Promising Photoluminescence Enhancement of Tris(8-hydroxyquinoline)aluminum by Simultaneous Localized and Propagating Surface Plasmons of Ag Nanostructures
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
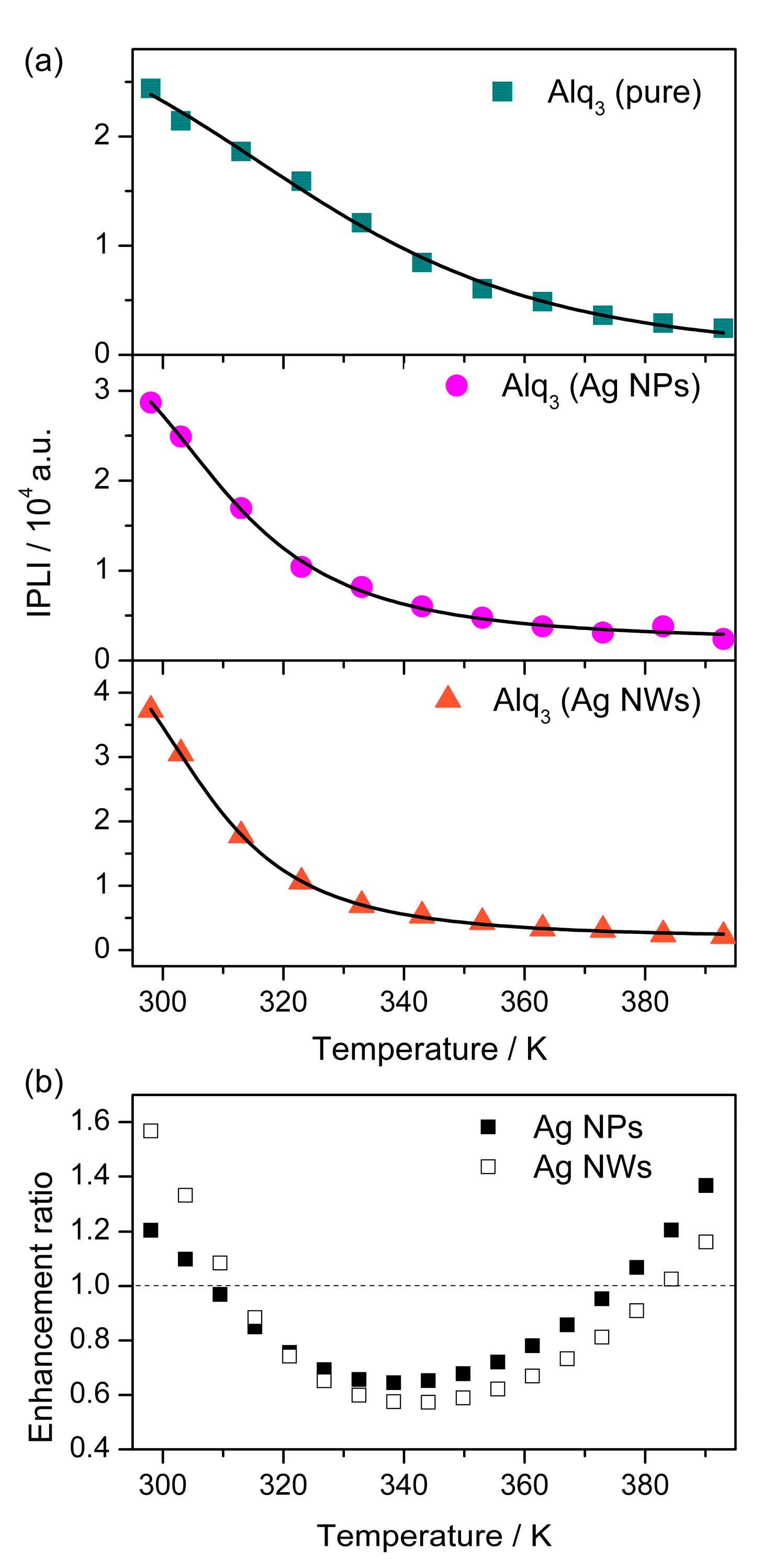
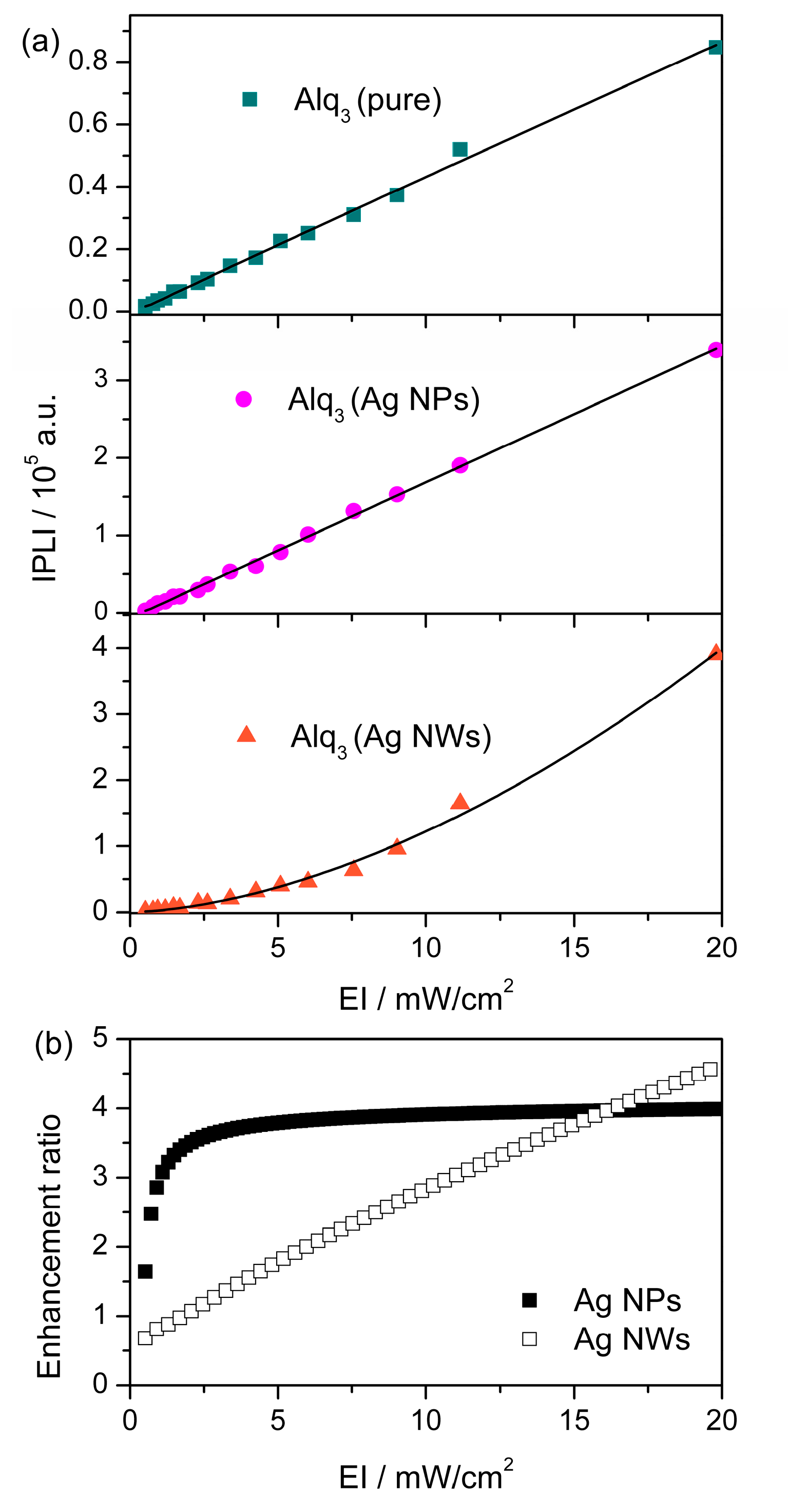
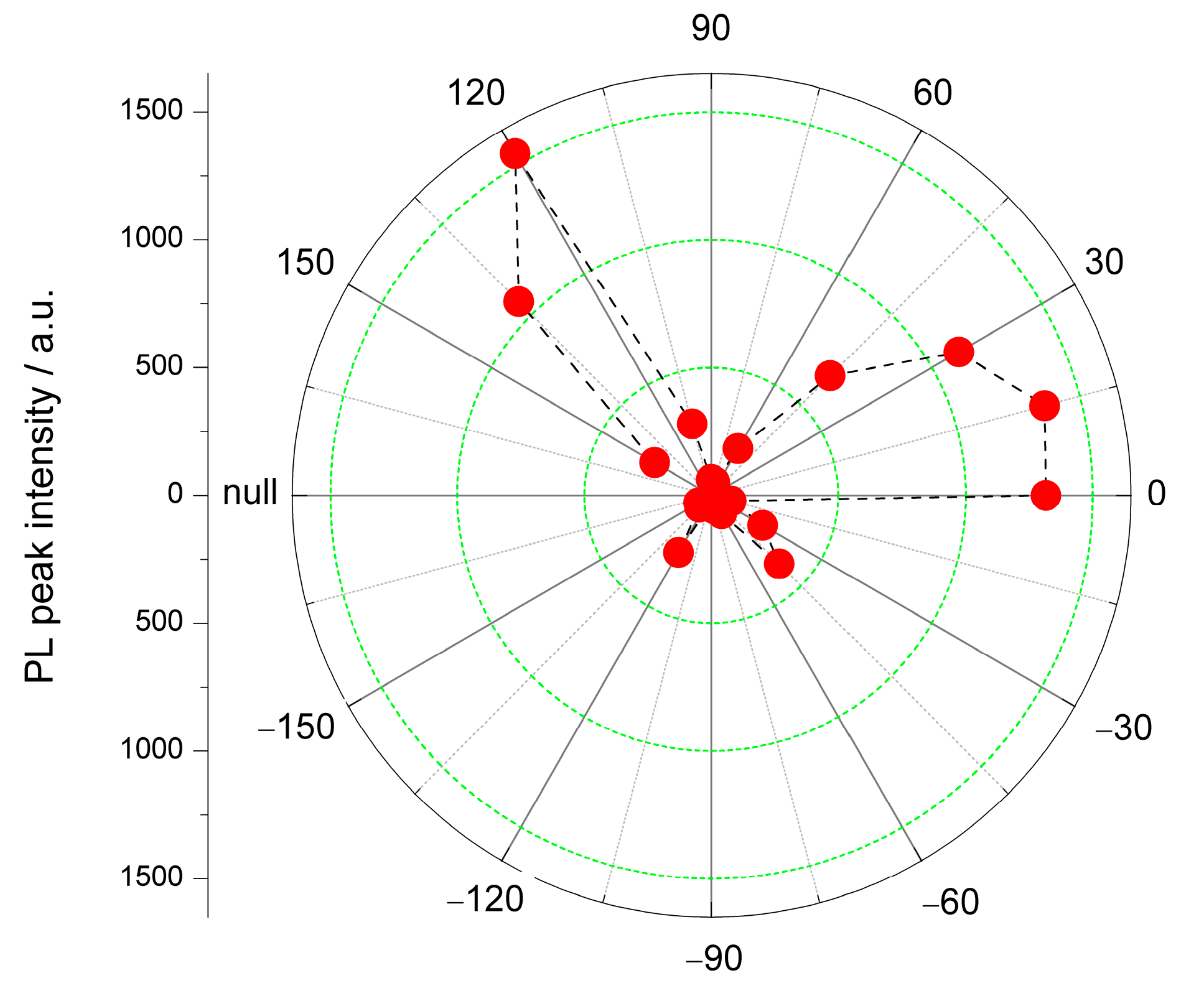
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, C.W.; Van Slyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Van Slyke, S.A.; Chen, C.H.; Tang, C.W. Organic electroluminescent devices with improved stability. Appl. Phys. Lett. 1996, 69, 2160–2162. [Google Scholar] [CrossRef]
- Naka, S.; Okada, H.; Onnagawa, H.; Yamaguchi, Y.; Tsutsui, T. Carrier transport properties of organic materials for EL device operation. Synth. Met. 2000, 111, 331–333. [Google Scholar] [CrossRef]
- Paydavosi, S.; Aidala, K.E.; Brown, P.R.; Hashemi, P.; Supran, G.J.; Osedach, T.P.; Hoyt, J.L.; Bulović, V. Detection of charge storage on molecular thin films of tris(8-hydroxyquinoline) aluminum (Alq3) by Kelvin force microscopy: A candidate system for high storage capacity memory cells. Nano Lett. 2012, 12, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Krieg, U.; Lichtenstein, T.; Brand, C.; Tegenkamp, C.; Pfnür, H. Origin of metallicity in atomic Ag wires on Si(557). New J. Phys. 2015, 17, 043062. [Google Scholar] [CrossRef]
