Thermal-Hydraulic-Mechanical Coupling Simulation of CO2 Enhanced Coalbed Methane Recovery with Regards to Low-Rank but Relatively Shallow Coal Seams
Abstract
1. Introduction
2. Theory of Modelling
2.1. Fundamental Assumption
2.2. Governing Model of the Hydraulic Field
2.3. Governing Model of the Mechanical Field
2.4. Governing Model of the Thermal Field
2.5. Permeability Evolution Equation
2.6. Physical Parameters
2.7. Cross Coupling of the Thermal-Hydraulic-Mechanical Fields
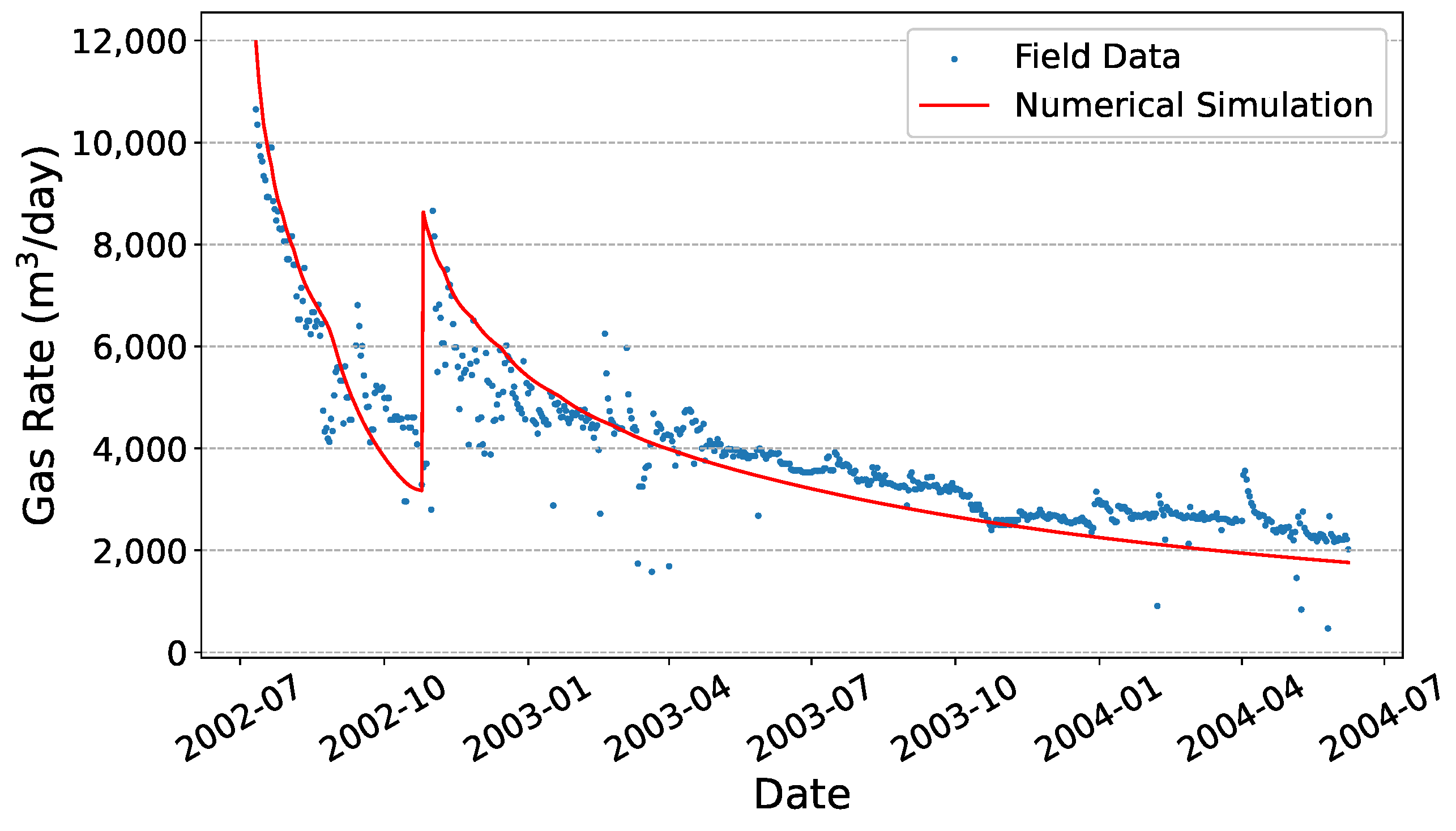
2.8. Model Validation
3. Simulation Scheme
3.1. Simulation Program
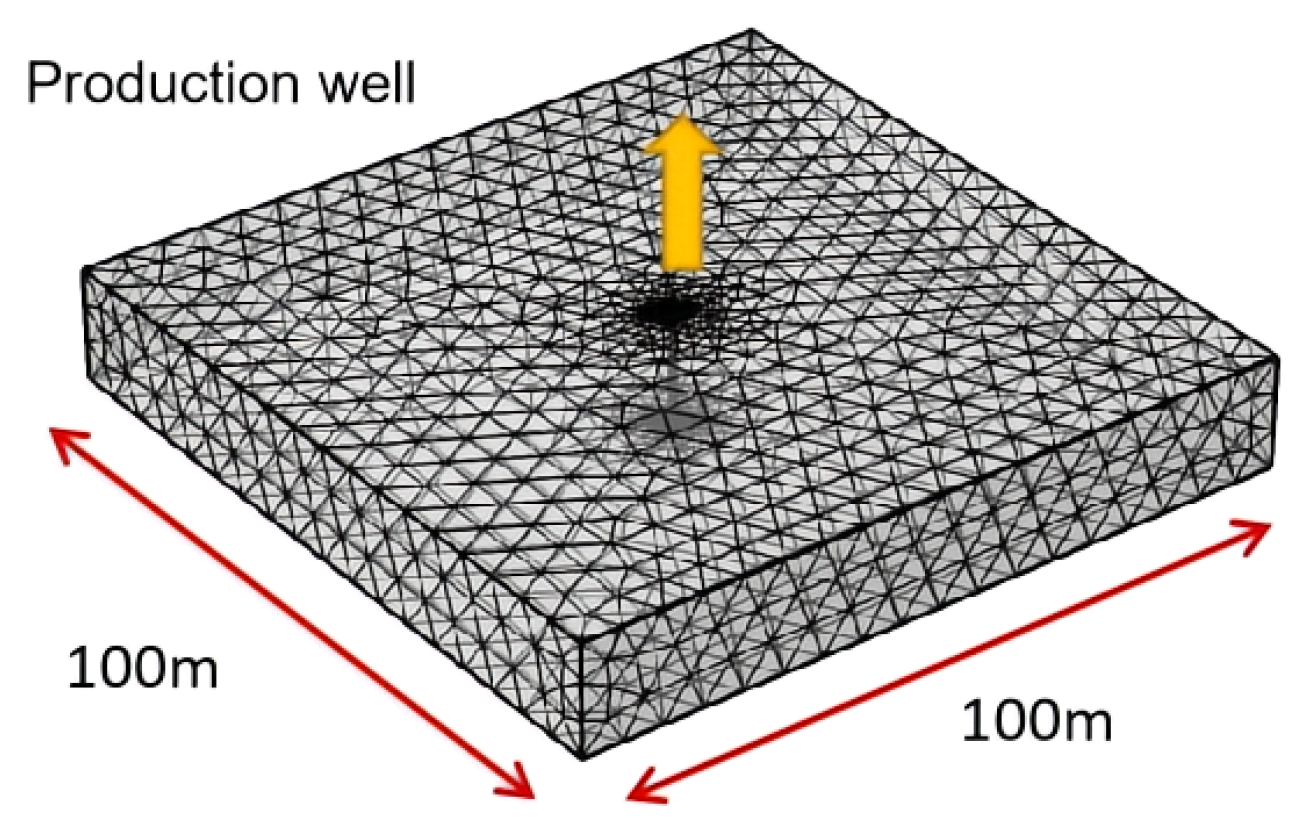
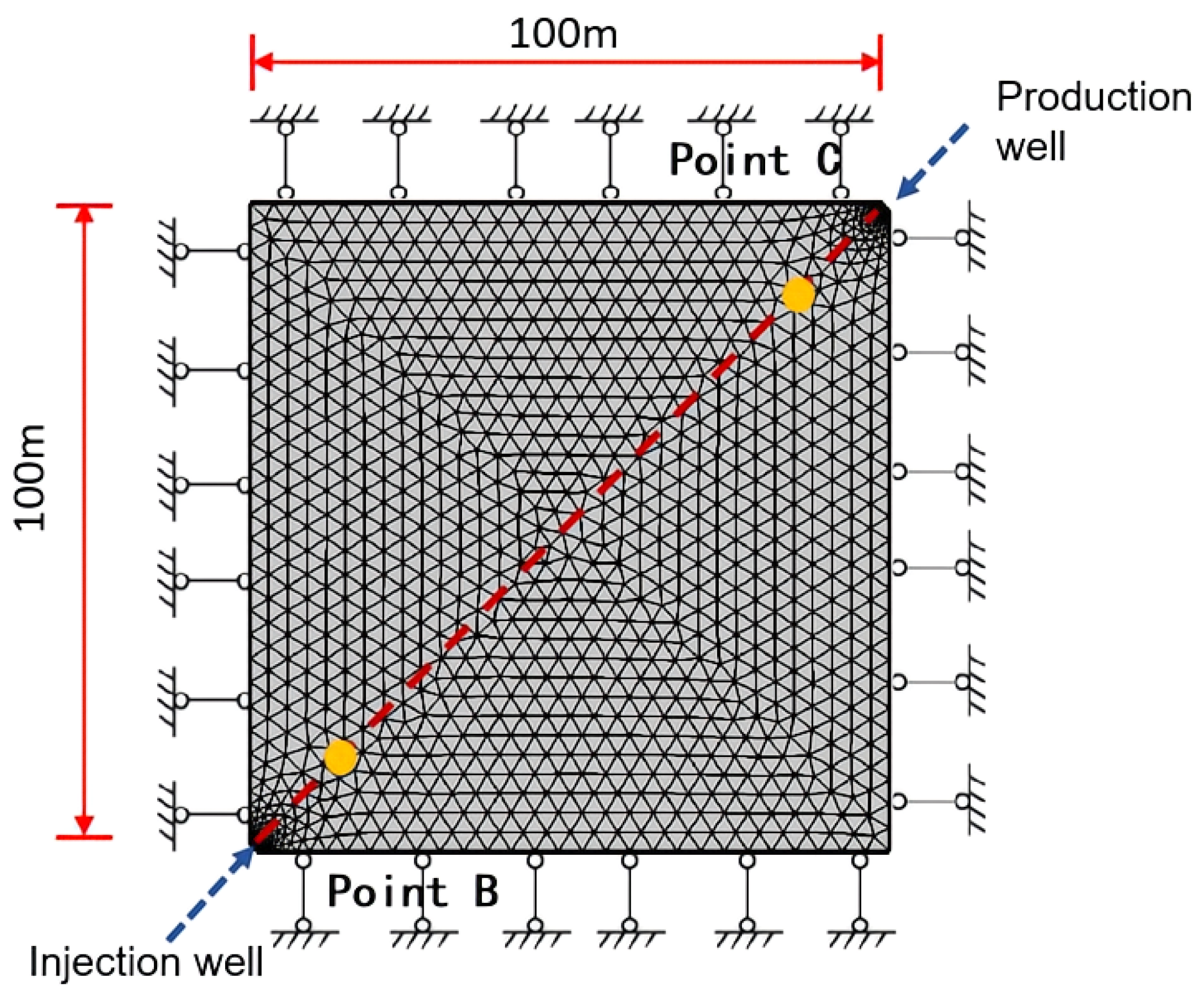
3.2. Basic Model
4. Results and Discussions
4.1. Effect of CO Injection on Coalbed Methane Production
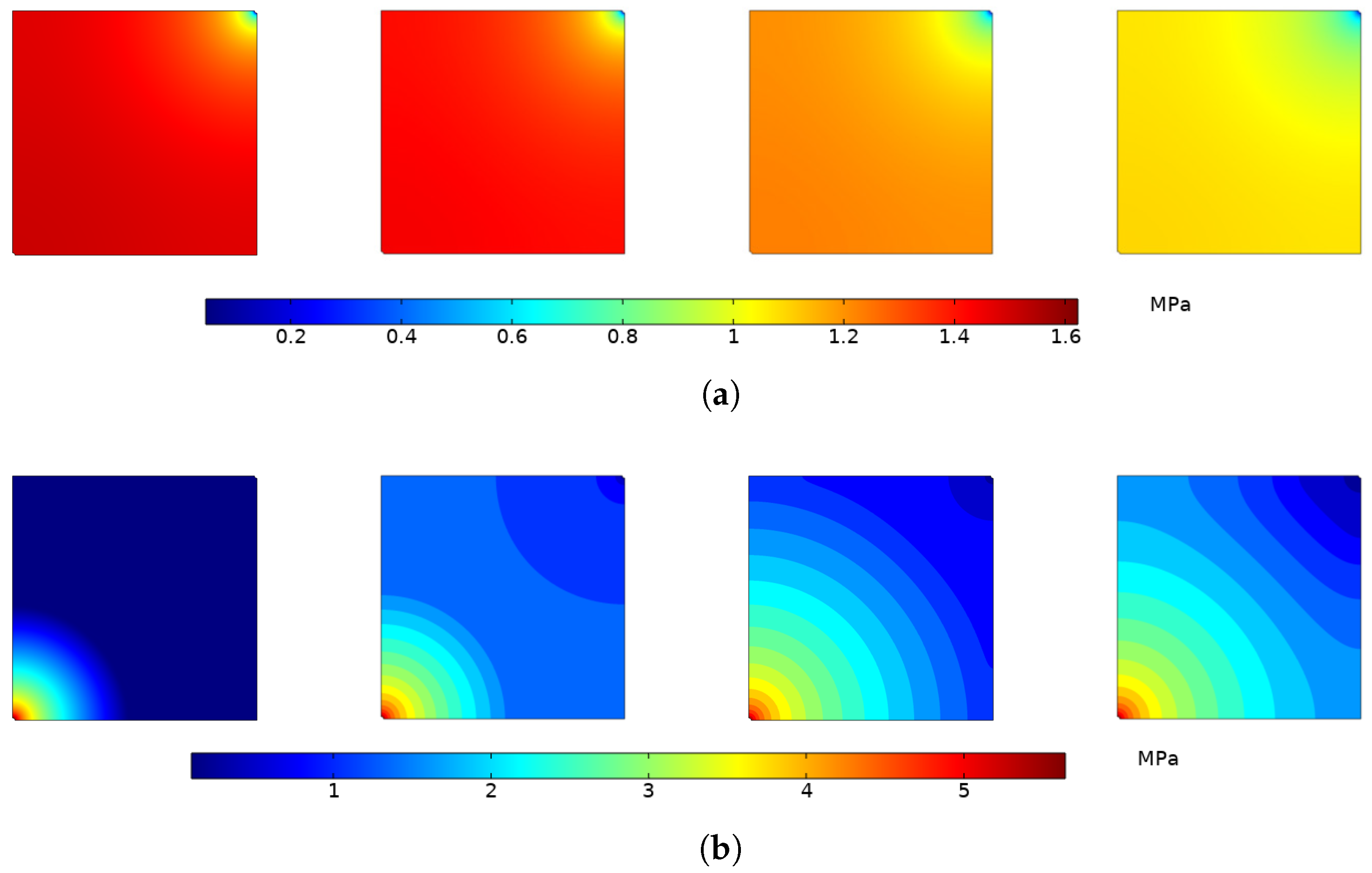
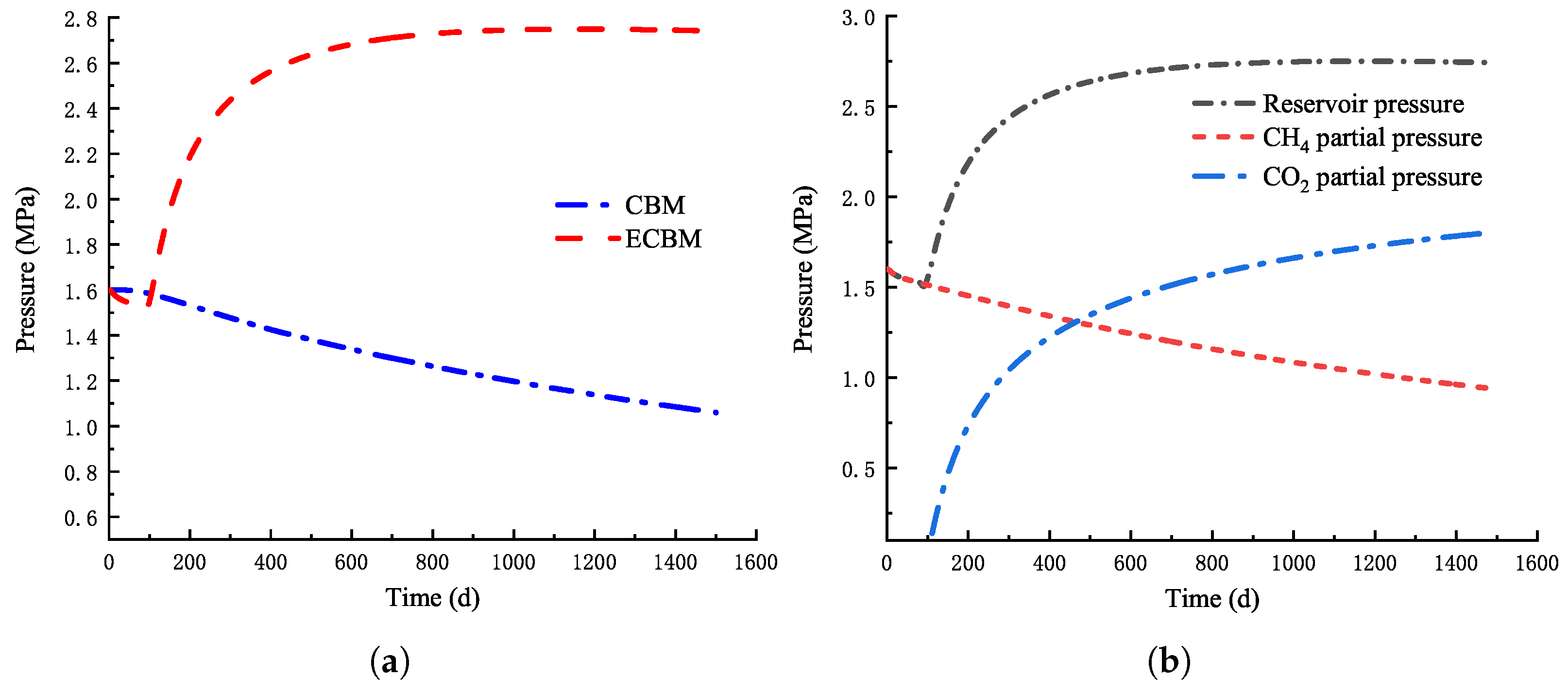
4.1.1. Variation of Reservoir Pressure
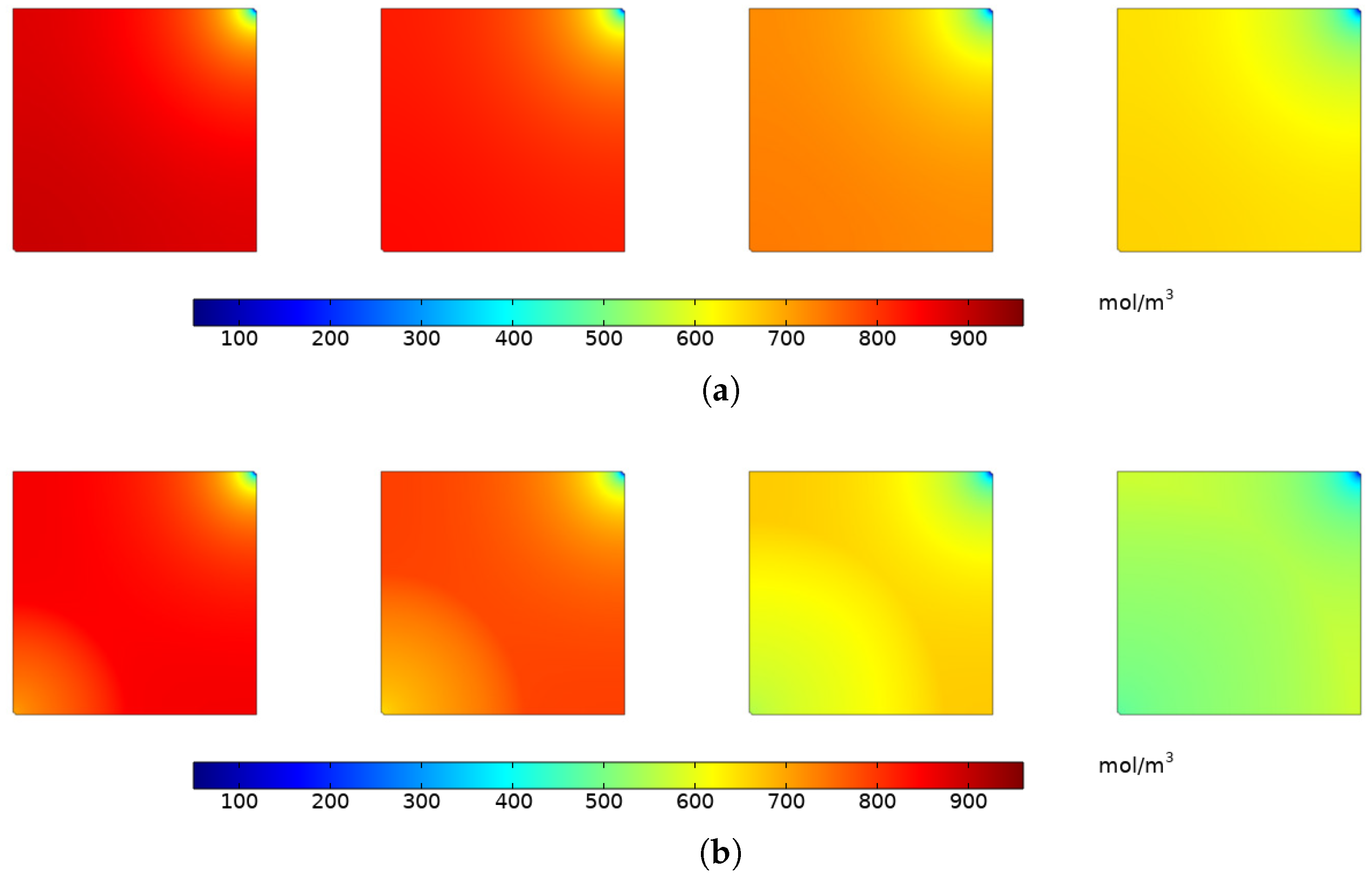
4.1.2. Variation of CH Concentration
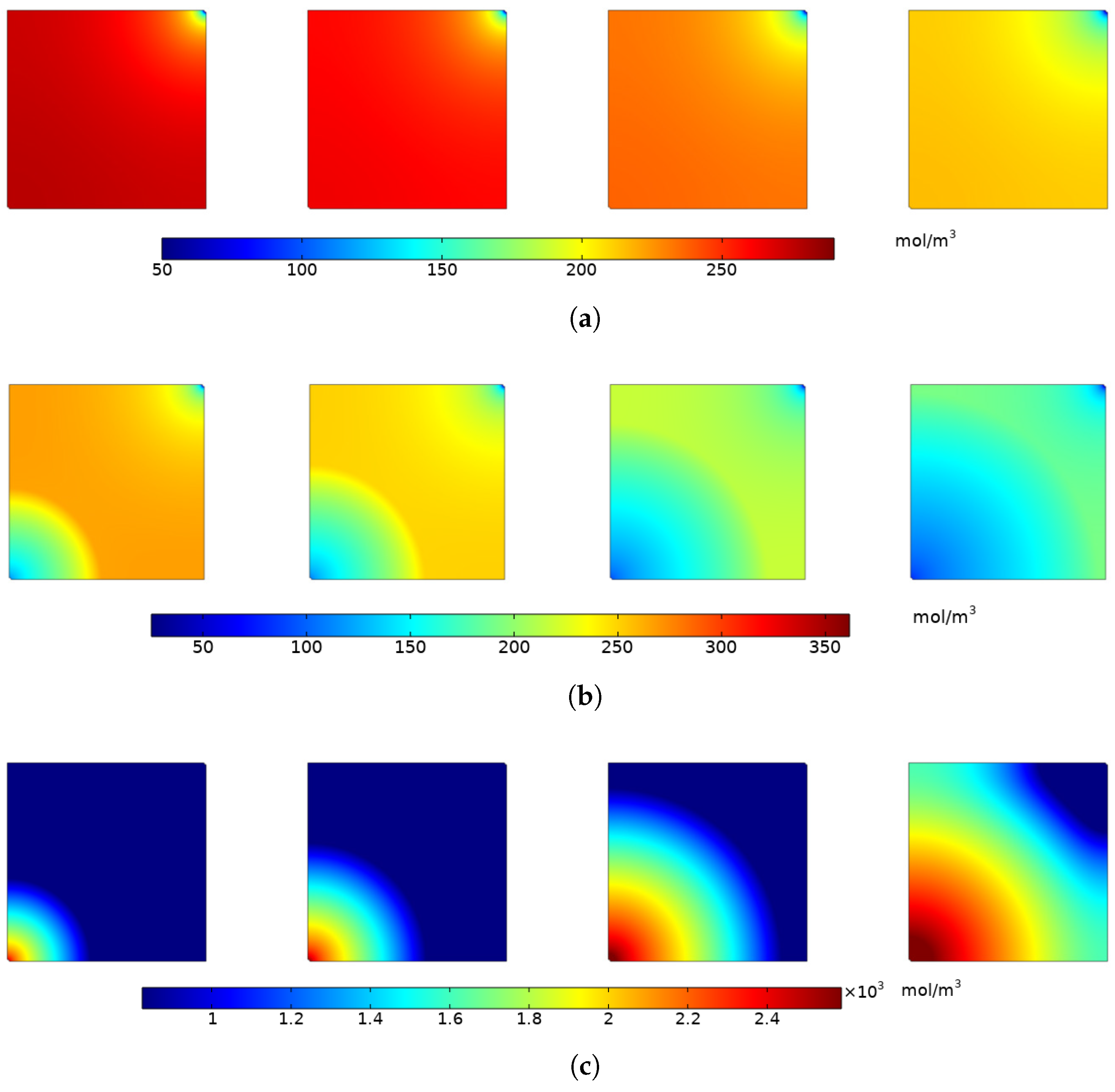
4.1.3. Variation of Adsorbed Gas Concentration
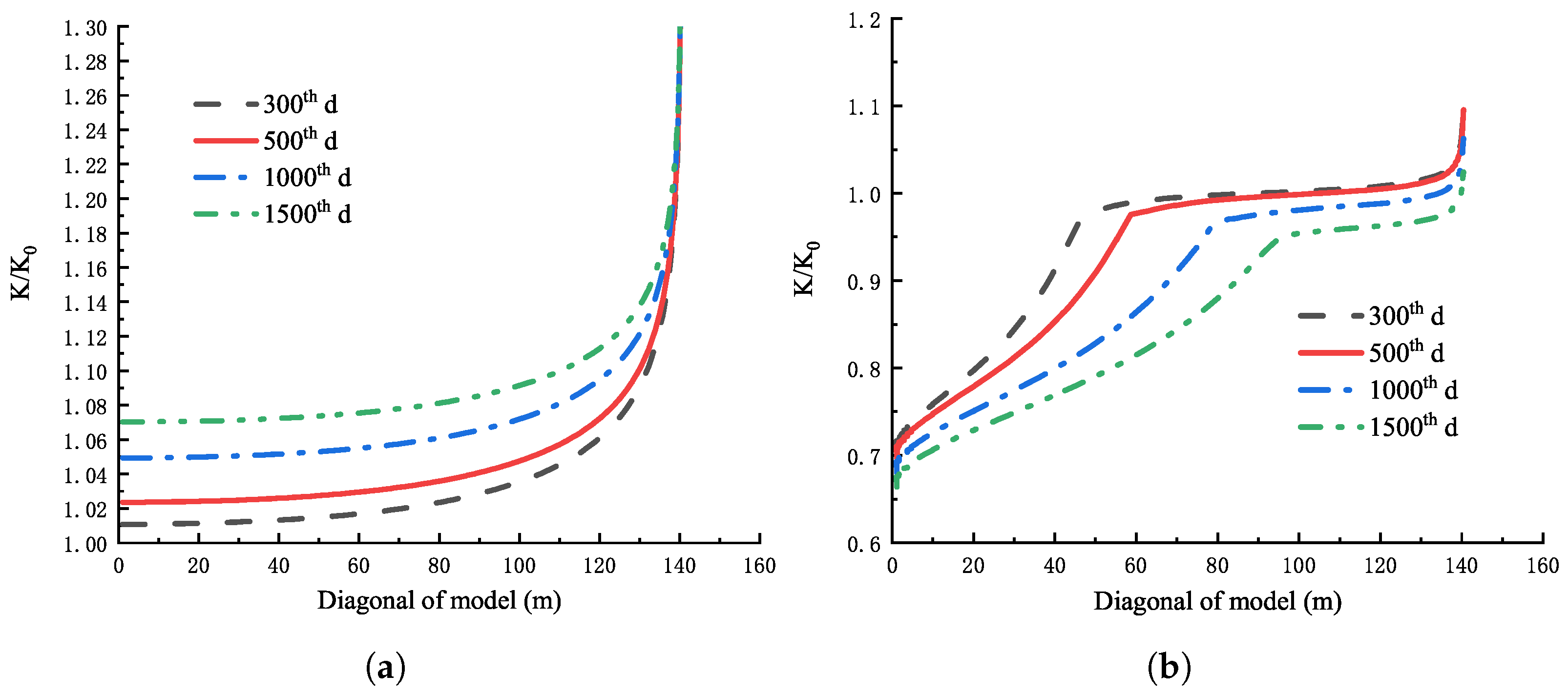
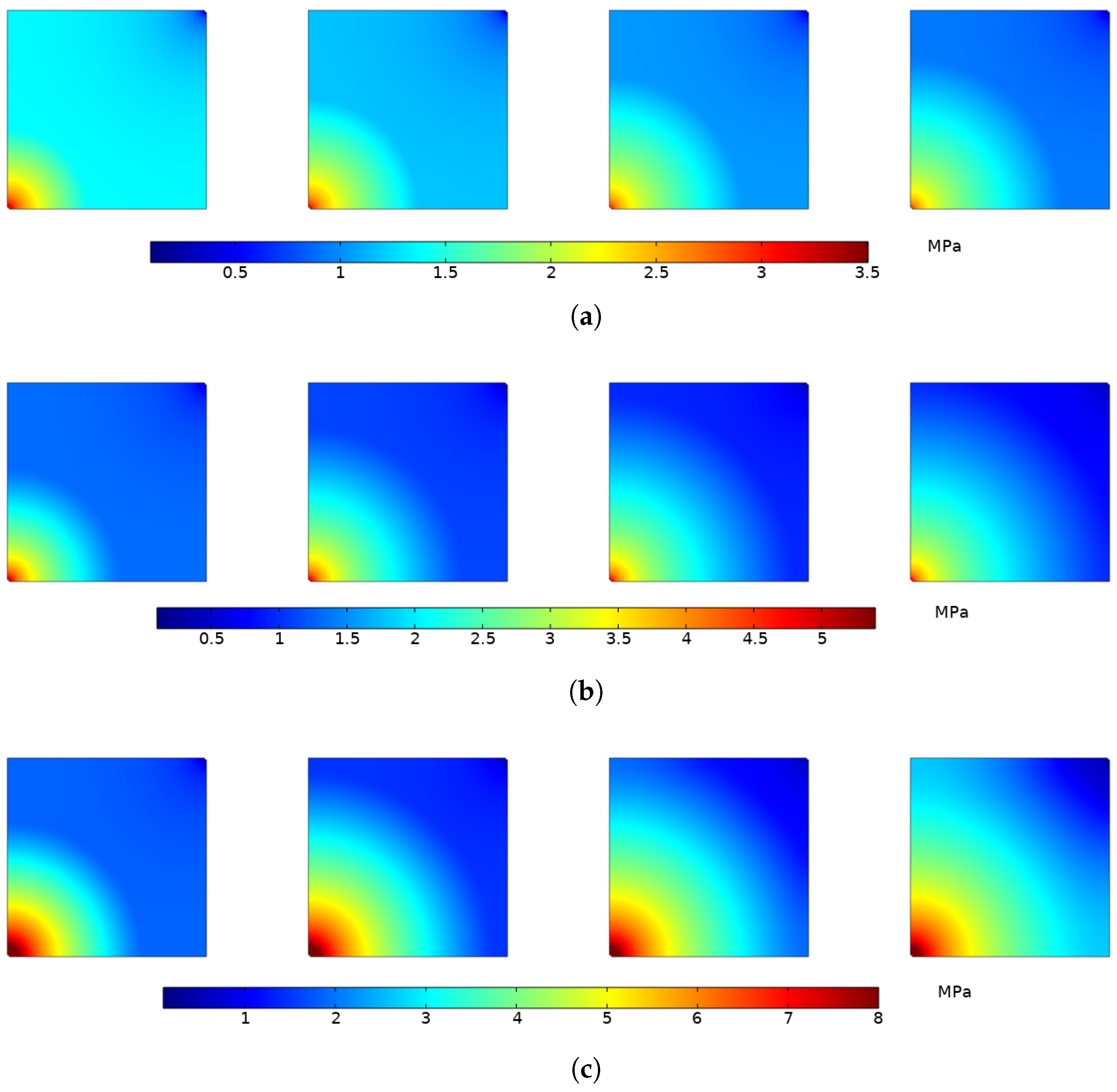
4.1.4. Variation of Reservoir Permeability
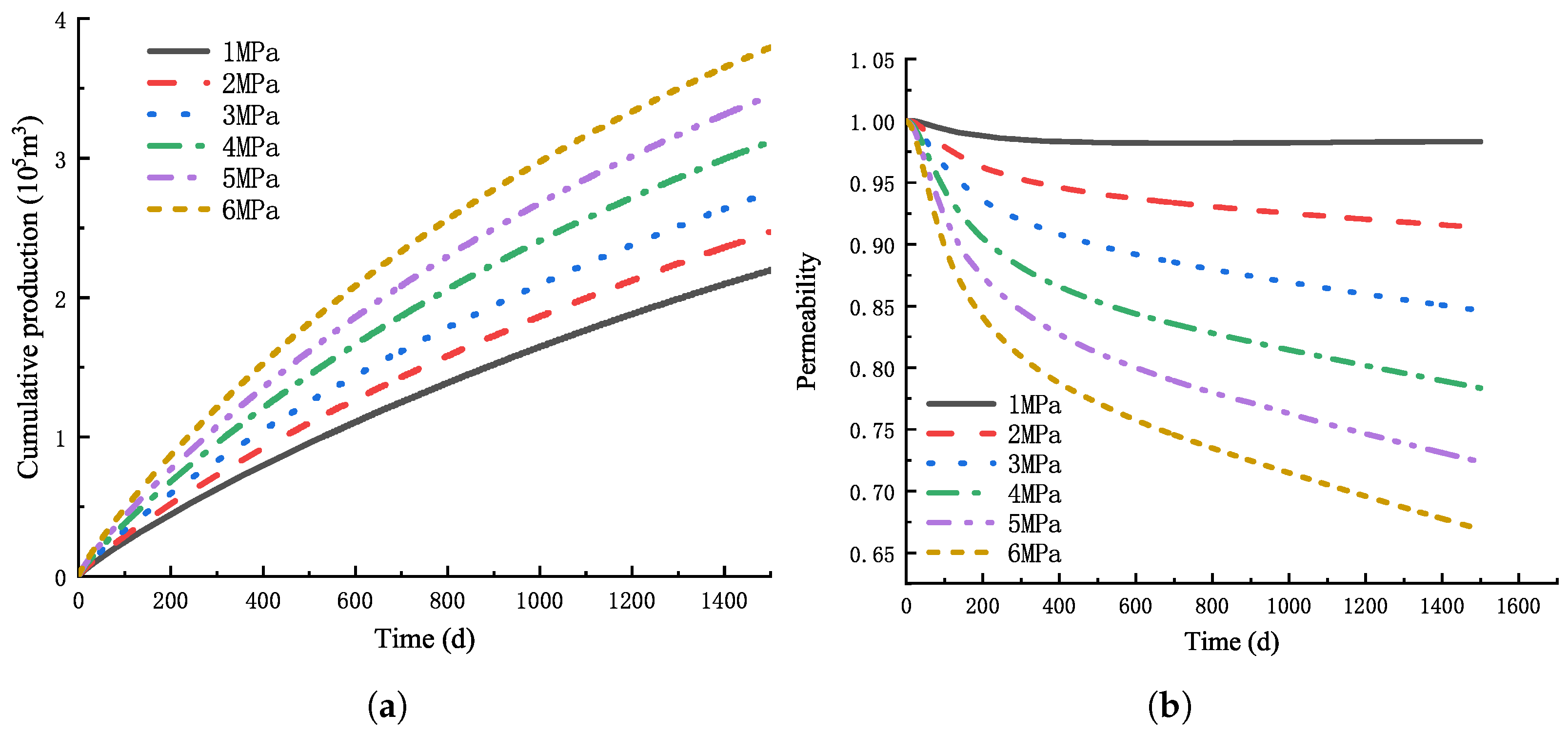
4.2. Effect of Different Injection Pressure on CO-ECBM
4.2.1. Variation of Reservoir Pressure
4.2.2. Optimal Injection Pressure Selection
4.3. Effect of Different Injection Temperatures on CO-ECBM
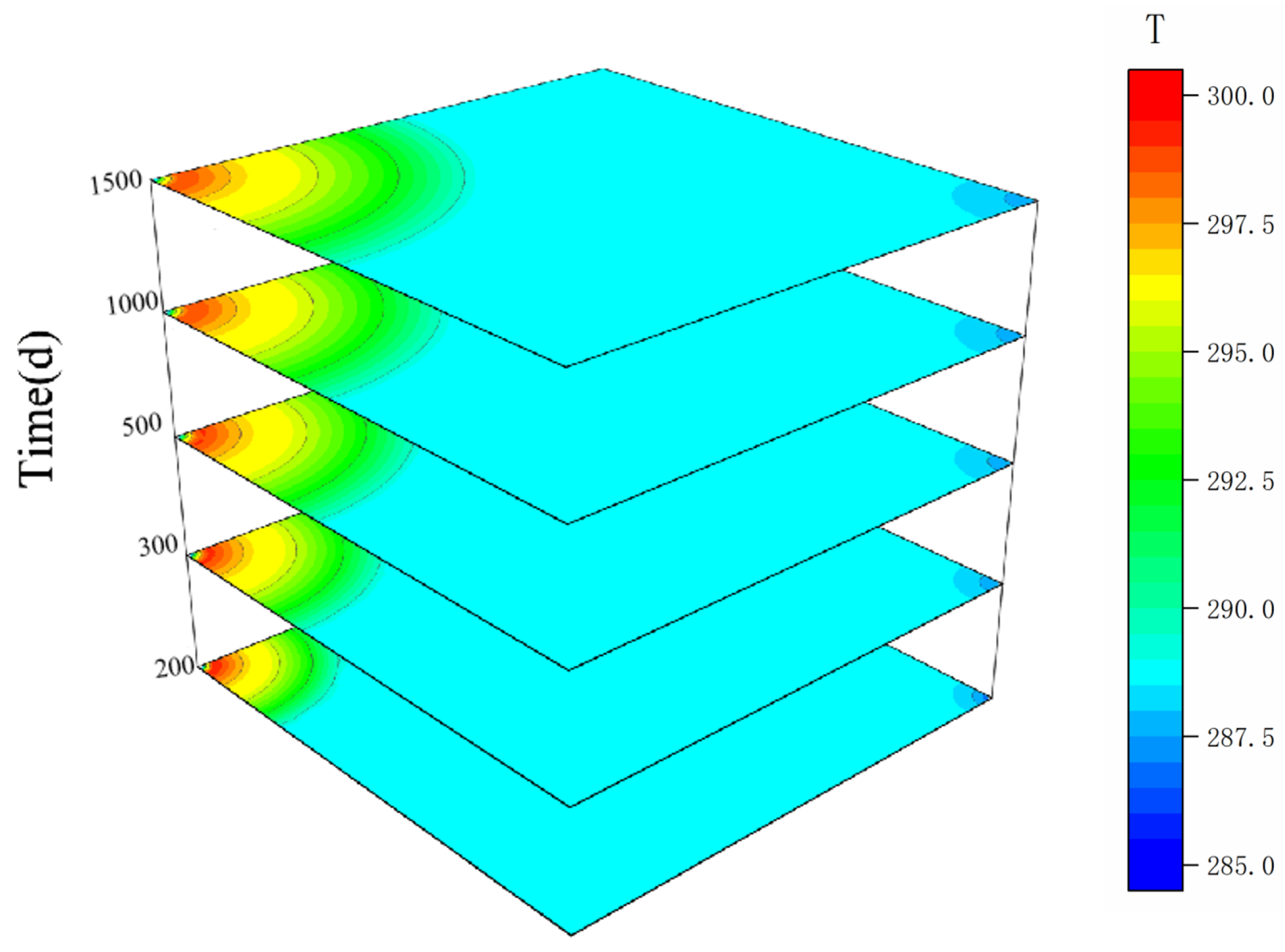
4.3.1. Variation of Reservoir Temperature
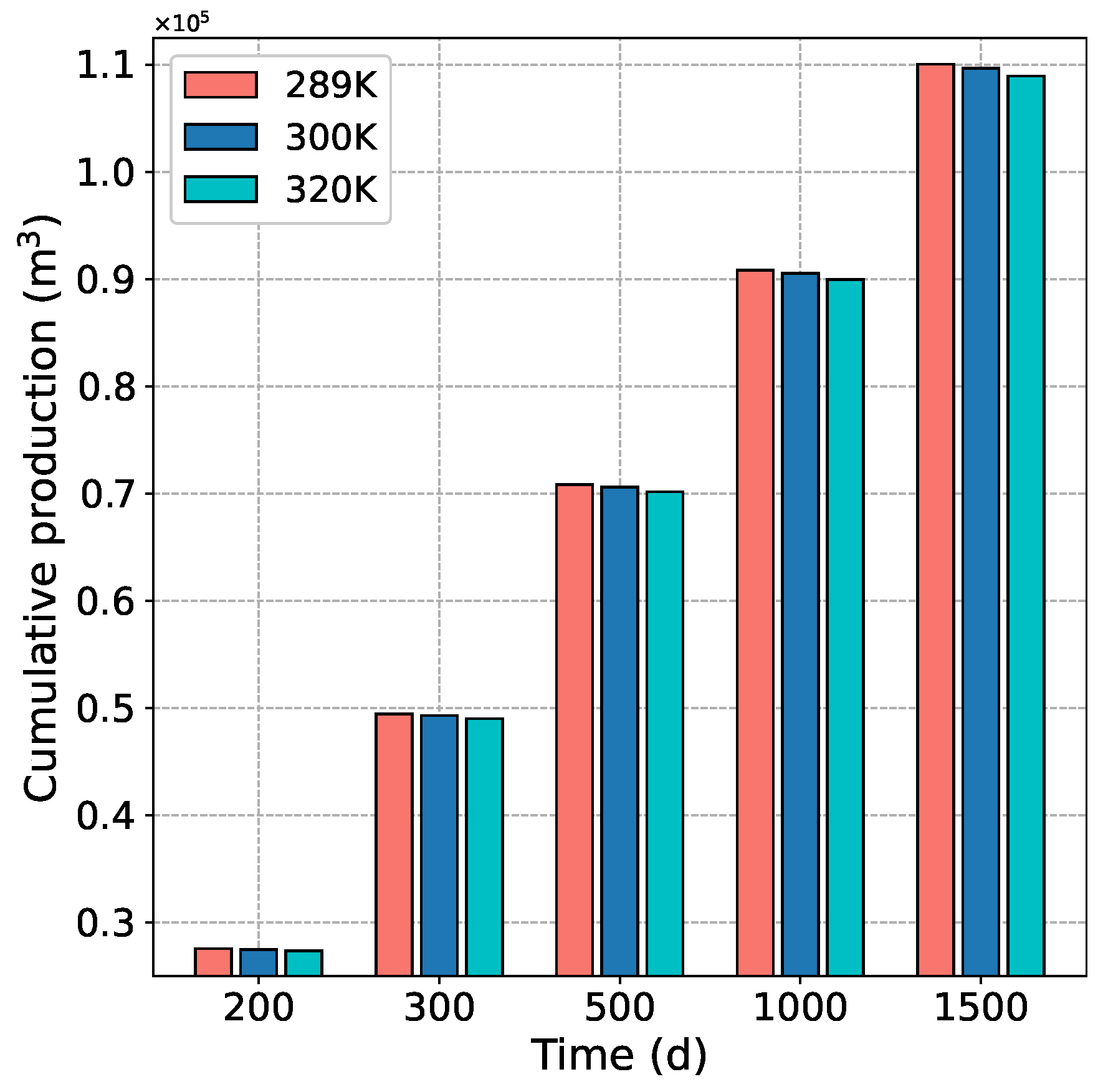
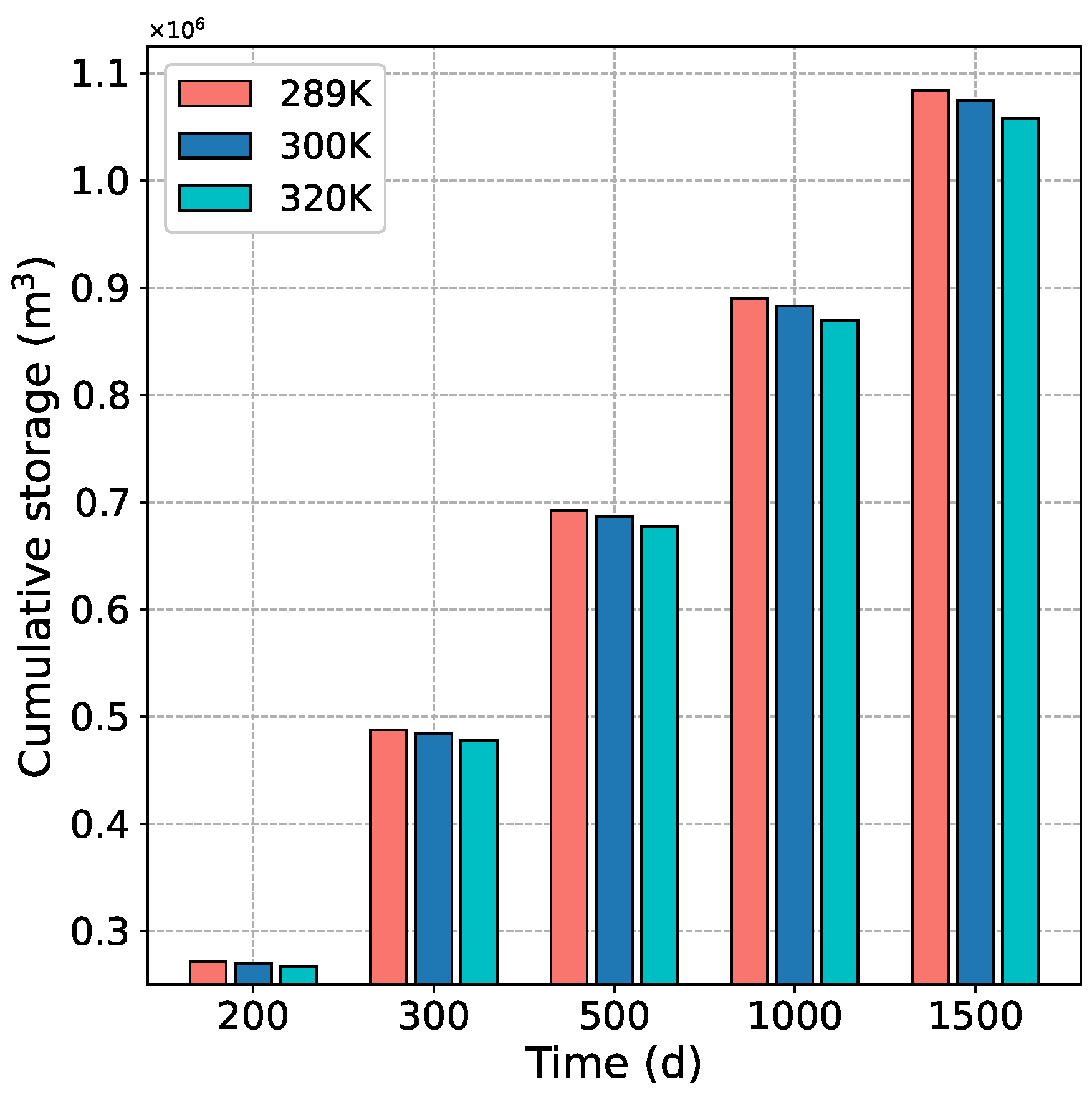
4.3.2. Effect of Temperature on CH Production and CO Storage
5. Conclusions
- During direct mining, the reservoir pressure shows a downward trend, and the downward trend gradually slows down and tends to be stable. During CO injection, the reservoir pressure increases with time, increases rapidly at the initial stage and gradually tends to be stable. The injection of CO has a significant enhancement effect on coalbed methane extraction.
- For direct mining, the increase in permeability caused by matrix shrinkage deformation dominates, and the reservoir permeability gradually increases with time, and the closer to the production well, the greater the permeability. For CO injection, the volumetric strain effect caused by adsorption and desorption is dominant in both production wells and injection wells. The permeability near the injection well decreases rapidly and the permeability near the production well increases.
