Enhanced Heterogeneous Activation of Peroxymonosulfate by Nitrogen–Sulfur Co-Doped Mofs-Derived Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Synthesis of Catalysts
2.2.1. Preparation of Sulfur-Containing Precursor ZIF-67-S
2.2.2. Preparation of Sulfur-Containing Precursor ZIF-8-S
2.2.3. Preparation of Precursor ZIF-67
2.2.4. Preparation of CoSN@C, CoN@C and SN@C
2.3. Experimental Procedures
2.4. Material Characterization and Analytical Methods
3. Results
3.1. Characterization of CoSN@C
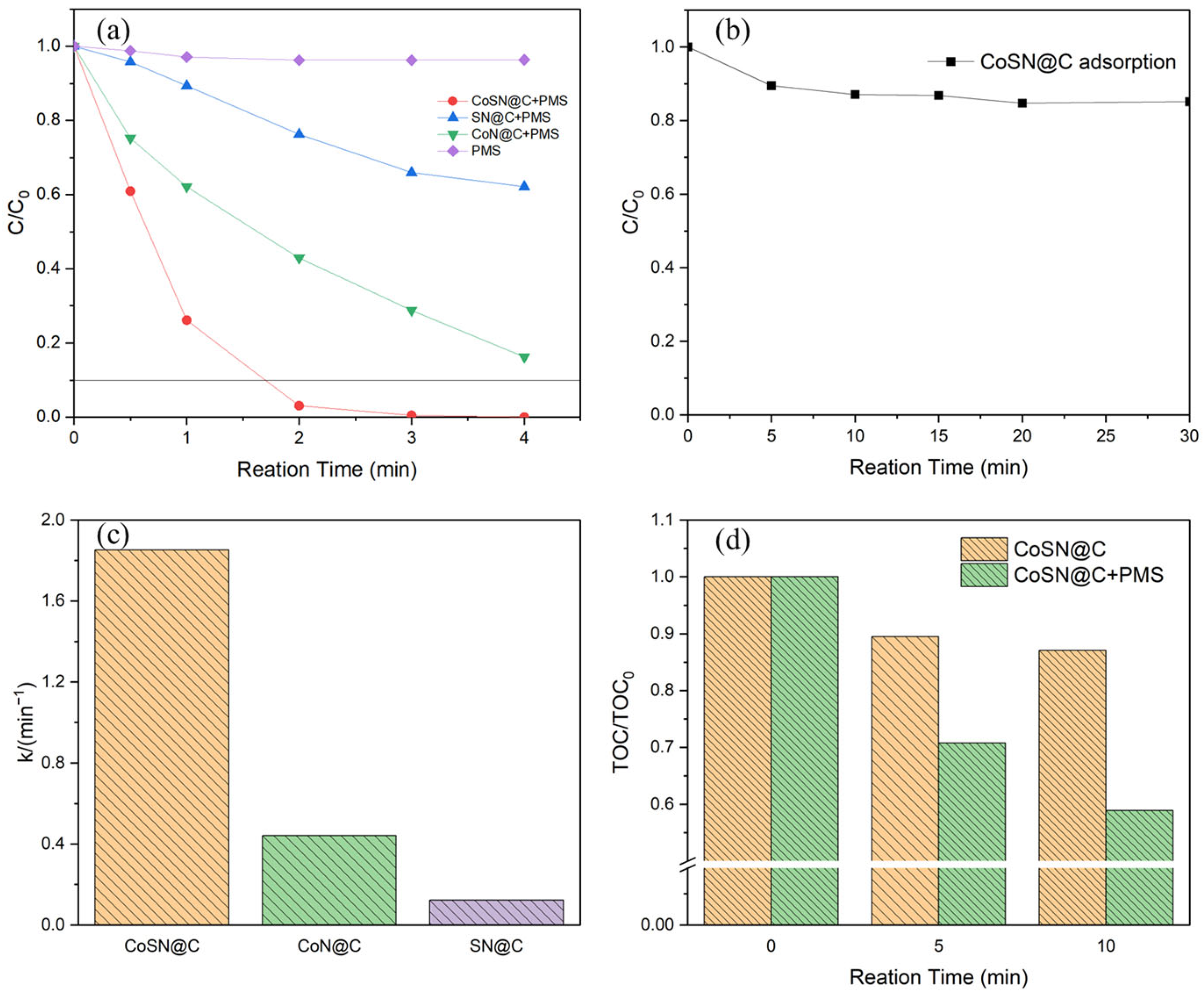
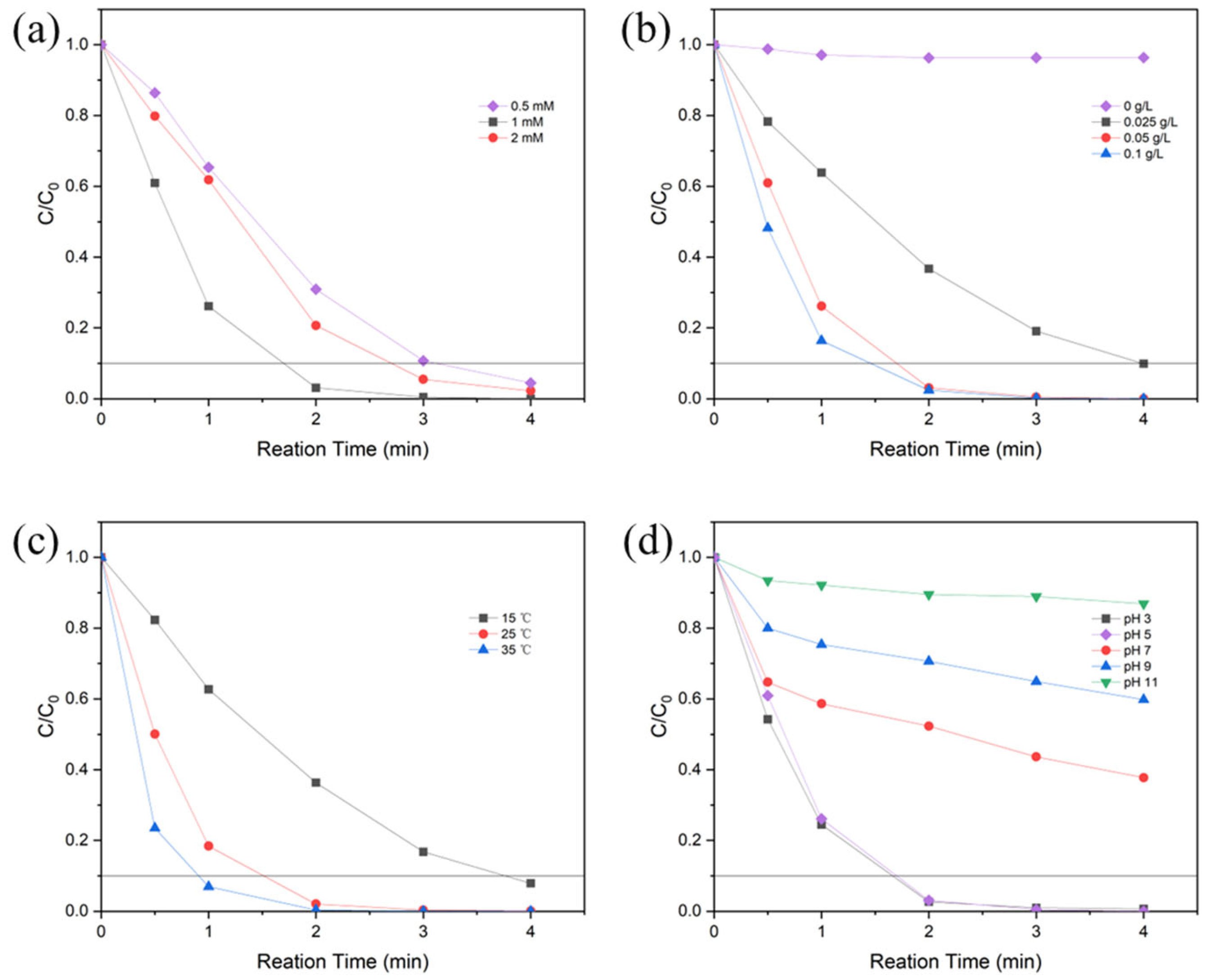
3.2. Catalytic Activity of CoSN@C
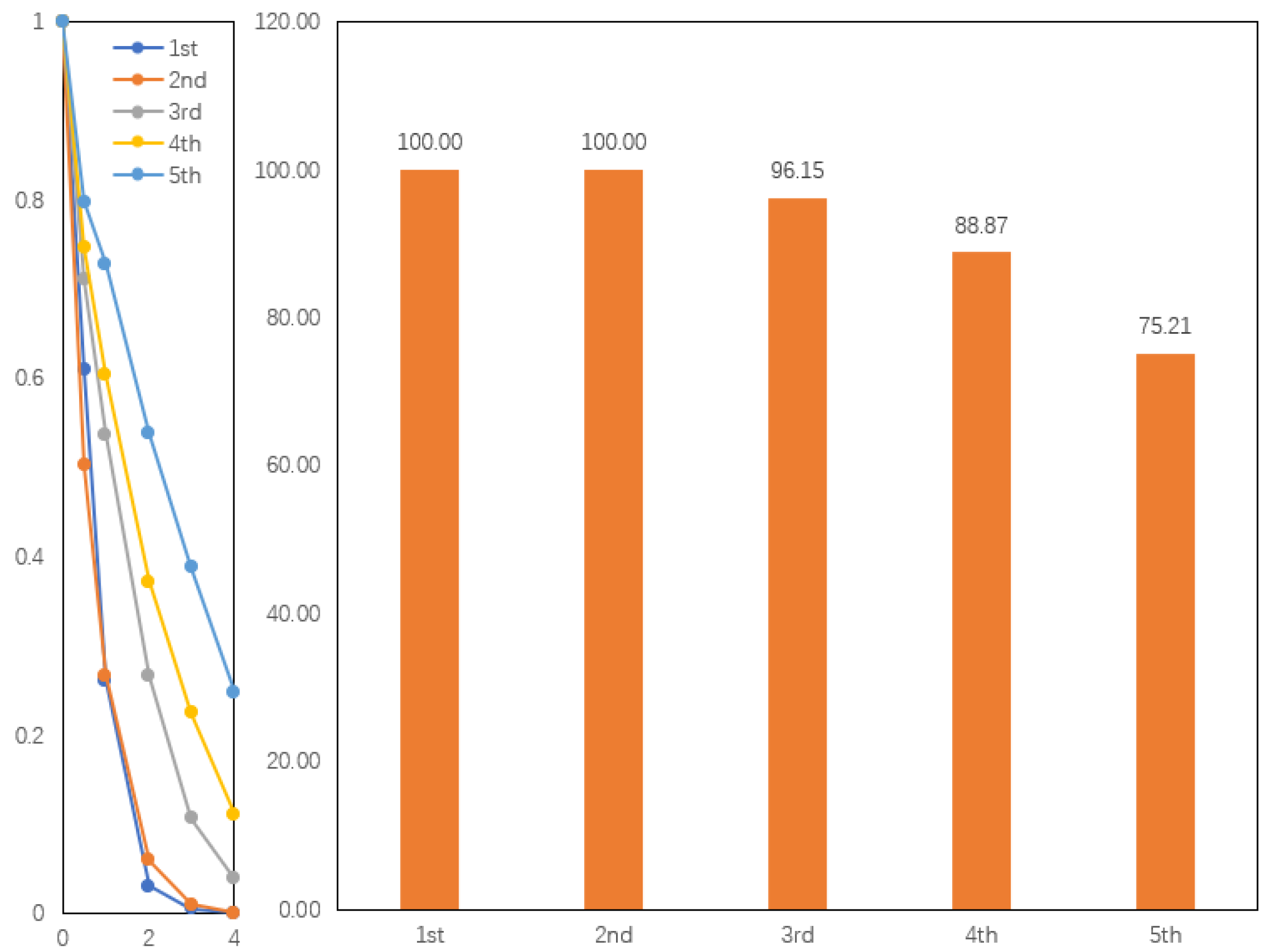
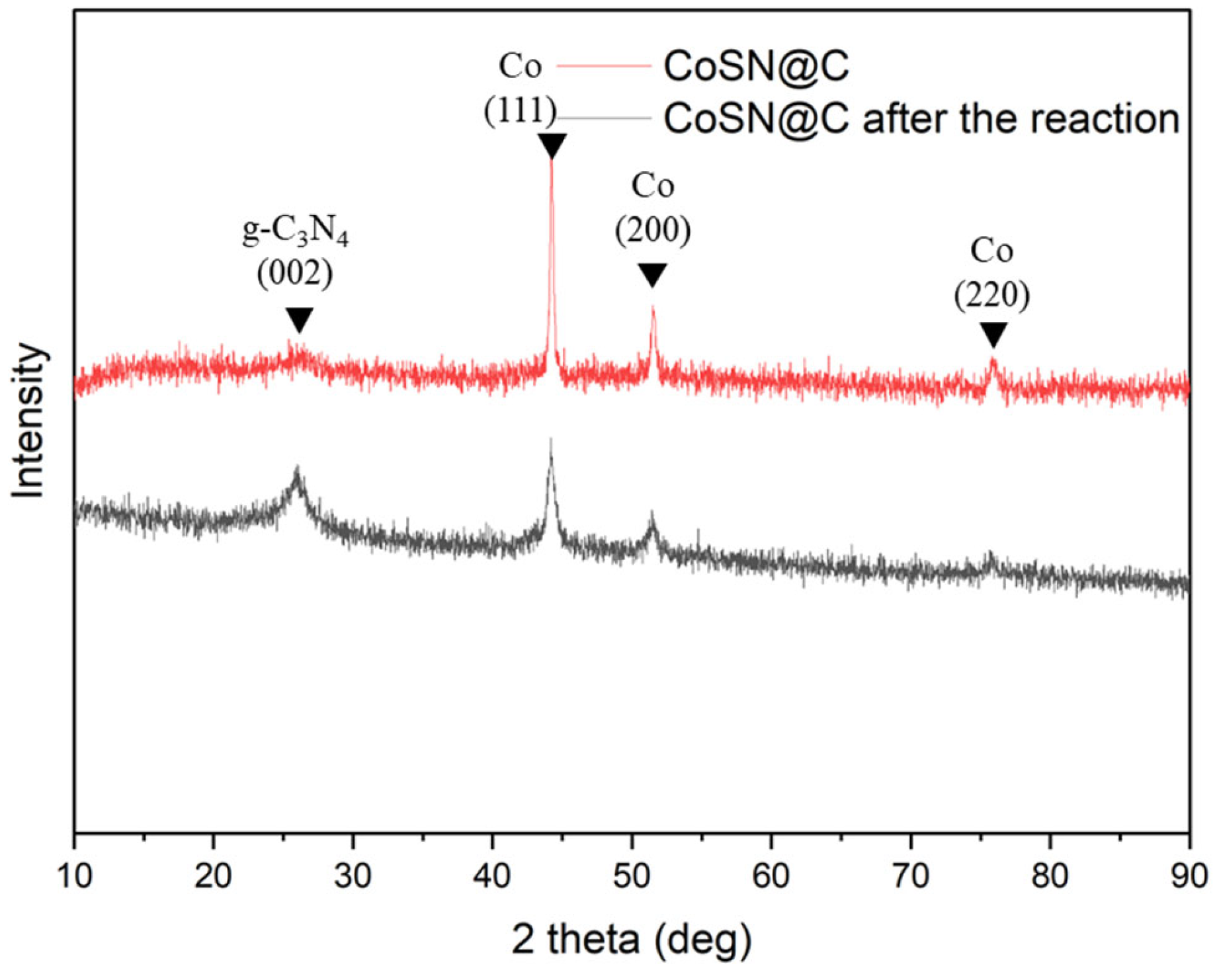
3.3. Cycling Stability and Failure Analysis of CoSN@C
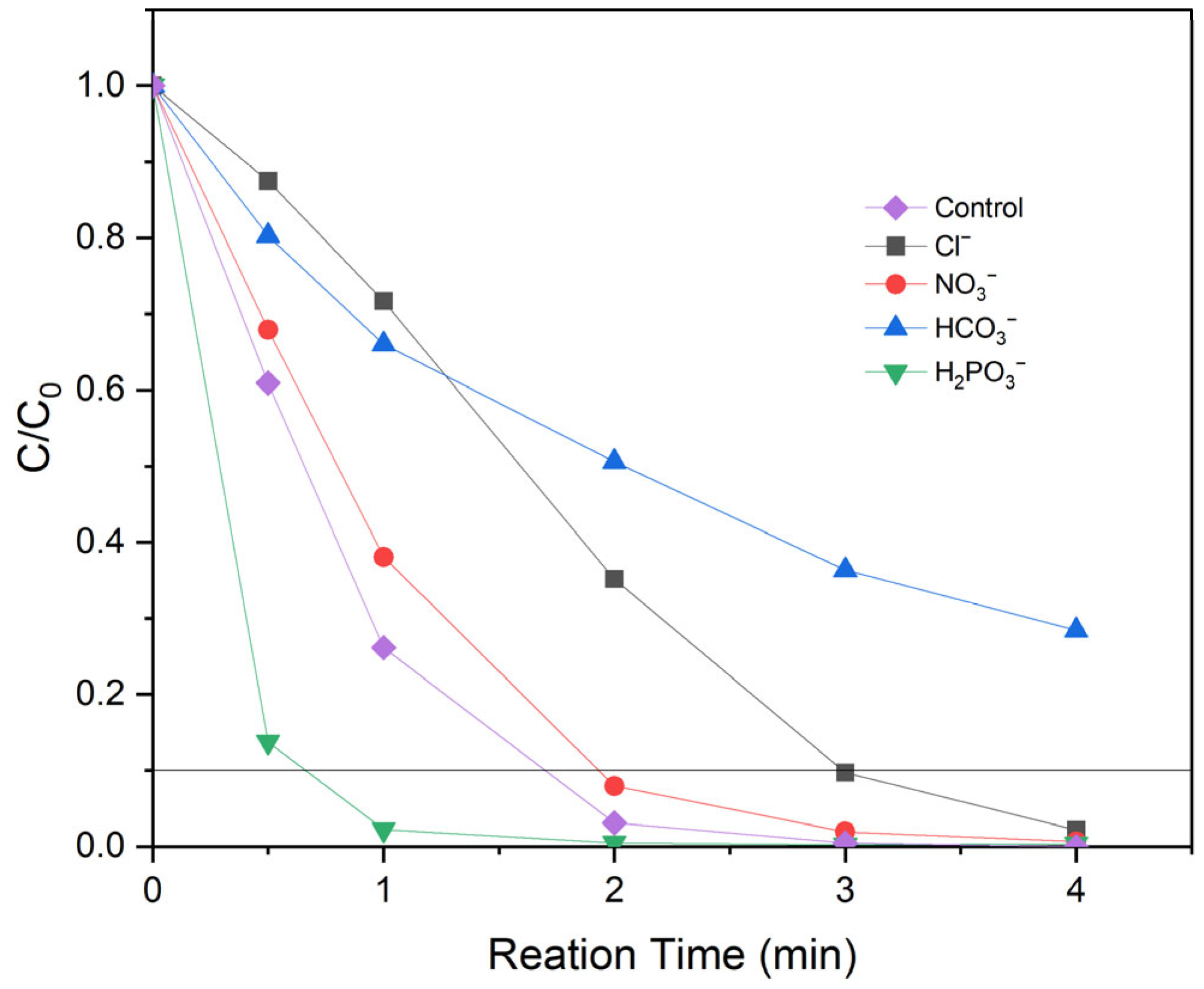
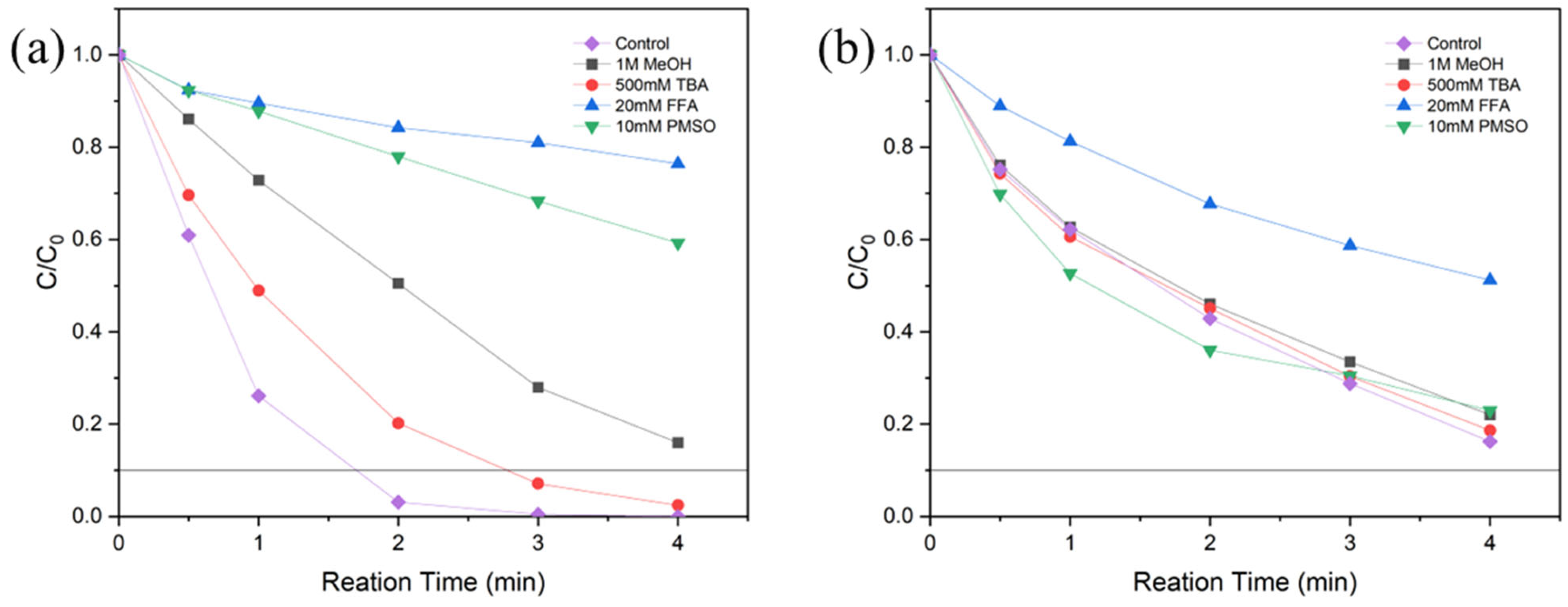
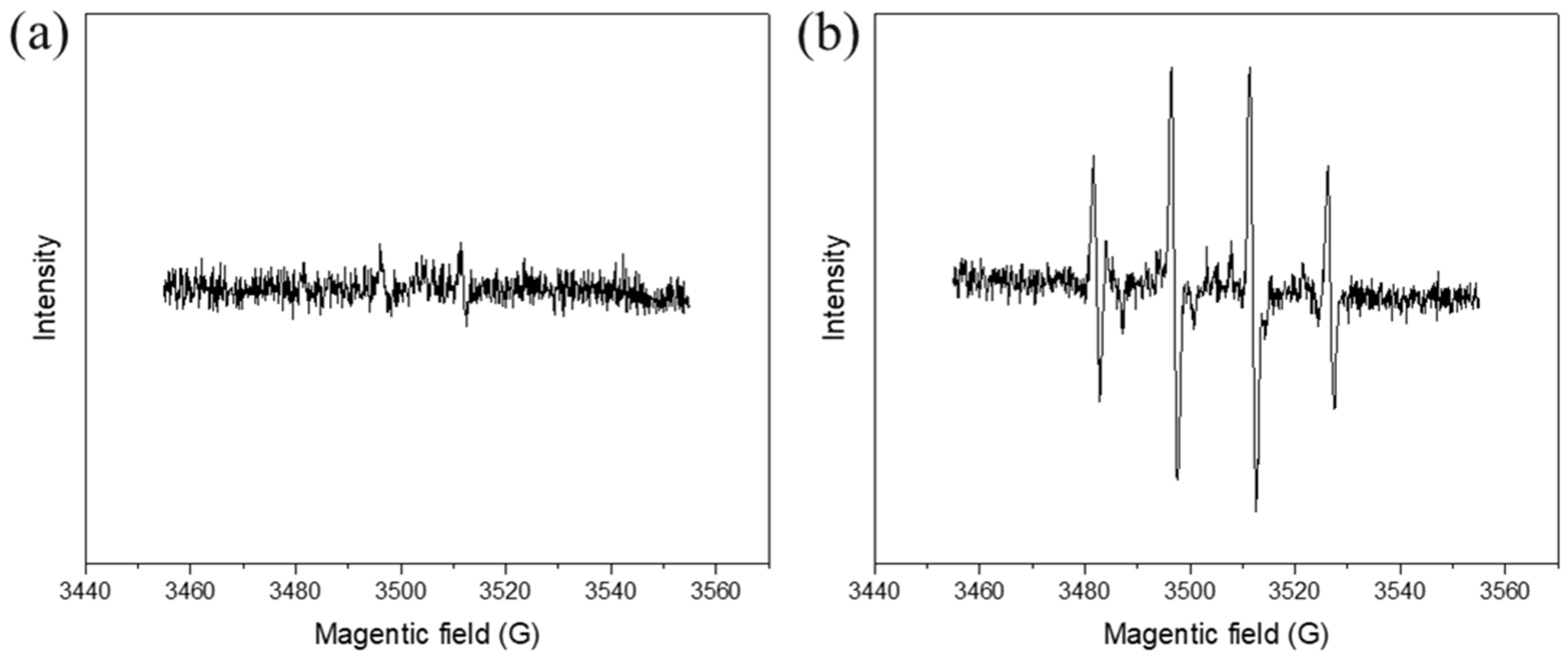
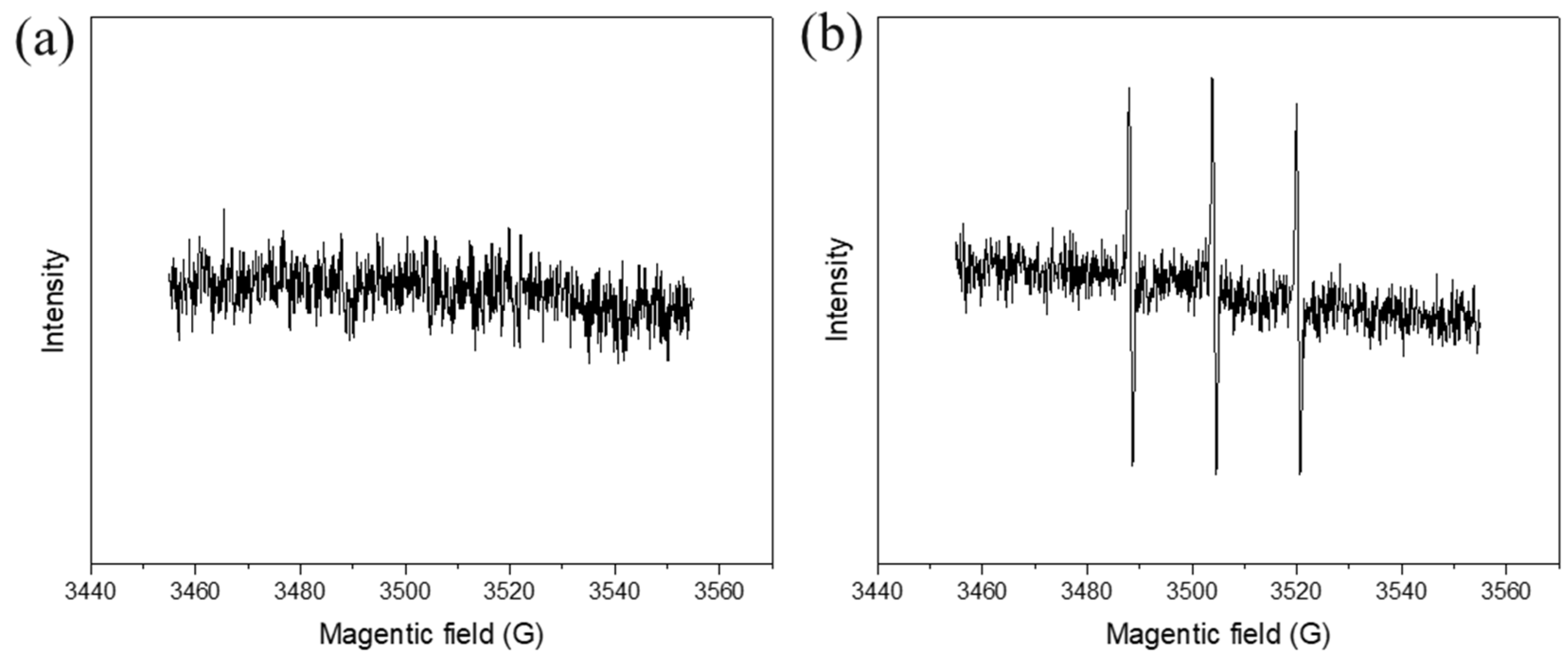
3.4. Identification of Reactive Species and Mechanism
4. Conclusions
- The sulfur-doped, nonhomogeneous catalyst CoSN@C was produced using a one-pot synthesis followed by carbonization, and it was employed for the degradation of RhB;
- Increasing the temperature can greatly increase the rate of RhB degradation; increasing the pH value will significantly decrease the rate of RhB degradation; the best results were achieved with 20 mg L−1 RhB, CoSN@C 0.05 g L−1, and 1 mM PMS;
- The results of radical capture experiments and EPR tests evidenced that a large amount of SO4·− and HO· radicals adsorbed on the catalyst surface and a certain amount of 1O2 were generated in the reaction, and that the radical and non-radical pathways collaborated to degrade RhB rapidly and efficiently;
