Thermocatalytic Decomposition of Dimethyl Methylphosphonate Based on CeO2 Catalysts with Different Morphologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of CeO2 Nanomaterials
2.2. Characterization of CeO2 Nanomaterials
2.3. Evaluation of Thermocatalytic Decomposition Performance
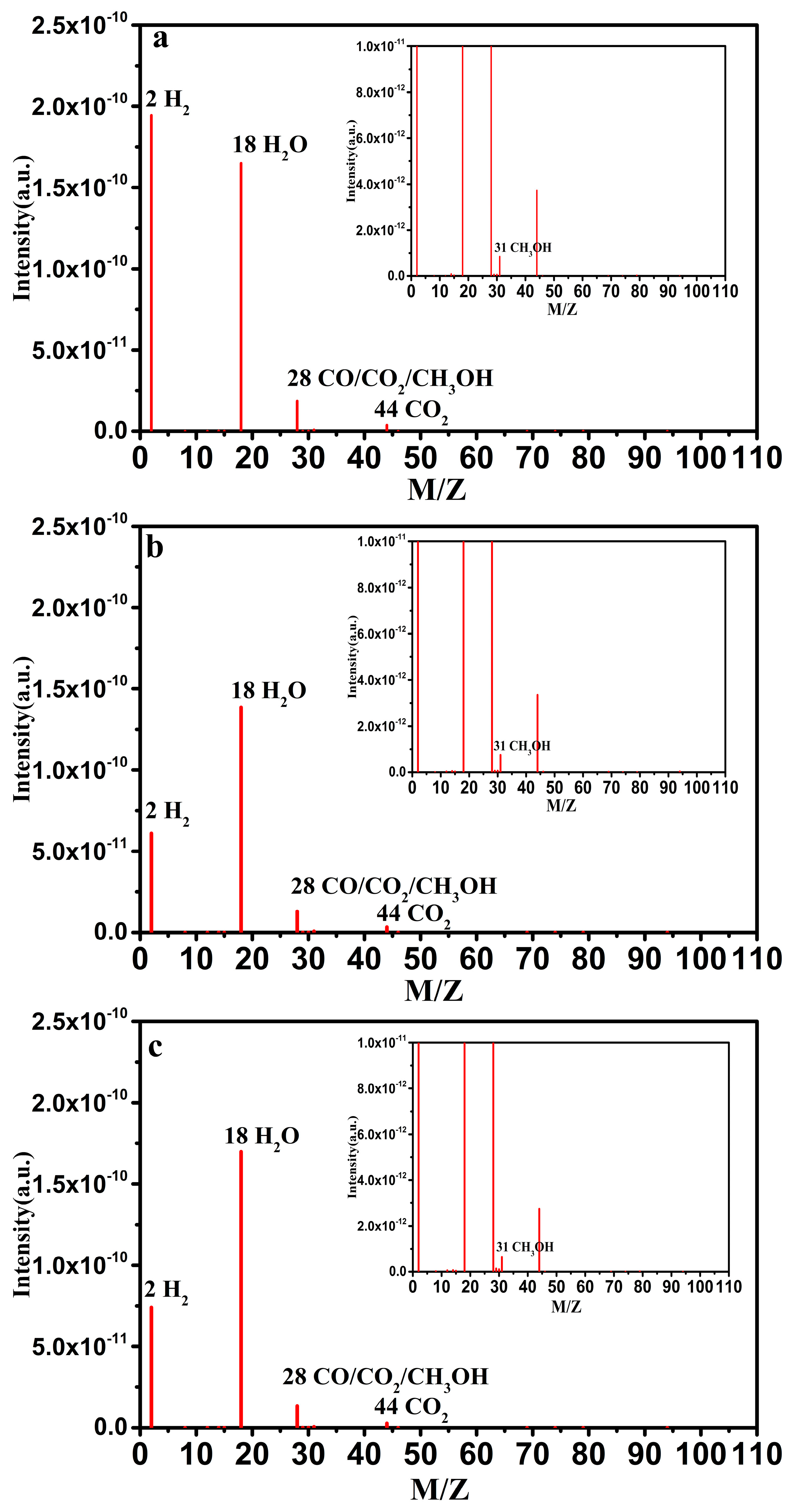
2.4. Thermocatalytic Decomposition Reaction Tail Gases Analysis
3. Results and Discussion
3.1. Characteristics of CeO2 Nanomaterials
3.2. Catalytic Performance Testing
3.3. Reaction Mechanism for Thermocatalytic Decomposition of DMMP
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jang, Y.J.; Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Update 1 of: Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2015, 115, PR1-76. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Review and Evaluation of Alternative Chemical Disposal Technologies; National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- Farquharson, S.; Inscore, F.E.; Christesen, S. Detecting Chemical Agents and Their Hydrolysis Products in Water. Surf. Enhanc. Raman Scatt. Phys. Appl. 2006, 103, 447–460. [Google Scholar] [CrossRef]
- Gustafson, R.L.; Martell, A.E. A Kinetic Study of the Copper(II) Chelate-catalyzed Hydrolysis of Isopropyl Methylphosphon-ofluoridate (Sarin). J. Am. Chem. Soc. 1962, 84, 2309–2316. [Google Scholar] [CrossRef]
- Ward, J.R.; Yang, Y.-C.; Wilson, R.B.; Burrows, W.D.; Ackerman, L.L. Base-catalyzed hydrolysis of 1,2,2-trimethylpropyl methylphosphonofluoridate—An examination of the saturation effect. Bioorg. Chem. 1988, 16, 12–16. [Google Scholar] [CrossRef]
- Yang, Y.C.; Baker, J.A.; Ward, J.R. Decontamination of chemical warfare agents. Chem. Rev. 1992, 92, 1729–1743. [Google Scholar] [CrossRef]
- Yang, Y.-C. Chemical Detoxification of Nerve Agent VX. Acc. Chem. Res. 1998, 32, 109–115. [Google Scholar] [CrossRef]
- Li, Y.X.; Schlup, J.R.; Klabunde, K.J. Fourier transform infrared photoacoustic spectroscopy study of the adsorption of or-ganophosphorus compounds on heat-treated magnesium oxide. Langmuir 1991, 7, 1394–1399. [Google Scholar] [CrossRef]
- Lin, S.T.; Klabunde, K.J. Thermally activated magnesium oxide surface chemistry. Adsorption and decomposition of phosphorus compounds. Langmuir 1985, 1, 600–605. [Google Scholar] [CrossRef]
- Nakamura, M.; Shi, M.; Okamoto, Y.; Takamuku, S. Photolysis of bis(methoxyphenyl) methylphosphonates. J. Photochem. Photobiol. A Chem. 1995, 85, 111–118. [Google Scholar] [CrossRef]
