Effects of Warming and Drought Stress on the Coupling of Photosynthesis and Transpiration in Winter Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
3. Measurements
3.1. Photosynthesis and Transpiration
3.2. Stomatal Morphology of Leaves
4. Model and Calculation Principle
4.1. Ball–Berry Model
4.2. Jarvis Model
4.3. Penman–Monteith Model
4.4. Model Calibration and Validation
4.5. Statistical Analysis
5. Results
5.1. Effects of Warming and Drought Stress on Stomatal Morphology of Winter Wheat Leaves
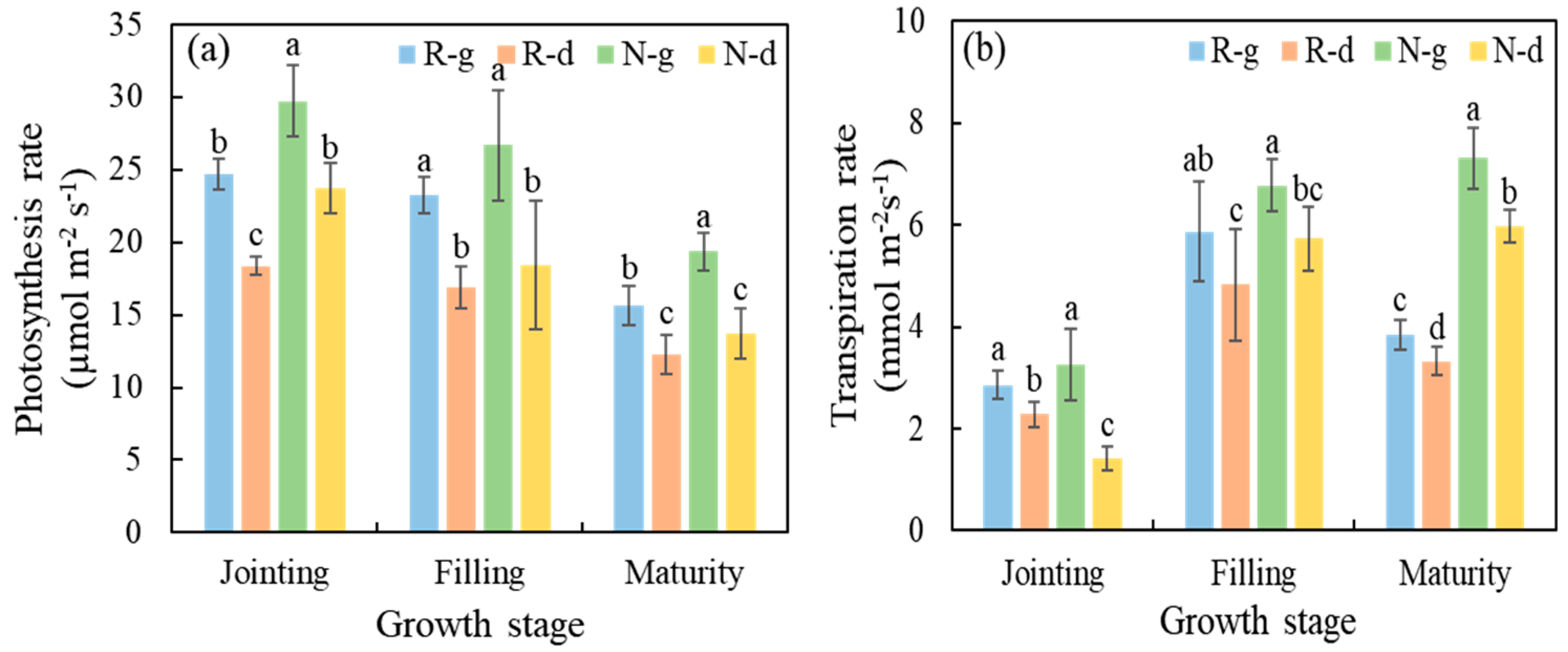
5.2. Effects of Warming and Drought Stress on Stomatal Conductance, Photosynthetic Rate, and Transpiration Rate of Winter Wheat
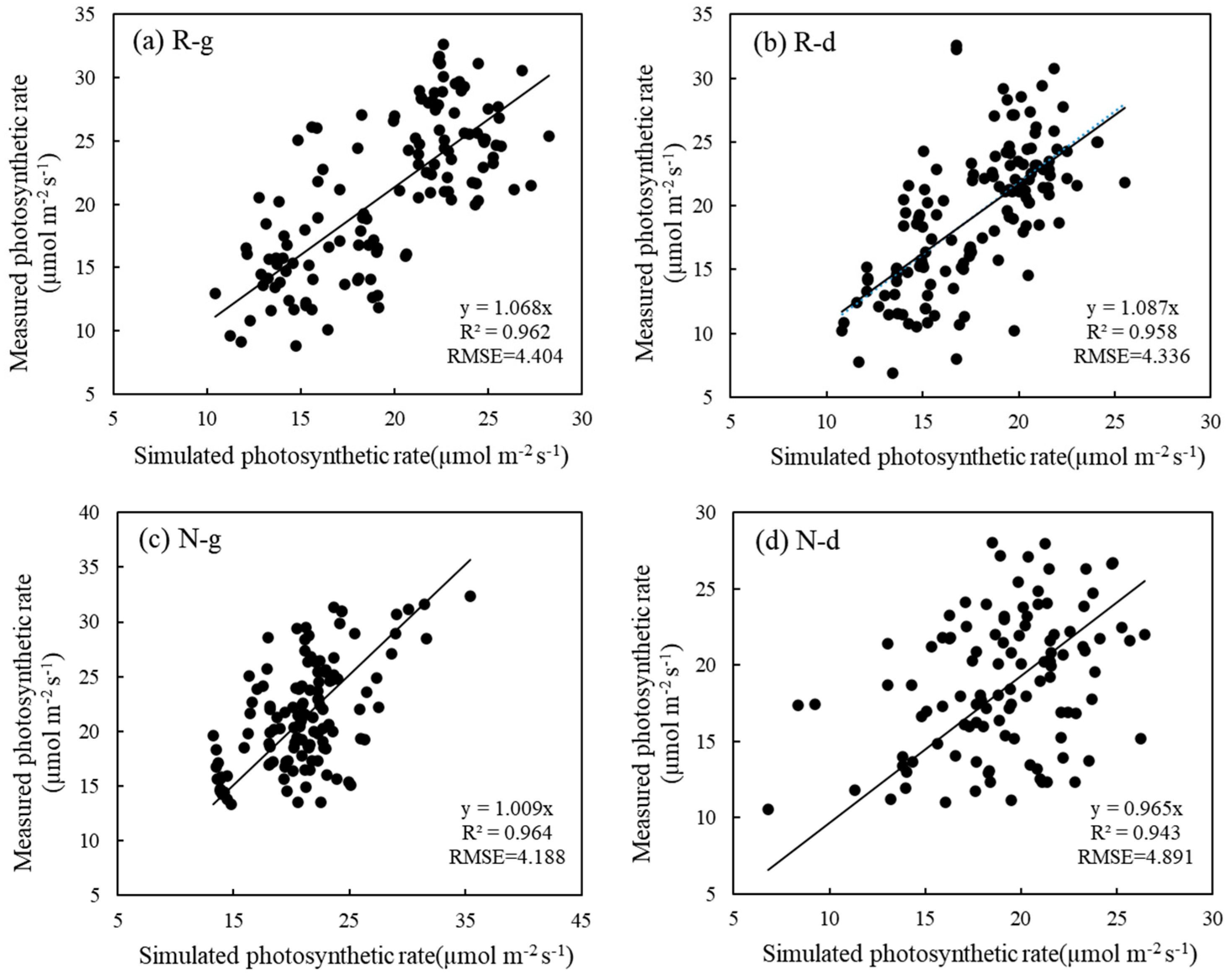
5.3. Effects of Warming and Drought Stress on Photosynthetic Rate Were Simulated Based on the Ball–Berry Model
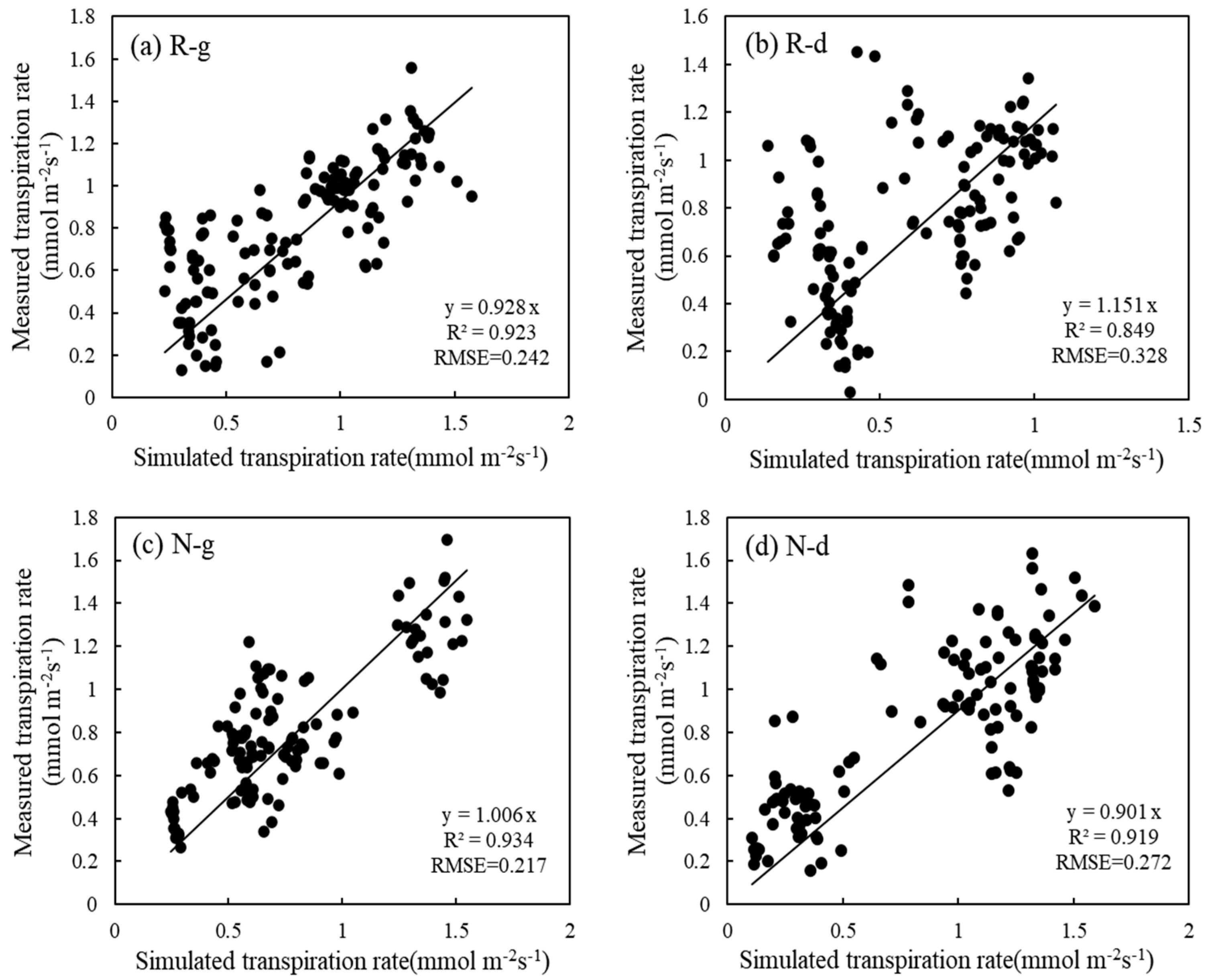
5.4. Effects of Warming and Drought Stress on Transpiration Rate Simulated by the Penman–Monteith Model
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, D.L.; Nadal, M. Linking water relations and hydraulics with photosynthesis. Plant J. Cell Mol. Biol. 2020, 101, 800–815. [Google Scholar] [CrossRef]
- Wang, X.X.; Du, T.T.; Huang, J.L.; Peng, S.B.; Xiong, D.L. Leaf hydraulic vulnerability triggers the decline in stomatal and mesophyll conductance during drought in rice. J. Exp. Bot. 2018, 69, 4033–4045. [Google Scholar] [CrossRef]
- Edwards, C.E.; Ewers, B.E.; McClung, C.R.; Lou, P.; Weinig, C. Quantitative variation in water-use efficiency across water regimes and its relationship with circadian, vegetative, reproductive, and leaf gas-exchange traits. Mol. Plant 2012, 5, 653–668. [Google Scholar] [CrossRef]
- Deng, J.M.; Wang, G.X.; Morris, E.C.; Wei, X.P.; Li, D.X.; Chen, B.M.; Zhao, C.M.; Liu, J.; Wang, Y. Plant Mass: Density Relationship along a Moisture Gradient in North-West China. J. Ecol. 2006, 94, 953–958. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Skirycz, A.; Inzé, D. More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Sharkey, T.D. Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ. 2005, 28, 269–277. [Google Scholar] [CrossRef]
