The Evolution of the Corrosion Mechanism of Structural Steel Exposed to the Urban Industrial Atmosphere for Seven Years
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Test Materials
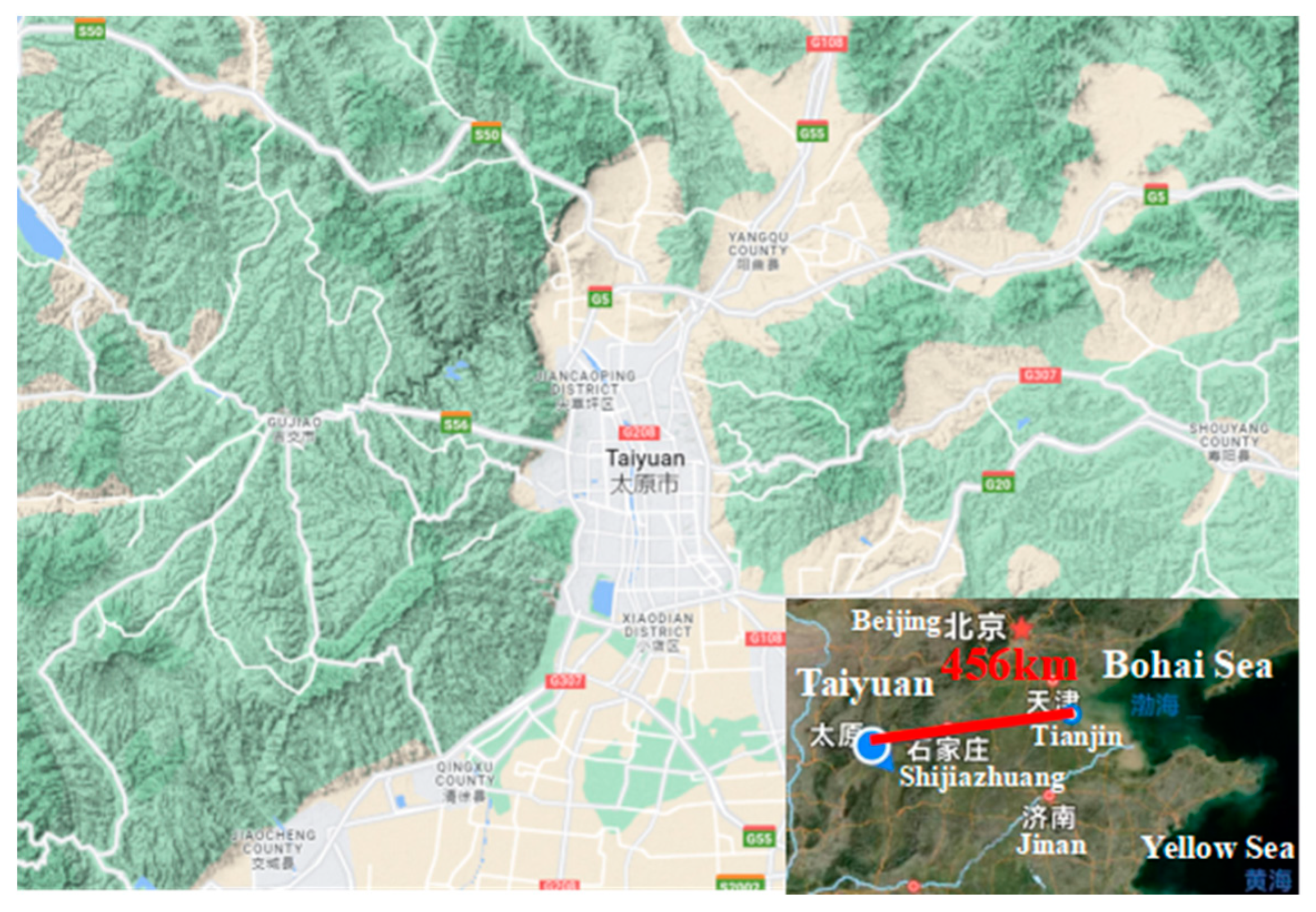
2.2. Urban Industrial Atmospheric Exposure Corrosion Test
2.3. Measurement of the Mass Loss
2.4. Characterization of Rust Layers
3. Results
3.1. Corrosion Kinetics
