Connections between Metallic Nanoparticles and Chlorin e6—An Overview of Physicochemical and Biological Properties and Prospective Medical Applications
Abstract
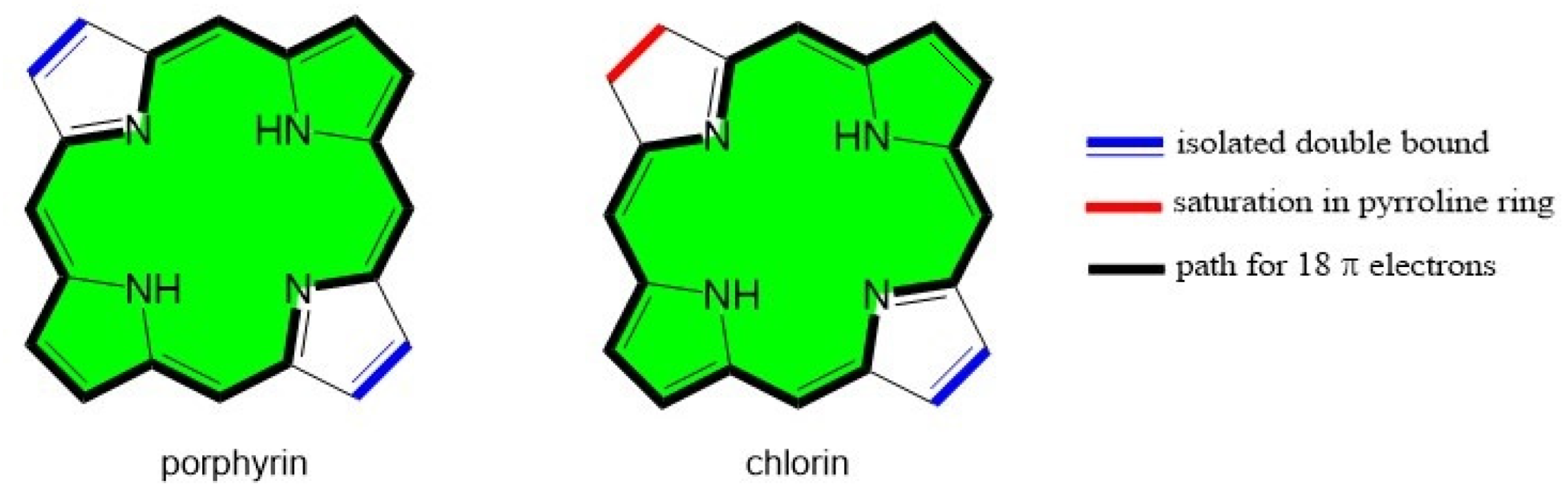
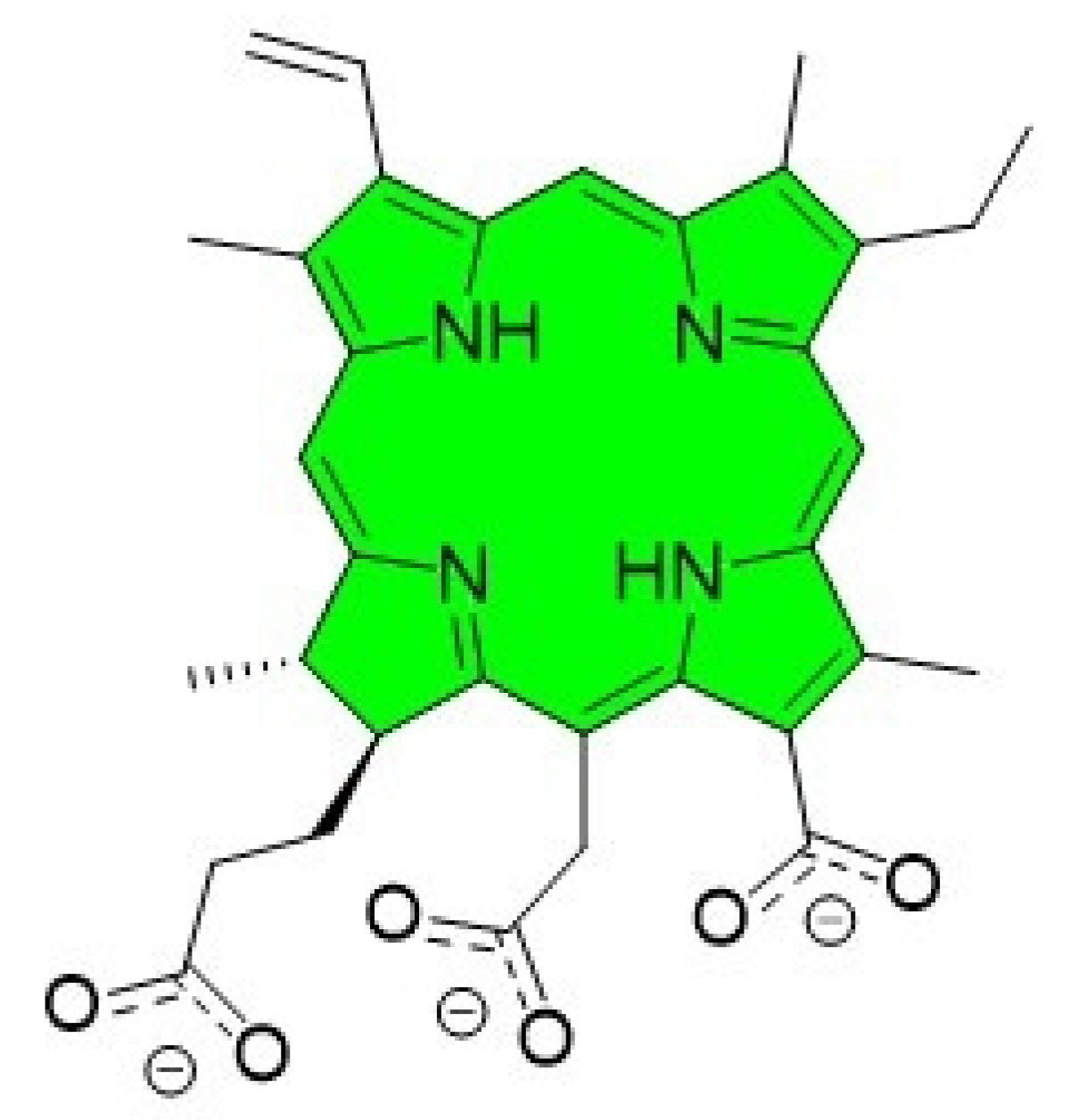
:1. Introduction
2. Metallic Nanoparticles in Conjugation with Chlorin Derivatives
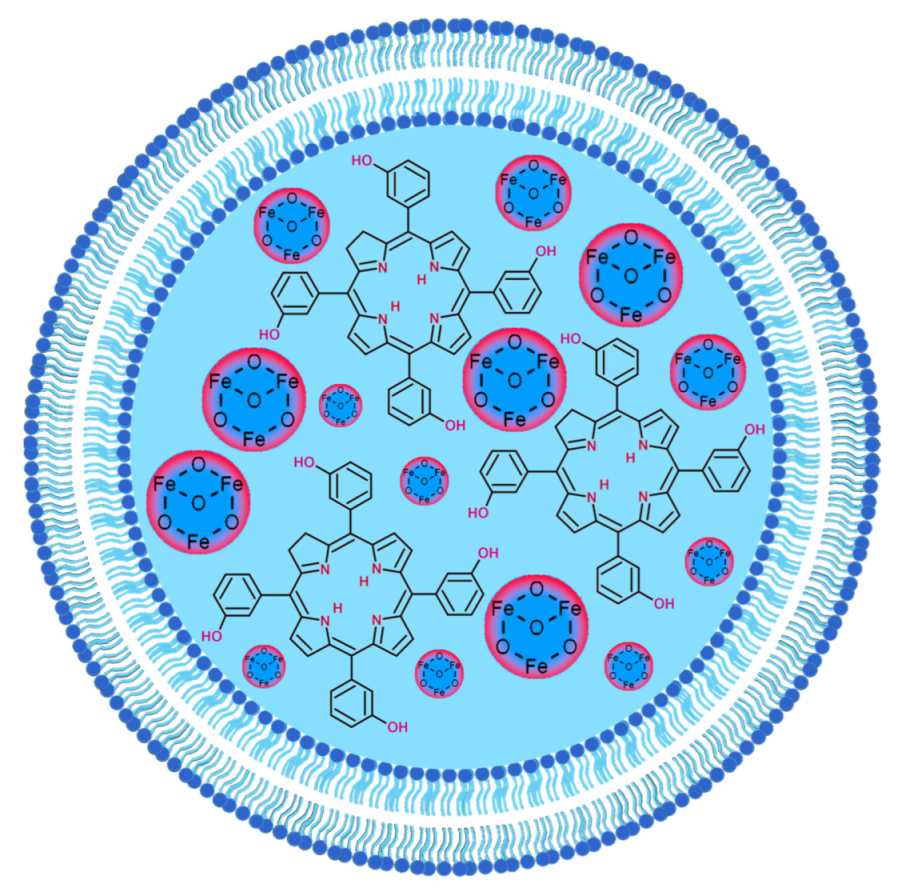
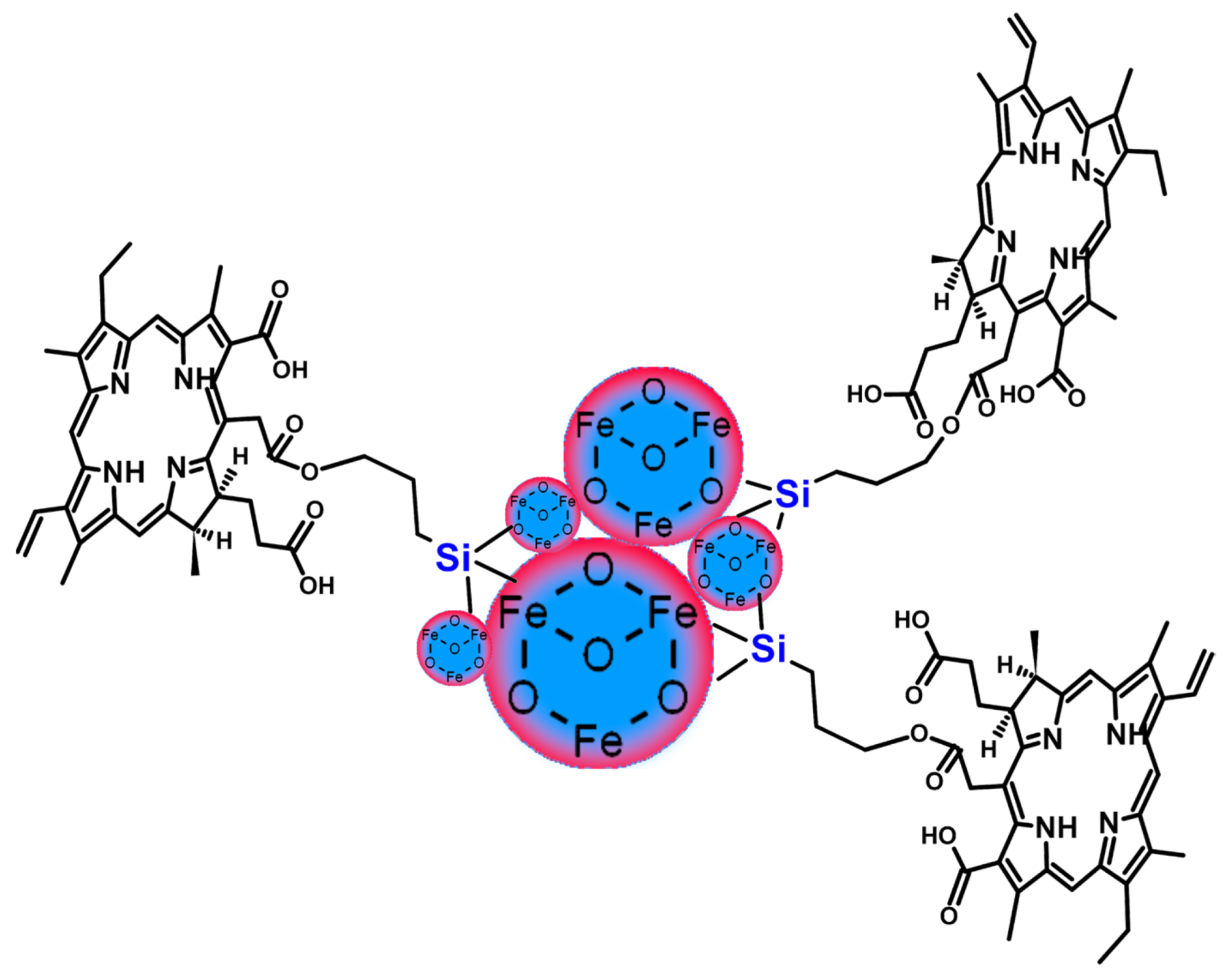
2.1. Iron Oxide and Other Magnetic Nanoparticles
- Most magnetic nanoparticles used were Fe3O4 conjugated with Ce6 via silica or dextran coatings or incorporated into a polymer structure.
