Abstract
PreCODE is a multifaceted protocol that applies lifestyle modifications to improve cognitive decline. NeuroQ is a blend of ingredients that have been individually reported to benefit cognition. The objective of this open-label exploratory study was to evaluate the efficacy of PreCODE with NeuroQ on cognition in healthy adults with risk factors for cognitive decline. Thirty participants ≥45 years at-risk of cognitive decline underwent PreCODE with NeuroQ. Cognitive function was assessed by CNS-Vital Signs (CNS-VS) at 30, 60 and 90 days. Quality of life (QoL), sleepiness, depression, and healthy habits were assessed with the Medical Outcomes Survey, Epworth Sleepiness Scale, Patient Health Questionnaire, and Life Habits Checklist, respectively. There was a 10, 12, and 14% increase in the neurocognitive index percentile scores from screening at Day 30, 60, and 90, respectively (p ≤ 0.01). The CNS-VS domains in psychomotor-, processing-, and motor-speed, complex- and sustained-attention, reaction time, cognitive flexibility, executive function, and working memory improved at Day 90 (p ≤ 0.04). Executive function, cognitive flexibility, processing speed, and working memory, improved by 12, 11, 10, and 7%, respectively, at Day 90. There were improvements in QoL, daytime sleepiness, depression, and lifestyle habits (p ≤ 0.014). NeuroQ was safe and well tolerated. PreCODE with NeuroQ improved cognitive function and QoL in adults at-risk of cognitive decline. Placebo- or comparator-controlled studies are warranted to confirm the effect on cognitive function.
1. Introduction
Early indications of cognitive decline are easily missed because they are often perceived as part of normal aging [1]. Natural aging is a significant risk factor for dementia and mild cognitive impairment (MCI) [2,3], and adults with MCI are at an increased risk of developing Alzheimer’s Disease (AD) [4,5]. Preventing cognitive decline is not only important to maintain the high functional capacity and better quality of life (QoL) of an individual, but it may also decrease the financial burden on the medical system. Annual mean medical costs significantly increase from cognitively normal to prevalent dementia, from $6042 to $11,678 [6], and the cost of caring for individuals with dementia is estimated to reach approximately $2 trillion dollars by 2030 [7]. Keeping the focus on prevention may also maintain a better QoL for family members that are not forced into a caregiver role [8,9].
Cognitive decline is associated with non-modifiable risk factors, including age, genetic polymorphisms, and family history, as well as modifiable risk factors such as physical inactivity, social isolation, metabolic dysfunction (hypertension, hypercholesterolemia, diabetes, obesity), poor diet, and high alcohol consumption [7,10]. The World Health Organization (WHO) recommends the implementation of interventions that delay or are capable of slowing cognitive decline with a focus on modifiable risk factors [7]. The current treatment strategies to combat cognitive decline include pharmaceuticals, exercise therapies, and cognitive training [11,12]. Pharmacological and exercise therapies have produced inconsistent and weak results [12,13], while brain training did not improve overall cognition [14]. Due to the number of risk factors involved and the complex pathophysiology of cognitive decline and dementias [5,12], multidimensional lifestyle interventions, such as lifestyle education, cognitive training, and physical activity, that focus on prevention may be beneficial [15,16]. There has been interest in multifactorial preventative options to provide a multidimensional benefit [16,17,18]. Several long-term studies have utilized various multidimensional approaches in the prevention of cognitive impairment in populations at-risk for cognitive decline [19,20,21,22]. Adults receiving a multidimensional lifestyle intervention improved or maintained their cognitive function more than the adults receiving general health advice [16]. Therefore, there is a need to understand whether shorter term interventions that may yield higher compliance could be beneficial.
Further, given the need for preventative strategies for cognitive decline, and the lack of effective pharmaceutical options, natural products and supplements have also received attention for their role in lifestyle intervention programs. Phosphatidylserine, turmeric, Ginkgo Biloba, Gotu Kola, propolis, and whole coffee fruit extracts have been studied individually and reported to have neuroprotective effects [23,24,25,26]. In older adults, phosphatidylserine has been shown to improve memory, verbal learning, verbal fluency, and sociability and Gotu Kola supplementation has been found to improve cognition [23,27,28]. Coffee fruit extract has increased subjective alertness and decreased task-related fatigue in healthy adults [29]. Previous pre-clinical models have examined the effect of two or more of these ingredients in combination on cognitive function [30,31]. In a 12 week, double-blind, randomized, placebo-controlled study, the same formulation of ingredients was found to be safe and significantly improved the neurocognitive index compared to placebo in a population of older Japanese adults [32].
As lifestyle modifications may offer different mechanisms for improving cognitive function, the addition of supplements with previously documented neuroprotective effects is certainly warranted. The nootropic supplement NeuroQ contains a blend of phosphatidylserine, turmeric, Ginkgo Biloba, Gotu Kola, propolis, and whole coffee fruit extracts. This open-label exploratory study evaluated the efficacy of a lifestyle modification protocol, known as PreCODE, in combination with NeuroQ as part of an overall intervention in supporting cognition in healthy adults with risk factors for cognitive decline.
2. Materials and Methods
This was a multi-center study conducted at the Kapoor Medical Center (Studio City, CA, USA) and LifeSeasons Medical Center (Flower Mound, TX, USA). Research ethics board approval was granted on 16 October 2019 (Pro00039253, Advarra, Columbia, Maryland). All participants provided written informed consent prior to the study procedures. The study was performed in accordance with the Declaration of Helsinki guidelines and its subsequent amendments and was conducted in compliance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline for Good Clinical Practice (GCP). The trial was registered at Clinicaltrials.gov (NCT04149639).
2.1. Participants
A total of 38 participants (13 males and 25 females) were enrolled based on risk factors for cognitive decline associated with aging.
Participants were ≥45 years of age and had one or more of the following risk factors for cognitive decline: self-reported apolipoprotein E4 allele; self-reported metabolic syndrome; age ≥ 60 years; self-reported family history of AD or dementia in a first-degree relative; or a self-reported lifestyle risk factor, including sedentary lifestyle (score of ≥3 on Life Habits Checklist (LHC) question 5), poor dietary habits (score of ≥3 on LHC questions 6–13), social support network (score of ≥3 on LHC question 19), stress management skills (score of ≥3 on LHC questions 14–18), or sleep habits (score of ≥7 on Epworth Sleepiness Scale SF-8). This information was collected using questionnaires and participant interviews, which were reviewed by the Medical Director (MD). The participants did not have significant cognitive impairment, as assessed by a Mini-Mental State Exam-2 (MMSE-2) score of ≥24; tested between the 24–75th percentile on one or more domains in the neurocognitive index (NCI); and had a low frequency of a depressed mood, as assessed by Patient Health Questionnaire (PHQ-9) score of ≤9.
Participants were excluded if they had an allergy to NeuroQ; a neuropsychological condition and/or marked cognitive impairment; tested below the 24th percentile or above the 75th percentile in all domains that comprise NCI; vitamin deficiencies affecting cognition below the normal clinical ranges (such as vitamin B12 or vitamin D); blood donation 30 days prior to screening, during, or 30-days after the study; participated in other clinical research trials 30 days prior to screening; type II diabetes, unstable hypertension, or current or pre-existing thyroid condition (stable dose of medication for >3 months was assessed by the MD); major surgery in the past 3 months or planned surgery during the trial (minor surgery was assessed by the MD); a significant cardiovascular event in the past 6 months (participants on a stable dose of medication may have been included after assessment by the MD); a history of or current diagnosis with kidney and/or liver diseases as assessed by the MD, except a history of kidney stones symptom-free for 6 months; cancer, except skin cancer excised with no chemotherapy or radiation with a negative follow-up (cancer in full remission for more than five years after diagnosis was acceptable); unstable metabolic disease or chronic diseases; current or history of any significant diseases of the gastrointestinal tract; blood/bleeding disorders; chronic use of cannabinoid products or currently taking medical cannabinoid products containing >0.3% tetrahydrocannabinol; alcohol or drug abuse within the last 12 months; high alcohol intake (>2 drinks per day); were cognitively impaired and/or unable to give informed consent; any other active or unstable medical condition, that, in the opinion of the MD, may have adversely affected the participant’s ability to complete the study or its measures or posed a significant risk to the participant. Additionally, participants taking benzodiazepines, sedatives, opiates, mood stabilizers, antidepressants, cholinergic or anticholinergics, or had regular use of prescription analgesic medication, St. John’s Wort, grapefruit, or first-generation antihistamines were excluded.
2.2. Intervention
The PreCODE protocol focuses on the prevention of cognitive decline for adults who want to minimize their modifiable risk factors by tailoring their individual needs to optimize their lifestyle-related health status. This protocol involved lifestyle modifications to diet, sleep optimization, exercise, and brain training. For the dietary modification, the participants were instructed to fast for 12–14 h, beginning three hours before bed. The participants were asked to ensure that they obtained 7.5–8.5 h of sleep each night and to supplement with melatonin or tryptophan as needed, exercise 30–60 min per day 4x/week for the first 30 days of the intervention, then increasing to 45–60 min per day 4–6x/week for the remaining 60 days of the intervention and to perform brain training (BrainHQ, Posit Science, San Francisco, CA, USA) 30–40 min per day, 4x/week. The brain training involved exercises of varying difficulty, depending on participant proficiency, and included subject areas such as attention, brain speed and memory, which were different from those completed for the study outcomes.
