Investigation of Annealing Process Effects on the Response and Stability of Sprayed Co2SnO4 Film under Ethanol Vapor
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Co2SnO4 Film Deposition
2.2. Characterization Techniques
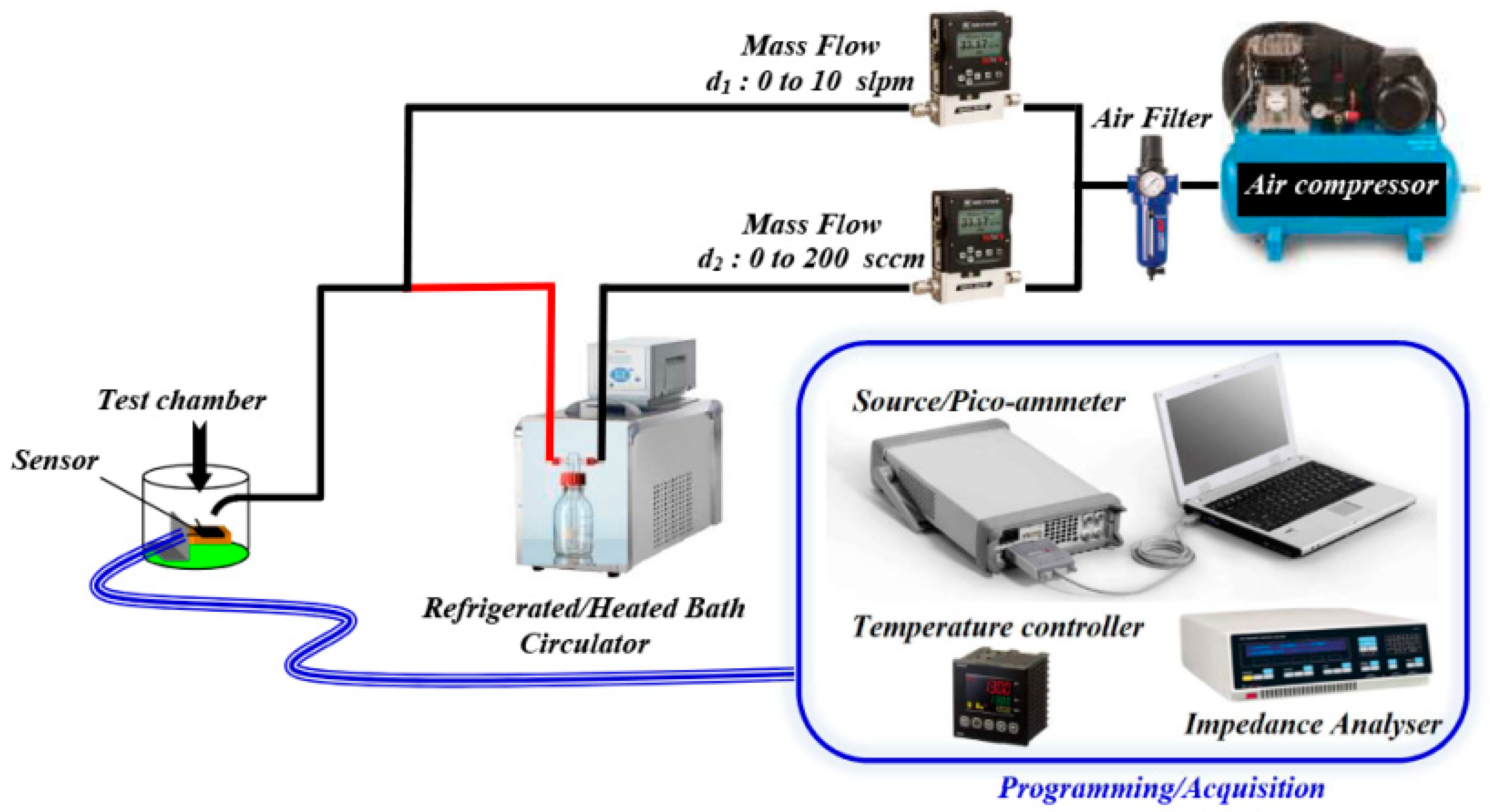
2.3. Experimental Setup for Ethanol Detection
3. Results and Discussion
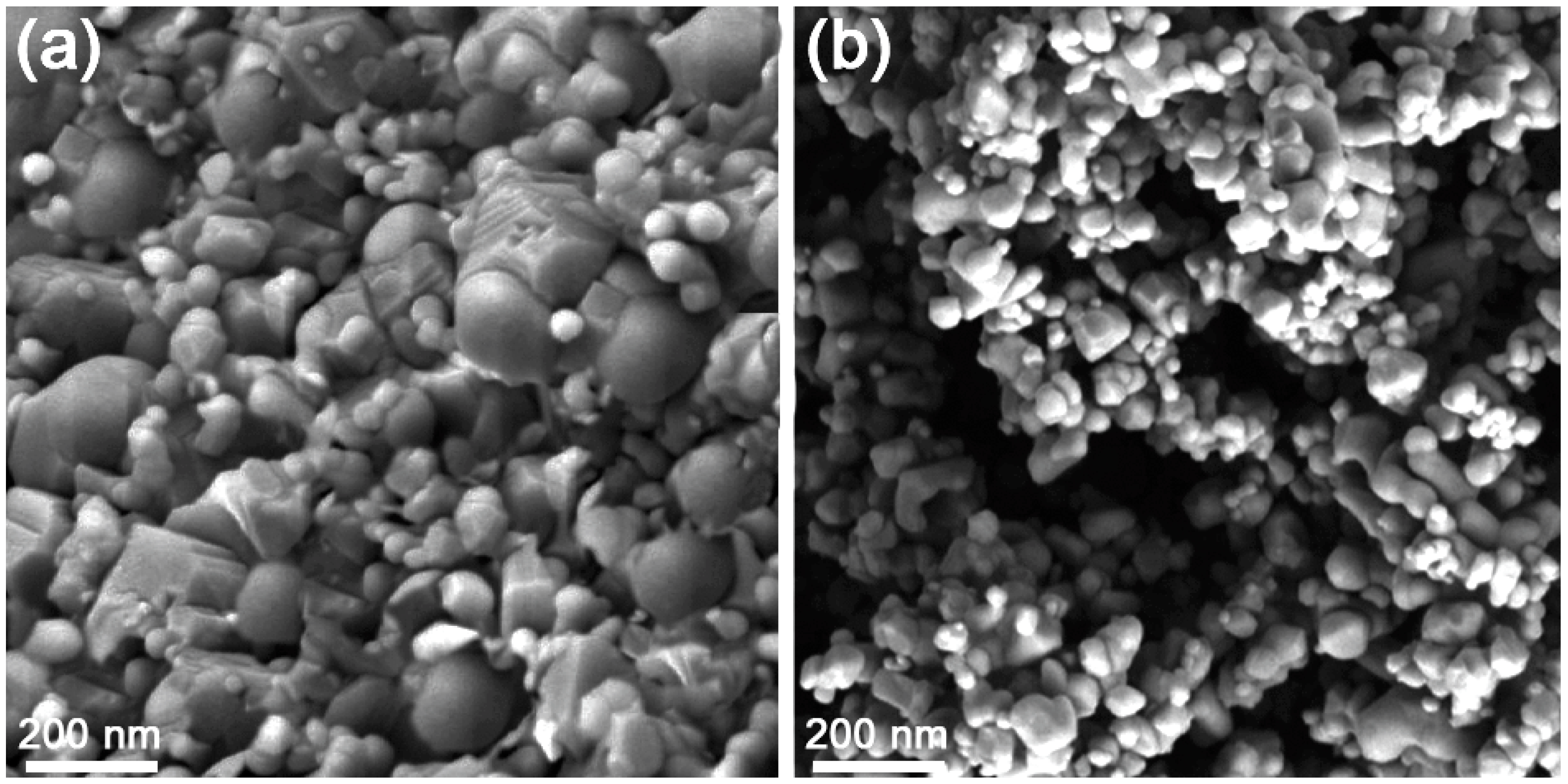
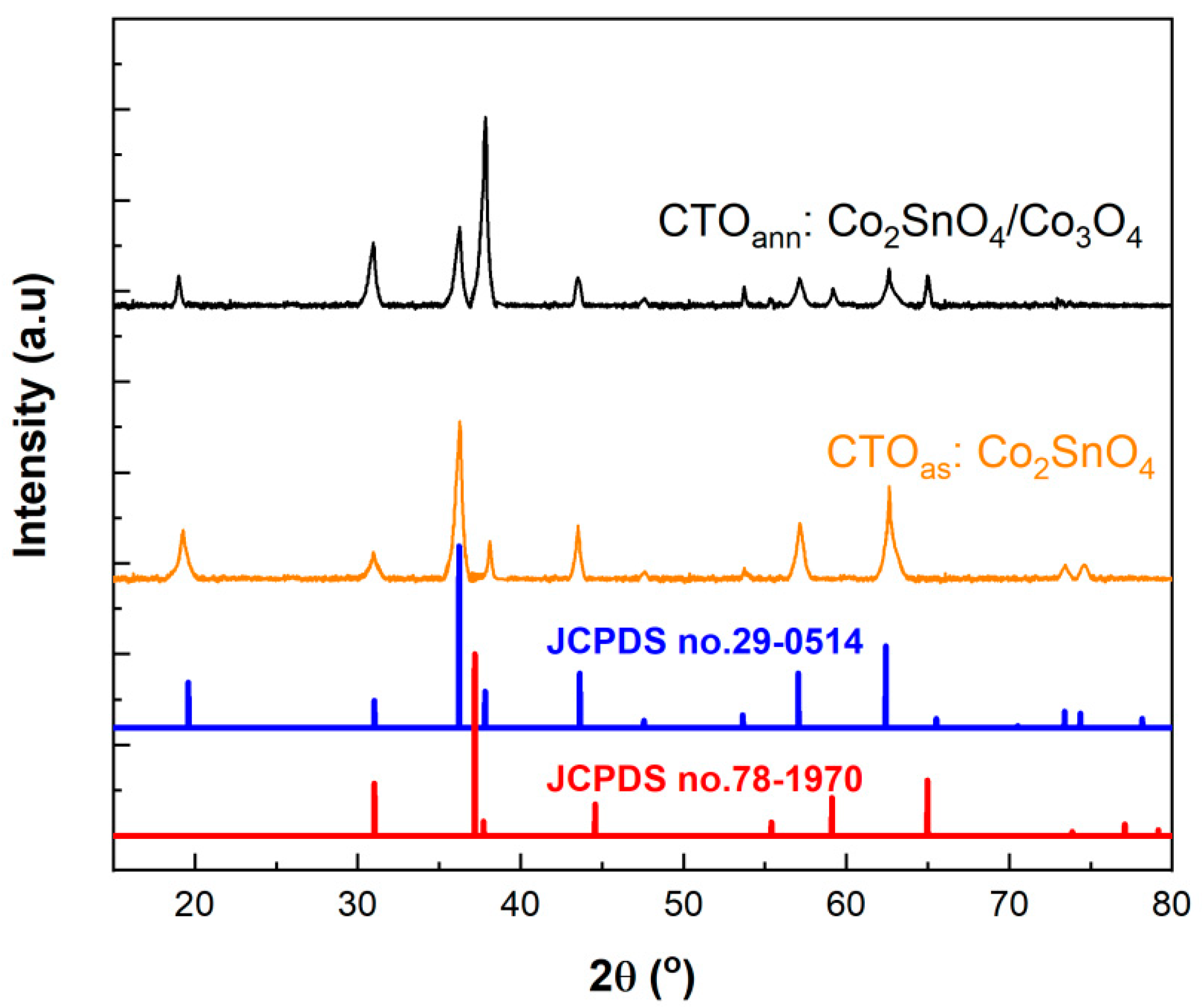
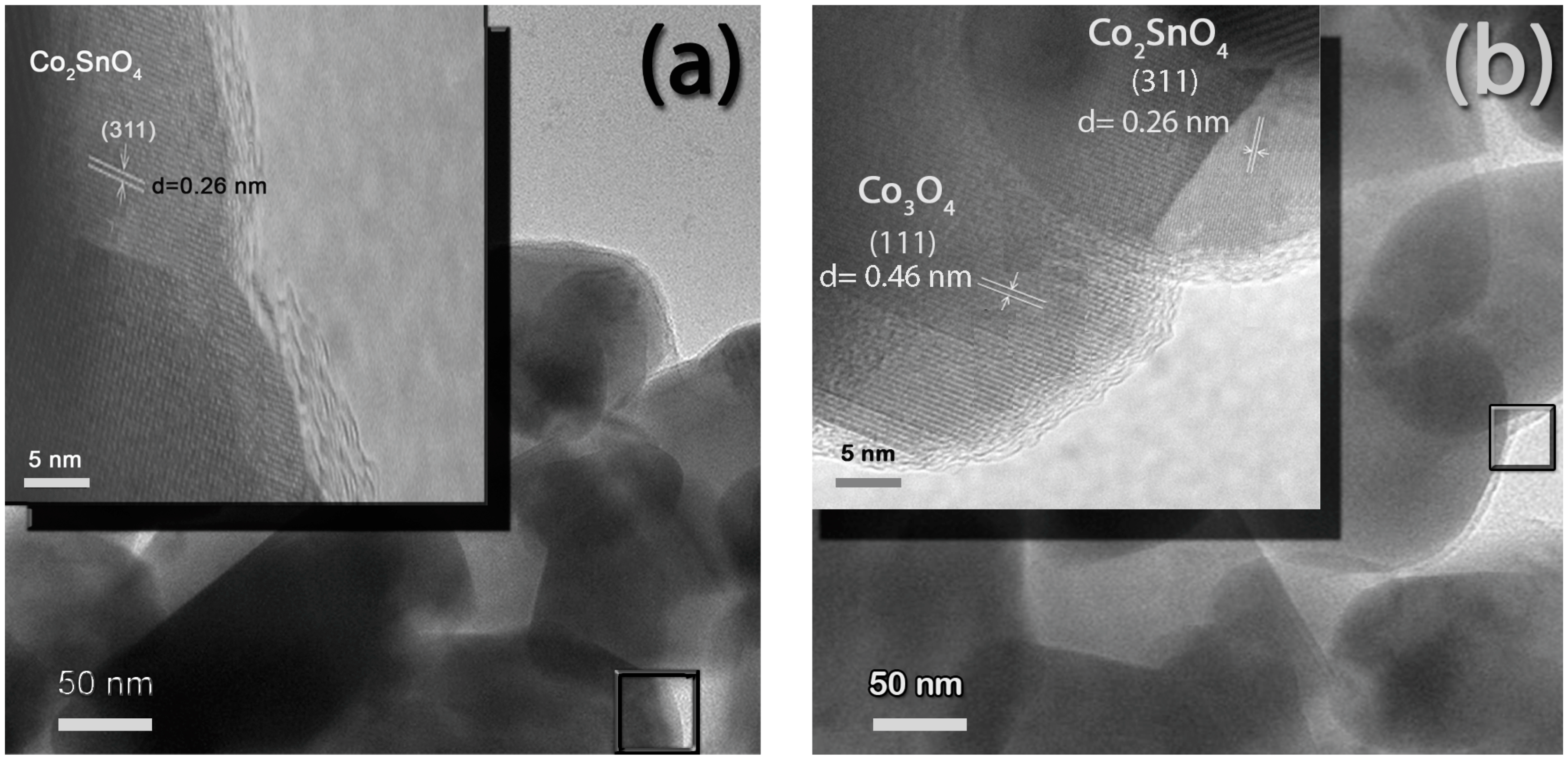
3.1. Characterization of Elaborated Films
3.2. Co2SnO4 and Co2SnO4/Co3O4 Sensing Results and Discussion under Ethanol
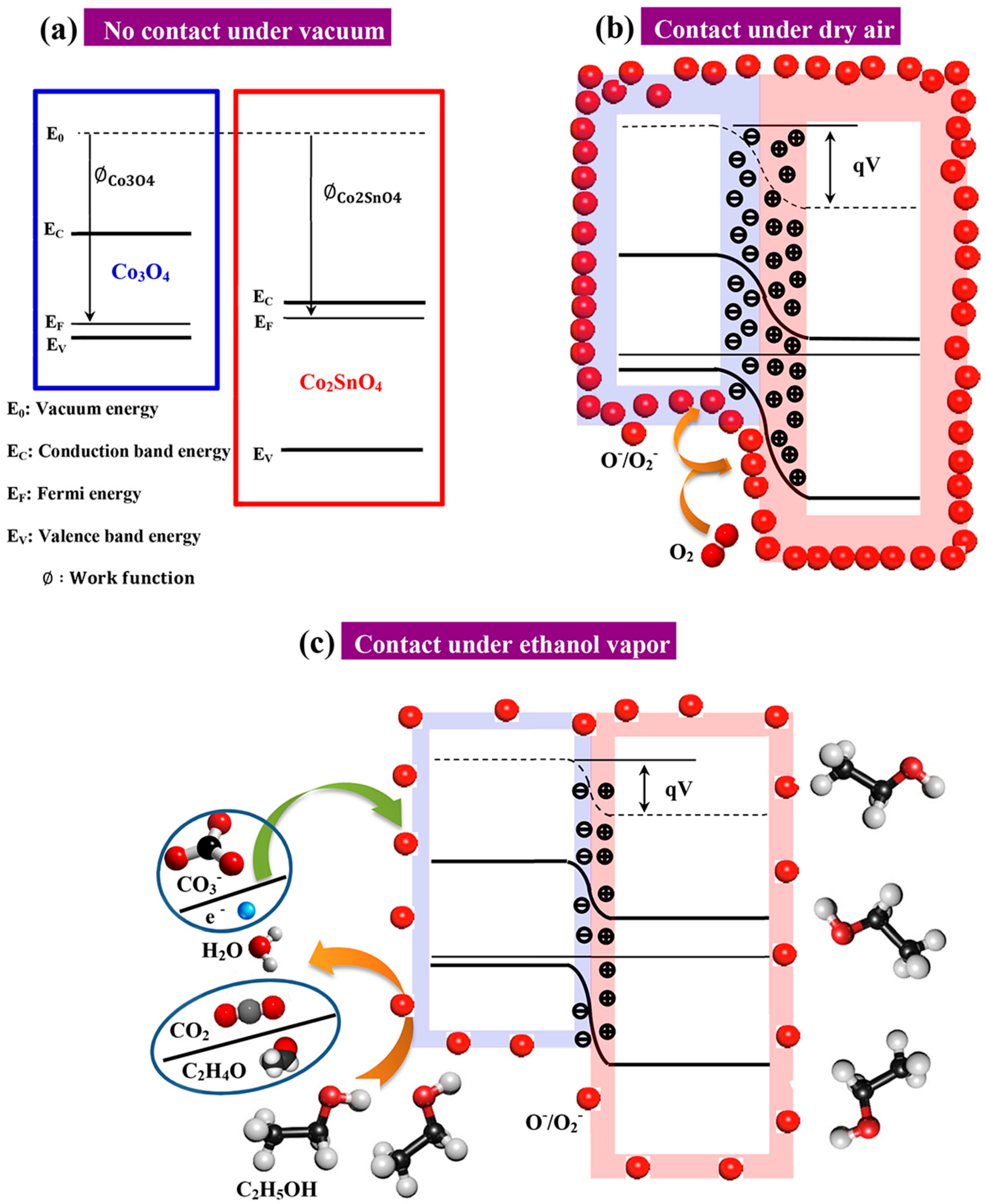
3.2.1. Detection Mechanism under Ethanol
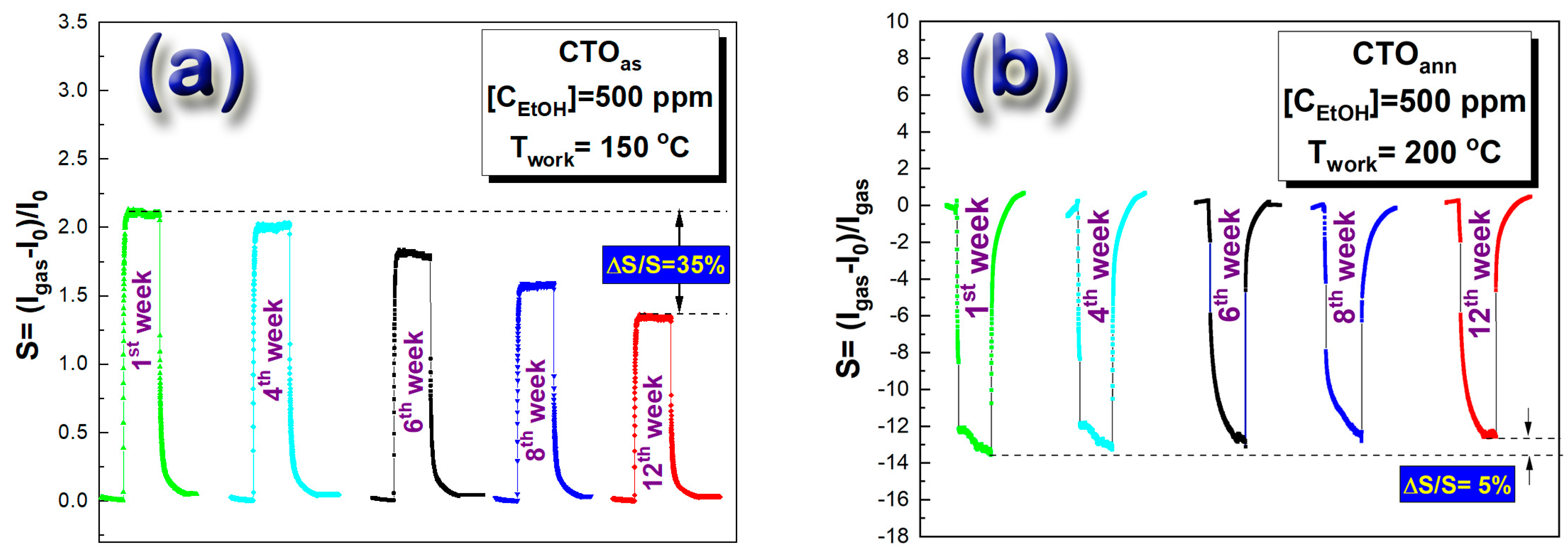
3.2.2. Response Stability under Ethanol
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaneti, Y.V.; Moriceau, J.; Liu, M.; Yuan, Y.; Quadir, M.Z.; Jiang, X.; Yu, A. Hydrothermal synthesis of ternary α-Fe2O3–ZnO–Au nanocomposites with high gas-sensing performance. Sens. Actuators B Chem. 2015, 209, 889–897. [Google Scholar] [CrossRef]
- Gawli, Y.; Badadhe, S.; Basu, A.; Guin, D.; Shelke, M.V.; Ogale, S. Evaluation of n-type ternary metal oxide NiMn2O4 nanomaterial for humidity sensing. Sens. Actuators B Chem. 2014, 191, 837–843. [Google Scholar] [CrossRef]
- Mohanta, D.; Ahmaruzzaman, M. Novel Ag-SnO2-βC3N4 ternary nanocomposite based gas sensor for enhanced low-concentration NO2 sensing at room temperature. Sens. Actuators B Chem. 2021, 326, 128910. [Google Scholar] [CrossRef]