- Lichtenstein, T.; Mamiyev, Z.; Braun, C.; Sanna, S.; Schmidt, W.G.; Tegenkamp, C.; Pfnür, H. Probing quasi-one-dimensional band structures by plasmon spectroscopy. Phys. Rev. B 2018, 97, 165421. [Google Scholar] [CrossRef] [Green Version]
- Sanna, S.; Lichtenstein, T.; Mamiyev, Z.; Tegenkamp, C.; Pfnür, H. How one-dimensional are atomic gold chains on a substrate? J. Phys. Chem. C 2018, 122, 25580–25588. [Google Scholar] [CrossRef]
- Raether, H. Surface plasmons on smooth surfaces. In Surface Plasmons on Smooth and Rough Surfaces and on Gratings; Springer Tracts in Modern Physics; Springer: Berlin/Heidelberg, Germany, 1988; pp. 4–39. [Google Scholar]
- Garcia, R.F.; Zeng, L.; Khadir, S.; Chakaroun, M.; Fischer, A.P.A.; Boudrioua, A. Enhanced electroluminescence of an organic light-emitting diode by localized surface plasmon using Al periodic structure. J. Opt. Soc. Am. B 2016, 33, 246–252. [Google Scholar] [CrossRef]
- Munkhbat, B.; Pohl, H.; Denk, P.; Klar, T.A.; Scharber, M.C.; Hrelescu, C. Performance boost of organic light-emitting diodes with plasmonic nanostars. Adv. Opt. Mater. 2016, 4, 772–781. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, T.W. Enhancement of the current efficiency of organic light-emitting devices due to the surface plasmonic resonance effect of dodecanethilol-functionalized Au nanoparticles. Org. Electron. 