Solvent-Dispersible Nanostructured MIMI: An Experimental and Computational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
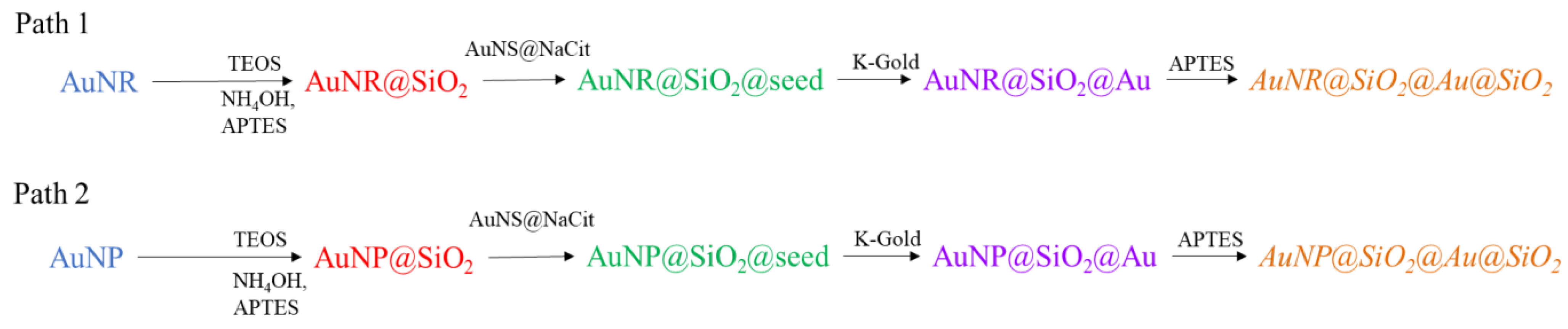
2.3. Synthetic Procedure
2.4. AuNR
2.5. AuNP@CTAB
2.6. AuNP@SiO2, AuNR@SiO2
2.7. AuNS@NaCit
2.8. AuNP@SiO2@seed, AuNR@SiO2@seed
2.9. K-Gold Solution
2.10. AuNP@SiO2@Au, AuNR@SiO2@Au
2.11. AuNP@SiO2@Au@SiO2, AuNR@SiO2@Au@SiO2
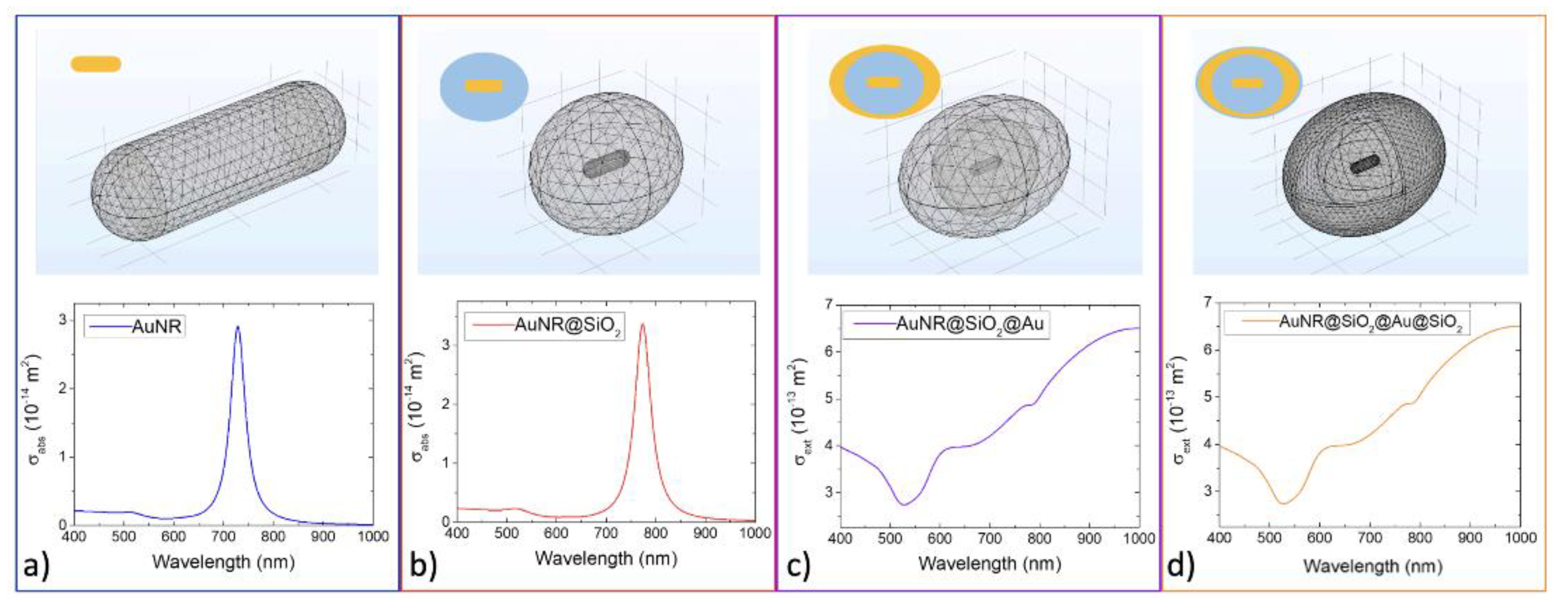
2.12. Theoretical Calculations
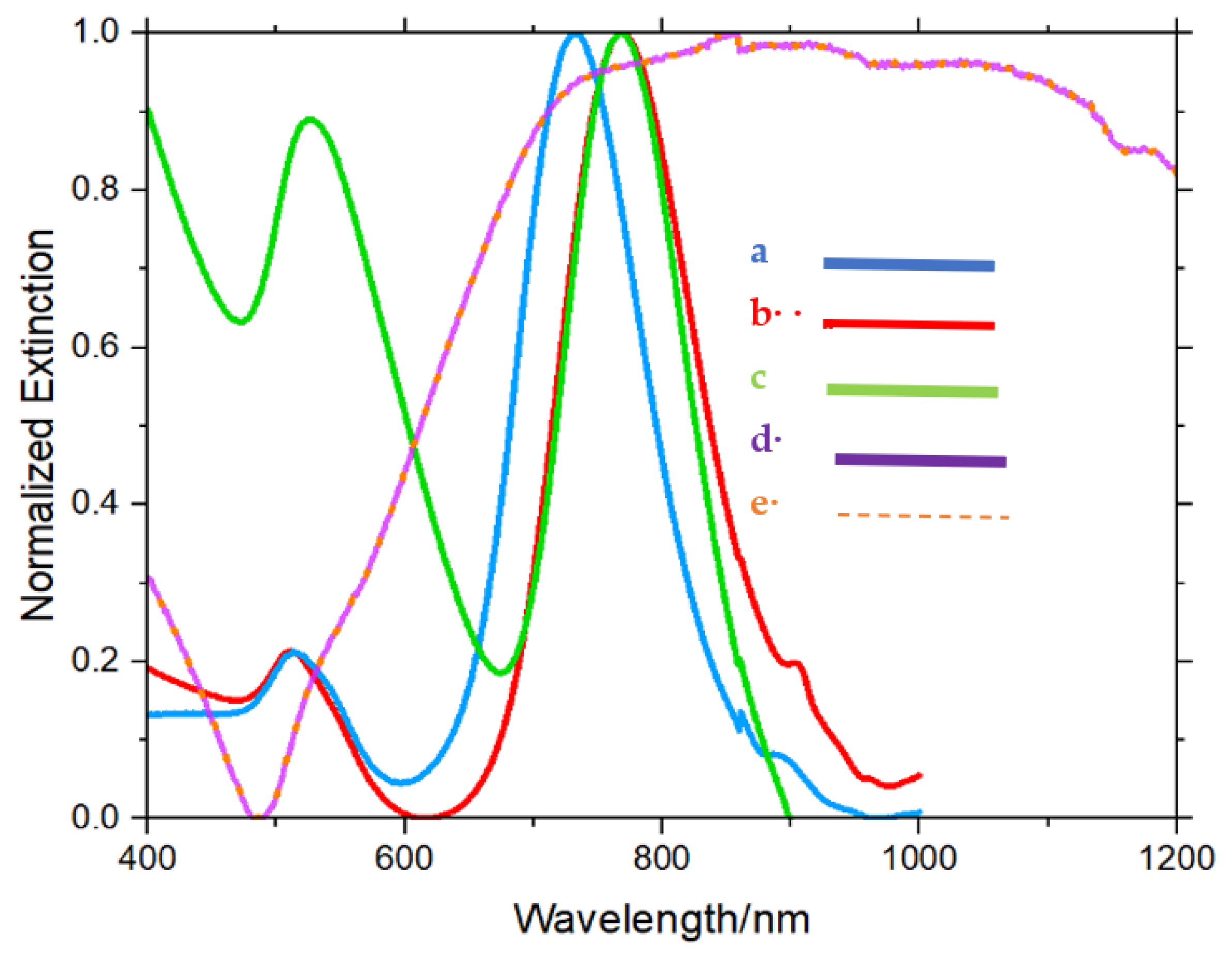
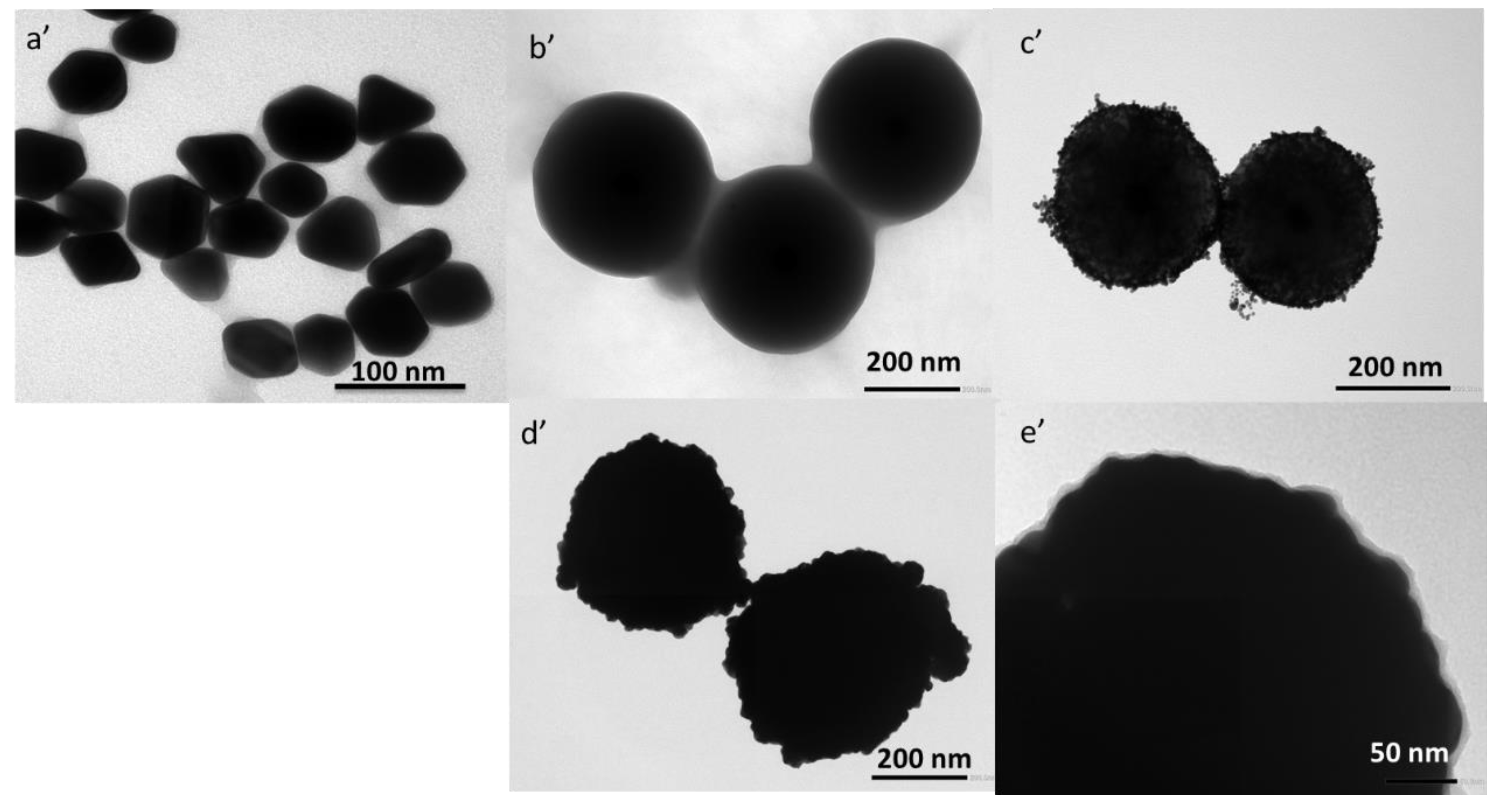
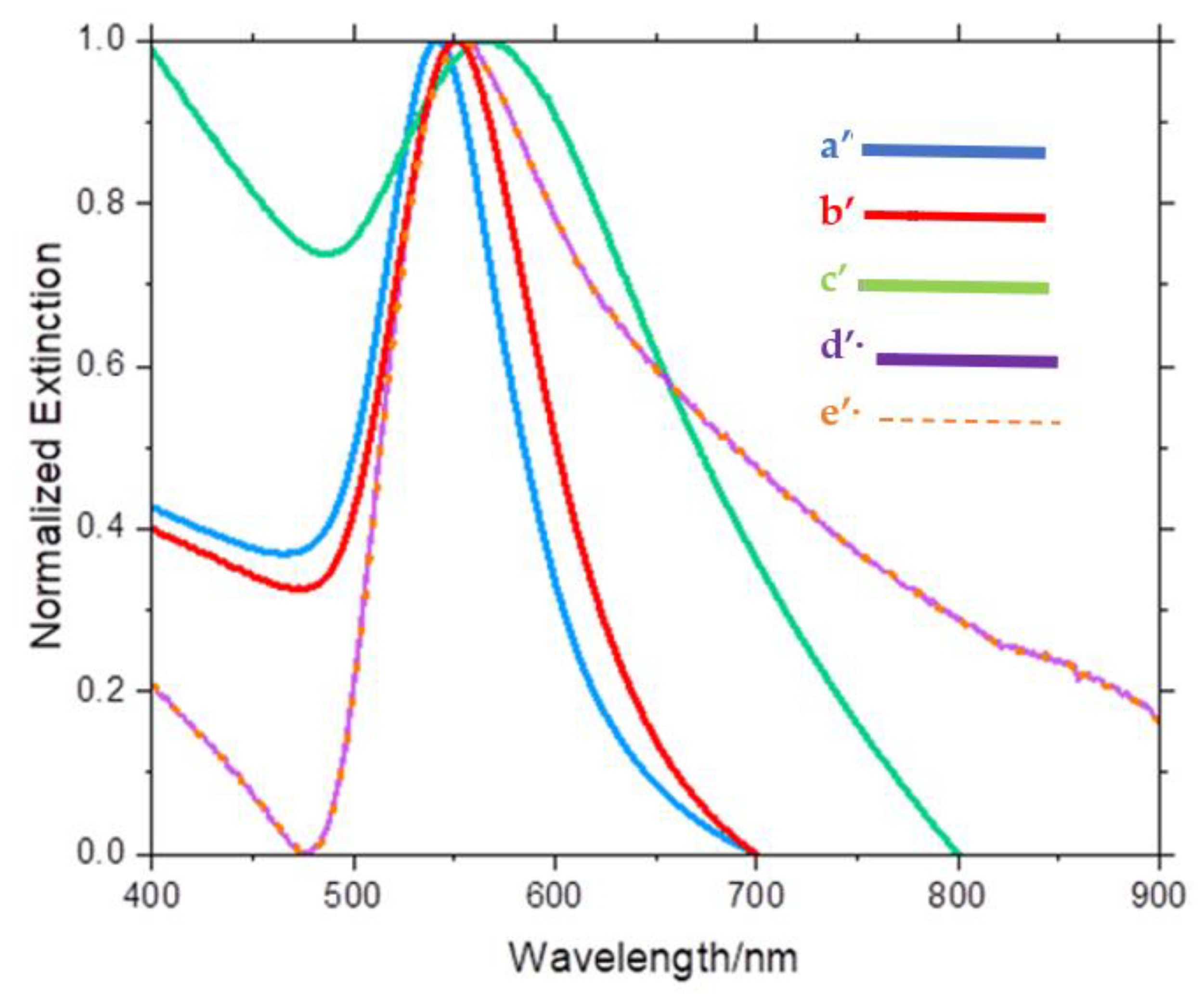
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roco, M.C. Nanotechnology: Convergence with modern biology and medicine. Curr. Opin. Biotechnol. 2003, 14, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roco, M.C. Principles of convergence in nature and society and their application: From nanoscale, digits, and logic steps to global progress. J. Nanoparticle Res. 2020, 22, 321. [Google Scholar] [CrossRef] [PubMed]
- Candreva, A.; De Rose, R.; Perrotta, I.D.; Guglielmelli, A.; La Deda, M. Light-Induced Clusterization of Gold Nanoparticles: A New Photo-Triggered Antibacterial against E. coli Proliferation. Nanomaterials 2023, 13, 746. [Google Scholar] [CrossRef]
- Fu, Y.; Fang, F.; Xu, Z. Nanofabrication and Characterization of Plasmonic Structures. Nanofabrication 2011, 4, 243–266. [Google Scholar] [CrossRef] [Green Version]
- Henzie, J.; Lee, J.; Lee, M.H.; Hasan, W.; Odom, T.W. Nanofabrication of plasmonic structures. Annu. Rev. Phys. Chem. 2009, 60, 147–165. [Google Scholar] [CrossRef] [Green Version]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Mohan Bhagyaraj, S.; Oluwafemi, O.S. Nanotechnology: The Science of the Invisible; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zeng, X.; Liang, J. Surface