Application Assessment of Electrical Cables during Smoldering and Flaming Combustion
Abstract
1. Introduction
- Nitrogen oxides (NOx: nitrogen oxide (NO) and nitrogen dioxide (NO2));
- Carbon oxide (CO) and carbon dioxide (CO2);
- Hydrocarbons, mainly polycyclic aromatic hydrocarbons (PAHs);
- Oxygen, hydrogen, fluorine, chlorine, sulfur, nitrogen, and bromine compounds, such as aldehydes, acrolein and benzopyrene, determined as volatile organic compounds;
- Sulfur oxides, mainly SO2; and
- Solid particles (PM) containing carbon black and a lot of toxic carbon, hydrogen and oxygen compounds, as well as isocyanates, as products of burning PUR foams.
- Flaming combustion;
- Smoldering combustion (cable insulation heated by a heating field);
- Smoldering combustion (cable heated from the inside by the flow of electric current in its wires).
2. Materials and Methods
2.1. Materials
2.2. Methods
- For each type of smoldering combustion (initiated by a heating field or by the flow of electric current through wires): 2 m test cable;
- For flaming combustion: 2 m test cable hung on a stand over a container of alcohol. Samples were placed above the flame.
- ΔI—the change in current due to the presence of smoke;
- Io—the current of the chamber without smoke;
- z—the aerosol particle concentration;
- η—the chamber constant that characterizes its sensitivity.
