Somatotropin Penetration Testing from Formulations Applied Topically to the Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Preparation
2.3. Measurement Method of STH
2.4. STH Permeation Study
2.4.1. Franz’s Vertical Diffusion Cells
2.4.2. Membranes for Permeation Studies
2.4.3. Determination of Permeation Parameters of STH
2.5. Rheological Properties
2.6. Sensory Evaluation
2.7. Stability Test
3. Results
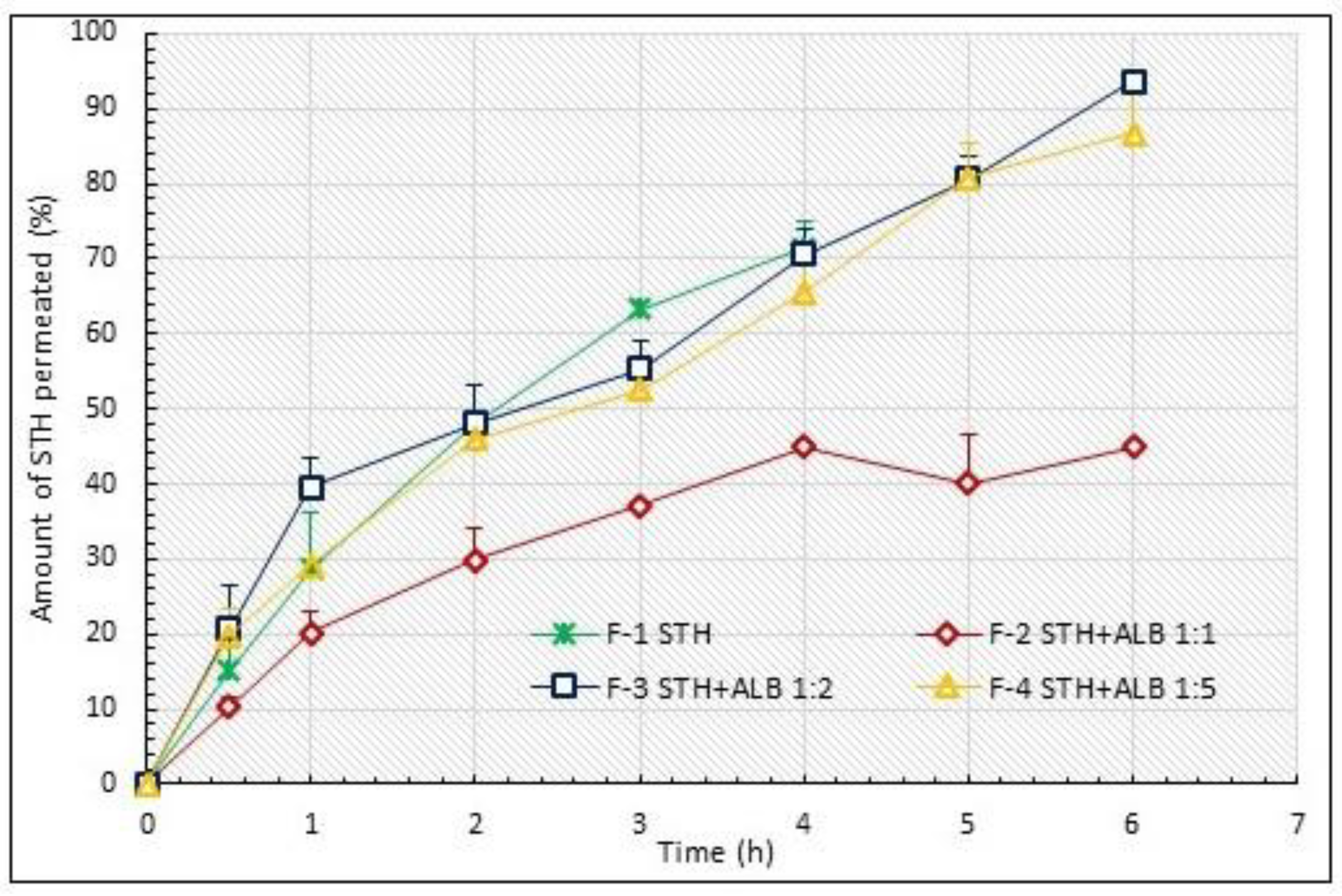
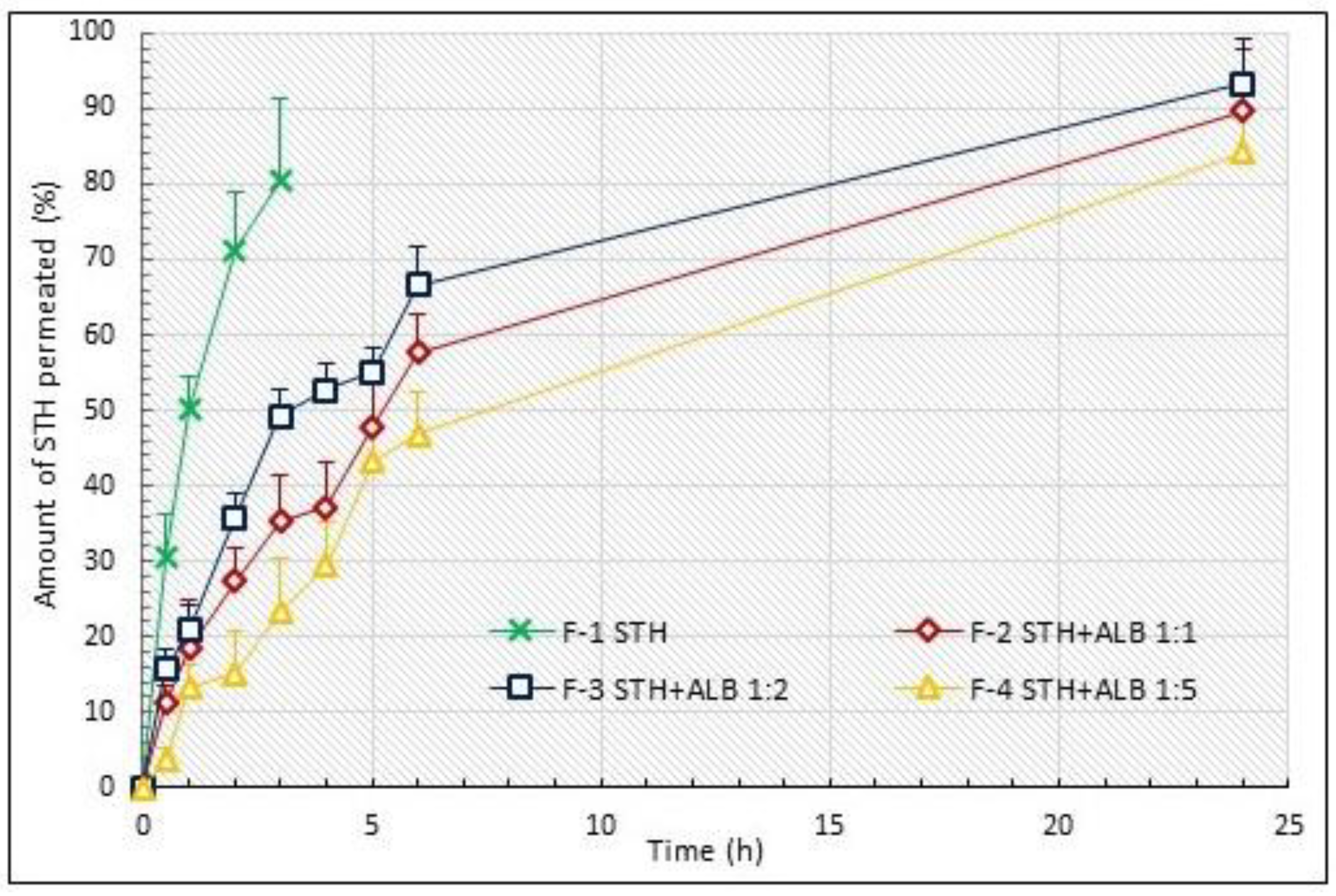
3.1. STH Permeation Study
3.2. Permeation Parameters of STH
3.3. Permeation Kinetics
3.4. Evaluation of the Similarity of the STH Permeation Profiles
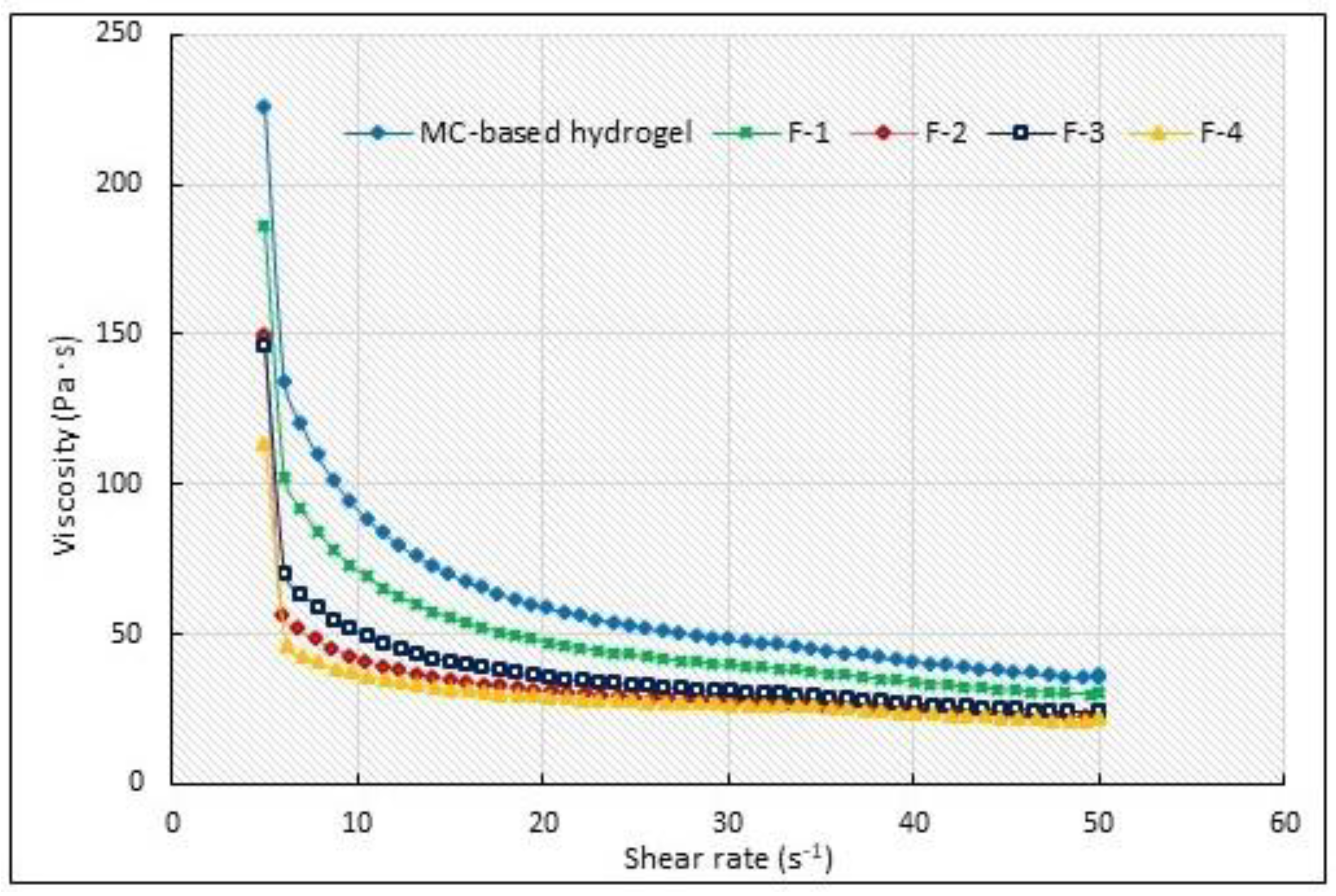
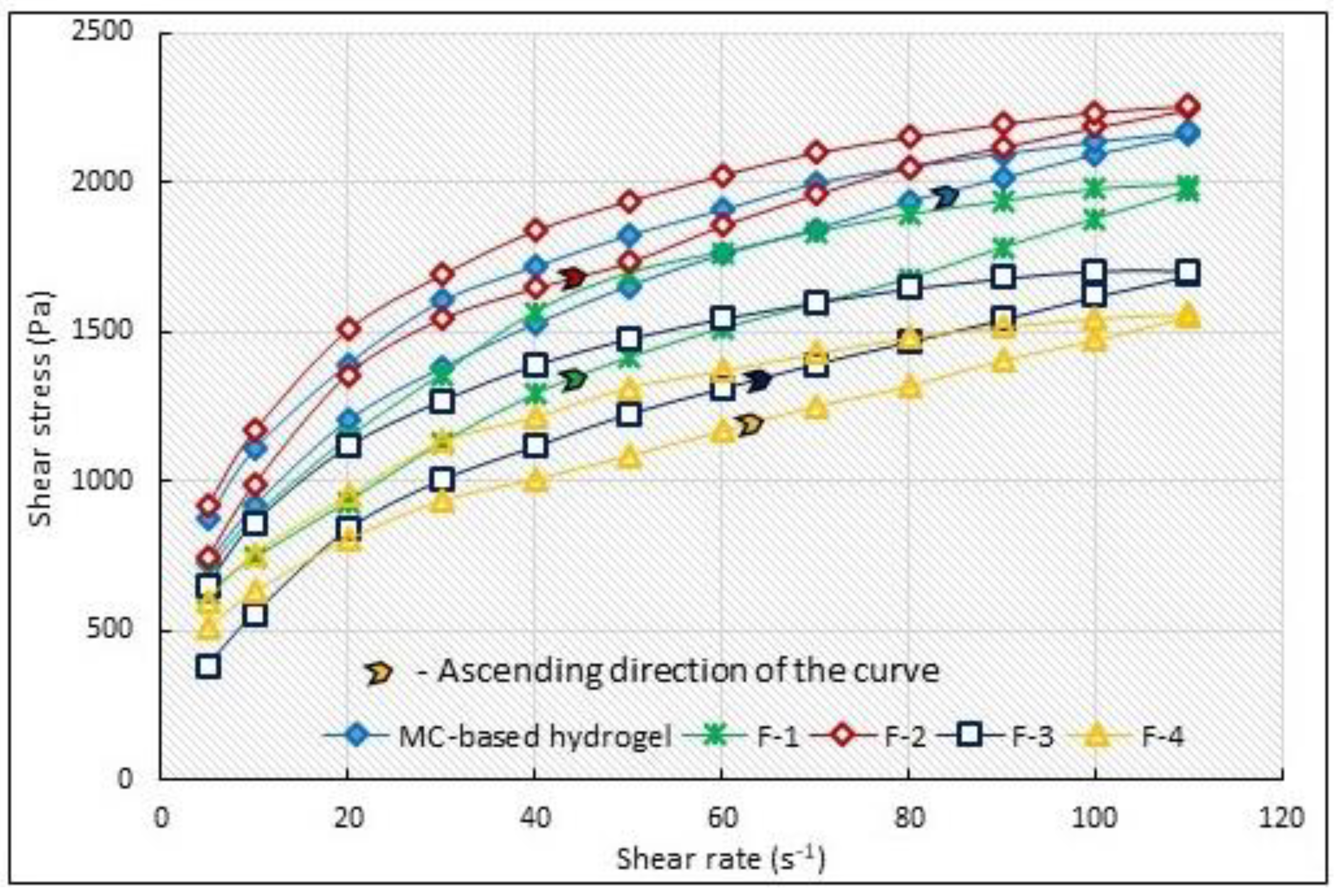
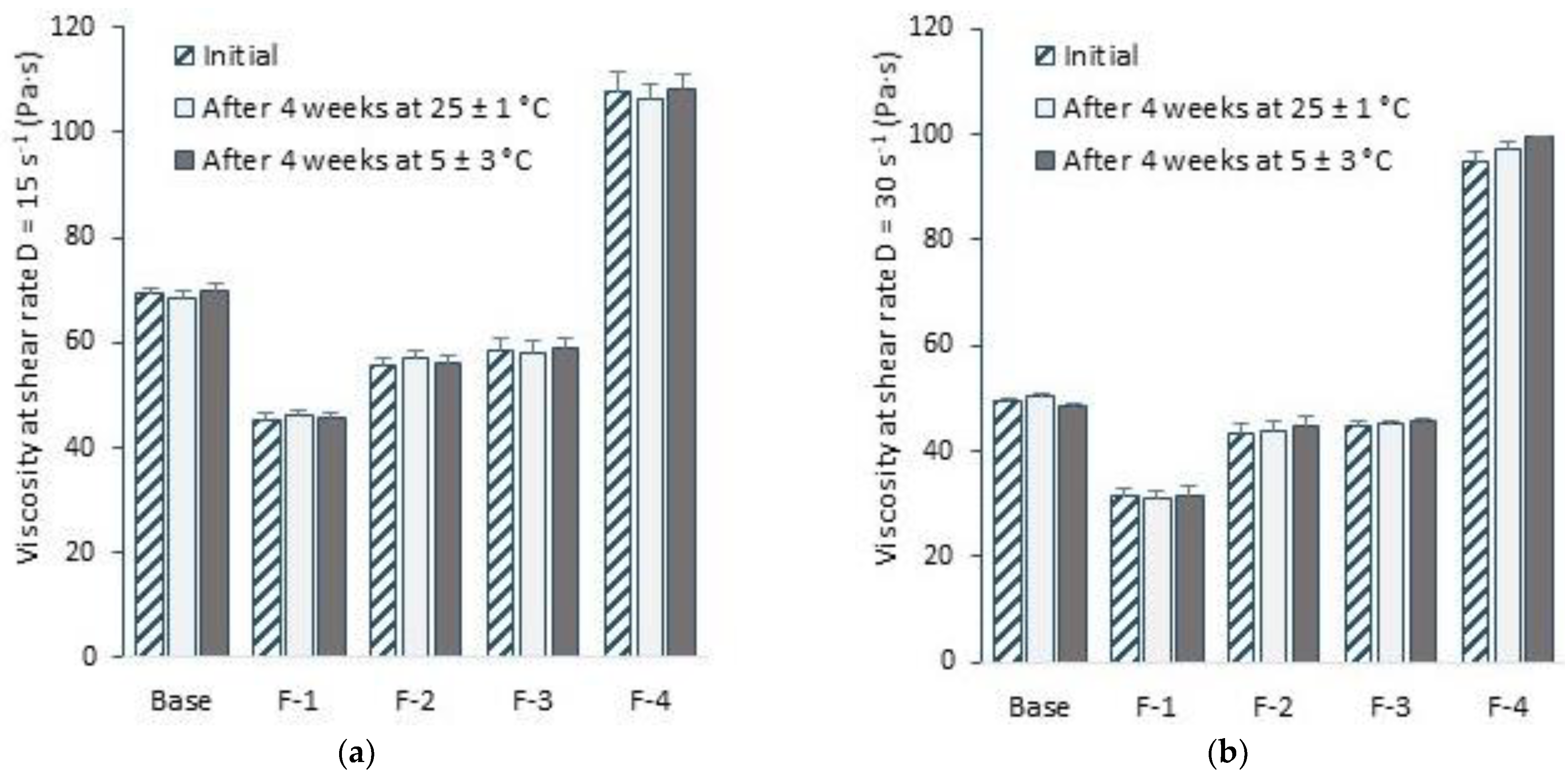
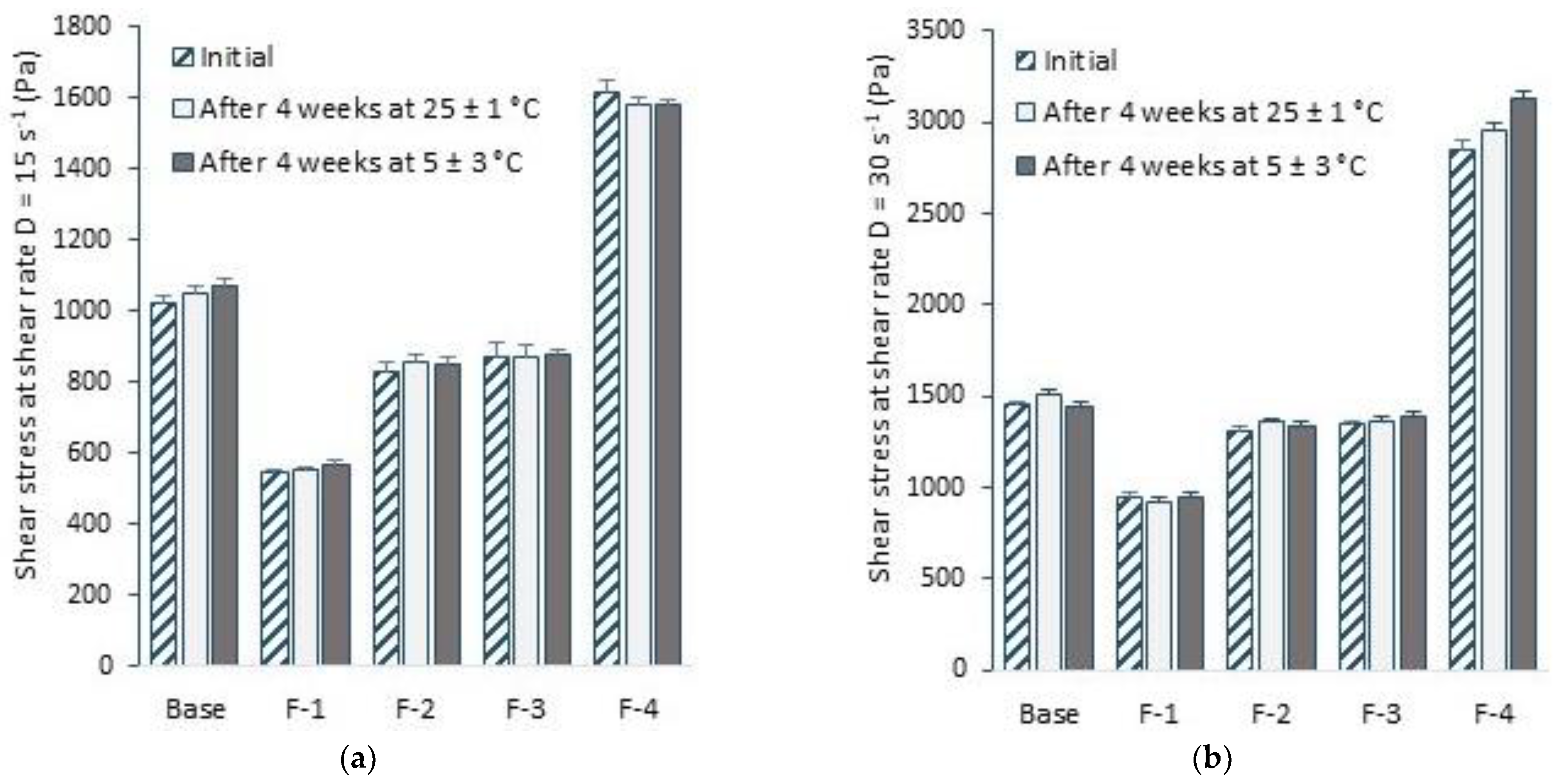
3.5. Selected Rheological Properties
3.6. Sensory Evaluation
3.7. Stability Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kato, Y.; Murakami, Y.; Sohmiya, M.; Nishiki, M. Regulation of human growth hormone secretion and its disorders. Intern. Med. 2002, 41, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashpole, N.M.; Sanders, J.E.; Hodges, E.L.; Yan, H.; Sonntag, W.E. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp. Gerontol. 2015, 68, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Korcz-Iżykowska, M.; Majewska, K.; Kędzia, A. Effects of growth hormone and recombinant human growth hormone on skin homeostasis. Endokrynol. Ped. 2018, 4, 245–250. [Google Scholar]
- Strous, G.J.; Almeida, A.D.S.; Putters, J.; Schantl, J.; Sedek, M.; Slotman, J.A.; Nespital, T.; Hassink, G.C.; Mol, J.A. Growth hormone receptor regulation in cancer and chronic diseases. Front. Endocrinol. 2020, 11, 597573. [Google Scholar] [CrossRef] [PubMed]
- Siebert, D.M.; Rao, A.L. The use and abuse of human growth hormone in sports. Sports Health 2018, 10, 419–426. [Google Scholar] [CrossRef]
- Romer, T. Growth hormone. Production and secretion. In Treatment with Hormones and Hormone Derivatives, 2nd ed.; Pawlikowski, M., Ed.; Medical Publishing House: Warsaw, Poland, 1996; p. 46. [Google Scholar]
