Performance of Automated Oral Cancer Screening Algorithm in Tobacco Users vs. Non-Tobacco Users
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
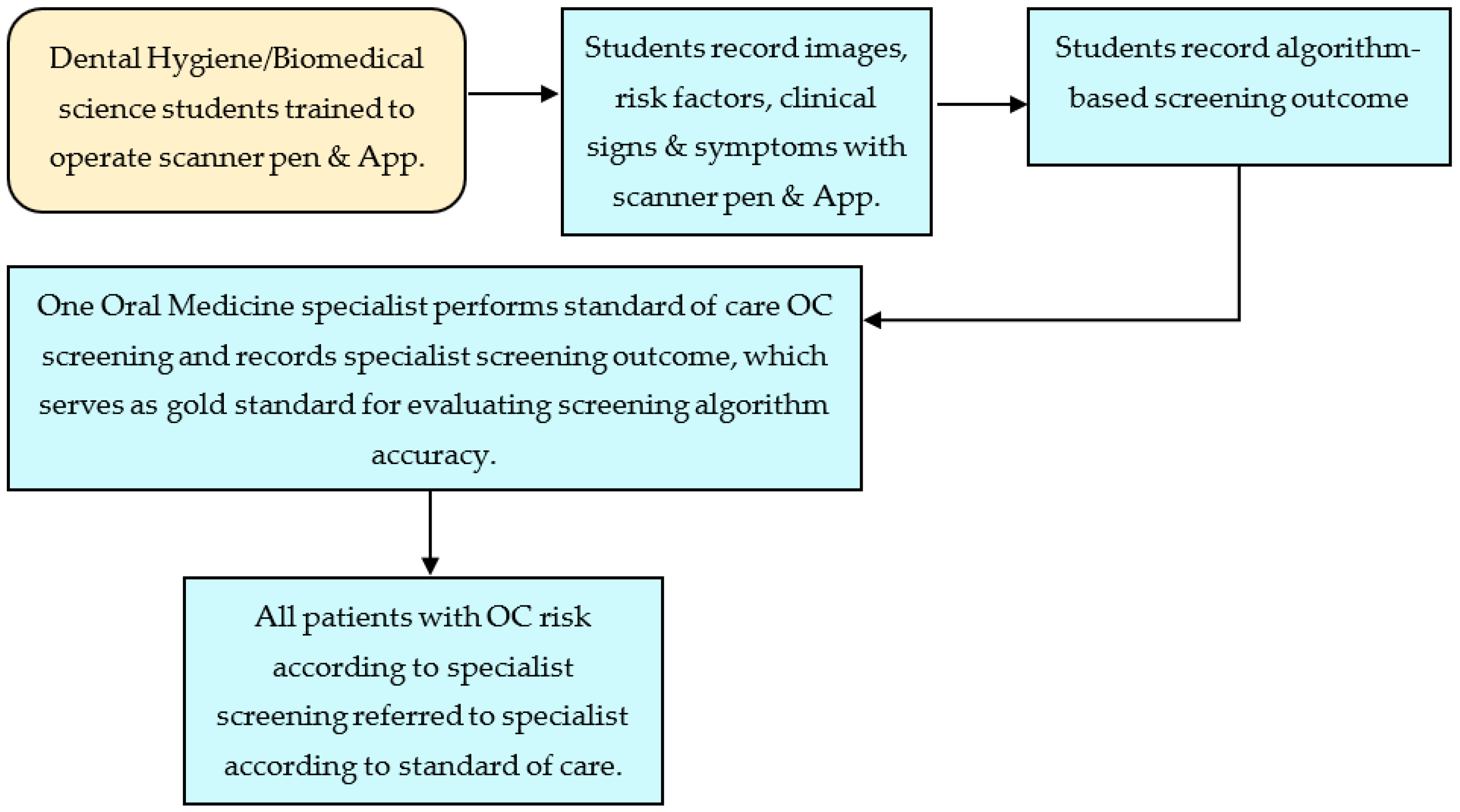
2.1. Overview
2.2. Subjects
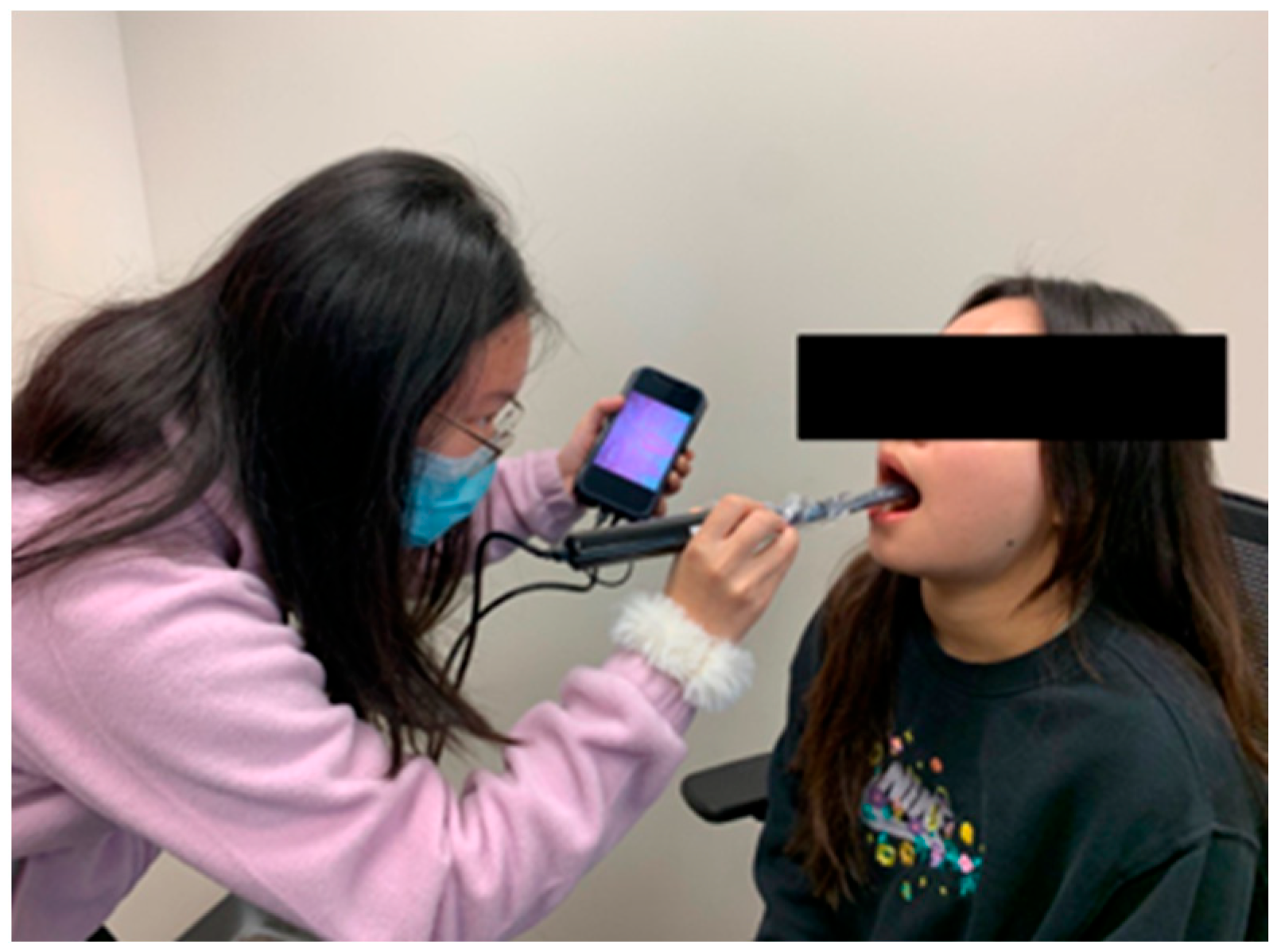
2.3. Protocol
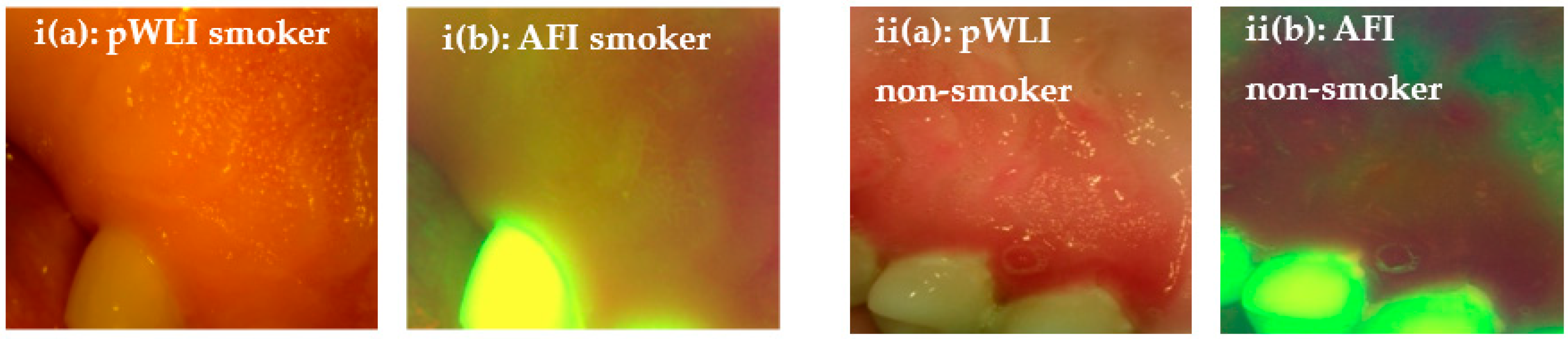
2.4. Intraoral Camera Platform
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oral Cancer Facts–Oral Cancer Foundation|Information and Resources about Oral Head and Neck Cancer. (n.d.). Retrieved 14 January 2023. Available online: https://oralcancerfoundation.org/facts/ (accessed on 28 February 2023).