- Io—the current of the chamber without smoke, with concentration of aerosol particles;
- In—the saturation current;
- β(d)—the ion attachment factor for particles of diameter d;
- x1 − x0—the electrode spacing;
- Vo—the voltage between electrodes at current Io;
- b+—the electrical mobility of the positive ion, 1.4/cm2 Vs;
- Di—the diffusion coefficient (for small ions 0.042 cm2/s);
- d—the mean particle diameter (homogeneous, isotropic particles).
- y—the smoke density;
- η—the ionization chamber constant;
- d—the particle diameter;
- nd(d)—the normal distribution of particle diameters.
- l—the length of the optical path measured in aerosol (m);
- po—the radiation power received under non-aerosol conditions (dB);
- p—the radiant power received in the presence of an aerosol (dB).
3. Results and Discussion
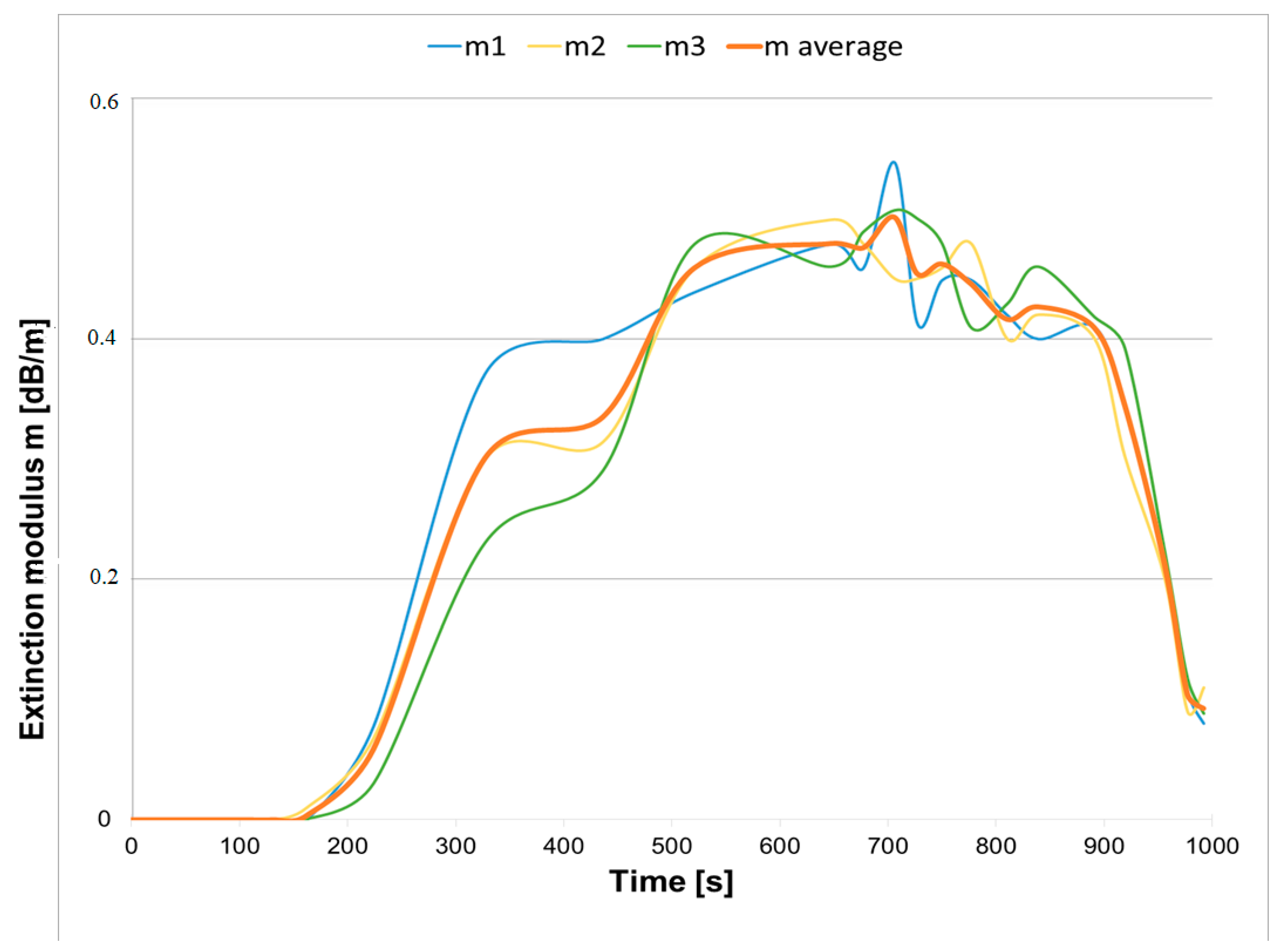
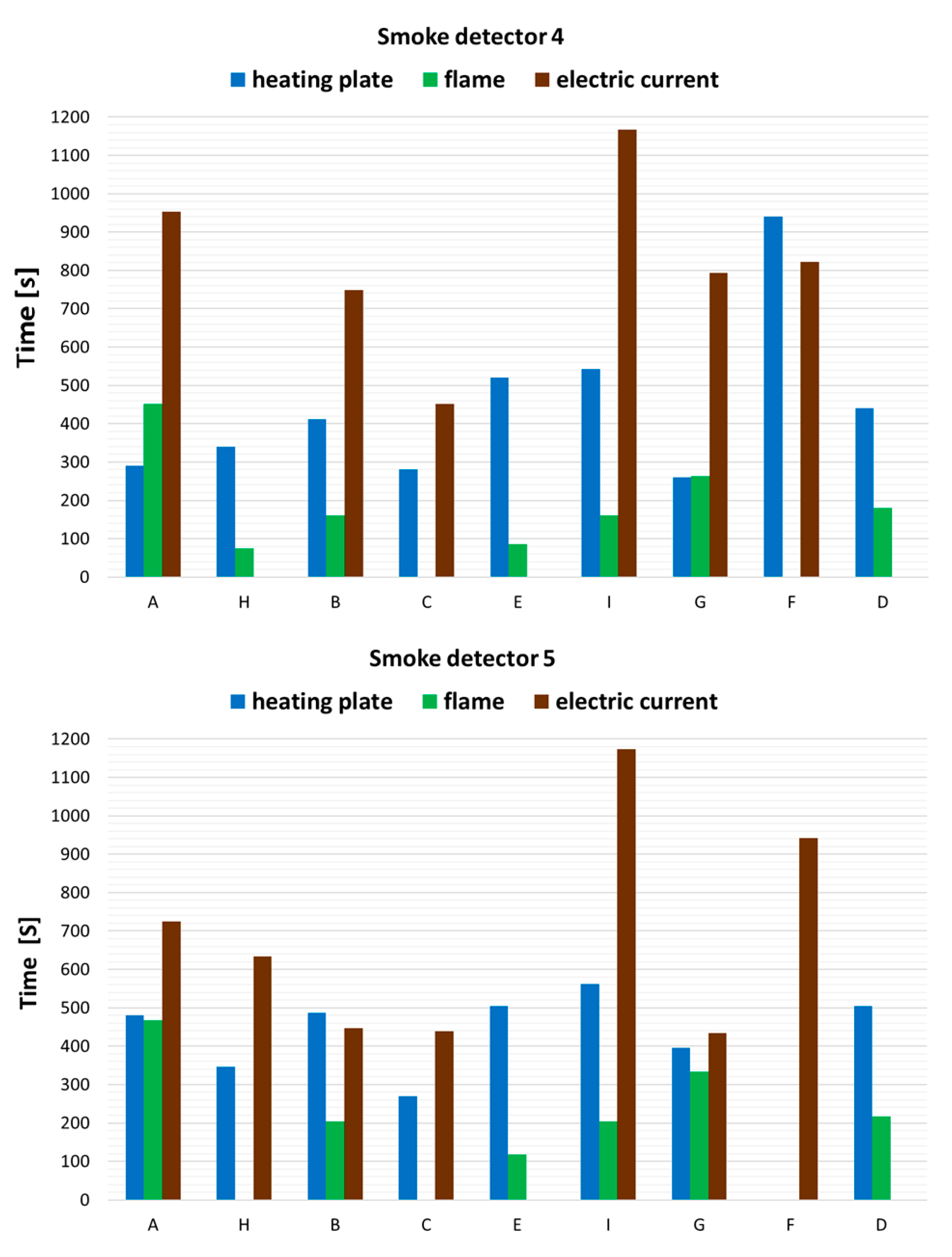
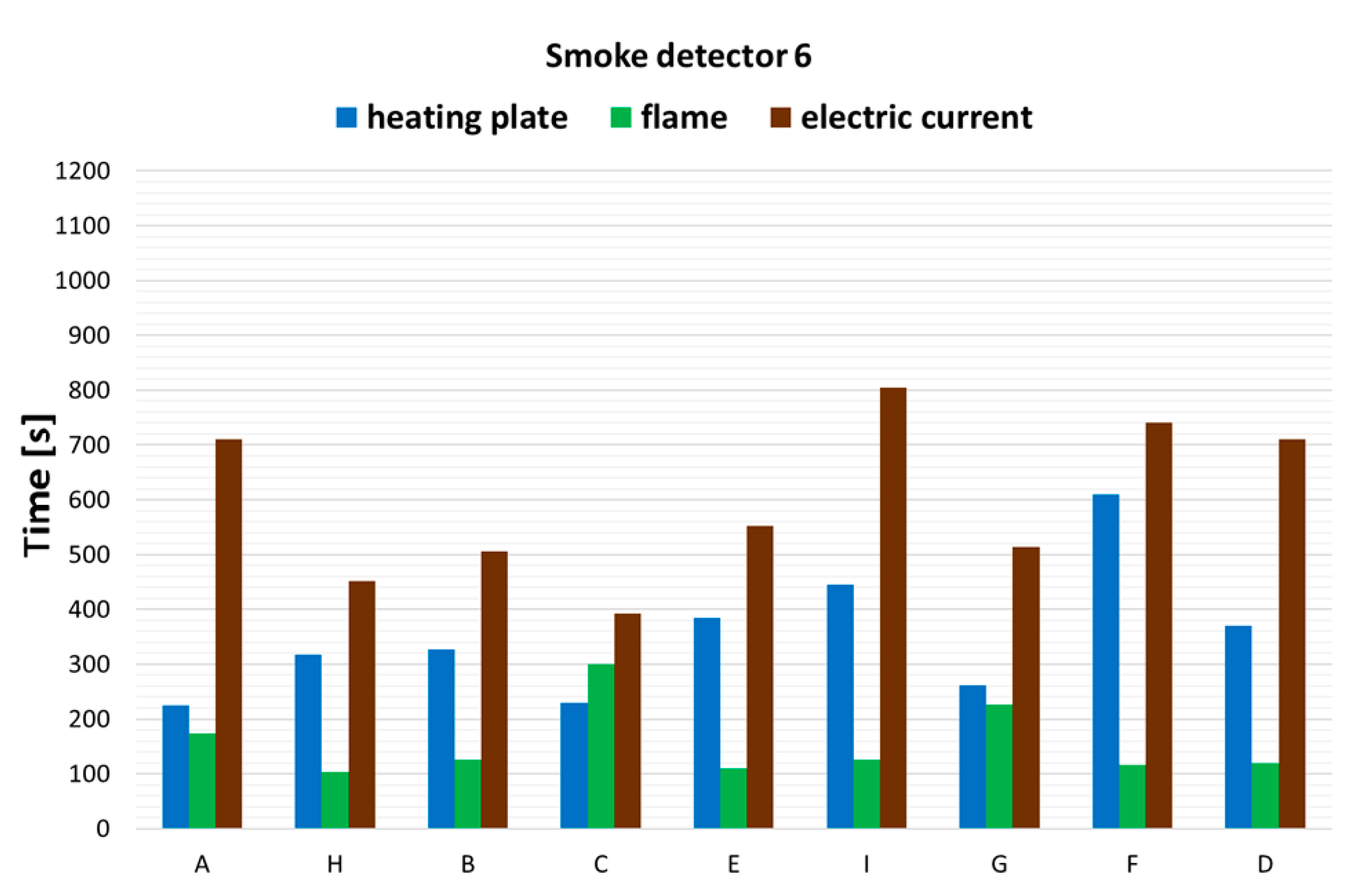
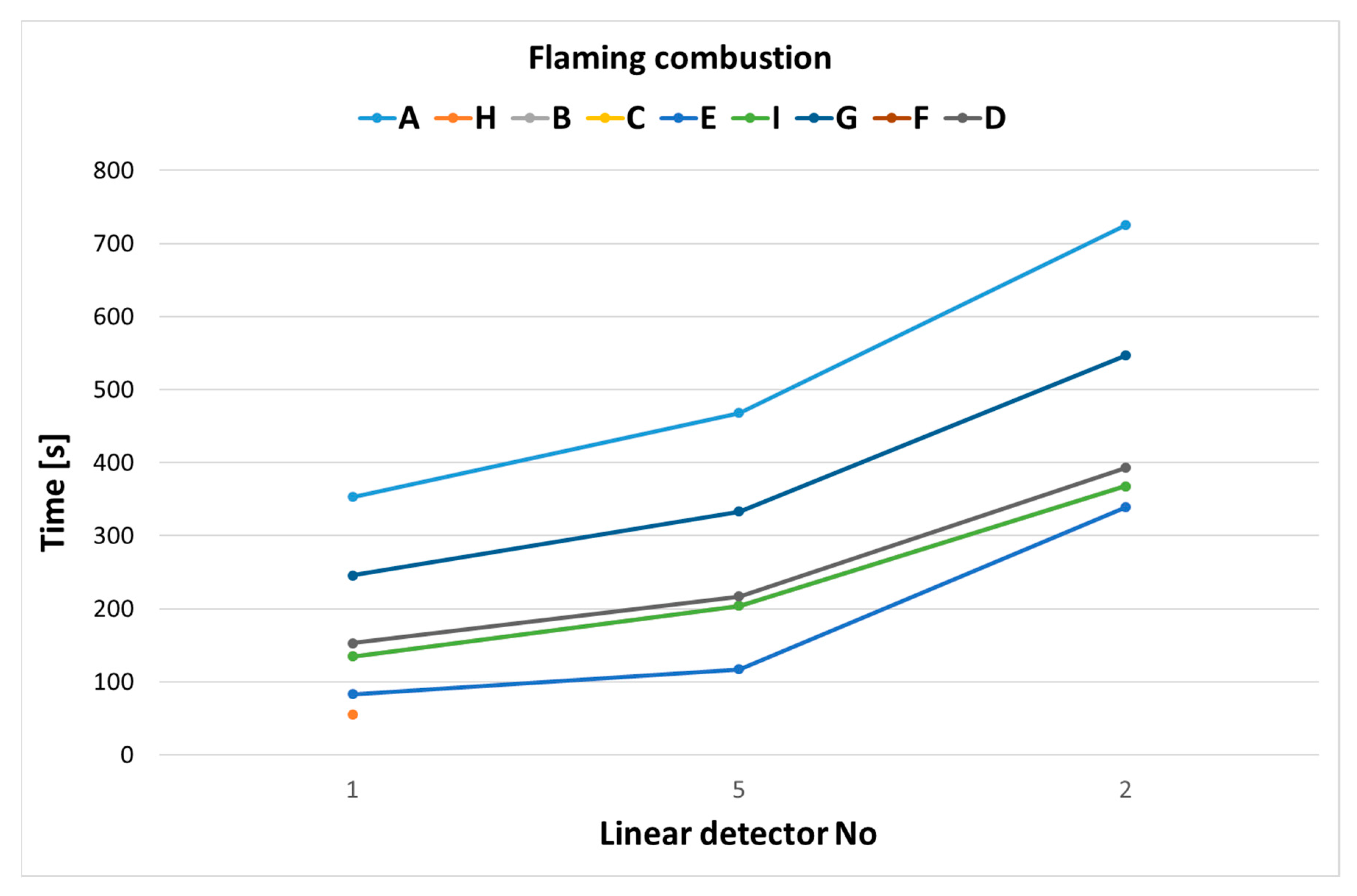
3.1. The Operation Times of the Detectors
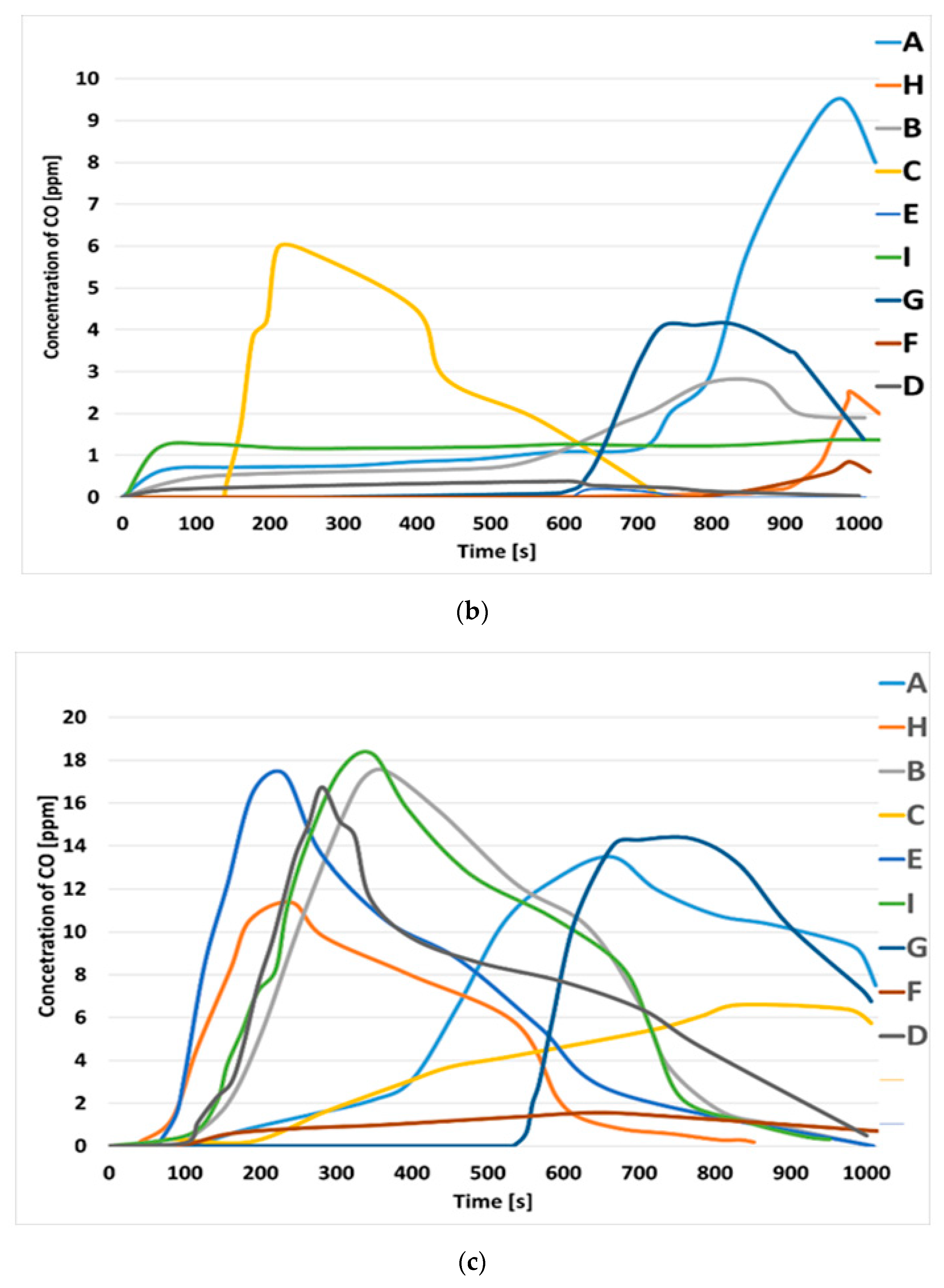
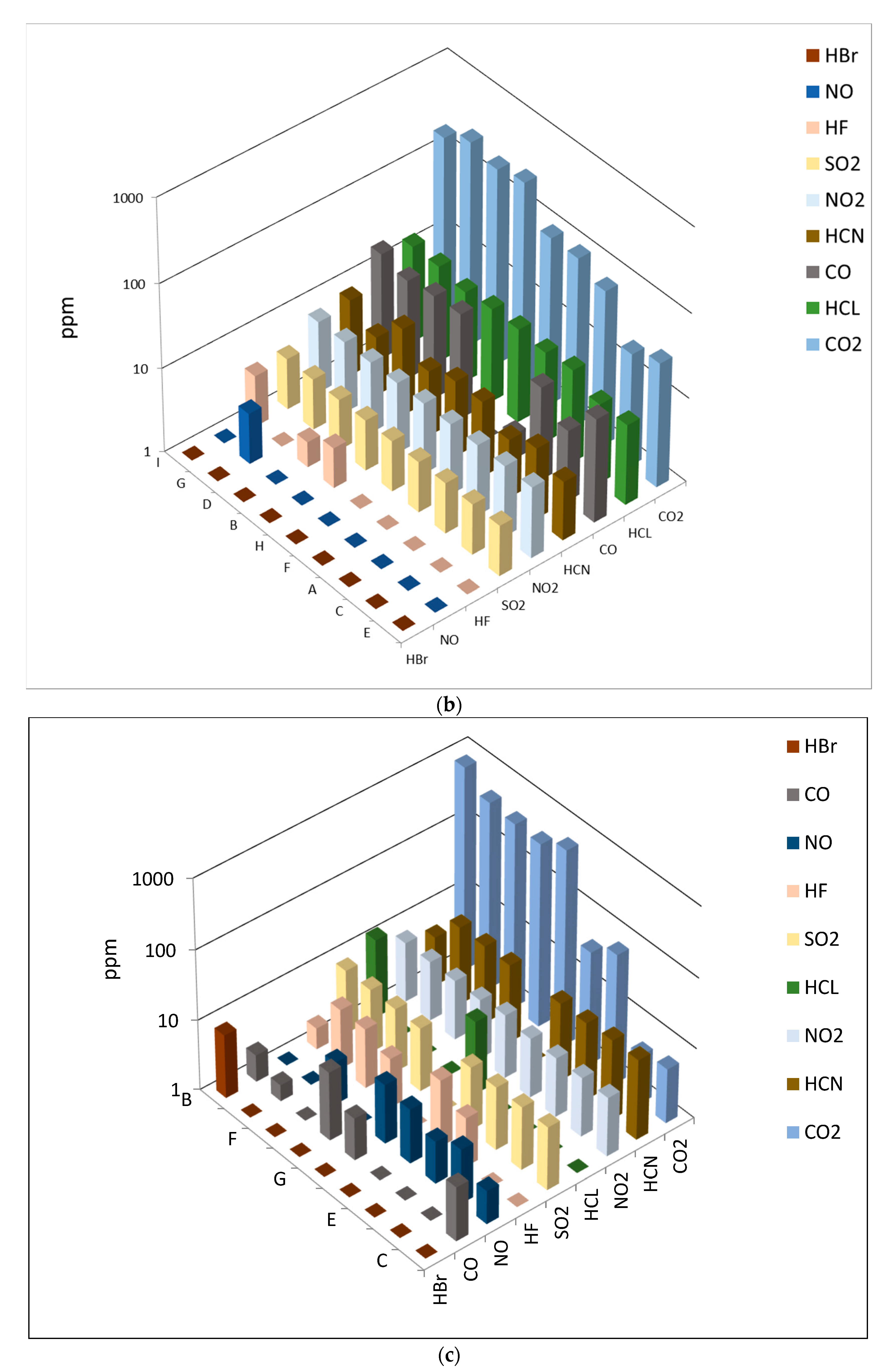
3.2. Toxic Gas Emission during Flaming and Smoldering Combustion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manan, N.; Idham, N.; Hasan, A.; Mariyah, N.; Faezah, N. Fire Risk Assessment of Residential Buildings Based on Fire Statistics from Selangor. J. Pharm. Sci. Innov. 2020, 7, 2321–2705. [Google Scholar]