All participants were instructed to take two capsules of NeuroQ at the same time of day for 90 days. The NeuroQ was formulated by Dr. Dale Bredesen [18,24] and provided by LifeSeasons (Lewisville, TX, USA). If a dose was missed, the participants were instructed to re-start their regular dosing the next day and not to exceed two capsules daily. NeuroQ contains 100 mg phosphatidylserine, 100 mg whole coffee fruit extract, 250 mg turmeric extract, 120 mg Ginkgo Biloba extract, 250 mg Gotu Kola extract, and 75 mg propolis extract. NeuroQ also contained non-medical ingredients including Hypromellose (vegetable cellulose), ascorbyl palmitate, and silica.
Participants received the same instructions regarding the PreCODE protocol and NeuroQ supplementation and started the combined intervention on Day 1. The 90-day intervention period was based on previous studies that demonstrated improvements in cognitive function outcomes with the ingredients found in the NeuroQ formulation. These studies ranged between 4 and 12 weeks [32,33,34,35,36]. The current study was designed to inform design and outcomes of future larger, randomized, controlled clinical trials.
2.3. Outcomes
2.3.1. CNS Vital Signs
The CNS-VS battery is a validated cognitive assessment tool comprised of eight normed neurocognitive tests: a verbal and visual memory test; a finger tap test, symbol digit coding, the Stroop Test, a shifting attention test, continuous performance, and four-part continuous performance [37]. Different combinations of these test scores generated five domain scores, including composite memory (comprised of verbal and visual memory tests), psychomotor speed (finger tap test and symbol digit coding), reaction time (the Stroop Test), complex attention (the Stroop Test, shifting attention test, continuous performance), and cognitive flexibility (the Stroop Test, shifting attention test). The mean of these domain scores generated the neurocognitive index (NCI), which was the primary outcome [38]. There were eight other unique tests that generated single scores, including: verbal memory; visual memory; working memory; processing speed; executive function; simple attention; sustained attention; and motor speed. The participant scores were standardized to an age-matched population to obtain normalized scores and ranked [37]. There was no practice CNS-VS administered in advance of the CNS-VS used as an outcome. Reliability has been previously reported in a normal population with reliability coefficients ranging between 0.54–0.74 for the CNS-VS domains [37].
2.3.2. Quality of Life Questionnaires
The 36-Item Short Form Survey (SF-36; RAND Corporation) was used to assess quality of life (QoL) [39]. The participants self-reported how limited they were in performing everyday activities due to physical functioning (10 items), role functioning (7 items), energy/fatigue (4 items), emotional wellbeing (5 items), social functioning (2 items), pain (2 items), and general health (5 items) [39].
The Epworth Sleepiness Scale was used to measure the participants’ sleepiness throughout the day during 8 low-energy activities [40]. The participants were instructed to rate, from 0 (would never doze or sleep) to 3 (high chance of dozing or sleeping), how likely they are to doze off or fall asleep during eight low-energy activities.
The Patient Health Questionnaire (PHQ-9) was used to measure the frequency and severity of a depressed mood or depression [41]. The participants were instructed to provide ratings from 0 (not at all) to 3 (nearly every day) for how often they felt each of the nine statements applied to them.
The Life Habits Checklist 2.0 (used with permission from Dr. David Haase) evaluated quantity and quality of sleep, exercise habits, eating habits, mindfulness, and interpersonal relationship habits. The questions were phrased as “I” statements, such as “I sleep in complete, or near complete darkness”. The participants were instructed to respond to each question on a 5-point scale, from 1 (“daily” doing the activity) to 5 (“never and have no intention of doing” the activity). The scores were summarized in six domains: neurodegenerative prevention score; brain health promotion score; healthy body rhythm score; food quality and dietary habits score; healthy shape and weight promotion score; and an overall aggregate score. This scale is part of the CNV-VS platform for which it can be used as an accompanying tool to CNV-VS in both research and clinical settings.
2.3.3. Mini-Mental State Exam-2 (MMSE-2)
The MMSE-2 was used to follow the course of the cognitive changes throughout the intervention [42]. Further, the MMSE-2 was used to confirm that, during the intervention period, the participants remained free of dementia or other significant cognitive impairment. The MMSE-2 was administered by the Medical Directors/Qualified Investigators at each study site (Drs. Margaret Apostol and Sandeep Kapoor). The results were not adjusted for age or education level.
2.3.4. Motivation to Make Lasting Changes to Their Lifestyle
This brief questionnaire consisted of five yes/no questions on whether the participants were motivated to continue each of the four individual lifestyle interventions, as well as the overall lifestyle intervention.
2.3.5. Safety
Safety was assessed by examining post-emergent adverse events and vital signs (heart rate and blood pressure) after 30, 60, and 90 days of the intervention. Clinical chemistry (comprehensive metabolic panel, complete blood count with differential and platelets), hematology (white blood cell count with differential, red blood cell (RBC) count, hemoglobin, hematocrit, platelet count, RBC indices [mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, red cell distribution width]), as well as serum chloride, magnesium, cobalamin, folate, thyroid-stimulating hormone, and total carbon dioxide were assessed by LabCorp (Dallas, TX, USA) following 82 ± 2 days of the intervention to allow for scheduling.
2.4. Experimental Protocol
At screening, eligibility was assessed by reviewing the inclusion and exclusion criteria, medical history, anthropometrics, vital signs, and completion of the MMSE-2, CNS-VS, and QoL questionnaires. For the inclusion criteria related to one or more risk factors for cognitive decline, all participants self-reported to the Medical Director/Qualified Investigator their genetic risk factors, family history, and lifestyle-associated risk factors. The eligible participants returned for a second screening visit, in a 12 h fasted state, for a safety blood panel, vitals, and weight, conducted >48 h before baseline.
The participants attended study visits at baseline, and Days 30, 60, and 90. Baseline consisted of a physical examination, vital signs, and weight measurement. Details on the PreCODE protocol were provided (see above) along with a study diary, and NeuroQ was dispensed. During the first 56 days of the intervention, the participants received weekly calls to ensure compliance and to answer any questions. The participants returned to the clinic after 30, 60, and 90 days for measurements of vital signs, weight, and completion of the CNS-VS and QoL questionnaires [37,39], and NeuroQ was re-dispensed on Days 30 and 60. Between two and five days before their Day 90 visit, the participants returned for their 12 h fasted safety blood panel. The participants also had a final physical examination at Day 90.
2.5. Statistical Analysis
An efficacy analysis based on the population was performed on the primary and secondary outcomes at screening and days 30, 60, and 90, in addition to changes between screening and days 30, 60, and 90. Within-group changes between days were evaluated using repeated measures mixed models with the visit number as the fixed effect and the participant as random effect. The secondary outcome, “motivation to make lasting changes to their lifestyle”, was evaluated with a binomial test for whether the proportion of respondents to “yes” were significantly different from the anticipated 50%, based on uniform chance. The secondary outcome, “MMSE-2 Score”, was measured at two timepoints, and the as appropriate within-group changes were evaluated using pairwise tests, conditional upon normality. Normality and log-normality were determined using the Shapiro-Wilk’s test. Normal and log-normal scores were tested using a paired t-test, while scores that were intractably non-normal were tested using Wilcoxon’s signed-rank test. Probabilities ≤ 0.05 were considered statistically significant. All statistical analyses were completed using the R Statistical Software Package Version 3.6.3 or newer for Microsoft Windows [43].
3. Results
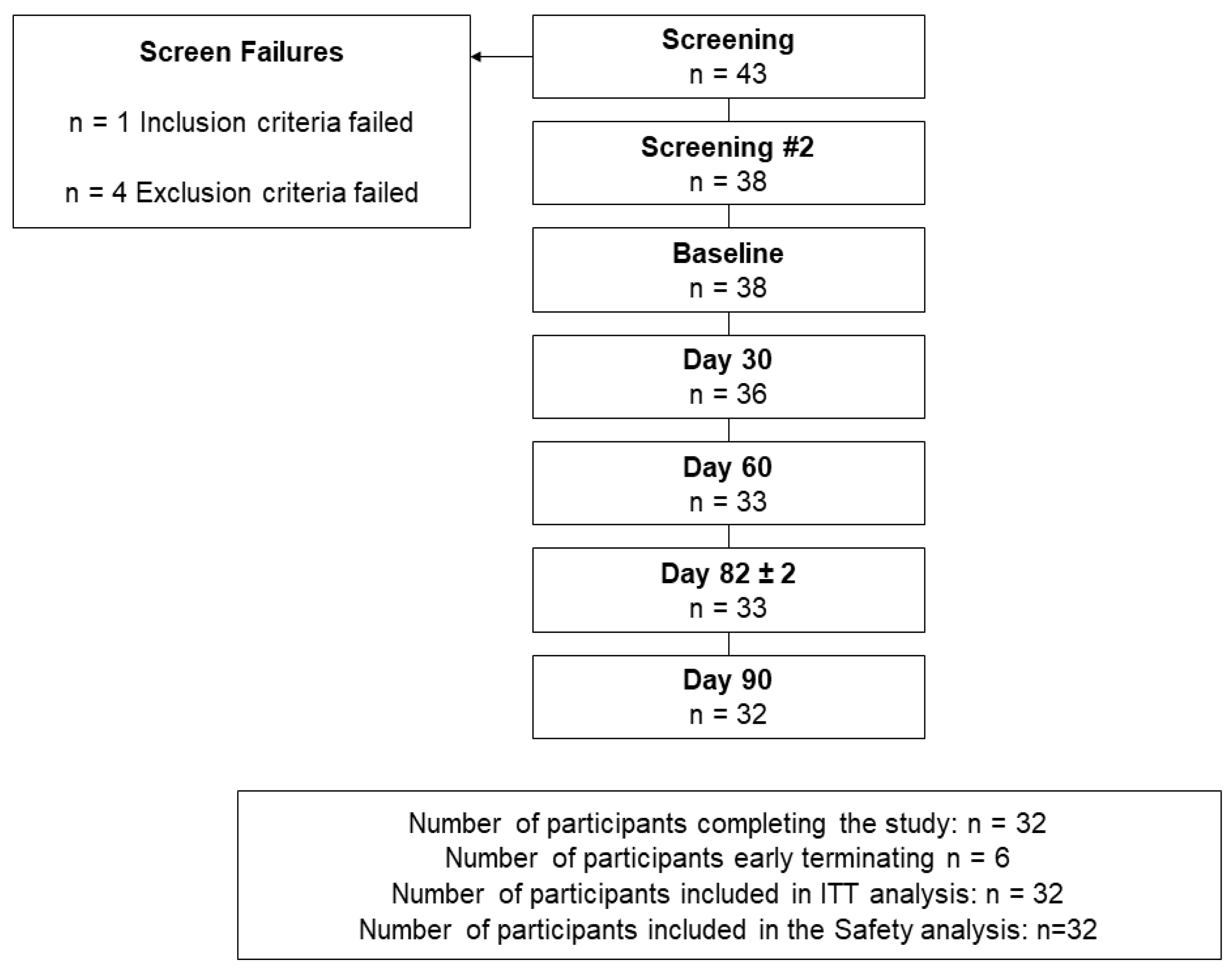
Forty-three volunteers were screened, and five were not eligible to participate for the following reasons; (1) did not meet the inclusion criteria based on the PHQ-9, as their score was >10, (2) history of spastic colon (exclusion of current or history of any significant diseases of the gastrointestinal tract), (3) history of pulmonary embolism and on a blood thinner (exclusion of blood/bleeding disorders) (4) used recreational cannabis for four years and excluded based on the exclusion of chronic use of cannabinoid products and (5) on opioids for chronic back pain, which was a concomitant medication not permitted during the study.
