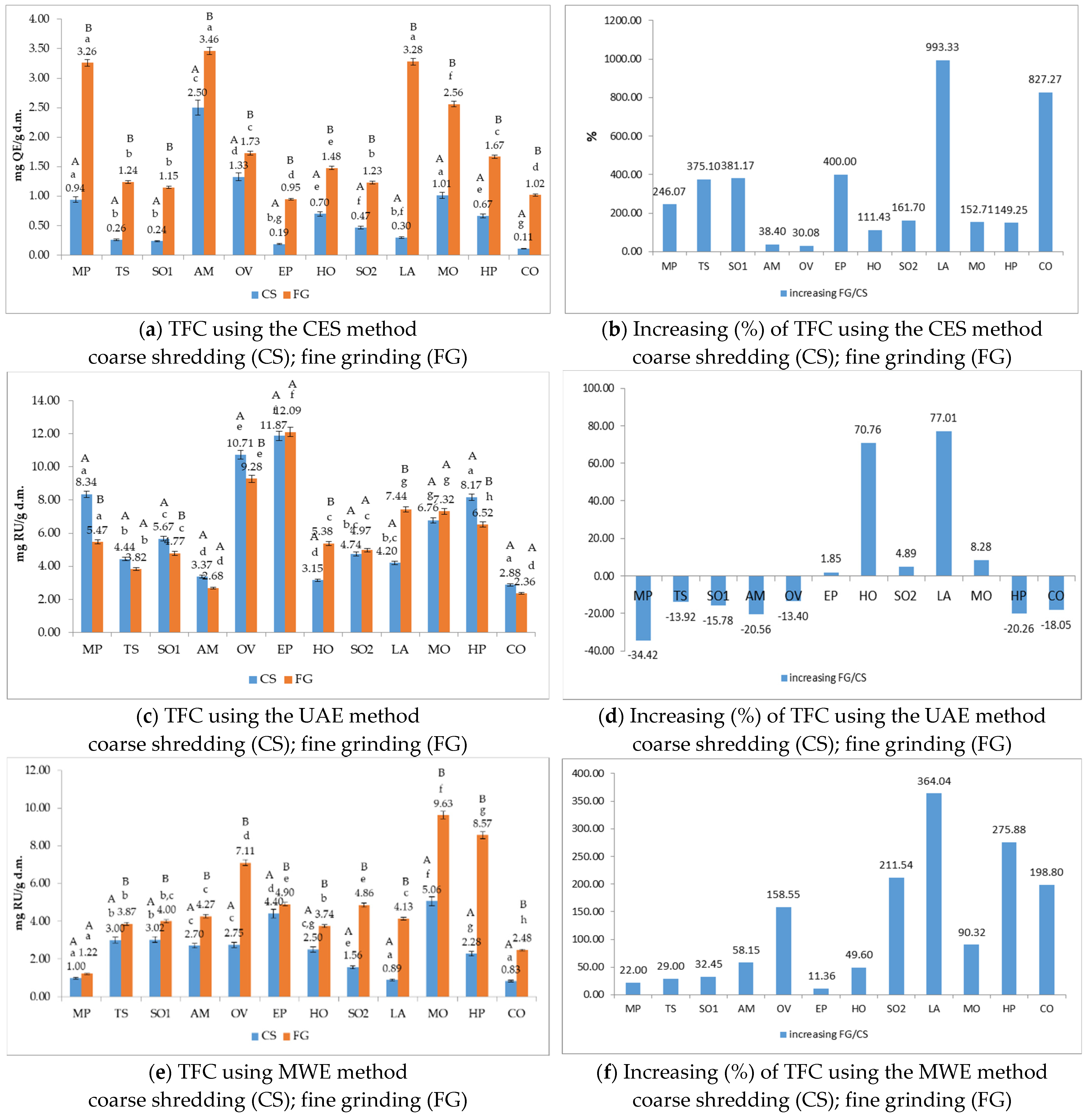
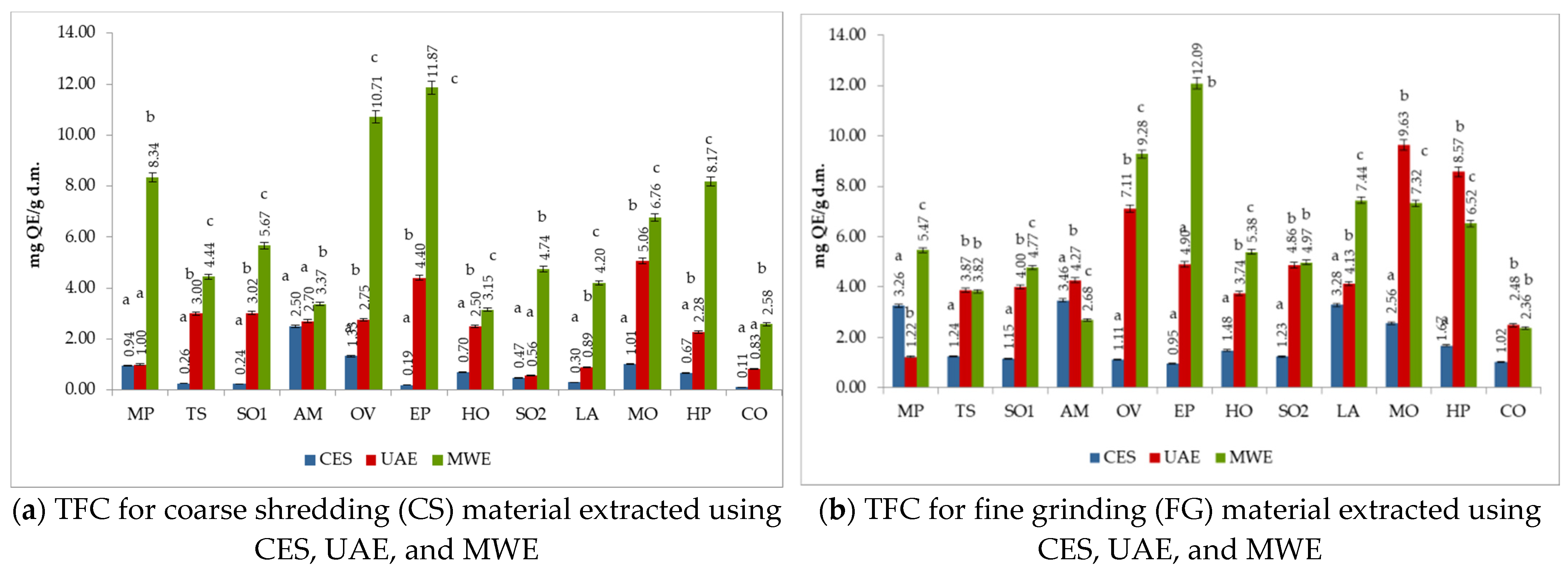
3.5. The Total Flavonoids Content (TFC) of Medicinal Plant Extracts
3.6. The Individual Polyphenols Detected by LC-MS
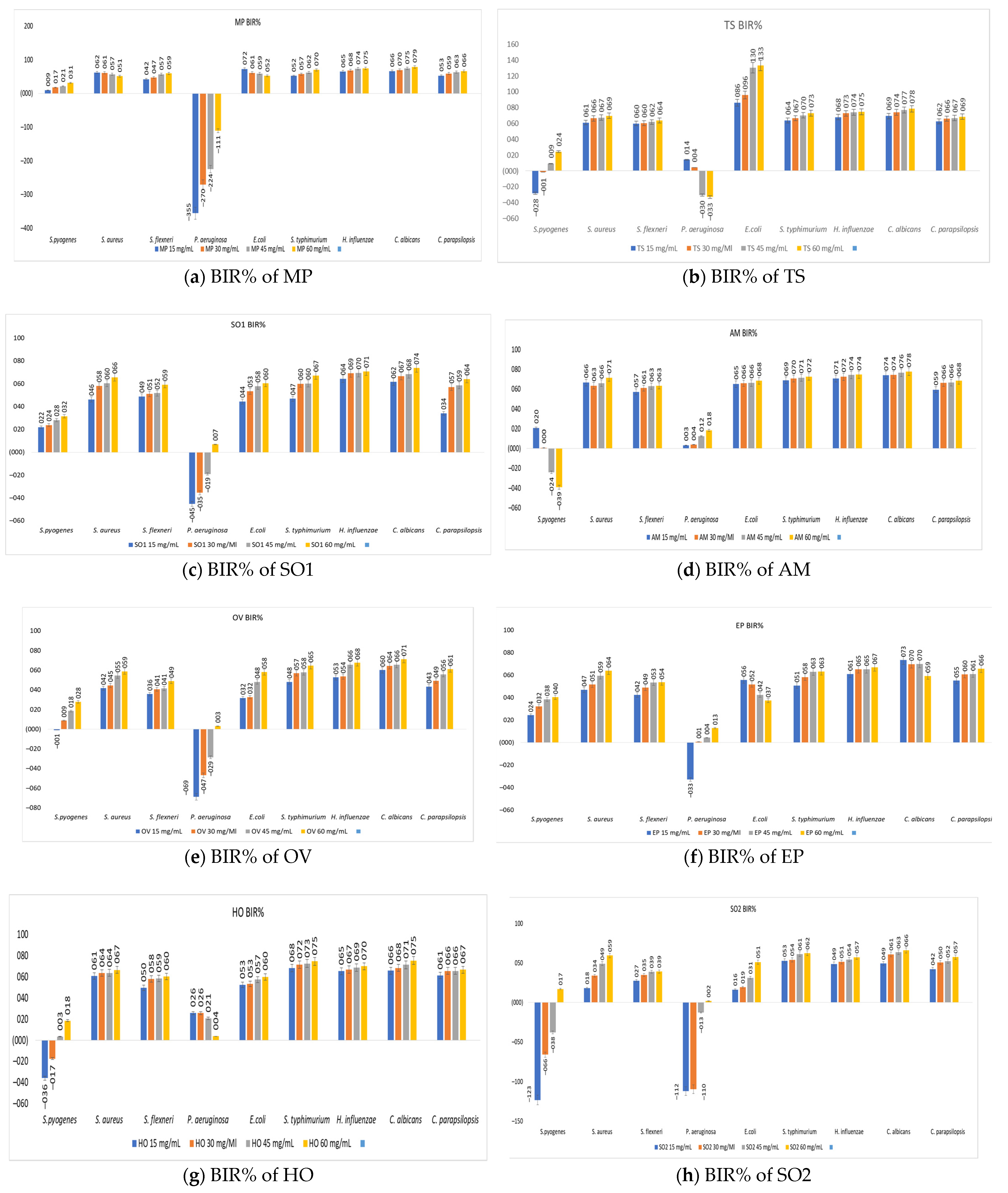
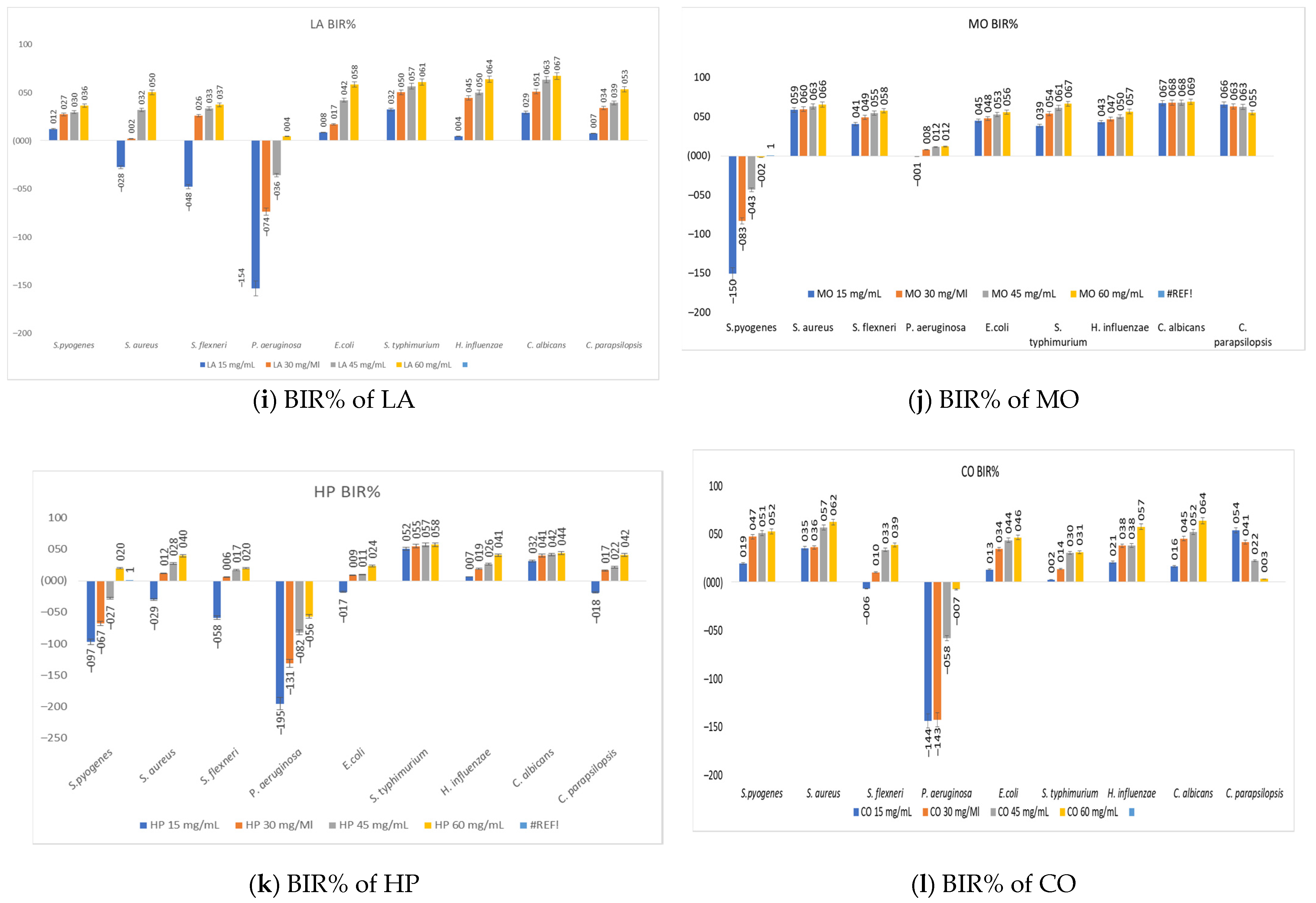
3.7. The Antimicrobial Activity of Medicinal Plant Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sagbo, I.J.; Hussein, A.A. Antidiabetic Medicinal Plants Used in the Eastern Cape Province of South Africa: An Updated Review. Processes 2022, 10, 1817. [Google Scholar] [CrossRef]
- Kumar, M.; Devi, H.; Prakash, S.; Rathore, S.; Thakur, M.; Puri, S.; Pundir, A.; Bangar, S.P.; Changan, S. Ethnomedicinal Plants Used in the Health Care System: Survey of the Mid Hills of Solan District, Himachal Pradesh, India. Plants 2021, 10, 1842. [Google Scholar] [CrossRef] [PubMed]
