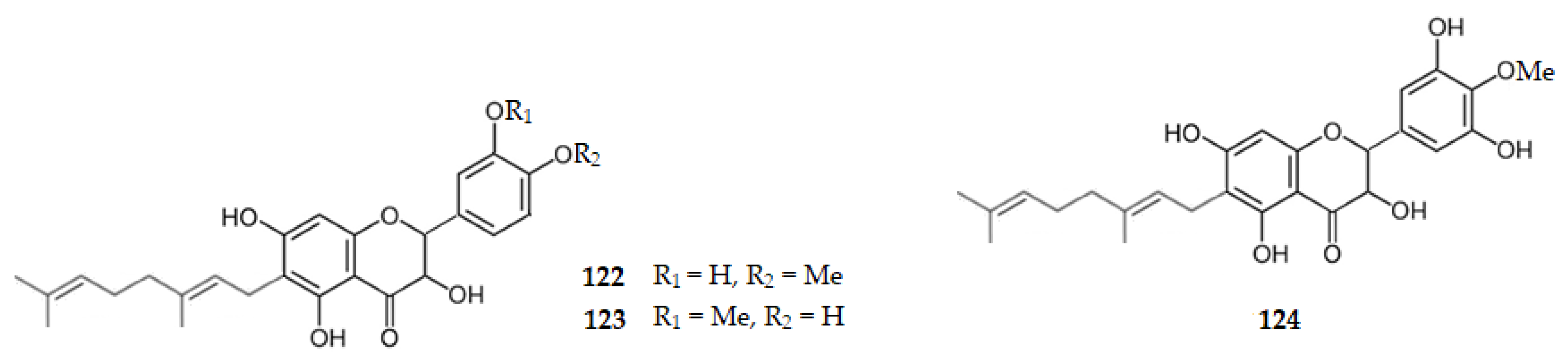Abstract
The increase in the occurrence of the multifactorial Alzheimer’s disease (AD) demands an urgent effort towards the development of effective anti-AD agents, such as the multitarget-directed ligands (MTDLs). In fact, AD is a genetic and an environmental disease, involving a diversity of etiopathogenic processes, and there is not yet a successful AD treatment. The major AD clinical indications (CIs) are extracellular amyloid plaques, intracellular neurofibrillary tangles (NFTs), abnormal inflammatory response, and neuron apoptosis and death caused by oxidative stress. The discovery of neuroprotective natural products, presenting good oral bioavailability, ability to cross the blood-brain barrier (BBB) and safety profile, is indeed a necessity, and some flavonoids are in clinical trials for AD treatment. In this review, the several flavonoids from natural sources that have shown activity on mechanisms associated with AD are presented. Although several reviews have been presented in the last few years, the main objective of this review is to recognize and discuss, for each CI, the scaffolds leading to the highest activity and so to attempt to achieve molecules targeting more than one CI, the MTDLs, which are potential leads for AD treatment. In conclusion, the most active flavonoids against several CIs of AD are flavanols and flavonols, which have a planar scaffold and structures presenting hydroxy groups at C5 and C7 on ring A and at C4′ of ring B. Thus, molecules linking flavanols to flavonols, with hydroxy groups at C5 and C7 on ring A and at C4′ of ring B, are also promising against CIs of AD and potential anti-AD agents.