- After four cycles, the CoSN@C catalyst maintained its high catalytic activity and structural stability, and the RhB degradation rate remained at 88.9%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Homaeigohar, S. The Nanosized Dye Adsorbents for Water Treatment. Nanomaterials 2020, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E.; Martinez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166, 603–643. [Google Scholar] [CrossRef]
- Ding, X.; Gutierrez, L.; Croue, J.-P.; Li, M.; Wang, L.; Wang, Y. Hydroxyl and sulfate radical-based oxidation of RhB dye in UV/H2O2 and UV/persulfate systems: Kinetics, mechanisms, and comparison. Chemosphere 2020, 253, 126655. [Google Scholar] [CrossRef] [PubMed]
- Al-Gheethi, A.A.; Azhar, Q.M.; Kumar, P.S.; Yusuf, A.A.; Al-Buriahi, A.K.; Mohamed, R.M.S.R.; Al-shaibani, M.M. Sustainable approaches for removing Rhodamine B dye using agricultural waste adsorbents: A review. Chemosphere 2022, 287, 132080. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R.; Yao, H.; Zhang, A.; Xiang, S.; Huang, L. Degradation of trimethoprim by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Environ. Sci. Pollut. Res. 2021, 28, 62572–62582. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Z.; Wang, S.; Liu, J.; Zhang, Y.; Wang, B.; Gong, Z.; Wang, M.; Dong, H.; Shi, J.; et al. Enhanced removal of methylparaben mediated by cobalt/carbon nanotubes (Co/CNTs) activated peroxymonosulfate in chloride-containing water: Reaction kinetics, mechanisms and pathways. Chem. Eng. J. 2021, 409, 128176. [Google Scholar] [CrossRef]
- Dai, Y.; Qi, C.; Cao, H.; Wen, Y.; Zhao, Y.; Xu, C.; Yang, S.; He, H. Enhanced degradation of sulfamethoxazole by microwave-activated peracetic acid under alkaline condition: Influencing factors and mechanism. Sep. Purif. Technol. 2022, 288, 120716. [Google Scholar] [CrossRef]