- Flexas, J.; Diaz-Espejo, A.; Berry, J.A.; Cifre, J.; Galmés, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbó, M. Analysis of leakage in IRGA’s leaf chambers of open gas exchange systems: Quantification and its effects in photosynthesis parameterization. J. Exp. Bot. 2007, 58, 1533–1543. [Google Scholar] [CrossRef]
- Lawler, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiol. Plant. 2004, 120, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.V.; Pisipati, S.R.; Ristic, Z.; Bukovnik, U.; Fritz, A.K. Impact of nighttime temperature on physiology and growth of spring wheat. Crop Sci. 2008, 48, 2372–2380. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Han, A.R.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Casson, S.; Gray, J.E. Influence of environmental factors on stomatal development. New Phytol. 2008, 178, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Kumagai, T.; Yoshifuji, N.; Sato, T.; Tanaka, N.; Tanaka, K.; Suzuki, M.; Tantasirin, C. Environmental control of canopy stomatal conductance in a tropical deciduous forest in northern Thailand. Agric. For. Meteorol. 2015, 202, 1–10. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, L.H.; Zhou, Q.; Huang, X.H. Stomatal and non-stomatal factors regulated the photosynthesis of soybean seedlings in the present of exogenous bisphenol A. Ecotoxicol. Environ. Saf. 2017, 145, 150–160. [Google Scholar] [CrossRef]
- Berry, J.A.; Beerling, D.J.; Franks, P.J. Stomata: Key players in the earth system, past and present. Curr. Opin. Plant Biol. 2010, 13, 233–239. [Google Scholar] [CrossRef]
- Yu, G.R.; Song, X.; Wang, Q.; Liu, Y.; Guan, D.; Yan, J.; Sun, X. Water-use efficiency of forest ecosystems ineastern China and its relations to climatic variables. New Phytol. 2008, 177, 927–937. [Google Scholar] [CrossRef]
- Zhao, F.H.; Yu, G.R. A review on the coupled carbon and water cycles in the terrestrial ecosystems. Prog. Geogr. 2008, 27, 32–38. [Google Scholar]