2016, 34, 270–274. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, R.; Mehta, D.S.; Kamalasanan, M.N. Sufcace plasmon enhanced blue organic light emitting diode with nearly 100% fluorescence efficiency. Org. Electron. 2012, 13, 1750–1755. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, D.H.; Yoo, K.H.; Kim, T.W. Efficiency enhancement of organic light-emitting devices due to the localized surface plasmonic resonant effect of Au nanoparticles embedded in ZnO nanoparticles. Appl. Phys. Lett. 2014, 105, 183303. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [Green Version]
- Hutter, E.; Fendler, J.H. Exploitation of localized surface plasmon resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Liu, F.; Rao, B.S.; Nunzi, J.-M. A dye functionalized silver-silica core-shell nanoparticle organic light emitting diode. Org. Electron. 2011, 12, 1279–1284. [Google Scholar] [CrossRef]
- Jung, J.S.; Lee, J.W.; Seo, M.R.; Lee, H.S.; Kim, J.; Lee, S.W.; Joo, J. Luminescence variation of organic Alq3 nanoparticles on surface of Au nanoparticles and graphene. Synth. Met. 2012, 162, 1852–1857. [Google Scholar] [CrossRef]
- Zou, S.-J.; Shen, Y.; Xie, F.-M.; Chen, J.-D.; Li, Y.-Q.; Tang, J.-X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Ditlbacher, H.; Hohenau, A.; Wagner, D.; Kreibig, U.; Rogers, M.; Hofer, F.; Aussenegg, F.R.; Krenn, J.R. Silver nanowires as surface plasmon resonators. Phys. Rev. Lett. 2005, 95, 257403. [Google Scholar] [CrossRef]
- Huang, T.-H.; Zhang, L.; Jiang, C.-Z.; Xu, T.-N.; Cai, C.-F.; Zhang, J.-R.; Liu, H.-Y. Plasmon enhanced optical properties of Ag nanowire decorated conjugated polymer. Opt. Mater. 2022, 126, 112214. [Google Scholar] [CrossRef]
- Masui, K.; Kiba, T.; Sato, A.; Yanome, K.; Kawamura, M.; Abe, Y.; Kim, K.H.; Kim, H.D.; Takayama, J.; Murayama, A. Photoluminescence enhancement of tris(8-hydroxyquinolinato)aluminum thin film by plasmonic Ag nanotriangle array fabricated by nanosphere lithography. Thin Solid Films 2018, 660, 907–912. [Google Scholar]
- Salah, N.; Habib, S.S.; Khan, Z.H. Highly luminescent material based on Alq3:Ag nanoparticles. J. Fluoresc. 2013, 23, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.B.; Khan, Z.H. Ag-incorporated Alq3 nanowires: Promising material for organic luminescent devices. J. Lumin. 2017, 188, 418–422. [Google Scholar] [CrossRef]
- Kiba, T.; Iijima, N.; Yanome, K.; Kawamura, M.; Abe, Y.; Kim, K.H.; Takase, M.; Higo, A.; Takayama, J.; Hiura, S.; et al. Emission enhancement of tris(8-quinolinolato)aluminum with Al nanotriangle arrays fabricated by nanosphere lithography. Appl. Surf. Sci. 2019, 478, 49–53. [Google Scholar] [CrossRef]
