Application of Fly Ash Obtained from the Incineration of Municipal Solid Waste in Agriculture
Abstract
:1. Introduction
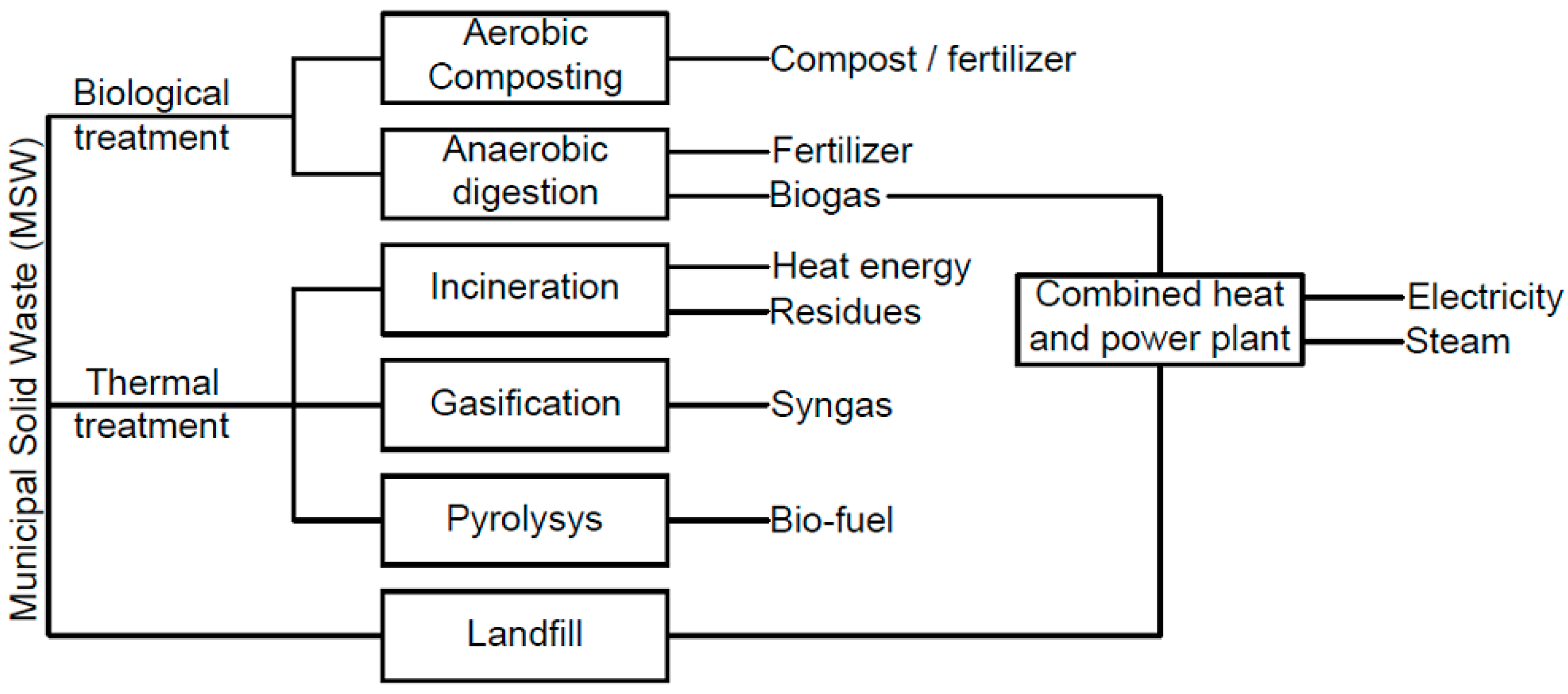
1.1. Description of the Municipal Solid Waste Incineration Process
1.2. Description of the Technological Flow of Municipal Solid Waste Incineration
- -
- drying and degassing of volatile substances (hydrocarbons and water) [26];
- -
- pyrolysis of organic substances at temperatures of 250–700 °C and gasification of coal residues at temperatures between 500–1000 °C.
- (a)
- The drying period: under the action of the heat radiated in the hearth, the air introduced, which in the vast majority of cases preheated, as well as the recirculated hot combustion gases, turn a large part of the moisture into water vapor, which is then removed in the mixture with combustion gases;
- (b)
- The transformation period: through the uniform application of heat, volatile substances and semicoke gases, which are in relatively large quantities, are removed from the waste. The characteristic of these gases is that they ignite at relatively low temperatures (250 °C). The combustion of waste will begin after igniting the released gases;
- (c)
- The burning period: if the appropriate conditions are met, the waste will burn continuously without the addition of auxiliary fuel. The combustion speed of the released gases depends on the thermal conductivity, the load capacity of the combustion grill, and the amount of air introduced into the hearth. The burning speed can be increased by reducing the amount of material on the burning grate and by preheating the air introduced into the incinerator;
- (d)
- The post-combustion period represents the last part of the combustion process in which the particles of matter falling from the combustion grate continue their combustion on an additional grate (post-combustion grate) mounted in the extension of the main one or are introduced into a vertical well mounted at the lower end of the combustion grate, and through the layer of material a current of air is introduced from the bottom up, possibly with the addition of steam. In some cases, a solution can be used in which the slag (matter subjected to post-combustion) is introduced into a rotating post-combustion drum with a very low rotation speed (4–8 rot/h).
