Risk Assessment of Bisphenol A in the Korean General Population
Abstract
:1. Introduction
2. Literature Search for Safety Levels of BPA
3. Exposure Level of BPA in Korea General Population
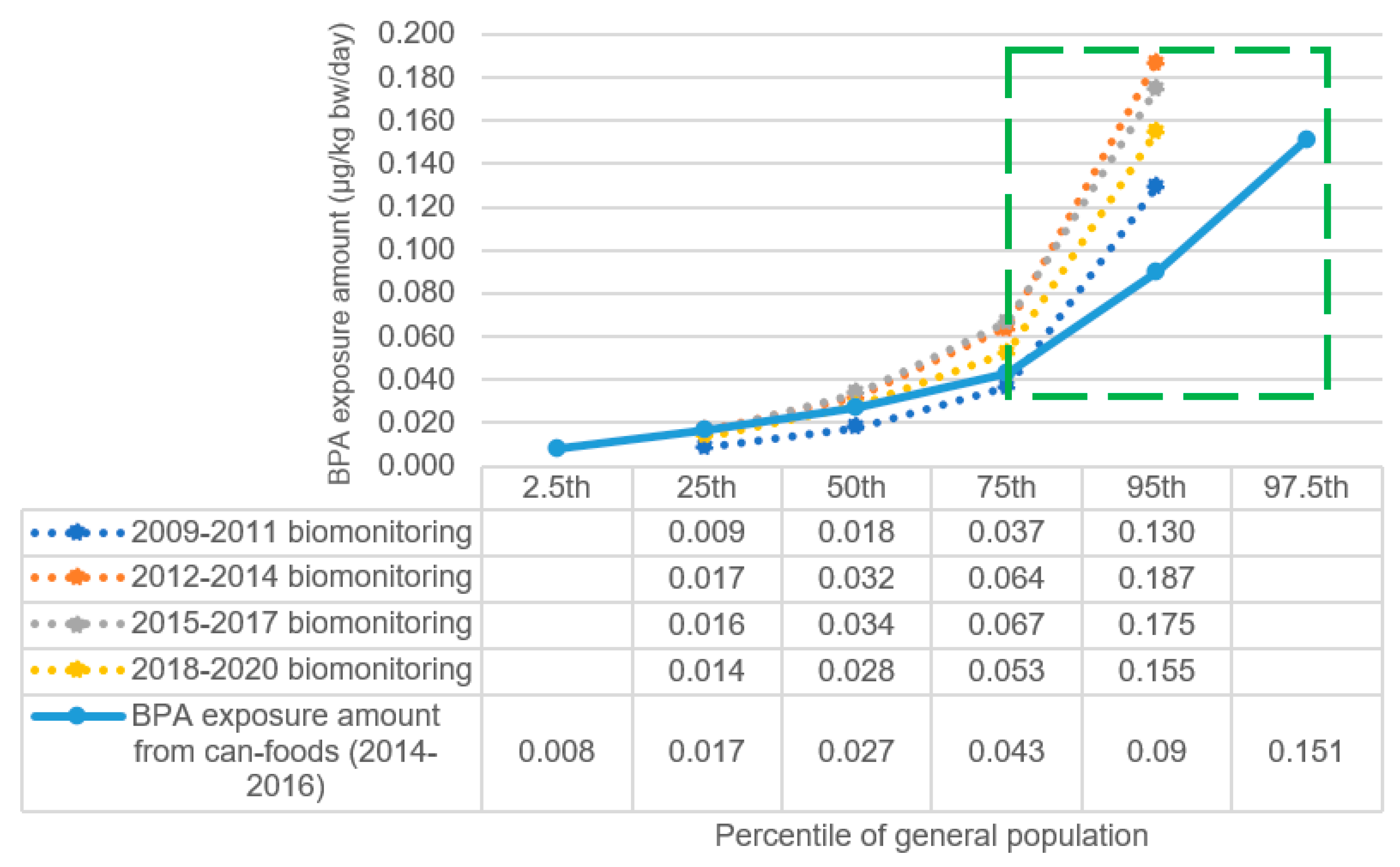
4. Risk Characterization of BPA
5. Further Study for BPA
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kortenkamp, A. Which chemicals should be grouped together for mixture risk assessments of male reproductive disorders? Mol. Cell. Endocrinol. 2020, 499, 110581. [Google Scholar] [CrossRef] [PubMed]
- Apel, P.; Kortenkamp, A.; Koch, H.M.; Vogel, N.; Rüther, M.; Kasper-Sonnenberg, M.; Conrad, A.; Brüning, T.; Kolossa-Gehring, M. Time course of phthalate cumulative risks to male developmental health over a 27-year period: Biomonitoring samples of the German Environmental Specimen Bank. Environ. Int. 2020, 137, 105467. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Martin, O.; Ermler, S.; Baig, A.; Scholze, M. Bisphenol A and declining semen quality: A systematic review to support the derivation of a reference dose for mixture risk assessments. Int. J. Hyg. Environ. Health 2022, 241, 113942. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef] [Green Version]
- Bisphenol A Production in the United States from 1990 to 2019. Available online: https://www.statista.com/statistics/974708/us-bisphenol-a-production-volume/ (accessed on 10 December 2022).
- Khan, S.; Ding, S.; Hong, A.; Ketama, A.; Chen, J. Plastic Chemical Bisphenol A Dampens Our Cardiovascular System: Evidence from Clinical and Animal Studies. Med. Res. Arch. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- ECHA (European Chemicals Agency). Seven New Substances Added to the Candidate List, Entry for Bisphenol-A Updated. Available online: https://echa.europa.eu/-/seven-new-substances-added-to-the-candidate-list-entry-for-bisphenol-a-updated-to-reflect-its-endocrine-disrupting-properties-for-the-environment (accessed on 10 December 2022).