- Jamal, A.; Agaku, I.T.; O’Connor, E.; King, B.A.; Kenemer, J.B.; Neff, L. Current cigarette smoking among adults—United States, 2005–2013. Morb. Mortal. Wkly. Rep. 2014, 63, 1108–1112. [Google Scholar] [PubMed]
- Bouquot, J.E.; Gorlin, R.J. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg. Oral Med. Oral Pathol. 1986, 61, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Woo, S.B. Leukoplakia—A Diagnostic and Management Algorithm. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2017, 75, 723–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.K.; Sethi, A.; Sareen, D.; Dhingra, S. Treatment Delay in Oral and Oropharyngeal Cancer in Our Population: The Role of Socio-Economic Factors and Health-Seeking Behaviour. Indian J. Otolaryngol. Head Neck Surg. 2011, 63, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indian Dental Association. (n.d.). Oral Cancer Foundation (OCF)|A ‘Cancer Free-India’. Retrieved 14 January 2023. Available online: http://ocf.org.in (accessed on 28 February 2023).
- SEER Cancer Statistics Review 1975–2002—Previous Version—SEER Cancer Statistics. (n.d.). SEER. Retrieved 14 January 2023. Available online: https://seer.cancer.gov/archive/csr/1975_2002/index.html (accessed on 28 February 2023).