2. The Chemical Composition of Fly Ash from the Incineration of Municipal Solid Waste
| Element | [35] | [38] | [39] | [40] |
|---|---|---|---|---|
| SiO2 | 20.33–44.90 | 13.60 | 44.40 | 4.48–24.84 |
| Al2O3 | 9.26–17.0 | 0.92 | 27.50 | 1.56–12.43 |
| CaO | 10.36–18.20 | 45.42 | 11.50 | 16.6–39.9 |
| Fe2O3 | 1.72–6.29 | 3.83 | 6.21 | 0.85–5.04 |
| MgO | 1.83–3.15 | 3.16 | 2.36 | 0.67–3.76 |
| K2O | 1.44–5.28 | 3.85 | 0.99 | 3.76–15.24 |
| Na2O | 2.03–7.70 | 4.16 | 1.38 | 2.71–9.17 |
| SO3 | - | 6.27 | 1.01 | 7.03–14.30 |
| P2O5 | 0.01–0.06 | 1.72 | 1.37 | 1.76 |
| MnO | 0.10–0.25 | - | - | - |
| TiO2 | 1.14–2.34 | 3.12 | 1.79 | 1.06 |
| Cr2O3 | - | 0.19 | - | - |
| CuO | - | 0.25 | - | - |
| ZnO | - | 2.32 | - | 0.71–3.11 |
| PbO | - | 0.57 | - | - |
| BaO | - | - | 0.42 | - |
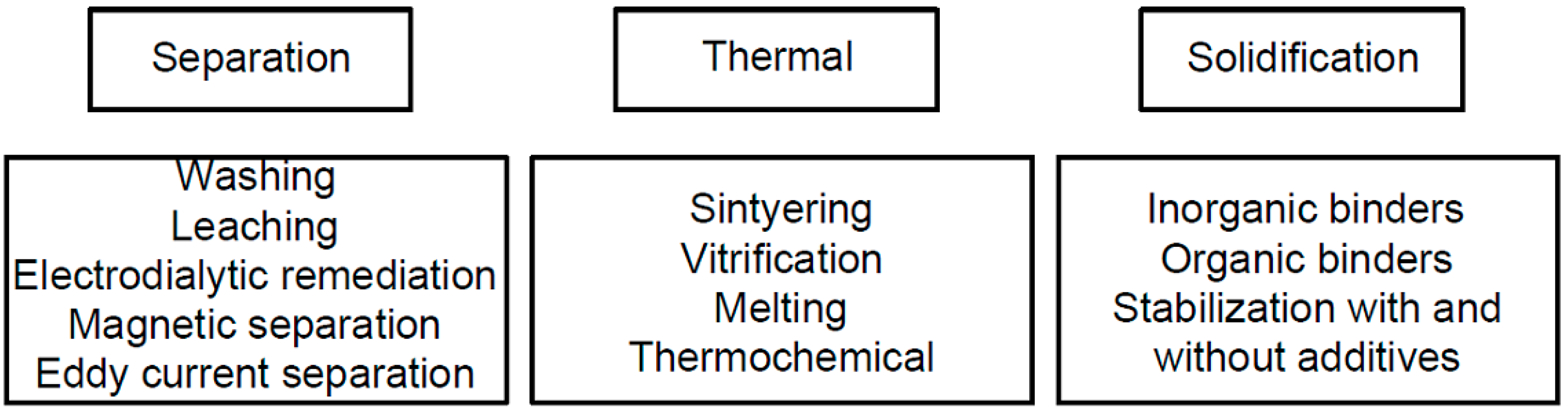
3. MSWI Treatment Methods
3.1. Separation Processes
3.2. Electrodialytic Remediation
3.3. Solidification/Stabilization Process
3.4. Thermal Methods
4. Physico-Chemical Properties of Fly Ash
5. The Impact of Using MSWI in Agriculture
6. The Effect of Using Fly Ash on Plant Development
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanhar, A.H.; Chen, S.; Wang, F. Incineration Fly Ash and Its Treatment to Possible Utilization: A Review. Energies 2020, 13, 6681. [Google Scholar] [CrossRef]
- Hirvonen, J.; Kosonen, R. Waste Incineration Heat and Seasonal Thermal Energy Storage for Promoting Economically Optimal Net-Zero Energy Districts in Finland. Buildings 2020, 10, 205. [Google Scholar] [CrossRef]
- Fabricius, A.L.; Renner, M.; Voss, M.; Funk, M.; Perfoll, A.; Gehring, F.; Graf, R.; Fromm, S.; Duester, L. Municipal waste incineration fy ashes: From a multi-element approach to market potential evaluation. Environ. Sci. Eur. 2020, 32, 88. [Google Scholar] [CrossRef] [PubMed]
- Neina, D.; Faust, S.; Joergensen, R.G. Characterization of charcoal and frewood ash for use in African peri-urban agriculture. Chem. Biol. Technol. Agric. 2020, 7, 20. [Google Scholar] [CrossRef]
- Ogunjuyigbe, A.S.O.; Ayodele, T.R.; Alao, M.A. Electricity generation from municipal solid waste in some selected cities of Nigeria: An assessment of feasibility, potential and technologies. Renew. Sustain. Energy Rev. 2017, 80, 149–162. [Google Scholar] [CrossRef]
- Tan, S.T.; Hashim, H.; Lim, J.S.; Ho, W.S.; Lee, C.T.; Yan, J. Energy and emissions benefits of renewable energy derived from municipal solid waste: Analysis of a low carbon scenario in Malaysia. Appl. Energy 2014, 136, 797–804. [Google Scholar] [CrossRef]
- Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials (accessed on 14 September 2022).