- Aleebrahim-Dehkordy, E.; Nasri, H.; Baradaran, A.; Nasri, P.; Tamadon, M.R.; Hedaiaty, M.; Beigrezaei, S.; Rafieian-Kopaei, M. Medicinal Plants, Effective Plant Compounds (Compositions) and their Effects on Stomach Cancer. Int. J. Prev. Med. 2017, 8, 96. [Google Scholar] [CrossRef]
- Pohl, P.; Bielawska-Pohl, A.; Dzimitrowicz, A.; Greda, K.; Jamroz, P.; Lesniewicz, A.; Szymczycha-Madeja, A.; Welna, M. Understanding element composition of medicinal plants used in herbalism-A case study by analytical atomic spectrometry. J. Pharm. Biomed. Anal. 2018, 159, 262–271. [Google Scholar] [CrossRef]
- Daur, I. Chemical composition of selected Saudi medicinal plants. Arab. J. Chem. 2015, 8, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M. Medicinal Plants. Plants 2021, 10, 1355. [Google Scholar] [CrossRef]
- Guimarães, R.; Milho, C.; Liberal, Â.; Silva, J.; Fonseca, C.; Barbosa, A.; Ferreira, I.C.F.R.; Alves, M.J.; Barros, L. Antibiofilm Potential of Medicinal Plants against Candida spp. Oral Biofilms: A Review. Antibiotics 2021, 10, 1142. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm Activity of Plant Polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef] [Green Version]
- Ampofo, E.K.; Amponsah, I.K.; Asante-Kwatia, E.; Armah, F.A.; Atchoglo, P.K.; Mensah, A.Y. Indigenous Medicinal Plants as Biofilm Inhibitors for the Mitigation of Antimicrobial Resistance. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8821905. [Google Scholar] [CrossRef]
- Available online: https://www.marketsandmarkets.com/Market-Reports/botanical-extracts-market-3151938.html (accessed on 20 December 2022).