- Pfaff, G. Wet chemical synthesis of BaSnO3 and Ba2SnO4 powders. J. Eur. Ceram. Soc. 1993, 35, 3017–3021. [Google Scholar] [CrossRef]
- Ishigaki, T.; Torisaka, A.; Nomizu, K.; Madhusudan, P.; Uematsu, K.; Toda, K.; Sato, M. Long phosphorescent Ca2SnO4 with minuscule rare earth dopant concentration. Dalton Trans. 2013, 42, 4781–4785. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, Y.; Zhao, X.; Liu, B.; Cao, M. Synthesis of Cd2SnO4–SnO2 hybrid micro-cubes with enhanced electrochemical performance for lithium-ion batteries. CrystEngComm 2014, 16, 922–929. [Google Scholar] [CrossRef]
- Lei, S.; Tang, K.; Chen, C.; Jin, Y.; Zhou, L. Preparation of Mn2SnO4 nanoparticles as the anode material for lithium secondary battery. Mater. Res. Bull. 2009, 44, 393–397. [Google Scholar] [CrossRef]
- Al-Shahrani, A.A. Sintering behavior and thermal property of Mg2SnO4. J. Mater. Sci.: Mater. Electron. 2005, 16, 193–196. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yan, B.; Xiong, D.; Li, D.; Lawes, S.; Sun, X. Recent Developments and Understanding of Novel Mixed Transition-Metal Oxides as Anodes in Lithium Ion Batteries. Adv. Energy Mater. 2016, 6, 1502175. [Google Scholar] [CrossRef]
- Aguilar-Martinez, J.; Pech-Canul, M.; Esneider, M.; Toxqui, A.; Shaji, S. Synthesis, structure parameter and reaction pathway for spinel-type Co2SnO4. Mater. Lett. 2012, 78, 28–31. [Google Scholar] [CrossRef]
- Chen, C.; Ru, Q.; Hu, S.; An, B.; Song, X.; Hou, X. Co2SnO4 nanocrystals anchored on graphene sheets as high-performance electrodes for lithium-ion batteries. Electrochim. Acta 2015, 151, 203–213. [Google Scholar] [CrossRef]