- Boguszewski, M.C.S.; Cardoso-Demartini, A.A.; Boguszewski, C.L.; Chemaitilly, W.; Higham, C.E.; Johannsson, G.; Yuen, K.C.J. Safety of growth hormone (GH) treatment in GH deficient children and adults treated for cancer and non-malignant intracranial tumors-a review of research and clinical practice. Pituitary 2021, 24, 810–827. [Google Scholar] [CrossRef]
- Harvey, S.; Martinez-Moreno, C.G. Growth hormone: Therapeutic possibilities—An overview. Int. J. Mol. Sci. 2018, 19, 2015. [Google Scholar] [CrossRef] [Green Version]
- Raoufinia, R.; Mota, A.; Keyhanvar, N.; Safari, F.; Shamekhi, S.; Abdolalizadeh, J. Overview of albumin and its purification methods. Adv Pharm Bull. 2016, 6, 495–507. [Google Scholar] [CrossRef] [Green Version]
- Steglich, M.; Lombide, R.; López, I.; Portela, M.; Fló, M.; Marín, M.; Alvarez, B.; Turell, L. Expression, purification and initial characterization of human serum albumin domain I and its cysteine 34. PLoS ONE 2020, 15, e0240580. [Google Scholar] [CrossRef]
- Zaman, R.; Islam, R.A.; Ibnat, N.; Othman, I.; Zaini, A.; Lee, C.Y.; Chowdhury, E.H. Current strategies in extending half-lives of therapeutic proteins. J. Control. Release 2019, 301, 176–189. [Google Scholar] [CrossRef]
- Siemiradzka, W.; Dolińska, B.; Ryszka, F. Modelling and control of corticotropin permeation from hydrogels across a natural membrane in the presence of albumin. Processes 2021, 9, 1674. [Google Scholar] [CrossRef]
- Jepps, O.G.; Dancik, Y.; Anissimov, Y.G.; Roberts, M.S. Modeling the human skin barier—towards a better understanding of dermal absorption. Adv. Drug Deliv. Rev. 2013, 65, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Half-life extended biotherapeutics. Expert Opin. Biol. Ther. 2016, 16, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Sterry, W. Nanoparticles–an efficient carrier for drug delivery into the hair follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- N’Da, D.D. Prodrug strategies for enhancing the percutaneous absorption of drugs. Molecules 2014, 19, 20780–20807. [Google Scholar] [CrossRef] [Green Version]