- Increasing the gas injection pressure can promote the production of CH and the storage of CO, but the increase of gas injection pressure will also lead to the rapid attenuation of near-well reservoir permeability and the weakening of injection capacity. When the gas injection pressure reaches 3MPa, the injection pressure continues to increase, and the recovery degree does not change much.
- When the injection temperature increases, the CO concentration is relatively reduced, and the replacement effect of CH is weakened, resulting in a slight decrease in CBM production and CO burial stock.
6. Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, G.; Hao, T.; Zhu, J. Discussion on action strategies of China’s carbon peak and carbon neutrality. Bull. Chin. Acad. Sci. 2022, 37, 423–434. [Google Scholar]
- Yao, J.; Han, H.; Yang, Y.; Song, Y.; Li, G. A Review of Recent Progress of Carbon Capture, Utilization, and Storage (CCUS) in China. Appl. Sci. 2023, 13, 1169. [Google Scholar] [CrossRef]
- Sarkodie, S.A.; Strezov, V. Effect of foreign direct investments, economic development and energy consumption on greenhouse gas emissions in developing countries. Sci. Total Environ. 2019, 646, 862–871. [Google Scholar] [CrossRef]
- Adedoyin, F.F.; Gumede, M.I.; Bekun, F.V.; Etokakpan, M.U.; Balsalobre-Lorente, D. Modelling coal rent, economic growth and CO2 emissions: Does regulatory quality matter in BRICS economies? Sci. Total Environ. 2020, 710, 136284. [Google Scholar] [CrossRef]
- Xian, Z. The Application Prospect of CCUS in China under the Target of Carbon Neutrality. China Sustain. Trib. 2020, 12, 22–24. [Google Scholar]
- Zhao, X.; Liao, X.; He, L. The evaluation methods for CO2 storage in coal beds, in China. J. Energy Inst. 2016, 89, 389–399. [Google Scholar] [CrossRef]
- Shu, S.; Liu, L.; Wang, W. Theory and Evaluation of the Effectiveness of Deep Coalbed CO2 Geological Storage and Enhanced Coalbed Methane Development, 1st ed.; Science Press: Beijing, China, 2020. [Google Scholar]
- Dutka, B. CO2 and CH4 sorption properties of granular coal briquettes under in situ states. Fuel 2019, 247, 228–236. [Google Scholar] [CrossRef]
- Sun, X.; Yao, Y.; Liu, D. The behavior and efficiency of methane displaced by CO2 in different coals and experimental conditions. J. Nat. Gas Sci. Eng. 2021, 93, 104032. [Google Scholar] [CrossRef]
