Thermographic Assessment of Skin Temperature Changes following Partial Body Cryostimulation (PBC) in Football Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Procedures
2.2.1. Blood Sampling and Analysis
2.2.2. Cryostimulation Procedure
2.2.3. Thermographic Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, C.; Archer, D.T.; Hogg, B.; Bush, M.; Bradley, P.S. The evolution of physical and technical performance parameters in the english premier league. Int. J. Sports Med. 2014, 35, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Faude, O.; Koch, T.; Mayer, T. Straight sprinting is the most frequent action in goal situations in professional football. J. Sports Sci. 2012, 30, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, M.; McCall, A.; Carling, C.; Legall, F.; Berthoin, S.; Dupont, G. Recovery in soccer: Part I—Post-match fatigue and time course of recovery. Sports Med. 2012, 42, 997–1015. [Google Scholar] [PubMed]
- Silva, J.R.; Rumpf, M.C.; Hertzog, M.; Castagna, C.; Farooq, A.; Girard, O.; Hader, K. Acute and Residual Soccer Match-Related Fatigue: A Systematic Review and Metaanalysis. Sports Med. 2018, 48, 539–583. [Google Scholar] [CrossRef]
- Marqués-Jiménez, D.; Calleja-González, J.; Arratibel, I.; Delextrat, A.; Terrados, N. Fatigue and Recovery in Soccer: Evidence and Challenges. Open Sports Sci. J. 2017, 10 (Suppl. S1), 52–70. [Google Scholar] [CrossRef]
- Hausswirth, C.; Le Meur, Y. Physiological and Nutritional Aspects of Post-Exercise Recovery Specific Recommendations for Female Athletes. Sports Med. 2011, 41, 861–882. [Google Scholar] [CrossRef]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A. Prevention, diagnosis, and treatment of the overtraining syndrome: Joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 2013, 45, 186–205. [Google Scholar] [CrossRef]
- Soligard, T.; Schwellnus, M.; Alonso, J.-M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.; Gleeson, M.; Hägglund, M.; Hutchinson, M.R.; et al. How much is too much? (Part 1) International Olympic Committee consensus statement on load in sport and risk of injury. Br. J. Sports Med. 2016, 50, 1030–1041. [Google Scholar] [CrossRef]
- Bishop, P.A.; Jones, E.; Woods, A.K. Recovery from training: A brief review. J. Strength Cond. Res. 2008, 22, 1015–1024. [Google Scholar] [CrossRef]
- Bouzigon, R.; Grappe, F.; Ravier, G.; Dugue, B. Whole- and partial-body cryostimulation/cryotherapy: Current technologies and practical applications. J. Therm. Biol. 2016, 61, 67–81. [Google Scholar] [CrossRef]
- Patel, K.; Bakshi, N.; Freehill, M.T.; Awan, T.M. Whole-Body Cryotherapy in Sports Medicine. Curr. Sports Med. Rep. 2019, 18, 136–140. [Google Scholar] [CrossRef]
- Bleakley, C.; Bieuzen, F.; Davison, G.; Costello, J. Whole-body cryotherapy: Empirical evidence and theoretical perspectives. Open Access J. Sports Med. 2014, 5, 25–36. [Google Scholar] [CrossRef]
