The Effect of Light Availability on Photosynthetic Responses of Four Aglaonema commutatum Cultivars with Contrasting Leaf Pigment
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Pigment and Colorimetric Analysis
2.4. Determination of Physiological and Biochemical Indexes
2.5. Determination of Light Response Curve
2.6. Data Analysis
3. Results
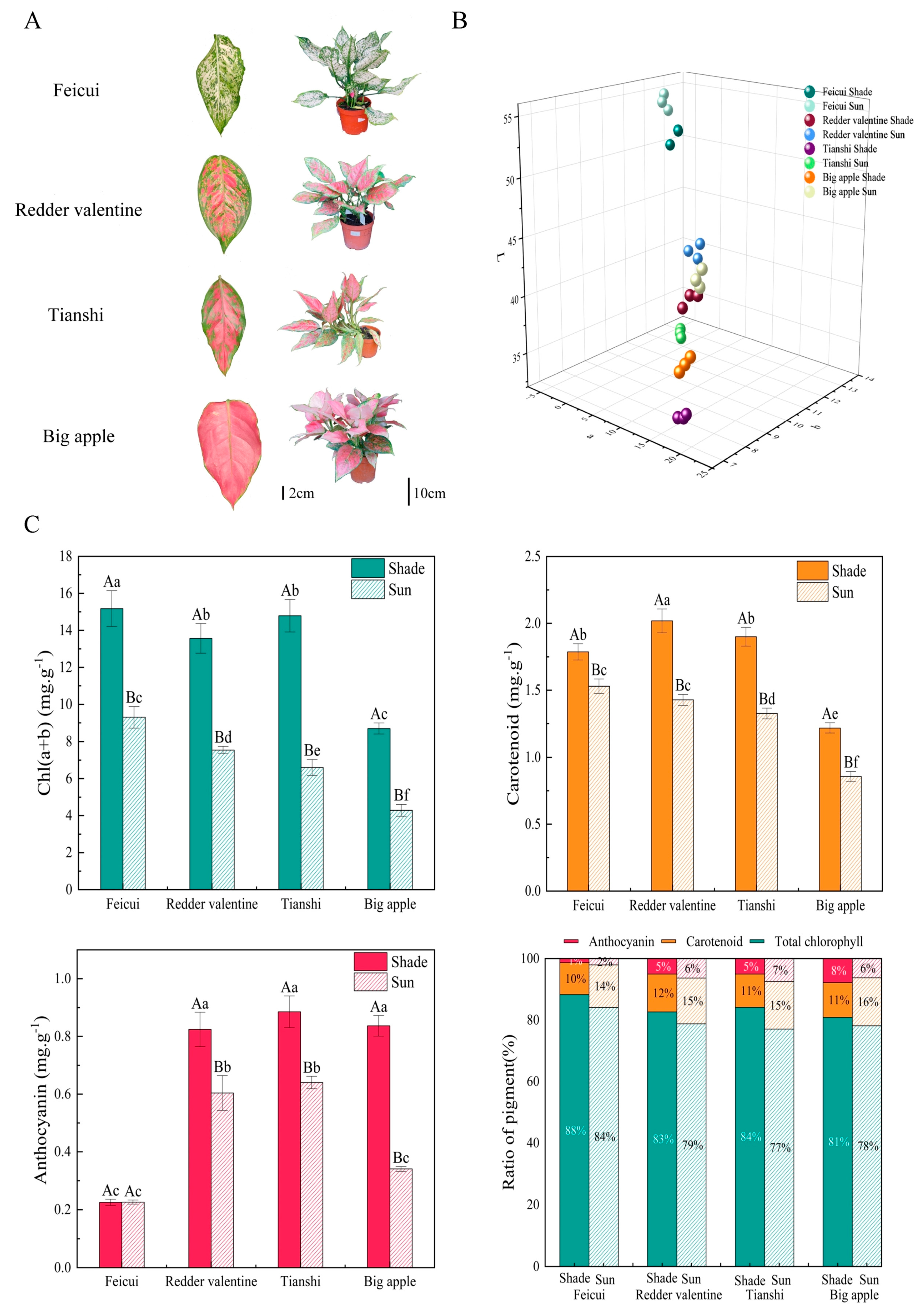
3.1. General Leaf Colorimetric Characteristics and Pigmentation
3.2. Physiochemical Variations among Cultivars × Irradiance
3.3. The Comparison of Photosynthetic Light Response
3.4. Correlation between Pigment Composition and Photosynthetic Light Response
4. Discussion
5. Conclusions and Further Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Rd | Dark respiration rate |
| Asat | Maximum net photosynthetic rate |
| Π | Light saturation point |
| Γ | Light compensation point |
| Φ | Apparent quantum yield |
| RWC | Relative water content |
| SLA | Specific leaf area |
| SLW | Specific leaf weight |
| A | Net photosynthetic rate |
| PPFD | Photosynthetic photon flux density |
| β | Coefficient of photoinhibition |
| γ | Coefficient of satiety |
| α | The initial slope |
| Chl | Chlorophyll |
| Car | Carotenoid |
| ACN | Anthocyanin |
| LHCll | Light-harvesting complex ii |
| UV-B | Ultraviolet-B radiation |
| FW | Fresh weight |
| DW | Dry weight |
| LAp | Projected leaf area |
| PMSS | Photosynthesis Model Simulation Software |
References
- Wataru, Y.; Hikosaka, K.; Way, D.A. Temperature Response of Photosynthesis in C3, C4, and Cam Plants: Temperature Acclimation and Temperature Adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Thomas, P.; Yang, C. The Hidden Function of Photosynthesis: A Sensing System for Environmental Conditions That Regulates Plant Acclimation Responses. Protoplasma 2012, 249, 125–136. [Google Scholar] [CrossRef]
- Mathur, S.; Jain, L.; Jajoo, A. Photosynthetic Efficiency in Sun and Shade Plants. Photosynthetica 2018, 56, 354–365. [Google Scholar] [CrossRef]
- Marek, Z.; Brestic, M.; Kalaji, H. Photosynthetic Responses of Sun-and Shade-Grown Barley Leaves to High Light: Is the Lower Psii Connectivity in Shade Leaves Associated with Protection against Excess of Light? Photosynth. Res. 2014, 119, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Hartmut, K.L.; Ač, A.; Marek, M.; Kalina, J.; Urban, O. Differences in Pigment Composition, Photosynthetic Rates and Chlorophyll Fluorescence Images of Sun and Shade Leaves of Four Tree Species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef]