1. Introduction
Alzheimer’s disease (AD) is a common disease in geriatric patients. Currently 50 million people are diagnosed with dementia and this number is expected to increase by 60% in 2030 and up to 180% in 2050 [1]. AD is the most common cause of dementia, accounting for 60 to 80% of cases. It is most common in people over the age of 65. Indeed, the risk of AD and other types of dementia increases with age, affecting an estimated 1 in 14 people over the age of 65 and 1 in every 6 people over the age of 80 [1]. Deficient cholinergic function, behavioral disorders, memory and intellectual function loss and neuronal death are symptoms of this disease. Some of the AD risk factors were identified as family medical history, way of living and psychosocial factors, elderliness, the apolipoprotein E (APOE) ε4 allele genotype and cardiovascular disease risk factors [2]. There are two subtypes of AD. The first one, called early onset of AD (EOAD), develops in people younger than 65 years old being associated with genetic mutations, especially of amyloid precursor protein (APP), presenilin 1 (PSEN1) and presenilin 2 (PSEN2) genes, that are involved in the production of the amyloid-beta (Aβ) peptides. The second one, called late onset of AD (LOAD), develops in people older than 65 years, being the most frequent AD, and has been consistently associated with only one gene, the APOE gene. The allele ε4 of APOE is a genetic risk factor [3,4], causing cognitive decline and cerebral amyloid in aged individuals [5]. APOE is formed in the neuroglial cells, astrocytes, producing Aβ plaques and development of cerebral amyloid angiopathology [6]. Furthermore, APOEε4 has been associated with tau pathology [7]. However, 50% of AD patients are not APOEε4 carriers [3,4], and up to 75% of APOEε4 homozygous carriers do not progress to AD. In fact, the genetic tendency to LOAD and the role of other risk factors is still unknown [8]. Nevertheless, the detailed studies of the mechanisms of action of these genes in AD pathogenesis suggest that AD is also an environmental complex disease [9], which efficacious treatment still remains to be developed [10] and the actions taken have been concerned mostly with the reduction of AD clinical indications (CIs). Extracellular plaques formed by Aβ peptide aggregation, intracellular neurofibrillary tangles (NFTs) resulting from hyperphosphorylated τ-protein, abnormal inflammatory response and neuron apoptosis and death caused by oxidative stress are the major CIs of AD [11,12,13]. Indeed, communication at synapses between neurons is impeded by cell death caused by Aβ plaques. Also, the transport of crucial molecules in neurons is hindered by NFTs [2].
The Aβ peptides result from APP cleavage and are the major components of Aβ plaques. Thus, the inhibition of APP cleavage will cause a decrease in Aβ plaques. APP can be cleaved by the amyloidogenic pathway involving the action of two enzymes, β-secretase (BACE1) and γ-secretase. BACE1 cleaves APP generating a soluble fragment, β-APP, and a longer 99-amino acids peptide fragment that is then cleaved by γ-secretase into amylogenic peptides of varying length, such as Aβ40, Aβ42, and Aβ43 [14]. The imbalance between these Aβ fragments generation and their clearance causes disequilibrium and consequently cell death. One way to combat AD is, therefore, to prevent the brain Aβ peptide deposition by the inhibition of BACE1.
The microtubules (MTs), which are involved in several important structural and regulatory functions, are stabilized by τ-protein. However, when hyperphosphorylated, they aggregate into paired helical and straight filaments resulting in the formation of the NFTs [15,16,17,18,19], losing MTs, which become destabilized. MTs result from the head-to-tail polymerization of α- and β-tubulin heterodimers [19], being always vibrating alternatively between growing and shrinking phases [20]. This dynamic nature of MTs results in the formation of several arrangements within cells. As MTs result from different isoforms of tubulin, their dynamism and their interaction with associated proteins (MAPs) regulates the morphology, stability, and their function in different cell types [21]. If this tuned process fails, AD or other neurodegenerative disorders will take place [22]. It has been concluded that stabilization of MTs may also potentially prevent AD progression. AD prevention also occurs by reducing hyperphosphorylation of τ-protein, avoiding MTs dysfunction. As τ-protein phosphorylation results from an equilibrium between τ-kinase and phosphatase activities, the aggregation and the formation of NFTs is restrained by kinase inhibitors [23,24,25]. Thus, inhibition of these protein kinases [26] constitutes another strategy to combat AD. The most important protein kinase that is involved in τ-phosphorylation is the glycogen synthase kinase-3 beta (GSK-3β) [10,14].
AD development may also be avoided by inhibiting inflammatory response of microglial cells [27,28]. When properly stimulated, microglia cells can transform themselves by modifying their shapes, releasing proinflammatory/neurotoxic factors, such as interleukin-1 (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), prostaglandin E2 (PGE2), cyclooxygenase-2 (COX-2), nitric oxide (NO) and reactive oxygen species (ROS) [29,30,31]. Accumulation of these proinflammatory/neurotoxic factors damages and causes degeneration of the nearby neurons. They release immune substances, increasing inflammatory neurotoxicity and causing irreversible neuroinflammation [32,33,34]. Another potential therapeutic strategy to combat AD is, therefore, the use of inhibitors of microglia response.