- Wang, R.Y.; Yang, X.G.; Zhao, H.; Liu, H.Y. Eco-physiological characteristics of wheat leaf photosynthesis and their responses to environmental factors in semiarid rain-fed region. Chin. J. Ecol. 2006, 25, 1161–1166. [Google Scholar]
- Yang, C.; Lu, S.W.; Chen, B.; Gu, J.C.; Li, S.N.; Guo, B.L.; Chu, C.Y. Coupling analysis of diurnal changes of leaf photosynthesis and transpiration of different apricot varieties. J. Inn. Mong. Agric. Univ. (Nat. Sci. Ed.) 2015, 36, 40–47. (In Chinese) [Google Scholar]
- Law, B.E.; Falge, E.; Gu, L.; Baldocchi, D.D.; Bakwin, P.; Berbigier, P.; Davis, K.; Dolman, A.J.; Falk, M.; Fuentes, J.D. Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agric. For. Meteorol. 2002, 113, 97–120. [Google Scholar] [CrossRef]
- Han, T. Study on Coupling Mechanism of Carbon and Water in Typical Plants in Arid Oasis; Lanzhou University: Lanzhou, China, 2020. [Google Scholar]
- Ball, J.T.; Woodrow, I.E.; Berry, J. A model predicting stomatal conductance and its contribution to the control ofphotosynthesis under different environmental conditions. In Progress in Photosynthesis Research; Springer: Dordrecht, The Netherlands, 1987; Volume 4, pp. 221–224. [Google Scholar]
- Leuning, R. A critical appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant Cell Environ. 1995, 18, 339–355. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Lange, O.; Kappen, L.; Buschbom, U.; Evenari, M. Stomatal responses to changes in temperature at increasing water stress. Planta 1973, 110, 29–42. [Google Scholar] [CrossRef]
- Ameye, M.; Wertin, T.M.; Bauweraerts, I.; McGuire, M.A.; Teskey, R.O.; Steppe, K. The effect of induced heat waves on Pinus taeda and Quercus rubra seedlings in ambient and elevated CO2 atmospheres. New Phytol. 2012, 196, 448–461. [Google Scholar] [CrossRef]
- Drake, J.E.; Tjoelker, M.G.; Varhammar, A.; Medlyn, B.E.; Reich, P.B.; Leigh, A.; Pfautsch, S.; Blackman, C.J.; Lopez, R.; Aspinwall, M.J.; et al. Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance Glob. Change Biol. 2018, 24, 2390–2402. [Google Scholar] [CrossRef]
- Ray, D.; Dey, S.K.; Das, G. Significance of the leaf area ratio in Hevea brasiliensis under high irradiance and low temperature stress. Photosynthetica 2004, 42, 93–97. [Google Scholar] [CrossRef]