- Hausswirth, C.; Louis, J.; Bieuzen, F.; Pournot, H.; Fournier, J.; Filliard, J.-R.; Brisswalter, J. Effects of whole-body cryotherapy vs. far-infrared vs. passive modalities on recovery from exercise-induced muscle damage in highly-trained runners. PLoS ONE 2011, 6, e27749. [Google Scholar] [CrossRef]
- Pournot, H.; Bieuzen, F.; Louis, J.; Mounier, R.; Fillard, J.R.; Barbiche, E.; Hausswirth, C. Time-course of changes in inflammatory response after whole-body cryotherapy multi exposures following severe exercise. PLoS ONE 2011, 6, e22748. [Google Scholar] [CrossRef]
- Bleakley, C.M.; Hopkins, J.T. Is it possible to achieve optimal levels of tissue cooling in cryotherapy? Phys. Ther. Rev. 2010, 15, 344–350. [Google Scholar] [CrossRef]
- Costello, J.T.; Culligan, K.; Selfe, J.; Donnelly, A.E. Muscle, Skin and Core Temperature after −110 °C Cold Air and 8 °C Water Treatment. PLoS ONE 2012, 7, e48190. [Google Scholar] [CrossRef]
- Lombardi, G.; Ziemann, E.; Banfi, G. Whole-body cryotherapy in athletes from therapy to stimulation. An updated review of the literature. Front. Physiol. 2017, 8, 258. [Google Scholar] [CrossRef]
- Costello, J.T.; Donnelly, A.E.; Karki, A.; Selfe, J. Effects of whole body cryotherapy and cold water immersion on knee skin temperature. Int. J. Sports Med. 2014, 35, 35–40. [Google Scholar] [CrossRef]
- Ziemann, E.; Olek, R.A.; Kujach, S.; Grzywacz, T.; Antosiewicz, J.; Garsztka, T.; Laskowski, R. Five-day whole-body cryostimulation, blood inflammatory markers, and performance in high-ranking professional tennis players. J. Athl. Train. 2012, 47, 664–672. [Google Scholar] [CrossRef]
- Piras, A.; Campa, F.; Toselli, S.; Di Michele, R.; Raffi, M. Physiological responses to partial-body cryotherapy performed during a concurrent strength and endurance session. Appl. Physiol. Nutr. Metab. 2018, 44, 59–65. [Google Scholar] [CrossRef]
- Selfe, J.; Alexander, J.; Costello, J.T.; May, K.; Garratt, N.; Atkins, S.; Dillon, S.; Hurst, H.; Davison, M.; Przybyla, D.; et al. The effect of three different (−135 °C) whole body cryotherapy exposure durations on elite rugby league players. PLoS ONE 2014, 9, e86420. [Google Scholar] [CrossRef] [PubMed]
- Bouzigon, R.; Dupuy, O.; Tiemessen, I.; De Nardi, M.; Bernard, J.-P.; Mihailovic, T.; Theurot, D.; Miller, E.D.; Lombardi, G.; Dugué, B.M. Cryostimulation for Post-exercise Recovery in Athletes: A Consensus and Position Paper. Front. Sports Act. Living 2021, 3, 688828. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Tong, T.K.; George, K.; Fu, F.H.; Lin, H.; Shi, Q. Resting and post-exercise serum biomarkers of cardiac and skeletal muscle damage in adolescent runners. Scand. J. Med. Sci. Sports 2011, 21, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, P.; Maffulli, N.; Buonauro, R.; Limongelli, F.M. Serum Enzyme Monitoring in Sports Medicine. Clin. Sports Med. 2008, 27, 1–18. [Google Scholar] [CrossRef]
- Andersson, H.; Raastad, T.; Nilsson, J.; Paulsen, G.; Garthe, I.; Kadi, F. Neuromuscular fatigue and recovery in elite female soccer: Effects of active recovery. Med. Sci. Sports Exerc. 2008, 40, 372–380. [Google Scholar] [CrossRef]
