Preventive Effects of Chlorogenic Acid on Alveolar Bone Loss in Ligature-Induced Periodontitis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
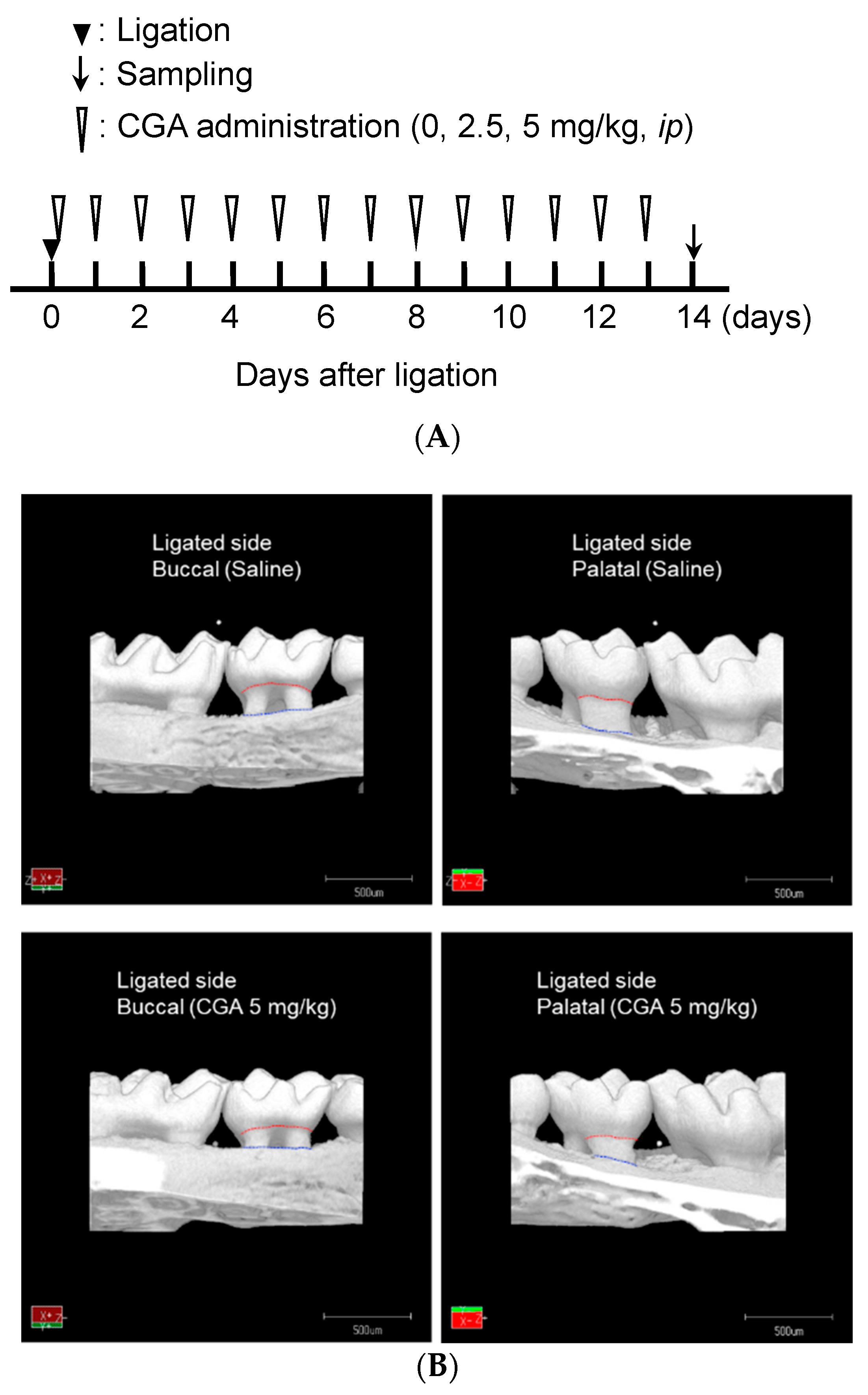
2.3. Creating a Ligature-Induced Periodontitis Model and Chemical Administration
2.4. Microcomputed Tomography (µCT) Analysis
2.5. Histological Analysis
2.6. Statistical Analysis
3. Results
3.1. Inhibitory Effects of CGA on Alveolar Bone Loss in the Ligature-Induced Periodontitis Model
3.2. Restorative Effects of CGA on Alveolar Bone Loss in the Ligature-Induced Periodontitis Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.K.; Singla, R.K.; Pandey, A.K. Chlorogenic acid: A dietary phenolic acid with promising pharmacotherapeutic potential. Curr. Med. Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Namioka, Y.; Azis, H.R.; Sano, T.; Aono, M.; Koshiyama, M.; Fujisawa, H.; Isoda, H. Prohydrojasmon Promotes the Accumulation of Phenolic Compounds in Red Leaf Lettuce. Plants 2021, 10, 1920. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.; Rupasinghe, H.P.; Forney, C.F.; Eaton, L. Characterization of Changes in Polyphenols, Antioxidant Capacity and Physico-Chemical Parameters during Lowbush Blueberry Fruit Ripening. Antioxidants 2013, 2, 216–229. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; Paulis, T.D.; Trugo, L.C.; Martin, P.R. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J. Agric. Food Chem. 2005, 53, 1505–1513. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Galardi, C.; Sani, G.; Cimato, A.; Heimler, D. Polyphenols in greenhouse and open-air-grown lettuce. Food Chem. 2002, 79, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Hamauzu, Y. Phenolic compounds and their antioxidant properties in different tissues of carrots (Daucus carota L.). Food Agric. Environ. 2004, 2, 95–100. [Google Scholar]
- Chen, Y.; Su, J.Y.; Yang, C.Y. Ultrasound-assisted aqueous extraction of chlorogenic acid and cynarin with the impact of inulin from burdock (Arctium lappa L.) roots. Antioxidants 2022, 11, 1219. [Google Scholar] [CrossRef]
- Awad, M.A.; Jager, A.; Westin, L.M.V. Flavonoid and chlorogenic acid levels in apple fruit: Characterisation of variation. Sci. Hortic. 2000, 83, 249–263. [Google Scholar] [CrossRef]
- Kang, J.; Thakali, K.M.; Jensen, G.S.; Wu, X. Phenolic acids of the two major Blueberry species in the US market and their antioxidant and anti-inflammatory activities. Plant Foods Human Nutr. 2015, 70, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Chai, S.S.; Cai, J.H.; Luo, J.H.; Zhao, B.X.; Wu, Z.M.; Liu, X.T.; Tian, H.; Zeng, Y. Inhibition of α-glucosidase by Cyclocarya paliurus based on HPLC fingerprinting integrated with molecular docking and molecular dynamics. Biomed. Chromatogr. 2022, 36, e5429. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M.; Oboh, G.; Bello, G.T.; Oyagbemi, A.A. Caffeic and chlorogenic acids modulate altered activity of key enzymes linked to hypertension in cyclosporine-induced hypertensive rats. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 169–177. [Google Scholar] [CrossRef]
