Influence of the Number of Microthreads on Marginal Bone Loss: A Five-Year Retrospective Clinical Study in Humans
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Patients
2.1.2. Implants
2.1.3. Clinical Evaluation
2.1.4. Radiographic Study
2.1.5. Radiographic Technique
2.1.6. Evaluation of Radiographs
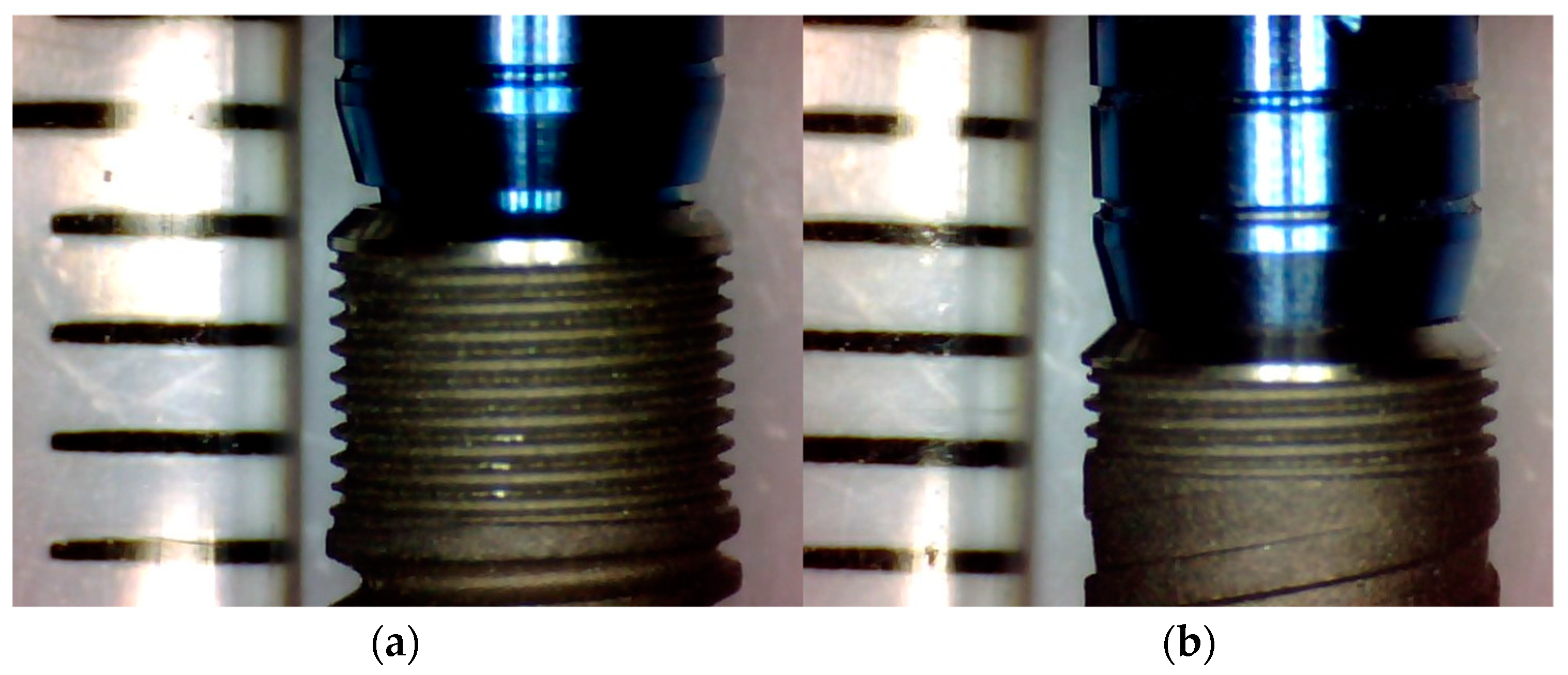
2.1.7. Measurement Program
2.1.8. Examiner Calibration
2.1.9. Inclusion Criteria
2.1.10. Exclusion Criteria
2.2. Method
- <0.40—Poor.
- 0.40–0.59—Sufficient.
- 0.60–0.74—Good.
- 0.75–1—Excellent.