plasmon resonance: An introduction to a surface spectroscopy technique. J. Chem. Educ. 2010, 87, 742–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Gong, M.; Fan, Y.; Feng, J.; Han, L.; Xin, H.L.; Cao, M.; Zhang, Q.; Zhang, D.; Lei, D.; et al. Collective Plasmon Coupling in Gold Nanoparticle Clusters for Highly Efficient Photothermal Therapy. ACS Nano 2022, 16, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Scheeler, S.P.; Mühlig, S.; Rockstuhl, C.; Hasan, S.B.; Ullrich, S.; Neubrech, F.; Kudera, S.; Pacholski, C. Plasmon coupling in self-assembled gold nanoparticle-based honeycomb islands. J. Phys. Chem. C 2013, 117, 18634–18641. [Google Scholar] [CrossRef]
- M.C. Daniel, D.A. Plasmon coupling in self-assembled gold nanoparticle-based honeycomb islands. Chem. Rev. 2004, 104, 293–346. [Google Scholar]
- Hanske, C.; Tebbe, M.; Kuttner, C.; Bieber, V.; Tsukruk, V.V.; Chanana, M.; König, T.A.F.; Fery, A. Strongly coupled plasmonic modes on macroscopic areas via template-assisted colloidal self-assembly. Nano Lett. 2014, 14, 6863–6871. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, R.H. Surface plasmons in solids. Surf. Sci. 1973, 34, 1–19. [Google Scholar] [CrossRef]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. Plasmonic sensors based on Metal-insulator-metal waveguides for refractive index sensing applications: A brief review. Phys. E Low-Dimensional Syst. Nanostructures 2020, 117, 113798. [Google Scholar] [CrossRef]
- Ghasemi, M.R.; Bayati, M.S. Proposal for metal–insulator–metal plasmonic power splitter and demultiplexer suitable for implementation in optical switches. IET Optoelectron. 2021, 15, 200–206. [Google Scholar] [CrossRef]
- Liang, Z.; Wen, Y.; Zhang, Z.; Liang, Z.; Xu, Z.; Lin, Y.S. Plasmonic metamaterial using metal-insulator-metal nanogratings for high-sensitive refraction index sensor. Results Phys. 2019, 15, 102602. [Google Scholar] [CrossRef]