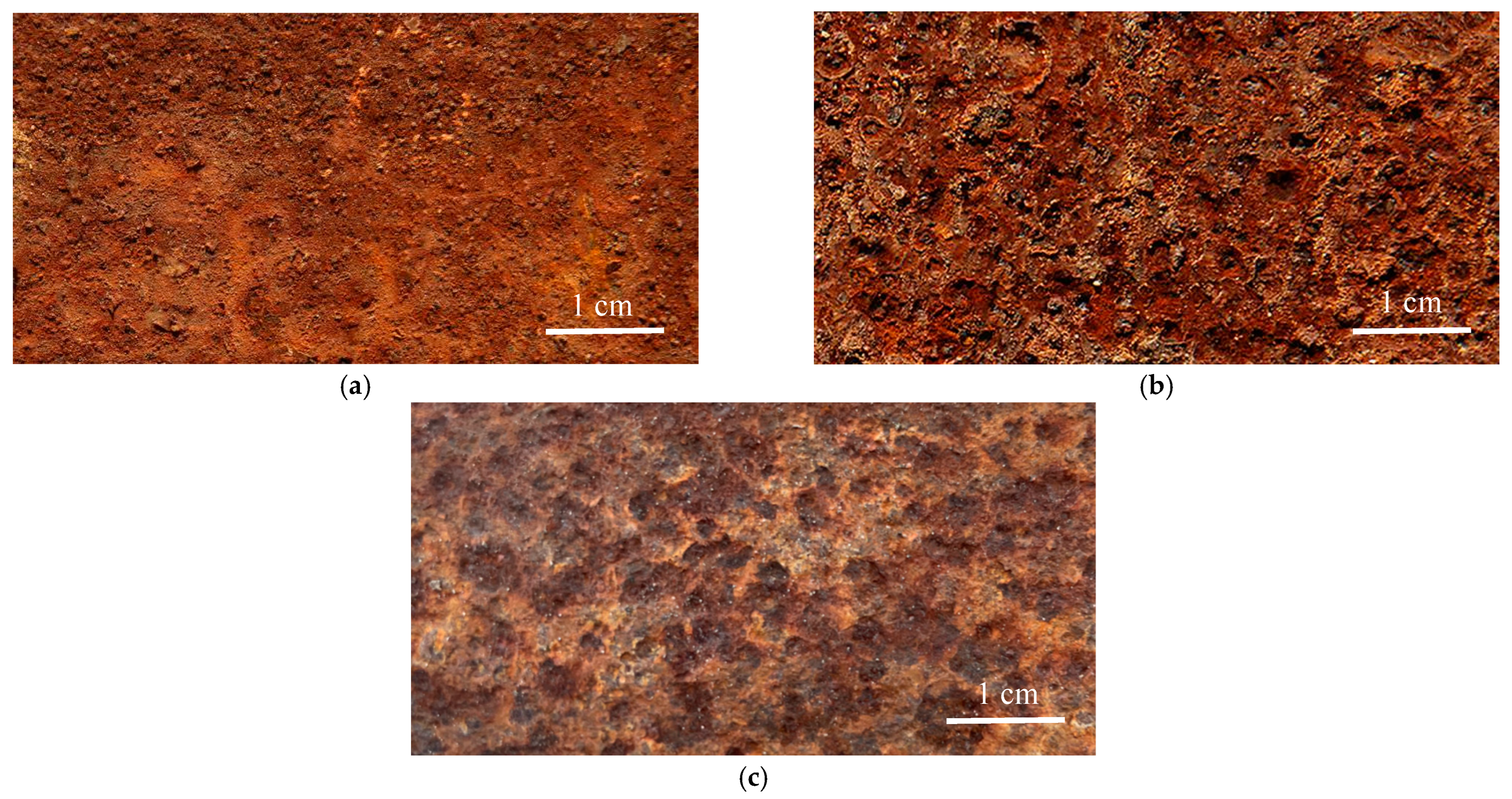
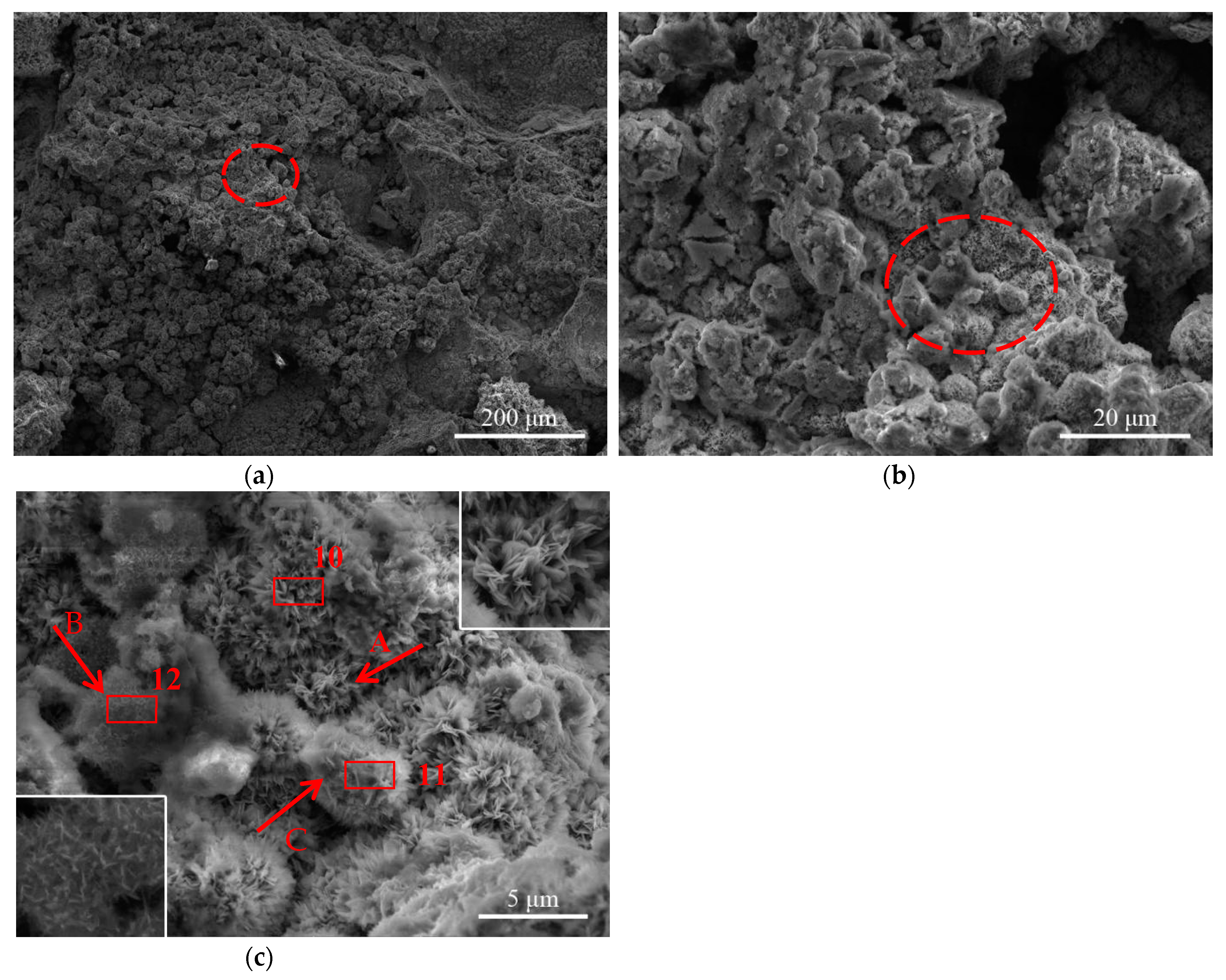
3.2. Morphologies of the Rust Layers
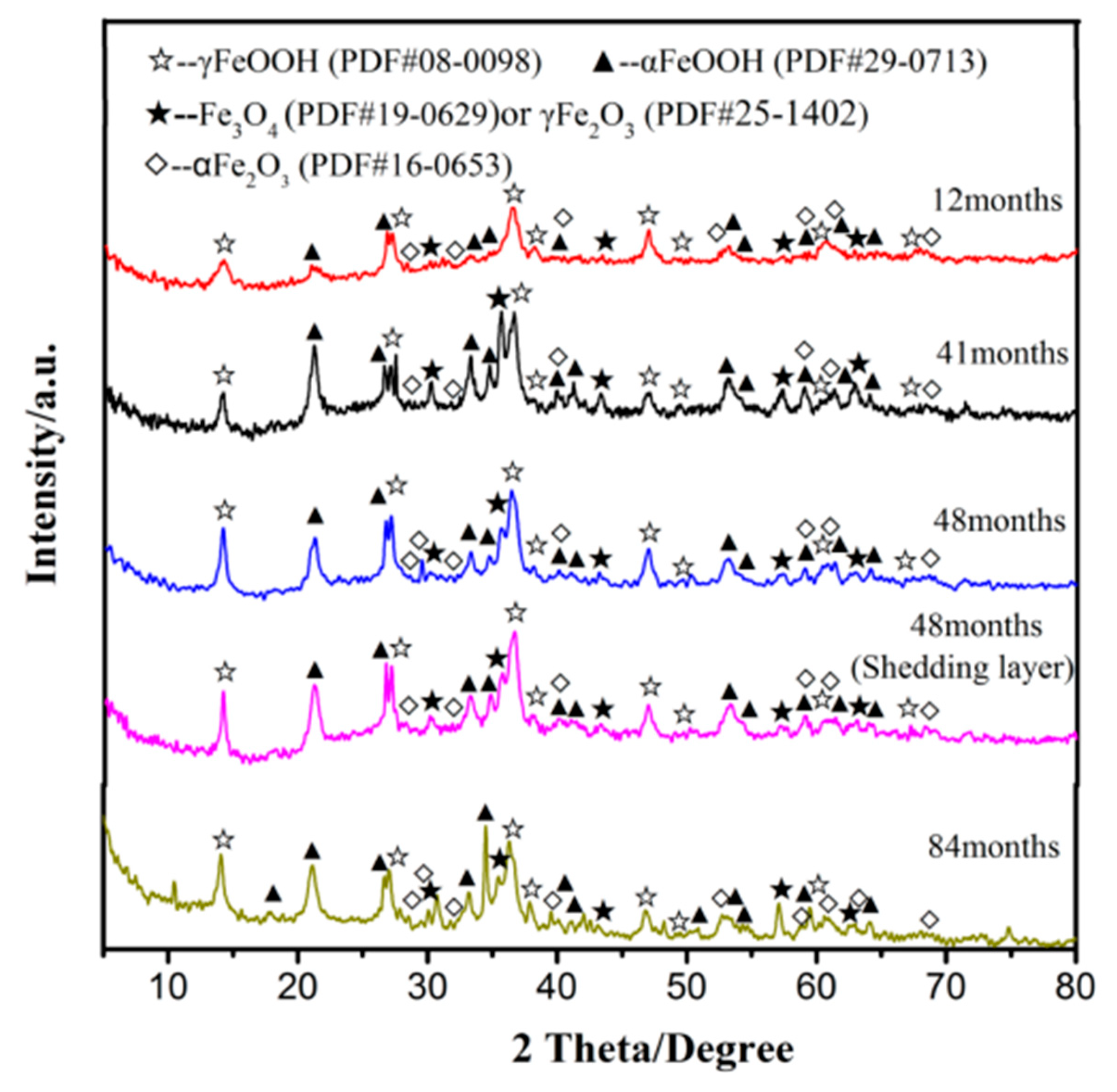
3.3. Phase Structures of the Rust Layers
4. Discussions
4.1. Prediction of Corrosion Rates
4.2. Evolution of Corrosion Products
5. Conclusions
- (1)
- The corrosion kinetics of Q235 steel exposed to the urban industrial atmosphere for a long term follow a two-stage corrosion power function law. The atmospheric corrosion evolution law for short-term exposure differs from that for long-term exposure. Therefore, the short-term corrosion test results fail to fully reflect the corrosion performance of Q235 steel in these environments. SO2 significantly affects the corrosion behavior of steel.