- Kiba, T.; Masui, K.; Inomata, Y.; Furumoto, A.; Kawamura, M.; Abe, Y.; Kim, K.H. Control of localized surface plasmon resonance of Ag nanoparticles by changing its size and morphology. Vacuum 2021, 192, 110432. [Google Scholar] [CrossRef]
- Huang, T.-H.; Chi, X.-C.; Xu, T.-N.; Zhang, J.-R.; Xu, H.-Y.; Zhu, Z.-Y.; Yu, R.-B.; Wang, Y.-H.; Zhang, H.-Z. Effect of Ag nanoparticles on the photoluminescence of poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylene-vinylene]. J. Photochem. Photobiol. A 2018, 356, 334–339. [Google Scholar] [CrossRef]
- Zong, R.-L.; Zhou, J.; Li, Q.; Du, B.; Li, B.; Fu, M.; Qi, X.-W.; Li, L.-T.; Buddhudu, S. Synthesis and optical properties of silver nanowire arrays embedded in anodic alumina membrane. J. Phys. Chem. B 2004, 108, 16713–16716. [Google Scholar] [CrossRef]
- Dong, S.H.; Wang, W.L.; Yin, S.W.; Li, C.Y.; Lu, J. Theoretical studies on charge transport character and optional properties of Alq3 and its difluorinated derivatives. Synth. Met. 2009, 159, 385–390. [Google Scholar] [CrossRef]
- Hoshi, T.; Kumagai, K.; Inoue, K.; Enomoto, S.; Nobe, Y.; Kobayashi, M. Electronic absorption and emission spectra of Alq3 in solution with special attention to a delayed fluorescence. J. Lumin. 2008, 128, 1353–1358. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Lin, X.F.; Qing, J.; Zhong, Z.F.; Ou, J.M.; Hu, C.L.; Chen, X.D.; Zhou, X.; Chen, Y.J. Enhancement of short-circuit current density in polymer bulk heterojunction solar cells comprising plasmonic silver nanowires. Appl. Phys. Lett. 2014, 104, 123302. [Google Scholar] [CrossRef]
- Zhang, W.K.; Chen, Y.J.; Gan, L.; Qing, J.; Zhou, X.; Huang, Y.Y.; Yang, Y.Z.; Zhang, Y.; Ou, J.M.; Chen, X.D.; et al. Localized surface plasmon resonance enhanced blue light-emission of polyfluorene copolymer. J. Phys. Chem. Solids 2014, 75, 1340–1346. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.-Y.; Gao, B.-R.; Jiang, Y.; Yang, Z.-Y.; Hao, Y.-W.; Chen, Q.-D.; Du, X.-B.; Sun, H.-B. Surface plasmon enhanced absorption dynamics of regioregular poly(3-hexylthiophene). Appl. Phys. Lett. 2011, 98, 251501. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, A.K.R. Characteristics of light sources. In Principles of Colour Appearance and Measurement; Woodhead Publishing Series in Textiles; Woodhead Publishing Limited: Cambridge, UK, 2014; Volume 1, pp. 1–52. [Google Scholar]
- Zawadzka, A.; Płóciennik, P.; Strzelecki, J.; Łukasiak, Z.; Sahraoui, B. Photophysical properties of Alq3 thin films. Opt. Mater. 2014, 36, 91–97. [Google Scholar] [CrossRef] [Green Version]