- Tamura, M.; Kagata, H. Analysis of metal-insulator-metal structure and its application to sensor. IEEE Trans. Microw. Theory Tech. 2010, 58, 3954–3960. [Google Scholar] [CrossRef]
- Shin, Y.J.; Son, J.H.; Lee, D.H.; Lee, M.H. Insulator-metal-insulator-metal-insulator and metal-insulator-metal vertical directional couplers with surface plasmon polariton waveguides. J. Nanosci. Nanotechnol. 2017, 17, 7093–7100. [Google Scholar] [CrossRef]
- Yan, M. Metal-insulator-metal light absorber: A continuous structure. J. Opt. 2013, 15, 025006. [Google Scholar] [CrossRef]
- Hung, C.C.; Oates, A.S.; Lin, H.C.; Chang, P.; Wang, J.L.; Huang, C.C.; Yau, Y.W. New understanding of Metal-Insulator-Metal (MIM) capacitor degradation behavior. Annu. Proc.-Reliab. Phys. 2007, 5, 630–631. [Google Scholar] [CrossRef] [Green Version]
- Ekanayake, S.R.; Ford, M.; Cortie, M. Metal-insulator-metal (MIM) nanocapacitors and effects of material properties on their operation. Mater. Forum 2004, 27, 15–20. [Google Scholar]
- Sreekanth, K.V.; De Luca, A.; Strangi, G. Negative refraction in graphene-based hyperbolic metamaterials. Appl. Phys. Lett. 2013, 103, 023107. [Google Scholar] [CrossRef]
- Le, K.Q.; Ngo, Q.M.; Nguyen, T.K. Nanostructured Metal-Insulator-Metal Metamaterials for Refractive Index Biosensing Applications: Design, Fabrication, and Characterization. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 388–393. [Google Scholar] [CrossRef]