- Sijian, Z.; Shuxun, S.; Yanbin, Y.; Dameng, L.; Shiqi, L. Characterization of CO2 adsorption and displacement of methane in coals: An NMR relaxation method. Coal J. 2022, 47, 3738–3745. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Yu, J. Experimental Study of the Radial Multi-Scale Dynamic Diffusion Model for Gas-Bearing Coal. Min. Miner. Depos. 2022, 16, 80–86. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, M.; Li, X.; Liu, Y. Impact research of CH4 replacement with CO2 in hydrous coal under high pressure injection. Min. Miner. Depos. 2022, 16, 121–126. [Google Scholar] [CrossRef]
- Michael, G.; Anders, D.E.; Law, B.E. Geochemical Evaluation of Upper Cretaceous Fruitland Formation Coals, San Juan Basin, New Mexico and Colorado. Org. Geochem. 1993, 20, 475–498. [Google Scholar] [CrossRef]
- Morse, D.G.; Mastalerz, M.; Drobniak, A.; Rupp, J.A.; Harpalani, S. Variations in Coal Characteristics and Their Possible Implications for CO2 Sequestration: Tanquary Injection Site, Southeastern Illinois, USA. Int. J. Coal Geol. 2010, 84, 25–38. [Google Scholar] [CrossRef]
- Eble, C.F.; Greb, S.F. Palynologic, Petrographic and Geochemical Composition of the Vancleve Coal Bed in Its Type Area, Eastern Kentucky Coal Field, Central Appalachian Basin. Int. J. Coal Geol. 2016, 158, 1–12. [Google Scholar] [CrossRef]
- Botnen, L.S.; Fisher, D.W.; Dobroskok, A.A.; Bratton, T.R.; Greaves, K.H.; Robert McLendon, T.; Steiner, G.; Sorensen, J.A.; Steadman, E.N.; Harju, J.A. Field Test of CO2 Injection and Storage in Lignite Coal Seam in North Dakota. Energy Procedia 2009, 1, 2013–2019. [Google Scholar] [CrossRef]
- Pashin, J.C.; Clark, P.E.; McIntyre-Redden, M.R.; Carroll, R.E.; Esposito, R.A.; Oudinot, A.Y.; Koperna, G.J. SECARB CO2 Injection Test in Mature Coalbed Methane Reservoirs of the Black Warrior Basin, Blue Creek Field, Alabama. Int. J. Coal Geol. 2015, 144–145, 71–87. [Google Scholar] [CrossRef]
- He, Q.; Mohaghegh, S.D.; Gholami, V. A Field Study on Simulation of CO2 Injection and ECBM Production and Prediction of CO2 Storage Capacity in Unmineable Coal Seam. J. Pet. Eng. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Gentzis, T.; Goodarzi, F.; Cheung, F.; Laggoun-Défarge, F. Coalbed Methane Producibility from the Mannville Coals in Alberta, Canada: A Comparison of Two Areas. Int. J. Coal Geol. 2008, 74, 237–249. [Google Scholar] [CrossRef]
- Weniger, P.; Franců, J.; Hemza, P.; Krooss, B.M. Investigations on the Methane and Carbon Dioxide Sorption Capacity of Coals from the SW Upper Silesian Coal Basin, Czech Republic. Int. J. Coal Geol. 2012, 93, 23–39. [Google Scholar] [CrossRef]