3. Results
3.1. Evaluator Reliability Analysis
3.2. Description of the Sample
3.3. B.L. Analysis between Groups
3.3.1. Comparison of B.L. between Groups at 3 Months after Loading
3.3.2. Comparison of B.L. between Groups at 5 Years after Loading
3.3.3. Comparison of Intragroup B.L. over Time (3 Months vs. 5 Years)
4. Discussion
- Collar design;
- Presence of microthreads;
- Beginning of microthreads;
- Number and length of microthreads.
4.1. Collar Design
4.2. Presence of Microthreads
4.3. Beginning of Microthreads
4.4. Number and Length of Microthreads
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrektsson, T.O.; Johansson, C.B.; Sennerby, L. Biological aspects of implant dentistry: Osseointegration. Periodontol. 2000 1994, 4, 58–73. [Google Scholar] [CrossRef]
- Smith, D.E.; Zarb, G.A. Criteria for success of osseointegrated endosseous implants. J. Prosthet. Dent. 1989, 62, 567–572. [Google Scholar] [CrossRef]
- Sanz, M.; Ivanoff, C.-J.; Weingart, D.; Wiltfang, J.; Gahlert, M.; Cordaro, L.; Ganeles, J.; Bragger, U.; Jackowski, J.; Martin, W.C.; et al. Clinical and Radiologic Outcomes after Submerged and Transmucosal Implant Placement with Two-Piece Implants in the Anterior Maxilla and Mandible: 3-Year Results of a Randomized Controlled Clinical Trial. Clin. Implant. Dent. Relat. Res. 2013, 17, 234–246. [Google Scholar] [CrossRef]
- Oh, T.-J.; Yoon, J.; Misch, C.E.; Wang, H.-L. The Causes of Early Implant Bone Loss: Myth or Science? J. Periodontol. 2002, 73, 322–333. [Google Scholar] [CrossRef]
- Nimbalkar, S.; Dhatrak, P.; Gherde, C.; Joshi, S. A review article on factors affecting bone loss in dental implants. Mater. Today Proc. 2020, 43, 970–976. [Google Scholar] [CrossRef]
- Pozzi, A.; Agliardi, E.; Tallarico, M.; Barlattani, A. Clinical and Radiological Outcomes of Two Implants with Different Prosthetic Interfaces and Neck Configurations: Randomized, Controlled, Split-Mouth Clinical Trial. Clin. Implant. Dent. Relat. Res. 2012, 16, 96–106. [Google Scholar] [CrossRef]
- Messias, A.; Nicolau, P.; Guerra, F. Titanium dental implants with different collar design and surface modifications: A systematic review on survival rates and marginal bone levels. Clin. Oral Implant. Res. 2018, 30, 20–48. [Google Scholar] [CrossRef] [Green Version]
- Sennerby, L.; Meredith, N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontol. 2000 2008, 47, 51–66. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Namgung, C.; Lee, J.-H.; Lim, Y.-J. The influence of thread geometry on implant osseointegration under immediate loading: A literature review. J. Adv. Prosthodont. 2014, 6, 547–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuhussein, H.; Pagni, G.; Rebaudi, A.; Wang, H.-L. The effect of thread pattern upon implant osseointegration. Clin. Oral Implant. Res. 2010, 21, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef]
- Siadat, H.; Panjnoosh, M.; Alikhasi, M.; Alihoseini, M.; Bassir, S.H.; Rokn, A.R. Does Implant Staging Choice Affect Crestal Bone Loss? J. Oral Maxillofac. Surg. 2012, 70, 307–313. [Google Scholar] [CrossRef]
- Yalçın, M.; Kaya, B.; Laçin, N.; Arı, E. Three-Dimensional Finite Element Analysis of the Effect of Endosteal Implants with Different Macro Designs on Stress Distribution in Different Bone Qualities. Int. J. Oral Maxillofac. Implant. 2019, 34, e43–e50. [Google Scholar] [CrossRef]
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant materials, designs, and surface topographies: Their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675–690. [Google Scholar]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. A 2005, 74, 49–58. [Google Scholar] [CrossRef]