- McGurk, M.; Chan, C.; Jones, J.; O’regan, E.; Sherriff, M. Delay in diagnosis and its effect on outcome in head and neck cancer. Br. J. Oral Maxillofac. Surg. 2005, 43, 281–284. [Google Scholar] [CrossRef]
- Dolan, R.W.; Vaughan, C.W.; Fuleihan, N. Symptoms in early head and neck cancer: An inadequate indicator. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 1998, 119, 463–467. [Google Scholar] [CrossRef]
- Vernham, G.A.; Crowther, J.A. Head and neck carcinoma–stage at presentation. Clin. Otolaryngol. Allied Sci. 1994, 19, 120–124. [Google Scholar] [CrossRef]
- Yao, M.; Epstein, J.B.; Modi, B.J.; Pytynia, K.B.; Mundt, A.J.; Feldman, L.E. Current surgical treatment of squamous cell carcinoma of the head and neck. Oral Oncol. 2007, 43, 213–223. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Ramadas, K.; Thomas, G.; Muwonge, R.; Thara, S.; Mathew, B.; Rajan, B.; Trivandrum Oral Cancer Screening Study Group. Effect of screening on oral cancer mortality in Kerala, India: A cluster-randomised controlled trial. Lancet 2005, 365, 1927–1933. [Google Scholar] [CrossRef]
- Siddiqi, A.D.; Britton, M.; Chen, T.A.; Carter, B.J.; Wang, C.; Martinez Leal, I.; Rogova, A.; Kyburz, B.; Williams, T.; Patel, M.; et al. Tobacco Screening Practices and Perceived Barriers to Offering Tobacco Cessation Services among Texas Health Care Centers Providing Behavioral Health Treatment. Int. J. Environ. Res. Public Health 2022, 19, 9647. [Google Scholar] [CrossRef]
- Moyer, V.A. U.S. Preventive Services Task Force. Screening for oral cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Tomar, S.L.; Hecht, S.S.; Jaspers, I.; Gregory, R.L.; Stepanov, I. Oral Health Effects of Combusted and Smokeless Tobacco Products. Adv. Dent. Res. 2019, 30, 4–10. [Google Scholar] [CrossRef]
- Shopland, D.R. Tobacco use and its contribution to early cancer mortality with a special emphasis on cigarette smoking. Environ. Health Perspect. 1995, 103 (Suppl. S8), 131–142. [Google Scholar] [CrossRef]
- IARC Working Group. Tobacco habits other than smoking; betel-quid and areca-nut chewing; and some related nitrosamines. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; IARC Working Group: Lyon, France, 1985; Volume 37, pp. 389–394. [Google Scholar]
- IARC. (n.d.). Tobacco Smoking. Retrieved 14 January 2023. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Tobacco-Smoking-1986 (accessed on 28 February 2023).
- Negri, E.; La Vecchia, C.; Franceschi, S.; Decarli, A.; Bruzzi, P. Attributable risks for oesophageal cancer in northern Italy. Eur. J. Cancer 1992, 28, 1167–1171. [Google Scholar] [CrossRef]
- Negri, E.; La Vecchia, C.; Franceschi, S.; Tavani, A. Attributable risk for oral cancer in northern Italy. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 1993, 2, 189–193. [Google Scholar]
- Blot, W.J.; McLaughlin, J.K.; Winn, D.M.; Austin, D.F.; Greenberg, R.S.; Preston-Martin, S.; Bernstein, L.; Schoenberg, J.B.; Stemhagen, A.; Fraumeni, J.F. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar]
- Hayes, R.B.; Bravo-Otero, E.; Kleinman, D.V.; Brown, L.M.; Fraumeni, J.F.; Harty, L.C.; Winn, D.M. Tobacco and alcohol use and oral cancer in Puerto Rico. Cancer Causes Control. 1999, 10, 27–33. [Google Scholar] [CrossRef]
- CDCTobaccoFree. (8 November 2022). Cigars. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/tobacco_industry/cigars/index.htm (accessed on 28 February 2023).
