Use of a Novel Mathematical Model to Assess the Effectiveness of Skin-to-Skin Care for the Prevention of Hypothermia in Low-Birth-Weight Neonates
Abstract
Featured Application
Abstract
1. Introduction
2. Method
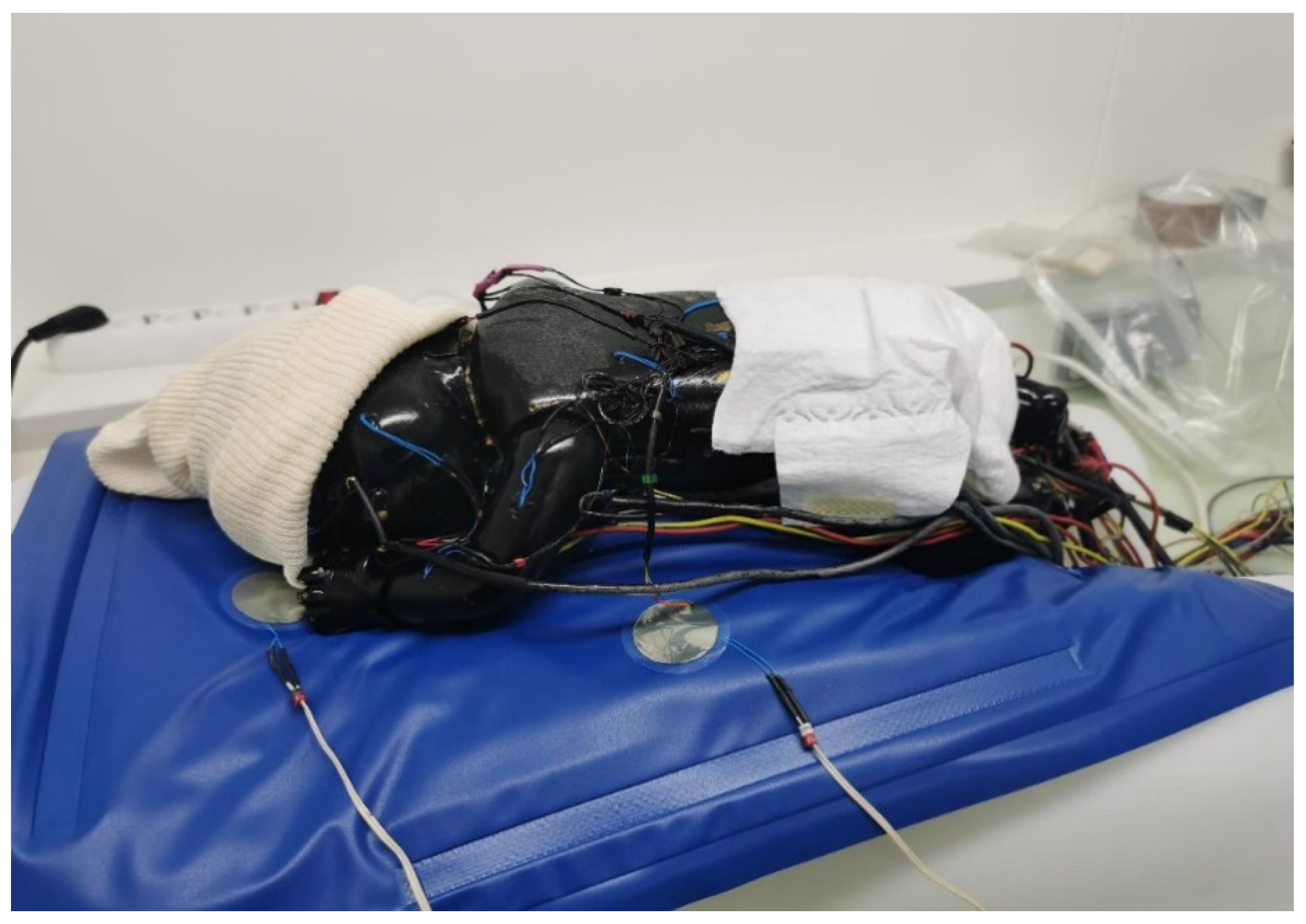
2.1. The Thermal Mannequin
2.2. Design of the Mathematical Model
2.3. Assessment of the Clothing-Associated Reduction in Thermal Insulation (Fcl, Fpcl)
2.4. The Experiments
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malaysian Very Low Birth Weight Study Group. A national study of risk factors associated with mortality in very low birthweight infants in the Malaysian neonatal intensive care units. J. Paediatr. Child Health 1997, 33, 18–25. [Google Scholar] [CrossRef]
- Laptook, A.R.; Salhab, W.; Bhaskar, B.; Neonatal Research Network. Admission temperature of low birth weight infants: Predictors and associated morbidities. Pediatrics 2007, 119, e643–e649. [Google Scholar] [CrossRef] [PubMed]
- Dahm, L.S.; James, L.S. Newborn temperature and calculated heat loss in the delivery room. Pediatrics 1972, 49, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Organisation Mondiale de la Santé. Bureau Régional du Pacifique Occidental. Premiers Soins Essentiels au Nouveau-né. Guide de Poche de Pratique Clinique. Bureau Régional de l’OMS pour le Pacifique Occidental. 2017. Available online: https://apps.who.int/iris/handle/10665/260404 (accessed on 28 February 2023).
- Kattwinkel, J.; Niermeyer, S.; Nadkarni, V.; Tibballs, J.; Phillips, B.; Zideman, D.; Van Reempts, P.; Osmond, M. Resuscitation of the newly born infant: An advisory statement from the Pediatric Working Group of the International Liaison Committee on Resuscitation. Circulation 1999, 40, 71–88. [Google Scholar] [CrossRef]
- World Health Organization. Maternal and Newborn Health/Safe Motherhood. Thermal Protection of the Newborn: A Practical Guide. World Health Organization. 1997. Report No.: WHO/RHT/MSM/97.2. Available online: https://apps.who.int/iris/handle/10665/6398 (accessed on 28 March 1997).
- Färdig, J.A. A comparison of skin-to-skin contact and radiant heaters in promoting neonatal thermoregulation. J. Nurse Midwifery 1980, 25, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Christensson, K.; Siles, C.; Moreno, L.; Belaustequi, A.; De La Fuente, P.; Lagercrantz, H.; Puyol, P.; Winberg, J. Temperature, metabolic adaptation and crying in healthy full-term newborns cared for skin-to-skin or in a cot. Acta Paediatr. Oslo Nor. 1992, 81, 488–493. [Google Scholar] [CrossRef]