- Fujioka, M.; Yamaguchi, S.; Nako, M. CO2-ECBM Field Tests in the Ishikari Coal Basin of Japan. Int. J. Coal Geol. 2010, 82, 287–298. [Google Scholar] [CrossRef]
- Wong, S.; Law, D.; Deng, X.; Robinson, J.; Kadatz, B.; Gunter, W.D.; Jianping, Y.; Sanli, F.; Zhiqiang, F. Enhanced Coalbed Methane and CO2 Storage in Anthracitic Coals—Micro-pilot Test at South Qinshui, Shanxi, China. Int. J. Greenh. Gas Control 2007, 1, 215–222. [Google Scholar] [CrossRef]
- Jianping, Y.; Zhang, B.; Wong, S. Test of and Evaluation on Elevation of Coalbed Methane Recovery Ratio by Injecting and Burying CO2 for 3#Coal Seam of North Section of Shizhuang, Qingshui Basin, Shanxi. Strateg. Study CAE 2012, 14, 38–44. [Google Scholar]
- Pan, Z.; Connell, L.; Shangzhi, M.; Sander, R.; Camilleri, M.; Down, D.I.; Carras, J.; Lu, M.; Xiaokang, F.; Wenzhong, Z.; et al. CO2 Injectivity in a Multi-lateral Horizontal Well in a Low Permeability Coal Seam: Results from a Field Trial. Energy Procedia 2013, 37, 5834–5841. [Google Scholar] [CrossRef]
- Vishal, V.; Singh, L.; Pradhan, S.P.; Singh, T.N.; Ranjith, P. Numerical modeling of Gondwana coal seams in India as coalbed methane reservoirs substituted for carbon dioxide sequestration. Energy 2013, 49, 384–394. [Google Scholar] [CrossRef]
- Siriwardane, H.J.; Bowes, B.D.; Bromhal, G.S.; Gondle, R.K.; Wells, A.W.; Strazisar, B.R. Modeling of CBM production, CO2 injection, and tracer movement at a field CO2 sequestration site. Int. J. Coal Geol. 2012, 96, 120–136. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Elsworth, D.; Chen, Z.; Connell, L.; Pan, Z. Dual Poroelastic Response of a Coal Seam to CO2 Injection. Int. J. Greenh. Gas Control 2010, 4, 668–678. [Google Scholar] [CrossRef]
- Yongkang, W. Experiment and Numerical Simulation Analysis of Displacing CH4 by CO2 Injection. Master’s Thesis, China University of Mining & Technology, Beijing, China, 2016. [Google Scholar]
- Fang, H.H.; Sang, S.X.; Liu, S.Q. Numerical simulation of enhancing coalbed methane recovery by injecting CO2 with heat injection. Pet. Sci. 2019, 16, 32–43. [Google Scholar] [CrossRef]
- Fang, H.; Sang, S.; Liu, S. Establishment of dynamic permeability model of coal reservoir and its numerical simulation during the CO2-ECBM process. J. Pet. Sci. Eng. 2019, 179, 885–898. [Google Scholar] [CrossRef]
- Kumar, H.; Elsworth, D.; Mathews, J.P.; Liu, J.; Pone, D. Effect of CO2 injection on heterogeneously permeable coalbed reservoirs. Fuel 2014, 135, 509–521. [Google Scholar] [CrossRef]