- Ascensão, A.; Rebelo, A.; Oliveira, E.; Marques, F.; Pereira, L.; Magalhães, J. Biochemical impact of a soccer match—Analysis of oxidative stress and muscle damage markers throughout recovery. Clin. Biochem. 2008, 41, 841–851. [Google Scholar] [CrossRef]
- Ispirlidis, I.; Fatouros, I.G.; Jamurtas, T.; Nikolaidis, M.G.; Michailidis, Y.; Douroudos, I.; Margonis, K.; Chatzinikolaou, A.; Kalistratos, E.; Katrabasas, I.; et al. Time-course of Changes in Inflammatory and Performance Responses Following a Soccer Game. Clin. J. Sport Med. 2008, 18, 423–431. [Google Scholar] [CrossRef]
- Trajkovic, N.; Sporis, G.; Vlahovic, T.; Madic, D.; Gusic, M. Post-Match Changes in Muscle Damage Markers among U-21 Soccer Players Monten. J. Sports Sci. Med. 2018, 7, 49–53. [Google Scholar]
- Hoffman, J.R.; Kang, J.; Ratamess, N.A.; Faigenbaum, A.D. Biochemical and Hormonal Responses during an Intercollegiate Football Season. Med. Sci. Sports Exerc. 2005, 37, 1237–1241. [Google Scholar] [CrossRef]
- Koch, A.J.; Pereira, R.; Machado, M. The creatine kinase response to resistance exercise. J. Musculoskelet. Neuronal Neuronal Interact. 2014, 14, 68–77. [Google Scholar]
- Hausswirth, C.; Schaal, K.; Le Meur, Y.; Bieuzen, F.; Filliard, J.-R.; Volondat, M.; Louis, J. Parasympathetic Activity and Blood Catecholamine Responses Following a Single Partial-Body Cryostimulation and a Whole-Body Cryostimulation. PLoS ONE 2013, 8, e72658. [Google Scholar] [CrossRef]
- Krustrup, P.; Ferguson, R.A.; Kjær, M.; Bangsbo, J. ATP and heat production in human skeletal muscle duringdynamic exercise: Higher efficiency of anaerobic than aerobic ATP resynthesis. J. Physiol. 2003, 549, 255–269. [Google Scholar] [CrossRef]
- Arfaoui, A.; Bertucci, W.; Letellier, T.; Polidori, G. Thermoregulation during incremental exercise in masters cycling. J. Sci. Cycl. 2014, 3, 33–41. [Google Scholar]
- Ferreira, J.J.A.; Mendonça, L.C.S.; Nunes, L.A.O.; Andrade Filho, A.C.C.; Rebelatto, J.R.; Salvini, T.F. Exercise-Associated Thermographic Changes in Young and Elderly Subjects. Ann. Biomed. Eng. 2008, 36, 1420–1427. [Google Scholar] [CrossRef]
- Formenti, D.; Ludwig, N.; Gargano, M.; Gondola, M.; Dellerma, N.; Caumo, A.; Alberti, G. Thermal Imaging of Exercise-Associated Skin Temperature Changes in Trained and Untrained Female Subjects. Ann. Biomed. Eng. 2013, 41, 863–871. [Google Scholar] [CrossRef]
- Ludwig, N.; Gargano, M.; Formenti, D.; Bruno, D.; Ongaro, L.; Alberti, G. Breathing training characterization by thermal imaging: A case study. Acta Bioeng. Biomech. 2012, 14, 41–47. [Google Scholar] [CrossRef]
- Balci, G.A.; Basaran, T.; Colakoglu, M. Analysing visual pattern of skin temperature during submaximal and maximal exercises. Infrared Phys. Technol. 2016, 74, 57–62. [Google Scholar] [CrossRef]
- Merla, A.; Matei, P.A.; Di Donato, L.; Romani, G.L. Thermal imaging of cutaneous temperature modifcations in runners during graded exercise. Ann. Biomed. Eng. 2010, 8, 158–163. [Google Scholar] [CrossRef]