- The conjugates of chlorins and magnetic nanoparticles allowed for efficient combined magnetothermal–photodynamic treatment after laser irradiation at 660 and 808 nm.
- The promising theranostic features of the Fe3O4-chlorin conjugates can be used for photodynamic therapy and MRI or fluorescent imaging.
- Different types of connections in the obtained conjugates were used, including core-shell structures, liposomes, polymeric nanoclusters, and covalent bonding.
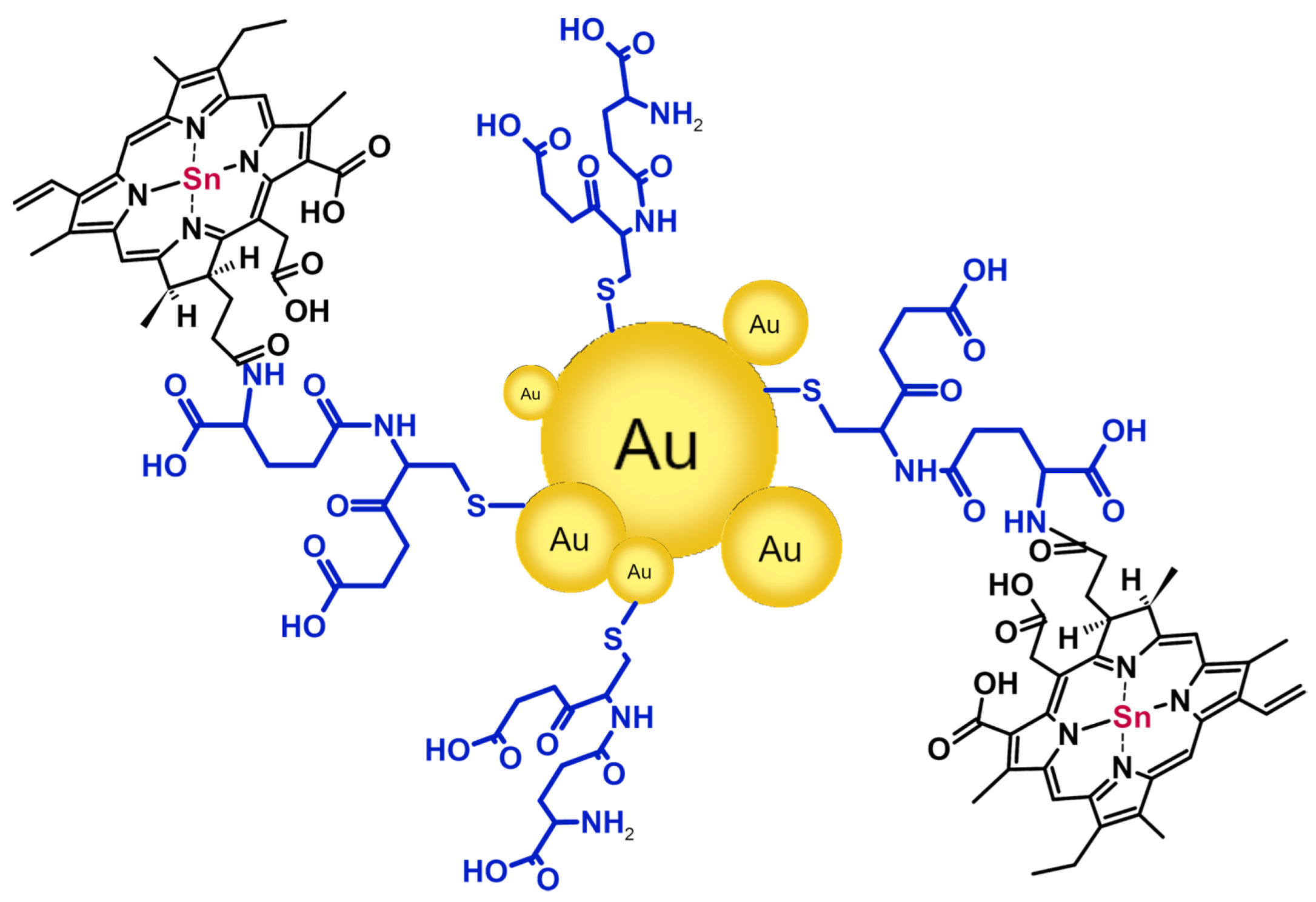
2.2. Gold Nanoparticles
- Gold nanoparticle@chlorin conjugates were studied regarding the PRET phenomenon and photothermal and photodynamic effects to assess their prospective biomedical applications.