- Ogawa, S.; Kimata, M. Metal-insulator-metal-based plasmonic metamaterial absorbers at visible and infrared wavelengths: A review. Materials 2018, 11, 458. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Sauvan, C.; Jouanin, A.; Collin, S.; Pelouard, J.L.; Lalanne, P. Quality factor of 3D Metal-Insulator-Metal nanoresonators. In Proceedings of the 2013 7th International Congress on Advanced Electromagnetic Materials in Microwaves and Optics, Talence, France, 16–21 September 2013; pp. 397–399. [Google Scholar] [CrossRef]
- Kharlamov, V.F.; Korostelev, D.A.; Bogoraz, I.G.; Milovidova, O.A. Electrical properties of the metal-insulator nanoparticles-metal structure. Phys. Solid State 2012, 54, 1281–1288. [Google Scholar] [CrossRef]
- Thissen, P.; Schindler, B.; Diesing, D.; Hasselbrink, E. Optical response of metal-insulator-metal heterostructures and their application for the detection of chemicurrents. New J. Phys. 2010, 12, 113014. [Google Scholar] [CrossRef]
- Periasamy, P.; O’Hayre, R.P.; Berry, J.J.; Parilla, P.A.; Ginley, D.S.; Packard, C.E. A novel way to characterize metal-insulator-metal devices via nanoindentation. In Proceedings of the 2011 37th IEEE Photovoltaic Specialists Conference, Seattle, WA, USA, 19–24 June 2011; pp. 001754–001757. [Google Scholar] [CrossRef]
- Hamon, C.; Sanz-Ortiz, M.N.; Modin, E.; Hill, E.H.; Scarabelli, L.; Chuvilin, A.; Liz-Marzán, L.M. Hierarchical organization and molecular diffusion in gold nanorod/silica supercrystal nanocomposites. Nanoscale 2016, 8, 7914–7922. [Google Scholar] [CrossRef] [Green Version]
- Hamon, C.; Novikov, S.; Scarabelli, L.; Basabe-Desmonts, L.; Liz-Marzán, L.M. Hierarchical self-assembly of gold nanoparticles into patterned plasmonic nanostructures. ACS Nano 2014, 8, 10694–10703. [Google Scholar] [CrossRef]
- Turković, A.; Dubček, P.; Fox, N.D. Self-organization of nanoparticles in a TiO2 thin film on a glass substrate. Vacuum 2005, 80, 108–112. [Google Scholar] [CrossRef]
- Caputo, G.; Pinna, N. Nanoparticle self-assembly using π-π Interactions. J. Mater. Chem. A 2013, 1, 2370–2378. [Google Scholar] [CrossRef]