- Christidis, I.; Zotter, H.; Rosegger, H.; Engele, H.; Kurz, R.; Kerbl, R. Infrared thermography in newborns: The first hour after birth. Gynakol. Geburtshilfliche Rundsch. 2003, 43, 31–35. [Google Scholar] [CrossRef]
- Christensson, K.; Bhat, G.J.; Amadi, B.C.; Eriksson, B.; Höjer, B. Randomised study of skin-to-skin versus incubator care for rewarming low-risk hypothermic neonates. Lancet Lond. Eng. 1998, 352, 1115. [Google Scholar] [CrossRef]
- Srivastava, S.; Gupta, A.; Bhatnagar, A.; Dutta, S. Effect of very early skin to skin contact on success at breastfeeding and preventing early hypothermia in neonates. Indian J. Public Health 2014, 58, 22–26. [Google Scholar] [CrossRef]
- Jia, Y.S.; Lin, Z.l.; Lv, H.; Li, Y.M.; Green, R.; Lin, J. Effect of a delivery room temperature on the admission temperature of premature infants: A randomized controlled trial. J. Perinatol. 2013, 3, 264–267. [Google Scholar] [CrossRef]
- Kardum, D.; Bell, E.F.; Grčić, B.F.; Müller, A. Duration of skin-to-skin care and rectal temperatures in late preterm and term infants. BMC Pregnancy Childbirth 2022, 22, 655. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H. Skin-to-skin care: Heat balance. Arch. Dis. Child. 1966, 75, F130–F132. [Google Scholar] [CrossRef] [PubMed]
- International Standards Organisation (ISO) 9920. Ergonomics of the Thermal Environment-Estimation of the Thermal Insulation and Evaporative Resistance of a Clothing Ensemble; 93571 Saint Denis La Plaine Cedex: Saint-Denis, France, 1995; pp. 313–372. [Google Scholar]
- Bauer, J.; Werner, C.; Gerss, J. Metabolic rate analysis of healthy preterm and full-term infants during the first weeks of life. Am. J. Clin. Nutr. 2009, 90, 1517–1524. [Google Scholar] [CrossRef]
- Silverman, W.; Agate, F.J. Variation in cold resistance among small newborn infants. Biol. Neonat. 1964, 6, 113–127. [Google Scholar] [CrossRef]
- Adamson, S.K.; Towell, M.E. Thermal homeostasis in the fetus and newborn. Anesthesiology 1965, 26, 531–548. [Google Scholar] [CrossRef]
- Pribylova, H.; Znamenacek, K. The effect of body temperature on the level of carbohydrate metabolites and oxygen consumption in the newborn. Pediatrics 1966, 37, 743–749. [Google Scholar] [CrossRef]