- Villalva, M.; Santoyo, S.; Salas-Pérez, L.; Siles-Sánchez, M.D.L.N.; Rodríguez García-Risco, M.; Fornari, T.; Reglero, G.; Jaime, L. Sustainable Extraction Techniques for Obtaining Antioxidant and Anti-Inflammatory Compounds from the Lamiaceae and Asteraceae Species. Foods 2021, 10, 2067. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Aćimović, M.; Šovljanski, O.; Pezo, L.; Travičić, V.; Tomić, A.; Zheljazkov, V.D.; Ćetković, G.; Švarc-Gajić, J.; Brezo-Borjan, T.; Sofrenić, I. Variability in Biological Activities of Satureja montana Subsp. montana and Subsp. variegata Based on Different Extraction Methods. Antibiotics 2022, 11, 1235. [Google Scholar] [CrossRef] [PubMed]
- Shams, K.A.; Abdel-Azim, N.S.; Saleh, I.A.; Hegazy, M.-E.F.; El-Missiry, M.M.; Hammouda, F.M. Green technology: Economically and environmentally innovative methods for extraction of medicinal & aromatic plants (MAP) in Egypt. J. Chem. Pharm. Res. 2015, 7, 1050–1074. [Google Scholar]
- Soquetta, M.B.; Terra, L.d.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Azmin, S.N.H.M.; Manan, Z.A.; Alwi, S.R.W.; Chua, L.S.; Mustaffa, A.A.; Yunus, N.A. Herbal Processing and Extraction Technologies. Sep. Purif. Rev. 2016, 45, 305–320. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International; AOAC: Rockville, MD, USA, 2000. [Google Scholar]
- Poșta, D.S.; Radulov, I.; Cocan, I.; Berbecea, A.A.; Alexa, E.; Hotea, I.; Iordănescu, O.A.; Băla, M.; Cântar, I.C.; Rózsa, S.; et al. Hazelnuts (Corylus avellana L.) from Spontaneous Flora of the West Part of Romania: A Source of Nutrients for Locals. Agronomy 2022, 12, 214. [Google Scholar] [CrossRef]
- Ciulca, S.; Roma, G.; Alexa, E.; Radulov, I.; Cocan, I.; Madosa, E.; Ciulca, A. Variation of Polyphenol Content and Antioxidant Activity in Some Bilberry (Vaccinium myrtillus L.) Populations from Romania. Agronomy 2021, 11, 2557. [Google Scholar] [CrossRef]
- Plustea, L.; Negrea, M.; Cocan, I.; Radulov, I.; Tulcan, C.; Berbecea, A.; Popescu, I.; Obistioiu, D.; Hotea, I.; Suster, G.; et al. Lupin (Lupinus spp.)-Fortified Bread: A Sustainable, Nutritionally, Functionally, and Technologically Valuable Solution for Bakery. Foods 2022, 11, 2067. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Cadariu, A.I.; Cocan, I.; Negrea, M.; Alexa, E.; Obistioiu, D.; Hotea, I.; Radulov, I.; Poiana, M.-A. Exploring the Potential of Tomato Processing Byproduct as a Natural Antioxidant in Reformulated Nitrite-Free Sausages. Sustainability 2022, 14, 11802. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Wayne, P., Ed.; CLSI: Malvern, VIC, Australia, 2018. [Google Scholar]
- Obistioiu, D.; Cocan, I.; Tîrziu, E.; Herman, V.; Negrea, M.; Cucerzan, A.; Neacsu, A.-G.; Cozma, A.L.; Nichita, I.; Hulea, A.; et al. Phytochemical Profile and Microbiological Activity of Some Plants Belonging to the Fabaceae Family. Antibiotics 2021, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Zain, U.; Musa, K.B.; Jameel, A.K.; Imam, B.B.; Riaz, U.; Naser, M.A.; Shumaila, N. Proximate and nutrient analysis of selected medicinal plants of Tank and South Waziristan area of Pakistan. Afr. J. Pharm. Pharmacol. 2013, 7, 179–184. [Google Scholar]
- Tomescu, A.; Rus, C.; Pop, G.; Alexa, E.; Radulov, I.; Imbrea, I.M.; Negrea, M. Researches regarding proximate and selected elements composition of some medicinal plants belonging to the lamiaceae family. Agron. Ser. Sci. Res. Lucr. Stiintifice Ser. Agron. 2015, 58, 211531074. [Google Scholar]
- Mainasara, M.M.; Bakar, M.F.A.; Waziri, A.H.; Musa, A.R. Comparison of Phytochemical, Proximate and Mineral Composition of Fresh and Dried Peppermint (Mentha piperita) Leaves. J. Sci. Technol. 2018, 10. Available online: https://penerbit.uthm.edu.my/ojs/index.php/JST/article/view/3002 (accessed on 20 December 2022). [CrossRef] [Green Version]
- Queralt, I.; Ovejero, M.; Carvalho, M.; Marques, A.; Llabrés, J. Quantitative determination of essential and trace element content of medicinal plants and their infusions by XRF and ICP techniques. X-Ray Spectrom. Int. J. 2005, 34, 213–217. [Google Scholar] [CrossRef]