- For Ce6, the laser irradiation wavelengths used for PDT purposes were in the range of 633–671 nm due to the different structures of obtained conjugates.
- Most conjugates between Ce6 and nanogold were based on covalent bonding via various molecules, such as glutathione, thiourea, oligonucleotide, and 3-mercaptopropionic acid.
- Gold nanoparticles were mainly synthesized via the chemical reduction of HAuCl4 by sodium citrate (the Turkevich method) or NaBH4.
- Depending on the synthetic procedure, various types of gold nanoparticles, including nanorods, nanocups, and nanoflowers, were obtained.
- Polymer coating of the gold nanoparticles allowed for the formation of inclusion complexes with Ce6.
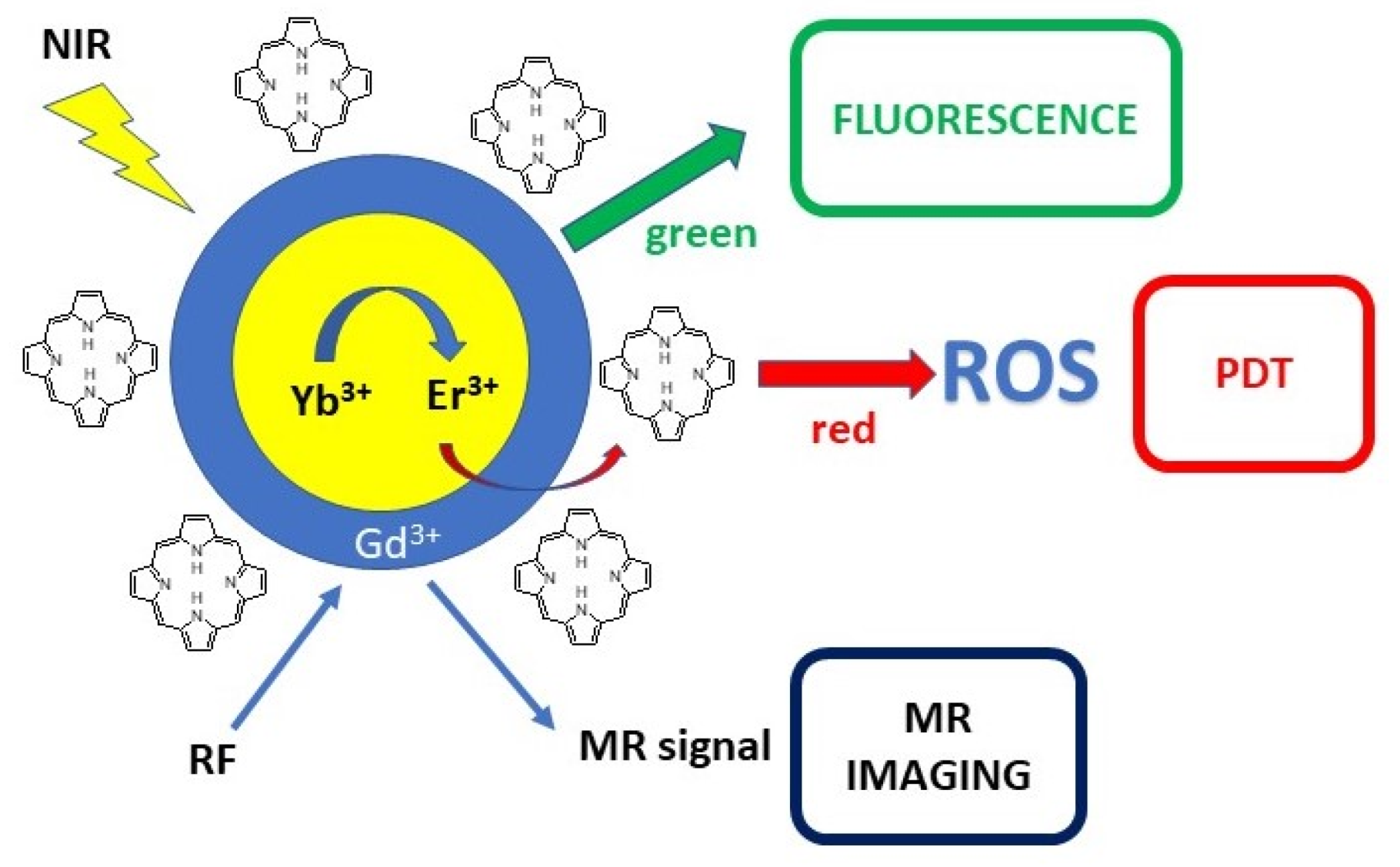
2.3. Upconverted Nanoparticles
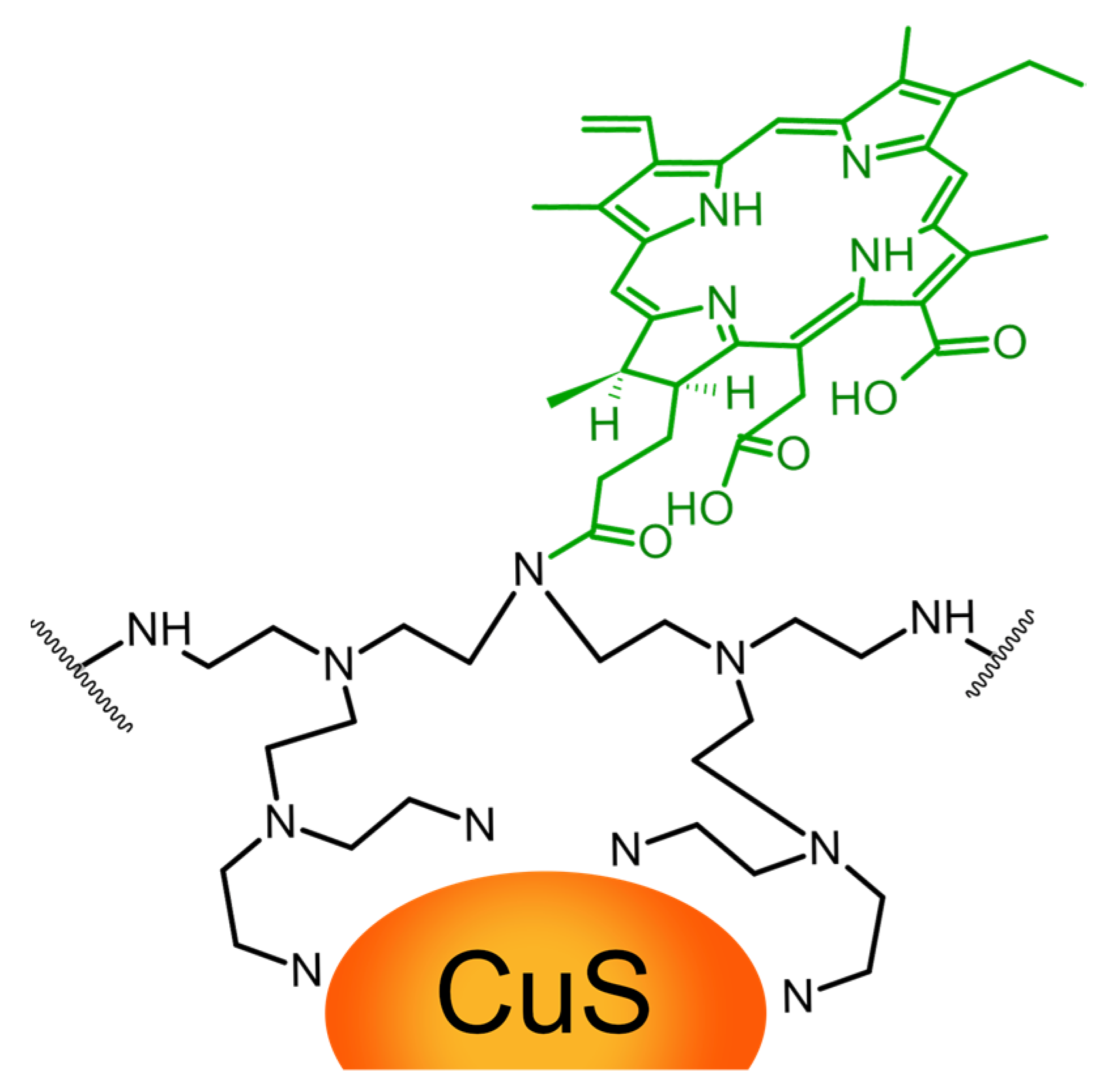
2.4. Other Metallic Nanoparticles
2.5. Carbon-Based Nanomaterials
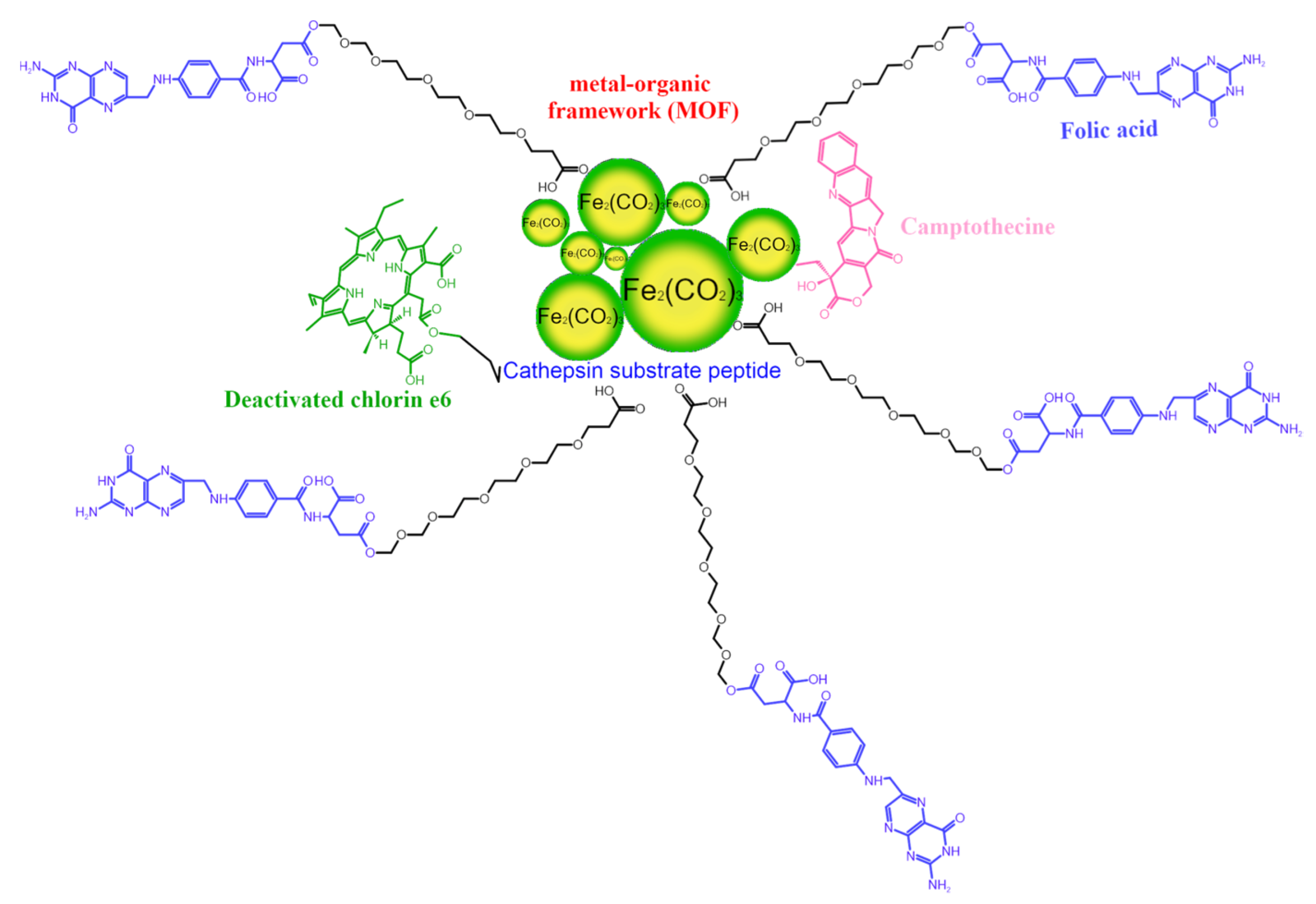
2.6. Metal-Organic Frameworks
2.7. Polymer-Based Nanoparticles
2.8. Bacteriochlorins
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in Photosensitizers and Light Delivery for Photodynamic Therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Borbas, K.E.; Chandrashaker, V.; Muthiah, C.; Hooi, L.K.; Holten, D.; Lindsey, J.S. Design, Synthesis, and Photophysical Characterization of Water-Soluble Chlorins. J. Org. Chem. 2008, 73, 3145–3158. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic Therapy (PDT) of Cancer: From Local to Systemic Treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.S.; Gomes, M.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Cavaleiro, J.A.S.; Almeida, A.; Tomé, J.P.C. Comparative Photodynamic Inactivation of Antibiotic Resistant Bacteria by First and Second Generation Cationic Photosensitizers. Photochem. Photobiol. Sci. 2012, 11, 1905–1913. [Google Scholar] [CrossRef]
- Yue, L.; Zheng, M.; Khan, I.M.; Wang, Z. Chlorin E6 Conjugated Chitosan as an Efficient Photoantimicrobial Agent. Int. J. Biol. Macromol. 2021, 183, 1309–1316. [Google Scholar] [CrossRef]
- Glowacka-Sobotta, A.; Ziental, D.; Sobotta, L. Chapter 12. Porphyrinoids Used for Photodynamic Inactivation against Bacteria. In Applications of Porphyrinoids as Functional Materials; Lang, H., Rueffer, T., Eds.; Smart Materials; Royal Society of Chemistry: Cambridge, UK, 2021; pp. 352–404. ISBN 978-1-83916-188-9. [Google Scholar]
- Nishie, H.; Kataoka, H.; Yano, S.; Yamaguchi, H.; Nomoto, A.; Tanaka, M.; Kato, A.; Shimura, T.; Mizoshita, T.; Kubota, E.; et al. Excellent Antitumor Effects for Gastrointestinal Cancers Using Photodynamic Therapy with a Novel Glucose Conjugated Chlorin E6. Biochem. Biophys. Res. Commun. 2018, 496, 1204–1209. [Google Scholar] [CrossRef]
- Martynenko, I.V.; Kuznetsova, V.A.; Orlova, O.; Kanaev, P.A.; Maslov, V.G.; Loudon, A.; Zaharov, V.; Parfenov, P.; Gun’Ko, Y.K.; Baranov, A.V.; et al. Chlorin E6-ZnSe/ZnS Quantum Dots Based System as Reagent for Photodynamic Therapy. Nanotechnology 2015, 26, 55102. [Google Scholar] [CrossRef]
- Alea-Reyes, M.E.; Soriano, J.; Mora-Espí, I.; Rodrigues, M.; Russell, D.A.; Barrios, L.; Pérez-García, L. Amphiphilic Gemini Pyridinium-Mediated Incorporation of Zn(II)Meso-Tetrakis(4-Carboxyphenyl)Porphyrin into Water-Soluble Gold Nanoparticles for Photodynamic Therapy. Colloids Surf. B Biointerfaces 2017, 158, 602–609. [Google Scholar] [CrossRef]
- Homayoni, H.; Ma, L.; Zhang, J.; Sahi, S.K.; Rashidi, L.H.; Bui, B.; Chen, W. Synthesis and Conjugation of Sr2MgSi2O7:Eu2+, Dy3+ Water Soluble Afterglow Nanoparticles for Photodynamic Activation. Photodiagnosis Photodyn. Ther. 2016, 16, 90–99. [Google Scholar] [CrossRef]
- Alea-Reyes, M.E.; Rodrigues, M.; Serrà, A.; Mora, M.; Sagristá, M.L.; González, A.; Durán, S.; Duch, M.; Plaza, J.A.; Vallés, E.; et al. Nanostructured Materials for Photodynamic Therapy: Synthesis, Characterization and in Vitro Activity. RSC Adv. 2017, 7, 16963–16976. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, D.C.; Monteiro, C.S.; Chaves, C.R.; Sáfar, G.A.M.; Moreira, R.L.; Pinheiro, M.V.B.; Martins, D.C.S.; Ladeira, L.O.; Krambrock, K. Hybrid Systems Based on Gold Nanostructures and Porphyrins as Promising Photosensitizers for Photodynamic Therapy. Colloids Surf. B Biointerfaces 2017, 150, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Chen, P.C.; Prasannan, A.; Vinayagam, J.; Huang, C.C.; Chou, P.Y.; Weng, C.C.; Tsai, H.C.; Lin, S.Y. Formation of Gold Decorated Porphyrin Nanoparticles and Evaluation of Their Photothermal and Photodynamic Activity. Mater. Sci. Eng. C 2016, 63, 678–685. [Google Scholar] [CrossRef]
- Koczorowski, T.; Glowacka-Sobotta, A.; Sysak, S.; Mlynarczyk, D.T.; Lesyk, R.; Goslinski, T.; Sobotta, L. BODIPY-Based Nanomaterials-Sensing and Biomedical Applications. Appl. Sci. Switz. 2022, 7815. [Google Scholar] [CrossRef]
- Di Corato, R.; Béalle, G.; Kolosnjaj-Tabi, J.; Espinosa, A.; Clément, O.; Silva, A.K.A.; Ménager, C.; Wilhelm, C. Combining Magnetic Hyperthermia and Photodynamic Therapy for Tumor Ablation with Photoresponsive Magnetic Liposomes. ACS Nano 2015, 9, 2904–2916. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Li, Z.; Lin, J.; Yang, D.; Gao, G.; Xu, C.; Bao, L.; Zhang, C.; Wang, K.; Song, H.; et al. Photosensitizer-Conjugated Magnetic Nanoparticles for in Vivo Simultaneous Magnetofluorescent Imaging and Targeting Therapy. Biomaterials 2011, 32, 3447–3458. [Google Scholar] [CrossRef]
