Essential Oils and Bacteriocin-Based Active Edible Coating: An Innovative, Natural and Sustainable Approach for the Control of Listeria monocytogenes in Seafoods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Natural Compounds and Strains
2.2. Minimal Inhibitory Concentration (MIC) and Fractional Inhibitory Concentration Index (FIC-Index) Determination
2.3. Shrimp Contamination and Coating
2.4. Anti-Listeria Activity Determination
2.5. Statistical Analysis
3. Results
3.1. Minimal Inhibitory Concentration (MIC) of the Single Natural Compounds and of the Most Advantageous Synergistic Mixtures
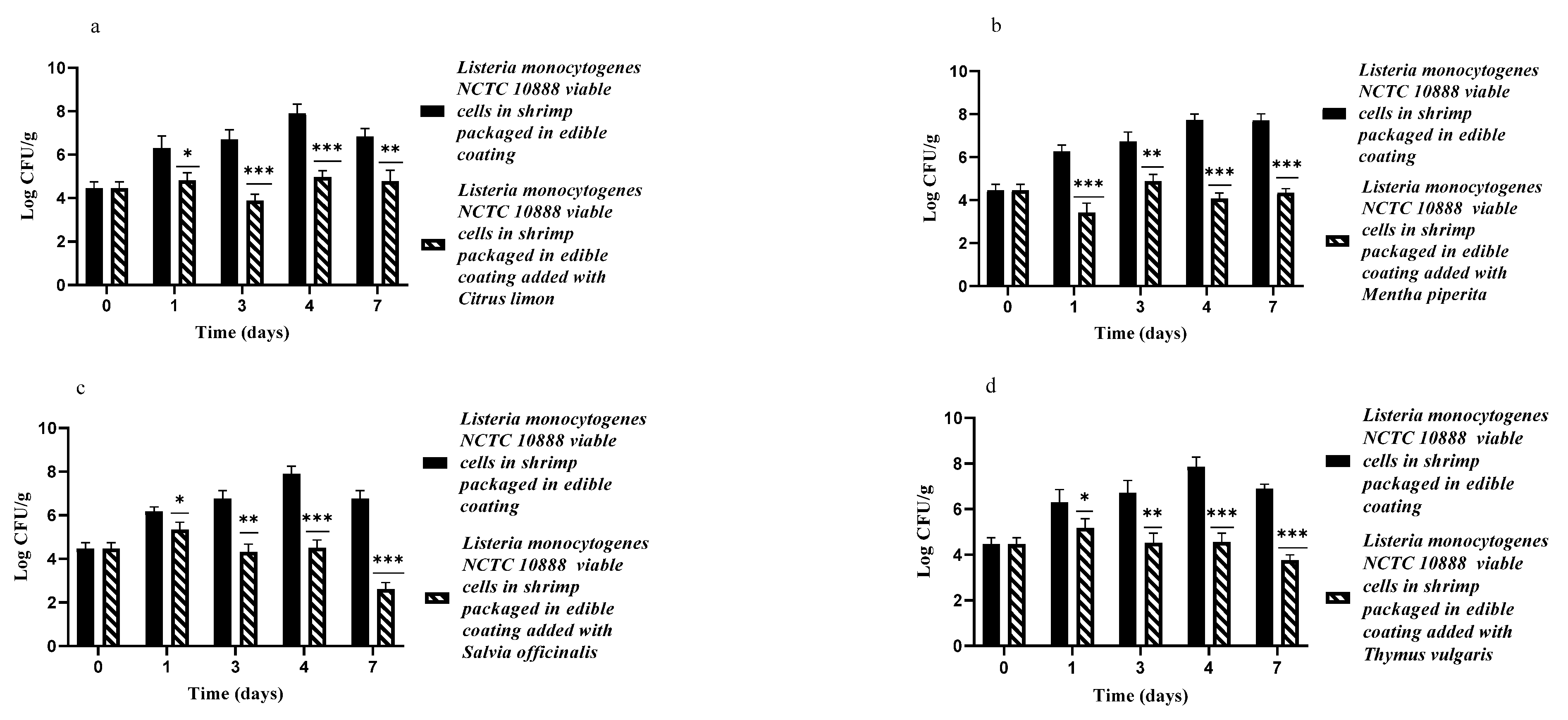
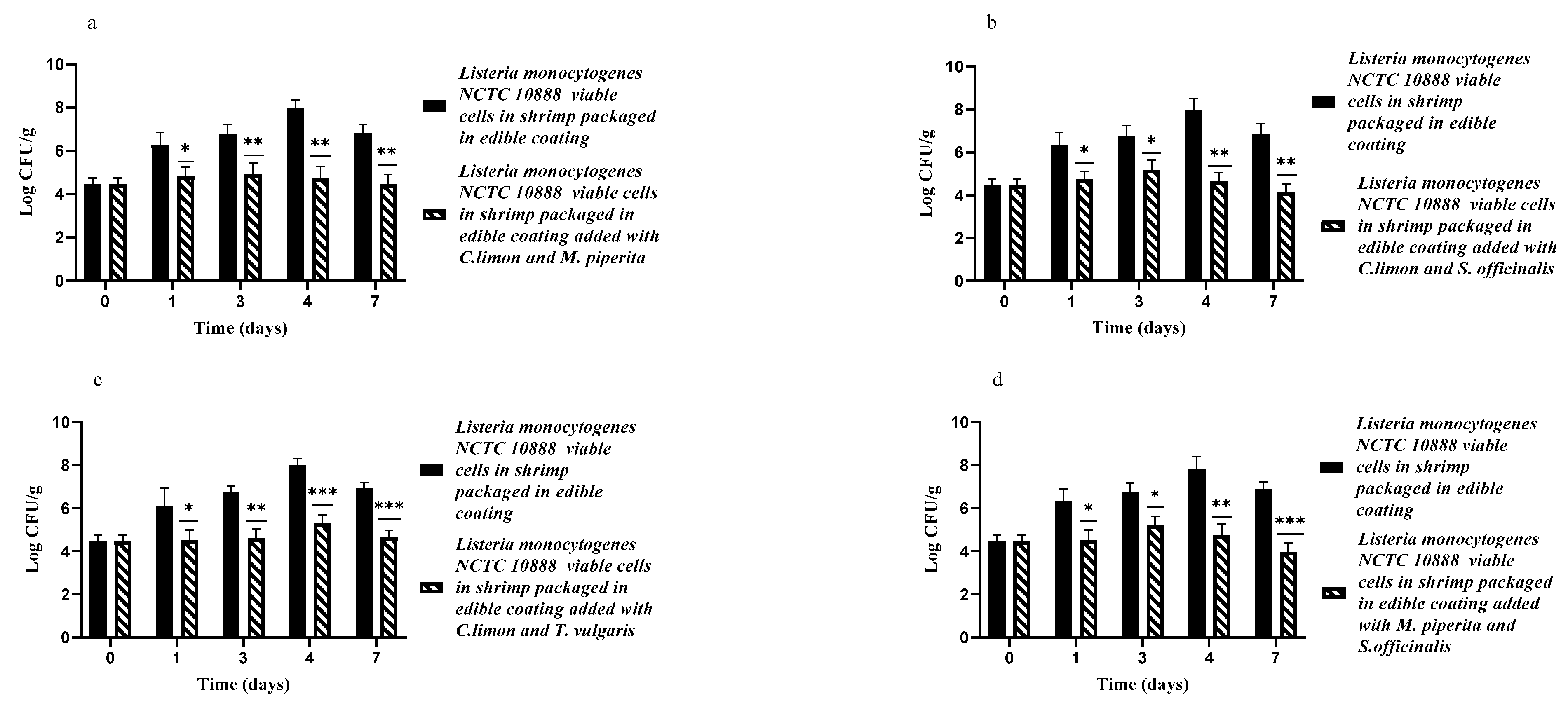