- Thota, S.; Narang, V.; Nayak, S.; Sambasivam, S.; Choi, B.; Sarkar, T.; Andersson, M.; Mathieu, R.; Seehra, M. On the nature of magnetic state in the spinel Co2SnO4. J. Phys. Condens. Matter. 2015, 27, 166001. [Google Scholar] [CrossRef]
- Qi, Y.; Du, N.; Zhang, H.; Wu, P.; Yang, D. Synthesis of Co2SnO4@C core–shell nanostructures with reversible lithium storage. J. Power Sources 2011, 196, 10234–10239. [Google Scholar] [CrossRef]
- Dinesh, S.; Barathan, S.; Premkumar, V.K.; Sivakumar, G.; Anandan, N. Hydrothermal synthesis of zinc stannate (Zn2SnO4) nanoparticles and its application towards photocatalytic and antibacterial activity. J. Mater. Sci.: Mater. Electron. 2016, 27, 9668–9675. [Google Scholar] [CrossRef]
- Alpuche-Aviles, M.; Wu, Y. Photoelectrochemical Study of the Band Structure of Zn2SnO4 Prepared by the Hydrothermal Method. J. Am. Chem. Soc. 2009, 131, 3216–3224. [Google Scholar] [CrossRef]
- Oh, L.; Kim, D.; Lee, J.; Shin, S.; Lee, J.; Park, I.; Ko, M.; Park, N.; Pyo, S.; Hong, K.; et al. Zn2SnO4-Based Photoelectrodes for Organolead Halide Perovskite Solar Cells. J. Phys. Chem. C 2014, 118, 22991–22994. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, M.; Hu, C. Novel Zn2SnO4 Hierarchical Nanostructures and Their Gas Sensing Properties toward Ethanol. J. Phys. Chem. C 2011, 115, 5522–5529. [Google Scholar] [CrossRef]