- Saitou, K.; Ochiai, R.; Kozuma, K.; Sato, H.; Koikeda, T.; Osaki, N.; Katsuragi, Y. Effect of Chlorogenic Acids on Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochiai, R.; Saitou, K.; Suzukamo, C.; Osaki, N.; Asada, T. Effect of Chlorogenic Acids on Cognitive Function in Mild Cognitive Im-pairment: A Randomized Controlled Crossover Trial. J. Alzheimers Dis. 2019, 72, 1209–1216. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef]
- Mitrea, D.R.; Malkey, R.; Florian, T.L.; Filip, A.; Clichici, S.; Bidian, C.; Moldovan, R.; Hoteiuc, O.A.; Toader, A.M.; Baldea, I. Daily oral administration of chlorogenic acid prevents the experimental carrageenan-induced oxidative stress. J. Physiol. Pharmacol. 2020, 71, 55–65. [Google Scholar] [CrossRef]
- Yoon, H.S.; Park, C.M. Alleviated Oxidative Damage by Taraxacum officinale through the Induction of Nrf2-MAPK/PI3K Mediated HO-1 Activation in Murine Macrophages RAW264.7 Cell Line. Biomolecules 2019, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Le Sage, F.; Meilhac, O.; Gonthier, M.P. Anti-inflammatory and antioxidant effects of polyphenols extracted from Antirhea borbonica medicinal plant on adipocytes exposed to Porphyromonas gingivalis and Escherichia coli lipopolysaccharides. Pharmacol. Res. 2017, 119, 303–312. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef]
- Petersen, P.E.; Ogawa, H. Strengthening the prevention of periodontal disease: The WHO approach. J. Periodontol. 2005, 76, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrungruang, K.; Jitpakdeebordin, S.; Charatkulangkun, O.; Gleebbua, Y. Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Treponema denticola/Prevotella intermedia Co-Infection Are Associated with Severe Periodontitis in a Thai Population. PLoS ONE 2015, 10, e0136646. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, J.; Mahendra, L.; Felix, J.; Romanos, G.E. Genetic analysis of Porphyromonas gingivalis (fimA), Aggregatibacter actinomycetemcomitans, and red complex in coronary plaque. J. Investig. Clin. Dent. 2014, 5, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Song, B.; Brandt, B.W.; Cheng, L.; Zhou, X.; Exterkate, R.A.M.; Crielaard, W.; Deng, D.M. Comparison of Red-Complex Bacteria Between Saliva and Subgingival Plaque of Periodontitis Patients: A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 2021, 11, 727732. [Google Scholar] [CrossRef]
- Yanushevich, O.O.; Ayvazova, R.A.; Shibaeva, A.V.; Rebrikov, D.V.; Trubnikova, E.V.; Kudykina, Y.K.; Zylnikova, M.V.; Zaripova, R.S.; Shevelev, A.B. Quantitative PCR studies of Aggregatibacter actinomycetemcomitans and Treponema denticola/Tanerella forsythensis Complex as Etiological Factors of Chronic Periodontitis. Bull. Exp. Biol. Med. 2016, 160, 495–497. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Mineoka, T.; Awano, S.; Rikimaru, T.; Kurata, H.; Yoshida, A.; Ansai, T.; Takehara, T. Site-specific development of periodontal disease is associated with increased levels of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia in subgingival plaque. J. Periodontol. 2008, 79, 670–676. [Google Scholar] [CrossRef]