- Huang, L.C.; Chang, H.C.; Chen, C.C.; Kuo, C.C. A ZigBee-based monitoring and protection system for building electrical safety. Energy Build. 2011, 43, 1418–1426. [Google Scholar] [CrossRef]
- Wnęk, W.; Boroń, S. Effect of smoke flow velocity on the fire protection systems. Przemysł Chemiczny. 2014, 93, 1946–1952. [Google Scholar]
- Kaczorek-Chrobak, K.; Fangrat, J. Influence of Constructional-Material Parameters on the Fire Properties of Electric Cables. Energies 2019, 12, 4569. [Google Scholar] [CrossRef]
- Fernandez-Pello, A.; Hasegawa, H.K.; Staggs, K.; Lipska-quinn, A.E.; Alvares, N.J. A Study of the Fire Performance of Electrical Cables. IAFSS 1991, 3, 237–247. [Google Scholar] [CrossRef]
- Torero, J. Flaming Ignition of Solid Fuels. In SFPE Handbook of Fire Protection Engineering, 5th ed.; Hurley, M.J., Gottuk, D.T., Hall, J.R., Jr., Harada, K., Kuligowski, E.D., Puchovsky, M., Torero, J.L., Watts, J.M., Jr., Wieczorek, C.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 633–661. [Google Scholar] [CrossRef]
- Ahrens, M. Home Structure Fires; National Fire Protection Association: Quincy, MA, USA, 2018. [Google Scholar]
- Carcillo, M.; Caro, A.-S.; Sonnier, R.; Ferry, L.; Gesta, E.; Lagreve, C. Fire behaviour of electrical cables in cone calorimeter: Influence of cables structure and layout. Fire Saf. J. 2018, 99, 12–21. [Google Scholar]