- Quesada, J.I.P.; Carpes, F.P.; Bini, R.R.; Palmer, R.S.; Pérez-Soriano, P.; de Anda, R.M.C.O. Relationship between skin temperature and muscle activation during incremental cycle exercise. J. Therm. Biol. 2015, 48, 28–35. [Google Scholar] [CrossRef]
- Quesada, J.P.; Lucas-Cuevas, A.G.; Gil-Calvo, M.; Giménez, J.V.; Aparicio, I.; de Anda, R.C.O.; Palmer, R.S.; Llana-Belloch, S.; Pérez-Soriano, P. Effects of graduated compression stockings on skin temperature after running. J. Therm. Biol. 2015, 52, 130–136. [Google Scholar] [CrossRef]
- Chudecka, M.; Lubkowska, A. Temperature changes of selected body’s surfaces of handball players in the course of training estimated by thermovision, and the study of the impact of physiological and morphological factors on the skin temperature. J. Therm. Biol. 2010, 35, 379–385. [Google Scholar] [CrossRef]
- Chudecka, M.; Lubkowska, A. The Use of Thermal Imaging to Evaluate Body Temperature Changes of Athletes During Training and a Study on the Impact of Physiological and Morphological Factors on Skin Temperature. Hum. Mov. 2012, 13, 33–39. [Google Scholar] [CrossRef]
- Sanz-López, F.; Martínez-Amat, A.; Hita-Contreras, F.; Valero-Campo, C.; Berzosa, C. Thermographic Assessment of Eccentric Overload Training Within Three Days of a Running Session. J. Strength Cond. Res. 2016, 30, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Côrte, A.C.R.E.; Lopes, G.H.R.; Moraes, M.; De Oliveira, R.M.; Brioschi, M.H.; Hernandez, A.J. The importance of thermography for injury prevention and performance improvement in olympic swimmers: A series of case study. Int. Phys. Med. Rehabil. J. 2018, 3, 137–141. [Google Scholar] [CrossRef]
- Korman, P.; Zieliński, J.; Kusy, K.; Straburzyńska-Lupa, A. Possible uses of infrared thermography in sport. Trends Sport Sci. 2016, 2, 57–62. [Google Scholar]
- Escamilla-Galindo, V.L.; Estal-Martínez, A.; Adamczyk, J.G.; Brito, C.J.; Arnaiz-Lastras, J.; Sillero-Quintana, M. Skin temperature response to unilateral training measured with infrared thermography. J. Exerc. Rehabil. 2017, 13, 526–534. [Google Scholar] [CrossRef]
- Rodríguez-Sanz, D.; Losa-Iglesias, M.E.; López-López, D.; Calvo-Lobo, C.; Palomo-López, P.; Becerro-de-Bengoa-Vallejo, R. Infrared thermography applied to lower limb muscles in elite soccer players with functional ankle equinus and non-equinus condition. PeerJ 2017, 5, e3388. [Google Scholar] [CrossRef]
- Rodriguez- Sanz, D.; Losa-Iglesias, M.E.; Becerro de Bengoa-Vallejo, R.; Palomo-Lopez, P.; Beltran-Alacreu, H.; Calvo-Lobo, C.; Navarro-Flores, E.; Lopez-Lopez, D. Skin temperature in youth soccer players with functional equinus and non-equinus condition after running. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2020–2024. [Google Scholar] [CrossRef]
- Polidori, R.; Taiar, F.; Legrand, F.; Beaumont, S.; Murer, F.; Bogard, F.; Boyer, C. Infrared thermography for assessing skin temperature differences between Partial Body Cryotherapy and Whole Body Cryotherapy devices at −140 °C. Infrared Phys. Technol. 2018, 93, 158–161. [Google Scholar] [CrossRef]