- He, P.; Xie, Z.; Chen, Y.; Dong, F.; Liu, H. Co2SnO4/activated carbon composite electrode for supercapacitor. Mater. Chem. Phys. 2012, 137, 576–579. [Google Scholar] [CrossRef]
- Balasubramaniam, G.S.R.; Bhuvaneshwari, S.; Wu, J.J.; Abdullah, M.A.; Sambandam, A. Sonochemical synthesis of Co2SnO4 nanocubes for super capacitor applications. Ultrason Sonochem. 2018, 41, 435–440. [Google Scholar]
- Choi, K.I.; Kim, H.R.; Kim, K.M.; Liu, D.; Gao, G.; Lee, J.H. C2H5OH sensing characteristics of various Co3O4 nanostructures prepared by solvothermal reaction. Sens. Actuators B Chem. 2010, 146, 183–189. [Google Scholar] [CrossRef]
- Sun, C.; Su, X.; Xiao, F.; Niu, C.; Wang, J. Synthesis of nearly monodisperse Co3O4 nanocubes via a microwave-assisted solvothermal process and their gas sensing properties. Sens. Actuators B Chem. 2011, 157, 681–685. [Google Scholar] [CrossRef]
- Wen, Z.; Zhu, L.; Mei, W.; Hu, L.; Li, Y.; Sun, L.; Cai, H. Rhombus-shaped Co3O4 nanorod arrays for high-performance gas sensor. Sens. Actuators B Chem. 2013, 186, 172–179. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.; Liu, Q.; Chen, R.; Zhang, H.; Yu, J.; Song, D. Core-shell structure of ZnO/Co3O4 composites derived from bimetallic organic frameworks with superior sensing performance for ethanol gas. Appl. Surf. Sci. 2019, 475, 700–709. [Google Scholar] [CrossRef]
- Chen, X.; Liang, R.; Qin, C.; Ye, Z.; Zhu, L. Coaxial electrospinning Fe2O3@Co3O4 double-shelled nanotubes for enhanced ethanol sensing performance in a wide humidity range. J. Alloys Compd. 2022, 891, 161868. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Liang, Q.; Zhang, J.; Liu, J. Nanoporous Co3O4–TiO2 Heterojunction Nanosheets for Ethanol Sensing. ACS Appl. Nano Mater 2022, 5, 4779–4786. [Google Scholar] [CrossRef]