- O’Shea, K.E.; Beightol, S.; Garcia, I.; Aguilar, M.; Kalen, D.V.; Cooper, W.J. Photocatalytic decomposition of organophospho-nates in irradiated TiO2 suspensions. J. Photochem. Photobiol. A Chem. 1997, 107, 221–226. [Google Scholar] [CrossRef]
- Segal, S.R.; Suib, S.L.; Tang, X.; Satyapal, S. Photoassisted Decomposition of Dimethyl Methylphosphonate over Amorphous Manganese Oxide Catalysts. Chem. Mater. 1999, 11, 1687–1695. [Google Scholar] [CrossRef]
- Cao, L.; Segal, S.R.; Suib, S.L.; Tang, X.; Satyapal, S. Thermocatalytic Oxidation of Dimethyl Methylphosphonate on Supported Metal Oxides. J. Catal. 2000, 194, 61–70. [Google Scholar] [CrossRef]
- Segal, S.R.; Cao, L.; Suib, S.L.; Tang, X.; Satyapal, S. Thermal Decomposition of Dimethyl Methylphosphonate over Manganese Oxide Catalysts. J. Catal. 2001, 198, 66–76. [Google Scholar] [CrossRef]
- Wang, L.; Denchy, M.; Blando, N.; Hansen, L.; Bilik, B.; Tang, X.; Hicks, Z.; Bowen, K.H. Thermal Decomposition of Dimethyl Methylphosphonate on Size-Selected Clusters: A Comparative Study between Copper Metal and Cupric Oxide Clusters. J. Phys. Chem. C 2021, 125, 11348–11358. [Google Scholar] [CrossRef]
- Walenta, C.A.; Xu, F.; Tesvara, C.; O’Connor, C.R.; Sautet, P.; Friend, C.M. Facile Decomposition of Organophosphonates by Dual Lewis Sites on a Fe3O4(111) Film. J. Phys. Chem. C 2020, 124, 12432–12441. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Schoenitz, M.; Dreizin, E.L. Vapor-phase decomposition of dimethyl methylphosphonate (DMMP), a sarin surrogate, in presence of metal oxides. Def. Technol. 2020, 17, 1095–1114. [Google Scholar] [CrossRef]
- Sheinker, V.N.; Mitchell, M.B. Quantitative Study of the Decomposition of Dimethyl Methylphosphonate (DMMP) on Metal Oxides at Room Temperature and Above. Chem. Mater. 2002, 14, 1257–1268. [Google Scholar] [CrossRef]
- Zhanpeisov, N.U.; Zhidomirov, G.M.; Yudanov, I.V.; Klabunde, K.J. Cluster Quantum Chemical Study of the Interaction of Dimethyl Methylphosphonate with Magnesium Oxide. J. Phys. Chem. 1994, 98, 10032–10035. [Google Scholar] [CrossRef]
- Holdren, S.; Tsyshevsky, R.; Fears, K.; Owrutsky, J.; Wu, T.; Wang, X.; Eichhorn, B.W.; Kuklja, M.M.; Zachariah, M.R. Adsorption and Destruction of the G-Series Nerve Agent Simulant Dimethyl Methylphosphonate on Zinc Oxide. ACS Catal. 2018, 9, 902–911. [Google Scholar] [CrossRef]