- Qi, C.; Wen, Y.; Zhao, Y.; Dai, Y.; Li, Y.; Xu, C.; Yang, S.; He, H. Enhanced degradation of organic contaminants by Fe(III)/peroxymonosulfate process with l-cysteine. Chin. Chem. Lett. 2022, 33, 2125–2128. [Google Scholar] [CrossRef]
- Dai, Y.; Cao, H.; Qi, C.; Zhao, Y.; Wen, Y.; Xu, C.; Zhong, Q.; Sun, D.; Zhou, S.; Yang, B.; et al. L-cysteine boosted Fe(III)-activated peracetic acid system for sulfamethoxazole degradation: Role of L-cysteine and mechanism. Chem. Eng. J. 2023, 451, 138588. [Google Scholar] [CrossRef]
- Su, R.; Xie, C.; Alhassan, S.I.; Huang, S.; Chen, R.; Xiang, S.; Wang, Z.; Huang, L. Oxygen Reduction Reaction in the Field of Water Environment for Application of Nanomaterials. Nanomaterials 2020, 10, 1719. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Chen, H.; Li, W.; Ao, Z.; Wu, Y.N.; Guan, X. Single-Atom Fe Catalyst Outperforms Its Homogeneous Counterpart for Activating Peroxymonosulfate to Achieve Effective Degradation of Organic Contaminants. Environ. Sci. Technol. 2021, 55, 7034–7043. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Xi, S.; Miao, S.; Ding, J.; Cai, W.; Liu, S.; Yang, X.; Yang, H.; Gao, J.; et al. Single Cobalt Atoms Anchored on Porous N-Doped Graphene with Dual Reaction Sites for Efficient Fenton-like Catalysis. J. Am. Chem. Soc. 2018, 140, 12469–12475. [Google Scholar] [CrossRef]
- Wei, Z.-X.; Zhu, Y.-T.; Liu, J.-Y.; Zhang, Z.-C.; Hu, W.-P.; Xu, H.; Feng, Y.-Z.; Ma, J.-M. Recent advance in single-atom catalysis. Rare Met. 2021, 40, 767–789. [Google Scholar] [CrossRef]