A total of 38 participants, with a mean age of 64.5 (45.0 to 97.0) years, were eligible and were enrolled in the study (Figure 1, Table 1).
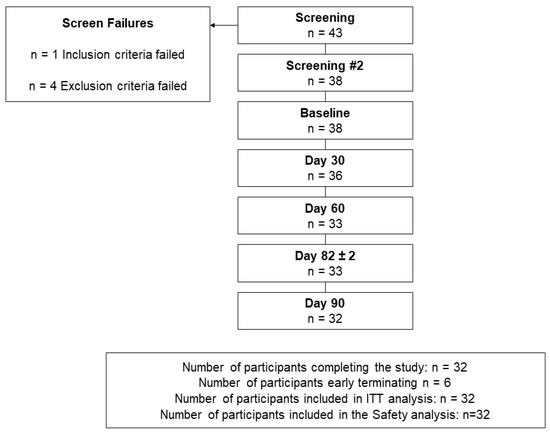
Figure 1.
Disposition of participants.

Table 1.
Participant demographic and anthropometric data.
3.1. CNS Vital Signs
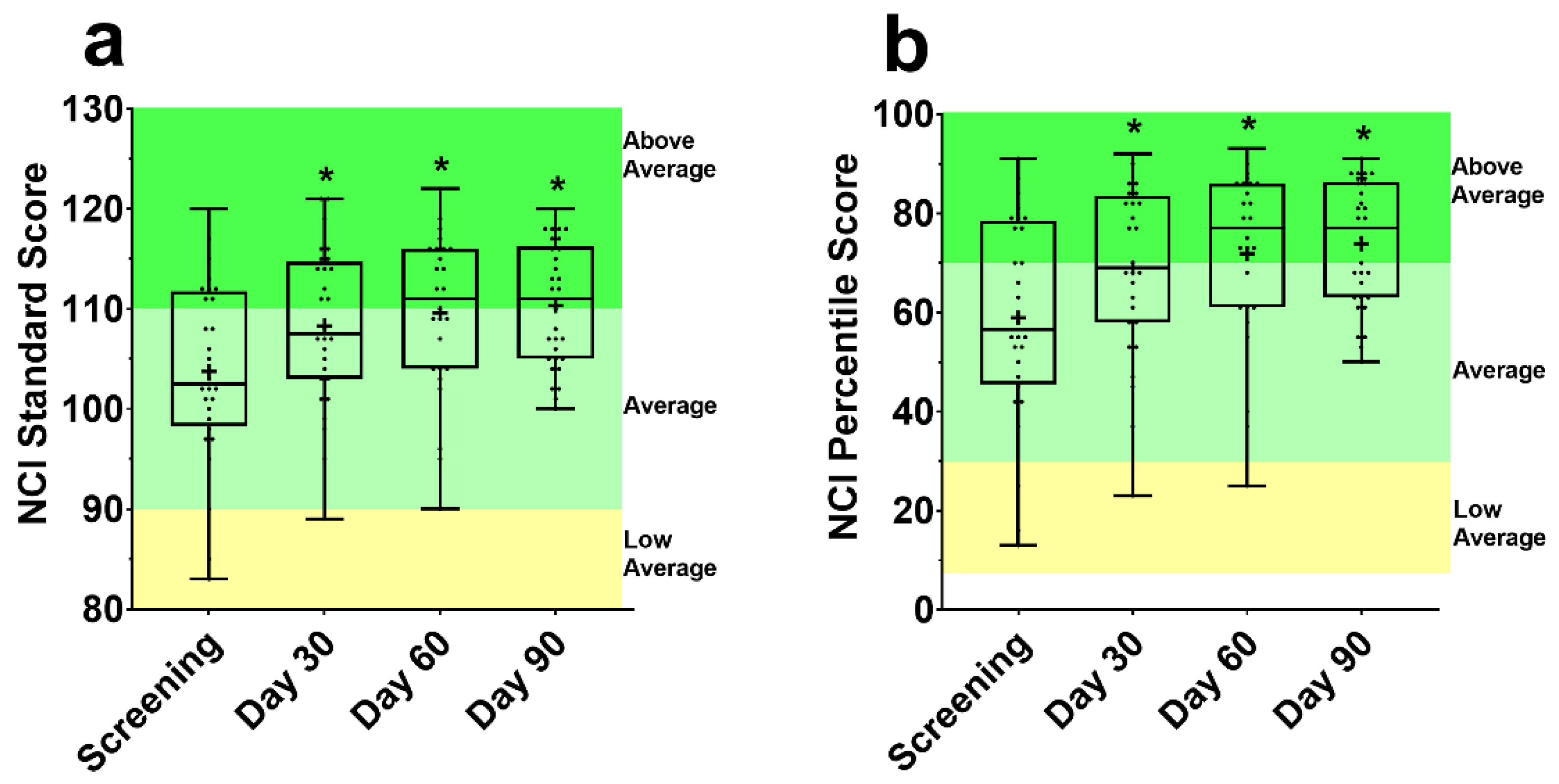
There was an improvement in the NCI standard score (Figure 2a) and percentile score (Figure 2b) from screening at Day 30, 60, and 90 (all p ≤ 0.001). This corresponded to a significant increase in the percentile score, by 10.0%, 11.6% and 13.6% at Day 30, 60, and 90 from screening, respectively.
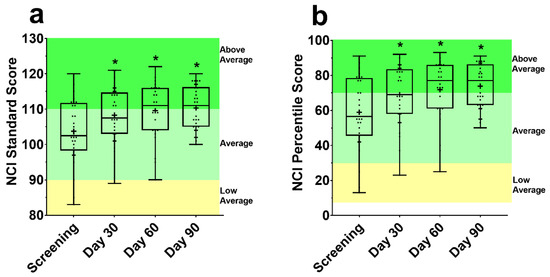
Figure 2.
Standard (a) and percentile (b) scores for the CNS Vital Signs (VS) Neurocognitive Index (NCI). Each box is made up of the 25th, 50th, and 75th quartile with the whiskers extending to the minimum and maximum value. Dots are individual data points, and the cross is the mean. Above average, average, and low average classification from CNS-VS Interpretation Guide [44]. NCI score CNS-VS panel was assessed using a repeated measures mixed effects model with visit number as the fixed effect and participant as the random effect. * Significantly different from screening (p < 0.05).