- Gorini, F.; Bustaffa, E.; Coi, A.; Iervasi, G.; Bianchi, F. Bisphenols as environmental triggers of thyroid dysfunction: Clues and evidence. Int. J. Environ. Res. Public Health 2020, 17, 2654. [Google Scholar] [CrossRef]
- Glausiusz, J. The plastics puzzle. Nature 2014, 508, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuszczak, E.; Komarowska, M.D.; Debek, W.; Hermanowicz, A. The impact of bisphenol A on fertility, reproductive system, and development: A review of the literature. Int. J. Endocrinol. 2019, 2019, 4068717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björnsdotter, M.K.; de Boer, J.; Ballesteros-Gómez, A. Bisphenol A and replacements in thermal paper: A review. Chemosphere 2017, 182, 691–706. [Google Scholar] [CrossRef]
- Baek, K.; Park, J.-T.; Kwak, K. Association of Urinary Bisphenols Concentration with Asthma in Korean Adolescents: Data from the Third Korean National Environmental Health Survey. Toxics 2021, 9, 291. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.J.; Jeon, J.; Jung, K.J.; Jee, S.H. Inverse associations of bisphenol A and phthalate metabolites with serum bilirubin levels in Korean population. Environ. Sci. Pollut. Res. 2019, 26, 26685–26695. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, Y.J. Bisphenols and thyroid hormone. Endocrinol. Metab. 2019, 34, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Yu, S.H.; Lee, C.B.; Park, Y.J.; Yoo, H.J.; Kim, D.S. Effects of bisphenol A on cardiovascular disease: An epidemiological study using National Health and Nutrition Examination Survey 2003–2016 and meta-analysis. Sci. Total Environ. 2021, 763, 142941. [Google Scholar] [CrossRef]
- National Institute of Food and Drug Safety Evaluation (NIFDS). Risk Assessment Report: Bisphenols, Parabens, Phthalates. 2020. Available online: https://www.nifds.go.kr/en/wpge/m_25/cont_02/cont_02_07_02.do (accessed on 20 October 2022).