- Guerra, F.; Wagner, W.; Wiltfang, J.; Rocha, S.; Moergel, M.; Behrens, E.; Nicolau, P. Platform switch versus platform match in the posterior mandible–1-year results of a multicentre randomized clinical trial. J. Clin. Periodontol. 2014, 41, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Nevins, M.; Camelo, M.; Nevins, M.L.; Schupbach, P.; Kim, D.M. Connective tissue attachment to laser-microgrooved abutments: A human histologic case report. Int. J. Periodontics Restor. Dent. 2012, 32, 385–392. [Google Scholar]
- King, G.N.; Hermann, J.S.; Schoolfield, J.D.; Buser, D.; Cochran, D.L. Influence of the Size of the Microgap on Crestal Bone Levels in Non-Submerged Dental Implants: A Radiographic Study in the Canine Mandible. J. Periodontol. 2002, 73, 1111–1117. [Google Scholar] [CrossRef] [Green Version]
- Hermann, J.S.; Buser, D.; Schenk, R.K.; Cochran, D.L. Crestal Bone Changes Around Titanium Implants. A Histometric Evaluation of Unloaded Non-Submerged and Submerged Implants in the Canine Mandible. J. Periodontol. 2000, 71, 1412–1424. [Google Scholar] [CrossRef] [Green Version]
- Hermann, J.S.; Cochran, D.L.; Nummikoski, P.V.; Buser, D. Crestal Bone Changes Around Titanium Implants. A Radiographic Evaluation of Unloaded Nonsubmerged and Submerged Implants in the Canine Mandible. J. Periodontol. 1997, 68, 1117–1130. [Google Scholar] [CrossRef]
- Hermann, J.S.; Schoolfield, J.D.; Schenk, R.K.; Buser, D.; Cochran, D.L. Influence of the Size of the Microgap on Crestal Bone Changes Around Titanium Implants. A Histometric Evaluation of Unloaded Non-Submerged Implants in the Canine Mandible. J. Periodontol. 2001, 72, 1372–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaillancourt, H.; Pilliar, R.M.; McCammond, D. Finite element analysis of crestal bone loss around porous-coated dental implants. J. Appl. Biomater. 1995, 6, 267–282. [Google Scholar] [CrossRef]
- Song, D.-W.; Lee, D.-W.; Kim, C.-K.; Park, K.-H.; Moon, I.-S. Comparative Analysis of Peri-Implant Marginal Bone Loss Based on Microthread Location: A 1-Year Prospective Study After Loading. J. Periodontol. 2009, 80, 1937–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravald, N.; Dahlgren, S.; Teiwik, A.; Gröndahl, K. Long-term evaluation of Astra Tech and Brånemark implants in patients treated with full-arch bridges. Results after 12-15 years. Clin. Oral Implant. Res. 2013, 24, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Vroom, M.G.; Sipos, P.; De Lange, G.L.; Gründemann, L.J.; Timmerman, M.F.; Loos, B.G.; Van Der Velden, U. Effect of surface topography of screw-shaped titanium implants in humans on clinical and radiographic parameters: A 12-year prospective study. Clin. Oral Implant. Res. 2009, 20, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar] [CrossRef]
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on implants and osseointegration. Periodontol. 2000 2019, 79, 178–189. [Google Scholar] [CrossRef]
- Lesmes, D.; Laster, Z. Innovations in Dental Implant Design for Current Therapy. Oral Maxillofac. Surg. Clin. North Am. 2011, 23, 193–200. [Google Scholar] [CrossRef]
- Bora, U.; Joshi, S.; Dhatrak, P.; Mutha, R. An effect of micro-threaded design of implant neck on stress distribution to improve the survival rate of dental implants. AIP Conf. Proc. 2021, 2358, 070004. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Bieling, K.; Becker, J. Crestal bone changes at nonsubmerged implants (Camlog) with different machined collar lengths: A histomorphometric pilot study in dogs. Int. J. Oral Maxillofac. Implant. 2008, 23, 335–342. [Google Scholar]
- Bae, H.E.K.; Chung, M.-K.; Cha, I.-H.; Han, D.-H. Marginal tissue response to different implant neck design. J. Korean Acad. Prosthodont. 2008, 46, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Rothamel, D.; Heinz, M.; Ferrari, D.; Eissing, A.; Holtmann, H.; Schorn, L.; Fienitz, T. Impact of machined versus structured implant shoulder designs on crestal bone level changes: A randomized, controlled, multicenter study. Int. J. Implant. Dent. 2022, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, L.A. The biomechanics of force distribution in implant-supported prostheses. Int. J. Oral Maxillofac. Implant. 1993, 8, 19–31. [Google Scholar]