- Wang, G.; Wang, K.; Jiang, Y.; Wang, S. Reservoir permeability evolution during the process of CO2-enhanced coalbed methane recovery. Energies 2018, 11, 2996. [Google Scholar] [CrossRef]
- Gang, B.A.I.; Xihua, Z.; Shiping, W.E.I.; Chaojun, F.A.N.; Xueming, L.I. Simulation and Test of Enhancement of Gas Drainage through CO2 Injection into Coal Seam of Low Permeability. Coal Geol. Explor. 2019, 47, 77–84. [Google Scholar]
- Su, E.; Liang, Y.; Zou, Q.; Xu, M.; Sasmito, A.P. Numerical analysis of permeability rebound and recovery during coalbed methane extraction: Implications for CO2 injection methods. Process. Saf. Environ. Prot. 2021, 149, 93–104. [Google Scholar] [CrossRef]
- Yudong, H.; Saipeng, H.; Jian, H.; Xingbin, L.; Lianfu, H.; Changfeng, F. Numerical Simulation of the Effect of Injected CO2 Temperature and Pressure on CO2-Enhanced Coalbed Methane. Appl. Sci. 2020, 10, 1385. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y. Some Suggestions on the Construction of an Integrated Gas Supply Security Index in China. Nat. Gas Ind. 2015, 35, 125–128. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Elsworth, D.; Connell, L.D.; Pan, Z. Impact of CO2 Injection and Differential Deformation on CO2 Injectivity under In-Situ Stress Conditions. Int. J. Coal Geol. 2010, 81, 97–108. [Google Scholar] [CrossRef]
- Yin, G.; Deng, B.; Li, M.; Zhang, D.; Wang, W.; Li, W.; Shang, D. Impact of Injection Pressure on CO2–Enhanced Coalbed Methane Recovery Considering Mass Transfer between Coal Fracture and Matrix. Fuel 2017, 196, 288–297. [Google Scholar] [CrossRef]
- Yin, G.Z.; Li, M.H.; Li, S.Z.; Li, W.P.; Yao, J.W.; Zhang, Q.G. 3D Numerical Simulation of Gas Drainage from Boreholes Based on Solid Gas Coupling Model of Coal Containing Gas. J. China Coal Soc. 2013, 38, 535–541. [Google Scholar]
- Fan, Y.; Deng, C.; Zhang, X.; Li, F.; Wang, X.; Qiao, L. Numerical Study of CO2-enhanced Coalbed Methane Recovery. Int. J. Greenh. Gas Control 2018, 76, 12–23. [Google Scholar] [CrossRef]
- Tao, Y.; Xu, J.; Cheng, M.; Li, S.; Peng, S. Theoretical Analysis and Experimental Study on Permeability of Gas-Bearing Coal. Yanshilixue Yu Gongcheng Xuebao/Chin. J. Rock Mech. Eng. 2009, 28, 3363–3370. [Google Scholar]
- Kong, X.; Wang, E.; Liu, Q.; Li, Z.; Li, D.; Cao, Z.; Niu, Y. Dynamic Permeability and Porosity Evolution of Coal Seam Rich in CBM Based on the Flow-Solid Coupling Theory. J. Nat. Gas Sci. Eng. 2017, 40, 61–71. [Google Scholar] [CrossRef]