- Karl, L.H.; Babani, F.; Langsdorf, G. Chlorophyll Fluorescence Imaging of Photosynthetic Activity in Sun and Shade Leaves of Trees. Photosynth. Res. 2007, 93, 235–244. [Google Scholar] [CrossRef]
- Junzeng, X.; Lv, Y.; Liu, X.; Wei, Q.; Qi, Z.; Yang, S.; Liao, L. A General Non-Rectangular Hyperbola Equation for Photosynthetic Light Response Curve of Rice at Various Leaf Ages. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Qiang, L.; Jia, W.; Li, F. Determination of the Most Effective Design for the Measurement of Photosynthetic Light-Response Curves for Planted Larix Olgensis Trees. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Kate, M.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar]
- Demmig-Adams, B.; Adams, W.W., III; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using Chlorophyll Fluorescence to Assess the Fraction of Absorbed Light Allocated to Thermal Dissipation of Excess Excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Henny, R.J. Aglaonema. In Crc Handbook of Flowering; CRC Press: Boca Raton, FL, USA, 2019; pp. 12–14. [Google Scholar]
- Songjun, Z.; Li, J.; Wu, K.; Li, L.; Hua, M.G.; Fang, L. Acmyb1 Interacts with Acbhlh1 to Regulate Anthocyanin Biosynthesis in Aglaonema Commutatum. Front. Plant Sci. 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Halyna, T.; Opryshko, M.; Gyrenko, O.; Buyun, L.; Kurhaluk, N. Antibacterial Activity of Ethanolic Extracts of Aglaonema Commutatum Schott and Its Cultivars against Enterococcus Faecalis Strains. Agrobiodivers. Improv. Nutr. Health Life Qual. 2022, 2, 171–179. [Google Scholar] [CrossRef]
- Richard, T.P.; Conover, C.A. Nitrogen and Potassium Fertilization of Aglaonema Commutatum Schott Cvs. Fransher and Pseudobracteatum1. HortScience 1977, 12, 570–571. [Google Scholar] [CrossRef]
- Barakat, A.A.; Gaber, M.K. Micropropagation and Ex Vitro Acclimatization of Aglaonema Plants. Sciences 2018, 8, 1425–1436. [Google Scholar]
- ALdeen, A.M.T.; Abd El-Aal, M.S. Enhancement of Aglaonema Commutatum Propagation Using Thidiazuron and Naphthalene Acetic Acid in Vitro. Lond. J. Med. Health Res. 2021, 21, 7–14. [Google Scholar]