3.2. Edible Antimicrobial Coating Added with EOs by Themselves or in Association against L. monocytogenes NCTC10888 on Artificially Contaminated Shrimp Samples
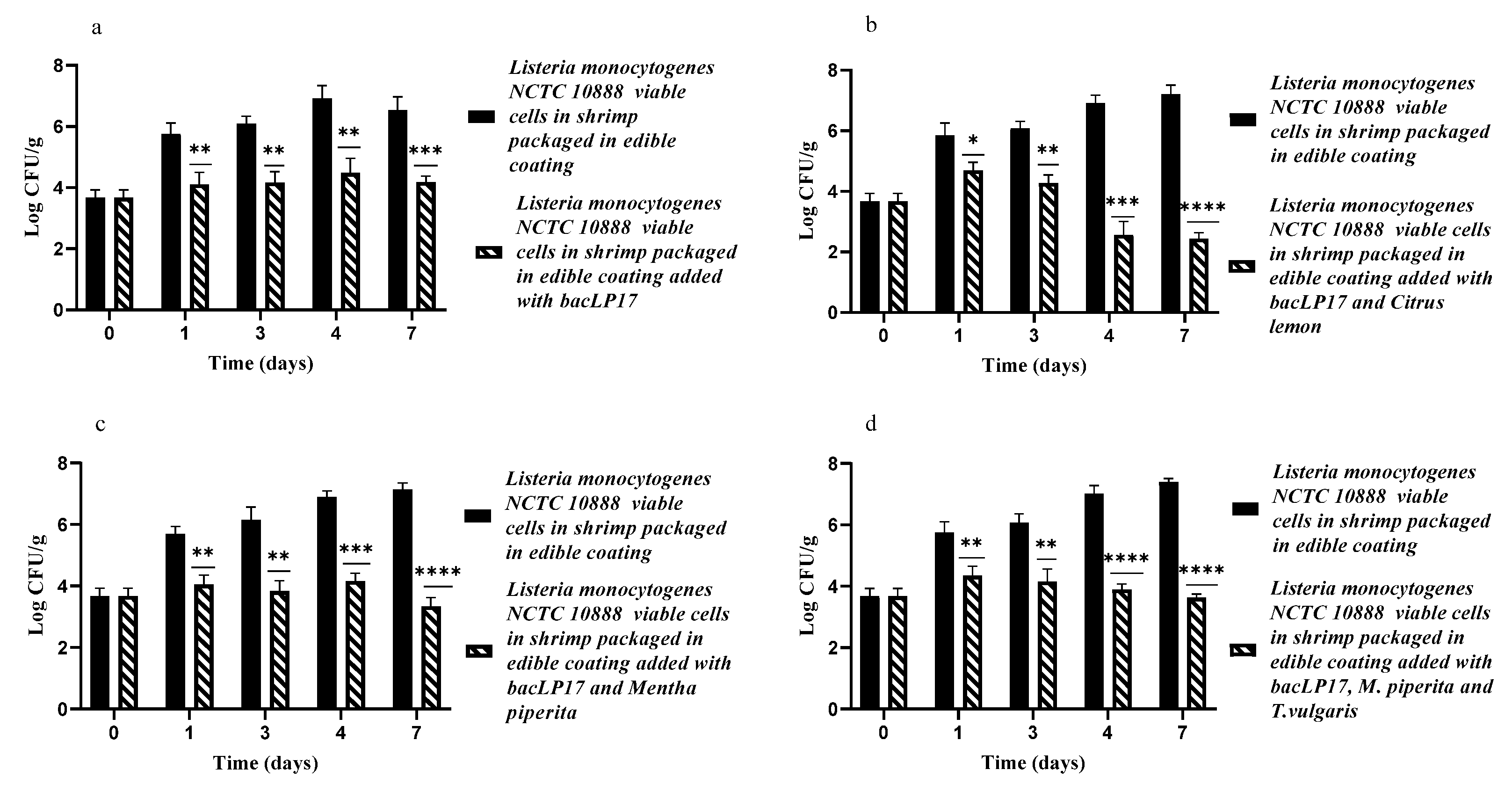
3.3. Edible Antimicrobial Coating Added with a Mixture of EOs and bacLP17 against L. monocytogenes NCTC10888 on Artificially Contaminated Shrimp Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Union. Regulation (EC) No. 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008R1333 (accessed on 31 December 2008).
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Centre for Disease Prevention and Control. Listeriosis. In ECDC. Annual Epidemiological Report for 2021; ECDC: Stockholm, Sweden, 2022; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER%20listeriosis%20-%202021.pdf (accessed on 1 December 2022).
- FDA/USDA/CDC (US Food and Drug Administration, United States Department of Agriculture and Centers for Disease Control and Prevention). Quantitative assessment of the relative risk to public health from foodborne Listeria monocytogenes among selected categories of ready-to-eat foods. 2003. Available online: https://www.fda.gov/food/cfsan-risk-safety-assessments/quantitative-assessment-relative-risk-public-health-foodborne-listeria-monocytogenes-among-selected (accessed on 1 September 2003).