- Candreva, A.; Morrone, E.; La Deda, M. Gold Sea Urchin-Shaped Nanoparticles: Synthesis and Characterization of Energy Transducer Candidates. Plasmonics 2022, 18, 291–298. [Google Scholar] [CrossRef]
- Song, W.; Yoshitake, M.; Yamauchi, Y.; Lykhach, Y. Fabrication of nano-scale metal-insulator-metal cathode: Control of film structure and thickness. In Proceedings of the IVESC 2004. The 5th International Vacuum Electron Sources Conference Proceedings (IEEE Cat. No. 04EX839), Beijing, China, 6–10 September 2004; pp. 131–133. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; Alapan, Y.; Elkabbash, M.; Ilker, E.; Hinczewski, M.; Gurkan, U.A.; De Luca, A.; Strangi, G. Extreme sensitivity biosensing platform based on hyperbolic metamaterials. Nat. Mater. 2016, 15, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Khanikaev, A.B.; Adato, R.; Arju, N.; Yanik, A.A.; Altug, H.; Shvets, G. Fano-resonant asymmetric metamaterials for ultrasensitive spectroscopy and identification of molecular monolayers. Nat. Mater. 2012, 11, 69–75. [Google Scholar] [CrossRef]
- Zeng, S.; Sreekanth, K.V.; Shang, J.; Yu, T.; Chen, C.K.; Yin, F.; Baillargeat, D.; Coquet, P.; Ho, H.P.; Kabashin, A.V.; et al. Graphene-Gold Metasurface Architectures for Ultrasensitive Plasmonic Biosensing. Adv. Mater. 2015, 27, 6163–6169. [Google Scholar] [CrossRef]
- Liu, N.; Weiss, T.; Mesch, M.; Langguth, L.; Eigenthaler, U.; Hirscher, M.; Sönnichsen, C.; Giessen, H. Planar metamaterial analogue of electromagnetically induced transparency for plasmonic sensing. Nano Lett. 2010, 10, 1103–1107. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Evans, P.; Pastkovsky, S.; Hendren, W.; Wurtz, G.A.; Atkinson, R.; Pollard, R.; Podolskiy, V.A.; Zayats, A.V. Plasmonic nanorod metamaterials for biosensing. Nat. Mater. 2009, 8, 867–871. [Google Scholar] [CrossRef]