- Amirshaghaghi, A.; Yan, L.; Miller, J.; Daniel, Y.; Stein, J.M.; Busch, T.M.; Cheng, Z.; Tsourkas, A. Chlorin E6-Coated Superparamagnetic Iron Oxide Nanoparticle (SPION) Nanoclusters as a Theranostic Agent for Dual-Mode Imaging and Photodynamic Therapy. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.Y.; Fu, Y.; Li, Y.; Jang, M.S.; Lee, J.H.; Lee, D.S. Polymer Ligand-Assisted Fabrication of Multifunctional and Redox-Responsive Self-Assembled Magnetic Nanoclusters for Bimodal Imaging and Cancer Treatment. J. Mater. Chem. B 2018, 6, 5562–5569. [Google Scholar] [CrossRef]
- Yu, T.T.; Peng, X.C.; Wang, M.F.; Han, N.; Xu, H.Z.; Li, Q.R.; Li, L.G.; Xu, X.; Ma, Q.L.; Liu, B.; et al. Harnessing Chlorin E6 Loaded by Functionalized Iron Oxide Nanoparticles Linked with Glucose for Target Photodynamic Therapy and Improving of the Immunogenicity of Lung Cancer. J. Cancer Res. Clin. Oncol. 2022, 148, 867–879. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Korngold, E.; Weissleder, R.; Jaffer, F.A. A Light-Activated Theranostic Nanoagent for Targeted Macrophage Ablation in Inflammatory Atherosclerosis. Small 2010, 6, 2041–2049. [Google Scholar] [CrossRef] [Green Version]
- Işıklan, N.; Hussien, N.A.; Türk, M. Multifunctional Aptamer-Conjugated Magnetite Graphene Oxide/Chlorin E6 Nanocomposite for Combined Chemo-Phototherapy. Colloids Surf. Physicochem. Eng. Asp. 2022, 645. [Google Scholar] [CrossRef]
- Hu, H.; Li, R.; Huang, P.; Mo, Z.; Xu, Q.; Hu, T.; Yao, S.; Dai, X.; Xu, Z. BSA-Coated β-FeOOH Nanoparticles Efficiently Deliver the Photosensitizer Chlorin E6 for Synergistic Anticancer PDT/CDT. Colloids Surf. B Biointerfaces 2023, 222, 1–10. [Google Scholar] [CrossRef]
- Ling, D.; Park, W.; Park, S.J.; Lu, Y.; Kim, K.S.; Hackett, M.J.; Kim, B.H.; Yim, H.; Jeon, Y.S.; Na, K.; et al. Multifunctional Tumor PH-Sensitive Self-Assembled Nanoparticles for Bimodal Imaging and Treatment of Resistant Heterogeneous Tumors. J. Am. Chem. Soc. 2014, 136, 5647–5655. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, J.; Li, Y.; Hu, T.; Fan, L.; Xi, J.; Han, J.; Guo, R. Yolk-Shell Fe3O4@Carbon@Platinum-Chlorin E6 Nanozyme for MRI-Assisted Synergistic Catalytic-Photodynamic-Photothermal Tumor Therapy. J. Colloid Interface Sci. 2022, 628, 1033–1043. [Google Scholar] [CrossRef]
- Magro, M.; Bramuzzo, S.; Baratella, D.; Ugolotti, J.; Zoppellaro, G.; Chemello, G.; Olivotto, I.; Ballarin, C.; Radaelli, G.; Arcaro, B.; et al. Self-Assembly of Chlorin-E6 on γ-Fe 2 O 3 Nanoparticles: Application for Larvicidal Activity against Aedes Aegypti. J. Photochem. Photobiol. B 2019, 194, 21–31. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Fan, H.; Yan, J.; Meng, D.; Hou, S.; Ji, Y.; Wu, X. Formation of Plasmon Quenching Dips Greatly Enhances 1O2 Generation in a Chlorin E6–Gold Nanorod Coupled System. Nano Res. 2018, 11, 1456–1469. [Google Scholar] [CrossRef]
- Tian, S.; He, J.; Lyu, D.; Li, S.; Xu, Q.H. Aggregation Enhanced Photoactivity of Photosensitizer Conjugated Metal Nanoparticles for Multimodal Imaging and Synergistic Phototherapy below Skin Tolerance Threshold. Nano Today 2022, 45, 101534. [Google Scholar] [CrossRef]
- Gao, F.; Sun, M.; Xu, L.; Liu, L.; Kuang, H.; Xu, C. Biocompatible Cup-Shaped Nanocrystal with Ultrahigh Photothermal Efficiency as Tumor Therapeutic Agent. Adv. Funct. Mater. 2017, 27, 1–6. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Y.; Wu, Y.; Song, D.; Qian, J.; Zhu, B. Chlorin E6 and Polydopamine Modified Gold Nanoflowers for Combined Photothermal and Photodynamic Therapy. J. Mater. Chem. B 2020, 8, 2128–2138. [Google Scholar] [CrossRef]
- Gil-Tomás, J.; Dekker, L.; Narband, N.; Parkin, I.P.; Nair, S.P.; Street, C.; Wilson, M. Lethal Photosensitisation of Bacteria Using a Tin Chlorin E6-Glutathione-Gold Nanoparticle Conjugate. J. Mater. Chem. 2011, 21, 4189–4196. [Google Scholar] [CrossRef]
- Vieira, L.; Castilho, M.L.; Ferreira, I.; Ferreira-Strixino, J.; Hewitt, K.C.; Raniero, L. Synthesis and Characterization of Gold Nanostructured Chorin E6 for Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2017, 18, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Xu, L.; Ma, W.; Wu, X.; Kuang, H.; Wang, L.; Xu, C. Hierarchical Plasmonic Nanorods and Upconversion Core-Satellite Nanoassemblies for Multimodal Imaging-Guided Combination Phototherapy. Adv. Mater. 2016, 28, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.L.; Jesus, V.P.S.; Vieira, P.F.A.; Hewitt, K.C.; Raniero, L. Chlorin E6-EGF Conjugated Gold Nanoparticles as a Nanomedicine Based Therapeutic Agent for Triple Negative Breast Cancer. Photodiagnosis Photodyn. Ther. 2021, 33, 102186. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.L.L.; Cheah, J.U.J.; Neo, D.J.H.; Goh, W.I.; Kanchanawong, P.; Soo, K.C.; Thong, P.S.P.; Kah, J.C.Y. Exploiting the Protein Corona around Gold Nanorods for Low-Dose Combined Photothermal and Photodynamic Therapy. J. Mater. Chem. B 2017, 5, 254–268. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Lin, J.; Wang, S.; Zhou, Z.; Li, Z.; Wang, Z.; Zhang, C.; Yue, X.; Niu, G.; Yang, M.; et al. Photosensitizer-Conjugated Silica-Coated Gold Nanoclusters for Fluorescence Imaging-Guided Photodynamic Therapy. Biomaterials 2013, 34, 4643–4654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Guo, F.; Wang, J.; Tan, F.; Li, N. Photosensitizer-Loaded PH-Responsive Hollow Gold Nanospheres for Single Light-Induced Photothermal/Photodynamic Therapy. ACS Appl. Mater. Interfaces 2015, 7, 17592–17597. [Google Scholar] [CrossRef]