- Klote, J.H.; Milke, J.A.; Turnbull, P.G.; Kashef AFerreira, M.J. Handbook of Smoke Control Engineering; ASHRAE: Atlanta, GA, USA, 2012. [Google Scholar]
- Blomqvist, P.; Rosell, L.; Simonson, M. Emissions from Fires Part I: Fire Retarded and Non-Fire Retarded TV-Sets. Fire Technol. 2004, 40, 39–58. [Google Scholar] [CrossRef]
- Blomqvist, P.; Rosell, L.; Simonson, M. Emissions from Fires Part II: Simulated Room Fires Part 2. Fire Technol. 2004, 40, 59–73. [Google Scholar] [CrossRef]
- Lyon, R.E. Solid State Thermochemistry of Flaming Combustion; Report 1999; Office of Aviation Research Washington USA: Washington, DC, USA.
- Ohlemiller, T.J. Modeling of Smoldering Combustion Propagation. Prog. Energy Combust. Sci. 1985, 11, 277–310. [Google Scholar] [CrossRef]
- Rein, G. Smouldering Combustion Phenomena in Science and Technology. Int. Rev. Chem. Eng. 2009, 1, 3–18. [Google Scholar]
- Stec, A.A.; Rhodes, J. Bench Scale Generation of Smoke Particulates and Hydrocarbons from Burning Polymers. In Fire Safety Science, Proceedings of the Tenth International Symposium 2011, Christchurch, New Zealand, 19–24 June 2011; International Association For Fire Safety Science: Tsukuba, Japan, 2011; pp. 629–640. [Google Scholar] [CrossRef]
- Braun, E.; Shields, J.R.; Harris, R.H. Flammability Characteristics of Electrical Cables Using the Cone Calorimeter; NIST Report, NISTIR 88 4003; Department of Commerce: Washington, DC, USA, 1989.