- (2)
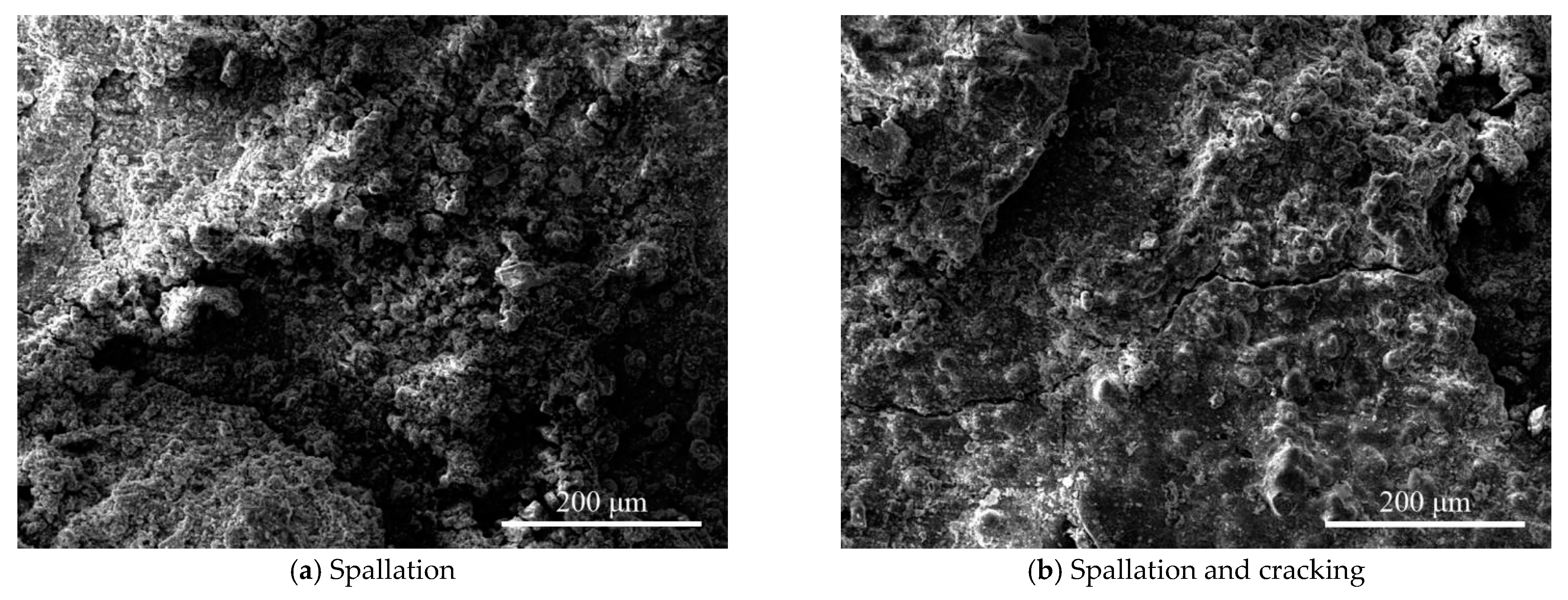
- The morphology of the corrosion products of Q235 steel in the urban industrial atmosphere changes from loose and flat to granular, and finally to compact and smooth. Although the outer rust layer was easy to fall off, the thickness of the inner and outer rust layers increases with increased exposure time. The various forms of corrosion products connect and agglomerate with each other and possess cavities, cracks, and spallation, reducing the rust layer’s protection.
- (3)
- The rust layer crystalline phase contains α-FeOOH, γ-FeOOH, Fe3O4/γ-Fe2O3, and α-Fe2O3 in the urban industrial atmosphere. Corrosion products in the initial period are mainly γ-FeOOH, followed by α-FeOOH, and only a small amount of Fe3O4/γ-Fe2O3. With the increase in exposure time, α-FeOOH and Fe3O4/γ-Fe2O3 in the rust layer increases. The concentration of SO2 in the atmosphere affects the formation of α-Fe2O3. In long-term atmospheric corrosion, the increase in Fe3O4/γ-Fe2O3 content is beneficial to improving the corrosion rate.
- (4)
- In the urban industrial atmosphere, γ-FeOOH typically has bird’s nest structures, worm’s nest structures, globular structures with crystalline substructures, and hair-like structures; α-FeOOH has laminar petal structures, cloud-like structures, laminar structures with whiskers, and cotton ball structures.
- (5)
- The corrosion rate is impacted by the composition of the rust layer and can be measured with the α/γ ratio. When the exposure time is no more than 48 months, α/γ decreases with the increasing exposure time, indicating weak rust layer protection and a high corrosion rate. After 84 months of corrosion, α/γ increases, indicating increased rust layer protection and a decreased corrosion rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quintana, P.; Veleva, L.; Cauich, W.; Pomes, R.; Peña, J.L. Study of the composition and morphology of initial stage of corrosion products formed on Zn plates exposed to the atmosphere of southeast Mexico. Appl. Surf. Sci. 1996, 99, 325–334. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- Asami, K.; Kikuchi, M. In-depth distribution of rusts on a plain carbon steel and weathering steels exposed to coastal–industrial atmosphere for 17 Years. Corros. Sci. 2003, 45, 2671–2688. [Google Scholar] [CrossRef]
- de la Fuente, D.; Diaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef] [Green Version]
- Hou, B.Y. Corrosion Control Technology of Marine Steel Structure in Splash Zone; Science Press: Beijing, China, 2011; pp. 1–2. (In Chinese) [Google Scholar]
- Bhaskaran, R.; Palaniswamy, N.S.; Rengaswamy, N.; Jayachandran, M. ASM Hand Book. ASM Int. Met. Park Ohio 2005, 13B, 619. [Google Scholar]
- Schmitt, G.; Schütze, M.; Hays, G.F.; Burns, W.; Jacobson, G. Global Needs for Knowledge Dissemination, Research, and Development in Materials Deterioration and Corrosion Control; World Corrosion Organization: New York, NY, USA, 2009. [Google Scholar]
- Arroyave, C.; Lopez, F.A.; Morcillo, M. The early atmospheric corrosion stages of carbon steel in acidic fogs. Corros. Sci. 1995, 37, 1751–1761. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Yu, G.C.; Wang, Z.Y.; Wang, J. Characterisation of initial atmospheric corrosion carbon steels by field exposure and laboratory simulation. Corros. Sci. 2007, 49, 2920–2935. [Google Scholar] [CrossRef]
- Han, W.; Pan, C.; Wang, Z.Y.; Yu, G.C. A study on the initial corrosion behavior of carbon steel exposed to outdoor wet-dry cyclic condition. Corros. Sci. 2014, 88, 89–100. [Google Scholar] [CrossRef]
- Yan, S.T.; Jin, Y.; Xu, F.F.; Wen, L. Influence of deposited atmospheric particulates on initial corrosion behavior of mild steel. Corros. Sci. Prot. Technol. 2019, 31, 467–474. (In Chinese) [Google Scholar]
- Song, X.X.; Huang, S.P.; Wang, C.; Wang, Z.Y. The Initial corrosion behavior of carbon steel exposed to the coastal-Industrial atmosphere in Hongyanhe. Acta Metall. Sin. 2020, 56, 1355–1365. (In Chinese) [Google Scholar]
- Rodríguez, J.J.S.; Hernandez, F.J.S.; Gonzalez, J.E.G. The effect of environmental and meteorological variables on atmospheric corrosion of carbon steel, copper, zinc and aluminium in a limited geographic zone with different types of environment. Corros. Sci. 2003, 45, 799–815. [Google Scholar] [CrossRef]
- Lan, T.T.N.; Thoa, N.T.P.; Nishimura, R.; Tsujino, Y.; Yokoi, M.; Maeda, Y. Atmospheric corrosion of carbon steel under field exposure in the southern part of Vietnam. Corros. Sci. 2006, 48, 179–192. [Google Scholar] [CrossRef]
- Corvo, F.; Betancourt, N.; Mendoza, A. The Influence of airborne salinity on the atmospheric corrosion of steel. Corros. Sci. 1995, 37, 1889–1901. [Google Scholar] [CrossRef]