- da Silva, M.A.T.; Dias, I.F.L.; Duarte, J.L.; Laureto, E.; Silvestre, I.; Cury, L.A.; Guimarães, P.S.S. Identification of the optically active vibrational modes in the photoluminescence of MEH-PPV films. J. Chem. Phys. 2008, 128, 094902. [Google Scholar] [CrossRef] [PubMed]
- Leroux, M.; Grandjean, N.; Beaumont, B.; Nataf, G.; Semond, F.; Massies, J.; Gibart, P. Temperature quenching of photoluminescence intensities in undoped and doped GaN. J. Appl. Phys. 1999, 86, 3721–3728. [Google Scholar] [CrossRef]
- Li, J.; Ong, H.C. Temperature dependence of surface plasmon mediated emission from metal-capped ZnO films. Appl. Phys. Lett. 2008, 92, 121107. [Google Scholar] [CrossRef]
- Wang, Y.J.; He, H.P.; Zhang, Y.L.; Sun, L.W.; Hu, L.; Wu, K.W.; Huang, J.Y.; Ye, Z.Z. Metal enhanced photoluminescence from Al-capped ZnMgO films: The roles of plasmonic coupling and non-radiative recombination. Appl. Phys. Lett. 2012, 100, 112103. [Google Scholar] [CrossRef]
- Zhou, X.B.; Jiang, M.M.; Wu, Y.T.; Ma, K.J.; Liu, Y.; Wan, P.; Kan, C.X.; Shi, D.N. Hybrid quadrupole plasmon induced spectrally pure ultraviolet emission from a single AgNPs@ZnO:Ga microwire based heterojunction diode. Nanoscale Adv. 2020, 2, 1340–1351. [Google Scholar] [CrossRef]
- Wedge, S.; Hooper, I.R.; Sage, I.; Barnes, W.L. Light emission through a corrugated metal film: The role of cross-coupled surface plasmon polaritons. Phys. Rev. B 2004, 69, 245418. [Google Scholar] [CrossRef] [Green Version]