- Maru, K.; Kalla, S.; Jangir, R. Dye contaminated wastewater treatment through metal-organic framework (MOF) based materials. New J. Chem. 2022, 46, 3054–3072. [Google Scholar] [CrossRef]
- Zhong, Q.; Xu, C.; Liu, Y.; Ji, Q.; Xu, Z.; Sun, D.; Zhou, S.; Yang, B.; Dai, Y.; Qi, C.; et al. Defect-engineered FeSe2−x@C with porous architecture for enhanced peroxymonosulfate-based advanced oxidation processes. Appl. Catal. B Environ. 2022, 309, 121259. [Google Scholar] [CrossRef]
- He, Y.; Hwang, S.; Cullen, D.A.; Uddin, M.A.; Langhorst, L.; Li, B.; Karakalos, S.; Kropf, A.J.; Wegener, E.C.; Sokolowski, J.; et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: Carbon-shell confinement strategy. Energy Environ. Sci. 2019, 12, 250–260. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Wang, W.; Cao, D. Recent Progress in MOF-Derived, Heteroatom-Doped Porous Carbons as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. Adv. Funct. Mater. 2018, 28, 1704537. [Google Scholar] [CrossRef]
- Mi, X.; Wang, P.; Xu, S.; Su, L.; Zhong, H.; Wang, H.; Li, Y.; Zhan, S. Almost 100% Peroxymonosulfate Conversion to Singlet Oxygen on Single-Atom CoN2+2 Sites. Angew. Chem. Int. Ed. 2021, 60, 4588–4593. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Xu, X.; Gao, B.; Wang, S.; Duan, X. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 2021, 50, 5281–5322. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, B.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Peng, W. Facile Synthesis of Atomic Fe-N-C Materials and Dual Roles Investigation of Fe-N4 Sites in Fenton-Like Reactions. Adv. Sci. 2021, 8, 2101824. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xing, T.; Lou, Y.; Chen, J. Controlling ZIF-67 crystals formation through various cobalt sources in aqueous solution. J. Solid State Chem. 2016, 235, 107–112. [Google Scholar] [CrossRef]