- Gordon, W.O.; Tissue, B.M.; Morris, J.R. Adsorption and decomposition of dimethyl methylphosphonate on Y2O3 nano-particles. J. Phys. Chem. C 2007, 111, 3233–3240. [Google Scholar] [CrossRef]
- Mitchell, M.B.; Sheinker, V.N.; Mintz, E.A. Adsorption and Decomposition of Dimethyl Methylphosphonate on Metal Oxides. J. Phys. Chem. B 1997, 101, 11192–11203. [Google Scholar] [CrossRef]
- Trotochaud, L.; Head, A.R.; Büchner, C.; Yu, Y.; Karslıoğlu, O.; Tsyshevsky, R.; Holdren, S.; Eichhorn, B.; Kuklja, M.M.; Bluhm, H. Room temperature decomposition of dimethyl methylphosphonate on cuprous oxide yields atomic phosphorus. Surf. Sci. 2019, 680, 75–87. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Tuller, H. Ionic conduction in nanocrystalline materials. Solid State Ionics 2000, 131, 143–157. [Google Scholar] [CrossRef]
- Powell, B.R.; Bloink, R.L.; Eickel, C.C. Preparation of Cerium Dioxide Powders for Catalyst Supports. J. Am. Ceram. Soc. 1988, 71, C-104. [Google Scholar] [CrossRef]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active Nonmetallic Au and Pt Species on Ceria-Based Water-Gas Shift Catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, P.; Suzuki, T.; Anderson, H.U. Nanocrystalline undoped ceria oxygen sensor. Sens. Actuators B Chem. 2003, 95, 73–77. [Google Scholar] [CrossRef]
- Si, R.; Zhang, Y.-W.; Li, S.-J.; Lin, B.-X.; Yan, C.-H. Urea-Based Hydrothermally Derived Homogeneous Nanostructured Ce1−xZrxO2 (x = 0–0.8) Solid Solutions: A Strong Correlation between Oxygen Storage Capacity and Lattice Strain. J. Phys. Chem. B 2004, 108, 12481–12488. [Google Scholar] [CrossRef]
- Mitchell, M.B.; Sheinker, V.N.; Tesfamichael, A.B.; Gatimu, E.N.; Nunley, M. Decomposition of Dimethyl Methylphosphonate (DMMP) on Supported Cerium and Iron Co-Impregnated Oxides at Room Temperature. J. Phys. Chem. B 2002, 107, 580–586. [Google Scholar] [CrossRef]
- Mitchell, M.B.; Sheinker, V.N.; Cox, W.W.; Gatimu, E.N.; Tesfamichael, A.B. The Room Temperature Decomposition Mechanism of Dimethyl Methylphosphonate (DMMP) on Alumina-Supported Cerium Oxide − Participation of Nano-Sized Cerium Oxide Domains. J. Phys. Chem. B 2004, 108, 1634–1645. [Google Scholar] [CrossRef]
- Chen, D.A.; Ratliff, J.S.; Hu, X.; Gordon, W.O.; Senanayake, S.D.; Mullins, D.R. Dimethyl methylphosphonate decomposition on fully oxidized and partially reduced ceria thin films. Surf. Sci. 2010, 604, 574–587. [Google Scholar] [CrossRef]