- Chilingar, G.V. Relationship Between Porosity, Permeability, and Grain-Size Distribution of Sands and Sandstones. In Developments in Sedimentology; Deltaic and Shallow Marine Deposits; van Straaten, L.M.J.U., Ed.; Elsevier: Amsterdam, The Netherlands, 1964; Volume 1, pp. 71–75. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Huber, M.L.; McLinden, M.O. NIST reference fluid thermodynamic and transport properties–REFPROP. NIST Stand. Ref. Database 2002, 23, v7. [Google Scholar]
- Gou, S.; Li, H.; Li, J.; Zhao, Y. Effective Means to Alleviate the Greenhouse Effect: Case Study of History Match Simulations on a Brief CO2 Injection into Less-Deep Low-Rank Coal Seams. Arab. J. Geosci. 2021, 14, 803. [Google Scholar] [CrossRef]
- Niu, Q.; Cao, L.; Sang, S.; Zhou, X.; Wang, W.; Yuan, W.; Ji, Z.; Wang, H.; Nie, Y. Study on the anisotropic permeability in different rank coals under influences of supercritical CO2 adsorption and effective stress and its enlightenment for CO2 enhance coalbed methane recovery. Fuel 2020, 262, 116515. [Google Scholar] [CrossRef]
- Palmer, I.; Mansoori, J. How permeability depends on stress and pore pressure in coalbeds: A new model. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 6–9 October 1996. [Google Scholar]
- Gao, T.; Zhao, D.; Wang, C.; Feng, Z. Energy variation in coal samples with different particle sizes in the process of adsorption and desorption. J. Pet. Sci. Eng. 2020, 188, 106932. [Google Scholar] [CrossRef]
- Perrot, P. A to Z of Thermodynamics; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Maxwell, J.C. Theory of Heat; Longmans, Green, and Company: Harlow, UK, 1872. [Google Scholar]
- Younes, K.; Grasset, L. Comparison of thermochemolysis and classical chemical degradation and extraction methods for the analysis of carbohydrates, lignin and lipids in a peat bog. J. Anal. Appl. Pyrolysis 2018, 134, 61–72. [Google Scholar] [CrossRef]
- Younes, K.; Grasset, L. Analysis of molecular proxies of a peat core by thermally assisted hydrolysis and methylation-gas chromatography combined with multivariate analysis. J. Anal. Appl. Pyrolysis 2017, 124, 726–732. [Google Scholar] [CrossRef]
- Younes, K.; Laduranty, J.; Descostes, M.; Grasset, L. Molecular biomarkers study of an ombrotrophic peatland impacted by an anthropogenic clay deposit. Org. Geochem. 2017, 105, 20–32. [Google Scholar] [CrossRef]
- Younes, K.; Grasset, L. The application of DFRC method for the analysis of carbohydrates in a peat bog: Validation and comparison with conventional chemical and thermochemical degradation techniques. Chem. Geol. 2020, 545, 119644. [Google Scholar] [CrossRef]