- Choi, C.-W.; Jeong, J.-Y.; Hwang, M.-S.; Jung, K.-K.; Lee, K.-H.; Lee, H.-M. Establishment of the Korean tolerable daily intake of bisphenol a based on risk assessments by an expert committee. Toxicol. Res. 2010, 26, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Hwang, M.-S.; Ko, A.; Jeong, D.-H.; Lee, J.-M.; Moon, G.; Lee, K.-S.; Kho, Y.-H.; Shin, M.-K.; Lee, H.-S. Risk assessment based on urinary bisphenol A levels in the general Korean population. Environ. Res. 2016, 150, 606–615. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Stakeholder Meeting on the Draft Scientific Opinion on Re-Evaluation of Bisphenol A (BPA). 2022. Available online: https://www.efsa.europa.eu/en/events/stakeholder-meeting-draft-scientific-opinion-re-evaluation-bisphenol-bpa (accessed on 15 October 2022).
- European Food Safety Authority. Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Abdulhameed, A.-S.A.R.; Lim, V.; Bahari, H.; Khoo, B.Y.; Abdullah, M.N.H.; Tan, J.J.; Yong, Y.K. Adverse Effects of Bisphenol A on the Liver and Its Underlying Mechanisms: Evidence from In Vivo and In Vitro Studies. BioMed Res. Int. 2022, 2022, 8227314. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.K.; Elobeid, M.A.; Virk, P.; Omer, S.A.; ElAmin, M.; Daghestani, M.H.; AlOlayan, E.M. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxidative Med. Cell. Longev. 2012, 2012, 194829. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, S.; Kani, S.N.M.; Rezazadeh, L.; Pouramir, M.; Ghasemi-Kasman, M.; Moghadamnia, A.A. Low dose administration of Bisphenol A induces liver toxicity in adult rats. Biochem. Biophys. Res. Commun. 2017, 494, 107–112. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Gao, H.; Yang, B.-J.; Li, N.; Feng, L.-M.; Shi, X.-Y.; Zhao, W.-H.; Liu, S.-J. Bisphenol A and hormone-associated cancers: Current progress and perspectives. Medicine 2015, 94, e211. [Google Scholar] [CrossRef]
- Ohore, O.E.; Zhang, S. Endocrine disrupting effects of bisphenol A exposure and recent advances on its removal by water treatment systems. A review. Sci. Afr. 2019, 5, e00135. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Jochmanová, I.; Lazúrová, Z.; Rudnay, M.; Bačová, I.; Mareková, M.; Lazúrová, I. Environmental estrogen bisphenol A and autoimmunity. Lupus 2015, 24, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Cariati, F.; D’Uonno, N.; Borrillo, F.; Iervolino, S.; Galdiero, G.; Tomaiuolo, R. Bisphenol a: An emerging threat to male fertility. Reprod. Biol. Endocrinol. 2019, 17, 6. [Google Scholar] [CrossRef] [Green Version]
- Loganathan, S.N.; Kannan, K. Occurrence of bisphenol A in indoor dust from two locations in the eastern United States and implications for human exposures. Arch. Environ. Contam. Toxicol. 2011, 61, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Gómez, A.; Rubio, S.; Pérez-Bendito, D. Analytical methods for the determination of bisphenol A in food. J. Chromatogr. A 2009, 1216, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Yolton, K.; Dietrich, K.N.; Hornung, R.; Ye, X.; Calafat, A.M.; Lanphear, B.P. Prenatal bisphenol A exposure and early childhood behavior. Environ. Health Perspect. 2009, 117, 1945–1952. [Google Scholar] [CrossRef] [Green Version]
- Von Goetz, N.; Wormuth, M.; Scheringer, M.; Hungerbühler, K. Bisphenol A: How the most relevant exposure sources contribute to total consumer exposure. Risk Anal. Int. J. 2010, 30, 473–487. [Google Scholar] [CrossRef]
- Rancière, F.; Lyons, J.G.; Loh, V.H.; Botton, J.; Galloway, T.; Wang, T.; Shaw, J.E.; Magliano, D.J. Bisphenol A and the risk of cardiometabolic disorders: A systematic review with meta-analysis of the epidemiological evidence. Environ. Health 2015, 14, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Li, M.; Liu, A.; Wu, C.; Li, D.; Deng, Q.; Zhang, B.; Du, J.; Gao, X.; Hong, Y. Bisphenol A and the risk of obesity a systematic review with meta-analysis of the epidemiological evidence. Dose-Response 2020, 18, 1559325820916949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.-C.; Moon, K.; Thayer, K.A.; Navas-Acien, A. Environmental chemicals and type 2 diabetes: An updated systematic review of the epidemiologic evidence. Curr. Diabetes Rep. 2013, 13, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef] [Green Version]