- Gandy, G.M.; Adamson, K.; Cunningham, N.; Silverman, W.A.; James, L.S. Thermal environment and acid base homeostasis in human infants during the first few hours of life. J. Clin. Investig. 1964, 43, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, R.J.; Hardy, J.D.; Fessenden, E. Electrical analog simulation of temperature regulation in man. In Temperature: Its Measurement and Control in Science and Industry; Hardy, J.D., Ed.; Part 3; Biology and Medicine: New York, NY, USA, 1963; pp. 627–635. [Google Scholar]
- Houdas, Y.; Guieu, J.D. Le système thermorégulateur de l’Homme: Système régulé ou système asservi? Arch. Sci. Physiol. 1973, 27, 311–338. [Google Scholar]
- Lenzi, P.; Libert, J.P.; Franzini, C.; Cianci, T.; Guidalotti, P.L. Short-term thermoregulatory adjustments involving opposite regional temperature changes. J. Therm. Biol. 1986, 11, 151–156. [Google Scholar] [CrossRef]
- Libert, J.P.; Tourneux, P. The thermostatic control of body temperature. In Vegetative Functions and Their Interactions in Neonates; Chardon, K., Ed.; Research Sing Post Press: Ahmedabad, India, 2010. [Google Scholar]
- Fenton, T.R.; Kim, J.H. A systematic review and metanalysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Chessex, P.; Reichman, B.L.; Verellen, G.J.; Putet, G.; Smith, J.M.; Heim, T.; Swyer, P.R. Influence of postnatal age, energy intake, and weight gain on energy metabolism in the very low-birth-weight infant. J. Pediatr. 1981, 99, 761–766. [Google Scholar] [CrossRef] [PubMed]
- International Standards Organisation (ISO) 7933:1989 Modified. Analytical Determination and Interpretation of Thermal Stress Using Calculation of Required Sweat Rate; AFNOR, 93571 Saint Denis La Plaine Cedex: Saint-Denis, France, 1997; pp. 259–290. [Google Scholar]
- Hanson, R.d.G. Respiratory heat loss at increased -core-temperature. J. Applied Physiol. 1974, 37, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Gagge, A.P. A new physiological variable associated with sensible and insensible perspiration. Am. J. Physiol. 1937, 120, 277–287. [Google Scholar] [CrossRef]
- Hannouch, A. Analyses Numériques et Expérimentales du Transfert Thermique dans un Incubateur Néonatal avec un Mannequin Imprimé en 3D. Thèse de Doctorat, University of Angers, Angers, France, 2021. Available online: https://www.theses.fr/2021ANGE0051 (accessed on 23 November 2021).