- Jong-Yi, F.; Hsu, Y.-R.; Chen, F.-C. Development of an Efficient Micropropagation Procedure for Aglaonema ‘Lady Valentine’ through Adventitious Shoot Induction and Proliferation. Plant Biotechnol. 2013, 30, 423–431. [Google Scholar] [CrossRef] [Green Version]
- BO, I.; Zhila, A. Special Features of Generative Reproduction of Aglaonema Commutatum Schott (Araceae Juss.) under Introduction. Plant Introd. 2010, 47, 96–101. [Google Scholar] [CrossRef]
- Torres, T.T.; Nascimento, M.; Leite, R.D.S.; Guimarães, D.S. Spectrophotometric Determinations of Chloroplastidic Pigments in Physalis Angulata, L. Leaves Using Different Methodologies. J. Agric. Sci. 2017, 9, 117. [Google Scholar] [CrossRef]
- Jian-jiang, Z.; Seki, T.; Kinoshita, S.; Yoshida, T. Effect of Light Irradiation on Anthocyanin Production by Suspended Culture of Perilla Frutescens. Biotechnol. Bioeng. 1991, 38, 653–658. [Google Scholar] [CrossRef]
- Li, H.S.; Sun, Q.; Zhao, S.J.; Zhang, W.H. Techniques of Plant Physiological Biochemical Experiment. Princ. Tech. Plant Physiol. Biochem. Exp. 2000, 200, 119–124. [Google Scholar]
- Jagadish, S.V.; Muthurajan, R.; Rang, Z.; Malo, R.; Heuer, S.; Bennett, J.; Craufurd, P. Spikelet Proteomic Response to Combined Water Deficit and Heat Stress in Rice (Oryza Sativa Cv. N22). Rice 2011, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, D.W.; Seymour, R.S.; Halteman, W.A.; Greenwood, M.S. Canopy Dynamics and the Morphological Development of Abies Balsamea: Effects of Foliage Age on Specific Leaf Area and Secondary Vascular Development. Tree Physiol. 1995, 15, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zi-Piao, Y.E. A Review on Modeling of Responses of Photosynthesis to Light and Co2. Chin. J. Plant Ecol. 2010, 34, 727. [Google Scholar] [CrossRef]
- Meng, Z.; Cao, T.; Ni, L.; Xie, P.; Li, Z. Carbon, Nitrogen and Antioxidant Enzyme Responses of Potamogeton Crispus to Both Low Light and High Nutrient Stresses. Environ. Exp. Bot. 2010, 68, 44–50. [Google Scholar] [CrossRef]
- Evandro, N.S.; Ribeiro, R.V.; Ferreira-Silva, S.L.; Vieira, S.A.; Ponte, L.F.A.; Silveira, J.A.G. Coordinate Changes in Photosynthesis, Sugar Accumulation and Antioxidative Enzymes Improve the Performance of Jatropha Curcas Plants under Drought Stress. Biomass Bioenergy 2012, 45, 270–279. [Google Scholar] [CrossRef]