- Castaño, J.G.; Botero, C.A.; Restrepo, A.H.; Agudelo, E.A.; Correa, E.; Echeverría, F. Atmospheric corrosion of carbon steel in Colombia. Corros. Sci. 2010, 52, 216–223. [Google Scholar] [CrossRef]
- Corvo, F.; Mendoza, A.R.; Autie, M.; Betancourt, N. Role of water adsorption and salt content in atmospheric corrosion products of steel. Corros. Sci. 1997, 39, 815–820. [Google Scholar] [CrossRef]
- Song, Z.B.; Wang, Z.C.; Wang, J.G.; Peng, B.; Zhang, C.Z. Atmospheric corrosion behavior of Q235 Steel in northern Hebei region. Mater. Mech. Eng. 2021, 45, 46–51. (In Chinese) [Google Scholar]
- Cole, I.S.; Ganther, W.D.; Sinclair, J.D.; Lau, D.; Paterson, D.A. A study of the wetting of metal surfaces in order to understand the processes controlling atmospheric corrosion. J. Electrochem. Soc. 2004, 151, B627–B635. [Google Scholar] [CrossRef]
- Mansfeld, F.; Kenkel, J.V. Electrochemical measurement of time-of-wetness and atmospheric corrosion rates. Corrosion 1977, 33, 13–16. [Google Scholar] [CrossRef]
- Dean, S.; Reiser, D.B. Analysis of long-term atmospheric corrosion results from ISOCORRAG Program. In Outdoor Atmospheric Corrosion, ASTM STP 1421; Towsend, H.E., Ed.; American Society for Testing and Materials: Philadelphia, PA, USA, 2002; pp. 3–18. [Google Scholar]
- Ma, Y.T.; Li, Y.; Wang, F.H. The atmospheric corrosion kinetics of low carbon steel in a tropical marine environment. Corros. Sci. 2010, 52, 1796–1800. [Google Scholar] [CrossRef]
- Melchers, R.E.; Jeffrey, R. The critical involvement of anaerobic bacterial activity in modelling the corrosion behaviour of mild steel in marine environments. Electrochim. Acta 2008, 54, 80–85. [Google Scholar] [CrossRef]
- Melchers, R.E. Long-term corrosion of cast irons and steel in marine and atmospheric environments. Corros. Sci. 2013, 68, 186–194. [Google Scholar] [CrossRef]
- Liang, C.F.; Hou, W.T. Sixteen-year atmospheric corrosion exposure study of steel. J. Chin. Soc. Corros. Prot. 2005, 25, 1–6. (In Chinese) [Google Scholar]
- Liang, C.F.; Hou, W.T. Eight-year atmospheric corrosion exposure study of steel. Corros. Sci. Prot. Technol. 1995, 7, 183–186. (In Chinese) [Google Scholar]
- Mao, H.R.; Qu, G.C.; Huang, S.Y.; Zhang, Q.Y. Atmospheric corrosion of carbon steels and low alloy steel exposed in Guangzhou. Environ. Technol. 2001, 3, 5–7. (In Chinese) [Google Scholar]
- Zhi, Y.J.; Yang, T.; Fu, D.M. An improved deep forest model for forecast the outdoor atmospheric corrosion rate of low-alloy steels. J. Mater. Sci. Technol. 2020, 49, 202–210. [Google Scholar] [CrossRef]
- Song, X.X.; Wang, K.Y.; Zhou, L.J.; Chen, Y.J.; Ren, K.X.; Wang, J.Y.; Zhang, C. Multi-factor mining and corrosion rate prediction model construction of carbon steel under dynamic atmospheric corrosion environment. Eng. Fail. Anal. 2022, 134, 105987. [Google Scholar] [CrossRef]
- Oh, S.J.; Cook, D.; Townsend, H. Atmospheric corrosion of different steels in marine, rural and industrial environments. Corros. Sci. 1999, 41, 1687–1702. [Google Scholar] [CrossRef]
- Fonna, S.; Ibrahim, I.B.M.; Gunawarman; Huzni, S.; Ikhsan, M.; Thalib, S. Investigation of corrosion products formed on the surface of carbon steel exposed in Banda Aceh’s atmosphere. Heliyon 2021, 7, e06608. [Google Scholar] [CrossRef] [PubMed]
- Antunes, R.A.; Costa, I.; de Faria, D.L.A. Characterization of corrosion products formed on steels in the first months of atmospheric exposure. Mater. Res. 2003, 6, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.J.; Cook, D.C.; Carpio, J.J. Characterization of the corrosion products formed on carbon steel in a marine environment. J. Korean Phys. Soc. 2000, 36, 106–110. [Google Scholar]
- Wang, Z.G.; Hai, C.; Jiang, J.; Lan, X.S.; Du, C.W.; Li, X.G. Corrosion behavior of Q235 steels in atmosphere at Deyang District for one Year. J. Chin. Soc. Corros. Prot. 2021, 41, 871–876. (In Chinese) [Google Scholar]
- Tian, Q.Q.; Hai, C.; Wang, Z.G.; Wang, N.H.; Lan, X.S.; Du, C.W. Study on corrosion behavior of Q235 carbon steel in typical atmospheric pollution environment in Leshan, Sichuan province. J. Southwest Minzu Univ. 2020, 46, 478–486. (In Chinese) [Google Scholar]
- Wang, C.; Cao, G.W.; Pan, C.; Wang, Z.Y.; Liu, M.R. Atmospheric corrosion of carbon steel and weathering steel in three environments. J. Chin. Soc. Corros. Prot. 2016, 36, 39–46. (In Chinese) [Google Scholar]
- Mi, F.Y.; Wang, X.D.; Liu, Z.P.; Wang, B.; Peng, Y.; Tao, D.P. Industrial atmospheric corrosion resistance of P-RE weathering steel. J. Iron Steel Res. Int. 2011, 18, 67–73. [Google Scholar] [CrossRef]
- Díaz, I.; Cano, H.; Lopesino, P.; de la Fuente, D.; Chico, B.; Jiménez, J.A.; Medina, S.F.; Morcillo, M. Five-year atmospheric corrosion of Cu, Cr and Ni weathering steels in a wide range of environments. Corros. Sci. 2018, 141, 146–157. [Google Scholar] [CrossRef]