- Schwarz, F.; Messias, A.; Sanz-Sánchez, I.; De Albornoz, A.C.; Nicolau, P.; Taylor, T.; Beuer, F.; Schär, A.; Sader, R.; Guerra, F.; et al. Influence of implant neck and abutment characteristics on peri-implant tissue health and stability. Oral reconstruction foundation consensus report. Clin. Oral Implant. Res. 2019, 30, 588–593. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.-H.; Peng, M.-D.; Li, Q. The effect of implant neck microthread design on stress distribution of peri-implant bone with different level: A finite element analysis. J. Dent. Sci. 2020, 15, 466–471. [Google Scholar] [CrossRef]
- Shin, Y.-K.; Han, C.-H.; Heo, S.-J.; Kim, S.; Chun, H.-J. Radiographic evaluation of marginal bone level around implants with different neck designs after 1 year. Int. J. Oral Maxillofac. Implant. 2006, 21, 789–794. [Google Scholar]
- Hansson, S. The implant neck: Smooth or provided with retention elements. A biomechanical approach. Clin. Oral Implant. Res. 1999, 10, 394–405. [Google Scholar] [CrossRef]
- Hansson, S. A conical implant-abutment interface at the level of the marginal bone improves the distribution of stresses in the supporting bone. An axisymmetric finite element analysis. Clin. Oral Implant. Res. 2003, 14, 286–293. [Google Scholar] [CrossRef]
- Merİç, G.; Erkmen, E.; Kurt, A.; Eser, A.; Özden, A.U. Biomechanical effects of two different collar implant structures on stress distribution under cantilever fixed partial dentures. Acta Odontol. Scand. 2011, 69, 374–384. [Google Scholar] [CrossRef]
- Bratu, E.A.; Tandlich, M.; Shapira, L. A rough surface implant neck with microthreads reduces the amount of marginal bone loss: A prospective clinical study. Clin. Oral Implant. Res. 2009, 20, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, H.-J.; Wichmann, M.; Schlegel, K.A.; Nkenke, E.; Eitner, S. Radiographic evaluation of marginal bone levels adjacent to parallel-screw cylinder machined-neck implants and rough-surfaced microthreaded implants using digitized panoramic radiographs. Clin. Oral Implant. Res. 2009, 20, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, R.; Patil, Y.B.; Asopa, S.J.; Deepa, D.; Goel, A.; Jyoti, D.; Somayaji, N.S. Influence of implant neck design on crestal bone loss: A comparative study. Niger. J. Surg. 2020, 26, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Koodaryan, R.; Hafezeqoran, A. Evaluation of Implant Collar Surfaces for Marginal Bone Loss: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2016, 2016, 4987526. [Google Scholar] [CrossRef] [Green Version]
- Abrahamsson, I.; Berglundh, T. Tissue Characteristics at Microthreaded Implants: An Experimental Study in Dogs. Clin. Implant. Dent. Relat. Res. 2006, 8, 107–113. [Google Scholar] [CrossRef]
- Golmohammadi, S.; Eskandari, A.; Movahhedy, M.R.; Shirmohammadi, A.; Amid, R. The effect of microthread design on magnitude and distribution of stresses in bone: A three-dimensional finite element analysis. Dent. Res. J. 2018, 15, 347–353. [Google Scholar] [CrossRef]
- Filho, L.C.D.C.; Faot, F.; Madruga, M.D.M.; Marcello-Machado, R.M.; Bordin, D.; Cury, A.A.D.B. Effect of implant macrogeometry on peri-implant healing outcomes: A randomized clinical trial. Clin. Oral Investig. 2018, 23, 567–575. [Google Scholar] [CrossRef]
- Lee, D.-W.; Choi, Y.-S.; Park, K.H.; Kim, C.-S.; Moon, I.-S. Effect of microthread on the maintenance of marginal bone level: A 3-year prospective study. Clin. Oral Implant. Res. 2007, 18, 465–470. [Google Scholar] [CrossRef]
- Aslroosta, H.; Akbari, S.; Naddafpour, N.; Adnaninia, S.T.; Khorsand, A.; Esfahani, N.N. Effect of microthread design on the preservation of marginal bone around immediately placed implants: A 5-years prospective cohort study. BMC Oral Health 2021, 21, 541. [Google Scholar] [CrossRef]