The decline of cognitive functions in AD patients is associated with the deficiency of the brain neurotransmitter acetylcholine (ACh). Upon action of the acetylcholinesterase enzyme (AChE), ACh breaks down giving acetate and choline, which is uptaken into the presynaptic neuron and carried out by choline carriers. The signal transduction at the neuromuscular junction finishes rapidly [35]. On the other hand, ACh binds to several receptors in the synaptic cleft. One of them, nicotinic ACh receptors (nAChRs) in the central nervous system (CNS), controls the liberation of other neurotransmitters which are involved in cognitive processes and memory [36,37]. Inhibition of AChE prevents the hydrolysis of Ach, increasing its concentration and duration of action, which is clinically beneficial for AD patients. Thus, AChE inhibitors are widely used for AD treatment [36].
AD pathology can, therefore, progress through different pathways which can even be related. For example, AChE accelerates deposition of Aβ peptide [38] interacting also with Aβ deposits producing an AChE-Aβ complex, a very toxic substance, which in turn increases the intracellular calcium load and decreases mitochondrial membrane potential. The AChE-Aβ complex formation causes the neuronal cells death [39]. AChE also stimulates the protein kinase C (PKC) which inhibits GSK-3β. Thus, the above mechanisms may work altogether through interaction between genetic, molecular and cellular events [40].
Several compounds have been identified to combat AD, and multitarget compounds represent an effective strategy for the treatment of this multifactorial disease [41,42,43]. For example, preventing the appearance of the Aβ plaques and cholinergic deficit are considered major contributing factors for AD. Thus, compounds which act simultaneously against BACE1 and AChE are multitarget compounds to combat AD.
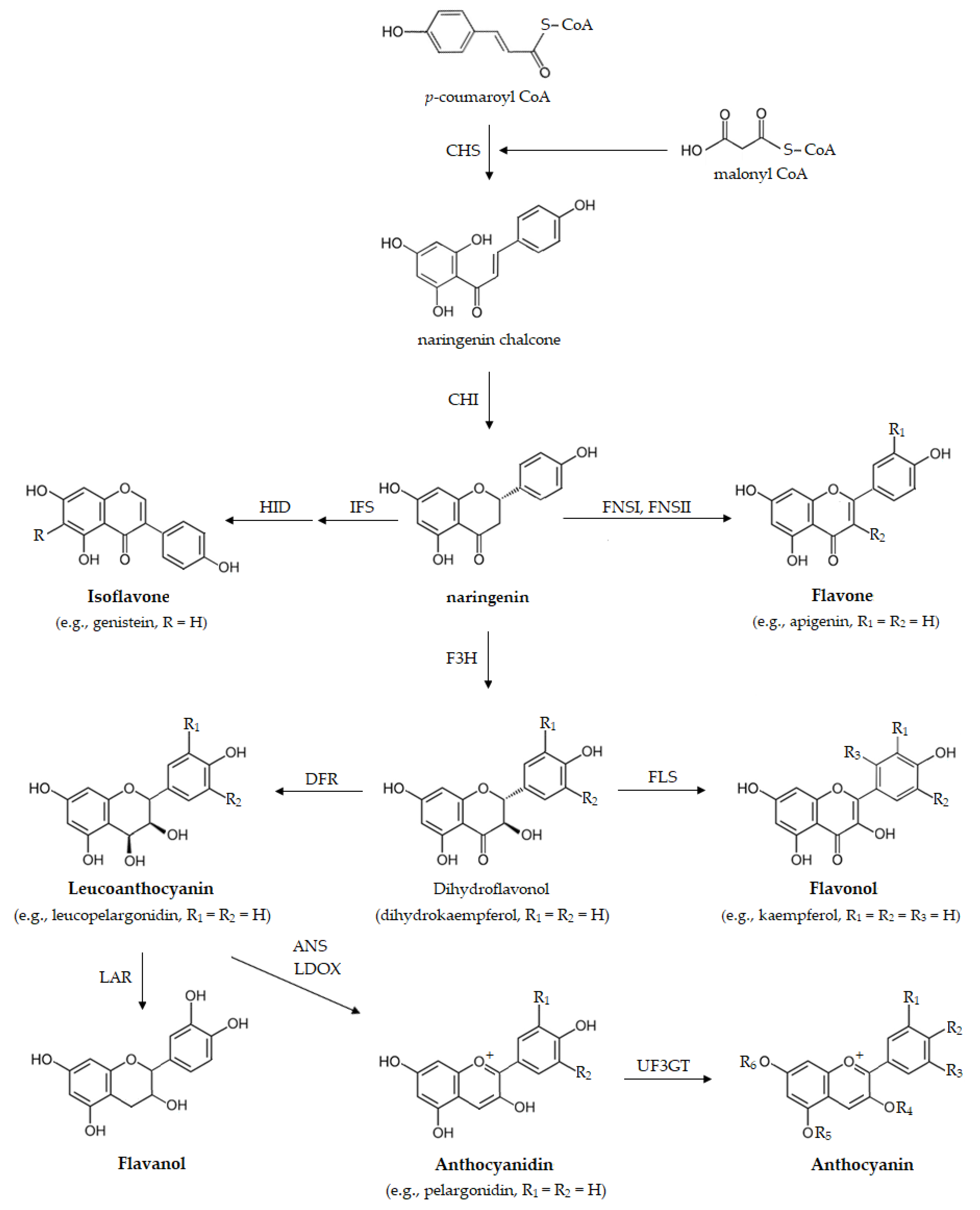