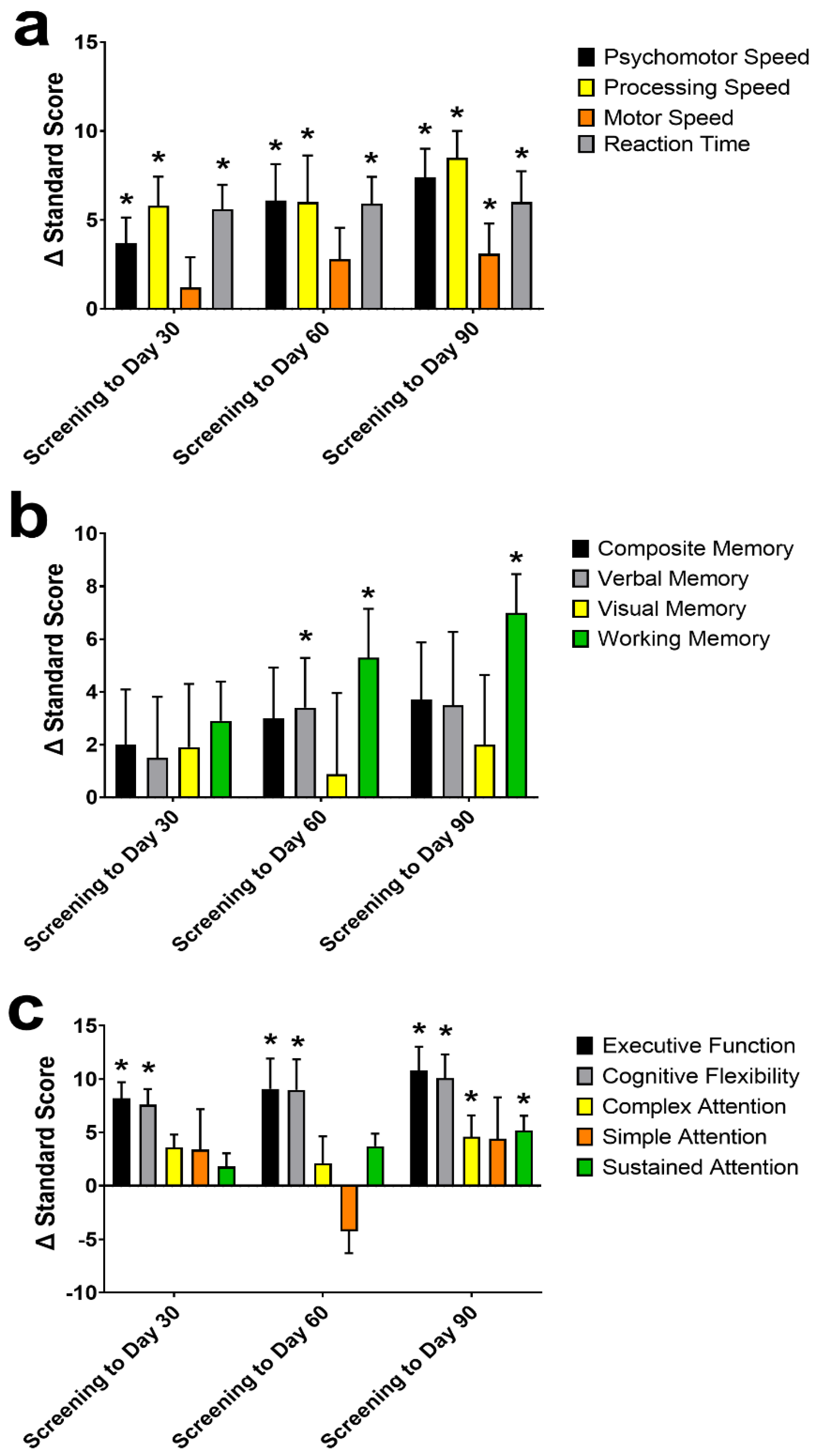
Of the individual cognitive domains examined in the CNS-VS (Figure 3), psychomotor speed, processing speed, motor speed, reaction time, working memory, complex attention, sustained attention, cognitive flexibility, and executive function standard scores improved from screening to Day 90 (all p ≤ 0.028). At Day 30, psychomotor speed, processing speed, reaction time, cognitive flexibility, and executive function all significantly improved from screening (all p ≤ 0.012). At Day 60, all the domains from Day 30, plus verbal memory and working memory, were greater than at screening (all p ≤ 0.040).
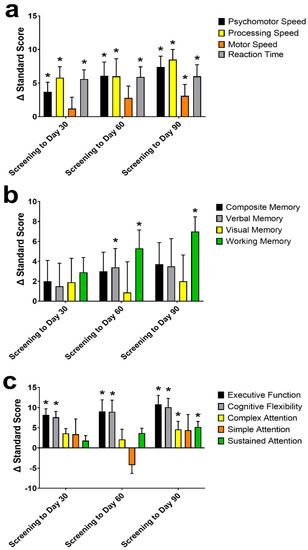
Figure 3.
The change (Δ) in the CNS Vital Signs (VS) individual domains standard score from screening to Day 30, 60, and 90. Individual domains of psychomotor-, processing-, and motor-speed, and reaction time (a); composite-, verbal-, visual-, and working-memory (b), and executive function, cognitive flexibility, complex-, simple-, and sustained-attention (c) Data are mean ± standard error of the mean. CNS-VS domains were assessed using a repeated measures mixed effects model with visit number as the fixed effect and participant as the random effect. * Significantly different from screening (p < 0.05).
3.2. Quality of Life Questionnaires
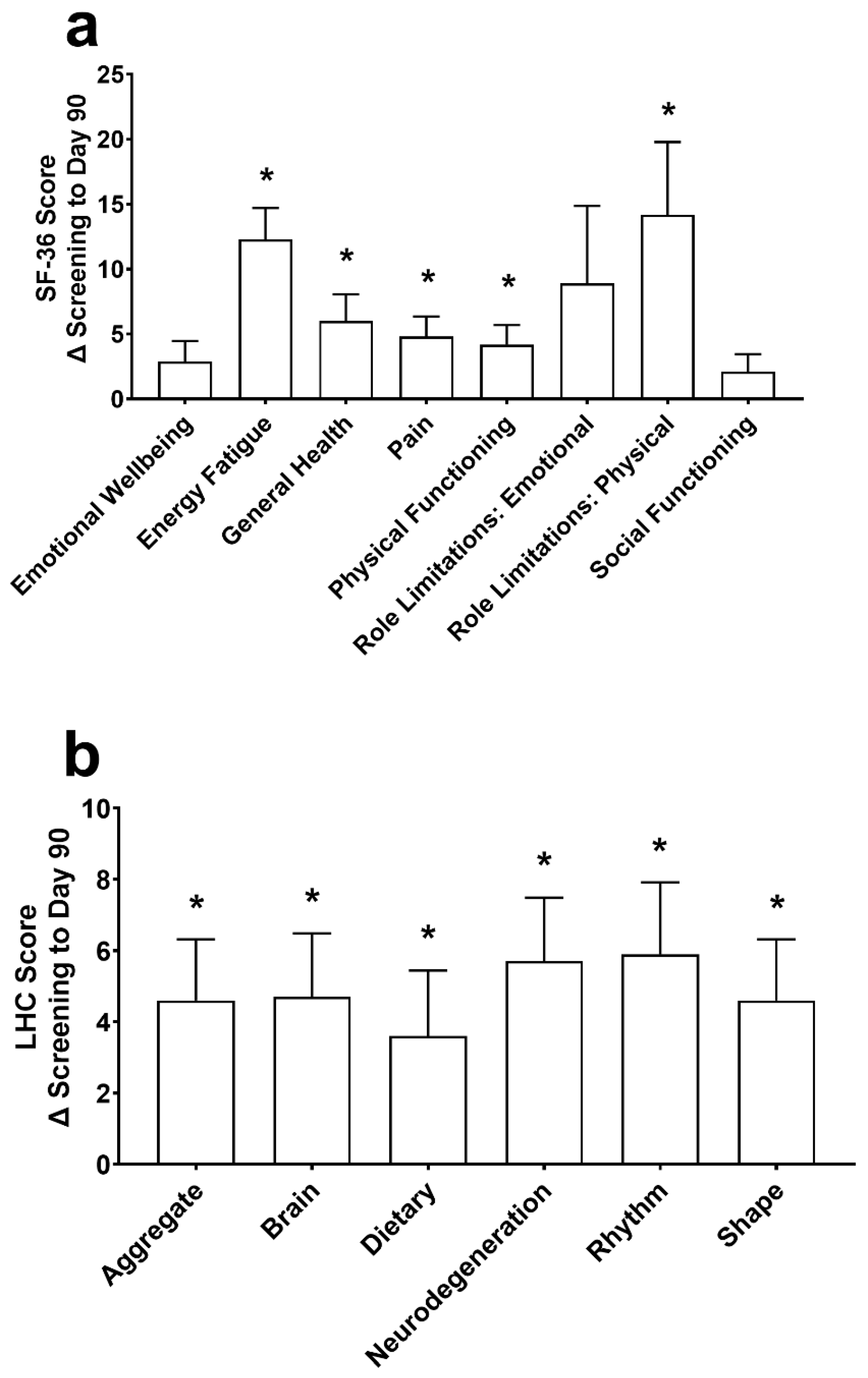
At Day 90, improvements in the SF-36 (Figure 4a) were found for energy/fatigue, general health, pain, physical functioning, and role limitations due to physical health (all p ≤ 0.007). Energy/fatigue was increased by 5.8 ± 12.5 at Day 30 (p = 0.020), and emotional wellbeing, energy/fatigue, general health, and role limitations due to physical health were significantly increased by 2.9 ± 5.6, 5.0 ± 12.7, 4.0 ± 8.7, and 9.5 ± 38.0, respectively, at Day 60 (all p ≤ 0.027). There were no changes in emotional wellbeing, role limitations due to emotional problems, and social functioning (all p ≥ 0.239) at Day 90.
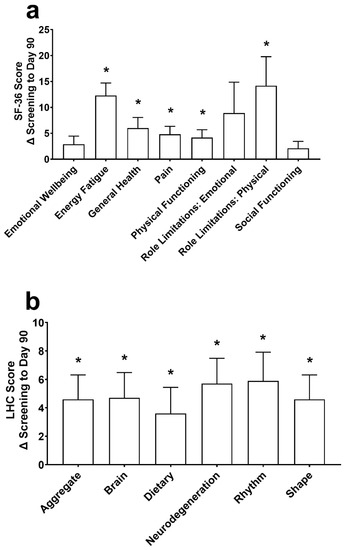
Figure 4.