- Gao, C.; Goebl, J.; Yin, Y. Seeded growth route to noble metal nanostructures. J. Mater. Chem. C 2013, 1, 3898–3909. [Google Scholar] [CrossRef]
- Xia, Y.; Gilroy, K.D.; Peng, H.C.; Xia, X. Seed-Mediated Growth of Colloidal Metal Nanocrystals. Angew. Chemie-Int. Ed. 2017, 56, 60–95. [Google Scholar] [CrossRef] [PubMed]
- Millstone, J.E.; Wei, W.; Jones, M.R.; Yoo, H.; Mirkin, C.A. Iodide ions control seed-mediated growth of anisotropic gold Nanoparticles. Nano Lett. 2008, 8, 2526–2529. [Google Scholar] [CrossRef]
- Leng, W.; Pati, P.; Vikesland, P.J. Room temperature seed mediated growth of gold nanoparticles: Mechanistic investigations and life cycle assesment. Environ. Sci. Nano 2015, 2, 440–453. [Google Scholar] [CrossRef] [Green Version]
- Candreva, A.; Parisi, F.; Bartucci, R.; Guzzi, R.; Maio, D. Synthesis and Characterization of Hyper-Branched Nanoparticles with Magnetic and Plasmonic Properties. ChemistrySelect 2022, 7, 202201375. [Google Scholar] [CrossRef]
- Scarabelli, L.; Grzelczak, M.; Liz-Marzán, L.M. Tuning gold nanorod synthesis through prereduction with salicylic acid. Chem. Mater. 2013, 25, 4232–4238. [Google Scholar] [CrossRef] [Green Version]
- Scarabelli, L.; Sánchez-Iglesias, A.; Pérez-Juste, J.; Liz-Marzán, L.M. A “Tips and Tricks” Practical Guide to the Synthesis of Gold Nanorods. J. Phys. Chem. Lett. 2015, 6, 4270–4279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candreva, A.; Lewandowski, W.; La Deda, M. Thickness control of the Silica Shell: A way to tune the Plasmonic Properties of isolated and assembled Gold Nanorods. J. Nanoparticle Res. 2022, 24, 19. [Google Scholar] [CrossRef]