- Zhang, F.; Yamasaki, S.; Nanzyo, M. Application of waste ashes to agricultural land-effect of incineration temperature on chemical characteristics. Sci. Total Environ. 2001, 264, 205–214. [Google Scholar] [CrossRef]
- Petropavlovskaya, V.K.; Shishkov, A.N.; Zamaraev, S.A.; Goncharova, A.V.; Zolnikov, D.A. Considering different methods of thermal utilization of municipal solid waste. IOP Conf. Ser. Earth Environ. Sci. 2022, 990, 012067. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/990/1/012067/pdf (accessed on 20 September 2022). [CrossRef]
- Gong, B.; Deng, Y.; Yang, Y.; He, Y.; Sun, X.; Li-Ya, G.; Zhang, K.; Yang, W. Stabilization of lead in incineration fly ash by moderate thermal treatment with sodium hydroxide addition. PLoS ONE 2017, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Gu, W.; Liu, D.; Wang, C. Energy recovery potential from incineration using municipal solid waste based on multi-scenario analysis in Beijing. Environ. Sci. Pollut. Res. 2021, 28, 27119–27131. [Google Scholar] [CrossRef]
- Seo, Y.J. Current MSW Management and Waste-to-Energy Status in the Republic of Korea; Columbia University: New York, NY, USA, 2013. [Google Scholar]
- Lam, C.H.K.; Ip, A.W.M.; Barford, J.P.; McKay, G. Use of Incineration MSW Ash: A Review. Sustainability 2010, 2, 1943–1968. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Municipal_waste_statistics#Municipal_waste_treatment (accessed on 5 September 2022).
- Bell, L.; Bremmer, J. Burning Waste for Energy It Doesn’t Stack-Up. Exposing the Push towards Unsustainable Waste to Energy Technology in Australia. 2013. Available online: https://ntn.org.au/wp-content/uploads/2013/11/NTN-waste-to-energy-incineration-report-2013.1-1.pdf (accessed on 29 September 2022).
- Quina, M.J.; Bordado, J.M.; Rosa, M.; Quinta-Ferreira, R.M. Air Pollution Control in Municipal Solid Waste Incinerators. In The Impact of Air Pollution on Health, Economy, Environment and Agricultural Source; InTech: Nappanee, IN, USA, 2011; pp. 331–358. [Google Scholar] [CrossRef] [Green Version]
- Margallo, M.; Taddei, M.B.; Hernández-Pellón, A.; Aldaco, R.; Irabien, A. Environmental Sustainability Assessment of the Management of Municipal Solid Waste Incineration Residues: A Review of the Current Situation. Clean Technol. Environ. Policy 2015, 17, 1333–1353. [Google Scholar] [CrossRef]
- Vateva, I.; Laner, D. Grain-Size Specific Characterisation and Resource Potentials of Municipal Solid Waste Incineration (MSWI) Bottom Ash: A German Case Study. Resources 2020, 9, 66. [Google Scholar] [CrossRef]
- He, X.; Zhu, S.; Hwang, J.Y. Physical and chemical properties of MSWI fly ash. In Characterization of Minerals, Metals, and Materials; Springer: Cham, Switzerland, 2016; pp. 451–459. [Google Scholar] [CrossRef]
- Dwivedi, A.; Jain, M.K. Fly ash—Waste management and overview: A Review. Recent Res. Sci. Technol. 2014, 6, 30–35. [Google Scholar]
- Wang, P.; Hu, Y.; Cheng, H. Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China. Environ. Pollut. 2019, 252, 461–475. [Google Scholar] [CrossRef]
- Rautaray, S.K.; Ghosh, B.C.; Mittra, B.N. Effect of fly ash, organic wastes and chemical fertilizers on yield, nutrient uptake, heavy metal content and residual fertility in a rice–mustard cropping sequence under acid lateritic soils. Bioresour. Technol. 2003, 90, 275–283. [Google Scholar] [CrossRef]
- Sahu, G.; Bag, A.G.; Chatterjee, N.; Mukherjee, A.K. Potential use of flyash in agriculture: A way to improve soil health. J. Pharmacogn. Phytochem. 2017, 6, 6–873. [Google Scholar]
- Amoo, O.M.; Fagbenle, R.L. Renewable municipal solid waste pathways for energy generation and sustainable development in the Nigerian context. Int. J. Energy Environ. Eng. 2013, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.; Fanning, M.; Venn, M.; Whitting, K. Review of state of the art waste to energy technologies. Stage two—Case studies. In Waste Management Branch; Department of Environment and Conservation: Perth, Australia, 2013; Available online: http://www.wtert.com.br/home2010/arquivo/noticias_eventos/WSP%20Waste%20to%20Energy%20Technical%20Report%20Stage%20Two.pdf (accessed on 14 September 2022).