- Kaczorek-Chrobak, K.; Fangrat, J.; Papis, B.K. Calorimetric Behaviour of Electric Cables. Energies 2021, 14, 1007. [Google Scholar] [CrossRef]
- Tewarson, A.; Khan, M.M. Flame propagation for polymers in cylindrical configuration and vertical orientation. Symp. Int. Combust. 1989, 22, 1231–1240. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, H.; Tong, L. Experimental study on the fire protection properties of PVC sheath for old and new cables. J. Hazard. Mater. 2010, 179, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, E. Flame Retardants for Plastics. In Handbook of Building Materials for Fire Protection; Harper, C.A., Ed.; McGraw-Hill: New York, NY, USA, 2004; pp. 4.1–4.88. [Google Scholar]
- Gong, T.; Xie, Q.; Huang, X. Fire behaviors of flame-retardant cables part I: Decomposition, swelling and spontaneous ignition. Fire Saf. J. 2018, 95, 113–121. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M. Effect of the plasticizer concentration and heating rate on the thermal decomposition behaviour of PVC plastisols. Kinetic analysis. Polym. Degrad. Stab. 1988, 60, 1–10. [Google Scholar] [CrossRef]
- EN 50399:2011/A1:2016; Common Test Methods for Cables under Fire Conditions—Heat Release and Smoke Production Measurement on Cables during Flame Spread Test—Test Apparatus, Procedures, Results. CEN: Brussels, Belgium, 2011.