- Murphy, C.J.; Thompson, L.B.; Chernak, D.J.; Yang, J.A.; Sivapalan, S.T.; Boulos, S.P.; Huang, J.; Alkilany, A.M.; Sisco, P.N. Gold nanorod crystal growth: From seed-mediated synthesis to nanoscale sculpting. Curr. Opin. Colloid Interface Sci. 2011, 16, 128–134. [Google Scholar] [CrossRef]
- Candreva, A.; Di Maio, G.; Parisi, F.; Scarpelli, F.; Crispini, A.; Godbert, N.; Ricciardi, L.; Nucera, A.; Rizzuto, C.; Barberi, R.C.; et al. Luminescent Self-Assembled Monolayer on Gold Nanoparticles: Tuning of Emission According to the Surface Curvature. Chemosensors 2022, 10, 176. [Google Scholar] [CrossRef]
- Candreva, A.; Parisi, F.; Di, G.; Francesca, M.; Iolinda, S.; Godbert, N.; La, M. Post—Synthesis heating, a key step to tune the LPR band of gold nanorods covered with CTAB or embedded in a silica shell. Gold Bull. 2022, 55, 195–205. [Google Scholar] [CrossRef]
- Candreva, A.; Di Maio, G.; La Deda, M. A quick one-step synthesis of luminescent gold nanospheres. Soft Matter 2020, 16, 10865–10868. [Google Scholar] [CrossRef] [PubMed]
- Hinman, J.G.; Stork, A.J.; Varnell, J.A.; Gewirth, A.A.; Murphy, C.J. Seed mediated growth of gold nanorods: Towards nanorod matryoshkas. Faraday Discuss. 2016, 191, 9–33. [Google Scholar] [CrossRef]
- ZIMMERMAN, W.B.J. Introduction To Comsol Multiphysics. Multiphys. Model. Finite Elem. Methods 2006, 6, 1–26. [Google Scholar] [CrossRef]
- Yushanov, S.P.; Crompton, J.S.; Koppenhoefer, K.C. Scattering of Electromagnetic Waves Overview. In Proceedings of the 2013 COMSOL Conference, Boston, MA, USA, 9‒11 October 2013. [Google Scholar]
- Palermo, G.; Cataldi, U.; Condello, A.; Caputo, R.; Bürgi, T.; Umeton, C.; De Luca, A. Flexible thermo-plasmonics: An opto-mechanical control of the heat generated at the nanoscale. Nanoscale 2018, 10, 16556–16561. [Google Scholar] [CrossRef]
- Palermo, G.; Strangi, G. Thermoplasmonic-biosensing demonstration based on the photothermal response of metallic nanoparticles. J. Appl. Phys. 2020, 128, 164302. [Google Scholar] [CrossRef]