- Du, X.-D.; Wang, C.-C.; Liu, J.-G.; Zhao, X.-D.; Zhong, J.; Li, Y.-X.; Li, J.; Wang, P. Extensive and selective adsorption of ZIF-67 towards organic dyes: Performance and mechanism. J. Colloid Interface Sci. 2017, 506, 437–441. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, X.; Wang, X.; Kaneti, Y.V.; Chen, Y.; Liu, J.; Jiang, J.-S.; Yamauchi, Y.; Hu, M. Spontaneous Weaving of Graphitic Carbon Networks Synthesized by Pyrolysis of ZIF-67 Crystals. Angew. Chem. Int. Ed. 2017, 56, 8435–8440. [Google Scholar] [CrossRef]
- Karmakar, A.; Hazra, S.; Pombeiro, A.J.L. Urea and thiourea based coordination polymers and metal-organic frameworks: Synthesis, structure and applications. Coord. Chem. Rev. 2022, 453, 214314. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Li, H.; Zhang, Q.; Liu, H. Sulfur-functionalized metal-organic frameworks: Synthesis and applications as advanced adsorbents. Coord. Chem. Rev. 2020, 408, 213191. [Google Scholar] [CrossRef]
- Luo, J.; Bo, S.; An, Q.; Xiao, Z.; Wang, H.; Cai, W.; Zhai, S.; Li, Z. Designing ordered composites with confined Co–N/C layers for efficient pollutant degradation: Structure-dependent performance and PMS activation mechanism. Microporous Mesoporous Mater. 2020, 293, 109810. [Google Scholar] [CrossRef]
- Li, N.; Li, R.; Duan, X.; Yan, B.; Liu, W.; Cheng, Z.; Chen, G.; Hou, L.A.; Wang, S. Correlation of Active Sites to Generated Reactive Species and Degradation Routes of Organics in Peroxymonosulfate Activation by Co-Loaded Carbon. Environ. Sci. Technol. 2021, 55, 16163–16174. [Google Scholar] [CrossRef]