- Fangrat, J. Combustibility of building products versus fire safety. Bull. Pol. Acad. Sci. Tech. Sci. 2016, 64, 4. [Google Scholar] [CrossRef]
- Fazzini, T.; Perkins, R.; Grossman, D. Ionization and phtoelectric smoke alarms in rural Alaskan homes. West. J. Med. 2000, 173, 89–92. [Google Scholar] [CrossRef]
- Miller, T.; Bergen, G.; Ballesteros, M.; Bhattacharya, S.; Gielen, A.; Sheppard, M. Increasing smoke alarm operability through theory-based health education: A radomised trial. J. Epidemiol. Community Health 2014, 68, 1168–1174. [Google Scholar] [CrossRef]
- Ahrens, M. Smoke Alarm Presence and Performance in U.S. Home Fires. Fire Technol. 2011, 47, 699–720. [Google Scholar] [CrossRef]
- Moinuddin, K.; Bruck, D.; Shi, L. An experimental study on timely activation of smoke alarms and their effective notification in typical residential buildings. Fire Saf. J. 2017, 93, 1–11. [Google Scholar] [CrossRef]
- Kumar, A.; Gaur, A.; Singh, A.; Kumar, K.S.; Kulkarni, S.; Lala, K.; Kapoor, V.; Srivastava, S.C. Mukhopadhyay, Fire sensing technologies: A review. IEEE Sens. J. 2019, 19, 3191–3202. [Google Scholar]
- Sesseng, C. Mapping of Gas Concentrations, Effect of Deadair Space and Effect of Alternative Detection Technology in Smouldering Fires. SPFR Rep. A 2016, 16, 20053. [Google Scholar]
- Fleming, J.M. Smoke Detector Technology and the Investigation of Fatal Fires May 2000; Boston Fire Department: Boston, MA, USA, 2000.
- Mosisa Wako, F.; Pio, G.; Salzano, E. Reduced Combustion Mechanism for Fire with Light Alcohols. Fire 2021, 4, 86. [Google Scholar] [CrossRef]
- Fangrat, J.; Kaczorek-Chrobak, K.; Papis, B.K. Fire Behavior of Electrical Installations in Buildings. Energies 2020, 13, 6433. [Google Scholar] [CrossRef]
- Rehman, A.; Qureshi, M.A.; Ali, T.; Irfan, M.; Abdullah, S.; Yasin, S.; Draz, U.; Glowacz, A.; Nowakowski, G.; Alghamdi, A.; et al. Smart Fire Detection and Deterrent System for Human Savior by Using Internet of Things (IoT). Energies 2021, 14, 5500. [Google Scholar] [CrossRef]
- EN 13501-6:2018; Fire Classification of Construction Products and Building Elements—Part 6: Classification Using Data from rEaction to Fire Tests on Power, Control and Communication Cables. CEN: Brussels, Belgium, 2018.