The change (Δ) in the 36-Item Short Form Survey (SF-36; (a)) and the Life Habits Checklist (LHC; (b)) from screening to Day 90. Data are mean ± standard error of the mean. SF-36 and LHC were assessed using a repeated measures mixed effects model with visit number as the fixed effect and participant as the random effect. * Significantly different from screening (p < 0.05).
There was an increase in the aggregate score (p < 0.001) and each individual domain of the Life Habits Checklist, including the neurodegenerative prevention score, brain health promotion score, healthy body rhythm score, food quality and dietary habits score, and healthy shape and weight promotion score (p ≤ 0.014; Figure 4b) from screening to Day 90. At Day 30, the aggregate, healthy body rhythm, and healthy shape were different from screening by 2.6 ± 8.1, 3.9 ± 11.7 and 2.6 ± 8.1, respectively (all p ≤ 0.044). At Day 60, the aggregate, brain, dietary, neurodegeneration, and healthy shape domains were increased from screening by 3.9 ± 7.9, 4.0 ± 8.0, 4.7 ± 10.0, 4.1 ± 9.0, and 3.9 ± 7.9, respectively (all p ≤ 0.013). The healthy body rhythm score increased by 3.5 ± 10.5 from screening at Day 60 (p = 0.051).
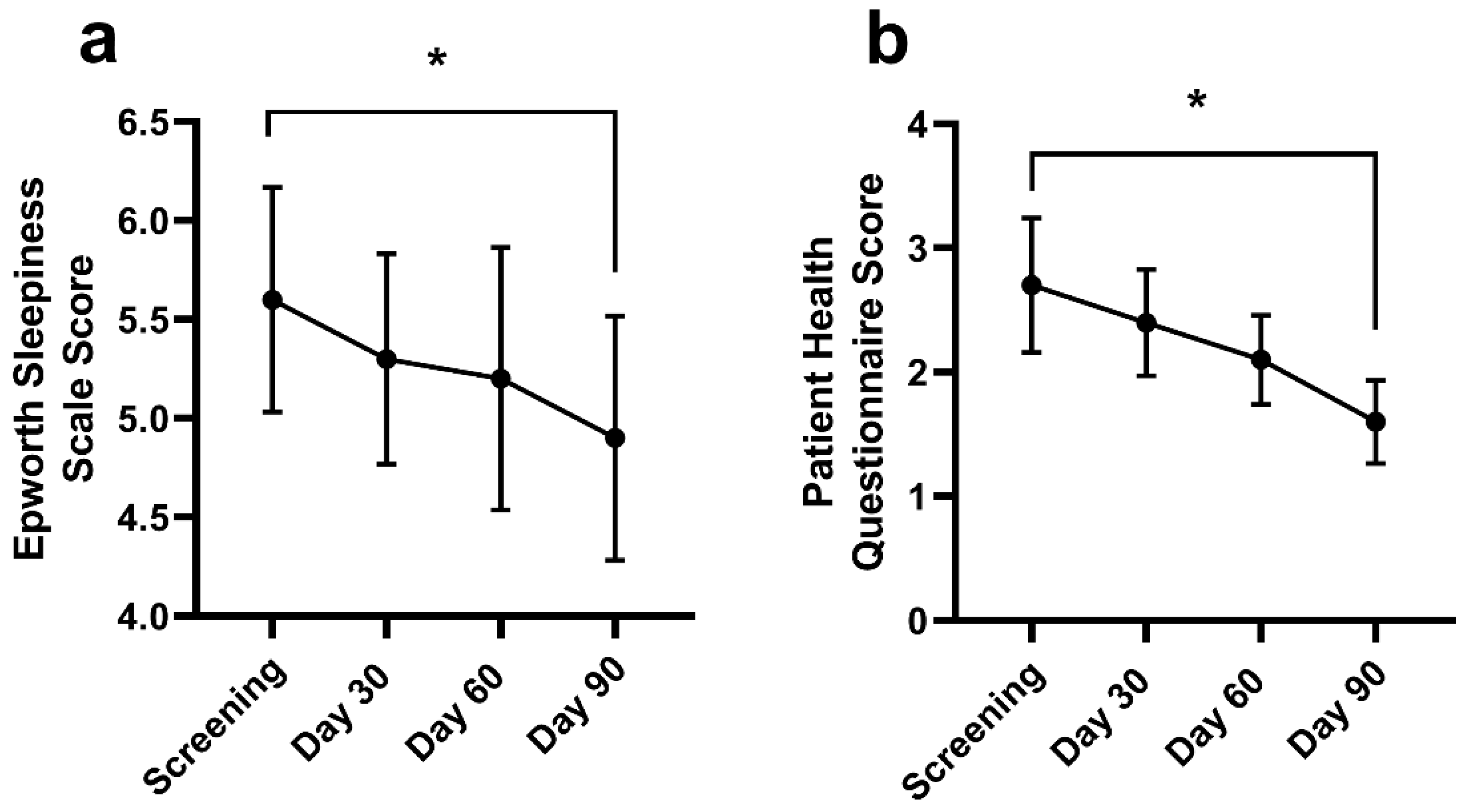
Daytime sleepiness throughout the day reduced from screening (5.6 ± 3.5) at Day 90 (4.9 ± 3.5; p = 0.005) (Figure 5a). There was no change in the Epworth Sleepiness scale at Day 30 (5.3 ± 3.1; p = 0.124) or Day 60 (5.2 ± 3.7; p = 0.133). The frequency and severity of a depressed mood or depression (Figure 5b) decreased from screening (2.7 ± 3.3) at Day 90 (1.6 ± 1.9; p = 0.003). There were no changes in PHQ-9 at Day 30 (2.4 ± 2.5; p = 0.302) or Day 60 (2.1 ± 2.0; p = 0.266).
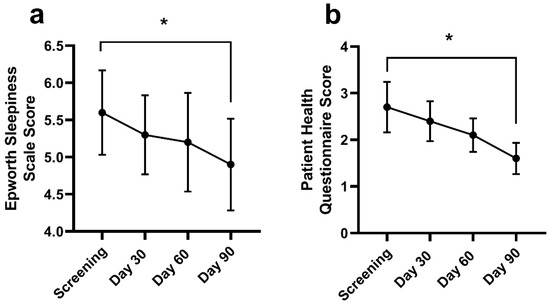
Figure 5.
The Epworth Sleepiness Scale (ESS; (a)) and Patient Health Questionnaire (PHQ; (b)) at screening, Days 30, 60, and 90. Data are mean ± standard deviation. The ESS and PHQ were assessed using a repeated measures mixed effects model with visit number as the fixed effect and participant as the random effect. * Significantly different from screening at Day 90 (p < 0.05).
3.3. Mini-Mental State Exam-2
There was no difference in the MMSE-2 scores from screening (28.8 ± 1.5) to Day 90 (28.9 ± 2.8; p = 0.549). The scores were categorized as “normal” both prior to and following the treatment protocol.
3.4. Motivation to Make Lasting Lifestyle Changes
The 33 participants that completed the study were asked if they were motivated to make lasting lifestyle changes. Twenty-three were motivated to continue to optimize sleep (69.7%; p = 0.035 vs. no), 17 to continue fasting (51.5%; p = 1.000 vs. no), 19 to continue exercising (57.6%; p = 0.487 vs. no), and 15 to continue brain training (45.5%; p = 0.728 vs. no). Overall, 19 participants stated that they were motivated to make lasting lifestyle changes (57.6%; p = 0.487 vs. no).
3.5. Safety
Post-emergent AEs were categorized as “unlikely” or “not related” to NeuroQ and were resolved by the end of the intervention. There was a statistically significant increase in chloride (p < 0.001) and decrease in total carbon dioxide (p = 0.019) at Day 82; however, these were deemed “not clinically relevant” by the MDs. There were no differences in the magnesium, vitamin B12, folate, or TSH levels from screening to Day 82.
4. Discussion
This exploratory intervention combined PreCODE with NeuroQ to determine the effects on cognitive function in adults at-risk of cognitive decline. Both the multidimensional lifestyle modifications and the ingredients in NeuroQ have previously shown beneficial and protective effects on cognitive function in adults at-risk of cognitive decline or with MCI or AD [15,16,17,18,24,25]. Following this 90-day intervention, adults at-risk of cognitive decline demonstrated significant improvements in their cognitive function, quality of life, daytime sleepiness, mood, and healthy lifestyle habits.
There are several potential mechanisms whereby the lifestyle modifications used in the current study and the ingredients that comprise NeuroQ may elicit their positive effects on cognitive function. Pre-clinical and clinical studies have shown that exercise induces neurogenesis in the hippocampal region of the brain, preserves white and gray matter, modulates neurotransmission systems, and increases neurotrophic factors [45]. Further, a fasting state promotes the production of ketones from the neuronal cells, which upregulate the expression of the brain-derived neurotrophic factor (BDNF), which in turn promotes mitochondrial biogenesis, synaptic plasticity, and cellular stress resistance [46]. Previous evidence showed that engagement in complex mental activity enhances resting neural activity, structural connectivity, and increases cerebral blood flow [47], while sleep restores the brain synaptic plasticity, which facilitates learning processes [48]. NeuroQ is comprised of turmeric, Gotu Kola, gingko biloba, propolis, and coffee fruit, which are all independently known to have antioxidant properties [24,26,49]. Accumulating evidence suggests that sources rich in antioxidants increase the expression of mitochondrial and antioxidant response genes, contributing to cognitive improvement [50]. Moreover, supplementation with polyphenolic compounds enhances neuronal communication, neuroprotective adaptations, and reduces stress signals [51,52]. Further, phosphatidylserine supplementation is reported to slow biochemical alterations and structural deterioration in nerve cells, and the incorporation of phosphatidylserine into the cell membrane influences the metabolism of neurotransmitters [23].