- Ereifej, K.; Ranya, E.; Taha, R.; Ali, M.A.; Muhammad, H.A. Minerals, proximate composition and their correlations of medicinal plants from Jordan. J. Med. Plants Res. 2012, 6, 5757–5762. [Google Scholar]
- Gjorgieva, D.; Kadifkova-Panovska, T.; Bačeva, K.; Stafilov, T. Metalic trace elements in medicinal plants from Macedonia. Middle-East J. Sci. Res. 2011, 7, 109–114. [Google Scholar]
- Cocan, I.; Alexa, E.; Danciu, C.; Radulov, I.; Galuscan, A.; Obistioiu, D.; Morvay, A.A.; Sumalan, R.M.; Poiana, M.A.; Pop, G.; et al. Phytochemical screening and biological activity of Lamiaceae family plant extracts. Exp. Ther. Med. 2017, 15, 1863–1870. [Google Scholar] [CrossRef] [Green Version]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive Compounds and Aroma Profile of Some Lamiaceae Edible Flowers. Plants 2020, 9, 691. [Google Scholar] [CrossRef]
- Guemari, F.; Laouini, S.E.; Rebiai, A.; Bouafia, A.; Meneceur, S.; Tliba, A.; Majrashi, K.A.; Alshareef, S.A.; Menaa, F.; Barhoum, A. UV-Visible Spectroscopic Technique-Data Mining Tool as a Reliable, Fast, and Cost-Effective Method for the Prediction of Total Polyphenol Contents: Validation in a Bunch of Medicinal Plant Extracts. Appl. Sci. 2022, 12, 9430. [Google Scholar] [CrossRef]
- Niculae, M.; Hanganu, D.; Oniga, I.; Benedec, D.; Ielciu, I.; Giupana, R.; Sandru, C.D.; Ciocârlan, N.; Spinu, M. Phytochemical Profile and Antimicrobial Potential of Extracts Obtained from Thymus marschallianus Willd. Molecules 2019, 24, 3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiridon, I.B.R.; Teaca, C.A. Total phenolic content and antioxidant activity of plants used in traditional Romanian herbal medicine. Cent. Eur. J. Biol. 2011, 6, 388–396. [Google Scholar] [CrossRef]
- Derakhshani, Z.; Hassani, A.; Pirzad, A.; Abdollahi, R.; Dalkani, M. Evaluation of phenolic content and antioxidant capacity in some medicinal herbs cultivated in Iran. Bot. Serbica 2012, 36, 117–122. [Google Scholar]
- Maleš, I.; Dragović-Uzelac, V.; Jerković, I.; Zorić, Z.; Pedisić, S.; Repajić, M.; Garofulić, I.E.; Dobrinčić, A. Non-Volatile and Volatile Bioactives of Salvia officinalis L., Thymus serpyllum L. and Laurus nobilis L. Extracts with Potential Use in the Development of Functional Beverages. Antioxidants 2022, 11, 1140. [Google Scholar] [CrossRef]
- Doymaz, I.; Karasu, S. Effect of air temperature on drying kinetics, colour changes and total phenolic content of sage leaves (Salvia officinalis). Qual. Assur. Saf. Crop. Foods 2018, 10, 269–276. [Google Scholar] [CrossRef]
- Francik, S.; Francik, R.; Sadowska, U.; Bystrowska, B.; Zawiślak, A.; Knapczyk, A.; Nzeyimana, A. Identification of Phenolic Compounds and Determination of Antioxidant Activity in Extracts and Infusions of Salvia Leaves. Materials 2020, 13, 5811. [Google Scholar] [CrossRef]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Hamrouni-Sellami, I.; Rahali, F.Z.; Rebey, I.B.; Bourgou, S.; Limam, F.; Marzouk, B. Total Phenolics, Flavonoids, and Antioxidant Activity of Sage (Salvia officinalis L.) Plants as Affected by Different Drying Methods. Food Bioprocess Technol. 2013, 6, 806–817. [Google Scholar] [CrossRef]
- Pavić, V.; Jakovljević, M.; Molnar, M.; Jokić, S. Extraction of carnosic acid and carnosol from sage (Salvia officinalis L.) leaves by supercritical fluid extraction and their antioxidant and antibacterial activity. Plants 2019, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Hassan, R.A.; Abotaleb, S.T.; Hamed, H.B.; Eldeen, M.S. Antioxidant and Antimicrobial Activities of Melissa officinalis L. (Lemon Balm) Extracts. J. Agric. Chem. Biotechnol. 2019, 10, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Goyal, S.P.H.; Guleria, K.; Tewari, G. Variation in Antioxidant Activity and Antioxidant Constituents of Thymus Serpyllum L Grown in Different Climatic Conditions of Uttarakhand Himalayas. DLSJ 2021, 6, 109–116. [Google Scholar] [CrossRef]