- Yu, M.; Guo, F.; Wang, J.; Tan, F.; Li, N. A PH-Driven and Photoresponsive Nanocarrier: Remotely-Controlled by near-Infrared Light for Stepwise Antitumor Treatment. Biomaterials 2016, 79, 25–35. [Google Scholar] [CrossRef]
- Haimov, E.; Weitman, H.; Polani, S.; Schori, H.; Zitoun, D.; Shefi, O. Meso-Tetrahydroxyphenylchlorin-Conjugated Gold Nanoparticles as a Tool to Improve Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 2319–2327. [Google Scholar] [CrossRef]
- Li, B.; Fu, Y.; Xie, M.; Feng, L.; Niu, X.; Que, L.; You, Z. Gold-Based Nanoparticles Realize Photothermal and Photodynamic Synergistic Treatment of Liver Cancer and Improve the Anaerobic Tumor Microenvironment under near-Infrared Light. Front. Bioeng. Biotechnol. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Magalhães, J.A.; Fernandes, A.U.; Junqueira, H.C.; Nines, B.C.; Cursino, T.A.F.; Formaggio, D.M.D.; Baptista, M.S.; Tada, D.B. Bimetallic Nanoparticles Enhance Photoactivity of Conjugated Photosensitizer. Nanotechnology 2020, 31, 095102. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Liu, Y.; Zhang, J.; Bao, C.; Liang, S.; Wang, Q.; Yang, Y.; Fu, H.; Wang, K.; et al. Gold Nanoclusters-Based Nanoprobes for Simultaneous Fluorescence Imaging and Targeted Photodynamic Therapy with Superior Penetration and Retention Behavior in Tumors. Adv. Funct. Mater. 2015, 25, 1314–1325. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Cheng, K.; Xuan, Y.; Yang, X.Q.; An, J.; Hu, Y.G.; Liu, B.; Zhao, Y. Di A PH/Ultrasonic Dual-Response Step-Targeting Enterosoluble Granule for Combined Sonodynamic-Chemotherapy Guided: Via Gastrointestinal Tract Imaging in Orthotopic Colorectal Cancer. Nanoscale 2021, 13, 4278–4294. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, M.; Czarczynska-Goslinska, B.; Ziental, D.; Michalak, M.; Güzel, E.; Sobotta, L. Excited State and Reactive Oxygen Species against Cancer and Pathogens: A Review on Sonodynamic and Sono-Photodynamic Therapy. ChemMedChem 2022, 17. [Google Scholar] [CrossRef]
- Wijesiri, N.; Ozkaya-Ahmadov, T.; Wang, P.; Zhang, J.; Tang, H.; Yu, X.; Ayres, N.; Zhang, P. Photodynamic Inactivation of Multidrug-Resistant Staphylococcus Aureus Using Hybrid Photosensitizers Based on Amphiphilic Block Copolymer-Functionalized Gold Nanoparticles. ACS Omega 2017, 2, 5364–5369. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, J.; Yin, M.; Ren, J.; Miyoshi, D.; Sugimoto, N.; Qu, X. Reduced Graphene Oxide Upconversion Nanoparticle Hybrid for Electrochemiluminescent Sensing of a Prognostic Indicator in Early-Stage Cancer. Small 2014, 10, 330–336. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Drug Delivery with Upconversion Nanoparticles for Multi-Functional Targeted Cancer Cell Imaging and Therapy. Biomaterials 2011, 32, 1110–1120. [Google Scholar] [CrossRef]
- Park, Y.I.; Kim, H.M.; Kim, J.H.; Moon, K.C.; Yoo, B.; Lee, K.T.; Lee, N.; Choi, Y.; Park, W.; Ling, D.; et al. Theranostic Probe Based on Lanthanide-Doped Nanoparticles for Simultaneous in Vivo Dual-Modal Imaging and Photodynamic Therapy. Adv. Mater. 2012, 24, 5755–5761. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Zhang, M.; Wu, F.; Zheng, T.; Sheng, B.; Liu, Y.; Shen, J.; Zhou, N.; Sun, Y. A Theranostic Nanocomposite with Integrated Black Phosphorus Nanosheet, Fe3O4@MnO2-Doped Upconversion Nanoparticles and Chlorin for Simultaneous Multimodal Imaging, Highly Efficient Photodynamic and Photothermal Therapy. Chem. Eng. J. 2020, 391, 123525. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Manivasagan, P.; Moorthy, M.S.; Bui, N.Q.; Lee, K.D.; Oh, J. Chlorin E6 Conjugated Copper Sulfide Nanoparticles for Photodynamic Combined Photothermal Therapy. Photodiagnosis Photodyn. Ther. 2017, 19, 128–134. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, L.; Wang, D.; Lin, M.; Sun, H.; Zhang, H.; Sun, H.; Yang, B. Hollow Pd Nanospheres Conjugated with Ce6 to Simultaneously Realize Photodynamic and Photothermal Therapy. ACS Appl. Bio Mater. 2018, 1, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Huang, W.W.; Dehvari, K.; Ling, Y.C.; Ghule, A.V.; Tsai, S.L.; Chang, J.Y. Photosensitizer–Conjugated Cu-In-S Heterostructured Nanorods for Cancer Targeted Photothermal/Photodynamic Synergistic Therapy. Mater. Sci. Eng. C 2019, 97, 793–802. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, J.; Zhu, W.; Dong, Z.; Liu, Z.; Cheng, L. Albumin-Assisted Synthesis of Ultrasmall FeS2 Nanodots for Imaging-Guided Photothermal Enhanced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, L.; Hou, M.; Chen, Q.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Phase-Change Material Packaged within Hollow Copper Sulfide Nanoparticles Carrying Doxorubicin and Chlorin E6 for Fluorescence-Guided Trimodal Therapy of Cancer. ACS Appl. Mater. Interfaces 2019, 11, 417–429. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Cui, W.; Gong, H.; Liang, C.; Shi, X.; Li, Z.; Sun, B.; Liu, Z. Combined Photothermal and Photodynamic Therapy Delivered by PEGylated MoS2nanosheets. Nanoscale 2014, 6, 11219–11225. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hou, M.; Zhang, L.; Qian, D.; Yang, Q.; Xu, Z.; Kang, Y.; Xue, P. PEGylated Mesoporous Bi2S3 Nanostars Loaded with Chlorin E6 and Doxorubicin for Fluorescence/CT Imaging-Guided Multimodal Therapy of Cancer. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 1–12. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Lin, W. A Chlorin-Based Nanoscale Metal-Organic Framework for Photodynamic Therapy of Colon Cancers. J. Am. Chem. Soc. 2015, 137, 7600–7603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Luo, L.; Zeng, L.; Xing, J.; Xia, Y.; Sun, S.; Zhang, L.; Yu, Z.; Yao, J.; Yu, Z.; et al. Porous Gold Nanoshells on Functional NH2-MOFs: Facile Synthesis and Designable Platforms for Cancer Multiple Therapy. Small 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Halliwell, B.; Clement, M.V.; Long, L.H. Hydrogen Peroxide in the Human Body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Youssef, Z.; Jouan-Hureaux, V.; Colombeau, L.; Arnoux, P.; Moussaron, A.; Baros, F.; Toufaily, J.; Hamieh, T.; Roques-Carmes, T.; Frochot, C. Titania and Silica Nanoparticles Coupled to Chlorin E6 for Anti-Cancer Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2018, 22, 115–126. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Song, J.; Liu, Y.; Li, J.; Wang, X.; Tang, R. Construction of Multifunctional Mesoporous Silicon Nano-Drug Delivery System and Study of Dual Sensitization of Chemo-Photodynamic Therapy in Vitro and In Vivo. J. Colloid Interface Sci. 2022, 628, 271–285. [Google Scholar] [CrossRef]
- Fan, J.; Qin, Y.; Xiao, C.; Yuan, L.; Long, Y.; Zhao, Y.; Nguyen, W.; Chen, S.; Chen, W.; Liu, X.; et al. Biomimetic PLGA-Based Nanocomplexes for Improved Tumor Penetration to Enhance Chemo-Photodynamic Therapy against Metastasis of TNBC. Mater. Today Adv. 2022, 16, 100289. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, D.; Zou, J.; Shao, J.; Tang, H.; Xu, H.; Si, W.; Dong, X. Tumor Microenvironment Responsive Oxygen-Self-Generating Nanoplatform for Dual-Imaging Guided Photodynamic and Photothermal Therapy. ChemistrySelect 2018, 3, 4366–4373. [Google Scholar] [CrossRef]
- Ren, G.; Wang, Z.C.; Tian, Y.; Li, J.; Ma, Y.; Zhou, L.; Zhang, C.; Guo, L.; Diao, H.; Li, L.; et al. Targeted Chemo-Photodynamic Therapy toward Esophageal Cancer by GSH-Sensitive Theranostic Nanoplatform. Biomed. Pharmacother. 2022, 153. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Lei, J.; Shen, H.; Ju, H. Multifunctional Metal-Organic Framework Nanoprobe for Cathepsin B-Activated Cancer Cell Imaging and Chemo-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; He, C.; Guo, N.; Chan, C.; Ni, K.; Weichselbaum, R.R.; Lin, W. Chlorin-Based Nanoscale Metal-Organic Framework Systemically Rejects Colorectal Cancers via Synergistic Photodynamic Therapy and Checkpoint Blockade Immunotherapy. J. Am. Chem. Soc. 2016, 138, 12502–12510. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Wang, L.; Liu, M.; Lei, P.; Liu, F.; Xie, Z. Nanoscale Mixed-Component Metal-Organic Frameworks with Photosensitizer Spatial-Arrangement-Dependent Photochemistry for Multimodal-Imaging-Guided Photothermal Therapy. Chem. Mater. 2018, 30, 6867–6876. [Google Scholar] [CrossRef]
- Park, K.; Ahn, J.W.; Kim, J.H.; Kim, J.W. Tumor-Associated Macrophage-Targeted Photodynamic Cancer Therapy Using a Dextran Sulfate-Based Nano-Photosensitizer. Int. J. Biol. Macromol. 2022, 218, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Cai, H.; Wei, Q.; Tang, X.; Zhang, Q.; Kopytynski, M.; Yang, J.; Yi, Y.; Zhang, H.; Gong, Q.; et al. Enhanced Chemo-Photodynamic Therapy of an Enzyme-Responsive Prodrug in Bladder Cancer Patient-Derived Xenograft Models. Biomaterials 2021, 277, 121061. [Google Scholar] [CrossRef] [PubMed]
- Pantiushenko, I.V.; Rudakovskaya, P.G.; Starovoytova, A.V.; Mikhaylovskaya, A.A.; Abakumov, M.A.; Kaplan, M.A.; Tsygankov, A.A.; Majouga, A.G.; Grin, M.A.; Mironov, A.F. Development of Bacteriochlorophyll A-Based near-Infrared Photosensitizers Conjugated to Gold Nanoparticles for Photodynamic Therapy of Cancer. Biochem. Mosc. 2015, 80, 752–762. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, Z.; Meng, X.; Zhao, W.; Shi, Z.; Yang, Z.; Dong, H.; Zhang, X. A Bacteriochlorin-Based Metal–Organic Framework Nanosheet Superoxide Radical Generator for Photoacoustic Imaging-Guided Highly Efficient Photodynamic Therapy. Adv. Sci. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alghamdi, M.S.; Alotaibi, A.S.; Alzhrani, R.; Alwuthaynani, F.; Althobaiti, Y.S.; Almalki, A.H.; Sau, S.; Iyer, A.K. Progress in Clinical Trials of Photodynamic Therapy for Solid Tumors and the Role of Nanomedicine. Cancers 2020, 12, 2793. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.T.; Cen, Y.; Xu, L.; Li, S.Y.; Cheng, H. Recent Progress in Carrier-Free Nanomedicine for Tumor Phototherapy. Adv. Healthc. Mater. 2022, 2202307, 1–32. [Google Scholar] [CrossRef] [PubMed]

| Type of NPs | Size of NPs | Preparation Method | Wavelength [nm] | Light Dose/Light Power | Cell Type | Cell Viability | Ref. |
|---|---|---|---|---|---|---|---|
| Fe3O4 (loaded in liposomes) | 150 nm | alkaline co-precipitation of FeCl2 and FeCl3 | 650 | 5 or 10 J/cm2 | human adenocarcinoma SKOV-3 cells | 0.2% at 5 J/cm2 and 0% at 10 J/cm2 | [16] |