- Alam, B.; Nair, D.B.; Jacob, J. Low temperature stress modifies the photochemical efficiency of a tropical tree species Hevea brasiliensis: Effects of varying concentration of CO2 and photonflux density. Photosynthetica 2005, 43, 247–252. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Shaheen, M.R.; Ayyub, C.M.; Amjad, M.; Waraich, E.A. Morpho-physiological evaluation of tomato genotypes under high temperature stress conditions. J. Sci. Food Agric. 2016, 96, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Von Caemmerer, S.; Evans, J.R. Temperature responses of mesophyll conductance differ greatly between species. Plant Cell Environ. 2015, 38, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot. 2017, 68, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Tuzet, A.; Perrier, A.; Leuning, R. A coupled model of stomatal conductance, photosynthesis and transpiration. Plant Cell Environ. 2003, 26, 1097–1116. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, Y.Q.; Liu, Y.F.; Shi, P.L. Simulation of the stomatal conductance of winter wheat in response to light, temperature and CO2 changes. Ann. Bot. 2004, 93, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Von Caemmerer, S.; Lawson, T.; Oxborough, K.; Baker, N.R.; Andrews, T.J.; Raines, C.A. Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J. Exp. Bot. 2004, 55, 1157–1166. [Google Scholar] [CrossRef]
- Wang, J.L.; Yu, G.R.; Wang, B.L.; Hua, Q.; Jin, X. Response of photosynthetic rate and stomatal conductance of rice to light intensity and CO2 concentration in Northern China. Acta Phytoecol. Sin. 2005, 29, 16–25. [Google Scholar]
- Hu, X.B.; Lu, X.J.; Yu, Y.; He, K.N. Simulation of canopy conductance of Picea crassifolia based on thermal diffusion method. J. For. Sci. 2018, 54, 8–18. [Google Scholar]
- Leuning, R. Modelling Stomatal Behaviour and and Photosynthesis of Eucalyptus grandis. Funct. Plant Biol. 1990, 17, 159–175. [Google Scholar] [CrossRef]
- Aphalo, P.J.; Jarvis, P.G. An analysis of Ball’s empirical model of stomatal conductance. Ann. Bot. 1993, 72, 321–327. [Google Scholar] [CrossRef]