- Miao, J.; Geng, W.; Alvarez, P.J.J.; Long, M. 2D N-Doped Porous Carbon Derived from Polydopamine-Coated Graphitic Carbon Nitride for Efficient Nonradical Activation of Peroxymonosulfate. Environ. Sci. Technol. 2020, 54, 8473–8481. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Zhang, T.; Silva, T.L.; Almeida, V.C.; Asefa, T. Bone char-derived metal-free N- and S-co-doped nanoporous carbon and its efficient electrocatalytic activity for hydrazine oxidation. Appl. Catal. B Environ. 2018, 225, 30–39. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, R.; Wang, C.; Zhang, W.; Yan, X.; Sun, X.; Wang, L.; Li, J. Confined pyrolysis of metal-organic frameworks to N-doped hierarchical carbon for non-radical dominated advanced oxidation processes. J. Mater. Chem. A 2019, 7, 12547–12555. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Govindan, K.; Raja, M.; Noel, M.; James, E.J. Degradation of pentachlorophenol by hydroxyl radicals and sulfate radicals using electrochemical activation of peroxomonosulfate, peroxodisulfate and hydrogen peroxide. J. Hazard. Mater. 2014, 272, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liu, H.; Feng, M.; Guo, L.; Zhai, Z.; Fang, Y.; Zhang, X.; Sharma, V.K. Nitrogen-sulfur co-doped industrial graphene as an efficient peroxymonosulfate activator: Singlet oxygen-dominated catalytic degradation of organic contaminants. Appl. Catal. B: Environ. 2019, 251, 335–345. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, H.; Xiong, Z.; Liu, Y.; Yao, G.; Lai, B. Heterogeneous activation of peroxymonosulfate by CoMgFe-LDO for degradation of carbamazepine: Efficiency, mechanism and degradation pathways. Chem. Eng. J. 2020, 391, 123604. [Google Scholar] [CrossRef]