- Available online: http://www.jascoint.co.jp/asia/products/spectroscopy/ftir/ftir4700.html (accessed on 10 February 2022).
- Bukowski, R.; Peacock, R.; Averill, J.; Bryner, N.; Walton, W.; Reneke, P.; Kuligowski, E. Performance of Home Smoke Alarms Analysis of the Response of Several Available Technologies in Residential Fire Settings. NIST Technical Note 1455-1. 2008. Available online: https://www.nist.gov/system/files/documents/2017/04/28/NIST_TN_1455-1_Feb2008.pdf (accessed on 11 February 2022).
- Gałązka, E.; Piechocka, E. Metody Obliczeniowe Wybranych Parametrów Palności, Wybuchowości i Dymotwórczości Substancji Chemicznych; SGSP: Warszawa, Poland, 2004. [Google Scholar]
- PN-EN 54:7; 2004/A2:2009 Fire Alarm Systems. Part 7: Smoke Detectors. Point Detectors Working with Scattered Light, Trans-mitted Light or Ionization. CEN: Brussels, Belgium, 2018.

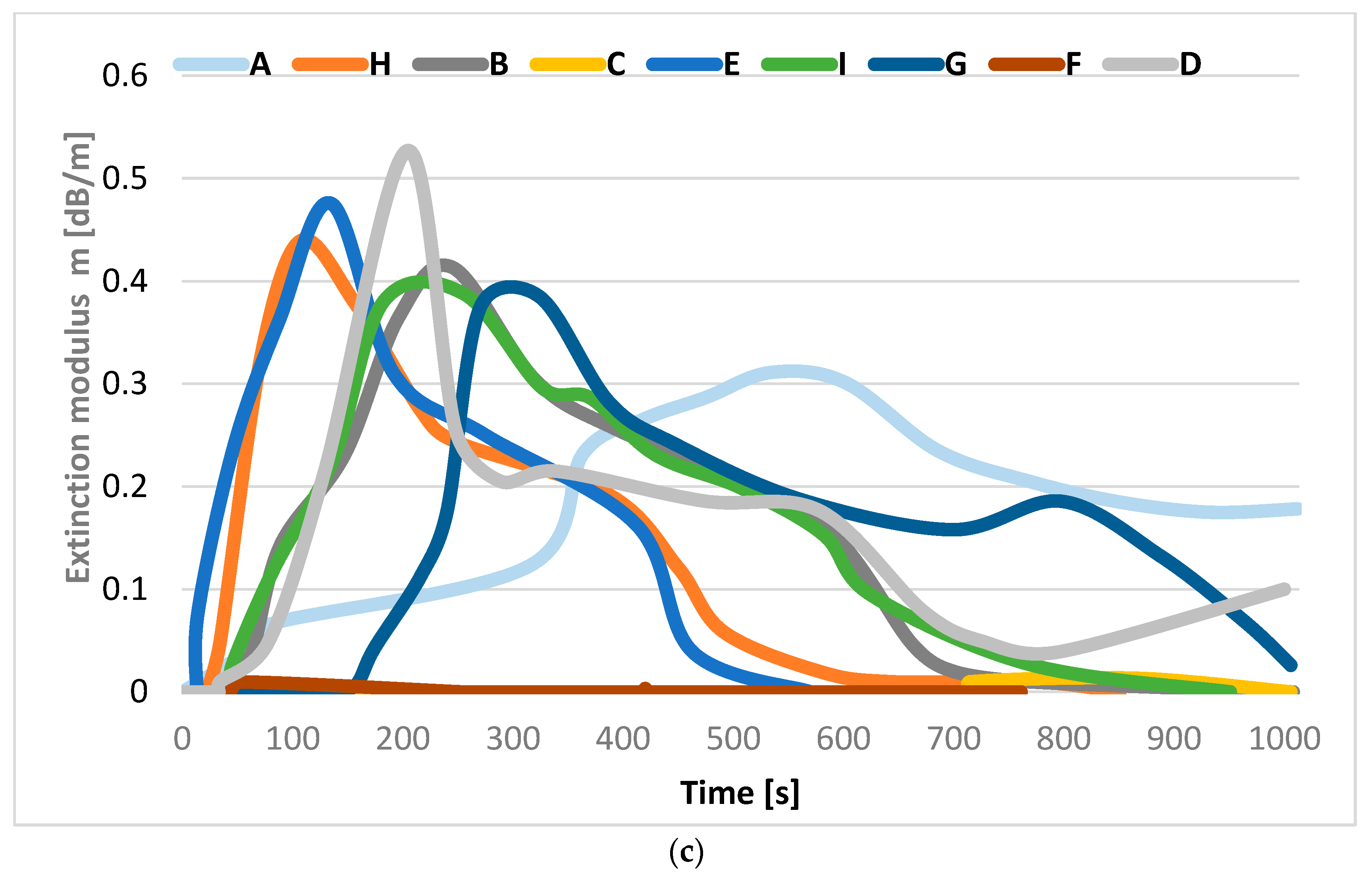

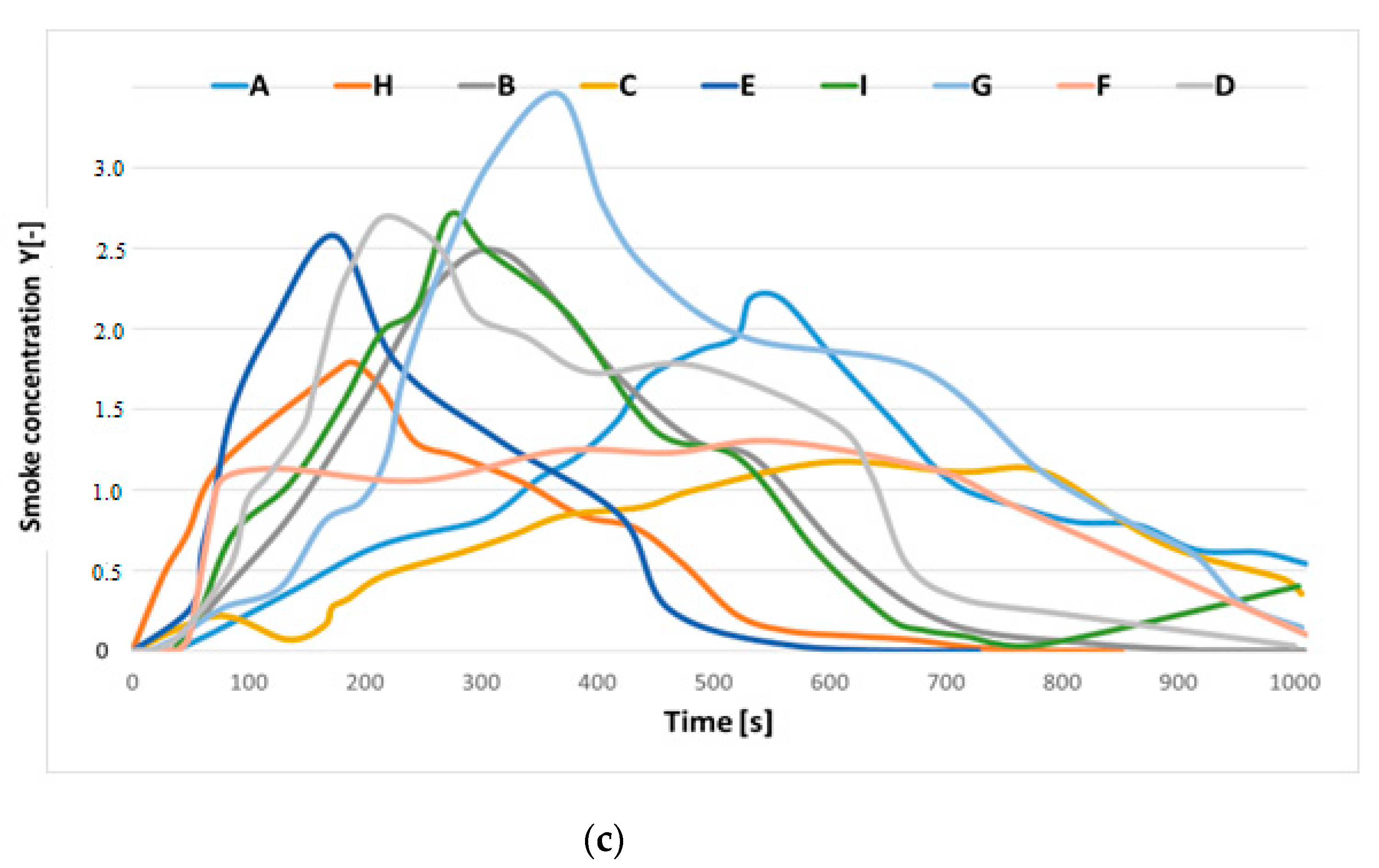





| Cable Marking | Insulation and/or Sheath Material | Number and Rated Diameter of Wires (mm2) | Cable Diameter (mm) | Information about Cable |
|---|---|---|---|---|
| Cable A | Halogen-free plastic | 5 × 2 × 0.8 | 10.9 | Control cable with multi-wire copper conductors, in halogen-free plastic insulation and in halogen-free plastic coating |
| Cable B | Plasticized PVC | 3 × 4.0 | 10.3 | Control cable with multi-wire copper wires, PVC insulation and PVC coating |
| Cable C | Mica tape banding and halogen-free plastic | 2 × 2 × 1.4 | 11.6 | Telecommunication cable resistant to fire, with copper wires insulated with mica tape and halogen-free insulation with a high oxygen index and a halogen-free plastic coating |
| Cable D | Plasticized PVC | 1 × 2 × 1.4 | 10.0 | Telecommunication cable, with single-wire copper conductors, PVC insulation and flame-retardant PVC coating |
| Cable E | Plasticized PVC | 5 × 2 × 0.8 | 10.9 | Multi-wire cables, PVC insulation, copper braid shield |
| Cable F | Silicone | 3 × 1.5 | 8.5 | Recommended applications include foundries, steel mills, glass factories, baking equipment, burners, heating and lighting systems |