Ubiquitously presented in plants, flavonoids, a class of polyphenolic compounds, are present in plant-based foods, and so are consumed by humans through the diet. Dietary flavonoids are considered able to cross the blood-brain barrier (BBB) and are known for their CNS-related activity [44]. Flavonoids are biosynthesized through the phenylpropanoid-acetate pathway (Figure 1) [44] as a response to protect themselves against foreign agents such as UV radiation, parasites, or virus, as well as to regulate enzymes involved in cell metabolism [44,45,46,47]. Flavonoids themselves are subdivided in different classes [48]. Those flavonoids with a C6-C3-C6 carbon skeleton may have the structure of a chromane or that of a chromene, with the aromatic ring being designated as ring A and the pyran or the 3,4-dihydro-2H-pyran as ring C. Ring B is the substituent linked to ring C. The class of flavonoids in restricted sense has ring B linked to chromane/chromene position 2, whereas that of isoflavonoids has ring B connected to position 3. Chalcones and dihydrochalcones are flavonoids with a C6-C3-C6 skeleton but ring C is absent (Figure 2). Depending on the oxidation of ring C, flavonoids can be named as flavans, such as the catechins, or flavanones, 3-hydroxyflavanones, flavones and flavonols, when ring C is a pyrone. The several flavonoids differ greatly due to diverse substitutions on the different scaffolds including glycosylation, hydrogenation, hydroxylation, malonylation and alkylation. Usually the scaffolds comprising conjugation, glycosylation or methylation are responsible for several biological properties and for the hydrophilicity of the compounds [47].
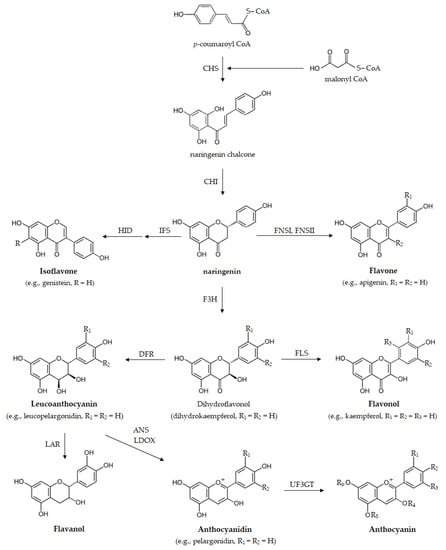
Figure 1.
Biosynthetic pathway of flavonoids illustrated for naringenin and derivatives. ANS, anthocyanidin synthase; CHI, chalcone isomerase; CHR, chalcone reductase; CHS, chalcone synthase; DFR, dihydroflavonol 4-reductase; F3H, flavanone 3-hydroxylase; FLS, flavonol synthase; FNS, flavone synthase; HID, 2-hydroxyisoflavanone dehydratase; IFS, isoflavone synthase; LAR, leucoanthocyanidin reductase; LDOX, leucoanthocyanidin dioxygenase; UF3GT, UDP-glucose flavonoid 3-O-glucosyltransferase.