- Adoamnei, E.; Mendiola, J.; Vela-Soria, F.; Fernández, M.F.; Olea, N.; Jørgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Urinary bisphenol A concentrations are associated with reproductive parameters in young men. Environ. Res. 2018, 161, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Miao, M.; Liang, H.; Shi, H.; Ruan, D.; Li, Y.; Wang, J.; Yuan, W. Exposure of environmental Bisphenol A in relation to routine sperm parameters and sperm movement characteristics among fertile men. Sci. Rep. 2018, 8, 17548. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; Food and Agriculture Organization of the United Nations. Toxicological and Health Aspects of Bisphenol A: Final Report, Including Report of Stakeholder Meeting on Bisphenol A. In Proceedings of the Joint FAO/WHO Expert Meeting and Stakeholder Meeting on Bisphenol A, Ottawa, ON, Canada, 1–5 November 2010; Available online: https://apps.who.int/iris/handle/10665/44624 (accessed on 15 September 2022).
- U.S. FDA. 2014 Updated Safety Assessment of Bisphenol A for Use in Food Contact Application. 2014. Available online: https://www.fda.gov/food/food-additives-petitions/bisphenol-bpa-use-food-contact-application (accessed on 10 October 2022).
- U.S. Environmental Protection Agency. A Review of the Reference Dose and Reference Concentration Process. 2002. Available online: https://www.epa.gov/risk/review-reference-dose-and-reference-concentration-processes-document (accessed on 10 October 2022).
- Health Canada. Bureau of Chemical Safety Food Directorate Health Products and Food Branch. In Health Risk Assessment of Bisphenol A from Food Packaging Applications; Government of Canada: Ottawa, ON, Canada, 2008. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/packaging-materials/bisphenol/health-risk-assessment-bisphenol-food-packaging-applications.html (accessed on 15 September 2022).
- Health Canada Press. Government of Canada Acts to Protect Newborns and Infants from Bisphenol A in Polycarbonate Plastic Baby Bottles. 2009. Available online: https://www.ctvnews.ca/bisphenol-a-can-build-up-in-babies-and-infants-1.372908?cache=almzxqnumb%3FclipId%3D68597 (accessed on 30 September 2022).
- LaKind, J.S.; Goodman, M.; Mattison, D.R. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: A systematic review of epidemiologic research. Crit. Rev. Toxicol. 2014, 44, 121–150. [Google Scholar] [CrossRef]
- Wang, Y.; Du, X.; Wang, D.; Wang, J.; Du, J. Effects of bisphenol A exposure during pregnancy and lactation on hippocampal function in newborn rats. Int. J. Med. Sci. 2020, 17, 1751. [Google Scholar] [CrossRef]
- Tyl, R.; Myers, C.; Marr, M.; Thomas, B.; Keimowitz, A.; Brine, D.; Veselica, M.; Fail, P.; Chang, T.; Seely, J. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. 2002, 68, 121–146. [Google Scholar] [CrossRef] [Green Version]
- Tyl, R.W.; Myers, C.B.; Marr, M.C.; Sloan, C.S.; Castillo, N.P.; Veselica, M.M.; Seely, J.C.; Dimond, S.S.; Van Miller, J.P.; Shiotsuka, R.N. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 2008, 104, 362–384. [Google Scholar] [CrossRef]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [Google Scholar] [CrossRef] [Green Version]
- Willhite, C.C.; Ball, G.L.; McLellan, C.J. Derivation of a bisphenol A oral reference dose (RfD) and drinking-water equivalent concentration. J. Toxicol. Environ. Health Part B 2008, 11, 69–146. [Google Scholar] [CrossRef]
- National Institute of Technology and Evaluation. Summary of the Interim Report: Bisphenol A. 2003. Available online: https://www.nite.go.jp/data/000010110.pdf (accessed on 12 October 2022).