- Bu, X.; Ma, F.; Wu, Q.; Wu, H.; Yuan, Y.; Hu, L.; Han, C. Metal-organic frameworks-derived Co3O4/Ti3C2Tx Mxene nanocomposites for high performance ethanol sensing. Sens. Actuators B Chem. 2022, 369, 132232. [Google Scholar] [CrossRef]
- Mhamdi, A.; Labidi, A.; Souissi, B.; Kahlaoui, M.; Yumak, A.; Boubaker, K.; Amlouk, A.; Amlouk, M. Impedance spectroscopy and sensors under ethanol vapors application of sprayed vanadium-doped ZnO compounds. J. Alloys Compd. 2015, 639, 648–658. [Google Scholar] [CrossRef]
- Umar, A.; Al-Hazmi, F.; Dar, G.N.; Zaidi, S.A.; Al-Tuwirqi, R.M.; Alnowaiserb, F.; Al-Ghamdi, A.A.; Hwang, S. Ultra-sensitive ethanol sensor based on rapidly synthesized Mg(OH)2 hexagonal nanodisks. Sens. Actuators B Chem. 2012, 166, 97–102. [Google Scholar] [CrossRef]
- Tiemann, M. Porous Metal Oxides as Gas Sensors. Chem. Eur. J. 2007, 13, 8376–8388. [Google Scholar] [CrossRef]
- Chakraborty, G.; Pugazhenthi, G.; Katiyar, V. Exfoliated graphene-dispersed poly (lactic acid)-based nanocomposite sensors for ethanol detection. Polym. Bull. 2019, 76, 2367–2386. [Google Scholar] [CrossRef]
- Labidi, A.; Gillet, E.; Delamare, R.; Maaref, M.; Aguir, K. Ethanol and ozone sensing characteristics of WO3 based sensors activated by Au and Pd. Sens. Actuators B Chem. 2006, 120, 338–345. [Google Scholar] [CrossRef]