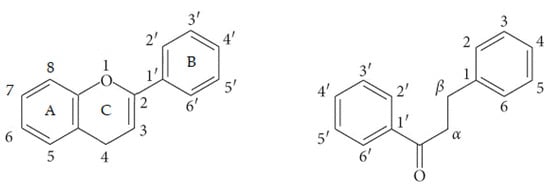
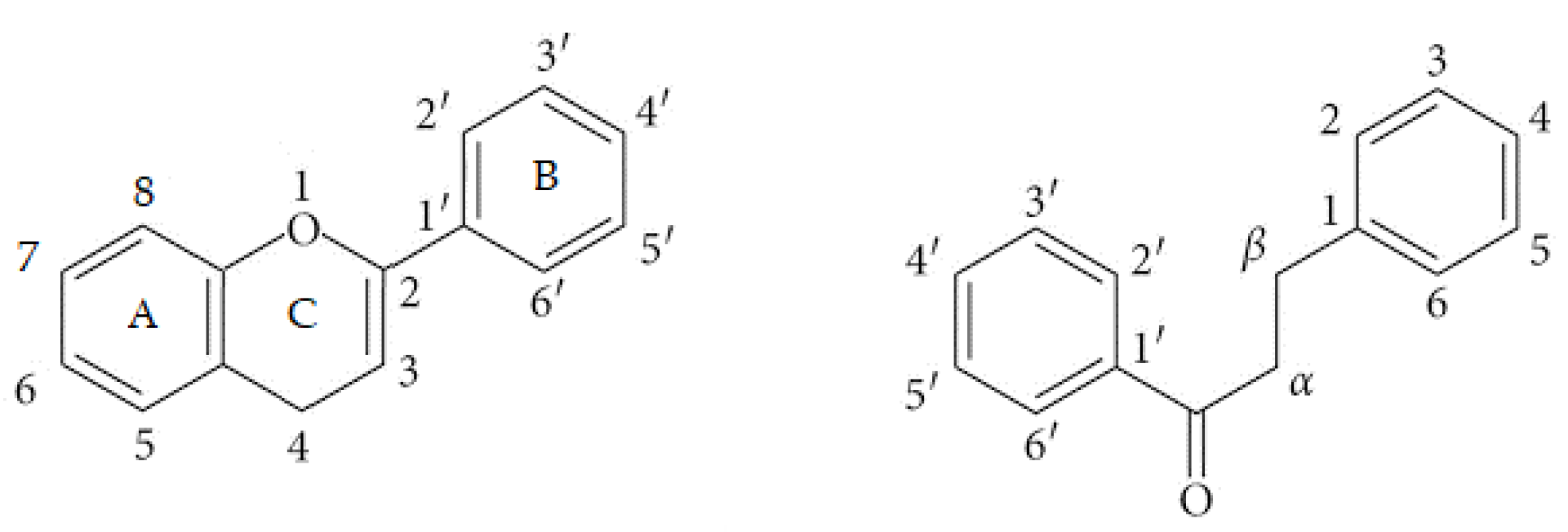
Figure 2.
Atom numbering used in flavonoids with a C6-C3-C6 structure, shown in a flavonoid with a pyran ring (left) or an open chain form (right).
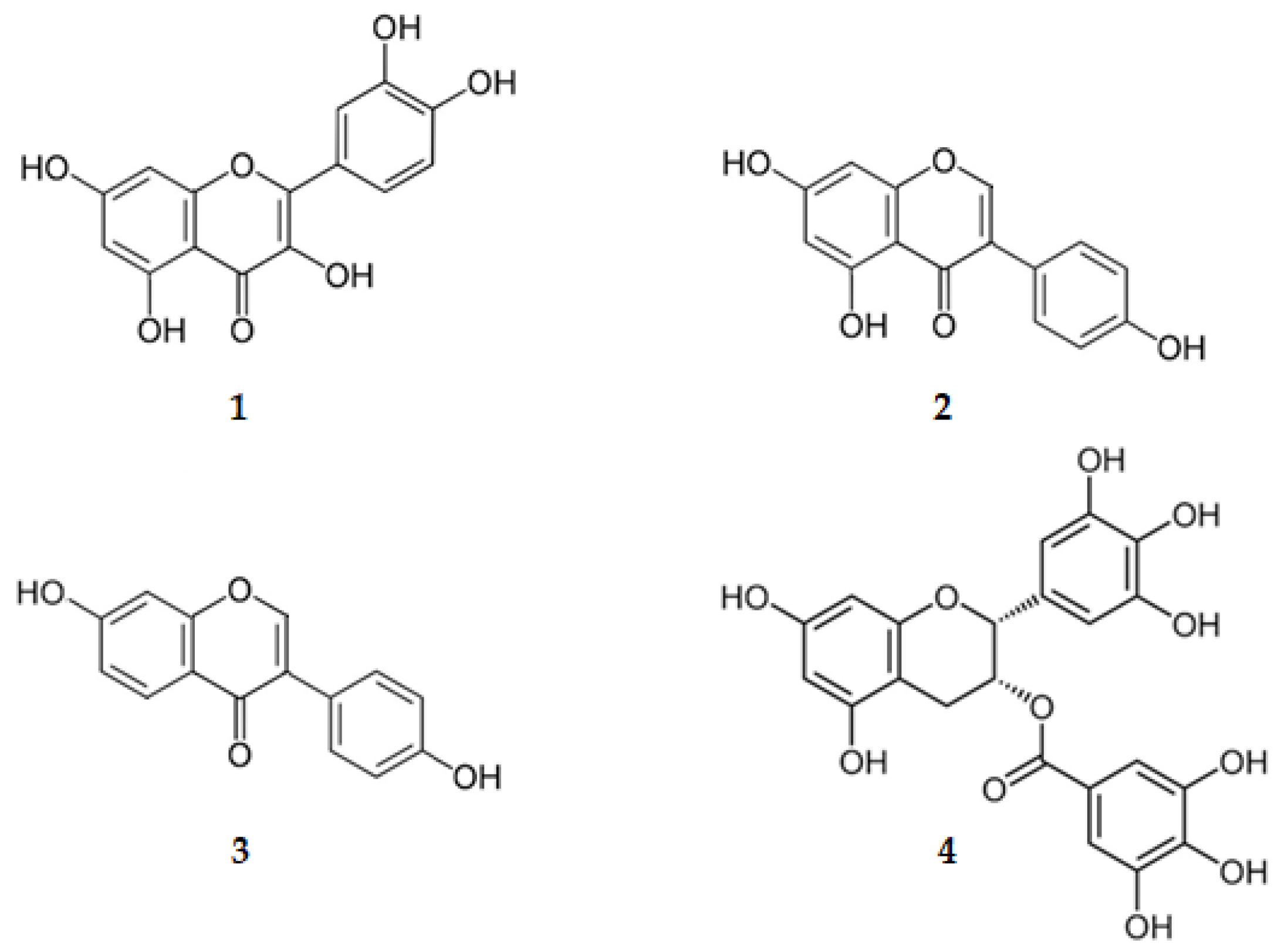
Some flavonoids are in clinical trials for the treatment of several signs and symptoms of AD [49]. Examples are quercetin (3,3′,4′,5,7-pentahydroxyflavone, (1), genistein (4′,5,7-trihydroxyisoflavone, (2), daidzein (4′,7-dihydroxyisoflavone, (3) and epigallocatechin-3-gallate, the (2R,3R)-3′,4′,5,5′,7-pentahydroxyflavan-3-yl 3,4,5-trihydroxybenzoate (EGCG, (4) (Figure 3 and Table 1).
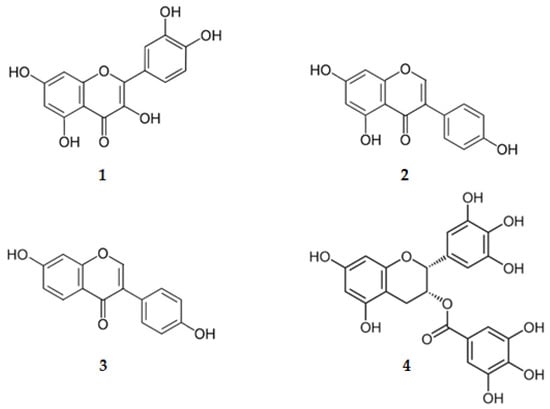
Figure 3.
Structure of flavonoids on clinical trials.

Table 1.
Flavonoids under clinical trials [49].
On this review, the several flavonoids from natural sources are presented, which have shown activity on the mechanisms highlighted below (Figure 4 and Table 2). Although several reviews have been presented on the last years, the main objective of this review is to recognize and discuss, for each CI, the scaffolds leading to the highest activity (lower IC50) and so to attempt to achieve molecules targeting more than one CI, the multitarget-directed ligands (MTDLs), which have the potential to become a lead in AD treatment. In addition, the log10 value of the partition coefficient (LogP) of flavonoids discussed in this review is also presented in Table 2. This constant is a measure of lipophilicity of a compound that can be used as a quick and simple prediction of its BBB permeability.