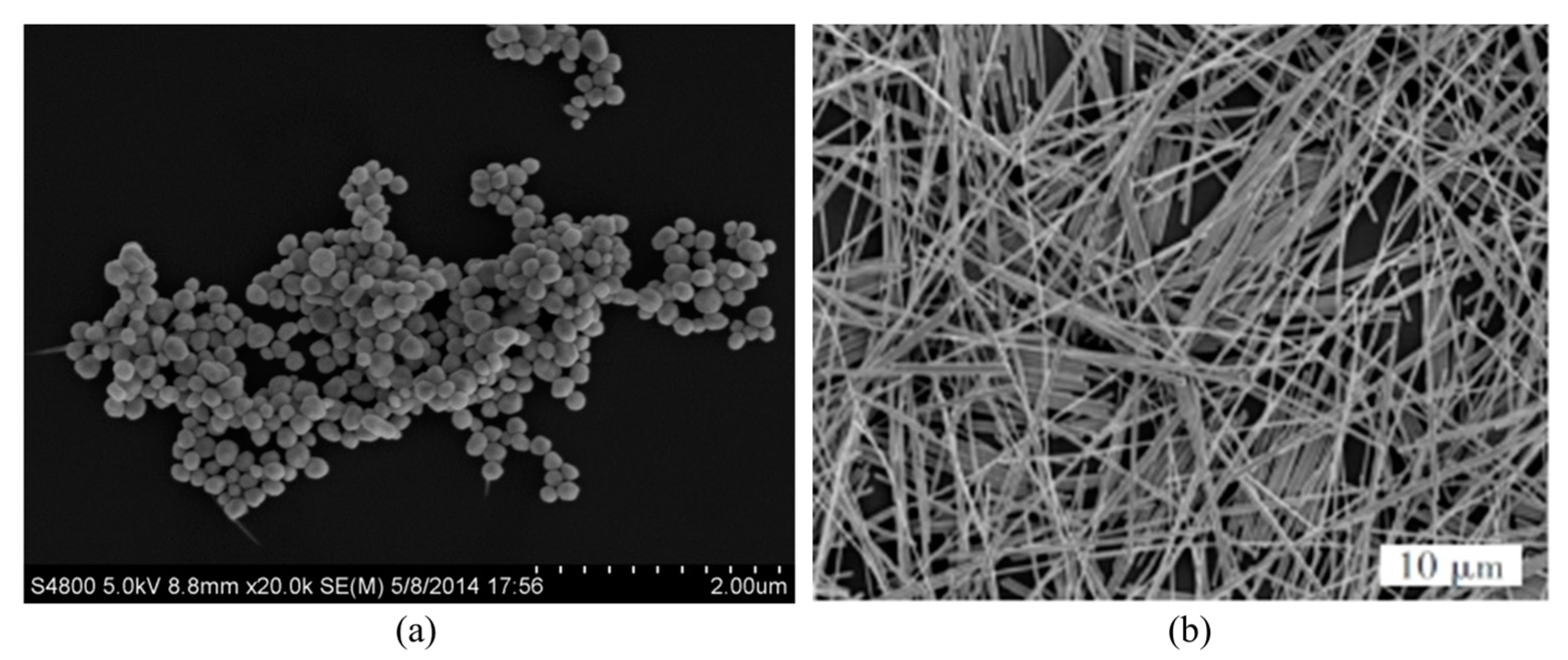
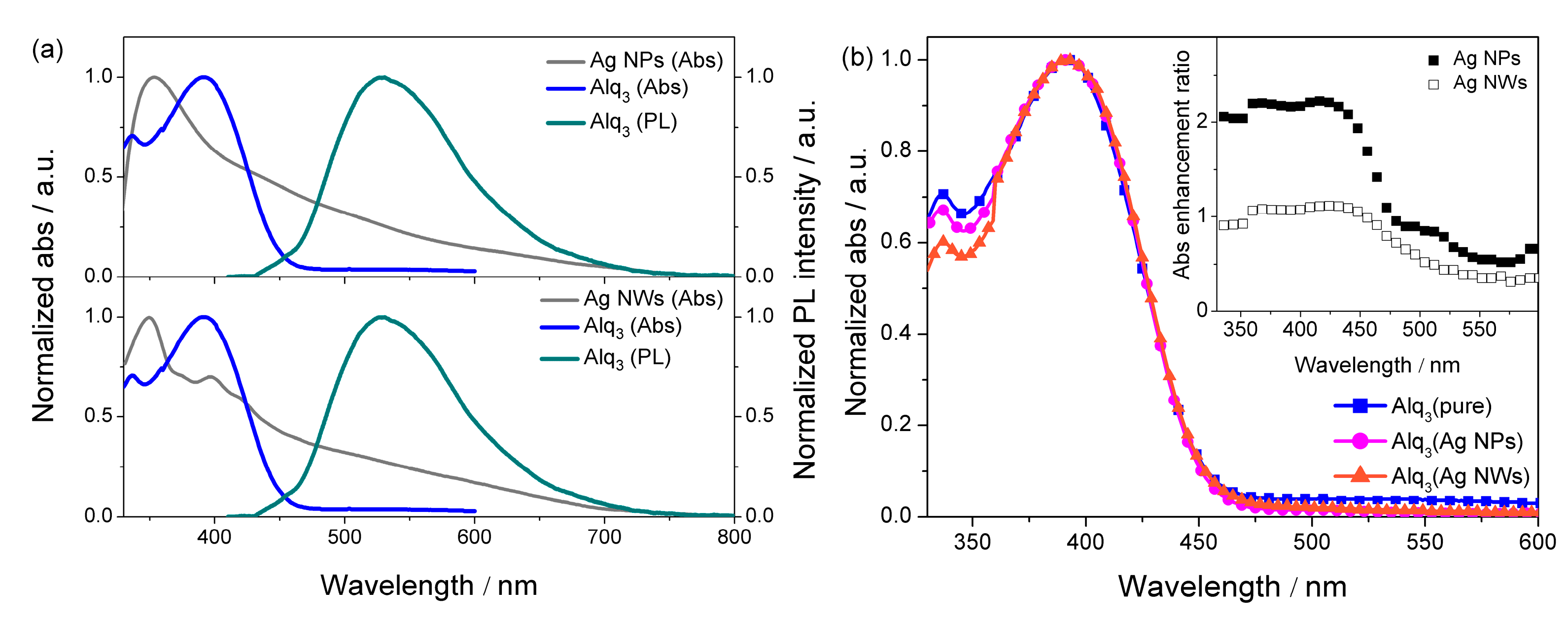
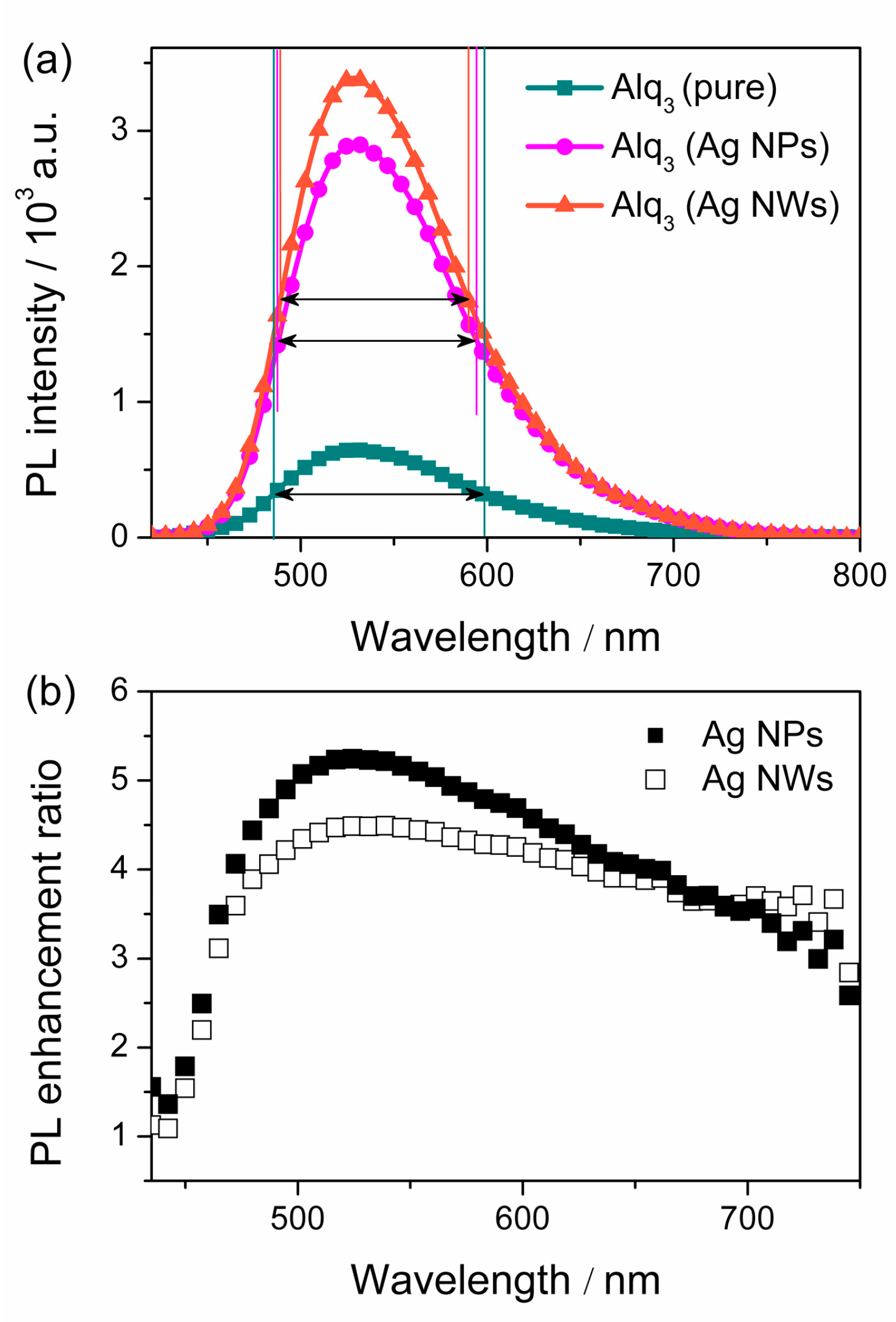
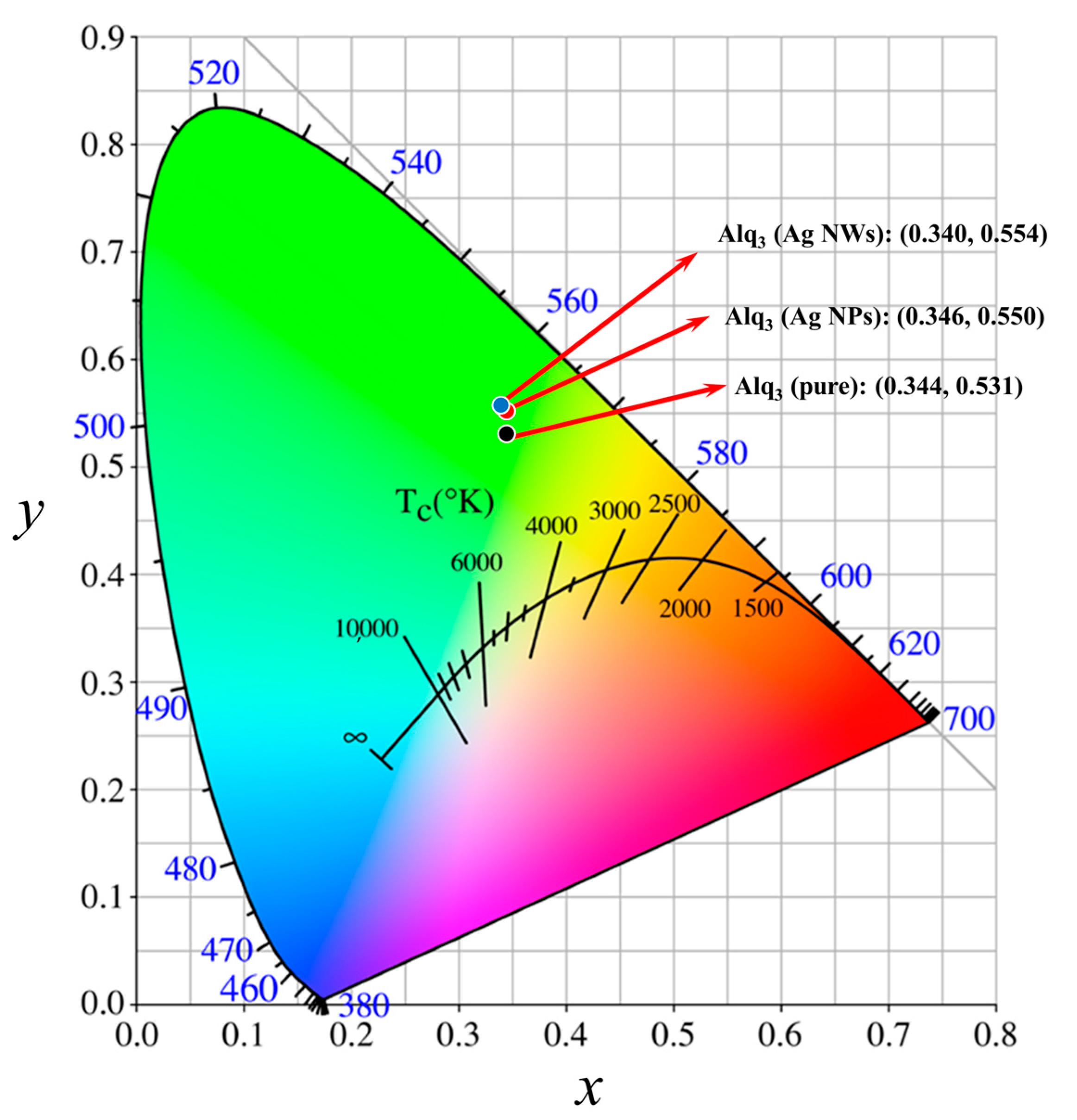

| Sample | λabs/nm | λPL/nm | FWHM/nm |
|---|---|---|---|
| Alq3 (pure) | 336 391 | 529 | 112 |
| Alq3 (Ag NPs) | 336 391 | 529 | 107 |
| Alq3 (Ag NWs) | 336 391 | 529 | 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.-H.; Jiang, C.-Z.; Xu, T.-N.; Tian, Z.-Y. Promising Photoluminescence Enhancement of Tris(8-hydroxyquinoline)aluminum by Simultaneous Localized and Propagating Surface Plasmons of Ag Nanostructures. Appl. Sci. 2023, 13, 3786. https://doi.org/10.3390/app13063786
Huang T-H, Jiang C-Z, Xu T-N, Tian Z-Y. Promising Photoluminescence Enhancement of Tris(8-hydroxyquinoline)aluminum by Simultaneous Localized and Propagating Surface Plasmons of Ag Nanostructures. Applied Sciences. 2023; 13(6):3786. https://doi.org/10.3390/app13063786
Chicago/Turabian StyleHuang, Tian-Hao, Cheng-Zi Jiang, Tian-Ning Xu, and Zhen-Yu Tian. 2023. "Promising Photoluminescence Enhancement of Tris(8-hydroxyquinoline)aluminum by Simultaneous Localized and Propagating Surface Plasmons of Ag Nanostructures" Applied Sciences 13, no. 6: 3786. https://doi.org/10.3390/app13063786