| Fe3O4 | 20 nm | coprecipitation of Fe2+ and Fe3+ in the presence of NH3 × H2O | 632.8 | 30 mW/cm2 | MGC803 cells | 40% at 25.2 µM; 80% at 42 µM | [17] |
| Fe3O4 | 92 nm | thermal decomposition method | 665 | 5 J/cm2 | 4T1 cells | <10% | [18] |
| Fe3O4 | 90 nm | thermal decomposition method | 660 | N/A | MDA-MB-231 cells | approx. 20% | [19] |
| Fe3O4 | 220 ± 30 nm | reaction of tris(acetylacetonate) iron with tri(ethylene) glycol at 300 °C for 2 h | 690 | 0.5 W/cm2 | Lewis cells | approx. 0.5% | [20] |
| Dextran-coated Fe3O4 | 49.9 nm | crosslinking of the dextran coating around the iron oxide core | 650 | 150 mW/cm2 (6 J/cm2) | murine macrophage RAW 264.7 cells | 0% (LD50 of 14 nM) | [21] |
| Aptamer and GO-functionalized Fe3O4 | 10 nm (bare Fe3O4) | thermal decomposition method | 660 and 808 | 100 mW/cm2 for 660 nm | MCF-7 tumor cells | 16.33% | [22] |
| FeOOH | 197.5 ± 8.2 nm | hydrothermal method | 660 | 5.8 mW/cm2 | murine breast cancer cells 4T1 | 3% | [22] |
| Magnetic nanogrenades | 60 nm | thermal decomposition of an iron−oleate complex in the presence of oleyl alcohol | 670 | 5 mW/cm2 | HCT116 cancer cells | approx. 10% | [24] |
| Fe3O4 | 15 nm (bare Fe3O4) | thermal decomposition of an iron−oleate complex in the presence of ethanol and 1-octadecene | 660 and 808 | 346 mW/cm2 for 660 nm and 1 W/cm2 | CT26 colon cancer cells | 3.01% | [25] |
| γ-Fe2O3 | 11 ± 2 nm | chemical reduction of FeCl3 by NaBH4 in alkaline media | constant white light (no specific wavelength) | N/A | mosquito larvae | average mortality after 5 h—74.3 ± 36.8% | [26] |
| Type of NPs | Size of NPs | Preparation Method | Wavelength [nm] | Light Dose/Light Power | Cell Type | Cell Viability | Ref. |
|---|---|---|---|---|---|---|---|
| Gold | 52.2 ± 6.3 nm | chemical reduction of HAuCl4 by NaBH4 | 660 | 53 mW/cm2 | MDA-MB-231 cancer cells | approx. 25% | [27] |
| Gold | 13 nm | aqueous reduction of HAuCl4 with trisodium citrate using the Turkevich–Frens method | 660 | 194 mW/cm2 | HeLa cells | 24% | [28] |
| Gold nanocups | 124 ± 4 nm | chemical reduction of HAuCl4 by ascorbic acid in PbS nanooctahedron suspension | 660 and 808 | 10 mW/cm2 for 660 nm and 0.5 W/cm2 for 808 nm | HeLa cells | 10.3 ± 1.4% | [29] |
| Gold nanoflowers | 80 nm | chemical reduction of HAuCl4 by ascorbic acid and further treatment with NH2OH·HCl | 660 and 808 | 100 mW/cm2 for 660 nm and 2 W/cm2 for 808 nm | HeLa cells | 13% | [30] |
| Glutathione coated gold | 5.6 ± 1 nm | chemical reduction of HAuCl4 by NaBH4 in acidified methanol | 20 W halogen bulb cold light (620–700 nm) | N/A | Staphylococcus aureus Streptococcus pyogenes | 2 log reduction at conc. of 2.88 × 10−8 M | [31] |
| Gold | 18 ± 4 nm | aqueous reduction of HAuCl4 with trisodium citrate using the Turkevich–Frens method | 660 | 25 mW/cm2 (25 J/cm2) | human breast carcinoma cells MDA-MB-468 | 0% in conc. range 0.16–1.67 µM | [32] |
| Gold nanorods dimers/upconverted nanoparticles | ~40 nm | gold seed-mediated growth method | 808 and 980 | 0.2 W/cm2 for 808 nm and 2 mW/cm2 for 980 | HeLa cells | 0% | [33] |
| EGF-functionalized gold NPs | 21 nm | conventional sodium citrate reduction of gold chloride | 660 | 25 mW/cm2 (25 J/cm2) | human breast carcinoma cells MDA-MB-468 | 14% | [34] |
| Human serum corona protein-coated gold nanorods | 46.5 ± 1.2 nm by 19.0 ± 0.7 nm | chemical reduction of HAuCl4 by NaBH4 | 665 | 250 mW/cm2 | squamous cell carcinoma (OSCC) cells | 4.8% | [35] |
| Gold | 67.93 ± 8.5 nm | chemical reduction of HAuCl4 by ascorbic acid in bovine serum | 671 | 2 W/cm2 | human breast carcinoma cells MDA-MB-435 | approx. 5% | [36] |
| PEGylated gold nanoparticles | 119.2 nm | sacrificial galvanic replacement of cobalt nanoparticles based on chloroauric acid | 670 and 808 | 2 W/cm2 for 808 nm | HeLa cells | approx. 25% | [37,38] |
| Gold | 12 ± 1 nm | aqueous reduction of HAuCl4 with citrate using the Turkevich–Frens method | 650 | 1 J/cm2 | SH-SY5Y human neuroblastoma cells | 0% at Ce6 conc. 0.64 µM | [39] |
| CeO2-coated gold nanorods | 55 nm | chemical reduction of HAuCl4 by NaBH4 in presence of CTAB, AgNO3, and NH2OH × HCl | 660 and 808 | N/A | HepG2 cells | <20% | [41] |
| Bimetallic gold–platinum NPs | 19 ± 8 nm | aqueous reduction of HAuCl4 with trisodium citrate using the Turkevich–Enustun method | 660 | 102 J/cm2 | murine melanoma cell line B16F10-Nex2 | 14% | [41] |
| Folic acid functionalized gold nanoclusters | 6.1 ± 1.2 nm | modified TBAB-reduction method (aqueous reduction of HAuCl4 with tetrabutylammonium borohydride) | 633 | 100 mW/cm2 | human gastric carcinoma cell line MGC-803 cells | 10% | [42] |
| Silica-coated gold nanoparticles | 61.21 nm | chemical reduction of HAuCl4 by sodium citrate in presence of CTAB | Not applicable—sonodynamic therapy | Not applicable—sonodynamic therapy | orthotopic colorectal tumor C26 and 3T3 cells | <50% | [44] |
| Poly(NIPAAm-b-styrene/gold nanoparticles copolymer | ~40 nm | aqueous reduction of HAuCl4 with trisodium citrate | white light | 408 mW/cm2 (73 J/cm2) | Staphylococcus aureus | 7 log reduction | [45] |
| Type of NPs | Size of NPs | Preparation Method | Wavelength [nm] | Light Dose/Light Power | Cell Type | Cell Viability | Ref. |