- Rusănescu, C.O.; Popescu, I.N.; David, L. Relative humidity monitoring. (Advances in Environmental and Geological Science and Engineering) 3rd. In Proceedings of the International Conference on Environmental and Geological Science and Engineering (EG’ 10), Singapore, 3–5 September 2010; pp. 175–180. [Google Scholar]
- Luo, Y.; Wu, Y.; Ma, S.; Zheng, S.; Zhang, Y.; Chu, P.K. Utilization of coal fly ash in China: A mini-review on challenges and future directions. Environ. Sci. Pollut. Res. 2021, 28, 18727–18740. [Google Scholar] [CrossRef]
- Srivastava, N.; Kustagi, G.K. An Eco-friendly method for reducing the risk of fly ash using Sesbania cannabina (Dhaincha). Res. J. Recent Sci. 2015, 4, 63–67. [Google Scholar]
- Hyks, J. Leaching from Municipal Solid Waste Incineration Residues. Ph.D. Thesis, Technical University of Denmark, Kongens Lyngby, Denmark, 2008. [Google Scholar]
- Basu, M.; Pande, M.; Bhadoria, P.B.S.; Mahapatra, S.C. Potential fly-ash utilization in agriculture: A global review. Prog. Nat. Sci. 2009, 19, 1173–1186. [Google Scholar] [CrossRef]
- Order, no. 756 of 3 November 1997 for the Approval of the Regulation Regarding the Assessment of Environmental Pollution. Available online: https://biosol.ro/wp-content/uploads/linkuri/ord-756-din-03-11-1997-pentru-aprobarea-Reglementarii-privind-evaluarea-poluarii-mediului.pdf (accessed on 24 September 2022).
- U.S. EPA. Clean Water Act; U.S. Environmental Protection Agency: Washington, DC, USA, 1993; Volume 58. Available online: https://www.epa.gov/sites/default/files/2018-12/documents/plain-english-guide-part503-biosolids-rule.pdf (accessed on 26 January 2023).
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N. Impact of fly ash incorporation in soil systems. Agric. Ecosyst. Environ. 2010, 136, 16–27. [Google Scholar] [CrossRef]
- Alba, N.; Gasso, S.; Lacorte, T.; Baldasano, J.M. Characterization of municipal solid waste incineration residues from facilities with different air pollution control systems. J. Air Waste Manage. Assoc. 1997, 47, 1170–1179. [Google Scholar] [CrossRef]
- Sabbas, T.; Polettini, A.; Pomi, R.; Astrup, T.; Hjelmar, O.; Mostbauer, P.; Cappai, G.; Magel, G.; Salhofer, S.; Speiser, C.; et al. Management of Municipal Solid Waste Incineration Residues. Waste Manag. 2003, 23, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Zacco, A.; Borgese, L.; Gianoncelli, A.; Struis, R.; Depero, L.; Bontempi, E. Review of fly ash inertisation treatments and recycling. Environ. Chem. Lett. 2014, 12, 153–175. [Google Scholar] [CrossRef]
- Pan, J.R.; Huang, C.; Kuo, J.J.; Lin, S.H. Recycling MSWI bottom and fly ash as raw materials for Portland cement. Waste Manag. 2008, 28, 1113–1118. [Google Scholar] [CrossRef]
- Hauashdh, A.; Mohamed, R.M.S.R.; Jailani, J.; Abd Rahman, J. Stabilization of Peat Soil Using Fly Ash, Bottom Ash and Portland Cement: Soil Improvement and Coal Ash Waste Reduction Approach. IOP Conf. Ser. Earth Environ. Sci. 2020, 498, 012011. [Google Scholar] [CrossRef]
- Sinha, S.; Gupta, A.K. Translocation of metals from fly ash amended soil in the plant of Sesbania cannabina L. Ritz: Effect on antioxidants. Chemosphere 2005, 61, 1204–1214. [Google Scholar] [CrossRef]
- Sun, X.; Li, J.; Zhao, X.; Zhu, B.; Zhang, G. A review on the management of municipal solid waste fly ash in American, The Tenth International Conference on Waste Management and Technology (ICWMT). Procedia Environ. Sci. 2016, 31, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, C.N.; Kamali, M.; Gibbs, B.F. Bioleaching of heavy metals from a low-grade mining ore using Aspergillus niger. J. Hazard. Mater. 2004, 110, 7–84. [Google Scholar] [CrossRef] [PubMed]
- Funari, V.; Mäkinen, J.; Salminen, J.; Braga, R.; Dinelli, E.; Revitzer, H. Metal removal from Municipal Solid Waste Incineration fly ash: A comparison between chemical leaching and bioleaching. Waste Manag. 2016, 60, 397–406. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Jensen, P.E.; Kirkelund, G.M. Phosphorous Recovery from Sewage Sludge Ash Suspended in Water in a Two-Compartment Electrodialytic. Cell. Waste Manag. 2016, 51, 142–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boom, A.; Degrez, M.; Hubaux, P.; Lucion, C. MSWI boiler fly ashes: Magnetic separation for material recovery. Waste Manag. 2011, 31, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; He, P.; Shao, L.; Zhang, H. Metal distribution characteristic of MSWI bottom ash in view of metal recovery. J. Environ. Sci. 2016, 52, 178–189. [Google Scholar] [CrossRef]
- Joseph, A.M.; Snellings, R.; Van den Heede, P.; Matthys, S.; De Belie, N. The Use of Municipal Solid Waste Incineration Ash in Various Building Materials: A Belgian Point of View. Materials 2018, 11, 141. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Kim, J.; Nam, B.H. Investigation on Impacts of Municipal Solid Waste Incineration Bottom Ash on Cement Hydration. ACI Mater. J. 2017, 5, 114. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, D. Municipal solid waste incineration bottom ash: A competent raw material with new possibilities. Innov. Infrastruct. Solut. 2021, 6, 201. [Google Scholar] [CrossRef]
- Swamy, T.N.; Dash, N.; Nahak, G.; Deo, B.; Sahu, R.K. Effect of Coal fly ash On Growth, Biochemistry, Cytology and Heavy Metal Content of Allium cepa L. N. Y. Sci. J. 2010, 3, 5. [Google Scholar]
- Rosen, C.J.; Olson, D.; Bierman, P.M. Swiss Chard and Alfalfa Responses to Soils Amended with Municipal Solid Waste Incinerator Ash: Growth and Elemental Composition. J. Agric. Food Chem. 1994, 42, 1361–1368. [Google Scholar] [CrossRef]
- Rusănescu, C.O.; Murad, E.; Jinescu, C.; Rusănescu, M. The impact of biochar on the soil. Rev. Chim. 2018, 8, 2197–2202. [Google Scholar] [CrossRef]
- Raj, S.; Mohan, S. Approach for Improved Plant Growth Using Fly Ash Amended. Int. J. Emerg. Technol. Adv. Eng. 2014, 4, 6. Available online: https://ijetae.com/files/Volume4Issue6/IJETAE_0614_110.pdf (accessed on 14 September 2022).