- Adams, A.K.; Nelson, R.A.; Bell, E.F.; Egoavil, C.A. Use of infrared thermographic calorimetry to determine energy expenditure in preterm infants. Am. J. Clin. Nutr. 2000, 71, 969–977. [Google Scholar] [CrossRef]
- Museux, N.; Cardot, V.; Bach, V.; Delanaud, S.; Degrugilliers, L.; Agourram, B.; Elabbassi, E.B.; Libert, J.P. A reproducible means of assessing the metabolic heat status of preterm neonates. Med. Phys. 2008, 35, 89–100. [Google Scholar] [CrossRef]
- Bazett, H.C. The regulation of body temperature. In Physiology of Heat Regulation and the Science of Clothing; Newburgh, L.H., Ed.; W.B. Saunders Company: Philadelphia, PA, USA; London, UK, 1949; Chapter 4; pp. 109–192. [Google Scholar]
- Hey, E.N.; Katz, G. Temporary loss of a metabolic response to cold stress in infants of low birthweight. Arch. Dis. Child. 1969, 44, 323–330. [Google Scholar] [CrossRef]
- Asakura, H.; Nakai, A.; Power, G.G.; Araki, T. Short term effects of different thermal conditions during uteroplacental ischaemia on fetal growth of Sprague-Dawley rats. Reprod Fertile Dev. 2002, 14, 355–361. [Google Scholar] [CrossRef]
- Yoneyama, Y.; Wakatsuki, M.; Sawka, R.; Kamoi, S.; Takahashi, H.; Shin, S.; Power, G.G.; Araki, T. Plasma adenosine concentration in appropriate and small-for-gestational-age fetus. Am. J. Obstet. Gynecol. 1974, 170, 684–688. [Google Scholar] [CrossRef]