- Zeng, T.; Li, S.; Hua, J.; He, Z.; Zhang, X.; Feng, H.; Song, S. Synergistically enhancing Fenton-like degradation of organics by in situ transformation from Fe3O4 microspheres to mesoporous Fe, N-dual doped carbon. Sci. Total Environ. 2018, 645, 550–559. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.; Deng, L. Performance of CuO/Oxone system: Heterogeneous catalytic oxidation of phenol at ambient conditions. Chem. Eng. J. 2011, 178, 239–243. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B Environ. 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Wu, L.; Guo, Y.; Chen, C.; Wang, Z.; Xiao, D.; Fang, C.; Liu, J.; Zhao, J.; Lu, S. Peroxymonosulfate activation by phosphate anion for organics degradation in water. Chemosphere 2014, 117, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cai, M.; Liu, Y.; Zhang, L.; Feng, L. Effects of water matrices on the degradation of naproxen by reactive radicals in the UV/peracetic acid process. Water Res. 2019, 150, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, T.; Yang, C.; Ma, C.; Zhao, Z.; Wu, Z.; Cao, S.; Geng, W.; Wang, Y.; Yao, Y.; et al. Activity Trends and Mechanisms in Peroxymonosulfate-Assisted Catalytic Production of Singlet Oxygen over Atomic Metal-N-C Catalysts. Angew. Chem. Int. Ed. 2021, 60, 22513–22521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Jiang, X.H.; Zhong, Z.A.; Tian, L.; Sun, Q.; Cui, Y.T.; Lu, X.; Zou, J.P.; Luo, S.L. Carbon Nitride Supported High-Loading Fe Single-Atom Catalyst for Activation of Peroxymonosulfate to Generate (1) O2 with 100% Selectivity. Angew. Chem. Int. Ed. 2021, 60, 21751–21755. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, X.; Fu, L.; Peng, X.; Pan, C.; Mao, Q.; Wang, C.; Yan, J. Nonradicals induced degradation of organic pollutants by peroxydisulfate (PDS) and peroxymonosulfate (PMS): Recent advances and perspective. Sci. Total Environ. 2021, 765. [Google Scholar] [CrossRef]