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, e05077. [Google Scholar] [CrossRef] [Green Version]
- Carpentier, B.; Cerf, O. Review-Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.M.; Mclauchlin, J.; Bernardo, F.A. Listeria in ready-to-eat and unprocessed foods produced in Portugal. Food Microbiol. 2001, 18, 423–429. [Google Scholar] [CrossRef]
- Ericsson, H.; Eklöw, A.; Danielsson-Tham, M.L.; Loncarevic, S.; Mentzing, L.O.; Persson, I.; Unnerstad, H.; Tham, W. An outbreak of listeriosis suspected to have been caused by rainbow trout. J. Clin. Microbiol. 1997, 35, 2904–2907. [Google Scholar] [CrossRef] [Green Version]
- Brett, M.S.Y.; Short, P.; McLauchlin, J. A small outbreak of listeriosis associated with smoked mussels. Int. J. Food Microbiol. 1998, 43, 223–229. [Google Scholar] [CrossRef]
- Tham, W.; Ericsson, H.; Loncarevic, S.; Unnerstad, H.; Danielsson-Tham, M.L. Lessons from an outbreak of listeriosis related to vacuum-packed gravad and coldsmoked fish. Int. J. Food Microbiol. 2000, 62, 173–175. [Google Scholar] [CrossRef]
- ECDC (European Centre for Disease Prevention and Control); EFSA (European Food Safety Authority). Multi-Country OUTBREAK of Listeria Monocytogenes Clonal Complex 8 Infections Linked to Consumption of Cold-Smoked Fish Products—4 June 2019; ECDC/EFSA: Stockholm, Sweden; Parma, Italy, 2019. [Google Scholar]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes persistence in food associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef]
- Overney, A.; Jacques-Andre-Coquin, J.; Ng, P.; Carpentier, B.; Guillier, L.; Firmesse, O. Impact of environmental factors on the culturability and viability of Listeria monocytogenes under conditions encountered in food processing plants. Int. J. Food Microbiol. 2017, 244, 74–81. [Google Scholar] [CrossRef]
- Prakash, B.; Veeregowda, B.M.; Krishnappa, G. Biofilms: A survival strategy of bacteria. Curr. Sci. 2003, 85, 1299–1307. [Google Scholar]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Cortes, M.E.; Bonilla, J.C.; Sinisterra, R.D. Biofilm formation, control and novel strategies for eradication. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; Volume 2, pp. 896–905. [Google Scholar]
- Galie, S.; García-Gutierrez, C.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2019, 99, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef] [PubMed]
- Kalogianni, A.I.; Lazou, T.; Bossis, I.; Gelasakis, A.I. Natural Phenolic Compounds for the Control of Oxidation, Bacterial Spoilage, and Foodborne Pathogens in Meat. Foods 2020, 9, 794. [Google Scholar] [CrossRef]
- Abdelhamid, A.G.; El-Dougdoug, N.K. Controlling foodborne pathogens with natural antimicrobials by biological control and antivirulence strategies. Heliyon 2020, 6, e05020. [Google Scholar] [CrossRef]
- Deyno, S.; Mtewa, A.G.; Abebe, A.; Hymete, A.; Makonnen, E.; Bazira, J.; Alele, P.E. Essential oils as topical anti-infective agents: A systematic review and meta-analysis. Complement. Ther. Med. 2019, 47, 102224. [Google Scholar] [CrossRef]
- Deans, S.G.; Ritchie, G. Antibacterial properties of plant essential oils. Int. J. Food Microbiol. 1987, 5, 165–180. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents-myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [Green Version]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [Green Version]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, C.M. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Pop, O.L.; Pop, C.R.; Dufrechou, M.; Vodnar, D.C.; Socaci, S.A.; Dulf, F.V.; Minervini, F.; Suharoschi, R. Edible films and coatings functionalization by probiotic incorporation: A review. Polymers 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control. 2021, 126, 108063. [Google Scholar] [CrossRef]