- Jabri-Karoui, I.; Bettaieb, I.; Msaada, K.; Hammami, M.; Marzouk, B. Research on the phenolic compounds and antioxidant activities of Tunisian Thymus capitatus. J. Funct. Foods 2012, 4, 661–669. [Google Scholar] [CrossRef]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, G.; Csillag, I.; Sevastre, B.; Mot, A.C.; Silaghi-Dumitrescu, R.; Tilea, I. Evaluation of Antioxidant and Antimicrobial Activities and Phenolic Profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef]
- Slimestad, R.; Johny, A.; Thomsen, M.G.; Karlsen, C.R.; Rosnes, J.T. Chemical Profiling and Biological Activity of Extracts from Nine Norwegian Medicinal and Aromatic Plants. Molecules 2022, 27, 7335. [Google Scholar] [CrossRef]
- Stanisavljević, I.; Stojičević, S.; Veličković, D.; Veljković, V.; Lazić, M. Antioxidant and Antimicrobial Activities of Echinacea (Echinacea purpurea L.) Extracts Obtained by Classical and Ultrasound Extraction. Chin. J. Chem. Eng. 2009, 17, 478–483. [Google Scholar] [CrossRef]
- Duque-Soto, C.; Borrás-Linares, I.; Quirantes-Piné, R.; Falcó, I.; Sánchez, G.; Segura-Carretero, A.; Lozano-Sánchez, J. Potential Antioxidant and Antiviral Activities of Hydroethanolic Extracts of Selected Lamiaceae Species. Foods 2022, 11, 1862. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Generalić Mekinić, I.; Skroza, D.; Ljubenkov, I.; Šimat, V.; Smole Možina, S.; Katalinić, V. In vitro Antioxidant and Antibacterial Activity of Lamiaceae Phenolic Extracts: A Correlation Study. Food Technol. Biotechnol. 2014, 52, 119–127. [Google Scholar]
- Ivasenko, S.; Orazbayeva, P.; Skalicka–Wozniak, K.; Ludwiczuk, A.; Marchenko, A.; Ishmuratova, M.; Poleszak, E.; Korona-Glowniak, I.; Akhmetova, S.; Karilkhan, I.; et al. Antimicrobial Activity of Ultrasonic Extracts of Two Chemotypes of Thymus serpyllum L. of Central Kazakhstan and their Polyphenolic Profiles. Open Access Maced. J. Med. Sci. 2021, 9, 61–67. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef] [PubMed]
- Kopel, J.; McDonald, J.; Hamood, A. An Assessment of the In Vitro Models and Clinical Trials Related to the Antimicrobial Activities of Phytochemicals. Antibiotics 2022, 11, 1838. [Google Scholar] [CrossRef] [PubMed]
- Macedo, L.M.d.; Santos, É.M.D.; Ataide, J.A.; Silva, G.T.D.S.E.; Guarnieri, J.P.D.O.; Lancellotti, M.; Jozala, A.F.; Rosa, P.C.P.; Mazzola, P.G. Development and Evaluation of an Antimicrobial Formulation Containing Rosmarinus officinalis. Molecules 2022, 27, 5049. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.H.; Mohammed, S.A.S.; Rana, M.J.; Abuzaitoun, S.; Jondi, W.; Zatar, N. Antimicrobial activities of six plants used in Traditional Arabic Palestinian Herbal Medicine. Afr. J. Microbiol. Res. 2014, 8, 3501–3507. [Google Scholar] [CrossRef] [Green Version]
- Stojanović, G.; Radulović, N.; Hashimoto, T.; Palić, R. In vitro antimicrobial activity of extracts of four Achillea species: The composition of Achillea clavennae L. (Asteraceae) extract. J. Ethnopharmacol. 2005, 101, 185–190. [Google Scholar] [CrossRef]
- Ivanović, M.; Grujić, D.; Cerar, J.; Islamčević Razboršek, M.; Topalić-Trivunović, L.; Savić, A.; Kočar, D.; Kolar, M. Extraction of Bioactive Metabolites from Achillea millefolium L. with Choline Chloride Based Natural Deep Eutectic Solvents: A Study of the Antioxidant and Antimicrobial Activity. Antioxidants 2022, 11, 724. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Garcia-Oliveira, P.; Nuñez-Estevez, B.; Silva, A.; Finimundy, T.C.; Calhelha, R.; Nenadic, M.; Sokovic, M.; Barroso, F.; Simal-Gandara, J.; et al. Plants of the Family Asteraceae: Evaluation of Biological Properties and Identification of Phenolic Compounds. Chem. Proc. 2021, 5, 51. [Google Scholar]
- Dragan, T.; Velikovic, N.V.R.; Mihailo, S.; Risti, A.S. Chemical constituents and antimicrobial activity of the ethanol extracts obtained from the flower, leaf and stem of Salvia officinalis L. J. Serb. Chem. Soc. 2003, 68, 17–24. [Google Scholar]
- Dahiya, P.; Purkayastha, S. Phytochemical Screening and Antimicrobial Activity of Some Medicinal Plants Against Multi-drug Resistant Bacteria from Clinical Isolates. Indian J. Pharm. Sci. 2012, 74, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Pezzani, R.; Vitalini, S.; Iriti, M. Bioactivities of Origanum vulgare L.: An update. Phytochem. Rev. 2017, 16, 1253–1268. [Google Scholar] [CrossRef]