- Cheng, X.; Jin, Z.; Liu, M.; Li, X. Optimizing the nickel content in weathering steels to enhance their corrosion resistance in acidic atmospheres. Corros. Sci. 2017, 115, 135–142. [Google Scholar] [CrossRef]
- Yu, Q.; Dong, C.F.; Fang, Y.H.; Xiao, K.; Guo, C.Y.; He, G.; Li, X.G. Atmospheric corrosion of Q235 carbon steel and Q450 weathering steel in Turpan, China. J. Iron Steel Res. Int. 2016, 23, 1061–1070. [Google Scholar] [CrossRef]
- Dong, B.J.; Liu, W.; Zhang, T.Y.; Chen, L.J.; Fan, Y.M.; Zhao, Y.G.; Yang, W.J.; Banthukul, W. Corrosion failure analysis of low alloy steel and carbon steel rebar in tropical marine atmospheric environment: Outdoor exposure and indoor test. Eng. Fail. Anal. 2021, 129, 105720. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, X.; Hou, H.; Liu, B.; Li, X. Insight into the product film formed on Ni-advanced weathering steel in a tropical marine atmosphere. Appl. Surf. Sci. 2018, 436, 80–89. [Google Scholar] [CrossRef]
- Jia, J.; Wu, W.; Cheng, X.; Zhao, J. Ni-advanced weathering steels in Maldives for two years: Corrosion results of tropical marine field test. Constr. Build. Mater. 2020, 245, 118463. [Google Scholar] [CrossRef]
- Vassie, P.R.; Mckenzie, M. Electrode potentials for on-site monitoring of atmospheric corrosion of steel. Corros. Sci. 1985, 25, 1–13. [Google Scholar] [CrossRef]
- Yamashita, M.; Miyuki, H.; Matsuda, Y.; Nagano, H.; Misawa, T. The long term growth of the protective rust layer formed on weathering steel by atmospheric corrosion during a quarter of a century. Corros. Sci. 1994, 36, 283–299. [Google Scholar] [CrossRef]
- Kamimura, T.; Hara, S.; Miyuki, H.; Yamashita, M.; Uchida, H. Composition and protective ability of rust layer formed on weathering steel exposed to various environments. Corros. Sci. 2006, 48, 2799–2812. [Google Scholar] [CrossRef]
- Nishimura, T. Rust formation and corrosion performance of Si- and Al-bearing ultrafine grained weathering steel. Corros. Sci. 2008, 50, 1306–1312. [Google Scholar] [CrossRef]
- Tamura, H. The role of rusts in corrosion and corrosion protection of iron and steel. Corros. Sci. 2008, 50, 1872–1883. [Google Scholar] [CrossRef] [Green Version]
- Murayama, M.; Nishimura, T.; Tsuzaki, K. Nano-scale chemical analysis of rust on a 2% Si-bearing low alloy steel exposed in a coastal environment. Corros. Sci. 2008, 50, 2159–2165. [Google Scholar] [CrossRef]
- Samie, F.; Tidblad, J.; Kucera, V.; Leygraf, C. Atmospheric corrosion effects of HNO3—Comparison of laboratory-exposed copper, zinc and carbon steel. Atmos. Environ. 2007, 41, 4888–4896. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Díaz, I.; Cano, H.; de la Fuente, D. Atmospheric corrosion data of weathering steels. A Review. Corros. Sci. 2013, 77, 6–24. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, A.R.; Corvo, F. Outdoor and indoor atmospheric corrosion of carbon steel. Corros. Sci. 1999, 41, 75–86. [Google Scholar] [CrossRef]
- Corvo, F.; Torrens, A.D.; Betancourt, N.; Pérez, J.; Gonzalez, E. Indoor atmospheric corrosion in Cuba. A report about indoor localized corrosion. Corros. Sci. 2007, 49, 418–435. [Google Scholar] [CrossRef]
- Tidblad, J. Atmospheric corrosion of metals in 2010–2039 and 2070–2099. Atmos. Environ. 2012, 55, 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Guo, C.Y.; Xiao, K.; Silayiding, T.; Dong, C.F.; Li, X.G. Corrosion behavior of carbon steels Q235 and Q450 in dry hot atmosphere at Turpan District for four years. J. Chin. Soc. Corros. Prot. 2018, 38, 431–437. (In Chinese) [Google Scholar]
- Wu, H.Y.; Lei, H.G.; Chen, Y.F.; Qiao, J.Y. Comparison on corrosion behaviour and mechanical properties of structural steel exposed between urban industrial atmosphere and laboratory simulated environment. Constr. Build. Mater. 2019, 211, 228–243. [Google Scholar] [CrossRef]
- Wu, H.Y.; Lei, H.G.; Chen, Y.F. Study on corrosion models of structural steel exposed in urban industrial atmospheric and laboratory simulated environments based on the 3D profile. Thin-Walled Struct. 2021, 168, 108286. [Google Scholar] [CrossRef]
- Wu, H.Y.; Lei, H.G.; Chen, Y.G. Grey relational analysis of static tensile properties of structural steel subjected to urban industrial atmospheric corrosion and accelerated corrosion. Constr. Build. Mater. 2022, 315, 125706. [Google Scholar] [CrossRef]
- Wu, H.Y.; Lei, H.G.; Chen, Y.F. Comparison on mechanisms of high-cycle fatigue performance of structural steel exposed to urban industrial atmosphere and laboratory simulated corrosive environment based on infrared thermography. Int. J. Fatigue 2021, 145, 106098. [Google Scholar] [CrossRef]
- ISO 8407; Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens. ISO: Geneva, Switzerland, 2009.