- Bäsler, K.; Bergmann, S.; Heisig, M.; Naegel, A.; Zorn-Kruppa, M.; Brandner, J.M. The role of tight junctions in skin barrier function and dermal absorption. J. Control. Release 2016, 242, 105–118. [Google Scholar] [CrossRef]
- Maqbool, A. Semisolid dosage forms manufacturing: Tools, critical process parameters, strategies, optimization, and recent advances. Indo. Am. J. Pharm. Sci. 2017, 7, 882–893. [Google Scholar]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [Green Version]
- Siemiradzka, W.; Dolińska, B.; Ryszka, F. Influence of concentration on release and permeation process of model peptide substance-corticotropin-from Semisolid Formulations. Molecules 2020, 25, 2767. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, Y.N. Lonapegsomatropin: Pediatric First Approval. Pediatr. Drugs 2022, 24, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.C.J.; Miller, B.S.; Boguszewski, C.L.; Hoffman, A.R. Usefulness and potential pitfalls of long-acting growth hormone analogs. Front. Endocrinol. 2021, 12, 637209. [Google Scholar] [CrossRef]
- Sravanthi, S.; Kumari, M.M.; Sharma, J.V.C. A critique view on SKYTROFA®. IJRESM 2021, 4, 188–191. [Google Scholar]
- USA Food and Drug Administration. SKYTROFA® (Lonapegsomatropin-Tcgd): Highlights of Prescribing Information. 2021. Available online: https://www.accessdata.fda.gov/ (accessed on 12 October 2021).
- Maniatis, A.; Nadgir, U.; Hofman, P.; Saenger, P.; Chertok, E.; Aghajanova, E. Lonapegsomatropin (TransCon hGH) in children with growth hormone deficiency: Efficacy and safety of up to 2 years of treatment [poster]. In Proceedings of the PES 2021 virtual annual meeting, virtual, 30 April–3 May 2021. [Google Scholar]
- Siemiradzka, W.; Bułaś, L.; Dolińska, B. Permeation of albumin through the skin depending on its concentration and the substrate used in simulated conditions in vivo. Biomed. Pharm. 2022, 155, 113722. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta. Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Shoaib, M.H.; Tazeen, J.; Merchant, H.A.; Yousuf, R.I. Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak. J. Pharm. Sci. 2006, 19, 119–124. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.S.; Yang, H.M.; Kim, J.W.; Maeng, Y.J.; Lee, C.W.; Kang, Y.S.; Rang, M.J.; Kim, H.Y. Terminology development and panel training for sensory evaluation of skin care products including aqua cream. J. Sens. Stud. 2005, 20, 421–433. [Google Scholar] [CrossRef]