- Labidi, A.; Bejaoui, A.; Ouali, H.; Chaffar Akkari, F.; Hajjaji, A.; Gaidi, M.; Kanzari, M.; Bessaïs, B.; Maaref, M. Dry air effects on the copper oxides sensitive layers formation for ethanol vapor detection. Appl. Surf. Sci 2011, 257, 9941–9945. [Google Scholar] [CrossRef]
- Muzny, C.D.; Diky, V.; Kazakov, A.; Chirico, R.D.; Frenkel, M. Vapor Pressure. In CRC Handbook of Chemistry and Physics, 95th ed.; Haynes, W.M., Ed.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA; New York, NY, USA; Philadelphia, PA, USA, 2014; Volume 6, pp. 6–96. [Google Scholar]
- Khalifa, Z.S. Grain size reduction on nanostructured TiO2 thin films due to annealing. RSC Adv. 2017, 7, 30295–30302. [Google Scholar] [CrossRef] [Green Version]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Hellegouarc’h, F.; Arefi-Khonsari, F.; Planade, R.; Amouroux, J. PECVD prepared SnO2 thin films for ethanol sensors. Sens. Actuators B Chem. 2001, 73, 27–34. [Google Scholar] [CrossRef]
- Labidi, A. Novel ethanol sensing properties of sprayed ternary Co2SnO4 thin layer. Mater. Lett. 2021, 294, 129784. [Google Scholar] [CrossRef]
- Belaqziz, M.; Amjoud, M.; Gaddari, A.; Rhouta, B.; Mezzane, D. Enhanced room temperature ozone response of SnO2 thin film sensor. Superlattices Microstruct. 2014, 71, 185–189. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Chen, X.X.; Sun, R.; Xiong, Z.; Zheng, L.S. Hydrothermal syntheses and gas sensing properties of cubic and quasi-cubic Zn2SnO4. Mater. Chem. Phys. 2011, 129, 53–61. [Google Scholar] [CrossRef]