- Pradhan-Palikhe, P.; Mäntylä, P.; Paju, S.; Buhlin, K.; Persson, G.R.; Nieminen, M.S.; Sinisalo, J.; Pussinen, P.J. Subgingival bacterial burden in relation to clinical and radiographic periodontal parameters. J. Periodontol. 2013, 84, 1809–1817. [Google Scholar] [CrossRef]
- Kwak, S.C.; Lee, C.; Kim, J.Y.; Oh, H.M.; So, H.S.; Lee, M.S.; Rho, M.C.; Oh, J. Chlorogenic Acid Inhibits Osteoclast Differentiation and Bone Resorption by Down-Regulation of Receptor Activator of Nuclear Factor Kappa-B Ligand-Induced Nuclear Factor of Activated T Cells c1 Expression. Biol. Pharm. Bull. 2013, 36, 1779–1786. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xu, J.; Hu, J.F.; Hu, Q.Y.; Fang, X.; Sun, Z.J.; Xu, Z.; Zhang, L. Sustained release of chlorogenic acid-loaded nanomicelles alleviates bone loss in mouse periodontitis. Biomater. Sci. 2022, 10, 5583–5595. [Google Scholar] [CrossRef]
- Hu, X.; Wang, L.; He, Y.; Wei, M.; Yan, H.; Zhu, H. Chlorogenic Acid Promotes Osteogenic Differentiation of Human Dental Pulp Stem Cells Through Wnt Signaling. Stem. Cells Dev. 2021, 30, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, J.; Girnary, M.S.; Jing, L.; Miao, M.Z.; Zhang, S.; Sun, L.; Morelli, T.; Schoenfisch, M.H.; Inohara, N.; Offenbacher, S.; et al. An experimental murine model to study periodontitis. Nat. Protoc. 2018, 13, 2247–2267. [Google Scholar] [CrossRef]
- Abe, T.; Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 2013, 394, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Ara, T.; Nakatani, S.; Kobata, K.; Sogawa, N.; Sogawa, C. The Biological Efficacy of Natural Products against Acute and Chronic Inflammatory Diseases in the Oral Region. Medicines 2018, 5, 122. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Deng, W.-S.; Liu, Y.; Jiang, C.-H.; Sun, L.-C.; Sun, X.-F.; Xu, Q.; Zhou, H. Ellagic acid protects Lipopolysaccharide/D-galactosamine-induced acute hepatic injury in mice. Int. Immunopharmacol. 2014, 22, 341–345. [Google Scholar] [CrossRef]
- Beserra, A.; Calegari, P.; Souza Mdo, C.; Dos Santos, R.; Lima, J.; Silva, R.; Balogun, S.; Martins, D. Gastroprotective and ulcer-healing mechanisms of ellagic acid in experimental rats. J. Agric. Food Chem. 2011, 59, 6957–6965. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.; María Giner, R.; Ríos, J.; Recio, M. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Songkro, S.; Reanmongkol, W. Topical anti-inflammatory potential of standardized pomegranate rind extract and ellagic acid in contact dermatitis. Phytother. Res. 2014, 28, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Tsou, S.H.; Hu, S.W.; Yang, J.J.; Yan, M.; Lin, Y.Y. Potential Oral Health Care Agent from Coffee against Virulence Factor of Periodontitis. Nutrients 2019, 11, 2235. [Google Scholar] [CrossRef] [Green Version]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Li, L.; Chen, B.; Fang, Y.; Lin, W.; Zhang, T.; Feng, X.; Tao, X.; Wu, Y.; Fu, X.; et al. Chlorogenic acid exerts neuroprotective effect against hypoxia-ischemia brain injury in neonatal rats by activating Sirt1 to regulate the Nrf2-NF-κB signaling pathway. Cell Commun. Signal. 