- Moreira, D.G.; Costello, J.T.; Brito, C.J.; Adamczyk, J.G.; Ammer, K.; Bach, A.J.; Costa, C.M.; Eglin, C.; Fernandes, A.A.; Fernández-Cuevas, I.; et al. Thermographic imaging in sports and exercise medicine: A Delphi study and consensus statement on the measurement of human skin temperature. J. Therm. Biol. 2017, 69, 155–162. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Fuijmasa, I. Standardization of techniques for thermal imaging testing: The current situation. Biomed. Thermol. 1995, 15, 63–68. [Google Scholar]
- Sawasaki, N.; Iwase, S.; Mano, T. Effect of skin sympathetic response to local or systemic cold exposure on thermoregulatory functions in humans. Auton. Neurosci. 2001, 87, 274–281. [Google Scholar] [CrossRef]
- Stocks, J.M.; Taylor, N.A.S.; Tipton, M.J.; Greenleaf, J.E. Human physiological responses to cold exposure. Aviat. Space Environ. Med. 2004, 75, 444–457. [Google Scholar]
- Jutte, L.S.; Merrick, M.; Ingersoll, C.D.; Edwards, J.E. The relationship between intramuscular temperature, skin temperature, and adipose thickness during cryotherapy and rewarming. Arch. Phys. Med. Rehabil. 2021, 82, 845–850. [Google Scholar] [CrossRef]
- Wang, H.; Siemens, J. TRP ion channels in thermosensation, thermoregulation and metabolism. Temp. Multidiscip. Biomed. J. 2015, 2, 178–187. [Google Scholar] [CrossRef]
- Lezama-García, K.; Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Ghezzi, M.; Domínguez, A.; Gómez, J.; Geraldo, A.D.M.; Lendez, P.; Hernández-Ávalos, I.; et al. Transient Receptor Potential (TRP) and Thermoregulation in Animals: Structural Biology and Neurophysiological Aspects. Animals 2022, 12, 106. [Google Scholar] [CrossRef]
- Vardasca, R.; Ring, E.F.J.; Plassmann, P.; Jones, C.D. Thermal symmetry of the upper and lower extremities in healthy subjects. Thermol. Int. 2012, 22, 53–60. [Google Scholar]
- Uematsu, S.; Jankel, W.; Edwin, D.; Kim, W.; Kozikowski, A.; Long, D.M. Quantification of thermal asymmetry. Part 2: Application in low-back pain and sciatica. J. Neursurg. 1988, 69, 556–561. [Google Scholar] [CrossRef]
- Fonda, B.; De Nardi, M.; Sarabon, N. Effects of whole-body cryotherapy duration on thermal and cardio-vascular response. J. Therm. Biol. 2014, 42, 52–55. [Google Scholar] [CrossRef]
- Savic, M.; Fonda, B.; Sarabon, N. Actual temperature during and thermal response after whole-body cryotherapy in cryo-cabin. J. Therm. Biol. 2013, 38, 186–191. [Google Scholar] [CrossRef]
- Johnson, J.M.; Yen, T.C.; Zhao, K.; Kosiba, W.A. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am. J. Physiol. Circ. Physiol. 2005, 288, H1573–H1579. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Chelstrom, L.M.; Levitt, S.H.; Haumschild, D.J. Effects of temperature on blood circulation measured with the laser doppler method. Int. J. Radiat. Oncol. 1989, 17, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Dębiec-Bąk, A.; Skrzek, A.; Podbielska, H. Application of thermovision for estimation of the optimal and safe parameters of the whole body cryotherapy. J. Therm. Anal. Calorim. 2013, 111, 1853–1859. [Google Scholar] [CrossRef]