- Inoue, T.; Inanaga, S.; Sugimoto, Y.; El Siddig, K. Contribution of Pre-Anthesis Assimilates and Current Photosynthesis to Grain Yield, and Their Relationships to Drought Resistance in Wheat Cultivars Grown under Different Soil Moisture. Photosynthetica 2004, 42, 99–104. [Google Scholar] [CrossRef]
- Da-Quan, X.; Chen, Y.; Chen, G.-Y. Light-Harvesting Regulation from Leaf to Molecule with the Emphasis on Rapid Changes in Antenna Size. Photosynth. Res. 2015, 124, 137–158. [Google Scholar] [CrossRef]
- Qiansheng, L.; Deng, M.; Xiong, Y.; Coombes, A.; Zhao, W. Morphological and Photosynthetic Response to High and Low Irradiance of Aeschynanthus Longicaulis. Sci. World J. 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Richard, A.B.; Lee, D.W.; Norman, J.M. Epidermal Cells Functioning as Lenses in Leaves of Tropical Rain-Forest Shade Plants. Appl. Opt. 1985, 24, 1408–1412. [Google Scholar] [CrossRef]
- Hartmut, K.L.; Kuhn, G.; Prenzel, U.; Meier, D. Chlorophyll-Protein Levels and Degree of Thylakoid Stacking in Radish Chloroplasts from High-Light, Low-Light and Bentazon-Treated Plants. Physiol. Plant. 1982, 56, 183–188. [Google Scholar] [CrossRef]
- Kumar, R.G.; Guha, A.; Reddy, A.R. Elevated Atmospheric Co2 Mitigated Photoinhibition in a Tropical Tree Species, Gmelina Arborea. J. Photochem. Photobiol. B Biol. 2011, 103, 159–165. [Google Scholar] [CrossRef]
- Graham, J.H.; Baker, N.R.; Long, S.P. Growth in Elevated Co2 Can Both Increase and Decrease Photochemistry and Photoinhibition of Photosynthesis in a Predictable Manner. Dactylis Glomerata Grown in Two Levels of Nitrogen Nutrition. Plant Physiol. 2001, 127, 1204–1211. [Google Scholar] [CrossRef]
- Anupa, S. Growth, Physiological, and Biochemical Responses of Three Tropical Legumes to Enhanced Uv-B Radiation. Can. J. Bot. 1996, 74, 135–139. [Google Scholar] [CrossRef]
- Qiansheng, L.; Deng, M.; Chen, J.; Henny, R. Effects of Light Intensity and Paclobutrazol on Growth and Interior Performance of Pachira Aquatica Aubl. HortScience 2009, 44, 1291–1295. [Google Scholar] [CrossRef] [Green Version]
- Barbara, D.-A.; Adams, W.W., III. The Role of Xanthophyll Cycle Carotenoids in the Protection of Photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Leonardo, L.; Restrepo-Diaz, H.; Volder, A. Photosynthetic Light Response and Epidermal Characteristics of Sun and Shade Pecan Leaves. J. Am. Soc. Hortic. Sci. 2009, 134, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Sander, W.H.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; Van Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic Quantum Yield Dynamics: From Photosystems to Leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef] [Green Version]