- Guideline, I.H.T. Quality of biotechnological products: Stability testing of biotechnological/biological products. Annex to the ICH Harmonised Tripartite Guideline for the Stability Testing of New Drug Substances and Products. Dev. Biol. Stand. 1998, 93, 211–219. [Google Scholar]
- Al-Tabakha, M.M.; Fahelelbom, K.M.; Obaid, D.E.E.; Sayed, S. Quality attributes and in vitro bioequivalence of different brands of amoxicillin trihydrate tablets. Pharmaceutics 2017, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef]
- Cázares-Delgadillo, J.; Ganem-Rondero, A.; Kalia, Y.N. Human growth hormone: New delivery systems, alternative routes of administration, and their pharmacological relevance. Eur. J. Pharm. Biopharm. 2011, 78, 278–288. [Google Scholar] [CrossRef]
- Schuetz, Y.B.; Naik, A.; Guy, R.H.; Kalia, Y.N. Emerging strategies for the transdermal delivery of peptide and protein drugs. Expert Opin. Drug Deliv. 2005, 2, 533–548. [Google Scholar] [CrossRef]
- Flynn, G.L.; Amidon, G.L.; Choi, H.K. Transdermal delivery of bioactive peptides: The effect of N-decylmethyl sulfoxide, pH, and inhibitors on enkephalin metabolism and transport. Pharm. Res. 1990, 7, 1099–1106. [Google Scholar]
- Banerjee, P.S.; Ritschel, W.A. Transdermal permeation of vasopressin. I. Influence of pH, concentration, shaving and surfactant on in vitro permeation. Int. J. Pharm. 1989, 49, 189–197. [Google Scholar] [CrossRef]
- Shah, P.K.; Borchardt, R.T. A comparison of peptidase activities and peptide metabolism in cultured mouse keratinocytes and neonatal mouse epidermis. Pharm. Res. 1991, 8, 70–75. [Google Scholar] [CrossRef]
- Martin, R.J.; Denyer, S.P.; Hadgraft, J. Skin metabolism of topically applied compounds. Int. J. Pharm. 1987, 39, 23–32. [Google Scholar] [CrossRef]