- Srivastava, R.K.; Srivastava, A.K.; Gautam, P. Eco-friendly utilization of fly ash in agriculture: A review. Int. J. Environ. Pollut. Res. 2016, 4, 24–33. [Google Scholar]
- Quina, M.J.; Bordado, J.C.; Quinta-Ferreira, R.M. Treatment and use of air pollution control residues from MSW incineration: An overview. Waste Manag. 2008, 28, 2097–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousuf, A.; Manzoor, S.O.; Youssouf, M.; Malik, Z.A.; Khawaja, K.S. Fly Ash: Production and Utilization in India—An Overview. J. Mater. Environ. Sci. 2020, 11, 911–921. [Google Scholar]
- Ukwattage, N.L.; Ranjith, P.G.; Bouazza, M. The use of coal combustion fly ash as a soil amendment in agricultural lands (with comments on its potential to improve food security and sequester carbon). Fuel 2013, 109, 400–408. [Google Scholar] [CrossRef]
- Ferreira, C.; Ribeiro, A.; Ottosen, L. Possible applications for municipal solid waste fly ash. J. Hazard. Mater. 2003, B96, 201–216. [Google Scholar] [CrossRef]
- Ram, L.C.; Masto, R.E. Fly ash for soil amelioration: A review on the influence of ash blending with inorganic and organic amendments. Earth-Sci. Rev. 2014, 128, 52–74. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Pe´rez-Lo´pez, R.; Renard, F.; Nieto, J.M.; Charlet, L. Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash. J. Hazard. Mater. 2008, 181, 1347–1354. [Google Scholar] [CrossRef] [Green Version]
- Jala, S.; Goyal, D. Fly ash as a soil ameliorant for improving crop production—A review. Bioresour. Technol. 2006, 97, 1136–1147. [Google Scholar] [CrossRef]
- Zendehdel, P.; Seraj, S.; Ramezanianpour, A.A.; Nikravan, M. Evaluation of the application of municipal solid waste incinerator (MSWI) ash in civil engineering using sustainability approach. In Proceedings of the Sardinia 2017, Sixteenth International Waste Management and Landfill Symposium, Cagliari, Italy, 2–6 October 2017. [Google Scholar]
- Thien, L.V.; Chau, N.T.T.; Hong, L.T.T.; Trang, N.T.; Futamata, H. Properties of Fly Ashes from Thermal Power Stations in Relation to Use as Soil Amendments. Sains Malays. 2019, 48, 745–755. [Google Scholar] [CrossRef]
- Giordano, P.M.; Behel, A.D.; Lawrence, J.E.; Soileau, J.M.; Bradford, B.N. Mobility in Soil and Plant Availability of Metals Derived from Incinerated Municipal Refuse. Environ. Sci. Technol. 1983, 17, 193. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, D.; Molin, C.; Hupa, M. Thermal Treatment of Solid Residues from WtE Units: A Review. Waste Manag. 2015, 37, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.D.; Vajpayee, P.N.; Singh, N.; Rai, U.N.; Kumar, A.; Ali, M.B.; Kumar, B.; Yunus, M. Efficacy of various amendments for amelioration of fly-ash toxicity: Growth performance and metal composition of Cassia siamea Lamk. Chemosphere 2004, 54, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Ulmanu, M.; Anger, I.; Gament, E.; Olãnescu, G.; Predescu, C.; Sohaciu, M. Effect of a romanian zeolite on heavy metals transfer from polluted soil to corn, mustard and oat. UPB Sci. Bull. Ser. B 2006, 68, 67. [Google Scholar]
- Tripathi, R.D.; Dwivedi, S.; Shukla, M.K.; Mishra, S.; Srivastava, S.; Singh, R.; Rai, U.N.; Gupta, D.K. Role of blue green algae biofertilizer in ameliorating the nitrogen demand and fly-ash stress to the growth and yield of rice (Oryza sativa L.) plants. Chemosphere 2008, 70, 1919–1929. [Google Scholar] [CrossRef]
- Lopareva-Pohu, A.; Pourrut, B.; Waterlot, C.; Garçon, G.; Bidar, G.; Pruvot, C.; Shirali, P.; Douay, F. Assessment of fly ash-aided phytostabilisation of highly contaminated soils after an 8-year field trial part 1. Influence on soil parameters and metal extractability. Sci. Total Environ. 2011, 409, 647–654. [Google Scholar] [CrossRef]