- Louis, J.; Schaal, K.; Bieuzen, F.; Le Meur, Y.; Filliard, J.-R.; Volondat, M.; Brisswalter, J.; Hausswirth, C. Head Exposure to Cold during Whole-Body Cryostimulation: Influence on Thermal Response and Autonomic Modulation. PLoS ONE 2015, 10, e0124776. [Google Scholar] [CrossRef]
- Neves, E.B.; Salamunes, A.C.C.; de Oliveira, R.M.; Stadnik, A.M.W. Effect of body fat and gender on body temperature distribution. J. Therm. Biol. 2017, 70 Pt B, 1–8. [Google Scholar] [CrossRef]
- Salamunes, A.C.C.; Stadnik, A.M.W.; Neves, E.B. The effect of body fat percentage and body fat distribution on skin surface temperature with infrared thermography. J. Therm. Biol. 2017, 66, 1–9. [Google Scholar] [CrossRef]
- Johnson, J.M.; Minson, C.T.; Kellogg, D.L. Cutaneous Vasodilator and Vasoconstrictor Mechanisms in Temperature Regulation. Compr. Physiol. 2014, 4, 33–89. [Google Scholar] [CrossRef]
- Savastano, D.M.; Gorbach, A.M.; Eden, H.S.; Brady, S.M.; Reynolds, J.C.; Yanovski, J.A. Adiposity and human regional body temperature. Am. J. Clin. Nutr. 2009, 90, 1124–1131. [Google Scholar] [CrossRef]
- Ooijen, A.M.C.-V.; Westerterp, K.R.; Wouters, L.; Schoffelen, P.F.; Van Steenhoven, A.A.; Lichtenbelt, W.D.V.M. Heat Production and Body Temperature During Cooling and Rewarming in Overweight and Lean Men. Obesity 2006, 14, 1914–1920. [Google Scholar] [CrossRef]
- Montgomery, P.G.; Pyne, D.; Hopkins, W.G.; Dorman, J.C.; Cook, K.; Minahan, C. The effect of recovery strategies on physical performance and cumulative fatigue in competitive basketball. J. Sports Sci. 2008, 26, 1135–1145. [Google Scholar] [CrossRef]
- Ferreira-Junior, J.B.; Bottaro, M.; Vieira, A.; Siqueira, A.F.; Vieira, C.A.; Durigan, J.L.Q.; Cadore, E.L.; Coelho, L.G.M.; Simões, H.G.; Bemben, M.G. One session of partial-body cryotherapy (-110 degrees C) improves muscle damage recovery. Scand. J. Med. Sci. Sports. 2015, 25, e524–e530. [Google Scholar] [CrossRef]
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic markers in sports medicine. Adv. Clin. Chem. 2012, 56, 1–54. [Google Scholar] [CrossRef]
- Nybo, L.; Girard, O.; Mohr, M.; Knez, W.; Voss, S.; Racinais, S. Markers of Muscle Damage and Performance Recovery after Exercise in the Heat. Med. Sci. Sports Exerc. 2013, 45, 860–868. [Google Scholar] [CrossRef]
- Koury, J.C.; Daleprane, J.B.; Pitaluga-Filho, M.V.; de Oliveira, C.F.; Gonçalves, M.C.; Passos, M.C. Aerobic Conditioning Might Protect Against Liver and Muscle Injury Caused by Short-Term Military Training. J. Strength Cond. Res. 2016, 30, 454–460. [Google Scholar] [CrossRef]
- Banfi, G.; Morelli, P. Relation between body mass index and serum aminotransferases concentrations in professional athletes. J. Sports Med. Phys. Fit. 2008, 48, 197–200. [Google Scholar]