- Riesz, P.; Kondo, T. Free radical formation induced by ultrasound and its biological implications. Free Radic. Biol. Med. 1992, 13, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Hikima, T.; Hirai, Y.; Tojo, K. The effect of ultrasound application on skin metabolism of prednisolone 21-acetate. Pharm. Res. 1998, 15, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Svennebring, A. The connection between plasma protein binding and acute toxicity as determined by the LD50 value. Drug Dev. Res. 2016, 77, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery—New applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef]
- Tan, Y.L.; Ho, H.K. Navigating albumin-based nanoparticles through various drug delivery routes. Drug Discov. Today 2018, 23, 1108–1114. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A. The potential of pharmaceutical hydrogels in the formulation of topical administration hormone drugs. Polymers 2022, 14, 3307. [Google Scholar] [CrossRef]
- Heo, S.K.; Cho, Y.S.; Han, S.D.; Chang, J.K.; Yoon, E.J.; Ko, D.W.; Lim, C.B.; Chung, S.J.; Shim, C.K.; Kim, D.D. In vitro and in vivo evaluation of novel gel formulations of testosterone for transdermal delivery. J. Kor. Pharm. Sci. 2005, 35, 329–332. [Google Scholar]
- Ostróżka-Cieślik, A.; Maciążek-Jurczyk, M.; Pożycka, J.; Dolińska, B. Pre-formulation studies: Physicochemical characteristics and in vitro release kinetics of insulin from selected hydrogels. Pharmaceutics 2021, 13, 1215. [Google Scholar] [CrossRef]
- Şenyiğit, Z.A.; Coşkunmeriç, N.; Çağlar, E.Ş.; Öztürk, İ.; Atlıhan Gündoğdu, E.; Siafaka, P.I.; Üstündağ Okur, N. Chitosan-bovine serum albumin-Carbopol 940 nanogels for mupirocin dermal delivery: Ex-vivo permeation and evaluation of cellular binding capacity via radiolabeling. Pharm. Dev. Technol. 2021, 26, 852–866. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Zarei-Behjani, Z.; Bohlouli, M.; Khojasteh, A.; Ghasemi, N.; Salehi-Nik, N. Fabrication and optimization of bioactive cylindrical scaffold prepared by electrospinning for vascular tissue engineering. Iran. Polym. J. 2022, 31, 127–141. [Google Scholar] [CrossRef]