- Nucera, A.; Grillo, R.; Rizzuto, C.; Barberi, R.C.; Castriota, M.; Bürgi, T.; Caputo, R.; Palermo, G. Effect of the Combination of Gold Nanoparticles and Polyelectrolyte Layers on SERS Measurements. Biosensors 2022, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Ichimaru, S. Scattering of Electromagnetic Waves. Stat. Phys. Dense Plasmas 2019, 6, 39–52. [Google Scholar] [CrossRef]
- Novo, C.; Mulvaney, P. Charge-Induced Rayleigh Instabilities In Small Gold Rods. Nano Lett. 2007, 7, 520–524. [Google Scholar] [CrossRef]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Duyne, R.P. Van 2008 [review] Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 8–10. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candreva, A.; Di Maio, G.; Palermo, G.; Guglielmelli, A.; Strangi, G.; La Deda, M. Solvent-Dispersible Nanostructured MIMI: An Experimental and Computational Study. Appl. Sci. 2023, 13, 2982. https://doi.org/10.3390/app13052982
Candreva A, Di Maio G, Palermo G, Guglielmelli A, Strangi G, La Deda M. Solvent-Dispersible Nanostructured MIMI: An Experimental and Computational Study. Applied Sciences. 2023; 13(5):2982. https://doi.org/10.3390/app13052982
Chicago/Turabian StyleCandreva, Angela, Giuseppe Di Maio, Giovanna Palermo, Alexa Guglielmelli, Giuseppe Strangi, and Massimo La Deda. 2023. "Solvent-Dispersible Nanostructured MIMI: An Experimental and Computational Study" Applied Sciences 13, no. 5: 2982. https://doi.org/10.3390/app13052982
APA StyleCandreva, A., Di Maio, G., Palermo, G., Guglielmelli, A., Strangi, G., & La Deda, M. (2023). Solvent-Dispersible Nanostructured MIMI: An Experimental and Computational Study. Applied Sciences, 13(5), 2982. https://doi.org/10.3390/app13052982