- Younes, K.; Grasset, L. Carbohydrates as proxies in ombrotrophic peatland: DFRC molecular method coupled with PCA. Chem. Geol. 2022, 606, 120994. [Google Scholar] [CrossRef]




| Country | Project | Burial Depth (m) | Coal Rank | Permeability (md) | Reference |
|---|---|---|---|---|---|
| U.S.A | Allison Unit, San Juan Basin | 945 | Bituminous coal | 30–150 | |
| Pump Canyon, San Juan Basin | 918 | Bituminous coal | 146–550 | [13] | |
| Tanquary Farms test site, lllinois Basin | 274 | Bituminous coal | 2–7.0 | [14] | |
| Virginia Central Appalachian Basin Coal Test | 427–671 | Bituminous coal | 5–20 | [15] | |
| Lignite Field Validation Test, Williston Basin | 335 | Lignite | 1 | [16] | |
| Black Warrior Basin Coal Test | 548 437 237 | Bituminous coal | 0.1–0.2 10–16 | [17] | |
| Marshall County project, Northern Appalachian Basin | 400–530 | Bituminous coal | 1 | [18] | |
| Buchanan County, Central Appalachian Basin | 274–671 | Bituminous coal | 5–27 | ||
| Canada | FBV 4A Micro-Pilot Test, Western Canada Sedimentary Basin | Bituminous coal | 3.65 | [19] | |
| ARC ECBM | |||||
| CSEMP | 420 | Sub-Bituminous coal | |||
| Poland | RECOPOL, upper Silesian coalbasin, Poland | 1012–1076 | Bituminous coal | 0.4–1.5 | [20] |
| Japan | Yubari field test, Ishikari Coal Basin, Japan | 890 | Bituminous coal | 1 | [21] |
| China | Qinshui Basin ECBM 2004 | 472 | Anthracite | 12.0/0.95 | [22] |
| Qinshui Basin ECBM 2010 | 923 | Anthracite | 0.002–0.8 | [23] | |
| APP ECBM, Ordos Basin | 560 | Bituminous coal | 0.64 | [24] | |
| Qinshui Basin multiple wells | 972 | Anthracite | 0.002–0.8 |
| CH | ||||
|---|---|---|---|---|
| −12.08 | 1547 | −0.006677 | 1.501 × 10 | |
| 2.06 × 10 | 0.0002031 | 6.235 × 10 | −1.858 × 10 | |
| 0.0408 | 2.282 | 0.0001361 | 3.206 × 10 | |
| 1.205 × 10 | 2.65 × 10 | 3.108 × 10 | 9.262 × 10 | |
| −4.75 × 10 | −4.981 × 10 | 4.881 × 10 | 4.895 × 10 | |
| - | −1.045 × 10 | 1.269 × 10 | 2.735 × 10 | |
| - | −7.677 × 10 | −9.47 × 10 | −2.724 × 10 | |
| 0.9999 | 0.9995 | 0.9999 | 0.9999 |
| CO | ||||
|---|---|---|---|---|
| −1026 | 781.6 | −0.00621 | 5.872 × 10 | |
| 0.0002926 | −0.0009226 | −4.58 × 10 | −6.52 × 10 | |
| 8.73 | 0.1969 | 7.646 × 10 | 4.808 × 10 | |
| 5.988 × 10 | 6.334 × 10 | 2.761 × 10 | 4.66 × 10 | |
| −1.027 × 10 | 3.455 × 10 | 1.745 × 10 | 2.529 × 10 | |
| - | 9.827 × 10 | 5.218 × 10 | 1.044 × 10 | |
| - | −2.217 × 10 | −9.643 × 10 | −1.573 × 10 | |
| 0.9993 | 0.9867 | 0.8925 | 0.9994 |
| Plan | Model | Coupled Mode | Question |