- Robbie, K.; Sit, J.C.; Brett, M.J. Advanced techniques for glancing angle deposition. J. Vac. Sci. Technol. B 1998, 16, 1115–1122. [Google Scholar] [CrossRef]
- Tait, R.N.; Smy, T.; Brett, M.J. Modelling and characterization of columnar growth in evaporated films. Thin Solid Films 1993, 226, 196–201. [Google Scholar] [CrossRef]
- Balaji, G.; Rathinavel, S.; Vadivel, S. Design and fabrication of clad removed fiber optic based NiCo2O4 sensor for detection of ethanol and acetone gases. Optik 2021, 228, 166216. [Google Scholar] [CrossRef]
- Dirks, A.G.; Leamy, H.J. Columnar microstructure in vapor-deposited thin films. Thin Solid Films 1977, 47, 219–233. [Google Scholar] [CrossRef]
- Ben Nacer, S.; Jlidi, D.; Labidi, A.; Chaffar Akkari, F.; Touihri, S.; Maaref, M. Promising ethanol detection enhancement of Cu2O thin film deposited by GLAD technique. Measurement 2020, 151, 107208. [Google Scholar] [CrossRef]
- Zhu, C.L.; Chen, Y.J.; Wang, R.X.; Wang, L.J.; Cao, M.S.; Shi, X.L. Synthesis and enhanced ethanol sensing properties of α-Fe2O3/ZnO hetero nanostructures. Sens. Actuators B Chem. 2009, 140, 185–189. [Google Scholar] [CrossRef]

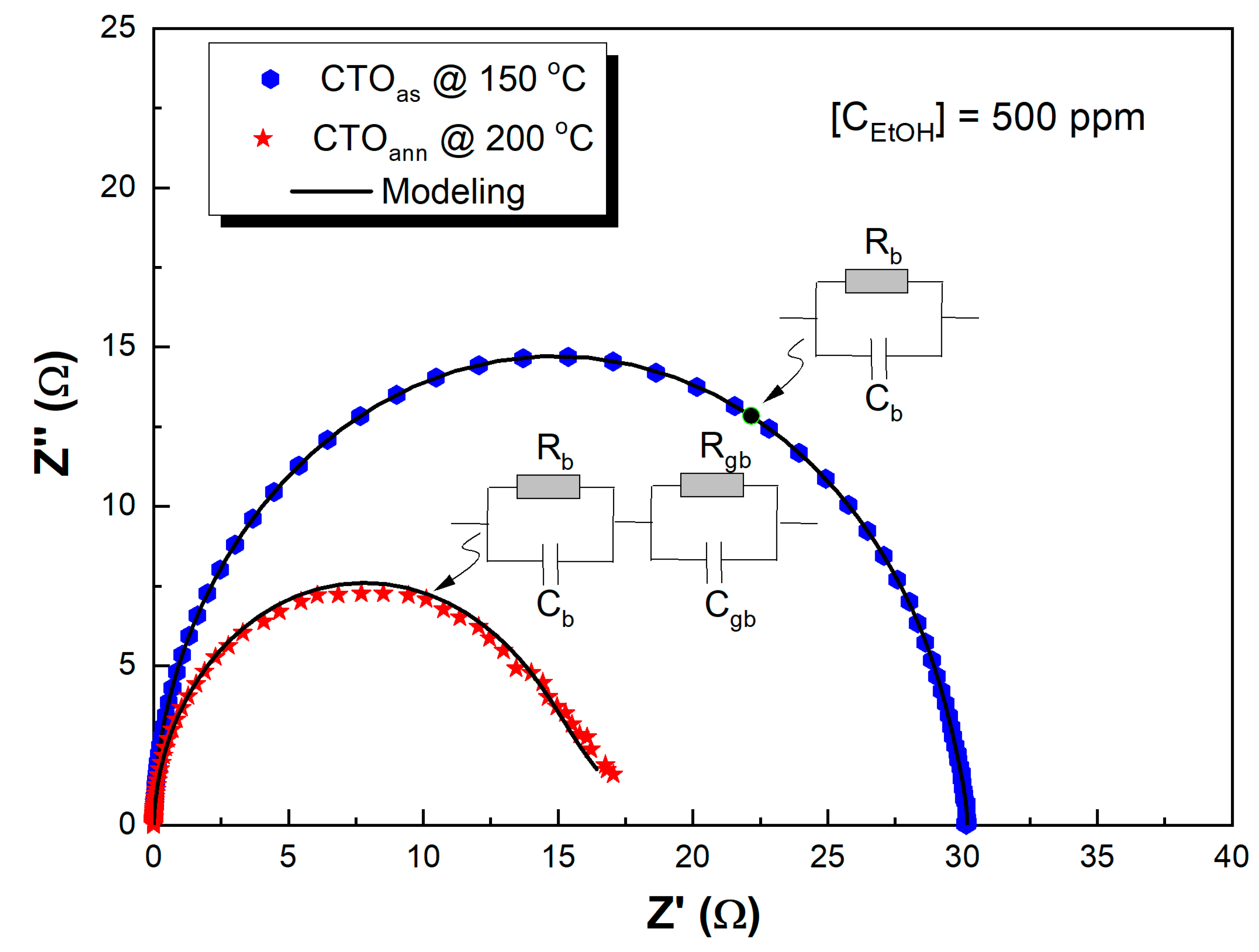
| Under 500 ppm of Ethanol | |||
|---|---|---|---|
| Film | CTOas | CTOann | |
| 1St Semicircle | Rb × 106 Ω | 6.44 | 1257 |
| Cb × 10−10 F | 1.812 | 1.88 | |
| N | 0.996 | 0.988 | |
| 2nd Semicircle | Rgb × 106 Ω | 79.25 | |
| Cgb × 10−7 F | 0.391 | ||
| N | 0.874 | ||