- Li, T.; Tsyshevsky, R.; Algrim, L.; McEntee, M.; Durke, E.M.; Eichhorn, B.; Karwacki, C.; Zachariah, M.R.; Kuklja, M.M.; Rodriguez, E.E. Understanding Dimethyl Methylphosphonate Adsorption and Decomposition on Mesoporous CeO2. ACS Appl. Mater. Interfaces 2021, 13, 54597–54609. [Google Scholar] [CrossRef]
- Qiao, Z.-A.; Wu, Z.; Dai, S. Shape-Controlled Ceria-based Nanostructures for Catalysis Applications. Chemsuschem 2013, 6, 1821–1833. [Google Scholar] [CrossRef]
- López, J.M.; Gilbank, A.L.; García, T.; Solsona, B.; Agouram, S.; Torrente-Murciano, L. The prevalence of surface oxygen vacancies over the mobility of bulk oxygen in nanostructured ceria for the total toluene oxidation. Appl. Catal. B Environ. 2015, 174–175, 403–412. [Google Scholar] [CrossRef]
- Torrente-Murciano, L.; Gilbank, A.; Puertolas, B.; Garcia, T.; Solsona, B.; Chadwick, D. Shape-dependency activity of nanostructured CeO2 in the total oxidation of polycyclic aromatic hydrocarbons. Appl. Catal. B Environ. 2013, 132–133, 116–122. [Google Scholar] [CrossRef]
- Arul, N.S.; Mangalaraj, D.; Chen, P.C.; Ponpandian, N.; Viswanathan, C. Strong quantum confinement effect in nano-crystalline cerium oxide. Mater. Lett. 2011, 65, 2635–2638. [Google Scholar] [CrossRef]
- Pouretedal, H.; Kadkhodaie, A. Synthetic CeO2 Nanoparticle Catalysis of Methylene Blue Photodegradation: Kinetics and Mechanism. Chin. J. Catal. 2010, 31, 1328–1334. [Google Scholar] [CrossRef]
- Mai, H.-X.; Sun, L.-D.; Zhang, Y.-W.; Si, R.; Feng, W.; Zhang, H.-P.; Liu, H.-C.; Yan, C.-H. Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef]
- Xu, J.; Harmer, J.; Li, G.; Chapman, T.; Collier, P.; Longworth, S.; Tsang, S.C. Size dependent oxygen buffering capacity of ceria nanocrystals. Chem. Commun. 2010, 46, 1887–1889. [Google Scholar] [CrossRef]
- Zhang, D.; Du, X.; Shi, L.; Gao, R. Shape-controlled synthesis and catalytic application of ceria nanomaterials. Dalton Trans. 2012, 41, 14455–14475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, L.; Chen, Z.; Wen, J.; Zhong, W.; Zou, S.; Fu, M.; Chen, L.; Ye, D. Highly efficient Cu/CeO2-hollow nanospheres catalyst for the reverse water-gas shift reaction: Investigation on the role of oxygen vacancies through in situ UV-Raman and DRIFTS. Appl. Surf. Sci. 2020, 516, 146035. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Liao, Y.; Fu, M.; Chen, L.; Wu, J.; Huang, B.; Ye, D. Catalytic oxidation of toluene over nanorod-structured Mn–Ce mixed oxides. Catal. Today 2013, 216, 220–228. [Google Scholar] [CrossRef]