- Kateh Shamshiri, M.; Momtazi-Borojeni, A.A.; Khodabandeh, S.M.; Rahimi, F. Lecithin soybean phospholipid nano-transfersomes as potential carriers for transdermal delivery of the human growth hormone. J. Cell Biochem. 2019, 120, 9023–9033. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, L.; Long, W.; Zhang, Q.; Zou, Y. Sustained transdermal delivery of human growth hormone from niosomal gel: In vitro and in vivo studies. J. Biomater. Sci. Polym. Ed. 2022, 33, 1198–1212. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Iwashita, K.; Shiraki, K. Viscosity control of protein solution by small solutes: A review. Curr. Protein Pept. Sci. 2018, 19, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Timasheff, S.N. Stabilization of protein structure by sugars. Biochemistry 1982, 21, 6536–6544. [Google Scholar] [CrossRef]
- Savary, G.; Gilbert, L.; Grisel, M.; Picard, C. Instrumental and sensory methodologies to characterize the residual film of topical products applied to skin. Skin Res. Technol. 2019, 25, 415–423. [Google Scholar] [CrossRef]
- Siddig, A.; Ramirex, R.; Wood, M.K.; Sun, X.; Smith, G. Formulation and comparative characterization of methylcellulose, carbomer and petrolatum gels for topical and transdermal delivery of Zosyn (piperacillin/tazobactam) antibiotics. J. Pharm. Pract. Pharm. Sci. 2020, 1, 96–114. [Google Scholar]
| Ingredient | STH (g) | ALB (g) | MC (g) | Glycerol (g) | Water (g) |
|---|---|---|---|---|---|
| Hydrogel Formulation | |||||
| F-1 | 0.1 | - | 6.00 | 10.00 | 83.9 |
| F-2 | 0.1 | 0.1 | 6.00 | 10.00 | 83.8 |
| F-3 | 0.1 | 0.2 | 6.00 | 10.00 | 83.7 |
| F-4 | 0.1 | 0.5 | 6.00 | 10.00 | 83.4 |
| MC-based hydrogel | - | - | 6.00 | 10.00 | 84.0 |
| Formulations | Total Permeated STH Amount (%) | AUC(0–6 h) * AUC(0–24 h) ** (% h−1) | Degree of Relative Availability EBA (%) |
|---|---|---|---|
| Spectra/Por® Dialysis Membrane permeation | |||
| F-1 | 71.44 ± 3.43 | 175.97 | 100.00 |
| F-2 | 44.96 ± 0.10 2 | 152.30 | 86.55 |
| F-3 | 93.59 ± 0.99 | 341.19 | 193.89 |
| F-4 | 86.78 ± 7.41 | 320.05 | 181.88 |
| Porcine skin permeation | |||
| F-1 | 80.37 ± 10.78 | 164.24 | 100.00 |