- Qin, H.; Arteaga, C.; Chowdhury, F.I.; Granda, E.; Yao, Y.; Han, Y.; Resco de Dios, V. Radiation and Drought Impact Residual Leaf Conductance in Two Oak Species with Implications for Water Use Models. Front. Plant Sci. 2020, 11, 1910. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, P.G. The Interpretation of the Variations in Leaf Water Potential and Stomatal Conductance Found in Canopies in the Field. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1976, 273, 593–610. [Google Scholar]
- Li, Y.X.; Lou, Y.S.; Zhang, F.C. Comparison of stomatal conductance models in winter wheat. Chin. J. Agrometeorol. 2011, 32, 106–110. (In Chinese) [Google Scholar]
- Liu, W.N. Studies on Stomatal Conductance and Its Regulation on Canopy Transpiration in the Beijing Mountain Area; Beijing Forestry University: Beijing, China, 2019. [Google Scholar]
- Long, S.P.; Osborne, C.P.; Humphries, S.W. Photosynthesis, rising atmospheric CO2 concentration and climate change. In Scope 56: Global Change; Bremeyer, A., Hall, D.O., Melillo, J., Eds.; Wiley: Chichester, UK, 1997. [Google Scholar]
- Arcus, V.L.; Prentice, E.J.; Hobbs, J.K.; Mulholland, A.J.; Van der Kamp, M.W.; Pudney, C.R.; Parker, E.J.; Schipper, L.A. On the temperature dependence of enzyme catalyzed rates. Biochemistry 2016, 55, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Bhusal, S.J.; Yoon, T.-M. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Porter, J.R.; Gawith, M. Temperatures and the growth and development of wheat: A review. Eur. J. Agron. 1999, 10, 23–36. [Google Scholar] [CrossRef]
- Wheeler, T.R.; Craufurd, P.Q.; Ellis, R.H.; Porter, J.R.; Vara Prasad, P.V. Temperature variability and the yield of annual crops. Agric. Ecosyst. Environ. 2000, 82, 159–167. [Google Scholar] [CrossRef]
- Verma, V.; Foulkes, M.J.; Worland, A.J.; Sylvester-Bradley, R.; Caligari, P.D.S.; Snape, J.W. Mapping quantitative trait loci for flag leaf senescence as a yield determinant in winter wheat under optimal and drought-stressed environments. Euphytica 2004, 135, 255–263. [Google Scholar] [CrossRef]