| Sensor Material | Fabrication Approach | Conc. (ppm) | Response “S” | Sens Temp. (°C) | τres/τrec (s) | Ref. |
|---|---|---|---|---|---|---|
| Co2SnO4 spinel thin film | Spray pyrolysis | 500 | 3.5 | 150 | 20/24 | [37] |
| SnO2 (triton) | Spin coating | 500 | 3 | RT | 15/720 | [38] |
| Zn2SnO4 spinel cube | Hydrothermal | 600 | 5.5 | 325 | 18/45 | [39] |
| α-Fe2O3 nanorods | Hydrothermal | 500 | 8 | 220 | −/− | [40] |
| Porous SiC | Electrochemical etching | Saturated vapors | 1.2 | RT | 85/57 | [41] |
| NiCo2O4 spinel nanoparticles | Hydrothermal | 500 | 0.6 | RT | 20/26 | [42] |
| TiO2 NWs | Electrospinning | 500 | 7.5 | 500 | 23/1 | [43] |
| Cu2O nano-columnar | GLAD technique | 500 | 8.12 | 200 | 60/180 | [44] |
| Zn2SnO4cuboctahedra | Chemical method | 600 | 0.01 | 325 | 18/45 | [45] |
| Co2SnO4/Co3O4 composite | Spray pyrolysis | 500 | 13.5 | 200 | 35/30 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labidi, A. Investigation of Annealing Process Effects on the Response and Stability of Sprayed Co2SnO4 Film under Ethanol Vapor. Appl. Sci. 2023, 13, 2797. https://doi.org/10.3390/app13052797
Labidi A. Investigation of Annealing Process Effects on the Response and Stability of Sprayed Co2SnO4 Film under Ethanol Vapor. Applied Sciences. 2023; 13(5):2797. https://doi.org/10.3390/app13052797
Chicago/Turabian StyleLabidi, Ahmed. 2023. "Investigation of Annealing Process Effects on the Response and Stability of Sprayed Co2SnO4 Film under Ethanol Vapor" Applied Sciences 13, no. 5: 2797. https://doi.org/10.3390/app13052797