- Popović, Z.V.; Dohčević-Mitrović, Z.; Konstantinović, M.J.; Šćepanović, M. Raman scattering characterization of na-nopowders and nanowires (rods). J. Raman Spectrosc. 2007, 38, 750–755. [Google Scholar] [CrossRef]
- Graven, W.M.; Weller, S.W.; Peters, D.L. Catalytic Conversion of Organophosphate Vapor over Platinum-Alumina. Ind. Eng. Chem. Process. Des. Dev. 1966, 5, 183–189. [Google Scholar] [CrossRef]
- Lee, K.; Houalla, M.; Hercules, D.; Hall, W. Catalytic Oxidative Decomposition of Dimethyl Methylphosphonate over Cu-Substituted Hydroxyapatite. J. Catal. 1994, 145, 223–231. [Google Scholar] [CrossRef]
- Gao, H.; Kong, W.; Zhou, S.; Wang, X.; He, Q.; Dong, Y. Thermal Catalytic Decomposition of Dimethyl Methyl Phosphonate Using CuO-CeO2/γ-Al2O3. Appl. Sci. 2022, 12, 10101. [Google Scholar] [CrossRef]
- Kong, W.; Zhou, S.; Wang, X.; He, Q.; Yang, P.; Yuan, Y.; Dong, Y. Catalytic Oxidative Decomposition of Dimethyl Methyl Phosphonate over CuO/CeO2 Catalysts Prepared Using a Secondary Alkaline Hydrothermal Method. Catalysts 2022, 12, 1277. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.; Chen, X.; Huang, C.; Yang, S. Biochar-loaded Ce3+-enriched ultra-fine ceria nanoparticles for phosphate adsorption. J. Hazard. Mater. 2020, 396, 122626. [Google Scholar] [CrossRef]
- Moravie, R.M.; Froment, F.; Corset, J. Vibrational spectra and possible conformers of dimethylmethylphosphonate by normal mode analysis. Spectrochim. Acta Part A Mol. Spectrosc. 1989, 45A, 1015–1024. [Google Scholar] [CrossRef]
- Rusu, C.N.; Yates, J.J.T. Adsorption and Decomposition of Dimethyl Methylphosphonate on TiO2. J. Phys. Chem. B 2000, 104, 12292–12298. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.-C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Mullins, D.R.; Overbury, S.H. Probing the Surface Sites of CeO2 Nanocrystals with Well-Defined Surface Planes via Methanol Adsorption and Desorption. ACS Catal. 2012, 2, 2224–2234. [Google Scholar] [CrossRef]
- Panayotov, D.A.; Morris, J.R. Thermal Decomposition of a Chemical Warfare Agent Simulant (DMMP) on TiO2: Adsorbate Reactions with Lattice Oxygen as Studied by Infrared Spectroscopy. J. Phys. Chem. C 2009, 113, 15684–15691. [Google Scholar] [CrossRef]