- Nelson, D.E.; Davis, R.M.; Chrismon, J.H.; Giovino, G.A. Pipe smoking in the United States, 1965–1991: Prevalence and attributable mortality. Prev. Med. 1996, 25, 91–99. [Google Scholar] [CrossRef]
- Winn, D.M.; Blot, W.J.; Shy, C.M.; Pickle, L.W.; Toledo, A.; Fraumeni, J.F. Snuff dipping and oral cancer among women in the southern United States. N. Engl. J. Med. 1981, 304, 745–749. [Google Scholar] [CrossRef]
- Epstein, J.B.; Güneri, P.; Boyacioglu, H.; Abt, E. The limitations of the clinical oral examination in detecting dysplastic oral lesions and oral squamous cell carcinoma. J. Am. Dent. Assoc. 2012, 143, 1332–1342. [Google Scholar] [CrossRef]
- Panzarella, V.; Pizzo, G.; Calvino, F.; Compilato, D.; Colella, G.; Campisi, G. Diagnostic delay in oral squamous cell carcinoma: The role of cognitive and psychological variables. Int. J. Oral Sci. 2014, 6, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, P.E. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century-the approach of the WHO Global Oral Health Programme: The World Oral Health Report 2003. Community Dent. Oral Epidemiol. 2003, 31, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Taybos, G. Oral changes associated with tobacco use. Am. J. Med. Sci. 2003, 326, 179–182. [Google Scholar] [CrossRef]
- Shuman, A.G.; Entezami, P.; Chernin, A.S.; Wallace, N.E.; Taylor, J.M.G.; Hogikyan, N.D. Demographics and efficacy of head and neck cancer screening. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2010, 143, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, E.K.; Fagan, P.; Cooper, L.; Canto, M.; Carroll, W.; Foster-Bey, J.; Hébert, J.R.; Lopez-Class, M.; Ma, G.X.; Nez Henderson, P.; et al. Working to Eliminate Cancer Health Disparities from Tobacco: A Review of the National Cancer Institute’s Community Networks Program. Nicotine Tob. Res. 2015, 17, 908–923. [Google Scholar] [CrossRef] [Green Version]
- Cicciù, M.; Herford, A.S.; Cervino, G.; Troiano, G.; Lauritano, F.; Laino, L. Tissue Fluorescence Imaging (VELscope) for Quick Non-Invasive Diagnosis in Oral Pathology. J. Craniofacial Surg. 2017, 28, e112–e115. [Google Scholar] [CrossRef]
- Farah, C.S.; McIntosh, L.; Georgiou, A.; McCullough, M.J. Efficacy of tissue autofluorescence imaging (VELScope) in the visualization of oral mucosal lesions. Head Neck 2012, 34, 856–862. [Google Scholar] [CrossRef]
- Song, B.; Sunny, S.; Uthoff, R.D.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Anbarani, A.; Wilder-Smith, P.; Kuriakose, M.A.; et al. Automatic classification of dual-modalilty, smartphone-based oral dysplasia and malignancy images using deep learning. Biomed. Opt. Express 2018, 9, 5318–5329. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ingole, N.; Roblyer, D.; Stepanek, V.; Richards-Kortum, R.; Gillenwater, A.; Shastri, S.; Chaturvedi, P. Evaluation of a low-cost, portable imaging system for early detection of oral cancer. Head Neck Oncol. 2010, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Poh, C.F.; MacAulay, C.E.; Zhang, L.; Rosin, M.P. Tracing the “At-Risk” Oral Mucosa Field with Autofluorescence: Steps Toward Clinical Impact. Cancer Prev. Res. 2009, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Lingen, M.W.; Abt, E.; Agrawal, N.; Chaturvedi, A.K.; Cohen, E.; D’Souza, G.; Gurenlian, J.; Kalmar, J.R.; Kerr, A.R.; Lambert, P.M.; et al. Evidence-based clinical practice guideline for the evaluation of potentially malignant disorders in the oral cavity: A report of the American Dental Association. J. Am. Dent. Assoc. 2017, 148, 712–727.e10. [Google Scholar] [CrossRef] [Green Version]