- Saddiqe Zeb, N.I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef] [PubMed]
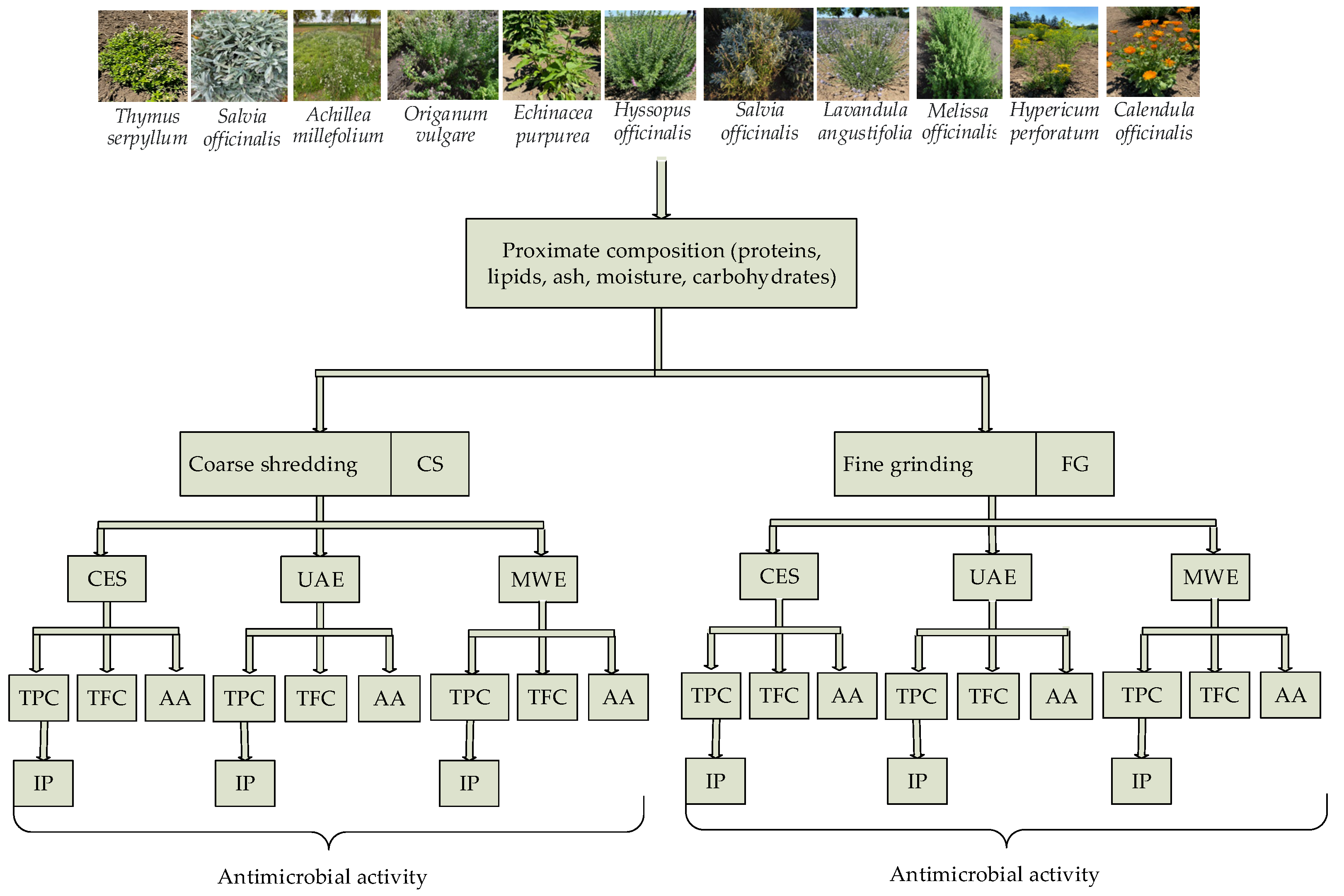
| Nr. Crt. | Botanical Name | Abreviation | Family | Popular Name | The Vegetal Part | Voucher No. |
|---|---|---|---|---|---|---|
| 1 | Mentha x piperita | MP | Lamiaceae | mint | aerial part | SNH.BUASTM—89/2 |
| 2 | Thymus serpyllum | TS | Lamiaceae | Wild thyme | aerial part | VSNH.BUASTM—101/5 |
| 3 | Salvia officinalis | SO1 | Lamiaceae | sage | aerial part | VSNH.BUASTM—98/1 |
| 4 | Achillea millefolium | AM | Asteraceae | yarrow | aerial part | VSNH.BUASTM—70/2 |
| 5 | Origanum vulgare | OV | Lamiaceae | oregano | aerial part | VSNH.BUASTM—93/1 |
| 6 | Echinacea purpurea | EP | Asteraceae | echinacea | aerial part | VSNH.BUASTM—124 |
| 7 | Hyssopus officinalis | HO | Lamiaceae | hyssop | aerial part | VSNH.BUASTM—83/1 |
| 8 | Salvia officinalis | SO2 | Lamiaceae | sage | seeds | VSNH.BUASTM—98/2 |
| 9 | Lavandula angustifolia | LA | Lamiaceae | lavender | aerial part | VSNH.BUASTM—85/5 |
| 10 | Melissa officinalis | MO | Lamiaceae | Lemon balm | aerial part | VSNH.BUASTM—88/3 |
| 11 | Hypericum perforatum | HP | Hypericaceae | St. John’s wort | aerial part | VSNH.BUASTM—82/4 |
| 12 | Calendula officinalis | CO | Asteraceae | marigold | aerial part | SNH.USABTM—71/1 |
| Sample | Chemical Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Moisture (g/100 g) | Proteins (g/100 g) | Lipids (g/100 g) | Ash (g/100 g) | Carbohydrates (g/100 g) | Energy Value (kcal/100 g) | Dry Residue (%) | |
| MP | 9.57 ± 0.22 a | 14.10 ± 0.34 a | 0.99 ± 0.03 a | 12.37 ± 0.28 a | 62.97 | 317.17 | 2.00 ± 0.01 |
| TS | 7.10 ± 0.16 b | 8.75 ± 0.20 b | 0.52 ± 0.01 b, c | 7.60 ± 0.18 b | 76.03 | 343.80 | 2.22 ± 0.03 |
| SO1 | 10.76 ± 0.24 c | 10.79 ± 0.26 c | 0.78 ± 0.02 c | 6.84 ± 0.17 c | 70.83 | 333.50 | 3.05 ± 0.02 |
| AM | 7.15 ± 0.15 b | 12.51 ± 0.31 d | 1.12 ± 0.03 a, e | 6.45 ± 0.16 c | 72.77 | 351.18 | 2.34 ± 0.02 |
| OV | 7.17 ± 0.16 b | 9.86 ± 0.23 e | 0.55 ± 0.01 b | 7.69 ± 0.19 b | 74.73 | 343.31 | 2.22 ± 0.01 |
| EP | 12.45 ± 0.31 d | 15.70 ± 0.38 f | 1.06 ± 0.03 a | 9.89 ± 0.25 d | 60.90 | 315.94 | 2.10 ± 0.02 |
| HO | 8.37 ± 0.22 e | 11.92 ± 0.28 d | 0.43 ± 0.01 c | 5.00 ± 0.12 e | 74.28 | 348.67 | 3.21 ± 0.03 |
| SO2 | 6.88 ± 0.16 b, f | 11.54 ± 0.27 c | 3.84 ± 0.10 d | 8.21 ± 0.20 f | 69.53 | 358.84 | 3.20 ± 0.01 |
| LA | 11.73 ± 0.27 d | 10.35 ± 0.24 c, e | 0.48 ± 0.01 b, c | 7.01 ± 0.17 c | 70.43 | 327.44 | 3.15 ± 0.01 |
| MO | 7.40 ± 0.18 b, | 21.34 ± 0.52 g | 0.90 ± 0.02 a | 12.82 ± 0.32 a | 57.54 | 323.62 | 2.24 ± 0.03 |
| HP | 6.19 ± 0.14 f | 13.69 ± 0.33 a | 1.21 ± 0.03 e | 3.57 ± 0.09 g | 75.34 | 367.01 | 3.20 ± 0.01 |
| CO | 8.09 ± 0.19 e | 19.25 ± 0.46 h | 1.08 ± 0.03 a | 8.97 ± 0.22 h | 62.61 | 337.16 | 2.42 ± 0.02 |
| Sample | Zn (ppm) | Fe (ppm) | Mn (ppm) | K (ppm) | Ca (ppm) | Mg (ppm) |
|---|---|---|---|---|---|---|
| MP | 22.21 ± 0.96 a | 416.77 ± 3.35 a | 46.10 ± 1.18 a | 5286.30 ± 27.07 a | 4256.71 ± 14.27 a | 381.14 ± 3.65 a |
| TS | 17.35 ± 0.81 b | 489.00 ± 3.64 b | 27.56 ± 1.07 b | 3400.10 ± 13.11 b | 4423.89 ± 13.77 b | 357.61 ± 3.46 b |
| SO1 | 24.32 ± 0.94 c | 271.85 ± 1.89 c | 26.53 ± 0.85 b | 7215.30 ± 19.44 c | 6115.06 ± 16.03 c | 462.88 ± 3.37 c |
| AM | 11.33 ± 0.65 d | 220.22 ± 1.38 d | 23.23 ± 0.79 c | 3459.00 ± 16.48 b | 4067.74 ± 14.86 d | 364.52 ± 3.92 b |
| OV | 17.47 ± 0.74 b | 286.80 ± 1.75 e | 22.28 ± 0.87 c | 3492.77 ± 17.54 b | 2748.35 ± 9.79 e | 361.54 ± 3.64 b |
| EP | 19.52 ± 0.87 e | 160.62 ± 1.00 f | 17.21 ± 0.77 d | 4825.90 ± 19.65 d | 4708.79 ± 15.83 f | 382.42 ± 3.82 a |
| HO | 14.25 ± 0.85 f | 198.53 ± 1.10 g | 23.22 ± 0.17 c | 8468.50 ± 20.00 e | 5664.99 ± 16.08 g | 536.67 ± 3.56 d |
| SO2 | 33.10 ± 1.17 g | 320.66 ± 2.15 h | 26.04 ± 0.97 b | 5013.20 ± 19.98 e | 5371.23 ± 16.40 h | 454.79 ± 3.47 e |
| LA | 35.07 ± 1.27 g | 298.25 ± 1.79 i | 22.26 ± 0.82 c | 9045.20 ± 21.96 f | 7575.43 ± 19.18 i | 605.26 ± 4.44 f |
| MO | 28.35 ± 1.14 h | 360.77 ± 2.42 j | 23.59 ± 0.81 c | 6029.80 ± 20.22 g | 5500.01 ± 16.58 j | 428.21 ± 3.58 g |