- Fernandes, A.D.A.; Pimenta, E.M.; Moreira, D.G.; Sillero-Quintana, M.; Marins, J.C.B.; Morandi, R.F.; Kanope, T.; Garcia, E. Effect of a professional soccer match in skin temperature of the lower limbs: A case study. J. Exerc. Rehabil. 2017, 13, 330–334. [Google Scholar] [CrossRef]
- Varley, I.; Lewin, R.; Needham, R.; Thorpe, R.T.; Burbeary, R. Association Between Match Activity Variables, Measures of Fatigue and Neuromuscular Performance Capacity Following Elite Competitive Soccer Matches. J. Hum. Kinet. 2017, 60, 93–99. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Chatzinikolaou, A.; Douroudos, I.I.; Nikolaidis, M.G.; Kyparos, A.; Margonis, K.; Michailidis, Y.; Vantarakis, A.; Taxildaris, K.; Katrabasas, I.; et al. Time-Course of Changes in Oxidative Stress and Antioxidant Status Responses Following a Soccer Game. J. Strength Cond. Res. 2010, 24, 3278–3286. [Google Scholar] [CrossRef]
- Russell, M.; Northeast, J.; Atkinson, G.; Shearer, D.A.; Sparkes, W.; Cook, C.J.; Kilduff, L.P. Between-Match Variability of Peak Power Output and Creatine Kinase Responses to Soccer Match-Play. J. Strength Cond. Res. 2015, 29, 2079–2085. [Google Scholar] [CrossRef]
- Vaile, J.; O’Hagan, C.; Stefanovic, B.; Walker, M.A.; Gill, N.; Askew, C. Effect of cold water immersion on repeated cycling performance and limb blood flow. Br. J. Sports Med. 2011, 45, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Gregson, W.; Black, M.A.; Jones, H.; Milson, J.; Morton, J.P.; Dawson, B.T.; Atkinson, G.; Green, D.J. Influence of Cold Water Immersion on Limb and Cutaneous Blood Flow at Rest. Am. J. Sports Med. 2011, 39, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, I.M.; Cronin, J.B.; Hing, W.A. Physiological response to water immersion: A method for sport recovery? Sports Med. 2006, 36, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.A.; Machado-Moreira, C.A. Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans. Extrem. Physiol. Med. 2013, 2, 4. [Google Scholar] [CrossRef]
- Smith, C.J.; Havenith, G. Body mapping of sweating patterns in male athletes in mild exercise-induced hyperthermia. Eur. J. Appl. Physiol. 2011, 111, 1391–1404. [Google Scholar] [CrossRef]
- O’Brien, C.; Cadarette, B.S. Quantification of head sweating during rest and exercise in the heat. Eur. J. Appl. Physiol. 2013, 113, 735–741. [Google Scholar] [CrossRef]
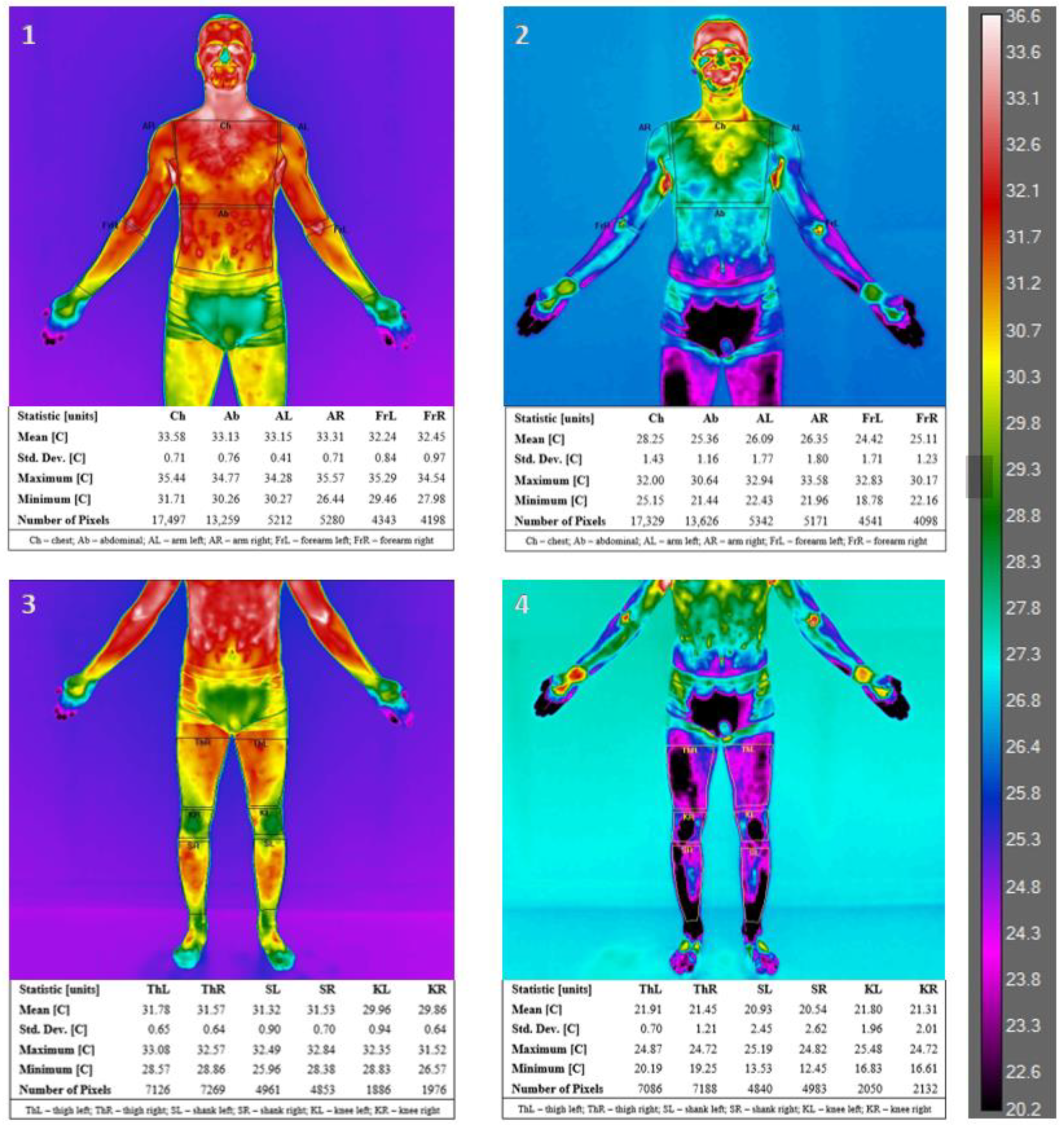
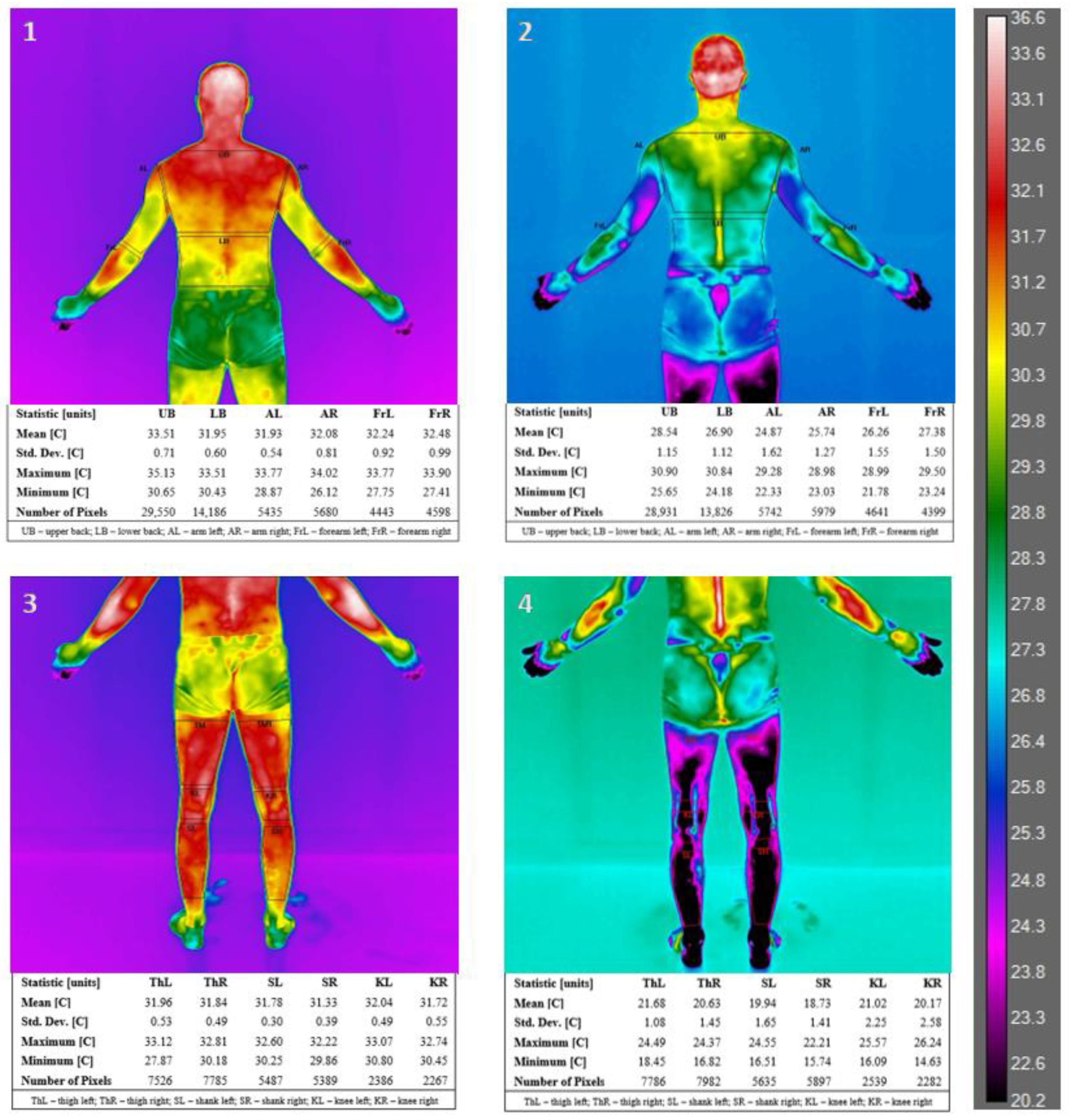
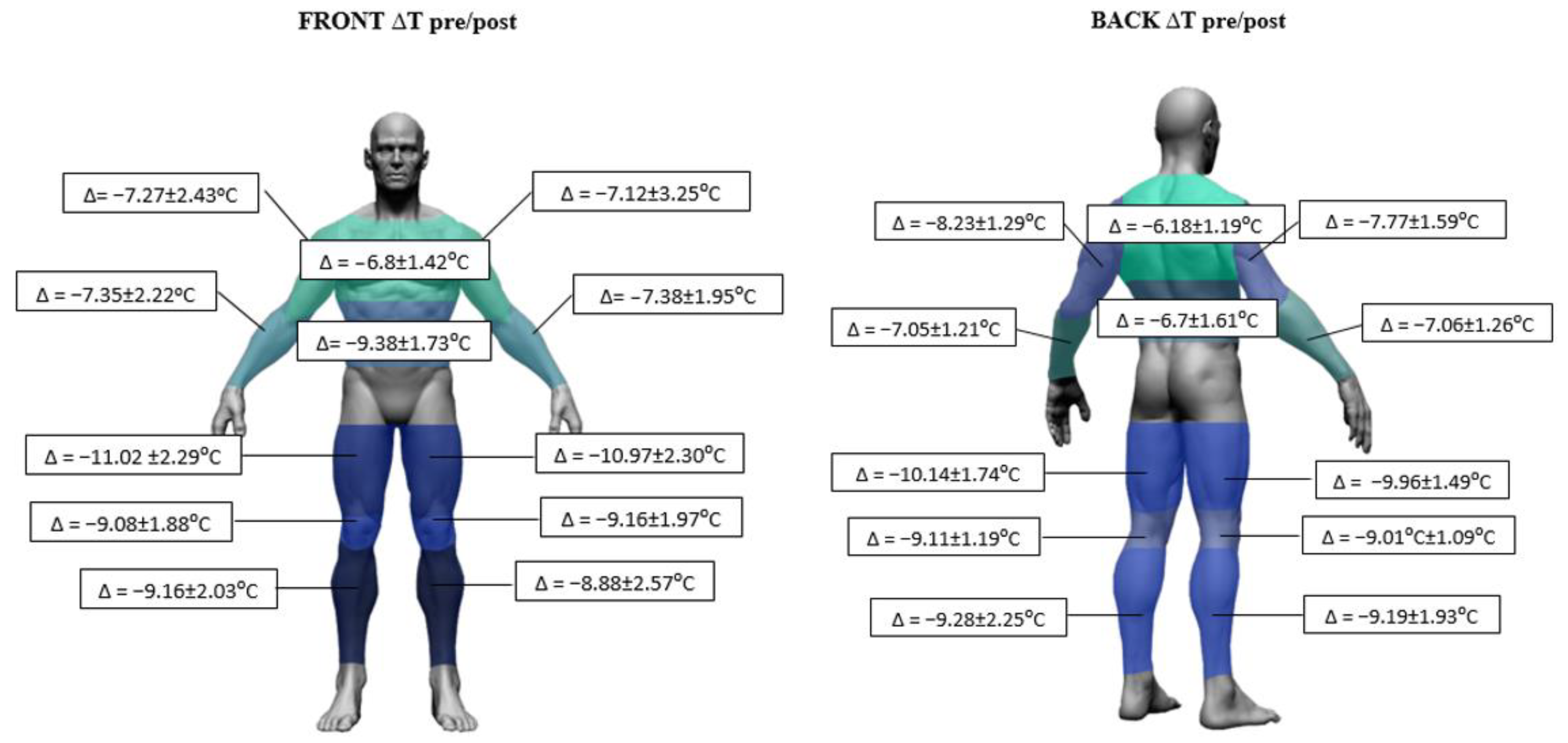
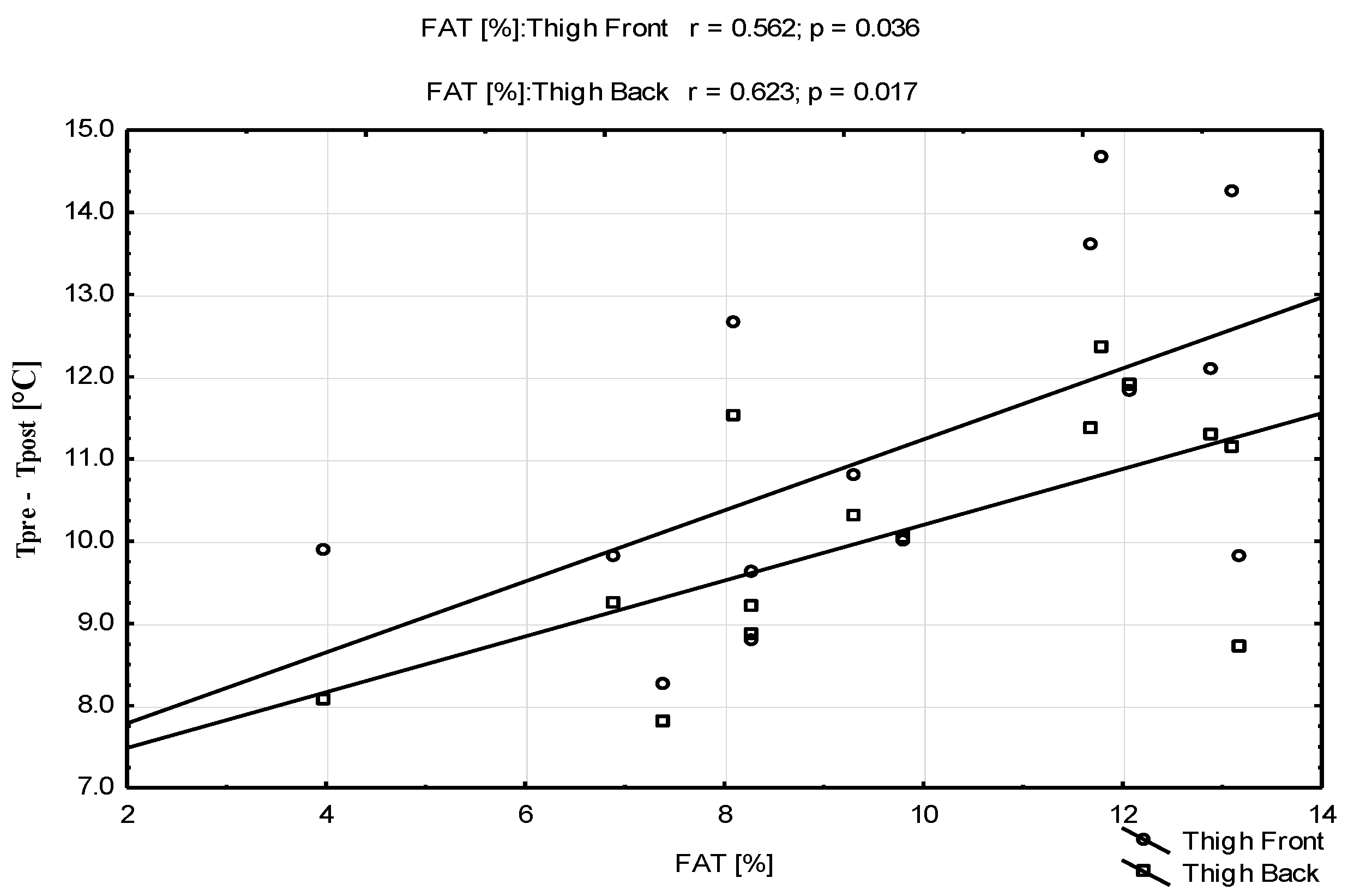
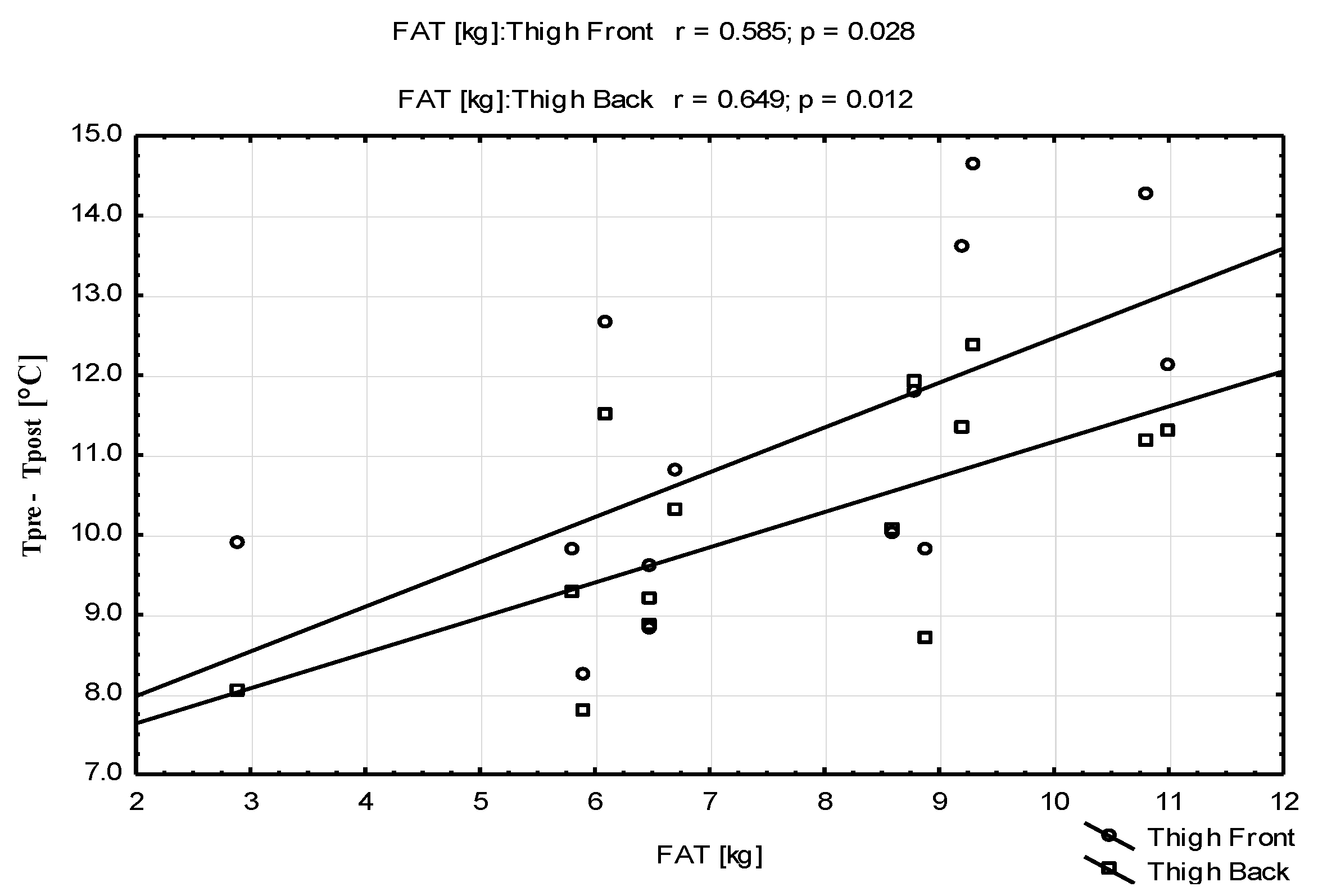
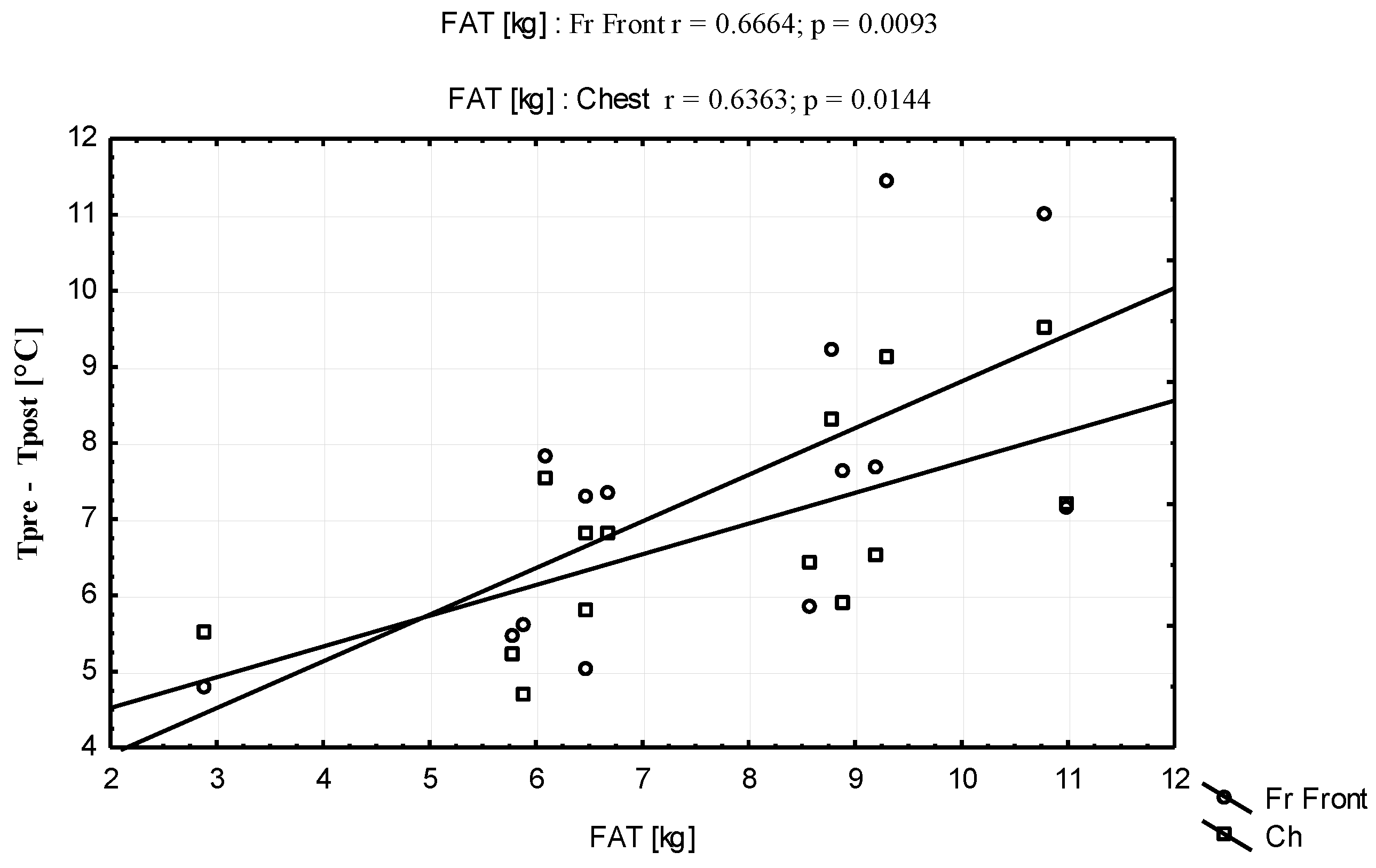


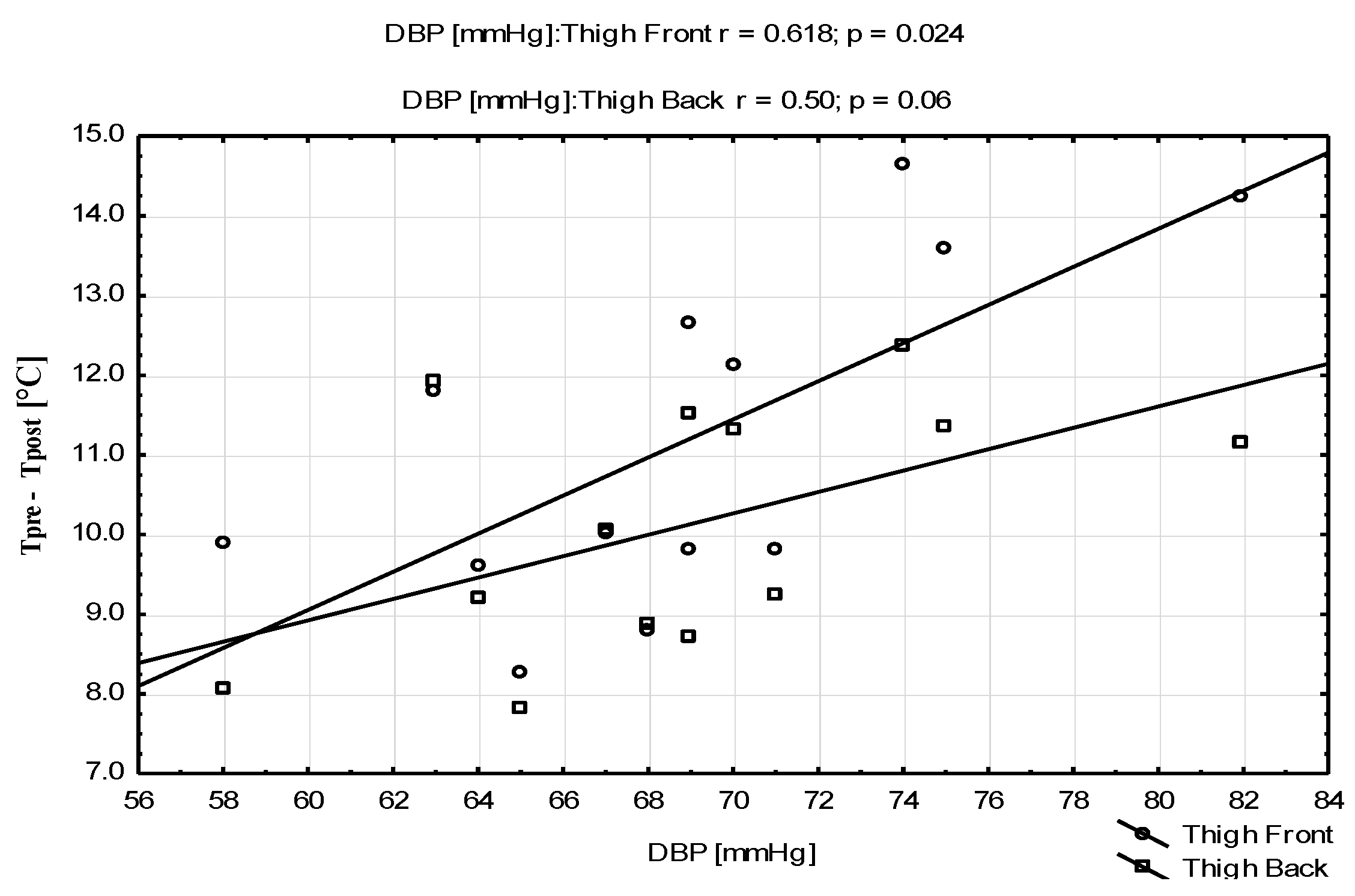
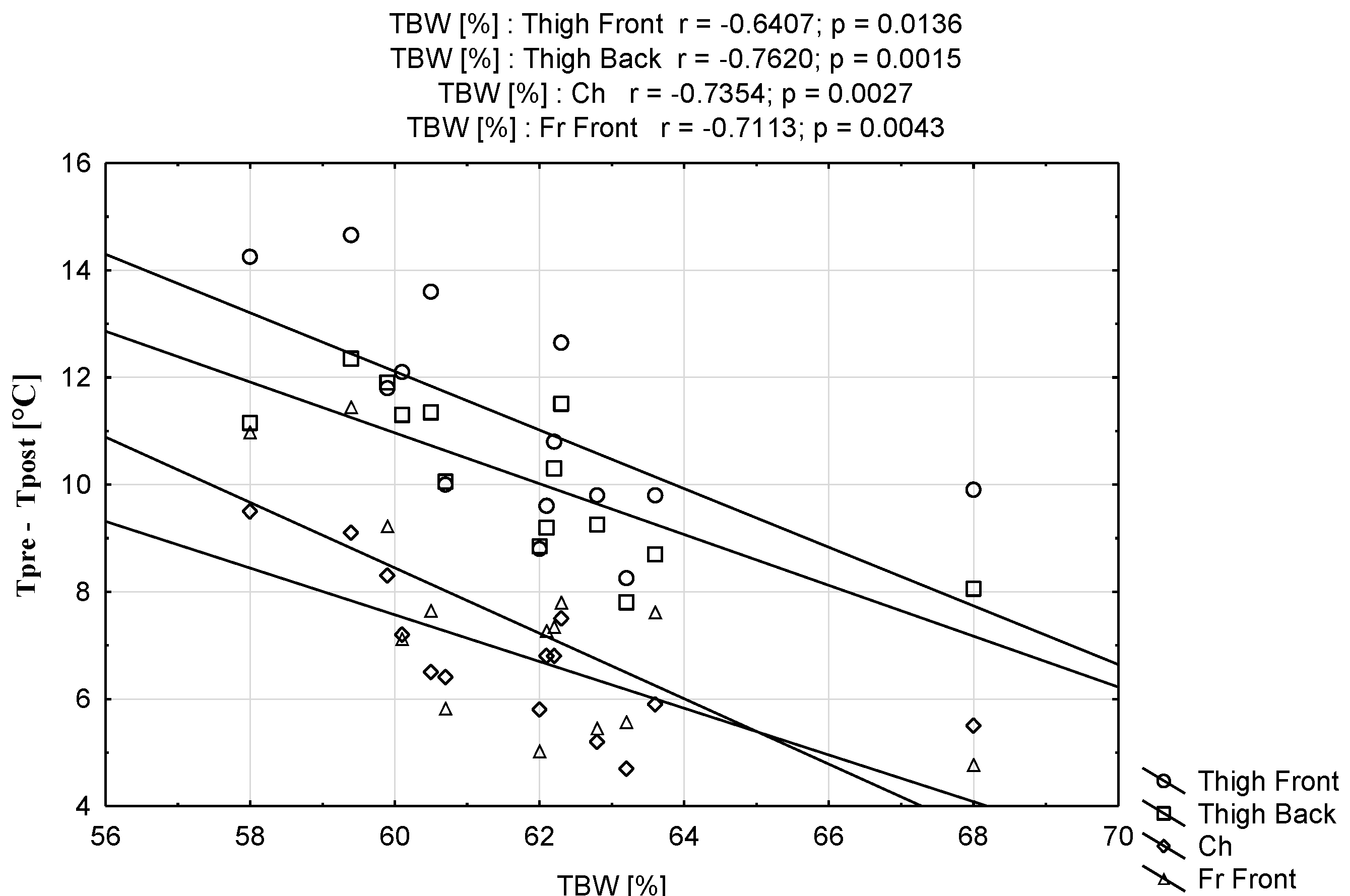
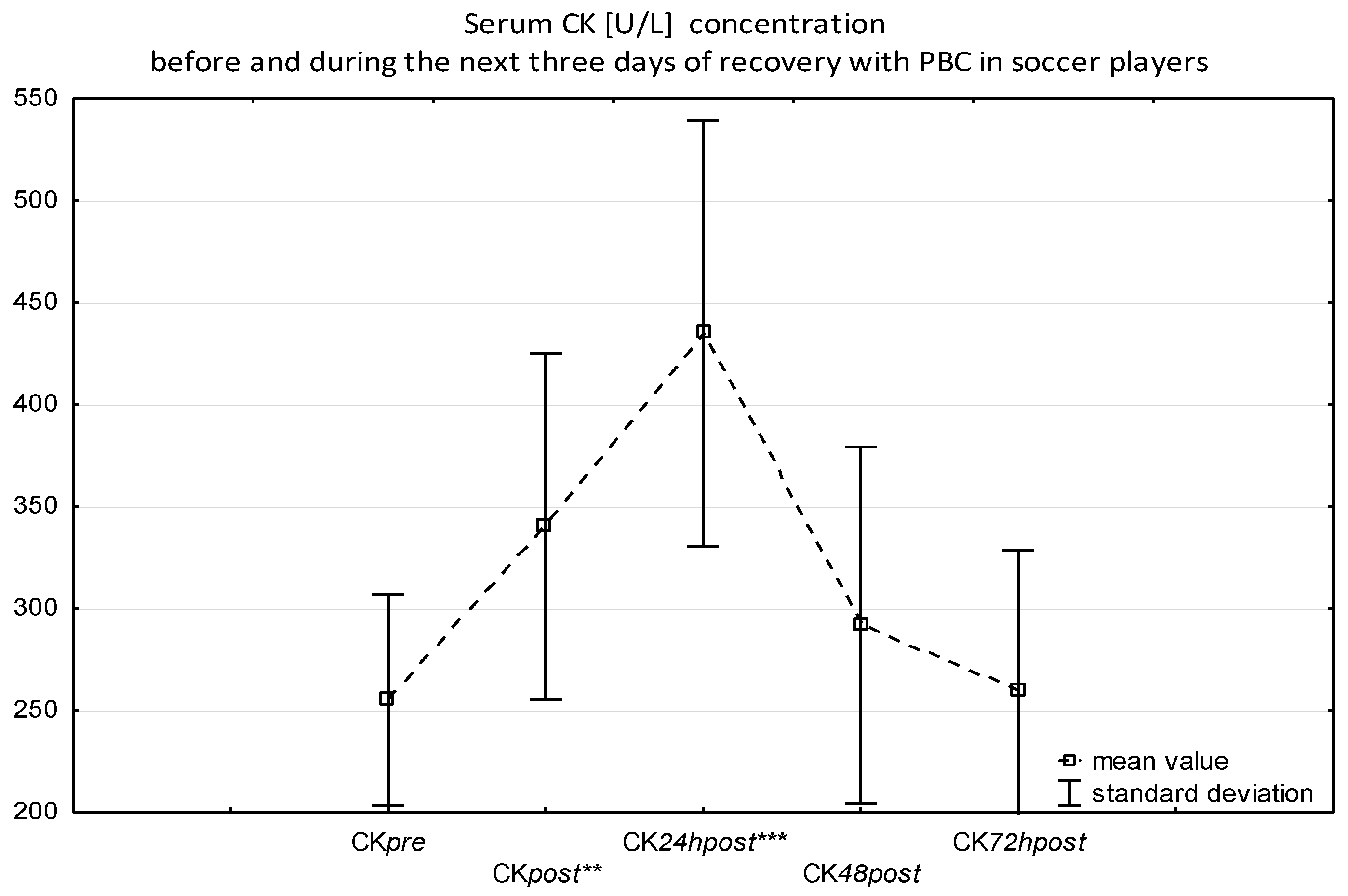
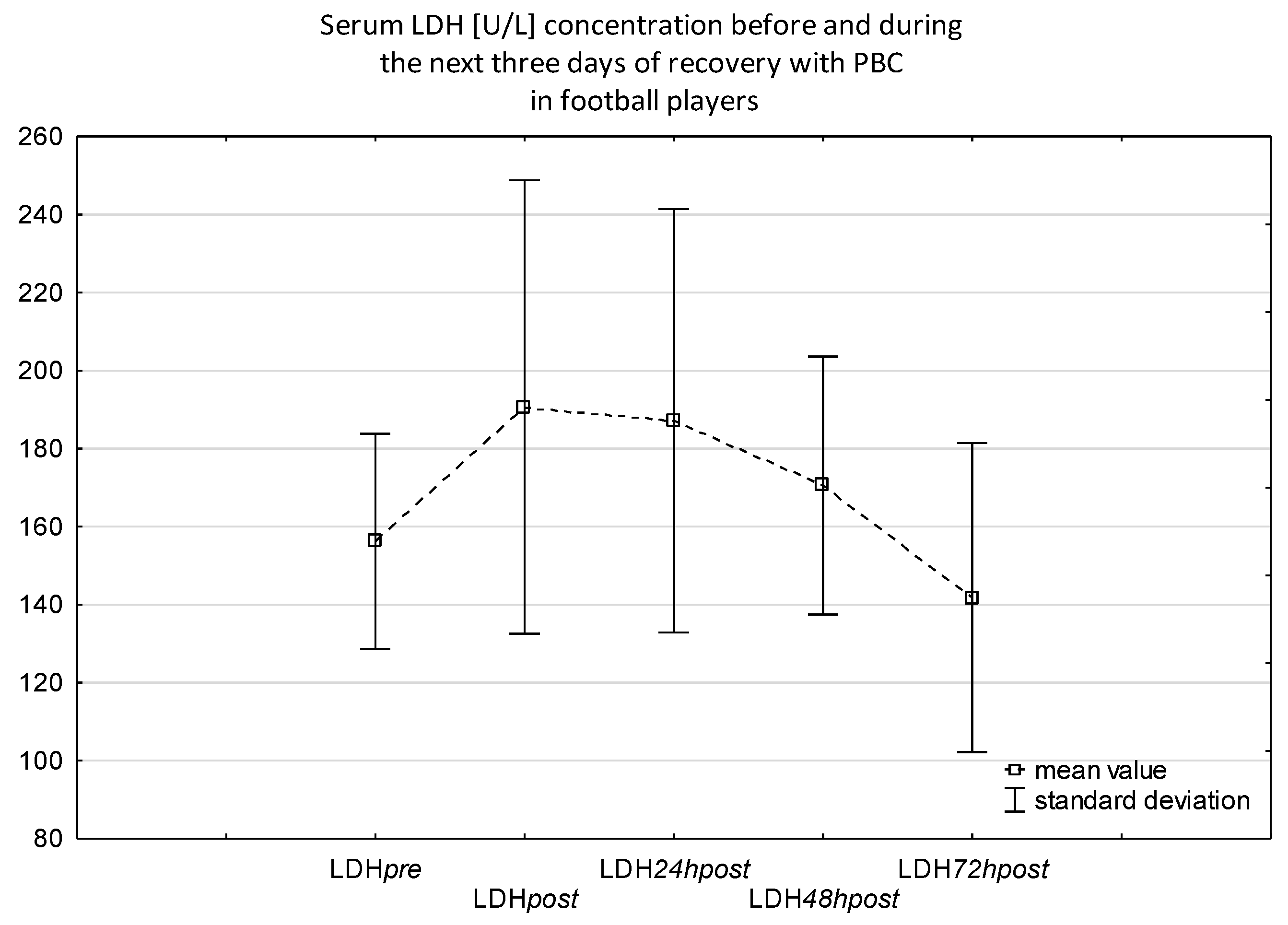

| Parameters | Mean ± SD | Range (Min–Max) |
|---|---|---|
| Age [years] | 26 ± 6 | 19–36 |
| Body mass [kg] | 77.4 ± 5.10 | 67.6–88.2 |
| Body hight [cm] | 181.6 ± 4.52 | 174–191 |
| BMI [kg/m2] | 23.4 ± 1.15 | 22–25.5 |
| FAT [%] | 9.6 ± 2.63 | 4–13.2 |
| FAT [kg] | 7.5 ± 2.15 | 2.9–11 |