The potential for practice effects using the CNS-VS is certainly a consideration due to the repeated administration of the CNS-VS. Practice effects refer to the improvement in performance with repeated testing due to the recognition or familiarity with the assessment materials or procedures. The test-retest reliability is the variability that can occur in the same person, using the same instrument, under the same testing conditions. Certainly, both practice effects and test-retest reliability need to be considered when interpreting repeated neuropsychological assessments as this may erroneously overestimate the efficacy of the intervention. As previously reported, there is the potential for practice effects in cognitive testing [53]. Thus, to address this limitation, the tool CNS-VS selected to assess cognitive decline was based on its demonstrated suitability for repeated administration [37,54,55]. Practice effects within the CNS-VS have been shown to be present between the first and second assessments, whether those are one week or three months apart, but diminish after the second assessment, whether those are one week or twelve months apart [55,56]. Therefore, in the context of the current study, it is possible to conclude that practice effects would not have had an influence beyond the second assessment at Day 30. Based on the latter, it is possible to surmise that the results of this study remain true to the intent and purpose of the hypothesis.
There were significant improvements in neurocognitive performance at Days 30, 60, and 90,. Based on 1069 age-matched scores, the six-point increase in the NCI corresponded to an improvement from the 56th to 74th percentile, or from “average” to “above average” [44]. The improvement in cognition is consistent with the findings from the FINGER study, a two-year double-blind randomized controlled trial examining lifestyle interventions compared with general health advice in participants at-risk of cognitive decline [16], as well as a one year intervention that investigated the impact of lifestyle interventions through goal setting in community dwelling adults [15]. While these previous interventions observed increases in cognition in one-to-two years, the current intervention of PreCODE with NeuroQ elicited increases in cognitive function in 30 days. In forgetful Japanese older adults with low BDNF, dietary supplementation with curcumin, propolis extract, gingko biloba extract, and phosphatidylserine for 12 weeks improved the NCI [32]. These findings, in addition to the previous research, indicate that supplementation and lifestyle modifications are an effective way to improve cognitive function in at-risk adults in as little as 30 days.
The improvements to the NCI were associated with increases in nine of the thirteen domains assessed by the CNS-VS. There were significant improvements in: working memory; reaction time; complex- and sustained-attention; processing-, psychomotor- and motor-speed; cognitive flexibility; and executive function scores at Day 90. The greatest improvements at Day 90 were seen in executive function, cognitive flexibility, processing speed, and working memory. Improvements to reaction time, cognitive flexibility, executive function, psychomotor speed, and processing speed were observed 30 days after beginning PreCODE with NeuroQ, indicating that this intervention can rapidly improve cognitive function in adults at-risk of cognitive decline, faster than what has been shown previously with lifestyle interventions alone [15,16]. In natural aging, processing speed, working memory, attention, and executive function are susceptible to age-related declines [1]; thus, an intervention that can improve these cognitive domains is of high value. In forgetful Japanese older adults with low BDNF concentrations, 12 weeks of supplementation with an herbal blend of ingredients improved the NCI, as well as cognitive flexibility, executive function, and shifting attention [32]. Interestingly, participants with BDNF concentrations that were greater than the first quartile did not experience the same benefit to their cognitive function as those with BDNF concentrations less than the first quartile [32]. While BDNF was not assessed in this study, PreCODE with NeuroQ improved cognitive function in a broader range of cognitive domains in the entire cohort of participants. Therefore, it is possible that PreCODE with NeuroQ acted synergistically, providing more benefit than supplementation or lifestyle alone. However, further research is needed to better understand the effects of the combination of PreCODE with NeuroQ, compared to either intervention alone.
Memory is one of the most common cognitive complaints associated with aging [1], and poor memory has been associated with poorer QoL [57]. Working memory, a measure of how well a participant can hold information in their consciousness using short-term memory processes [58], was improved by 15% at Day 90 by the current study intervention. Recent studies examining curcumin, an ingredient in NeuroQ, reported significant improvements in working memory among healthy older adults [59], and all participants in a Japanese population taking a supplement with the same formulation of ingredients to NeuroQ saw improvements in immediate and delayed memory tasks [32]. Interestingly, a two-year multidimensional lifestyle intervention did not observe greater improvements to memory compared with generic health advice [16]. Thus, the observed improvements in memory may be due to the supplementation, or the combined effect of PreCODE with NeuroQ, rather than lifestyle modification alone; however, this hypothesis needs to be examined in a placebo-controlled double-blind study. Impairments in executive function, cognitive flexibility, and working memory specifically are associated with mild cognitive impairment [60,61]. Therefore, improvements in these domains in a population at-risk of cognitive decline are a central component of cognitive performance and highly relevant for everyday activities in this population.
Executive function, commonly referred to as problem solving and reasoning, is [62] relevant to a population with mild cognitive impairment [44]. In the current study, this cognitive flexibility was improved by 11% at Day 90. As both inductive reasoning and mental flexibility begin to decline after age 45 and 70, respectively [1], this intervention may offer improvements to the cognitive domains that are negatively affected by natural aging.
The collective improvements observed in the QoL, and life habits of the participants are conducive to a healthy lifestyle, speaking to the breadth of the potential effects of PreCODE with NeuroQ, far beyond improvements in cognitive performance itself. Cognitive complaints are associated with higher rates of depression, anxiety, and stress, and decreased QoL and mental wellbeing [63,64]. Depressive symptoms are associated with an increased risk of developing cognitive impairment or dementia [65]. This intervention resulted in a 56% reduction in the depression score at Day 90, potentially decreasing the risk of developing cognitive impairment or dementia. There was an 11% reduction in participants reporting minimal or mild depression on the PHQ-9. Significant improvements in energy, general health, pain, physical functioning, and role limitations due to physical health were reported on the QoL. The combined intervention of lifestyle modifications and supplement increased the QoL by greater than previous reports on lifestyle modifications alone [15,66]. A 25% reduction in daytime sleepiness was reported by the participants. Daytime sleepiness is associated with cognitive decline in older adults [67] and may be an early indicator of cognitive decline [68]; therefore, a reduction in daytime sleepiness may indicate a decreased risk of developing MCI [67]. Alongside the improvements in depression, QoL, and sleep, the participants reported healthier life habits across all domains of the Life Habits Checklist, such as neurodegenerative prevention, brain health promotion, body rhythm, dietary habits, and body shape and weight promotion. The latter suggests that participants perceived this intervention to promote several aspects of their health.
The baseline MMSE-2 scores in this population were consistent with the healthy control data [69]. Therefore, the lack of change in the MMSE-2 score may be due to a ceiling effect, rather than a lack of efficacy of the intervention.
This study was limited by the lack of comparison against a placebo group or one not receiving the intervention. The placebo effect, in cognition studies, has been attributed to several phenomena, including the perception of treatment and anticipatory effects [70,71]. Thus, future studies on the latter are certainly needed. Further, future studies could consider exploring the potential benefits of the PreCODE protocol alone, and NeuroQ supplementation alone to identify their unique effects. The age range of the participants in the current study was between 45 and 97 years, suggesting a heterogenous population based on age, including younger adults who may not have had a high potential to observe improvements. The literature suggests that mild cognitive impairment may develop prior to onset and, therefore, the current study focused on an at-risk population rather than those with cognitive pathologies [72]. Furthermore, 80% of the participants were western European, which may limit the application to other populations. There was considerable heterogeneity in the age and BMI of this study population, allowing for the results of this study to be translatable to the larger population and informative to future studies. Based on the inclusion criteria for this study, participants could have one or more risk factor for cognitive decline. However, the statistical analysis did not control for the number of risk factors, nor for multiple comparisons, given the small sample size and exploratory nature of the study. This is a limitation of the current study and should be considered in future, larger randomized controlled trials as there may be a difference in efficacy between populations with different risk factors for cognitive impairment.
Previous reports on supplementation intervention are comparable to the current study’s duration, ranging between 4 and 12 weeks [32,33,34,35,36]; however, lifestyle interventions may require a longer intervention time [73]. Lifestyle changes take time, effort and consistency for sustained benefit, and longer studies investigating the potential effects of PreCODE in combination with NeuroQ are relevant.
To our knowledge, this is the first study that has utilized a dietary multidimensional lifestyle intervention plus supplementation to successfully improve cognitive function. The mechanisms of action between the supplement and lifestyle modifications may differ. The results indicated that the combined use of PreCODE with NeuroQ improved cognitive function. The safety profile and the efficacy results of this study point to a potential reduction in the risk of developing AD or MCI after a short 90-day protocol.
5. Conclusions
This 90-day open-label exploratory study demonstrated that PreCODE with NeuroQ significantly improved cognitive function in adults at-risk of cognitive decline as early as 30 days after intervention initiation. Quality of life, daytime sleepiness, and mood were also improved, as were all of the domains of the Life Habits Checklist, indicating that the intervention is conducive to a healthy lifestyle. PreCODE with NeuroQ was found to be safe and well tolerated in this study population. The results support the hypothesis that 90 days of PreCODE with NeuroQ can improve cognitive function in adults at-risk of cognitive decline and suggest a potential for reducing the risk of developing MCI or AD. Placebo- or comparator-controlled studies are warranted to confirm the role of PreCODE with NeuroQ on cognitive function.