- Raman, A.; Kuban, B.; Razvan, A. The application of infrared spectroscopy to the study of atmospheric rust systems—I. Standard spectra and illustrative applications to identify rust phases in natural atmospheric corrosion products. Corros. Sci. 1991, 32, 1295–1306. [Google Scholar] [CrossRef]
- Guo, J.; Yang, S.W.; Shang, C.J.; Wang, Y.; He, X.L. Influence of carbon content and microstructure on corrosion behavior of low alloy steels in a Cl- containing environment. Corros. Sci. 2008, 51, 242–251. [Google Scholar] [CrossRef]
- Antunes, R.A.; Costa, J.; Araujo, D.L. Characterization of atmospheric corrosion products formed on steels. Mater. Res. 2003, 6, 403. [Google Scholar] [CrossRef] [Green Version]
- Evans, U.R. Mechanism of Rusting Under Different Conditions. Br. Corros. J. 1972, 7, 10–14. [Google Scholar] [CrossRef]
- Bohnenkamp, K.; Burgmann, G.; Schwenk, W. Investigations of the atmospheric corrosion of plain carbon and low alloy steels in industrial, rural and sea air. Stahl Eisen 1973, 93, 1054–1060. [Google Scholar]
- Pourbaix, M.; Pourbaix, A. Recent progress in atmospheric corrosion testing. Corrosion 1989, 45, 71–83. [Google Scholar] [CrossRef]
- Almeida, E.; Morcillo, M.; Rosales, B.; Marrocos, M. Atmospheric corrosion of mild steel: Part I. Rural and urban atmospheres. Mater. Corros. 2000, 51, 859–864. [Google Scholar] [CrossRef]
- Almeida, E.; Morcillo, M.; Rosales, B. Atmospheric corrosion of mild steel: Part II. Marine atmospheres. Mater. Corros. 2000, 51, 865–874. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Yu, G.C.; Han, W. Atmospheric corrosion of carbon steel and low alloy steel in Polluted Environment. J. Iron Steel Res. 2003, 15, 47–51. (In Chinese) [Google Scholar]
- Zhou, Y.L.; Chen, J.; Liu, Z.Y. Corrosion Behavior of Rusted 550 MPa Grade Offshore Platform Steel. J. Iron Steel Res. Int. 2013, 20, 66–73. [Google Scholar] [CrossRef]
- Perdomo, J.; Chabica, M.E.; Song, I. Chemical and electrochemical conditions on steel under disbonded coatings: The e.ect of previously corroded surfaces and wet and dry cycles. Corros. Sci. 2001, 43, 515–532. [Google Scholar] [CrossRef]
- Lyon, S.B.; Thompson, G.E.; Johnson, J.B.; Wood, G.C.; Ferguson, J.M. Accelerated atmospheric corrosion testing using a cyclic wet/dry exposure test: Aluminum, galvanized steel and steel. Corrosion 1987, 43, 719–726. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Alcántara, J.; Díaz, I.; Wolthuis, R.; de la Fuente, D. SEM/Micro-Raman characterization of the morphologies of marine atmospheric corrosion products formed on mild steel. J. Electrochem. Soc. 2016, 163, C426–C439. [Google Scholar] [CrossRef]
- Alcántara, J.; Chico, B.; Simancas, J.; Díaz, I.; de la Fuente, D.; Morcillo, M. An attempt to classify the morphologies presented by different rust phases formed during the exposure of carbon steel to marine atmospheres. Mater. Charact. 2016, 118, 65–78. [Google Scholar] [CrossRef]
- Wang, J.; Wei, F.I.; Chang, Y.S.; Shih, H.C. The corrosion mechanisms of carbon steel and weathering steel in SO2 polluted atmospheres. Mater. Chem. Phys. 1997, 47, 1–8. [Google Scholar] [CrossRef]
- Weissenrieder, J.; Leygraf, C. In site studies of filiform corrosion of iron. J. Electrochem. Soc. 2004, 151, 165–171. [Google Scholar] [CrossRef]
- Yamashita, M.; Konishi, H.; Kozakura, T.; Uchida, H. In situ observation of initial rust formation process on carbon steel under Na2SO4 and NaCl solution films with wet/dry cycles using synchrotron radiation X-rays. Corros. Sci. 2005, 47, 2492–2498. [Google Scholar] [CrossRef]
- Singh, D.D.N.; Yadav, S.; Saha, J.K. Role of climatic conditions on corrosion characteristicsof structural steels. Corros. Sci. 2008, 50, 93–110. [Google Scholar] [CrossRef]
- Evans, U.R. The Economic Advantages of a Sound Painting Scheme. Trans. IMF 1960, 37, 1–6. [Google Scholar] [CrossRef]
- Evans, U. Mechanism of rusting. Corros. Sci. 1969, 9, 813–821. [Google Scholar] [CrossRef]
- Stratmann, M.; Hoffmann, K. IN Situ MÖßBbauer spectroscopic study of reactions within rust layers. Corros. Sci. 1989, 29, 1329–1352. [Google Scholar] [CrossRef]
- Dai, N.W.; Zhang, J.X.; Chen, Q.M.; Yi, B.; Cao, F.H.; Zhang, J.Q. Effect of the direct current electric field on the initial corrosion of steel in simulated industrial atmospheric environment. Corros. Sci. 2015, 99, 295–303. [Google Scholar] [CrossRef]