- Risch, S.J. New developments in packaging materials. In Food Packaging; American Chemical Society: Washington, DC, USA, 2000; Volume 753, pp. 1–7. [Google Scholar]
- Gontard, N.; Guilbert, S. Bio-packaging: Technology and properties of edible and/or biodegradable material of agricultural origin. In Food Packaging and Preservation; Springer: Boston, MA, USA, 1994. [Google Scholar]
- Diab, T.; Biliaderis, C.G.; Gerasopoulos, D.; Sfakiotakis, E. Physicochemical properties and application of pullulan edible films and coatings in fruit preservation. J. Sci. Food Agric. 2001, 81, 988–1000. [Google Scholar] [CrossRef]
- Pilet, M.F.; Leroi, F. Applications of protective cultures, bacteriocins and bacteriophages in fresh seafood and seafood products. In Protective Cultures, Antimicrobial Metabolites and Bacteriophages for Food and Beverage Biopreservation; WP, Woodhead Publ.: Oxford, UK, 2011. [Google Scholar]
- Ghanbari, M.; Jami, M.; Kneifel, W.; Domig, K.J. Antimicrobial activity and partial characterization of bacteriocins produced by lactobacilli isolated from Sturgeon fish. Food Control. 2013, 32, 379–385. [Google Scholar] [CrossRef]
- Jami, M.; Ghanbari, M.; Zunabovic, M.; Domig, K.J.; Kneifel, W. Listeria monocytogenes in Aquatic Food Products—A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 798–813. [Google Scholar] [CrossRef]
- Ben Embarek, P.K. Presence, detection and growth of Listeria monocytogenes in seafoods: A review. Int. J. Food Microbiol. 1994, 23, 17–34. [Google Scholar] [CrossRef]
- Huss, H.H. Control of indigenous pathogenic bacteria in seafood. Food Control. 1997, 8, 91–98. [Google Scholar] [CrossRef]
- Cordano, A.M.; Rocourt, J. Occurrence of Listeria monocytogenes in food in Chile. Int. J. Food Microbiol. 2001, 70, 175–178. [Google Scholar] [CrossRef]
- Gudmundsdottir, S.; Gudbjornsdottir, B.; Einarsson, H.; Kristinsson, K.G.; Kristjansson, M. Contamination of cooked peeled shrimp (Pandalus borealis) by Listeria monocytogenes during processing at two processing plants. J. Food Prot. 2006, 69, 1304–1311. [Google Scholar] [CrossRef]
- Norhana, M.N.W.; Poole, S.E.; Deeth, H.C.; Dykes, G.A. Prevalence, persistence and control of Salmonella and Listeria in shrimp and shrimp products: A review. Food Control. 2010, 21, 343–361. [Google Scholar] [CrossRef]
- Chen, B.Y.; Wang, C.Y.; Wang, C.L.; Fan, Y.C.; Weng, I.T.; Chou, C.H. Prevalence and persistence of Listeria monocytogenes in ready-to-eat tilapia sashimi processing plants. J. Food Prot. 2016, 79, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Tardugno, R.; Pellati, F.; Iseppi, R.; Bondi, M.; Bruzzesi, G.; Benvenuti, S. Phytochemical composition and in vitro screening of the antimicrobial activity of essential oils on oral pathogenic bacteria. Nat. Prod. Res. 2018, 32, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Stefani, S.; de Niederhäusern, S.; Bondi, M.; Sabia, C.; Messi, P. Characterization of anti-Listeria monocytogenes properties of two bacteriocin-producing Enterococcus mundtii isolated from fresh fish and seafood. Curr. Microbiol. 2019, 76, 1010–1019. [Google Scholar] [CrossRef]
- Iseppi, R.; Zurlini, C.; Cigognini, I.M.; Cannavacciuolo, M.; Sabia, C.; Messi, P. Eco-friendly edible packaging systems based on live-Lactobacillus kefiri MM5 for the control of Listeria monocytogenes in fresh vegetables. Foods 2022, 11, 2632. [Google Scholar] [CrossRef]
- M100-S20; Performance Standards for Antimicrobial Susceptibility Testing: Approved Standard. Clinical and Laboratory Standard Institutes (CLSI): Wayne, PA, USA, 2012.