2022, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, B.; Kim, O.; Yang, Y.; Liu, D.; Fu, W.; Ma, G.; Kim, Y.; Kim, O. Effect of Coffee on Lipopolysaccharide-Induced Immortalized Human Oral Keratinocytes. Foods 2022, 11, 2199. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, H.; Zhang, Y.; Zhang, Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Drug Des. Devel. Ther. 2020, 14, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Liu, Y.; Shen, H.; Fu, T.; Guo, Y.; Qiu, S. Chlorogenic acid attenuates inflammation in LPS-induced Human gingival fibroblasts via CysLT1R/Nrf2/NLRP3 signaling. Int. Immunopharmacol. 2022, 107, 108706. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [Green Version]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Maiorani, C.; Preda, C.; Chiesa, A.; Esposito, F.; Pascadopoli, M.; Scribante, A. Management of Gingival Bleeding in Periodontal Patients with Domiciliary Use of Toothpastes Containing Hyaluronic Acid, Lactoferrin, or Paraprobiotics: A Randomized Controlled Clinical Trial. Appl. Sci. 2021, 11, 8586. [Google Scholar] [CrossRef]
- Vale, G.C.; Mayer, M.P.A. Effect of probiotic Lactobacillus rhamnosus by-products on gingival epithelial cells challenged with Porphyromonas gingivalis. Arch. Oral Biol. 2021, 128, 105174. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, Y.; Shimada, K.; Horibe, K.; Seki, K.; Murai, Y.; Sogawa, C.; Murakami, S.; Nakamura, H.; Masuda, Y.; Sogawa, N. Preventive Effects of Chlorogenic Acid on Alveolar Bone Loss in Ligature-Induced Periodontitis in Mice. Appl. Sci. 2023, 13, 4129. https://doi.org/10.3390/app13074129
Nishida Y, Shimada K, Horibe K, Seki K, Murai Y, Sogawa C, Murakami S, Nakamura H, Masuda Y, Sogawa N. Preventive Effects of Chlorogenic Acid on Alveolar Bone Loss in Ligature-Induced Periodontitis in Mice. Applied Sciences. 2023; 13(7):4129. https://doi.org/10.3390/app13074129
Chicago/Turabian StyleNishida, Yuka, Katsumitsu Shimada, Kanji Horibe, Kousuke Seki, Yoshinori Murai, Chiharu Sogawa, Satoshi Murakami, Hiroaki Nakamura, Yuji Masuda, and Norio Sogawa. 2023. "Preventive Effects of Chlorogenic Acid on Alveolar Bone Loss in Ligature-Induced Periodontitis in Mice" Applied Sciences 13, no. 7: 4129. https://doi.org/10.3390/app13074129
APA StyleNishida, Y., Shimada, K., Horibe, K., Seki, K., Murai, Y., Sogawa, C., Murakami, S., Nakamura, H., Masuda, Y., & Sogawa, N. (2023). Preventive Effects of Chlorogenic Acid on Alveolar Bone Loss in Ligature-Induced Periodontitis in Mice. Applied Sciences, 13(7), 4129. https://doi.org/10.3390/app13074129