- Misawa, T.; Asami, K.; Hashimoto, K.; Shimodaira, S. The mechanism of atmospheric rusting and the protective amorphous rust on low alloy steel. Corros. Sci. 1974, 14, 279–289. [Google Scholar] [CrossRef]
- Francis, R.A. The morphology of corrosion products on steel. In Proceedings of the 10th International Congress on Metallic Corrosion; Oxford and IBH Publishing Co.: New Delhi, India, 1987; Volume 1, pp. 121–130. [Google Scholar]
- Stratmann, M.; Bohnenkamp, K.; Engell, H.-J. An electrochemical study of phase-transitions in rust layers. Corros. Sci. 1983, 23, 969–985. [Google Scholar] [CrossRef]
- Stratmann, M.; Bohnenkamp, K.; Ramchandran, T. The influence of copper upon the atmospheric corrosion of iron. Corros. Sci. 1987, 27, 905–926. [Google Scholar] [CrossRef]
- Morales, A.L.; Cartagena, D.; Rendon, J.L.; Valencia, A. The relation between corrosion rate and corrosion products from low carbon steel. Phys. Status Solidi B 2000, 220, 351–356. [Google Scholar] [CrossRef]
- Suzuki, I.; Hisamatsu, Y.; Masuko, N. Nature of atmospheric rust on iron. J. Electrochem. Soc. 1980, 127, 2210–2215. [Google Scholar] [CrossRef]
- Evans, U.R. The mechanism of rusting. Quart. Rev. 1967, 21, 29–43. [Google Scholar] [CrossRef]
- Kamimura, T.; Yamashita, M.; Uchida, H.; Miyuki, H. Correlation between corrosion rate and composition of crystalline corrosion products formed on weathering steels. J. Jpn. Inst. Met. 2001, 65, 922–928. [Google Scholar] [CrossRef] [Green Version]









| C | Si | Mn | S | P | O | N | Fe |
|---|---|---|---|---|---|---|---|
| 0.16 | 0.16 | 0.57 | 0.023 | 0.016 | 0.013 | 0.0021 | 99.0559 |
| Average Annual Temperature (°C) | Average Temperature of the Hottest Month (°C) | Average Temperature of the Coldest Month (°C) | Average Relative Humidity of the Hottest Month (%) | Average Relative Humidity of the Coldest Month (%) | Average Annual Rainfall (mm) | Average Annual Sunshine Time (h) |
|---|---|---|---|---|---|---|
| 11.3 | 23.3 | −2.7 | 72 | 50 | 449.2 | 2669.2 |
| Exposure Time | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|---|---|
| SO2 concentration | 80 | 73 | 71 | 68 | 54 | 29 | 22 | 18 |
| Exposure Time (Month) | Location | O | Fe | C | Si | S | Mn | P | Al | K | In |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Point 8 (Figure 4b) | 44.1 | 37.8 | 17.5 | 0.4 | 0.2 | —— | —— | —— | —— | —— |
| 12 | Point 13 (Figure 5c) | 41.1 | 42.8 | 15.2 | 0.2 | 0.5 | 0.1 | —— | —— | —— | —— |
| Point 14 (Figure 5c) | 57.9 | 29.6 | 12.2 | —— | 0.1 | 0.1 | —— | —— | —— | —— | |
| Point 17 (Figure 5f) | 51.3 | 35.3 | 12.8 | 0.1 | 0.2 | 0.2 | 0.1 | —— | —— | —— | |
| Point 19 (Figure 5f) | 53.8 | 19.3 | 20.1 | 3.4 | 0.4 | —— | 0.1 | 2.2 | 0.7 | —— | |
| 41 | Point 10 (Figure 6c) | 26.9 | 37.1 | 36 | —— | —— | —— | —— | —— | —— | —— |
| Point 11 (Figure 6c) | 8.4 | 91.6 | —— | —— | —— | —— | —— | —— | —— | —— | |
| Point 12 (Figure 6c) | 46.5 | 12.9 | 40.1 | —— | —— | —— | —— | —— | —— | 0.4 | |
| 84 | Point 41 (Figure 7e) | 43 | 20.5 | 29.7 | 4.5 | —— | —— | —— | 1.5 | 0.8 | —— |
| Point 43 (Figure 7e) | 51.6 | 16.7 | 30.3 | 1.4 | —— | —— | —— | —— | —— | —— | |
| Point 45 (Figure 7d) | 57.8 | 17.9 | 24.3 | —— | —— | —— | —— | —— | —— | —— |
| Time (Month) | α-FeOOH (Goethite) (%) | γ-FeOOH (Lepidocrocite) (%) | Fe3O4/γ-Fe2O3 (Magnetite/Maghemite) (%) | αFe2O3 (Hematite) (%) | α/γ |
|---|---|---|---|---|---|
| 12 | 31.0 | 50.2 | —— | 18.8 | 0.618 |
| 48 | 30.4 | 29.4 | 22.2 | 18.0 | 0.589 |
| 48 (Shedding layer) | 30.9 | 21.4 | 34.3 | 13.4 | 0.554 |
| 84 | 39.2 | 24.5 | 35.6 | 0.7 | 0.652 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Luo, Y.; Zhou, G. The Evolution of the Corrosion Mechanism of Structural Steel Exposed to the Urban Industrial Atmosphere for Seven Years. Appl. Sci. 2023, 13, 4500. https://doi.org/10.3390/app13074500
Wu H, Luo Y, Zhou G. The Evolution of the Corrosion Mechanism of Structural Steel Exposed to the Urban Industrial Atmosphere for Seven Years. Applied Sciences. 2023; 13(7):4500. https://doi.org/10.3390/app13074500
Chicago/Turabian StyleWu, Haiying, Yaozhi Luo, and Guangen Zhou. 2023. "The Evolution of the Corrosion Mechanism of Structural Steel Exposed to the Urban Industrial Atmosphere for Seven Years" Applied Sciences 13, no. 7: 4500. https://doi.org/10.3390/app13074500
APA StyleWu, H., Luo, Y., & Zhou, G. (2023). The Evolution of the Corrosion Mechanism of Structural Steel Exposed to the Urban Industrial Atmosphere for Seven Years. Applied Sciences, 13(7), 4500. https://doi.org/10.3390/app13074500