- Oya, A.; Asakura, H.; Koshino, T.; Araki, T. Thermographic demonstration of non-shivering thermogenesis in human newborns after birth: Its relation to umbilical gases. J. Perinat. Med. 1977, 25, 447–454. [Google Scholar] [CrossRef]
- Hey, E.N.; Katz, G. The range of thermal insulation in the tissues of the newborn baby. J. Physiol. Lond. 1970, 207, 667–681. [Google Scholar] [CrossRef]
- Berg, K.; Celander, O. Circulatory adaptation in the thermoregulation of fullterm and premature newborn infants. Acta Paediatr. Scand. 1971, 60, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.; Olegård, R.; Nilsson, K. Regional skin temperature, heat flow and conductance in preterm neonates nursed in low and in neutral environmental temperature. Acta Paediatr. 1996, 85, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J. Neonatal hypothermia. J. Neonat. Nurs. 2005, 11, 76–82. [Google Scholar] [CrossRef]
- Hammarlund, K.; Sedin, G. Water evaporation and heat exchange with the environment in newborn infants. Acta Paediatr. Scand. 1983, 305, 32–35. [Google Scholar] [CrossRef]

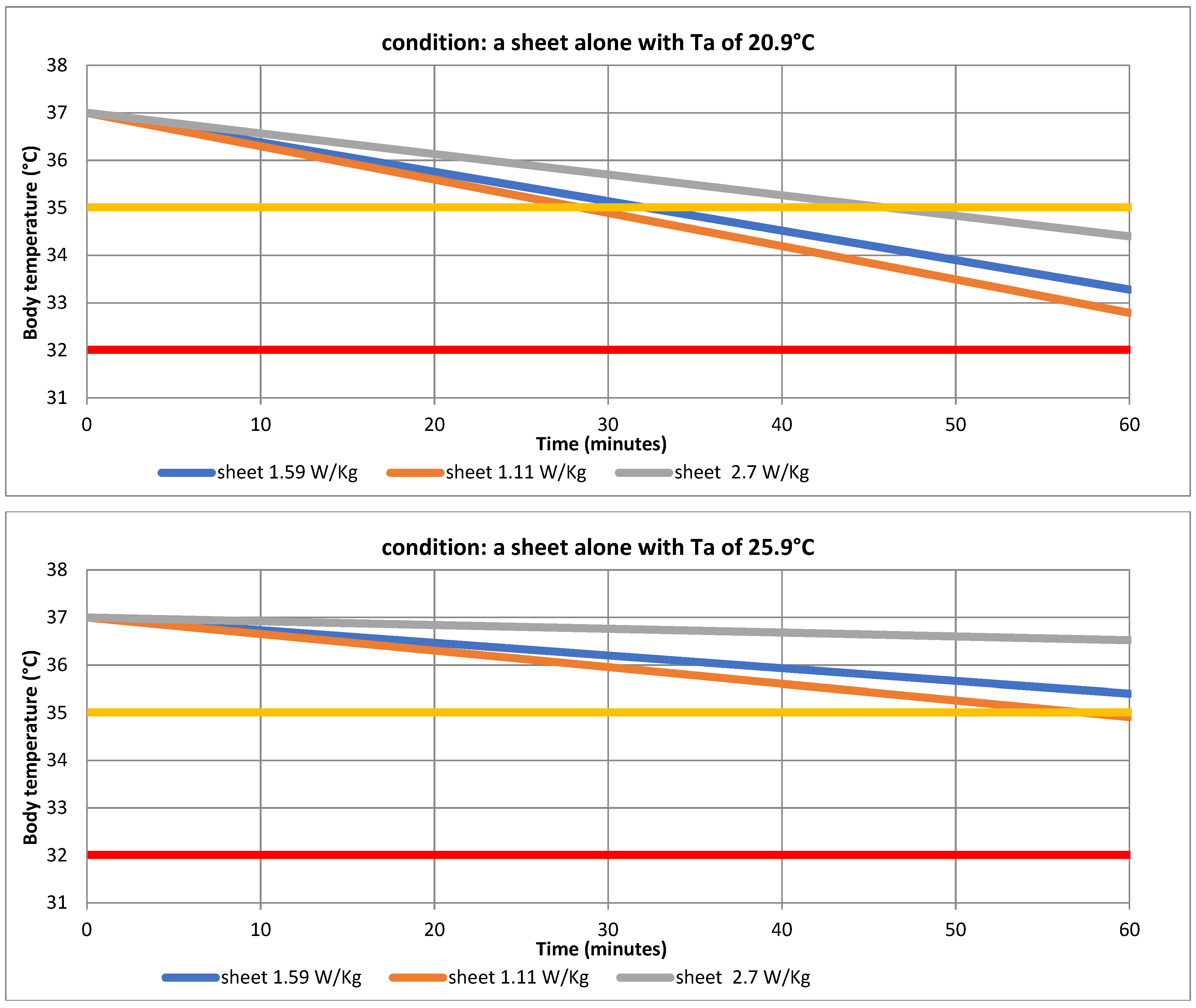
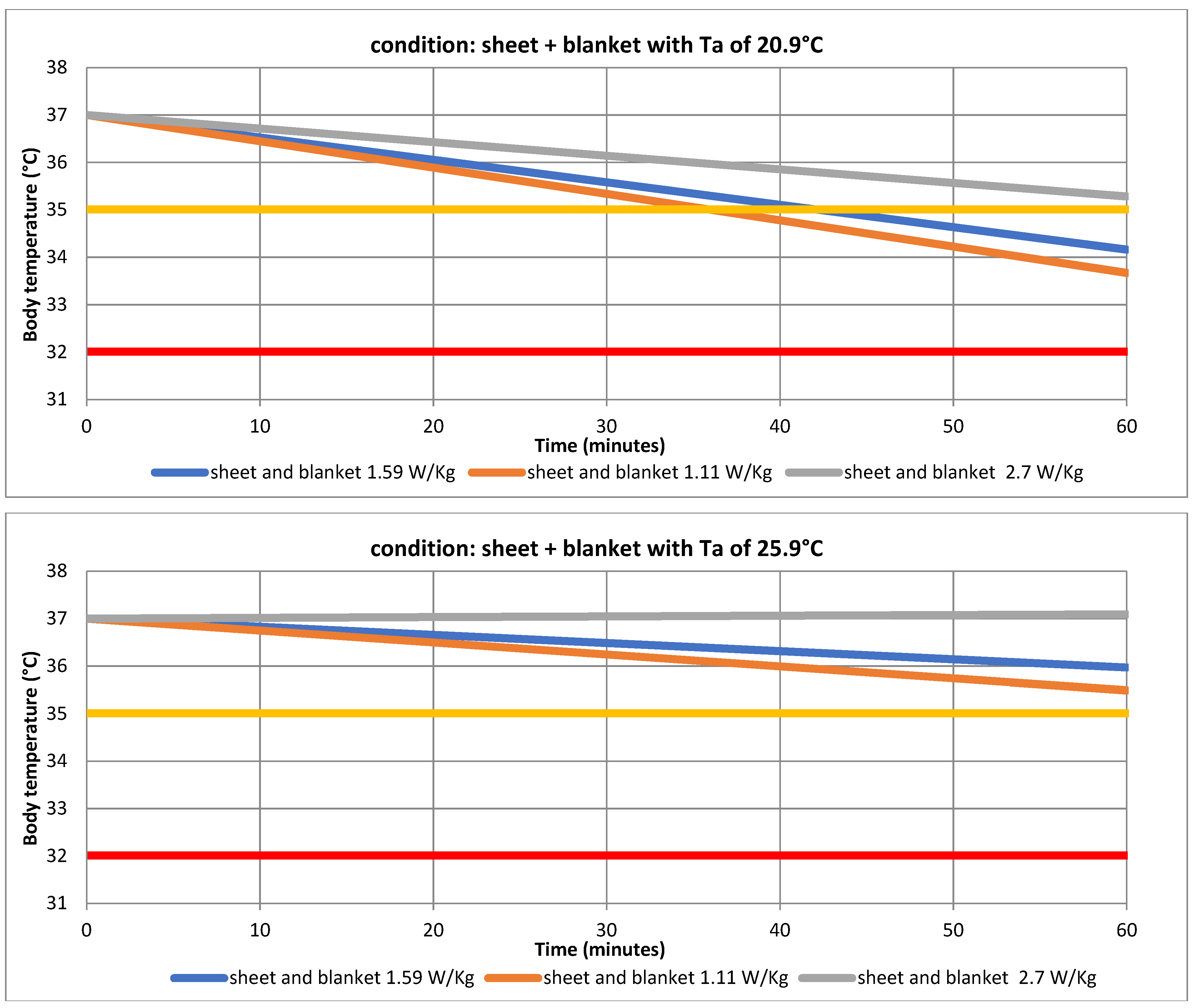
| Ta | 20.9 °C | 25.9 °C | ||
|---|---|---|---|---|
| Condition | Sheet | Sheet + Blanket | Sheet | Sheet + Blanket |
| (R + C)clo | −4.31 ± 0.14 | −3.76 ± 0.17 | −2.76 ± 0.11 | −2.30 ± 0.18 |
| (R + C)n | 8.92 ± 0.10 | 5.34 ± 0.10 | ||