- Tripathi, R.C.; Masto, R.E.; Ram, L.C. Bulk use of pond ash for cultivation of wheat– maize–eggplant crops in sequence on a fallow land Resources. Conserv. Recycl. 2009, 54, 134–139. [Google Scholar] [CrossRef]
- Ulmanu, M.; Matsi, T.; Gament, E.; Olănescu, G.; Predescu, C.; Sohaciu, M. The remedial treatment of soil polluted with heavy metals using fly ash. UPB Sci. Bull. B Chem. Mater. Sci. 2007, 69, 109–116. [Google Scholar]
- Rusănescu, C.O.; Rusănescu, M.; Jinescu, C.; Durbacă, I. Recovery of Treated Sludge. Rev.Chim. 2019, 70, 3477–3481. [Google Scholar] [CrossRef]
- Wang, T.; Liu, T.; Sun, C. Application of MSWI fly ash on acid soil and its effect on the environment. Waste Manag. 2008, 28, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.J. Reclamation and revegetation of fly ash disposal sites—Challenges and research needs. J. Environ. Manag. 2009, 90, 43–53. [Google Scholar] [CrossRef]
- Plank, C.O.; Martens, D.C. Boron availability as influenced by an application of fly ash to soil. Soil Sci. Soc. Am. Proc. 1974, 38, 974–977. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, J.S.; Kumar, A.; Tewary, D.D. Accumulation of heavy metals by chickpea grown in fly ash treated soil: Effect on antioxidants. Clean Soil Air Water 2010, 38, 1116–1123. [Google Scholar] [CrossRef]
- Wearing, C.; Birch, C.; Nairn, J. An assessment of tarong bottom ash for use on agricultural soils. Dev. Chem. Eng. Miner. Process. 2004, 12, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Kishor, P.; Ghosh, A.K.; Kumar, D. Use of fly ash in agriculture: A way to improve soil fertility. J. Agric. Res. 2010, 4, 1–14. [Google Scholar]
- Van Der Sloot, H.; Kosson, D.; Hjelmar, O. Characteristics, treatment and utilization of residues from municipal waste incineration. Waste Manag. 2001, 21, 753–765. [Google Scholar] [CrossRef]
- Yu, C.L.; Deng, Q.; Jian, S.; Li, J.; Dzantor, E.K.; Hui, D. Effects of fly ash application on plant biomass and element accumulations: A meta-analysis. Environ. Pollut. 2019, 250, 137–142. [Google Scholar] [CrossRef]
- He, H.; Dong, Z.; Peng, Q.; Wang, X.; Fan, C.; Zhang, X. Impacts of coal fly ash on plant growth and accumulation of essential nutrients and trace elements by alfalfa (Medicago sativa) grown in a loessial soil. J. Environ. Manag. 2017, 197, 428–439. [Google Scholar] [CrossRef]
- Fraißler, G.; Jöller, M.; Brunner, T.; Obernberger, I. Influence of dry and humid atmosphere on the thermal decomposition of calcium chloride and its impact on the remove of heavy metals by chlorination. Chem. Eng. Process. 2009, 48, 380–388. [Google Scholar] [CrossRef]
- Liu, J.Y.; Sun, S. Chlorination transformation and volatilization of heavy metals in fly ash from the incineration during the disposal process with higher temperature. Chin. J. Environ. Sci. 2012, 33, 3279–3287. [Google Scholar]
- Lai, K.M.; Ye, D.Y.; Wong, J.W.C. Enzyme activities in sandy soil amended with sewage sludge and coal fly ash. Water Air Soil Pollut. 1999, 113, 261–272. [Google Scholar] [CrossRef]
- Khan, M.R.; Singh, W.N. Effects of soil application of fly ash on the fusarial wilt on tomato cultivars. Int. J. Pest Manag. 2001, 47, 293–297. [Google Scholar] [CrossRef]
- El-Mogazi, D.; Lisk, D.J.; Weinstein, L.H. A review of physical, chemical, and biological properties of fly ash and effects on agricultural ecosystems. Sci. Total Environ. 1988, 74, 1–37. [Google Scholar] [CrossRef]
- Warren, C.J. Some limitations of sluiced fly ash as a liming material for acidic soils. Waste Manage. Res. 1992, 10, 317–327. [Google Scholar] [CrossRef]
- Pichtel, J.R.; Dick, W.A.; Sutton, P. Comparison of amendments and management practices for long-term reclamation of abandoned mine lands. J. Environ. Qual. 1994, 23, 766–772. [Google Scholar] [CrossRef]
- Ram, L.C.; Jha, S.K.; Tripathi, R.C.; Masto, R.E.; Selvi, V.A. Remediation of fly ash landfills through plantation. Remediat. J. 2008, 18, 71–90. [Google Scholar] [CrossRef]