| F-2 | 57.69 ± 5.12 1 | 1521.25 | 926.24 |
| F-3 | 66.60 ± 5.11 1 | 1687.99 | 1027.76 |
| F-4 | 46.76 ± 5.73 1 | 1324.13 | 806.22 |
| Formulations | Kinetics Release Models | Kinetics Parameters | |||
|---|---|---|---|---|---|
| Higuchi R2 | Korsmayer–Peppas R2 | First Order R2 | K (h−1) | Permeation Half-Life t50% (h) | |
| Membrane | Spectra/Por | ||||
| F-1 | 0.9999 | 0.9955 | 0.9996 | 0.314 ± 0.029 | 2.22 ± 0.21 |
| F-2 | 0.9197 | 0.9426 | 0.9688 | 0.100 ± 0.034 1 | 7.58 ± 1.75 1 |
| F-3 | 0.9733 | 0.9683 | 0.9620 | 0.451 ± 0.029 1 | 1.54 ± 0.10 1 |
| F-4 | 0.9864 | 0.9939 | 0.9616 | 0.351 ± 0.098 | 2.08 ± 0.61 |
| Average R2 | 0.9698 | 0.9751 | 0.9768 | ||
| Membrane | Porcine skin | ||||
| F-1 | 0.9772 | 0.9785 | 0.9885 | 0.601 ± 0.082 | 1.17 ± 0.16 |
| F-2 | 0.9634 | 0.9750 | 0.9869 | 0.108 ± 0.046 1 | 7.40 ± 1.47 1 |
| F-3 | 0.9113 | 0.9457 | 0.9770 | 0.131 ± 0.055 1 | 6.07 ± 0.80 1 |
| F-4 | 0.9663 | 0.9251 | 0.9841 | 0.077 ± 0.007 1 | 9.10 ± 0.83 1 |
| Average R2 | 0.9546 | 0.9561 | 0.9812 | ||
| Formulations | F-1 | F-2 | Permeation Profile |
|---|---|---|---|
| Cellulose membrane permeation profiles | |||
| F-2 vs. F-3 | 44.28 | 8.27 | Dissimilar |
| F-2 vs. F-4 | 40.25 | 11.86 | Dissimilar |
| F-3 vs. F-4 | 7.24 | 49.01 | Dissimilar |
| Porcine skin permeation profiles | |||
| F-2 vs. F-3 | 16.61 | 32.02 | Dissimilar |
| F-2 vs. F-4 | 25.35 | 31.70 | Dissimilar |
| F-3 vs. F-4 | 33.47 | 16.83 | Dissimilar |
| Formulations | Viscosity η (Pa·s) | |
|---|---|---|
| D = 15 s−1 | D = 30 s−1 | |
| MC-Hydrogel base | 69.17 ± 1.38 | 49.58 ± 0.52 |
| F-1 | 45.41 ± 1.19 1 | 31.25 ± 1.62 1 |
| F-2 | 55.60 ± 1.53 1,2 | 43.5 ± 1.82 1,2 |
| F-3 | 58.40 ± 2.32 1,2 | 44.8 ± 0.69 1,2 |
| F-4 | 107.70 ± 3.47 1,2 | 95.00 ± 1.74 1,2 |
| Parameter | Formulation Characteristic | ||||
|---|---|---|---|---|---|
| MC-Based Hydrogel | F-1 | F-2 | F-3 | F-4 | |
| Uniformity | homogeneous base; very smooth structure | homogeneous formulation; very smooth structure | homogeneous formulation; very smooth structure | homogeneous formulation; very smooth structure | homogeneous formulation; very smooth structure |