Author Contributions
Conceptualization, E.D.L., M.E. and J.L.; methodology, E.D.L. and M.E.; formal analysis, E.D.L. and M.E; investigation, M.A., A.P. and J.L.; data curation, A.P. and J.L.; writing—original draft preparation, E.D.L. and M.E.; writing—review and editing, M.A., A.P. and J.L.; visualization, E.D.L. and M.E.; supervision, M.A., M.E. and J.L.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research and APC was funded by LifeSeasons Inc., Texas, USA.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (IRB) Services, (Pro00039253, Advarra, Columbia, Maryland, approved on 16 October 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used in this study are available from the corresponding author on reasonable request.
Acknowledgments
The authors wish to thank our volunteers who participated in this study and for their compliance to the conduct of the study. We wish to thank Abdul Sulley for his assistance with the statistical analysis.
Conflicts of Interest
J.L. and A.P. are employees of LifeSeasons, Inc.; M.A. received funding to oversee conduct of the study as the Principal Investigator; E.D.L and M.E. have no financial interests in or conflict with the subject matter or materials discussed.
References
- Harada, C.N.; Love, M.C.N.; Triebel, K.L. Normal Cognitive Aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef]
- Prince, M.J.; Wimo, A.; Guerchet, M.M.; Ali, G.C.; Wu, Y.-T.; Prina, M. World Alzheimer Report 2015—The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Luck, T.; Luppa, M.; Briel, S.; Riedel-Heller, S.G. Incidence of Mild Cognitive Impairment: A Systematic Review. Dement. Geriatr. Cogn. Disord. 2010, 29, 164–175. [Google Scholar] [CrossRef]
- Janoutová, J.; Serý, O.; Hosák, L.; Janout, V. Is mild cognitive impairment a precursor of Alzheimer’s disease? Short review. Cent. Eur. J. Public Health 2015, 23, 365. [Google Scholar] [CrossRef]
- Petersen, R.C.; Negash, S. Mild Cognitive Impairment: An Overview. CNS Spectr. 2008, 13, 45–53. [Google Scholar] [CrossRef]
- Leibson, C.L.; Long, K.H.; Ransom, J.E.; Roberts, R.O.; Hass, S.L.; Duhig, A.M.; Smith, C.Y.; Emerson, J.A.; Pankratz, V.S.; Petersen, R.C. Direct medical costs and source of cost differences across the spectrum of cognitive decline: A population-based study. Alzheimer’s Dement 2015, 11, 917–932. [Google Scholar] [CrossRef]
- WHO. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Castro, D.M.; Dillon, C.; Machnicki, G.; Allegri, R.F. The economic cost of Alzheimer’s disease: Family or public-health burden? Dement. Neuropsychol. 2010, 4, 262–267. [Google Scholar] [CrossRef]
- El-Hayek, Y.H.; Wiley, R.E.; Khoury, C.P.; Daya, R.P.; Ballard, C.; Evans, A.R.; Karran, M.; Molinuevo, J.L.; Norton, M.; Atri, A. Tip of the Iceberg: Assessing the Global Socioeconomic Costs of Alzheimer’s Disease and Related Dementias and Strategic Implications for Stakeholders. J. Alzheimer’s Dis. 2019, 70, 323–341. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Brodziak, A.; Wolińska, A.; Kołat, E.; Różyk-Myrta, A. Guidelines for Prevention and Treatment of Cognitive Impairment in the Elderly. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 585. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J. Alzheimer’s Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef]
- Naqvi, R.; Liberman, D.; Rosenberg, J.; Alston, J.; Straus, S. Preventing cognitive decline in healthy older adults. Can. Med. Assoc. J. 2013, 185, 881–885. [Google Scholar] [CrossRef]
- Thompson, T.W.; Waskom, M.L.; Garel, K.-L.; Cardenas-Iniguez, C.; Reynolds, G.O.; Winter, R.; Chang, P.; Pollard, K.; Lala, N.; Alvarez, G.A.; et al. Failure of Working Memory Training to Enhance Cognition or Intelligence. PLoS ONE 2013, 8, e63614. [Google Scholar] [CrossRef]
- Clare, L.; Nelis, S.M.; Jones, I.R.; Hindle, J.V.; Thom, J.M.; Nixon, J.; Cooney, J.; Jones, C.L.; Edwards, R.T.; Whitaker, C.J. The Agewell trial: A pilot randomised controlled trial of a behaviour change intervention to promote healthy ageing and reduce risk of dementia in later life. BMC Psychiatry 2015, 15, 25. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- E Bredesen, D.; Sharlin, K.; Jenkins, D.; Okuno, M.; Youngberg, W.; Cohen, S.H.; Stefani, A.; Brown, R.L.; Conger, S.; Tanio, C.; et al. Reversal of Cognitive Decline: 100 Patients. J. Alzheimer’s Dis. Park. 2018, 8, 450. [Google Scholar] [CrossRef]
- Rao, R.V.; Kumar, S.; Gregory, J.; Coward, C.; Okada, S.; Lipa, W.; Kelly, L.; E Bredesen, D. ReCODE: A Personalized, Targeted, Multi-Factorial Therapeutic Program for Reversal of Cognitive Decline. Biomedicines 2021, 9, 1348. [Google Scholar] [CrossRef]
- Moon, S.Y.; Hong, C.H.; Jeong, J.H.; Park, Y.K.; Na, H.R.; Song, H.-S.; Kim, B.C.; Park, K.W.; Park, H.K.; Choi, M.; et al. Facility-based and home-based multidomain interventions including cognitive training, exercise, diet, vascular risk management, and motivation for older adults: A randomized controlled feasibility trial. Aging 2021, 13, 15898–15916. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Z.; Zhu, X.; Zheng, Z.; Li, J.; Fu, J.; Shao, Q.; Han, X.; Wang, X.; Wang, Z.; et al. Cognitive Benefit of a Multidomain Intervention for Older Adults at Risk of Cognitive Decline: A Cluster-Randomized Controlled Trial. Am. J. Geriatr. Psychiatry 2022, 31, 197–209. [Google Scholar] [CrossRef]
- Anstey, K.J.; Cherbuin, N.; Kim, S.; McMaster, M.; D’Este, C.; Lautenschlager, N.; Rebok, G.; McRae, I.; Torres, S.J.; Cox, K.L.; et al. An Internet-Based Intervention Augmented with a Diet and Physical Activity Consultation to Decrease the Risk of Dementia in At-Risk Adults in a Primary Care Setting: Pragmatic Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e19431. [Google Scholar] [CrossRef]
- Ng, P.E.M.; Nicholas, S.O.; Wee, S.L.; Yau, T.Y.; Chan, A.; Chng, I.; Yap, L.K.P.; Ng, T.P. Implementation and effectiveness of a multi-domain program for older adults at risk of cognitive impairment at neighborhood senior centres. Sci. Rep. 2021, 11, 3787. [Google Scholar] [CrossRef]
- Glade, M.J.; Smith, K. Phosphatidylserine and the human brain. Nutrition 2015, 31, 781–786. [Google Scholar] [CrossRef]
- Gregory, J.; Vengalasetti, Y.; Bredesen, D.; Rao, R. Neuroprotective Herbs for the Management of Alzheimer’s Disease. Biomolecules 2021, 11, 543. [Google Scholar] [CrossRef]
- Iriti, M.; Vitalini, S.; Fico, G.; Faoro, F. Neuroprotective Herbs and Foods from Different Traditional Medicines and Diets. Molecules 2010, 15, 3517–3555. [Google Scholar] [CrossRef]
- Nemzer, B.; Kalita, D.; Abshiru, N. Quantification of Major Bioactive Constituents, Antioxidant Activity, and Enzyme Inhibitory Effects of Whole Coffee Cherries (Coffea arabica) and Their Extracts. Molecules 2021, 26, 4306. [Google Scholar] [CrossRef]
- Olivera-Pueyo, J.; Pelegrín-Valero, C. Dietary supplements for cognitive impairment. Actas Esp. Psiquiatr. 2017, 45, 37–47. [Google Scholar]
- Wattanathorn, J.; Mator, L.; Muchimapura, S.; Tongun, T.; Pasuriwong, O.; Piyawatkul, N.; Yimtae, K.; Sripanidkulchai, B.; Singkhoraard, J. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J. Ethnopharmacol. 2008, 116, 325–332. [Google Scholar] [CrossRef]
- Reed, R.A.; Mitchell, E.S.; Saunders, C.; O’Connor, P.J. Acute Low and Moderate Doses of a Caffeine-Free Polyphenol-Rich Coffeeberry Extract Improve Feelings of Alertness and Fatigue Resulting from the Performance of Fatiguing Cognitive Tasks. J. Cogn. Enhanc. 2018, 3, 193–206. [Google Scholar] [CrossRef]
- Okuda, M.; Fujita, Y.; Sugimoto, H. The Additive Effects of Low Dose Intake of Ferulic Acid, Phosphatidylserine and Curcumin, Not Alone, Improve Cognitive Function in APPswe/PS1dE9 Transgenic Mice. Biol. Pharm. Bull. 2019, 42, 1694–1706. [Google Scholar] [CrossRef]
- Araujo, J.; Landsberg, G.M.; Milgram, N.W.; Miolo, A. Improvement of short-term memory performance in aged beagles by a nutraceutical supplement containing phosphatidylserine, Ginkgo biloba, vitamin E, and pyridoxine. Can. Vet. J. 2008, 49, 379–385. [Google Scholar]
- Asama, T. Cognitive Improvement and Safety Assessment of a Composite Dietary Supplement Containing Propolis Extract, Gingko biloba Extract, Phosphatidylserine and Curcumin in Healthy Mid—To Senior Age Japanese Adults―A Placebo—Controlled, Randomized, Parallel—Group, Double—Blind Human Clinical Study. Jpn. Pharmacol. Ther. 2020, 48, 1805–1819. [Google Scholar]
- Mix, J.A.; Crews, W. A double-blind, placebo-controlled, randomized trial of Ginkgo biloba extract EGb 761® in a sample of cognitively intact older adults: Neuropsychological findings. Hum. Psychopharmacol. Clin. Exp. 2002, 17, 267–277. [Google Scholar] [CrossRef]
- Richter, Y.; Herzog, Y.; Lifshitz, Y.; Hayun, R.; Zchut, S. The effect of soybean-derived phosphatidylserine on cognitive performance in elderly with subjective memory complaints: A pilot study. Clin. Interv. Aging 2013, 8, 557–563. [Google Scholar] [CrossRef]
- Stough, C.; Clarke, J.; Lloyd, J.; Nathan, P.J. Neuropsychological changes after 30-day Ginkgo biloba administration in healthy participants. Int. J. Neuropsychopharmacol. 2001, 4, 131–134. [Google Scholar] [CrossRef]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef]
- Gualtieri, C.T.; Johnson, L.G. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch. Clin. Neuropsychol. 2006, 21, 623–643. [Google Scholar] [CrossRef]
- Gualtieri, C.T.; Johnson, L.G. A computerized test battery sensitive to mild and severe brain injury. Am. J. Med. 2008, 10, 90. [Google Scholar]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; White, T.; Messer, M.A. MMSE-2: Mini-Mental State Examination; Psychological Assessments Resources: Lutz, FL, USA, 2010. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- CNS Vital Signs. Interpretation Guide. Available online: https://www.cnsvs.com/WhitePapers/CNSVS-BriefInterpretationGuide.pdf (accessed on 21 January 2022).