- Luo, S.; Li, Y.; Li, Y.; Zhu, Q.; Jiang, J.; Wu, C.; Shen, T. Gestational and lactational exposure to low-dose bisphenol A increases Th17 cells in mice offspring. Environ. Toxicol. Pharmacol. 2016, 47, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Larsson, K.; Lindh, C.H.; Jönsson, B.A.; Giovanoulis, G.; Bibi, M.; Bottai, M.; Bergström, A.; Berglund, M. Phthalates, non-phthalate plasticizers and bisphenols in Swedish preschool dust in relation to children’s exposure. Environ. Int. 2017, 102, 114–124. [Google Scholar] [CrossRef] [PubMed]
- von Goetz, N.; Pirow, R.; Hart, A.; Bradley, E.; Poças, F.; Arcella, D.; Lillegard, I.T.; Simoneau, C.; Van Engelen, J.; Husoy, T. Including non-dietary sources into an exposure assessment of the European Food Safety Authority: The challenge of multi-sector chemicals such as Bisphenol A. Regul. Toxicol. Pharmacol. 2017, 85, 70–78. [Google Scholar] [CrossRef]
- Jo, M.J.; Park, J.-H.; An, K.-A.; Choi, H.; Kang, Y.-s.; Hwang, M. Quantification of bisphenols in Korean urine using online solid-phase extraction-high-performance liquid chromatography-tandem mass spectrometry. Environ. Toxicol. Pharmacol. 2020, 80, 103491. [Google Scholar] [CrossRef]
- Ko, A.; Hwang, M.-S.; Park, J.-H.; Kang, H.-S.; Lee, H.-S.; Hong, J.-H. Association between urinary bisphenol A and waist circumference in Korean adults. Toxicol. Res. 2014, 30, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Remer, T.; Neubert, A.; Maser-Gluth, C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am. J. Clin. Nutr. 2002, 75, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johner, S.; Boeing, H.; Thamm, M.; Remer, T. Urinary 24-h creatinine excretion in adults and its use as a simple tool for the estimation of daily urinary analyte excretion from analyte/creatinine ratios in populations. Eur. J. Clin. Nutr. 2015, 69, 1336–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, K.-i.; Kotake, M. Estimation of daily bisphenol a intake of Japanese individuals with emphasis on uncertainty and variability. Environ. Sci. Int. J. Environ. Physiol. Toxicol. 2006, 13, 15–29. [Google Scholar]
- Lv, Y.; Rui, C.; Dai, Y.; Pang, Q.; Li, Y.; Fan, R.; Lu, S. Exposure of children to BPA through dust and the association of urinary BPA and triclosan with oxidative stress in Guangzhou, China. Environ. Sci. Process. Impacts 2016, 18, 1492–1499. [Google Scholar] [CrossRef]
- Korean Statistical Information Service. Korean National Environmental Health Survey (KoNEHS), 2015–2017. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=106&tblId=DT_106N_99_1100051&conn_path=I2 (accessed on 10 October 2022).
- Hartwig, A.; Arand, M.; Epe, B.; Guth, S.; Jahnke, G.; Lampen, A.; Martus, H.-J.; Monien, B.; Rietjens, I.M.; Schmitz-Spanke, S. Mode of action-based risk assessment of genotoxic carcinogens. Arch. Toxicol. 2020, 94, 1787–1877. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; Hong, Y.-C.; Yang, J.; Im, H. Research on Health Effect Follow-Up Survey and Reduced Exposure by Low Dose of Hazard Substance; Project Report No.18162MFDS121; National Institute of Food and Drug Safety Evaluation: Osong, Republic of Korea, 2018.