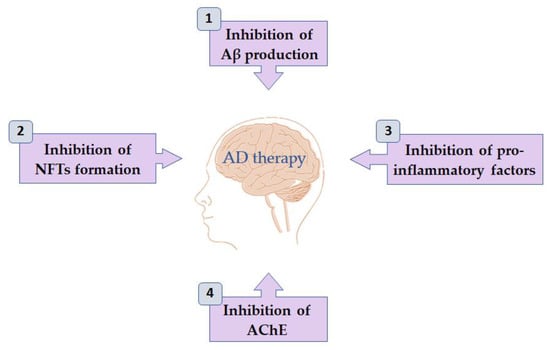
Figure 4.
Activities of flavonoids on mechanisms associated with Alzheimer’s disease.

Table 2.
Flavonoids from natural sources with neurological activities and their mode of action.
2. Activities of Flavonoids from Natural Sources
2.1. Inhibition of Aβ Production
In the amyloidogenic pathway, BACE1 is the enzyme that is involved in the rate-limiting step in the production of Aβ plaques. By inhibition of this protease the load of Aβ plaques in the neuronal cells will, therefore, be reduced by slowing or reversing the process [108].
2.1.1. Catechins and Flavanones
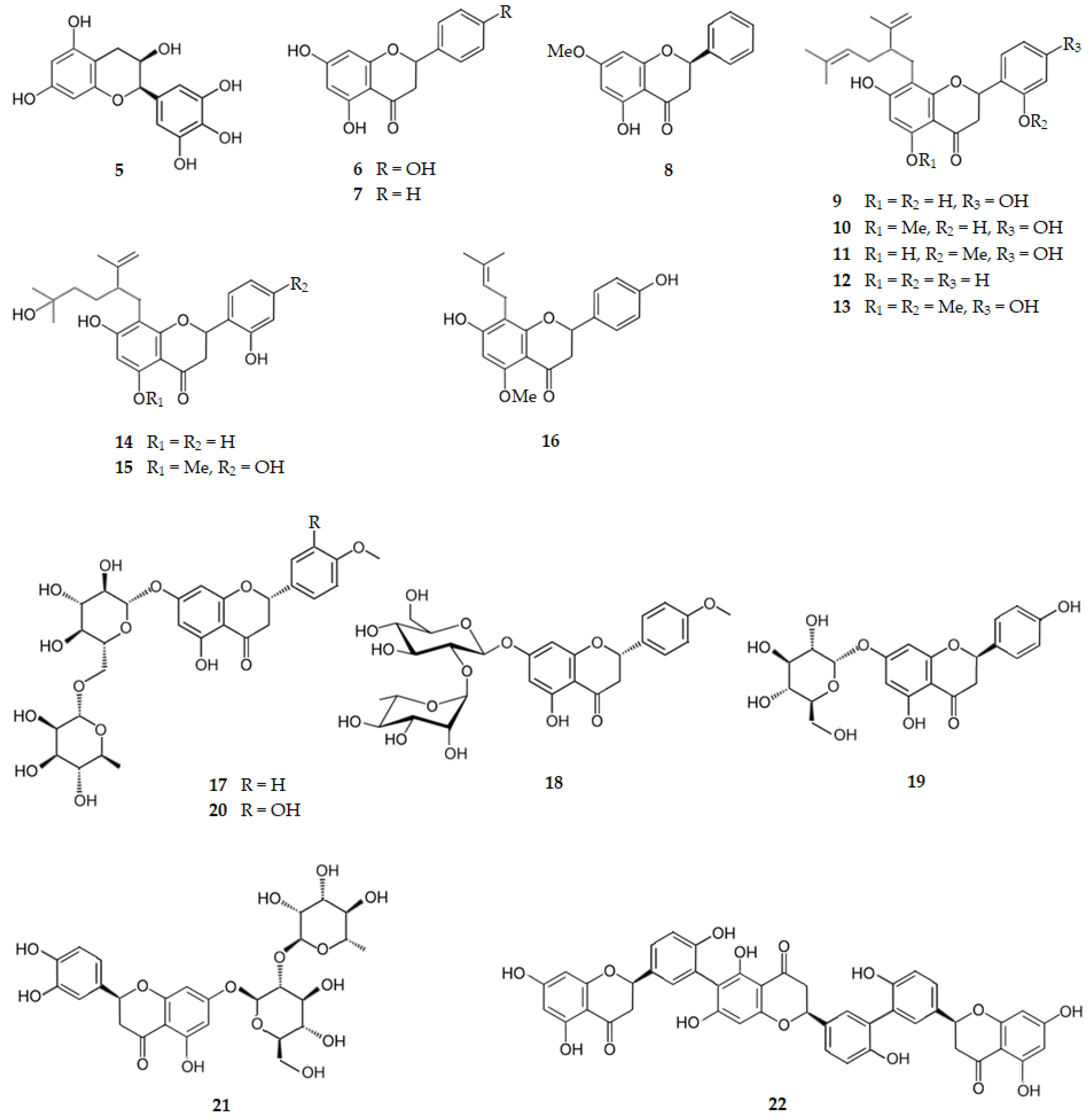
Green tea catechins present inhibitory activity of BACE1, with (−)-epigallocatechin (5) as the most potent one with an IC50 of 2.40 µM. That activity is attributed to the pyrogallol moiety linked to C2 of the catechin [50]. From Tamarix gallica L. [109], Drynariae rhizomethe [58] and several citrus fruits [49,109], naringenin, the (2S)-4′,5,7-trihydroxyflavanone (6), can be extracted, presenting a BACE1 IC50 of 38.06 μM) [59]. Pinocembrin, the 5,7-dihydroxyflavanone (7), existing in a variety of plants, mainly from Pinus heartwood, Eucalyptus, Populus, Euphorbia, Sparattosperma leucanthum and Turnera diffusa, presents a BACE1 IC50 of 27.01 µM [61]. Drynaria roosii (Nakaike), also called D. fortunei (Kunze) J. Sm., a large epiphytic fern of the family Polypodiaceae, known as “Gusuibu”, contains pinostrobin, the (2S)-5-hydroxy-7-methoxyflavanone (8), which presents a BACE1 IC50 of 4.35 μM [61]. These results suggest that the BACE1 inhibition is influenced by the presence of the pyrogallol moiety linked to C2 of the catechin.