- Artero, V.; Chandezon, F.; Co, D.; Dietzek, B. European and International Initiatives in the Field of Artificial Photosynthesis. Adv. Bot. Res. 2016, 79, 193–221. [Google Scholar] [CrossRef]

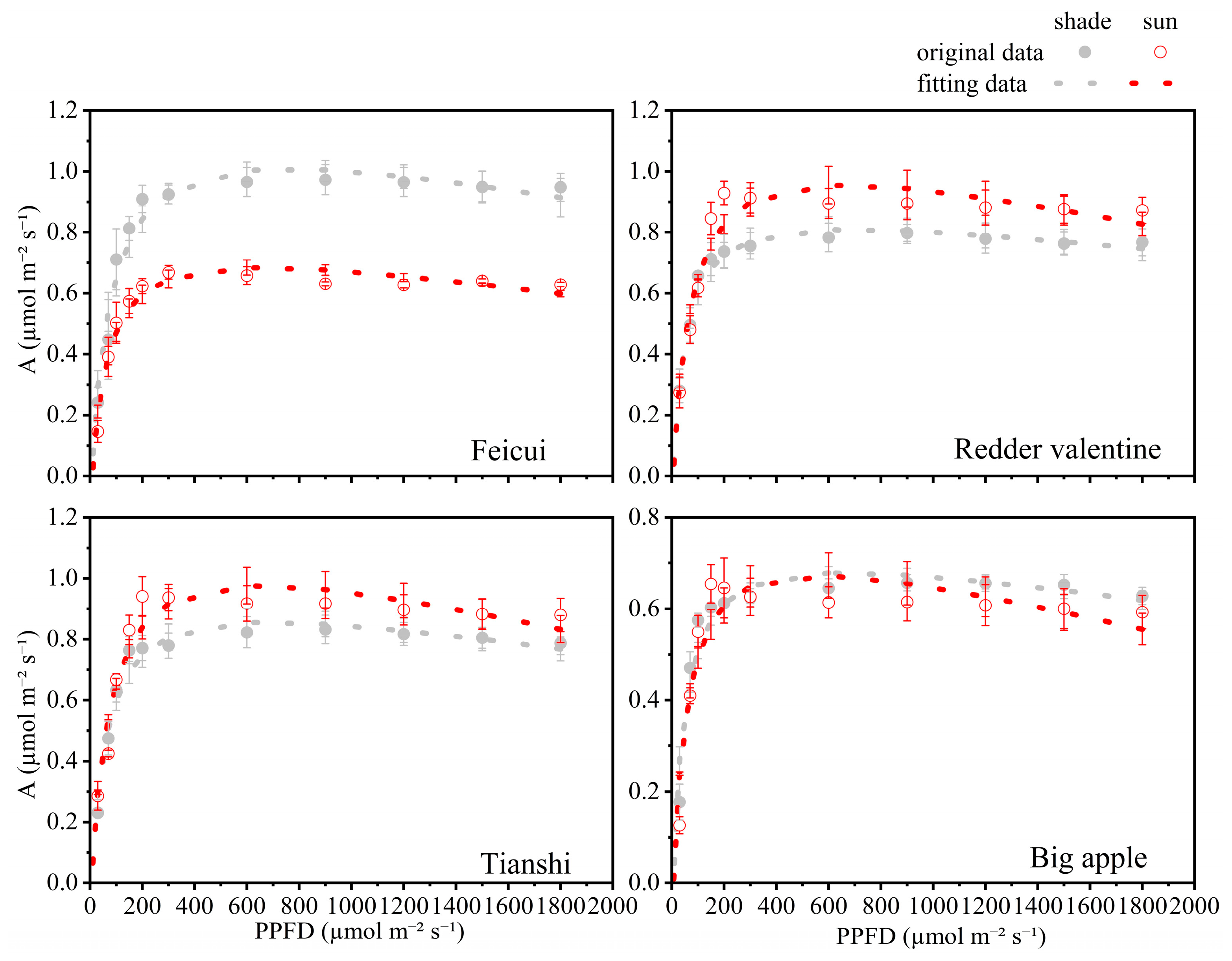

| Rd | Asat | Π | Γ | Φ | ||
|---|---|---|---|---|---|---|
| Feicui | shade | 0.0663 ± 0.007 b | 1.0092 ± 0.020 a | 760.1623 ± 39.382 a | 4.2592 ± 0.400 b | 0.0049 ± 0.000 a |
| sun | 0.0879 ± 0.020 a | 0.6852 ± 0.016 c | 657.819 ± 60.751 b | 6.4036 ± 0.445 a | 0.0035 ± 0.000 c | |
| Redder valentine | shade | 0.0558 ± 0.006 b | 0.8096 ± 0.030 b | 679.2483 ± 11.734 b | 2.9539 ± 0.409 c | 0.0037 ± 0.000 c |
| sun | 0.0575 ± 0.004 b | 0.9554 ± 0.043 a | 639.518 ± 13.489 b | 3.5211 ± 0.541 b | 0.0047 ± 0.001 b | |
| Tianshi | shade | 0.0527 ± 0.006 b | 0.8571 ± 0.031 b | 686.6397 ± 35.981 b | 3.2824 ± 0.334 b | 0.0044 ± 0.000 b |
| sun | 0.0596 ± 0.008 b | 0.9765 ± 0.042 a | 636.5493 ± 9.017 b | 3.7621 ± 0.491 b | 0.0047 ± 0.000 b | |
| Big apple | shade | 0.0655 ± 0.003 b | 0.6796 ± 0.009 c | 652.6023 ± 33.848 b | 3.9662 ± 0.676 b | 0.0035 ± 0.000 c |
| sun | 0.0732 ± 0.010 a | 0.6736 ± 0.034 c | 548.8977 ± 9.518 c | 4.9341 ± 0.604 a | 0.0043 ± 0.000 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, J.; Wu, C.; Li, X.; Huang, L.; Jiang, Y.; Zhang, B. The Effect of Light Availability on Photosynthetic Responses of Four Aglaonema commutatum Cultivars with Contrasting Leaf Pigment. Appl. Sci. 2023, 13, 3021. https://doi.org/10.3390/app13053021
Hui J, Wu C, Li X, Huang L, Jiang Y, Zhang B. The Effect of Light Availability on Photosynthetic Responses of Four Aglaonema commutatum Cultivars with Contrasting Leaf Pigment. Applied Sciences. 2023; 13(5):3021. https://doi.org/10.3390/app13053021
Chicago/Turabian StyleHui, Junai, Canhang Wu, Xiaomei Li, Leying Huang, Yongqiang Jiang, and Bipei Zhang. 2023. "The Effect of Light Availability on Photosynthetic Responses of Four Aglaonema commutatum Cultivars with Contrasting Leaf Pigment" Applied Sciences 13, no. 5: 3021. https://doi.org/10.3390/app13053021