- Use of the Margin of Exposure. Available online: https://foodsafetyportal.eu/hbgv/moe.html (accessed on 10 December 2022).
- Gingrich, J.; Pu, Y.; Ehrhardt, R.; Karthikraj, R.; Kannan, K.; Veiga-Lopez, A. Toxicokinetics of bisphenol A, bisphenol S, and bisphenol F in a pregnancy sheep model. Chemosphere 2019, 220, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Choi, J.W.; Ahn, Y.-A.; Kim, S. Pharmacokinetics of bisphenol S in humans after single oral administration. Environ. Int. 2018, 112, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Sirasanagandla, S.R.; Al-Huseini, I.; Sakr, H.; Moqadass, M.; Das, S.; Juliana, N.; Abu, I.F. Natural Products in Mitigation of Bisphenol A Toxicity: Future Therapeutic Use. Molecules 2022, 27, 5384. [Google Scholar] [CrossRef]
- Skledar, D.G.; Mašič, L.P. Bisphenol A and its analogs: Do their metabolites have endocrine activity? Environ. Toxicol. Pharmacol. 2016, 47, 182–199. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Yang, Y.; Yin, J.; Zhang, J.; Feng, Y.; Shao, B. Biotransformation of bisphenol AF to its major glucuronide metabolite reduces estrogenic activity. PloS ONE 2013, 8, e83170. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsberg, G.; Rice, D.C. Does rapid metabolism ensure negligible risk from bisphenol A? Environ. Health Perspect. 2009, 117, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Lucier, G.W.; Sonawane, B.; McDaniel, O. Glucuronidation and deglucuronidation reactions in hepatic and extrahepatic tissues during perinatal development. Drug Metab. Dispos. 1977, 5, 279–287. [Google Scholar]
- Paigen, K. Mammalian β-glucuronidase: Genetics, molecular biology, and cell biology. Prog. Nucleic Acid Res. Mol. Biol. 1989, 37, 155–205. [Google Scholar]
- Boucher, J.G.; Boudreau, A.; Ahmed, S.; Atlas, E. In vitro effects of bisphenol A β-D-glucuronide (BPA-G) on adipogenesis in human and murine preadipocytes. Environ. Health Perspect. 2015, 123, 1287–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, Y.; Goldblum, R.M.; Midoro-Horiuti, T. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: An animal model study. Environ. Health 2012, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.; Choi, Y.-J.; Lim, Y.-H.; Lee, K.-S.; Kim, B.-N.; Shin, C.H.; Lee, Y.A.; Kim, J.I.; Hong, Y.-C. Associations between thyroid hormone levels and urinary concentrations of bisphenol A, F, and S in 6-Year-old children in Korea. J. Prev. Med. Public Health 2021, 54, 37. [Google Scholar] [CrossRef] [PubMed]
- Koutaki, D.; Paltoglou, G.; Vourdoumpa, A.; Charmandari, E. The impact of bisphenol A on thyroid function in neonates and children: A systematic review of the literature. Nutrients 2022, 14, 168. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Vandenberg, L.N. Update on the health effects of bisphenol A: Overwhelming evidence of harm. Endocrinology 2021, 162, bqaa171. [Google Scholar] [CrossRef]

| Agencies | Endpoint | Point of Departure (Uncertainty Factor) | Tolerable Daily Intake |
|---|---|---|---|
| US EPA 1 | Reduced body weight | NOAEL 5 mg/kg bw/day (100) | 0.05 mg/kg bw/day |