- Mei, D.M.; Zhao, B.; Xu, H.; Wang, Y. Radiographic and clinical outcomes of rooted, platform-switched, microthreaded implants with a sandblasted, large-grid, and acid-etched surface: A 5-year prospective study. Clin. Implant. Dent. Relat. Res. 2017, 19, 1074–1081. [Google Scholar] [CrossRef]
- Araújo, M.G.; Silva, C.; Souza, A.B.; Sukekava, F. Socket healing with and without immediate implant placement. Periodontol. 2000 2019, 79, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Pesce, P.; Delucchi, F.; Ambrogio, G.; Canepa, C.; Carossa, M.; Pera, F. One-stage versus two-stage technique using two splinted extra-short implants: A multicentric split-mouth study with a one-year follow-up. Clin. Implant. Dent. Relat. Res. 2022, 24, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Lamazza, L.; Rapani, A.; Troiano, G.; Messina, M.; Antonelli, A.; Giudice, A.; Lombardi, T. Marginal bone changes around platform-switched conical connection implants placed 1 or 2 mm subcrestally: A multicenter crossover randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2023; Epub ahead of print. [Google Scholar] [CrossRef]




| BLM1 | Q | Mean | 0.5393 | |
| 95% confidence interval for mean | Lower limit | 0.1239 | ||
| Upper limit | 0.9547 | |||
| S | Mean | 0.5283 | ||
| 95% confidence interval for mean | Lower limit | 0.0326 | ||
| Upper limit | 1.0240 | |||
| BLD1 | Q | Mean | 0.4764 | |
| 95% confidence interval for mean | Lower limit | 0.0727 | ||
| Upper limit | 0.8801 | |||
| S | Mean | 0.6175 | ||
| 95% confidence interval for mean | Lower limit | 0.0129 | ||
| Upper limit | 1.2221 | |||
| BL1 | Q | Mean | 0.5079 | |
| 95% confidence interval for mean | Lower limit | 0.1253 | ||
| Upper limit | 0.8904 | |||
| S | Mean | 0.5729 | ||
| 95% confidence interval for mean | Lower limit | 0.0618 | ||
| Upper limit | 1.0840 | |||
| BLM2 | Q | Mean | 0.6871 | |
| 95% confidence interval for mean | Lower limit | 0.2225 | ||
| Upper limit | 1.1517 | |||
| S | Mean | 0.7042 | ||
| 95% confidence interval for mean | Lower limit | 0.1864 | ||
| Upper limit | 1.2220 | |||
| BLD2 | Q | Mean | 0.6229 | |
| 95% confidence interval for mean | Lower limit | 0.1617 | ||
| Upper limit | 1.0841 | |||
| S | Mean | 1.0217 | ||
| 95% confidence interval for mean | Lower limit | 0.5456 | ||
| Upper limit | 1.4978 | |||
| BL2 | Q | Mean | 0.6550 | |
| 95% confidence interval for mean | Lower limit | 0.2140 | ||
| Upper limit | 1.0960 | |||
| S | Mean | 0.8629 | ||
| 95% confidence interval for mean | Lower limit | 0.3922 | ||
| Upper limit | 1.3336 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jornet-García, A.; Sánchez-Pérez, A.; Planes-Nicolás, P.; Montoya-Carralero, J.M.; Moya-Villaescusa, M.J. Influence of the Number of Microthreads on Marginal Bone Loss: A Five-Year Retrospective Clinical Study in Humans. Appl. Sci. 2023, 13, 3936. https://doi.org/10.3390/app13063936
Jornet-García A, Sánchez-Pérez A, Planes-Nicolás P, Montoya-Carralero JM, Moya-Villaescusa MJ. Influence of the Number of Microthreads on Marginal Bone Loss: A Five-Year Retrospective Clinical Study in Humans. Applied Sciences. 2023; 13(6):3936. https://doi.org/10.3390/app13063936
Chicago/Turabian StyleJornet-García, Alfonso, Arturo Sánchez-Pérez, Pablo Planes-Nicolás, José M. Montoya-Carralero, and María J. Moya-Villaescusa. 2023. "Influence of the Number of Microthreads on Marginal Bone Loss: A Five-Year Retrospective Clinical Study in Humans" Applied Sciences 13, no. 6: 3936. https://doi.org/10.3390/app13063936
APA StyleJornet-García, A., Sánchez-Pérez, A., Planes-Nicolás, P., Montoya-Carralero, J. M., & Moya-Villaescusa, M. J. (2023). Influence of the Number of Microthreads on Marginal Bone Loss: A Five-Year Retrospective Clinical Study in Humans. Applied Sciences, 13(6), 3936. https://doi.org/10.3390/app13063936