| Fcl | 0.49 | 0.42 | 0.52 | 0.43 |
| Icl | 0.15 | 0.2 | 0.15 | 0.21 |
| Fpcl | 0.42 | 0.36 | 0.48 | 0.39 |
| Ta | 20.9 °C | 25.9 °C | ||
|---|---|---|---|---|
| Conditions | Sheet | Sheet + Blanket | Sheet | Sheet + Blanket |
| M = 1.11 W/Kg | ||||
| R + C + K | −4.48 ± 0.24 | −3.73 ± 0.16 | −2.52 ± 0.11 | −2.07 ± 0.10 |
| E | −0.69 ± 0.0009 | −0.54 ± 0.0004 | −0.59 ± 0.002 | −0.48 ± 0.002 |
| Eres | −0.0158 ± 0.0006 | −0.0182 ± 0.0010 | 0.0196 ± 0.0009 | −0.0222 ± 0.0003 |
| Cres | −0.003 ± 0.000 | −0.003 ± 0.000 | −0.0029 ± 0.000 | −0.0029 ± 0.000 |
| S | −4.08 ± 0.23 | −3.23 ± 0.16 | −2.03 ± 0.11 | −1.47 ± 0.10 |
| dTb/dt | −4.21 ± 0.24 | −3.33 ± 0.16 | −2.09 ± 0.11 | −1.51 ± 0.11 |
| Tb | 32.79 ± 0.24 | 33.67 ± 0.16 | 34.91 ± 0.11 | 35.49 ± 0.11 |
| M = 1.59 W/Kg | ||||
| Eres | −0.0226 ± 0.0008 | −0.026 ± 0.002 | −0.028 ± 0.001 | −0.0318 ± 0.000 |
| Cres | −0.0045 ± 0.000 | −0.0044 ± 0.000 | −0.0042 ± 0.000 | −0.0042 ± 0.000 |
| S | −3.61 ± 0.23 | −2.76 ± 0.16 | −1.56 ± 0.11 | −1.00 ± 0.11 |
| dTb/dt | −3.72 ± 0.24 | −2.84 ± 0.16 | −1.60 ± 0.11 | −1.03 ± 0.11 |
| Tb | 33.28 ± 0.24 | 34.16 ± 0.16 | 35.40 ± 0.11 | 35.97 ± 0.11 |
| M = 2.70 W/Kg | ||||
| Eres | −0.0384 ± 0.001 | 0.0442 ± 0.0030 | −0.0476 ± 0.0021 | −0.0539 ± 0.0008 |
| Cres | −0.0076 ± 0.000 | −0.0076 ± 0.000 | −0.0071 ± 0.000 | −0.0071 ± 0.000 |