| US FDA 2 | Reduced body weight and liver effects | NOAEL 5 mg/kg bw/day (1000) | 0.005 mg/kg bw/day |
| Irreversible reproductive effects | NOAEL 50 mg/kg bw/day (1000) | 0.05 mg/kg bw/day | |
| Reversible reproductive effects | NOAEL 50 mg/kg bw/day (100) | 0.5 mg/kg bw/day | |
| Japan 3 | Body weight | NOAEL 5 mg/kg bw/day (100) | 0.05 mg/kg bw/day |
| Reproduction | NOAEL 50 mg/kg bw/day (100) | 0.5 mg/kg bw/day | |
| Liver effects | NOAEL 23 mg/kg bw/day (500) | 0.046 mg/kg bw/day | |
| Republic of Korea 4 | Relative kidney weight increase | BMDL10 10.5 mg/kg bw/day (500) | 0.02 mg/kg bw/day |
| NSF-Int 5 | Systemic toxicity | NOAEL 5 mg/kg bw/day (300) | 0.016 mg/kg bw/day |
| EFSA 6 | Immunotoxicity | BMDL20 0.93 ng/kg bw/day (25) | 0.04 ng/kg bw/day |
| Canada 7 | Body weight reduction | NOEL 25 mg/kg bw/day (1000) | 0.025 mg/kg bw/day |
| Development and reproduction effects | NOAEL 5 mg/kg bw/day (200) | 0.025 mg/kg bw/day | |
| FAO/WHO 8 | Reproductive toxicity | NOAEL 5 mg/kg bw/day | - |
| Exposure Method | Age Group (Years) | |||
|---|---|---|---|---|
| 3–6 | 7–12 | 13–18 | 19–79 | |
| Aggregated exposure amount | 0.045 | 0.028 | 0.021 | 0.023 |
| Dietary exposure amount | 0.040 | 0.030 | 0.018 | 0.017 |
| Population | Urinary Concentration 1 (µg/g Creatinine) | Extrapolated Exposure Amount 2 (µg/kg bw/day) (Contribution Rate of Foods) | ||
|---|---|---|---|---|
| Mean | 95th Percentile | Mean | 95th Percentile | |
| 3–6 years old | 2.83 | 12.1 | 0.042 (95%) * | 0.201 (19.9%) * |
| 7–12 years old | 1.56 | 8.22 | 0.027 (100%) * | 0.160 (18.8%) * |
| 13–18 years old | 0.89 | 4.58 | 0.017 (100%) * | 0.104 (17.3%) * |
| ≥19 years old | 1.38 | 8.08 | 0.031 (54.8%) * | 0.181 (9.39%) * |
| Point of Departure (POD) | Margin of Exposure (MOE) | ||||
|---|---|---|---|---|---|
| 3–6 yrs | 7–12 yrs | 13–18 yrs | 19–79 yrs | ||
| Mean | NOAEL 5000 µg/kg bw/day (animal toxicity) | >119,000 | >185,100 | >294,100 | >161,200 |
| BMDL20 60 ng/kg bw/day (animal toxicity; immunotoxicity) | 22 | 34 | 55 | 30 | |
| EDI 0.5 µg/kg bw/day (epidemiological study; thyroid cancer) | 12 | 19 | 29 | 16 | |
| 95th | NOAEL 5000 µg/kg bw/day (animal toxicity) | >24,800 | >31,200 | >48,000 | >27,600 |
| BMDL20 60 ng/kg bw/day (animal toxicity; immunotoxicity) | 5 | 6 | 9 | 5 | |
| EDI 0.5 µg/kg bw/day (epidemiological study; thyroid cancer) | 2 | 3 | 5 | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, M.; Park, S.-J.; Lee, H.-J. Risk Assessment of Bisphenol A in the Korean General Population. Appl. Sci. 2023, 13, 3587. https://doi.org/10.3390/app13063587
Hwang M, Park S-J, Lee H-J. Risk Assessment of Bisphenol A in the Korean General Population. Applied Sciences. 2023; 13(6):3587. https://doi.org/10.3390/app13063587
Chicago/Turabian StyleHwang, Myungsil, Seon-Joo Park, and Hae-Jeung Lee. 2023. "Risk Assessment of Bisphenol A in the Korean General Population" Applied Sciences 13, no. 6: 3587. https://doi.org/10.3390/app13063587
APA StyleHwang, M., Park, S.-J., & Lee, H.-J. (2023). Risk Assessment of Bisphenol A in the Korean General Population. Applied Sciences, 13(6), 3587. https://doi.org/10.3390/app13063587