|---|---|---|---|---|---|---|---|
| NaYF4:Yb,Er/NaGdF4 core-shell nanoparticles | 33 nm (diameter) × 40 nm (length) | metal-oleate complex reduction with NaOH in methanol | 980 | N/A | U87MG glioblastoma cells | approx. 55% | [48] |
| Fe3O4/MnO2-doped upconversion NPs functionalized with black phosphorous | 0.31 nm for UCNPs; 50–100 nm black phosphorous | hydrothermal synthesis | 808 | 2 W/cm2 | HeLa cells | 0% | [49] |
| Type of NPs | Size of NPs | Preparation Method | Wavelength [nm] | Light Dose/Light Power | Cell Type | Cell Viability | Ref. |
|---|---|---|---|---|---|---|---|
| CuS | 6.5 nm | chemical reduction of copper(I) chloride in octadecene (ODE) | 670 and 808 | 100 mW/cm2 for 670 nm and 2 W/cm2 for 808 nm | MDA-MB-231 breast cancer cells | 16% | [50] |
| Pd nanospheres | 90 nm | hydrothermal synthesis | 660 and 808 | 0.5 W/cm2 for 660 nm and 2 W/cm2 for 808 nm | HeLa cells | 14% | [51] |
| Cu-In-S quantum dots | 30 nm | solvothermal method | 671 and 808 | 1 W/cm2 for 671 nm and 2 W/cm2 for 808 nm | B16F1 mouse melanoma cells | <15% | [52] |
| FeS2 nanodots | 7 nm | biomineralization of FeCl2 | 660 and 808 | 5 mW/cm2 for 660 nm and 0.8 W/cm2 for 808 nm | murine breast cancer (4T1) cells | 20% | [53] |
| CuS | 184.2 ± 4.8 nm | chemical reduction of copper(I) chloride by NaOH and further treatment with (NH4)2S | 660 and 808 | 0.5 W/cm2 for 660 nm and 2 W/cm2 for 808 nm | 4T1 mouse mammary tumor cell line | 1.68% | [54] |
| MoS2 | ~1 nm | exfoliation of the bulk MoS2 with n-butyl lithium | 660 and 808 | 5 mW/cm2 for 660 nm and 0.5 W/cm2 for 808 nm | 4T1 mouse mammary tumor cell line | approx. 15% | [55] |
| Bi2S3 nanospheres | ~186.2 nm | chemical reduction of Bi(NO3)3 by NaOH in the presence of HNO3 and ethylene glycol | 660 and 808 | 0.38 W/cm2 for 660 nm and 2 W/cm2 for 808 nm | HeLa cells | 6.26 ± 0.21% | [56] |
| Hf nanosheets | 100–200 nm × 3.3 − 7.5 nm | solvothermal method | 640 or 650 | 0.1 W/cm2 (90 J/cm2) | CT26 cells HT29 cells | 20% 25% | [57] |
| Pt nanoparticles in the [Zr6O4(OH)4] clusters | 60 nm | solvothermal method (MOF) encapsulation of Pt NPs in MOF structure | 670 and 808 | 50 mW/cm2 for 670 nm and 1 W/cm2 for 808 nm | human breast cancer MCF-7 cells | approx. 10% | [58] |
| TiO2 or SiO2 nanoparticles | ~30 nm | silanization process of TiO2 or SiO2 | 652 | 4.54 mW/cm2 (10 J/cm2) | glioblastoma U87 cells | 11% | [60] |
| mesoporous silicon NPs | ~211.4 nm | chemical reduction of tetraethyl orthosilicate by NaOH in the presence of hexadecyl trimethyl ammonium bromide | 660 | 100 mW/cm2 | human lung adenocarcinoma A549/DDP cells | 0.3% ± 0.4% | [61] |
| Type of NPs | Size of NPs | Preparation Method | Wavelength [nm] | Light Dose/Light Power | Cell Type | Cell Viability | Ref. |
|---|---|---|---|---|---|---|---|
| Graphene oxide quantum dots | 144.7 nm | N/A | 660 | 0.1 W/cm2 | MDA-MB-231 breast cancer cells | 29% | [62] |
| Carbon nanotubes with cross-linked MnO2 flakes | 113 nm | cross-linking of MnO2 flakes on carbon nanotubes | 660 and 808 | 40 mW/cm2 for 670 nm and 1 W/cm2 for 808 nm | HeLa cells | approx. 25% | [63] |
| Carbon dots | 90.56 ± 2.07 nm for GCDs-Ce6/Pt-EGF | one-step hydrothermal method using p-aminobenzamide and p-aminosalicylic acid | 660 | 0.5 W/cm2 | Human esophageal squamous cell carcinoma KYSE-150 cells | approx. 20% | [64] |
| Type of NPs | Size of NPs | Preparation Method | Wavelength [nm] | Light Dose/Light Power | Cell Type | Cell Viability | Ref. |
|---|---|---|---|---|---|---|---|
| FE2(CO2)3 MOFs | 95 nm | microwave-assisted synthesis | 660 | 100 mW/cm2 | HeLa cells | 0.73% | [65] |
| Hf4+ MOFs | 72.7 and 83.2 nm | solvothermal method | 650 | 100 mW/cm2 (90 J/cm2) | colorectal cancer CT26 and MC38 cells | approx. 10–15% | [66] |
| Hf-UiO-66 MOFs | 100–130 nm | solvothermal method | 635 | 0.8 W/cm2 | HepG2 and HeLa cells | approx. 35% | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koczorowski, T.; Glowacka-Sobotta, A.; Michalak, M.; Mlynarczyk, D.T.; Güzel, E.; Goslinski, T.; Sobotta, L. Connections between Metallic Nanoparticles and Chlorin e6—An Overview of Physicochemical and Biological Properties and Prospective Medical Applications. Appl. Sci. 2023, 13, 3933. https://doi.org/10.3390/app13063933
Koczorowski T, Glowacka-Sobotta A, Michalak M, Mlynarczyk DT, Güzel E, Goslinski T, Sobotta L. Connections between Metallic Nanoparticles and Chlorin e6—An Overview of Physicochemical and Biological Properties and Prospective Medical Applications. Applied Sciences. 2023; 13(6):3933. https://doi.org/10.3390/app13063933
Chicago/Turabian StyleKoczorowski, Tomasz, Arleta Glowacka-Sobotta, Maciej Michalak, Dariusz T. Mlynarczyk, Emre Güzel, Tomasz Goslinski, and Lukasz Sobotta. 2023. "Connections between Metallic Nanoparticles and Chlorin e6—An Overview of Physicochemical and Biological Properties and Prospective Medical Applications" Applied Sciences 13, no. 6: 3933. https://doi.org/10.3390/app13063933
APA StyleKoczorowski, T., Glowacka-Sobotta, A., Michalak, M., Mlynarczyk, D. T., Güzel, E., Goslinski, T., & Sobotta, L. (2023). Connections between Metallic Nanoparticles and Chlorin e6—An Overview of Physicochemical and Biological Properties and Prospective Medical Applications. Applied Sciences, 13(6), 3933. https://doi.org/10.3390/app13063933