- Huttunen, P.K.; Labadini, D.; Hafiz, S.S.; Gokalp, S.; Wolff, E.P.; Martell, S.M.; Foster, M. DRIFTS investigation of methanol oxidation on CeO2 nanoparticles. Appl. Surf. Sci. 2021, 554, 149518. [Google Scholar] [CrossRef]
- Wu, Z.; Mann, A.K.P.; Li, M.; Overbury, S.H. Spectroscopic Investigation of Surface-Dependent Acid–Base Property of Ceria Nanoshapes. J. Phys. Chem. C 2015, 119, 7340–7350. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, F.; Xiao, P.; Tao, H.; Wang, X.; Hu, Z.; Lü, Y. Great Influence of Anions for Controllable Synthesis of CeO2 Nanostructures: From Nanorods to Nanocubes. J. Phys. Chem. C 2008, 112, 17076–17080. [Google Scholar] [CrossRef]
- Bermudez, V.M. Quantum-Chemical Study of the Adsorption of DMMP and Sarin on γ-Al2O3. J. Phys. Chem. C 2007, 111, 3719–3728. [Google Scholar] [CrossRef]
- Albrecht, P.M.; Mullins, D.R. Adsorption and reaction of methanol over CeO(X)(100) thin films. Langmuir 2013, 29, 4559–4567. [Google Scholar] [CrossRef] [PubMed]
- Vayssilov, G.N.; Mihaylov, M.; Petkov, P.S.; Hadjiivanov, K.I.; Neyman, K.M. Reassignment of the vibrational spectra of carbonates, formates, and related surface species on ceria: A combined density functional and infrared spectroscopy investigation. J. Phys. Chem. C 2011, 115, 23435–23454. [Google Scholar] [CrossRef]
- Mi, R.; Li, D.; Hu, Z.; Yang, R.T. Morphology Effects of CeO2 Nanomaterials on the Catalytic Combustion of Toluene: A Combined Kinetics and Diffuse Reflectance Infrared Fourier Transform Spectroscopy Study. ACS Catal. 2021, 11, 7876–7889. [Google Scholar] [CrossRef]
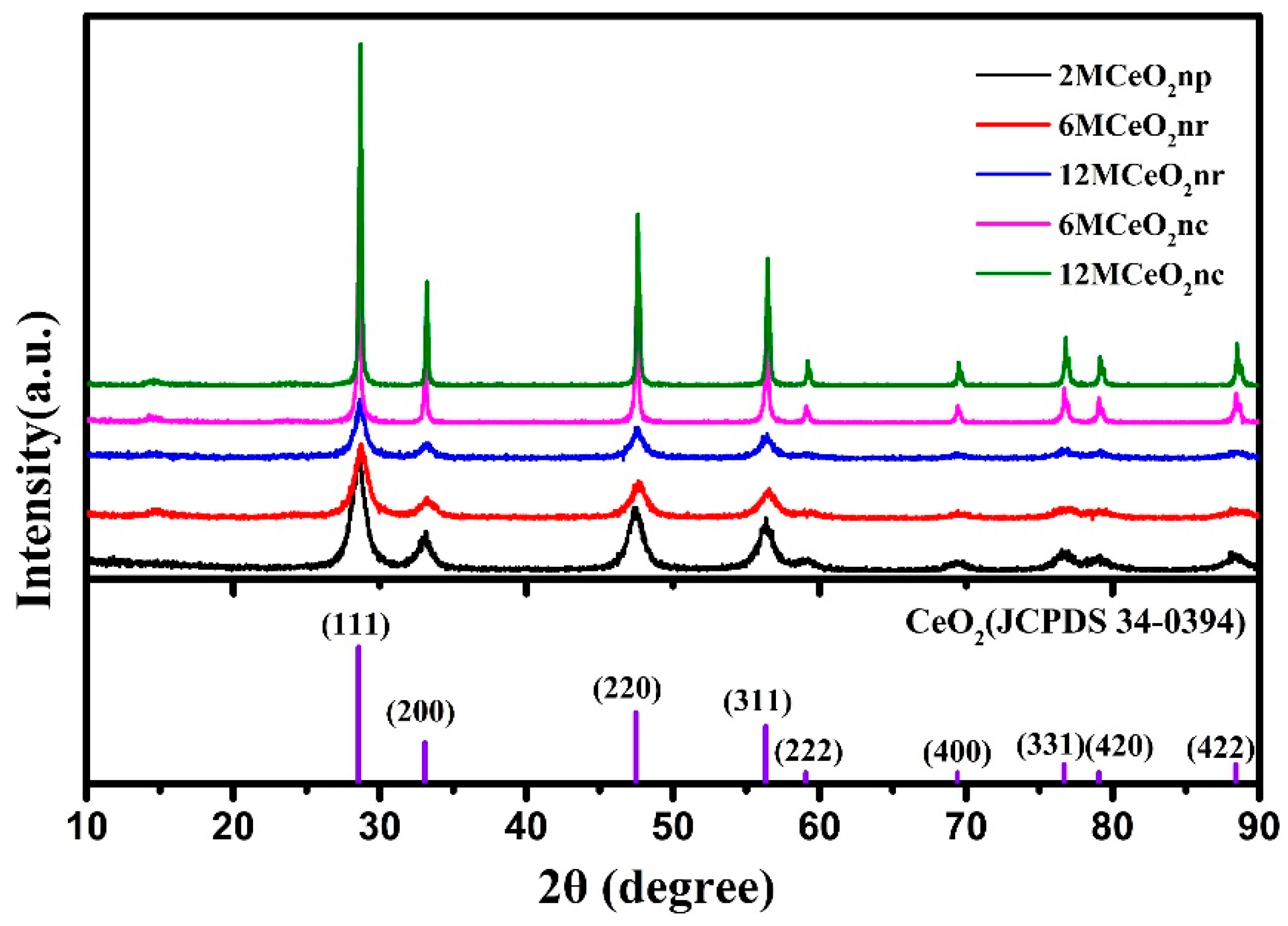

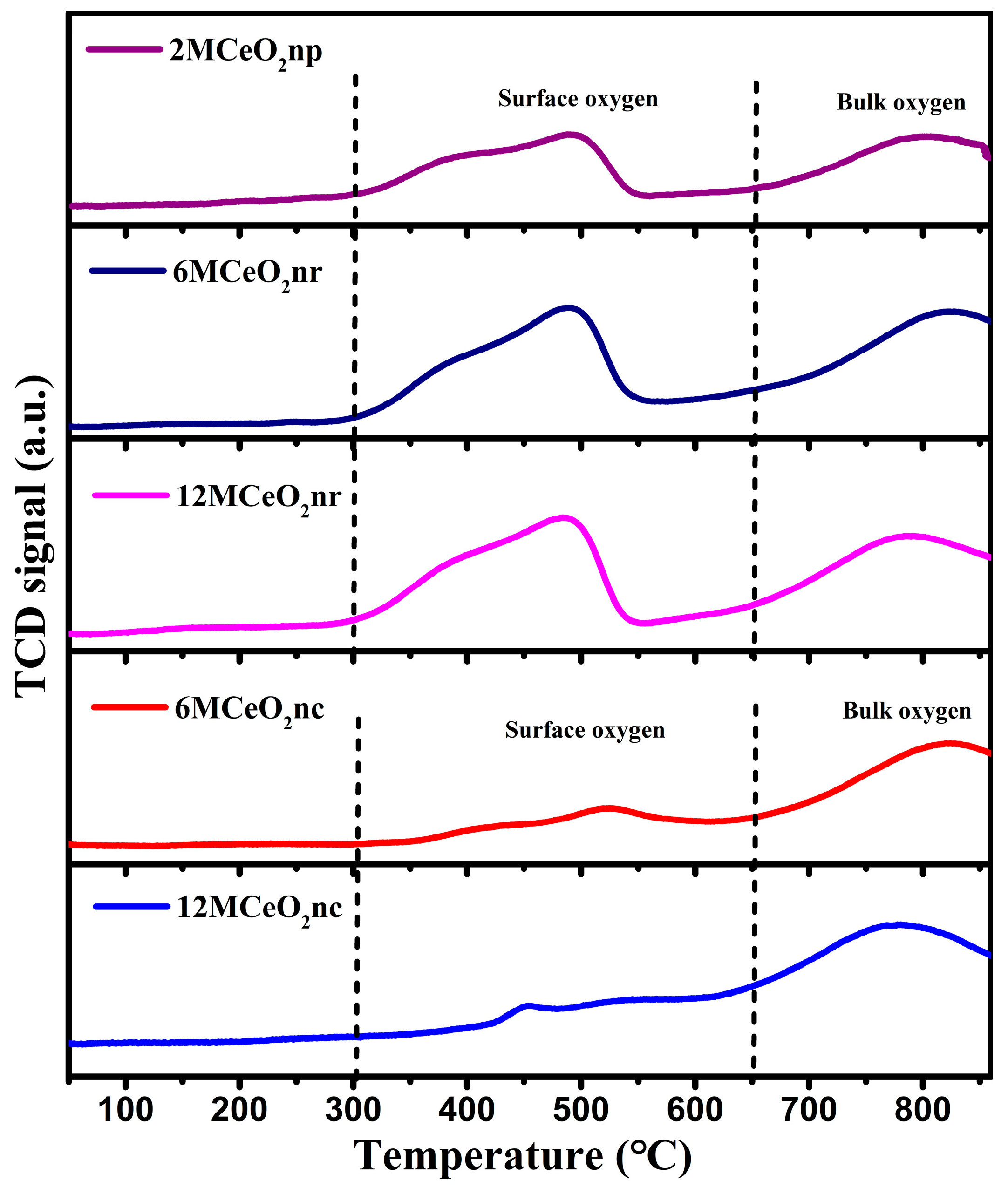



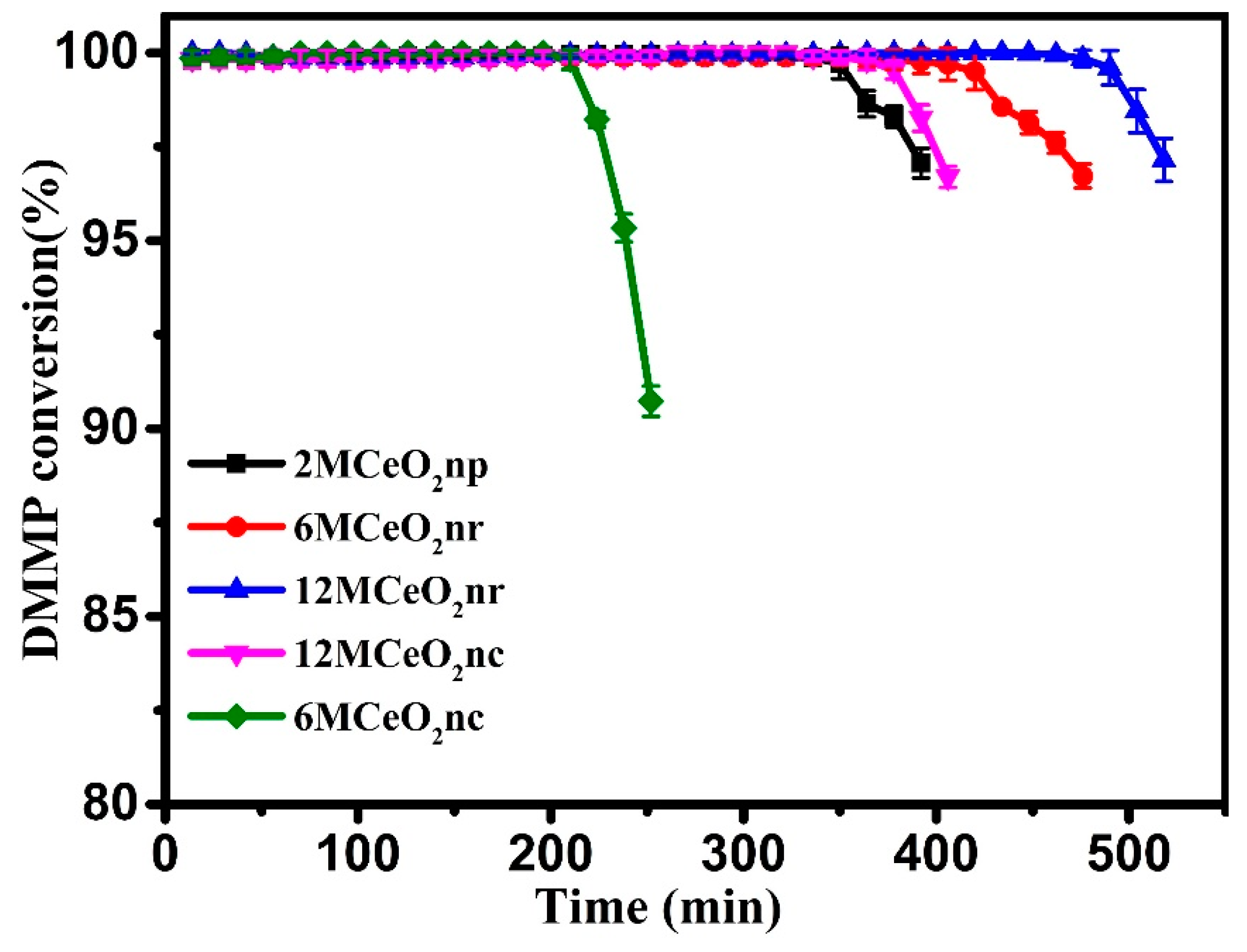
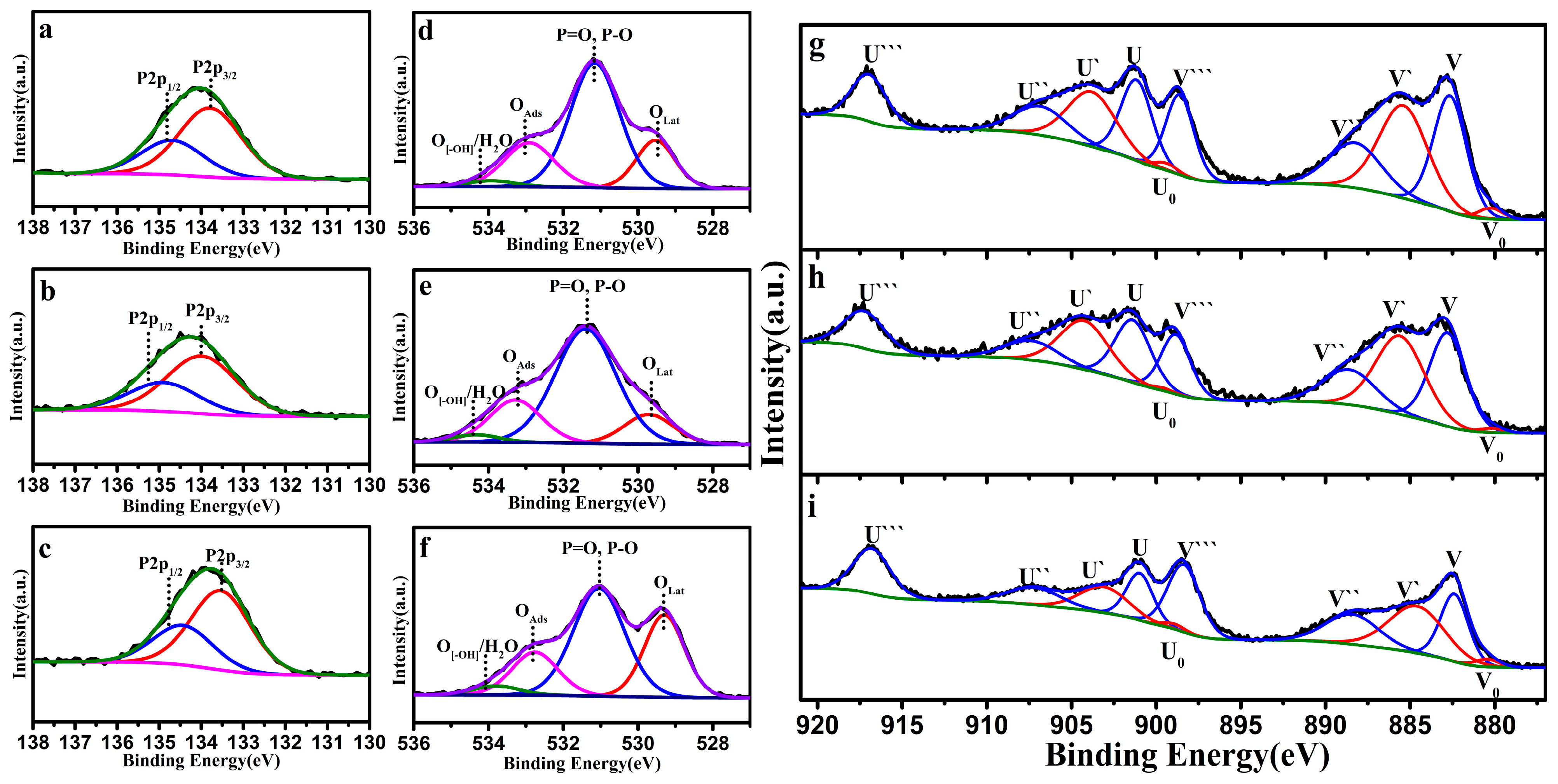


| Catalyst | Temperature (°C) | CNaOH (mol/L) | Morphology | Mean Length (nm) | Crystallinity Size (nm) | SBET (m2/g) |
|---|---|---|---|---|---|---|
| 2MCeO2np | 100 | 2 | Particles | 5.02 | 6.86 | 128.89 |
| 6MCeO2nr | 100 | 6 | Rods | 38.72 | 7.50 | 126.1 |
| 12MCeO2nr | 100 | 12 | Rods | 133.83 | 9.61 | 102.19 |
| 6MCeO2nc | 180 | 6 | Cubes | 18.78 | 39.56 | 31.13 |
| 12MCeO2nc | 180 | 12 | Cubes | 57.95 | 49.46 | 14.609 |
| Catalyst | XPS | Raman | ICP | Protection Time (min) | MSTC (gDMMP/gcat) | SSTC (gDMMP/m2) | |||
|---|---|---|---|---|---|---|---|---|---|
| Ce3+/(Ce3+ + Ce4+) | Oα/Oall | Oβ/Oall | FWHM460 | I600/I460 | Ce(wt%) | ||||
| 2MCeO2np | 19.64 | 58.94 | 41.06 | 27.27 | 0.0370 | 77.9 | 350 | 0.222 | 0.00072 |