| FFM [kg] | 70.0 ± 5.33 | 58.7–79.6 |
| MM [kg] | 66.6 ± 5.08 | 55.8–75.7 |
| TBW [kg] | 47.8 ± 3.20 | 43–53.5 |
| TBW [%] | 61.7 ± 2.24 | 58–68 |
| BM [kg] | 3.5 ± 0.21 | 3.2–3.9 |
| SBP [mmHg] | 127 ± 11 | 105–141 |
| DBP [mmHg] | 69 ± 7 | 58–87 |
| Body Area | Mean ± SD [°C] | Student’s t-Test Tpre vs. Tpost | |||||
|---|---|---|---|---|---|---|---|
| Right/Left | Tpre | Tpost | T | p | |||
| Upper Limb [UL] | A | R | front | 32.92 ± 0.59 | 25.97 ± 1.94 | 12.8828 | 0.0000 *** |
| L | 32.99 ± 0.63 | 26.00 ± 2.11 | 12.4208 | 0.0000 *** | |||
| R | back | 31.35 ± 0.76 | 23.76 ± 1.72 | 18.5029 | 0.0000 *** | ||
| L | 31.43 ± 0.82 | 23.26 ± 1.59 | 22.7050 | 0.0000 *** | |||
| Fr | R | front | 32.17 ± 0.55 | 25.14 ± 2.45 | 10.8496 | 0.0000 *** | |
| L | 32.01 ± 0.87 | 24.97 ± 2.44 | 12.0104 | 0.0000 *** | |||
| R | back | 32.43 ± 0.58 | 25.63 ± 1.35 | 19.4616 | 0.0000 *** | ||
| L | 32.40 ± 0.88 | 25.53 ± 1.42 | 21.7510 | 0.0000 *** | |||
| Lower Limb [LL] | Th | R | front | 30.92 ± 0.72 | 20.20 ± 2.60 | 15.3924 | 0.0000 *** |
| L | 30.98 ± 0.70 | 20.27 ± 2.66 | 15.2046 | 0.0000 *** | |||
| R | back | 31.67 ± 0.81 | 21.80 ± 1.86 | 21.2207 | 0.0000 *** | ||
| L | 31.47 ± 0.61 | 21.40 ± 1.90 | 17.8651 | 0.0000 *** | |||
| K | R | front | 29.64 ± 1.53 | 20.59 ± 2.33 | 15.8567 | 0.0000 *** | |
| L | 29.79 ± 1.56 | 20.94 ± 2.32 | 18.4414 | 0.0000 *** | |||
| R | back | 32.49 ± 0.51 | 23.30 ± 1.55 | 24.5938 | 0.0000 *** | ||
| L | 32.37 ± 0.46 | 23.29 ± 1.53 | 24.1189 | 0.0000 *** | |||
| S | R | front | 31.95 ± 0.89 | 23.10 ± 2.55 | 14.1431 | 0.0000 *** | |
| L | 31.81 ± 0.80 | 23.37 ± 3.16 | 10.6536 | 0.0000 *** | |||
| R | back | 31.57 ± 0.90 | 22.64 ± 2.54 | 14.2310 | 0.0000 *** | ||
| L | 31.46 ± 0.88 | 22.56 ± 3.04 | 12.6118 | 0.0000 *** | |||
| Trunk | Ch | 33.39 ± 0.60 | 26.79 ± 1.49 | 16.5471 | 0.0000 *** | ||
| Ab | 33.04 ± 0.94 | 23.70 ± 2.40 | 17.0142 | 0.0000 *** | |||
| UB | 33.59 ± 0.41 | 27.50 ± 1.24 | 18.4011 | 0.0000 *** | |||
| LB | 33.12 ± 0.61 | 24.25 ± 1.58 | 21.6113 | 0.0000 *** | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubkowska, A.; Knyszyńska, A. Thermographic Assessment of Skin Temperature Changes following Partial Body Cryostimulation (PBC) in Football Players. Appl. Sci. 2023, 13, 4123. https://doi.org/10.3390/app13074123
Lubkowska A, Knyszyńska A. Thermographic Assessment of Skin Temperature Changes following Partial Body Cryostimulation (PBC) in Football Players. Applied Sciences. 2023; 13(7):4123. https://doi.org/10.3390/app13074123
Chicago/Turabian StyleLubkowska, Anna, and Anna Knyszyńska. 2023. "Thermographic Assessment of Skin Temperature Changes following Partial Body Cryostimulation (PBC) in Football Players" Applied Sciences 13, no. 7: 4123. https://doi.org/10.3390/app13074123
APA StyleLubkowska, A., & Knyszyńska, A. (2023). Thermographic Assessment of Skin Temperature Changes following Partial Body Cryostimulation (PBC) in Football Players. Applied Sciences, 13(7), 4123. https://doi.org/10.3390/app13074123