- Hemaiswaryaa, S.; Kruthiventib, A.K.; Doblea, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Mc Carty, S.A.; Motes, M.L.; Mc Pearlson, R.M. Recovery of heat-stressed Listeria monocytogenes from experimentally and naturally contaminated shrimp. J. Food Prot. 1990, 53, 22–25. [Google Scholar] [CrossRef]
- El-Shenawy, M.A.; El-Shenawy, M.A. Incidence of Listeria monocytogenes in seafood enhanced by prolonged cold enrichment. J. Med. Res. Inst. 1995, 16, 32–40. [Google Scholar]
- Reis, D.R.; Ambrosi, A.; Luccio, M.D. Encapsulated essential oils: A perspective in food preservation. Future Foods 2022, 5, 100126. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Han, J.H. Antimicrobial food packaging. Food Technol. 2000, 54, 56–65. [Google Scholar] [CrossRef]
- Guerra, N.P.; Macias, C.L.; Agrasar, A.T.; Castro, L.P. Development of a bioactive packaging cellophane using Nisaplin as biopreservative agent. Lett. Appl. Microbiol. 2005, 40, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, G.; De Luca, E.; La Storia, A.; Villani, F.; Ercolini, D. Antimicrobial activity of a nisin-activated plastic film for food packaging. Lett. Appl. Microbiol. 2005, 41, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Ghrairi, T.; Hani, K. Enhanced bactericidal effect of enterocin A in combination with thyme essential oils against L. monocytogenes and E. coli O157:H7. J. Food Sci. Technol. 2015, 52, 2148–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The antimicrobial effect of thyme essential oil, nisin, and their combination against Listeria monocytogenes in minced beef during refrigerated storage. Food Microbiol. 2008, 25, 120–127. [Google Scholar] [CrossRef]
- Ghalfi, H.; Allaoui, A.; Destain, J.; Benkerroum, N.; Thonart, P. Bacteriocin activity by Lactobacillus curvatus CWBI-B28 to inactivate Listeria monocytogenes in cold-smoked salmon during 4 degrees C storage. J. Food Prot. 2006, 69, 1066–1071. [Google Scholar] [CrossRef]
- Turgis, M.; Vu, K.D.; Dupont, C.; Lacroix, M. Combined antimicrobial effect of essential oils and bacteriocins against foodborne pathogens and food spoilage bacteria. Food Res. Int. 2012, 48, 696–702. [Google Scholar] [CrossRef]

| C. lemon | M. piperita | S. officinalis | T. vulgaris | bacLP17 |
|---|---|---|---|---|
| 32 μL/mL | 32 μL/mL | 128 μL/mL | 8 μL/mL | 16 μL/mL |
| C. lemon M. piperita | C. lemon S. officinalis | C. lemon T. vulgaris | M. piperita S. officinalis | M. piperita T. vulgaris | S. officinalis T. vulgaris |
|---|---|---|---|---|---|
| 3 μL/mL +3 μL/mL | 1.5 μL/mL +6 μL/mL | 3 μL/mL +0.8 μL/mL | 1.5 μL/mL +6 μL/mL | 3 μL/mL +0.8 μL/mL | 6 μL/mL +0.8 μL/mL |
| C. lemon bacLP17 | M. piperita bacLP17 | S. officinalis bacLP17 | T. vulgaris bacLP17 |
|---|---|---|---|
| 3 μL/mL +0.8 μL/mL | 1.5 μL/mL +1.5 μL/mL | 12.5 μL/mL +0.4 μL/mL | 1.5 μL/mL +0.8 μL/mL |
| C. lemon M. piperita bacLP17 | C. lemon S. officinalis bacLP17 | C. lemon T. vulgaris bacLP17 | M. piperita S. officinalis bacLP17 | M.piperita T. vulgaris bacLP17 | S. officinalis T. vulgaris bacLP17 |
|---|---|---|---|---|---|
| 3 μL/mL +1.5 μL/mL +0.8 μL/mL | 3 μL/mL +3 μL/mL +1.5 μL/mL | 3 μL/mL +0.8 μL/mL +0.8 μL/mL | 3 μL/mL +3 μL/mL +0.8 μL/mL | 3 μL/mL +1.5 μL/mL +0.4 μL/mL | 6 μL/mL +0.4 μL/mL +1.5 μL/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iseppi, R.; Camellini, S.; Zurlini, C.; Cigognini, I.M.; Cannavacciuolo, M.; Messi, P. Essential Oils and Bacteriocin-Based Active Edible Coating: An Innovative, Natural and Sustainable Approach for the Control of Listeria monocytogenes in Seafoods. Appl. Sci. 2023, 13, 2562. https://doi.org/10.3390/app13042562
Iseppi R, Camellini S, Zurlini C, Cigognini IM, Cannavacciuolo M, Messi P. Essential Oils and Bacteriocin-Based Active Edible Coating: An Innovative, Natural and Sustainable Approach for the Control of Listeria monocytogenes in Seafoods. Applied Sciences. 2023; 13(4):2562. https://doi.org/10.3390/app13042562
Chicago/Turabian StyleIseppi, Ramona, Stefania Camellini, Chiara Zurlini, Ilaria Maria Cigognini, Mariarosaria Cannavacciuolo, and Patrizia Messi. 2023. "Essential Oils and Bacteriocin-Based Active Edible Coating: An Innovative, Natural and Sustainable Approach for the Control of Listeria monocytogenes in Seafoods" Applied Sciences 13, no. 4: 2562. https://doi.org/10.3390/app13042562