| Consistency | light; easy to spread | very light; easy to spread | very light; very easy to spread | very light; very easy to spread | very light; easy to spread |
| Pillow effect | small layer of formulation perceptible between the fingers | small layer of formulation perceptible between the fingers | small layer of formulation perceptible between the fingers | small layer of formulation perceptible between the fingers | small layer of formulation perceptible between the fingers |
| Adhesion | easy to apply; spreads enough | very easy to apply; spreads very lightly | very easy to apply; spreads very lightly | very easy to apply; spreads very lightly | easy to apply; spreads lightly |
| Stickiness | not sticky | not sticky | not sticky | not sticky | not sticky |
| Greasiness and greasing | no greasy film after application | no greasy film after application | no greasy film after application | no greasy film after application | no greasy film after application |
| Formulations | Average pH Value ± SD | ||
|---|---|---|---|
| Initially | After 4 Weeks at 25 ± 1 °C | After 4 Weeks at 5 ± 3 °C | |
| MC-based hydrogel | 4.58 ± 0.032 | 4.58 ± 0.053 | 4.56 ± 0.021 |
| F-1 | 5.52 ± 0.080 1 | 5.50 ± 0.078 1 | 5.51 ± 0.081 1 |
| F-2 | 5.60 ± 0.093 1 | 5.60 ± 0.087 1 | 5.59 ±0.093 1 |
| F-3 | 5.68 ± 0.021 1 | 5.68 ± 0.018 1 | 5.68 ± 0.025 1 |
| F-4 | 5.91 ± 0.026 1 | 5.90 ± 0.025 1 | 5.90 ± 0.032 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siemiradzka, W.; Franczyk, A.; Bułaś, L.; Dolińska, B. Somatotropin Penetration Testing from Formulations Applied Topically to the Skin. Appl. Sci. 2023, 13, 2588. https://doi.org/10.3390/app13042588
Siemiradzka W, Franczyk A, Bułaś L, Dolińska B. Somatotropin Penetration Testing from Formulations Applied Topically to the Skin. Applied Sciences. 2023; 13(4):2588. https://doi.org/10.3390/app13042588
Chicago/Turabian StyleSiemiradzka, Wioletta, Agata Franczyk, Lucyna Bułaś, and Barbara Dolińska. 2023. "Somatotropin Penetration Testing from Formulations Applied Topically to the Skin" Applied Sciences 13, no. 4: 2588. https://doi.org/10.3390/app13042588