| HP | 22.40 ± 0.93 a | 173.92 ± 1.21 k | 24.25 ± 0.89 c | 6083.30 ± 17.47 g | 1501.18 ± 10.17 k | 385.71 ± 3.14 a |
| CO | 26.03 ± 1.00 c | 209.86 ± 2.00 l | 33.60 ± 1.32 e | 6585.87 ± 20.54 h | 3687.16 ± 12.66 l | 428.21 ± 3.55 g |
| Compound | Rt | m/z | MP | TS | SO1 | AM | OV | EP | HO | SO2 | LA | MO | HP | CO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallic acid | 5.175 | 169 | 0.4 ± 0.02 a | 0.08 ± 0.001 b | 0.07 ± 0.002 b | 0.56 ± 0.02 c | 0.27 ± 0.01 d | 0.27 ± 0.01 d | 0.29 ± 0.01 d | 0.37 ± 0.01 a | 0.38 ± 0.01 a | 1.58 ± 0.05 e | 1.50 ± 0.05 e | 2.17 ± 0.12 f |
| Protocatechuic acid | 11.112 | 153 | nd | nd | 0.05 ± 0.002 a | 0.08 ± 0.002 b | 0.05 ± 0.002 a | 0.14 ± 0.002 c | 0.12 ± 0.02 c | 0.05 ± 0.002 a | 0.06 ± 0.002 a, b | 0.07 ± 0.003 b | 0.18 ± 0.005 d | 0.23 ± 0.01 e |
| Epicatechin | 22.205 | 289 | 0.22 ± 0.005 a | 0.53 ± 0.02 b | 0.30 ± 0.01 c | 0.18 ± 0.04 d | 0.39 ± 0.02 e, f | 0.38 ± 0.004 e, g | 0.41 ± 0.04 e, f | 0.40 ± 0.02 f | 0.39 ± 0.01 e, f | 0.35 ± 0.01 g | 0.30 ± 0.01 c | 0.18 ± 0.04 d |
| Rosmarinic acid | 29.289 | 359 | nd | 8.91 ± 0.28 a | 4.50 ± 0.17 b | 5.66 ± 0.15 c | 6.17 ± 0.17 d | 6.40 ± 0.19 d, e | 9.16 ± 0.28 a | 6.53 ± 0.18 e | 3.39 ± 0.16 f | 4.51 ± 0.17 b | 7.81 ± 0.20 g | 5.63 ± 0.20 c |
| Quercetin | 31.650 | 301 | 0.07 ± 0.002 a | 0.06 ± 0.001 a, b | 0.06 ± 0.002 a, b | 0.07 ± 0.002 a | nd | nd | 0.05 ± 0.001 b | 0.06 ± 0.002 a, b | 0.06 ± 0.002 a, b | 0.07 ± 0.002 a | 0.19 ± 0.005 c | 0.37 ± 0.01 d |
| Kaempferol | 34.535 | 285 | 0.05 ± 0.001 a | 0.14 ± 0.003 b | 0.09 ± 0.003 c, d | nd | 0.11 ± 0.002 c | nd | 0.07 ± 0.002 a, d | nd | nd | 0.42 ± 0.02 e | 0.05 ± 0.002 a, d | 0.67 ± 0.03 e |
| Plant Extract/Strains | S. pyogenes | S. aureus | S. flexneri | P. aeruginosa | E. coli | S. typhimurium | H. influenzae | C. albicans | C. parapsilopsis |
|---|---|---|---|---|---|---|---|---|---|
| MP | 15 | 15 | 15 | - | 15 | 15 | 15 | 15 | 15 |
| TS | 45 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| SO1 | 15 | 15 | 15 | 60 | 15 | 15 | 15 | 15 | 15 |
| AM | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| OV | - | 15 | 15 | 60 | 15 | 15 | 15 | 15 | 15 |
| EP | 15 | 15 | 15 | 30 | 15 | 15 | 15 | 15 | 15 |
| HO | 45 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| SO2 | 60 | 15 | 15 | 60 | 15 | 15 | 15 | 15 | 15 |
| LA | 15 | 30 | 30 | 60 | 15 | 15 | 15 | 15 | 15 |
| MO | - | 15 | 15 | 30 | 15 | 15 | 15 | 15 | 15 |
| HP | 60 | 30 | 30 | - | 30 | 15 | 15 | 15 | 30 |
| CO | 15 | 15 | 30 | - | 15 | 15 | 15 | 15 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horablaga, N.M.; Cozma, A.; Alexa, E.; Obistioiu, D.; Cocan, I.; Poiana, M.-A.; Lalescu, D.; Pop, G.; Imbrea, I.M.; Buzna, C. Influence of Sample Preparation/Extraction Method on the Phytochemical Profile and Antimicrobial Activities of 12 Commonly Consumed Medicinal Plants in Romania. Appl. Sci. 2023, 13, 2530. https://doi.org/10.3390/app13042530
Horablaga NM, Cozma A, Alexa E, Obistioiu D, Cocan I, Poiana M-A, Lalescu D, Pop G, Imbrea IM, Buzna C. Influence of Sample Preparation/Extraction Method on the Phytochemical Profile and Antimicrobial Activities of 12 Commonly Consumed Medicinal Plants in Romania. Applied Sciences. 2023; 13(4):2530. https://doi.org/10.3390/app13042530
Chicago/Turabian StyleHorablaga, Nicolae Marinel, Antoanela Cozma, Ersilia Alexa, Diana Obistioiu, Ileana Cocan, Mariana-Atena Poiana, Dacian Lalescu, Georgeta Pop, Ilinca Merima Imbrea, and Ciprian Buzna. 2023. "Influence of Sample Preparation/Extraction Method on the Phytochemical Profile and Antimicrobial Activities of 12 Commonly Consumed Medicinal Plants in Romania" Applied Sciences 13, no. 4: 2530. https://doi.org/10.3390/app13042530
APA StyleHorablaga, N. M., Cozma, A., Alexa, E., Obistioiu, D., Cocan, I., Poiana, M.-A., Lalescu, D., Pop, G., Imbrea, I. M., & Buzna, C. (2023). Influence of Sample Preparation/Extraction Method on the Phytochemical Profile and Antimicrobial Activities of 12 Commonly Consumed Medicinal Plants in Romania. Applied Sciences, 13(4), 2530. https://doi.org/10.3390/app13042530