- Macey, R.; Walsh, T.; Brocklehurst, P.; Kerr, A.R.; Liu, J.L.Y.; Lingen, M.W.; Ogden, G.R.; Warnakulasuriya, S.; Scully, C. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst. Rev. 2015, 2015, CD010276. [Google Scholar] [CrossRef] [Green Version]
- Lane, P.; Follen, M.; MacAulay, C. Has fluorescence spectroscopy come of age? A case series of oral precancers and cancers using white light, fluorescent light at 405 nm, and reflected light at 545 nm using the Trimira Identafi 3000. Gend. Med. 2012, 9 (Suppl. 1), S25–S35. [Google Scholar] [CrossRef]
- Rethman, M.P.; Carpenter, W.; Cohen, E.E.W.; Epstein, J.; Evans, C.A.; Flaitz, C.M.; Graham, F.J.; Hujoel, P.P.; Kalmar, J.R.; Koch, W.M.; et al. Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas. J. Am. Dent. Assoc. 2010, 141, 509–520. [Google Scholar] [CrossRef]
- Hegde, S.; Ajila, V.; Zhu, W.; Zeng, C. Artificial intelligence in early diagnosis and prevention of oral cancer. Asia-Pac. J. Oncol. Nurs. 2022, 9, 100133. [Google Scholar] [CrossRef]
- Ilhan, B.; Lin, K.; Guneri, P.; Wilder-Smith, P. Improving Oral Cancer Outcomes with Imaging and Artificial Intelligence. J. Dent. Res. 2020, 99, 241–248. [Google Scholar] [CrossRef]
- Munir, K.; Elahi, H.; Ayub, A.; Frezza, F.; Rizzi, A. Cancer Diagnosis Using Deep Learning: A Bibliographic Review. Cancers 2019, 11, 1235. [Google Scholar] [CrossRef] [Green Version]
- Jeyaraj, P.R.; Samuel Nadar, E.R. Computer-assisted medical image classification for early diagnosis of oral cancer employing deep learning algorithm. J. Cancer Res. Clin. Oncol. 2019, 145, 829–837. [Google Scholar] [CrossRef]
- Ramezani, K.; Tofangchiha, M. Oral Cancer Screening by Artificial Intelligence-Oriented Interpretation of Optical Coherence Tomography Images. Radiol. Res. Pract. 2022, 2022, e1614838. [Google Scholar] [CrossRef]
- Roblyer, D.; Kurachi, C.; Stepanek, V.; Schwarz, R.A.; Williams, M.D.; El-Naggar, A.K.; Lee, J.J.; Gillenwater, A.M.; Richards-Kortum, R. Comparison of multispectral wide-field optical imaging modalities to maximize image contrast for objective discrimination of oral neoplasia. J. Biomed. Opt. 2010, 15, 066017. [Google Scholar] [CrossRef] [Green Version]
- Fei, B.; Lu, G.; Wang, X.; Zhang, H.; Little, J.V.; Patel, M.R.; Griffith, C.C.; El-Diery, M.W.; Chen, A.Y. Label-free reflectance hyperspectral imaging for tumor margin assessment: A pilot study on surgical specimens of cancer patients. J. Biomed. Opt. 2017, 22, 086009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilhan, B.; Guneri, P.; Wilder-Smith, P. The contribution of artificial intelligence to reducing the diagnostic delay in oral cancer. Oral Oncol. 2021, 116, 105254. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Sunny, S.; Li, S.; Gurushanth, K.; Mendonca, P.; Mukhia, N.; Patrick, S.; Gurudath, S.; Raghavan, S.; Imchen, T.; et al. Mobile-based oral cancer classification for point-of-care screening. J. Biomed. Opt. 2021, 26, 065003. [Google Scholar] [CrossRef] [PubMed]