| Cable G | Plasticized PVC | 3 × 2.5 | 10.4 | Wire with copper conductors, PVC wire insulation, rubber coatings for housing applications |
| Cable H | PVC | 3 × 1.5 | 8.9 | Halogen-free, does not spread the flame, low smoke emission |
| Cable I | Rubber | 2 × 2.5 | 12.7 | Control cable with multi-wire copper conductors, rubber insulation |
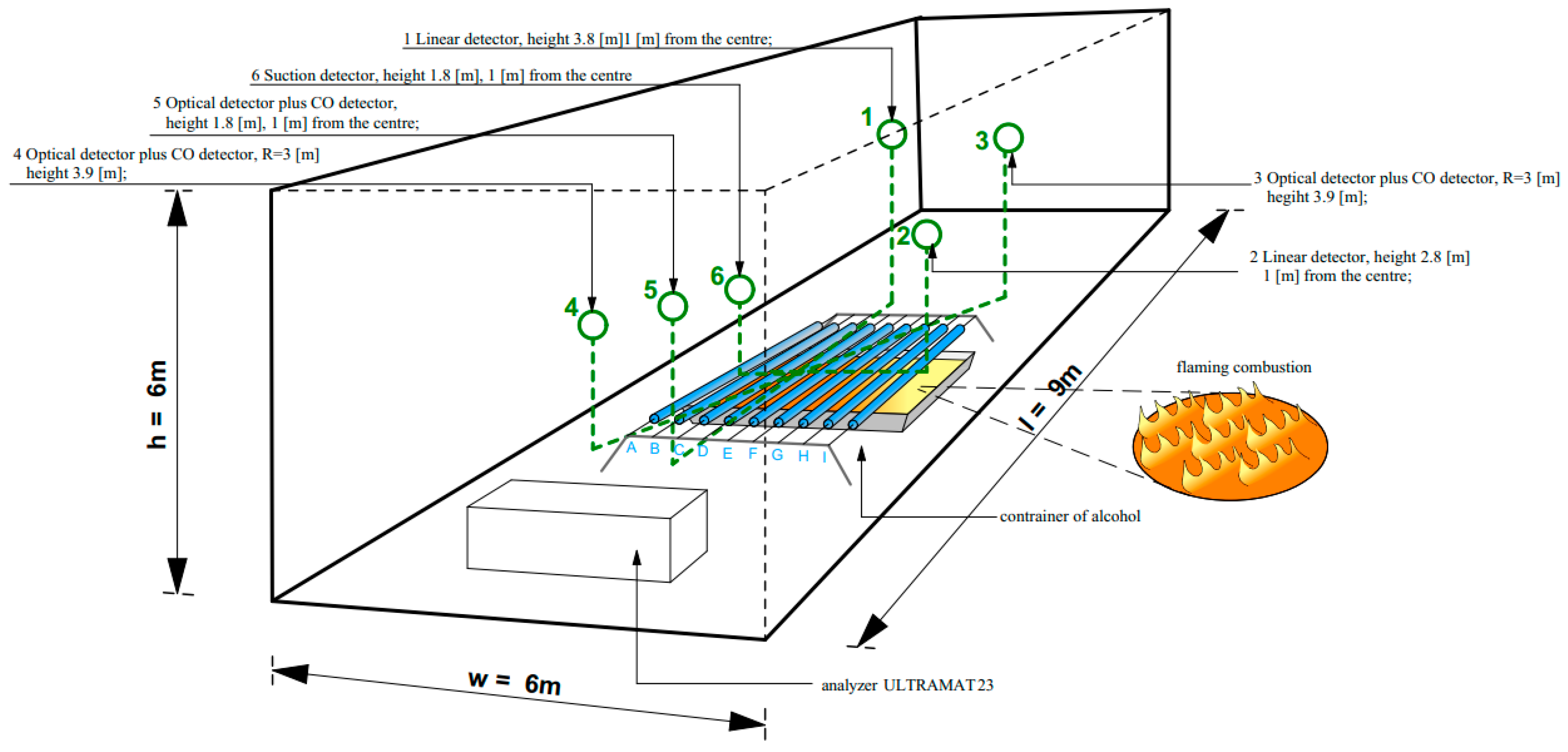
| No. | Detector Type | Installation Place in the Combustion Chamber |
|---|---|---|
| 1 | Linear detector | Height 3.8 m, 1 m from the center of the chamber |
| 2 | Linear detector | Height 2.8 m, 1 m from the center of the chamber |
| 3 | Optical detector plus CO detector | On radius R = 3 m, mounting height 3.9 m |
| 4 | Optical detector plus CO detector | On radius R = 3 m, mounting height 3.9 m |
| 5 | Optical detector plus CO detector | Height 1.8 m, 1 m from the center of the chamber |
| 6 | Suction detector (suction system) | Height 3.85 m, 1 m from the center of the chamber |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porowski, R.; Kowalik, R.; Ramiączek, P.; Bąk-Patyna, P.; Stępień, P.; Zielecka, M.; Popielarczyk, T.; Ludynia, A.; Chyb, A.; Gawdzik, J. Application Assessment of Electrical Cables during Smoldering and Flaming Combustion. Appl. Sci. 2023, 13, 3766. https://doi.org/10.3390/app13063766
Porowski R, Kowalik R, Ramiączek P, Bąk-Patyna P, Stępień P, Zielecka M, Popielarczyk T, Ludynia A, Chyb A, Gawdzik J. Application Assessment of Electrical Cables during Smoldering and Flaming Combustion. Applied Sciences. 2023; 13(6):3766. https://doi.org/10.3390/app13063766
Chicago/Turabian StylePorowski, Rafał, Robert Kowalik, Piotr Ramiączek, Paulina Bąk-Patyna, Paweł Stępień, Maria Zielecka, Tomasz Popielarczyk, Agata Ludynia, Angelika Chyb, and Jarosław Gawdzik. 2023. "Application Assessment of Electrical Cables during Smoldering and Flaming Combustion" Applied Sciences 13, no. 6: 3766. https://doi.org/10.3390/app13063766
APA StylePorowski, R., Kowalik, R., Ramiączek, P., Bąk-Patyna, P., Stępień, P., Zielecka, M., Popielarczyk, T., Ludynia, A., Chyb, A., & Gawdzik, J. (2023). Application Assessment of Electrical Cables during Smoldering and Flaming Combustion. Applied Sciences, 13(6), 3766. https://doi.org/10.3390/app13063766