| 6MCeO2nr | 25.32 | 51.08 | 48.92 | 33.40 | 0.0388 | 73.3 | 420 | 0.266 | 0.00089 |
| 12MCeO2nr | 25.15 | 62.74 | 37.26 | 41.26 | 0.0461 | 77.3 | 490 | 0.310 | 0.00128 |
| 6MCeO2nc | 19.04 | 70.10 | 29.90 | 12.41 | 0.0118 | 72.2 | 210 | 0.085 | 0.00179 |
| 12MCeO2nc | 20.82 | 60.70 | 39.30 | 16.55 | 0.0132 | 72.3 | 378 | 0.152 | 0.00688 |
| Catalyst | Reaction Condition | DMMP Concentration | Protection Time | Reference |
|---|---|---|---|---|
| 0.5% Pt-Al2O3 | 299 °C, flow rate 8.85 L/min | 3.5 g/m3 | 8 h | Graven et al. [46] |
| Cu2-HA | 400 °C, flow rate 100 mL/min | 3.58 g/m3 | 7.5 h | Lee et al. [47] |
| 1.6% Pt-TiO2 | 300 °C, flow rate 100 mL/min | 8 h | ||
| 10% V/Al2O3 | 400 °C, flow rate 50 mL/min | 1300 ppm | 12.5 h | Cao et al. [13] |
| 1% Pt/Al2O3 | 8.5 h | |||
| 10% Cu/Al2O3 | 7.5 h | |||
| Al2O3 | 4.0 h | |||
| 10% Fe/Al2O3 | 3.5 h | |||
| 10% Ni/Al2O3 | 1.5 h | |||
| 10% V/SiO2 | 25 h | |||
| CuO/γ-Al2O3 | 350 °C, flow rate 100 mL/min | 4.0 g/m3 | 1.8 h | Dong et al. [48] |
| CuO-1% CeO2/γ-Al2O3 | 2.1 h | |||
| CuO-5% CeO2/γ-Al2O3 | 3.9 h | |||
| CuO-10% CeO2/γ-Al2O3 | 1.8 h | |||
| CeO2 | 400 °C, flow rate 100 mL/min | 8.46 g/m3 | 2.33 h | Dong et al. [49] |
| 10% Cu/Ce | 4.2 h | |||
| 20% Cu/Ce | 4.43 h | |||
| 50% Cu/Ce | 5.36 h | |||
| 80% Cu/Ce | 2.33 h | |||
| CuO | 0.93 h | |||
| 2MCeO2np | 300 °C, flow rate 50 mL/min | 5.32 g/m3 | 5.8 h | This work |
| 6MCeO2nr | 7.0 h | |||
| 12MCeO2nr | 8.1 h | |||
| 6MCeO2nc | 3.5 h | |||
| 12MCeO2nc | 6.3 h |
| Catalyst | Before Decomposition Reaction | After Decomposition Reaction | ΔCe3+ | ΔCe3+/Time |
|---|---|---|---|---|
| Ce3+/(Ce3++ Ce4+) | Ce3+/(Ce3++ Ce4+) | |||
| 2MCeO2np | 19.64 | 34.99 | 15.35 | 0.044 |
| 12MCeO2nr | 25.15 | 35.01 | 9.86 | 0.020 |
| 12MCeO2nc | 20.82 | 30.57 | 9.75 | 0.026 |
| Samples | PO43− |
|---|---|
| 2MCeO2np | 3.508 (g/kg) |
| 12MCeO2nr | 1.450 (g/kg) |
| 12MCeO2nc | 1.720 (g/kg) |
| 2MCeO2np reaction tube tail residues | 0.2943 (mg/L) |
| 12MCeO2nr reaction tube tail residues | 0.0722 (mg/L) |
| 12MCeO2nc reaction tube tail residues | 0.1654 (mg/L) |
| Vibrational Mode | IR Frequencies (cm−1) | ||
|---|---|---|---|
| DMMP [51] | DMMP Gas Phase [21,52] | DMMP Liquid Phase This Work | |
| νa(P−CH3) | 2992 | 3014 | 2996 |
| νa(O−CH3) | 2957 | 2962 | 2958 |
| νs(P−CH3) | 2926 | 2924 | 2927 |
| νs(O−CH3) | 2852 | 2860 | 2854 |
| δa(O−CH3) | 1465 | 1467 | 1465 |
| δs(O−CH3) | 1450 | / | / |
| δs(P−CH3) | 1317 | 1314 | 1315 |