| S | −2.52 ± 0.23 | −1.67 ± 0.16 | −0.47 ± 0.11 | +0.09 ± 0.10 |
| dTb/dt | −2.60 ± 0.24 | −1.72 ± 0.16 | −0.48 ± 0.11 | +0.09 ± 0.11 |
| Tb | 34.40 ± 0.24 | 35.28 ± 0.16 | 36.52 ± 0.11 | 37.09 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delanaud, S.; Gossart, L.; Leclercq, M.; Libert, J.-P. Use of a Novel Mathematical Model to Assess the Effectiveness of Skin-to-Skin Care for the Prevention of Hypothermia in Low-Birth-Weight Neonates. Appl. Sci. 2023, 13, 4412. https://doi.org/10.3390/app13074412
Delanaud S, Gossart L, Leclercq M, Libert J-P. Use of a Novel Mathematical Model to Assess the Effectiveness of Skin-to-Skin Care for the Prevention of Hypothermia in Low-Birth-Weight Neonates. Applied Sciences. 2023; 13(7):4412. https://doi.org/10.3390/app13074412
Chicago/Turabian StyleDelanaud, Stéphane, Lisa Gossart, Maximilien Leclercq, and Jean-Pierre Libert. 2023. "Use of a Novel Mathematical Model to Assess the Effectiveness of Skin-to-Skin Care for the Prevention of Hypothermia in Low-Birth-Weight Neonates" Applied Sciences 13, no. 7: 4412. https://doi.org/10.3390/app13074412
APA StyleDelanaud, S., Gossart, L., Leclercq, M., & Libert, J.-P. (2023). Use of a Novel Mathematical Model to Assess the Effectiveness of Skin-to-Skin Care for the Prevention of Hypothermia in Low-Birth-Weight Neonates. Applied Sciences, 13(7), 4412. https://doi.org/10.3390/app13074412