- Luo, R.; Li, M.Q.; Wang, C.H.; Zhang, M.; Khan, M.A.N.; Sun, X.Y.; Shen, J.Y.; Han, W.Q.; Wang, L.J.; Li, J.S. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition. Water Res. 2019, 148, 416–424. [Google Scholar] [CrossRef]

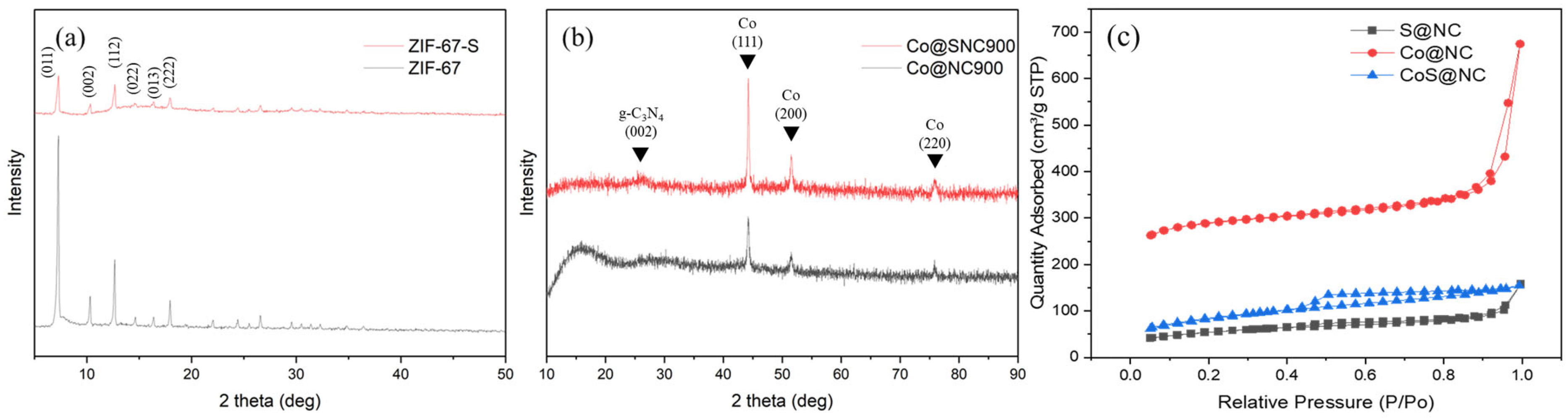
| Catalysts | Surface Area (m2 g−1) | Pore Size (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| CoSN@C | 293.30 | 3.26 | 0.24 |
| CoN@C | 876.66 | 4.76 | 1.04 |
| SN@C | 185.02 | 5.23 | 0.24 |
| Catalysts | k/(min−1) | R2 |
|---|---|---|
| CoSN@C | 1.852 | 0.99 |
| CoN@C | 0.442 | 0.98 |
| SN@C | 0.122 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Chu, H.; Ma, Q.; Chen, Y.; Fan, J. Enhanced Heterogeneous Activation of Peroxymonosulfate by Nitrogen–Sulfur Co-Doped Mofs-Derived Carbon. Appl. Sci. 2023, 13, 3182. https://doi.org/10.3390/app13053182
Zhang C, Chu H, Ma Q, Chen Y, Fan J. Enhanced Heterogeneous Activation of Peroxymonosulfate by Nitrogen–Sulfur Co-Doped Mofs-Derived Carbon. Applied Sciences. 2023; 13(5):3182. https://doi.org/10.3390/app13053182
Chicago/Turabian StyleZhang, Chuning, Huaqiang Chu, Qian Ma, Yanyan Chen, and Jianwei Fan. 2023. "Enhanced Heterogeneous Activation of Peroxymonosulfate by Nitrogen–Sulfur Co-Doped Mofs-Derived Carbon" Applied Sciences 13, no. 5: 3182. https://doi.org/10.3390/app13053182
APA StyleZhang, C., Chu, H., Ma, Q., Chen, Y., & Fan, J. (2023). Enhanced Heterogeneous Activation of Peroxymonosulfate by Nitrogen–Sulfur Co-Doped Mofs-Derived Carbon. Applied Sciences, 13(5), 3182. https://doi.org/10.3390/app13053182