| ν(P=O) | 1242 | 1276 | 1257 |
| ρ‖(O−CH3) | 1186 | 1188/1190 | 1187 |
| νa(C−O) | 1058 | 1070 | 1061 |
| νs(C−O) | 1034 | 1049 | 1034 |
| ρ‖(P−CH3) | 916 | 914 | 914 |
| ν(P−O) | 822 | 816 | 821 |
| ν(P−O) | 789 | / | 788 |
| ν(P−C) | 714 | / | 713 |
| Vibrational Mode | IR Frequencies (cm−1) | |||||
|---|---|---|---|---|---|---|
| 2MCeO2np | 12MCeO2nr | 12MCeO2nc | Reference [53] | Reference [54] | Reference [56] | |
| ν(OH) | 3701 | / | 3709 | 3710 | 3724 | 3720 |
| ν(OH) | 3659 | 3655 | / | 3660 | 3656/3651 | 3652 |
| ν(OH) | / | 3554 | 3594 | 3600 | 3616 | / |
| νs(P−CH3) | 2930 | 2928 | 2914 | / | / | / |
| νs(O−CH3) | 2854 | 2874 | 2876 | / | / | / |
| ν(Ce−O−CH3) [55] | 2813 | 2813 | 2813 | / | / | / |
| 2δ(C−H) | 2716 | 2716 | / | / | 2721 | / |
| νa(OCO) 1 | 1615 | 1620 | / | 1599 | 1609 | / |
| νa(OCO) 2 | 1535 | / | / | 1553 | 1549 | 1552/1550 |
| ν(CO3) 3 [57] | 1396 | / | / | 1354 | / | / |
| δ(C−H) | 1365 | 1363 | / | 1371 | 1367 | 1375/1372 |
| δs(P−CH3) | / | 1318 | 1318 | / | / | / |
| ν(CO3) 5 [57] | / | 1318 | 1318 | / | / | / |
| ν(CO3) 5 [57] | / | / | 1236 | / | / | / |
| ν(CO3) 4 [57] | 1294 | / | / | 1289/ | / | / |
| ν(P=O) [32,55] | 1262 | 1279 | 1272 | / | / | / |
| νa(P−O) [32,55] | 1213 | 1208/ | 1130 | / | / | / |
| νs(P−O) [32,55] | 1056 | 1169 | 1050 | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, W.; Wang, X.; Wang, K.; He, Q.; Zhou, S.; Yang, P.; Dong, Y. Thermocatalytic Decomposition of Dimethyl Methylphosphonate Based on CeO2 Catalysts with Different Morphologies. Appl. Sci. 2023, 13, 3093. https://doi.org/10.3390/app13053093
Kong W, Wang X, Wang K, He Q, Zhou S, Yang P, Dong Y. Thermocatalytic Decomposition of Dimethyl Methylphosphonate Based on CeO2 Catalysts with Different Morphologies. Applied Sciences. 2023; 13(5):3093. https://doi.org/10.3390/app13053093
Chicago/Turabian StyleKong, Weimin, Xuwei Wang, Kunpeng Wang, Qingrong He, Shuyuan Zhou, Piaoping Yang, and Yanchun Dong. 2023. "Thermocatalytic Decomposition of Dimethyl Methylphosphonate Based on CeO2 Catalysts with Different Morphologies" Applied Sciences 13, no. 5: 3093. https://doi.org/10.3390/app13053093
APA StyleKong, W., Wang, X., Wang, K., He, Q., Zhou, S., Yang, P., & Dong, Y. (2023). Thermocatalytic Decomposition of Dimethyl Methylphosphonate Based on CeO2 Catalysts with Different Morphologies. Applied Sciences, 13(5), 3093. https://doi.org/10.3390/app13053093