|---|---|---|---|
| A | H-M coupling | Effect of Injection on Exploitation | |
| H-M coupling | |||
| B | injection pressure: 2 MPa | T-H-M coupling | Effect of Injection Pressure on |
| injection pressure: 4 MPa | |||
| injection pressure: 6 MPa | |||
| C | injection temperature: 289 K | T-H-M coupling | The effect of injection well temperature on |
| injection temperature: 300 K | |||
| injection temperature: 320 K |
| Parameter (Unit) | Value | Parameter (Unit) | Value |
|---|---|---|---|
| Thermal expansion coefficienta (K) | 2.4 × 10 | Langmuir volumetric strain constant of CH (-) | 0.0128 |
| Universal gas constant (J/(K·mol)) | 8.314 | Langmuir volumetric strain constant of CO (-) | 0.0237 |
| Initial porosity (-) | 0.08 | Langmuir pressure of CH (MPa) | 3.82 |
| Initial permeability of coalbed (mD) | 0.5 | Langmuir pressure of CO (MPa) | 2.35 |
| Density of coal (kg/m) | 1785 | Langmuir volume constant of CH (m/kg) | 0.01237 |
| Coalbed temperature (K) | (289) | Langmuir volume constant of CO (m/kg) | 0.04607 |
| Specific heat capacity of coal (J/(K·kg)) | 1255 | Hydrodynamic dispersion coefficient of CH (m/s) | 3.6 × 10 |
| Initial coalbed pressure (MPa) | 1.6 | Hydrodynamic dispersion coefficient of CO (m/s) | 5.8 × 10 |
| Thermal conductivity of coal (W/(m·K)) | 0.12 | Bulk modulus of coal (MPa) | 2500 |
| Poisson’s ratio | 0.25 | Bulk modulus of coal skeleton (MPa) | 8134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Li, H.; Ji, K.; Huang, F. Thermal-Hydraulic-Mechanical Coupling Simulation of CO2 Enhanced Coalbed Methane Recovery with Regards to Low-Rank but Relatively Shallow Coal Seams. Appl. Sci. 2023, 13, 2592. https://doi.org/10.3390/app13042592
Ma Q, Li H, Ji K, Huang F. Thermal-Hydraulic-Mechanical Coupling Simulation of CO2 Enhanced Coalbed Methane Recovery with Regards to Low-Rank but Relatively Shallow Coal Seams. Applied Sciences. 2023; 13(4):2592. https://doi.org/10.3390/app13042592
Chicago/Turabian StyleMa, Qianqian, Hong Li, Kun Ji, and Fengjun Huang. 2023. "Thermal-Hydraulic-Mechanical Coupling Simulation of CO2 Enhanced Coalbed Methane Recovery with Regards to Low-Rank but Relatively Shallow Coal Seams" Applied Sciences 13, no. 4: 2592. https://doi.org/10.3390/app13042592
APA StyleMa, Q., Li, H., Ji, K., & Huang, F. (2023). Thermal-Hydraulic-Mechanical Coupling Simulation of CO2 Enhanced Coalbed Methane Recovery with Regards to Low-Rank but Relatively Shallow Coal Seams. Applied Sciences, 13(4), 2592. https://doi.org/10.3390/app13042592