- Haussmann, B.; Mahalakshmi, V.; Reddy, B.; Seetharama, N.; Hash, C.; Geiger, H. QTL mapping of stay-green in two sorghum recombinant inbred populations. Theor. Appl. Genet. 2002, 106, 133–142. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photo-synthesis of C 3 plants in response to progressive drought: Stomatal conductance as areference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef]
- Bhusal, N.; Bhusal, S.J.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Liu, B.; Liu, F.; Tian, L.; Cao, W.; Zhu, Y.; Asseng, S. Post-heading heat stress and yield impact in winter wheat of China. Glob. Chang. Biol. 2014, 20, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R.; Hayman, P.T.; Richards, R.A.; Passioura, J.B. Opportunities to reduce heat damage in rain-fed wheat crops based on plant breeding and agronomic management. Field Crops Res. 2018, 224, 126–138. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Gao, Y.; Hamani, A.K.M.; Fu, Y.; Liu, J.; Wang, H.; Wang, X. Effects of Warming and Drought Stress on the Coupling of Photosynthesis and Transpiration in Winter Wheat (Triticum aestivum L.). Appl. Sci. 2023, 13, 2759. https://doi.org/10.3390/app13052759
Li Q, Gao Y, Hamani AKM, Fu Y, Liu J, Wang H, Wang X. Effects of Warming and Drought Stress on the Coupling of Photosynthesis and Transpiration in Winter Wheat (Triticum aestivum L.). Applied Sciences. 2023; 13(5):2759. https://doi.org/10.3390/app13052759
Chicago/Turabian StyleLi, Qian, Yang Gao, Abdoul Kader Mounkaila Hamani, Yuanyuan Fu, Junming Liu, Hongbo Wang, and Xingpeng Wang. 2023. "Effects of Warming and Drought Stress on the Coupling of Photosynthesis and Transpiration in Winter Wheat (Triticum aestivum L.)" Applied Sciences 13, no. 5: 2759. https://doi.org/10.3390/app13052759
APA StyleLi, Q., Gao, Y., Hamani, A. K. M., Fu, Y., Liu, J., Wang, H., & Wang, X. (2023). Effects of Warming and Drought Stress on the Coupling of Photosynthesis and Transpiration in Winter Wheat (Triticum aestivum L.). Applied Sciences, 13(5), 2759. https://doi.org/10.3390/app13052759