- Adriano, D.C.; Page, A.L.; Elseewi, A.A.; Chang, A.; Straughan, I.A. Utilization and disposal of fly ash and other coal residues in terrestrial ecosystem: A review. J. Environ. Qual. 1980, 9, 333–344. [Google Scholar] [CrossRef]
- Adriano, D.C.; Weber, J.T. Influence of fly ash on soil physical properties and turf grass establishment. J. Environ. Qual. 2001, 30, 596–601. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Ram, L.C.; Jha, S.K.; Tripathi, R.C.; Singh, G. Role of CFRI’s fly ash soil amendment technology (FASAT) in improving the socio-economic condition of farmers via improvement in soil fertility and crop productivity. J. Ecophsiology Occup. Health 2003, 3, 127–142. [Google Scholar]
- Palumbo, A.V.; McCarthy, J.E.; Amonette, J.F.; Fisher, L.S.; Wullschlege, S.D.; Daniels, W.L. Properties for enhancing carbon sequestration and reclamation of degraded lands with fossil fuel combustion by-products. Adv. Environ. Res. 2004, 8, 425–438. [Google Scholar] [CrossRef]
- Ram, L.C.; Srivastava, N.K.; Tripathi, R.C.; Jha, S.K.; Sinha, A.K.; Singh, G.; Manoharan, V. Management of mine spoil for crop productivity with lignite fly ash and biological amendments. J. Environ. Manag. 2006, 79, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, B.; Bolan, N.S.; Kunhikrishnan, A. Effect of Clean Coal Combustion Products in Reducing Soluble Phosphorus in Soil I. Adsorption Study. Water Air Soil Pollut. 2013, 224, 1524. [Google Scholar] [CrossRef]
- Huotari Tillman-Sutela, E.; Moilanen, M.; Laiho, R. Recycling of ash—For the good of the environment? For. Ecol. Manag. 2015, 348, 226–240. [Google Scholar] [CrossRef]
- Malik, A.; Thapliyal, A. Eco-friendly fly ash utilization: Potential for land application. Critic. Rev. Environ. Sci. Technol. 2009, 39, 333–366. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Baldocchi, D.; Gershenson, A. Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Glob. Change Biol. 2007, 13, 2018–2035. [Google Scholar] [CrossRef] [Green Version]
- Taylor, E.M., Jr.; Schuman, G.E. Fly ash and lime amendment of acidic coal spoil to aid revegetation. J. Environ. Qual. 1988, 17, 120–124. [Google Scholar] [CrossRef]
- Lee, H.; Ho, S.H.; Lee, C.H.; Lee, Y.B.; Kim, P.J. Fly ash effect on improving soil properties and rice productivity in Korean paddy soils. Bioresour. Technol. 2006, 97, 1490–1497. [Google Scholar] [CrossRef]
- Rusănescu, C.O.; Rusănescu, M.; Voicu, G.; Paraschiv, G.; Biriș, S.S.; Popescu, I.N. The Recovery of Vermicompost Sewage Sludge in Agriculture. Agronomy 2022, 2, 2653. [Google Scholar] [CrossRef]
- Rusănescu, C.O.; Voicu, G.; Paraschiv, G.; Begea, M.; Purdea, L.; Petre, I.C.; Stoian, E.V. Recovery of Sewage Sludge in the Cement Industry. Energies 2022, 15, 2664. [Google Scholar] [CrossRef]
- Marin, E.; Rusănescu, C.O. Agricultural Use of Urban Sewage Sludge From the Wastewater Station in the Municipality of Alexandria in Romania. Water 2023, 15, 458. [Google Scholar] [CrossRef]
- Gu, H.; Li, F.P.; Guan, X.; Xu, Y.L.; Liu, Y.J.; Chen, X.T.; Wang, X.H.; Wang, Z. Effects of fly ash on heavy metal uptake of rice growing on multi-metal contaminated acidic soil. Adv. Mater. Res. 2013, 680, 94–99. [Google Scholar] [CrossRef]
- Scotti, A.; Silva, S.; Botteschi, G. Effect of fly ash on the availability of Zn, Cu, Ni and Cd to chicory. Agric. Eco. Environ. 1999, 72, 159–163. [Google Scholar] [CrossRef]
- Țucureanu, M.C.; Rusănescu, C.O.; Purdea, L. Polluting Emissions from Incineration and Waste Installations. Rev. Chim. 2019, 70, 2385–2387. [Google Scholar] [CrossRef]
- Thien, V.L.; Chau, N.T.; Futamata, H. Effect of fly ash amendment on sandy soil properties and peanut yields. Sci. Asia 2021, 47, 357–365. [Google Scholar] [CrossRef]