- Uthoff, R.D.; Song, B.; Sunny, S.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Spires, O.; Anbarani, A.; Wilder-Smith, P.; et al. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS ONE 2018, 13, e0207493. [Google Scholar] [CrossRef]
- Uthoff, R.D.; Song, B.; Sunny, S.; Patrick, S.; Suresh, A.; Kolur, T.; Gurushanth, K.; Wooten, K.; Gupta, V.; Platek, M.E.; et al. Small form factor, flexible, dual-modality handheld probe for smartphone-based, point-of-care oral and oropharyngeal cancer screening. J. Biomed. Opt. 2019, 24, 106003. [Google Scholar] [CrossRef]
- Song, B.; Li, S.; Sunny, S.; Gurushanth, K.; Mendonca, P.; Mukhia, N.; Patrick, S.; Gurudath, S.; Raghavan, S.; Tsusennaro, I.; et al. Classification of imbalanced oral cancer image data from high-risk population. J. Biomed. Opt. 2021, 26, 105001. [Google Scholar] [CrossRef]


| Eligibility Criteria | |
|---|---|
| Inclusion Criteria | Exclusion Criteria |
|
|
| Identifying Gender | Race/Ethnicity | Age |
|---|---|---|
| 137 Female | 124 White/Hispanic | Range: 37–89 years of age |
| 181 Male | 91 White/Non-Hispanic | Mean: 54 years of age |
| 66 Asian | Median: 41 years of age | |
| 19 more than one race | ||
| 14 African American | ||
| 4 Pacific Islander |
| VALUE | SE | LOWER CI | UPPER CI | ||
|---|---|---|---|---|---|
| Non-Smoker | Sensitivity | 0.622 | 0.022 | 0.600 | 0.644 |
| Specificity | 0.835 | 0.023 | 0.812 | 0.858 | |
| False Positive Rate | 0.165 | 0.024 | 0.142 | 0.188 | |
| False Negative Rate | 0.378 | 0.022 | 0.356 | 0.400 | |
| Agreement with Specialist | 0.733 | 0.010 | 0.726 | 0.742 | |
| Positive predictive values | 0.774 | 0.019 | 0.755 | 0.793 | |
| Negative predictive values | 0.710 | 0.012 | 0.700 | 0.720 | |
| Tobacco-Smoker | Sensitivity | 0.800 | 0.033 | 0.768 | 0.832 |
| Specificity | 0.864 | 0.041 | 0.824 | 0.904 | |
| False Positive Rate | 0.135 | 0.040 | 0.0959 | 0.174 | |
| False Negative Rate | 0.200 | 0.033 | 0.168 | 0.232 | |
| Agreement with Specialist | 0.833 | 0.026 | 0.807 | 0.858 | |
| Positive predictive values | 0.861 | 0.037 | 0.825 | 0.897 | |
| Negative predictive values | 0.805 | 0.026 | 0.780 | 0.830 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.M.; Song, B.; Wink, C.; Abouakl, M.; Takesh, T.; Hurlbutt, M.; Dinica, D.; Davis, A.; Liang, R.; Wilder-Smith, P. Performance of Automated Oral Cancer Screening Algorithm in Tobacco Users vs. Non-Tobacco Users. Appl. Sci. 2023, 13, 3370. https://doi.org/10.3390/app13053370
Yang SM, Song B, Wink C, Abouakl M, Takesh T, Hurlbutt M, Dinica D, Davis A, Liang R, Wilder-Smith P. Performance of Automated Oral Cancer Screening Algorithm in Tobacco Users vs. Non-Tobacco Users. Applied Sciences. 2023; 13(5):3370. https://doi.org/10.3390/app13053370
Chicago/Turabian StyleYang, Susan Meishan, Bofan Song, Cherie Wink, Mary Abouakl, Thair Takesh, Michelle Hurlbutt, Dana Dinica, Amber Davis, Rongguang Liang, and Petra Wilder-Smith. 2023. "Performance of Automated Oral Cancer Screening Algorithm in Tobacco Users vs. Non-Tobacco Users" Applied Sciences 13, no. 5: 3370. https://doi.org/10.3390/app13053370