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Gudden, J.; Vasquez, A.A.; Bloemendaal, M. The Effects of Intermittent Fasting on Brain and Cognitive Function. Nutrients 2021, 13, 3166. [Google Scholar] [CrossRef]
- Chapman, S.B.; Aslan, S.; Spence, J.S.; Hart, J.J.; Bartz, E.K.; Didehbani, N.; Keebler, M.W.; Gardner, C.M.; Strain, J.F.; DeFina, L.F. Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cereb. Cortex 2015, 25, 396–405. [Google Scholar] [CrossRef]
- Gorgoni, M.; D’Atri, A.; Lauri, G.; Rossini, P.M.; Ferlazzo, F.; De Gennaro, L. Is sleep essential for neural plasticity in humans, and how does it affect motor and cognitive recovery? Neural Plast. 2013, 2013, 103949. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2013, 19, 78–101. [Google Scholar] [CrossRef]
- Gray, N.E.; Harris, C.J.; Quinn, J.F.; Soumyanath, A. Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. J. Ethnopharmacol. 2016, 180, 78–86. [Google Scholar] [CrossRef]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P. Reversals of Age-Related Declines in Neuronal Signal Transduction, Cognitive, and Motor Behavioral Deficits with Blueberry, Spinach, or Strawberry Dietary Supplementation. J. Neurosci. 1999, 19, 8114–8121. [Google Scholar] [CrossRef]
- Lau, F.C.; Shukitt-Hale, B.; Joseph, J.A. The beneficial effects of fruit polyphenols on brain aging. Neurobiol. Aging 2005, 26, 128–132. [Google Scholar] [CrossRef]
- Hellmuth, J. Can we trust The End of Alzheimer’s? Lancet Neurol. 2020, 19, 389–390. [Google Scholar] [CrossRef]
- Cole, W.R.; Arrieux, J.P.; Schwab, K.; Ivins, B.J.; Qashu, F.M.; Lewis, S.C. Test-Retest Reliability of Four Computerized Neurocognitive Assessment Tools in an Active Duty Military Population. Arch. Clin. Neuropsychol. 2013, 28, 732–742. [Google Scholar] [CrossRef]
- Littleton, A.C.; Register-Mihalik, J.K.; Guskiewicz, K.M. Test-Retest Reliability of a Computerized Concussion Test: CNS Vital Signs. Sports Health 2015, 7, 443–447. [Google Scholar] [CrossRef]
- Rijnen, S.J.M.; Van der Linden, S.D.; Emons, W.H.M.; Sitskoorn, M.M.; Gehring, K. Test-retest reliability and practice effects of a computerized neuropsychological battery: A solution-oriented approach. Psychol. Assess. 2018, 30, 1652–1662. [Google Scholar] [CrossRef]
- Woods, D.L.; Wyma, J.M.; Yund, E.W.; Herron, T.J.; Reed, B. Factors influencing the latency of simple reaction time. Front. Hum. Neurosci. 2015, 9, 131. [Google Scholar] [CrossRef]
- Baddeley, A. Working Memory. Science 1992, 255, 556–559. [Google Scholar] [CrossRef]
- Cox, K.H.M.; White, D.J.; Pipingas, A.; Poorun, K.; Scholey, A. Further Evidence of Benefits to Mood and Working Memory from Lipidated Curcumin in Healthy Older People: A 12-Week, Double-Blind, Placebo-Controlled, Partial Replication Study. Nutrients 2020, 12, 1678. [Google Scholar] [CrossRef]
- Guarino, A.; Forte, G.; Giovannoli, J.; Casagrande, M. Executive functions in the elderly with mild cognitive impairment: A systematic review on motor and cognitive inhibition, conflict control and cognitive flexibility. Aging Ment. Health 2020, 24, 1028–1045. [Google Scholar] [CrossRef]
- Saunders, N.; Summers, M.J. Attention and working memory deficits in mild cognitive impairment. J. Clin. Exp. Neuropsychol. 2010, 32, 350–357. [Google Scholar] [CrossRef]
- Harvey, P.D. Domains of cognition and their assessment. Dialogues Clin. Neurosci. 2019, 21, 227–237. [Google Scholar] [CrossRef]
- Pusswald, G.; Tropper, E.; Kryspin-Exner, I.; Moser, D.; Klug, S.; Auff, E.; Dal-Bianco, P.; Lehrner, J. Health-Related Quality of Life in Patients with Subjective Cognitive Decline and Mild Cognitive Impairment and its Relation to Activities of Daily Living. J. Alzheimer’s Dis. 2015, 47, 479–486. [Google Scholar] [CrossRef]
- Stites, S.D.; Harkins, K.; Rubright, J.D.; Karlawish, J. Relationships Between Cognitive Complaints and Quality of Life in Older Adults with Mild Cognitive Impairment, Mild Alzheimer Disease Dementia, and Normal Cognition. Alzheimer Dis. Assoc. Disord. 2018, 32, 276–283. [Google Scholar] [CrossRef]
- Mourao, R.J.; Mansur, G.; Malloy-Diniz, L.F.; Castro Costa, E.; Diniz, B.S. Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: Systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2016, 31, 905–911. [Google Scholar] [CrossRef]
- Strandberg, T.; Levälahti, E.; Ngandu, T.; Solomon, A.; Kivipelto, M.; Lehtisalo, J.; Laatikainen, T.; Soininen, H.; Antikainen, R.; Jula, A.; et al. Health-related quality of life in a multidomain intervention trial to prevent cognitive decline (FINGER). Eur. Geriatr. Med. 2017, 8, 164–167. [Google Scholar] [CrossRef]
- Foley, D.; Monjan, A.; Masaki, K.; Ross, W.; Havlik, R.; White, L.; Launer, L. Daytime Sleepiness Is Associated with 3-Year Incident Dementia and Cognitive Decline in Older Japanese-American Men. J. Am. Geriatr. Soc. 2001, 49, 1628–1632. [Google Scholar] [CrossRef]
- Merlino, G.; Piani, A.; Gigli, G.; Cancelli, I.; Rinaldi, A.; Baroselli, A.; Serafini, A.; Zanchettin, B.; Valente, M. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: A population-based study. Sleep Med. 2010, 11, 372–377. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; Hochstetler, H.; Wang, S.; Walker, B.; Saykin, A.J.; Alzheimer’s Disease Neuroimaging. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for Assessment of Mild Cognitive Impairment in Llder Adults. BMC Geriatr. 2015, 15, 107. [Google Scholar] [CrossRef]
- Colagiuri, B.; Boakes, R.A. Perceived treatment, feedback, and placebo effects in double-blind RCTs: An experimental analysis. Psychopharmacology 2010, 208, 433–441. [Google Scholar] [CrossRef]
- Turi, Z.; Bjørkedal, E.; Gunkel, L.; Antal, A.; Paulus, W.; Mittner, M. Evidence for Cognitive Placebo and Nocebo Effects in Healthy Individuals. Sci. Rep. 2018, 8, 17443. [Google Scholar] [CrossRef]
- Kremen, W.S.; Jak, A.J.; Panizzon, M.S.; Spoon, K.M.; E Franz, C.; Thompson, W.K.; Jacobson, K.C.; Vasilopoulos, T.; Vuoksimaa, E.; Xian, H.; et al. Early identification and heritability of mild cognitive impairment. Int. J. Epidemiol. 2014, 43, 600–610. [Google Scholar] [CrossRef]
- Toman, J.; Klímová, B.; Vališ, M. Multidomain lifestyle intervention strategies for the delay of cognitive impairment in healthy aging. Nutrients 2018, 10, 1560. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).