- Weber, J.; Strączyńska, S.; Kocowicz, A.; Gilewska, M.; Bogacz, A.; Gwiżdż, M.; Debicka, M. Properties of soil materials derived from fly ash 11 years after revegetation of post-mining excavation. Catena 2015, 133, 250–254. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Eart. Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]

| Element | Unit | FA [30] | Soil [30] | Soil [31] | Soil [32] |
|---|---|---|---|---|---|
| Ca | % | 0.11–22.2 | 0.7–50 | - | - |
| S | % | 0.1–1.5 | 0.01–2.0 | - | - |
| Al | % | 0.1–17.3 | 4–30 | - | - |
| Si | % | - | - | - | - |
| Na | % | 0.01–2.03 | 0.04–3.0 | - | - |
| K | % | 0.15–3.5 | 0.04–3.0 | - | - |
| Cl | % | - | - | - | - |
| Mg | % | 0.04–7.6 | 0.06–0.6 | - | - |
| Fe | % | 36–1333 | 0.7–55 | - | - |
| As | mg/kg | 2.3–6300 | 0.1–40 | 5–15 | 20 |
| Ba | mg/kg | - | - | 200–400 | - |
| Cd | mg/kg | 0.7–130 | 0.01–7.0 | 1–3 | 1–3 |
| Co | mg/kg | 7–520 | 1–40 | 15–30 | - |
| Cr | mg/kg | 10–1000 | 5–3000 | 30–100 | 100 |
| Cu | mg/kg | 14–2800 | 2–100 | 20–100 | 50–140 |
| Mo | mg/kg | 7–160 | 0.2–5.0 | 2–5 | - |
| Ni | mg/kg | 6.3–4300 | 10–1000 | 20–75 | 30–75 |
| Pb | mg/kg | 3.1–5000 | 2–100 | 20–100 | 50–300 |
| Sr | mg/kg | - | - | - | - |
| V | mg/kg | - | - | 50–100 | - |
| Zn | mg/kg | 10–3500 | 10–300 | 100–300 | 150–300 |
| Sb | mg/kg | - | - | 5–20 | - |
| P | mg/kg | 0.004–0.8% | 0.005–0.2% | - | - |
| Hg | - | 0.02–1.0 | - | 0.1–1 | 1–5 |
| Mn | - | 58–3000 | 100–4000 | 900–1500 | - |
| TOC | g/kg | - | - | - | - |
| Ti | % | - | - | - | - |
| Ag | - | - | - | 2–20 | - |
| B | - | 10–618 | 2–100 | 1–2 | - |
| Se | - | 0.2–134 | 0.1–2.0 | 1–3 | - |
| Fly Ash (t/ha) | pH | EC | CEC | OC | OM | WHC |
|---|---|---|---|---|---|---|
| 0 | 7.0 | 210 | 2.35 | 0.58 | 0.99 | 33.68 |
| 1 | 7.5 | 265 | 1.78 | 0.54 | 0.93 | 34.69 |
| 2.5 | 8.1 | 265 | 2.56 | 0.60 | 1.03 | 33.75 |
| 5 | 8.0 | 280 | 2.48 | 0.58 | 0.99 | 36.13 |
| 10 | 8.10 | 320 | 3.0 | 0.64 | 1.10 | 39.93 |
| 15 | 8.2 | 346 | 2.82 | 0.73 | 1.26 | 42.93 |
| Fly Ash Concentration (%) | pH | EC μs/cm |
|---|---|---|
| 0 (Soil only) | 6.65 | 281 |
| 10 | 6.72 | 288 |
| 20 | 6.72 | 296 |
| 30 | 6.9 | 300 |
| 40 | 6.91 | 308 |
| 50 | 6.96 | 358 |
| 100 (Fly ash only) | 7.56 | 600 |
| Fly Ash (t/h) | 0 | 1 | 2.5 | 5 | 10 | 15 |
|---|---|---|---|---|---|---|
| Na | 1180 | 1215 | 1180 | 900 | 850 | 1025 |
| K | 3900 | 4280 | 6500 | 7050 | 8290 | 10,150 |
| P | 45.5 | 36.9 | 46.0 | 50.3 | 46.8 | 70.0 |
| Fe | 325 | 267 | 340 | 263 | 300 | 310 |
| Mn | 03 | 100 | 161 | 195 | 211 | 240 |
| Ni | 5.80 | 5.79 | 6.67 | 8.50 | 12.05 | 15.37 |
| Co | 5.15 | 6.10 | 6.68 | 7.36 | 10.22 | 17.31 |
| Zn | 36.0 | 39.6 | 46.0 | 49.8 | 51.0 | 67.0 |
| Cu | 5.06 | 5.73 | 5.97 | 7.50 | 10.0 | 14.38 |
| Pb | 8.3 | 9.50 | 13.46 | 12.97 | 17.58 | 20.00 |
| Cr | 0.0 | 0.02 | 0.20 | 1.06 | 1.39 | 1.89 |
| Cd | 0.0 | 0.0 | 0.0 | 0.005 | 0.02 | 0.06 |
| Physical Characteristics | Fly-Ash | Soil |
|---|---|---|
| Bulk density (g cc−1) | <1.0 | 1.33 |
| Water-holding capacity (%) | 35–40 | <20 |
| Porosity (%) | 50–60 | <25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusănescu, C.O.; Rusănescu, M. Application of Fly Ash Obtained from the Incineration of Municipal Solid Waste in Agriculture. Appl. Sci. 2023, 13, 3246. https://doi.org/10.3390/app13053246
Rusănescu CO, Rusănescu M. Application of Fly Ash Obtained from the Incineration of Municipal Solid Waste in Agriculture. Applied Sciences. 2023; 13(5):3246. https://doi.org/10.3390/app13053246
Chicago/Turabian StyleRusănescu, Carmen Otilia, and Marin Rusănescu. 2023. "Application of Fly Ash Obtained from the Incineration of Municipal Solid Waste in Agriculture" Applied Sciences 13, no. 5: 3246. https://doi.org/10.3390/app13053246
APA StyleRusănescu, C. O., & Rusănescu, M. (2023). Application of Fly Ash Obtained from the Incineration of Municipal Solid Waste in Agriculture. Applied Sciences, 13(5), 3246. https://doi.org/10.3390/app13053246