Eight flavanones were isolated from Sophora flavescens Ait. (Leguminosae), a plant widely distributed in Asia, Oceania, and the Pacific islands, namely: five lavandulyl flavanones, sophoraflavanone G (9), kurarinone (10), leachianone A (11), kushenol A (12), and (2S)-2′-methoxykurarinone (13), which present BACE1 IC50 values of 5.20, 3.30, 8.40, 2.60, and 6.70 μM, respectively; two hydrated lavandulyl flavanones, kushenol T (14) and kurarinol (15), with BACE1 IC50 values of 36.80 and 39.20 μM, respectively, and one isoprenyl flavanone, isoxanthohumol (16), with a BACE1 IC50 of 27.70 μM [63]. Thus, the lavandulyl substituent at C8 increases by ten times the BACE1 inhibition when compared with the hydrated lavandulyl flavanones or the isoprenyl flavanone, suggesting that the hydrophobic interactions are decreased on these two latter scaffolds.
The glycosylated flavanones didymin, the (2S)-5-hydroxy-4′-methoxy-7-[α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyloxy]flavan-4-one (17), and poncirin, the (2S)-5-hydroxy-4′-methoxy-7-[α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranosyloxy]flavan-4-one (18), existing in several citrus fruits, present BACE1 IC50 values of 2.34 and 3.96 μM, respectively [59]. Another glycosylated flavanone existing in citrus fruits is prunin, the (2S)-7-(β-d-glucopyranosyloxy)-4′,5-dihydroxyflavan-4-one (19), which exhibits a BACE1 IC50 of 13.41 μM [59]. Hesperidin, the (2S)-3′,5-dihydroxy-4′-methoxy-7-[α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyloxy]flavan-4-one (20), and neoeriocitrin, the 3′,4′,5-trihydroxy-7-(α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranosyloxy)flavan-4-one (21), existing also in several citrus fruits, present BACE1 IC50 values of 10.02 [65] and 22.49 μM [60], respectively, suggesting again that, when the number of hydroxy groups on the ring B of the flavanone increases, the BACE1 inhibition decreases. Selagintriflavonoid A (22), an uncommon triflavonoid consisting of three naringenin units, isolated from the plant Selaginella doederleinii that is widely distributed in southern China, exhibits a strong BACE1 inhibition (IC50 of 0.75 µM), and is thus a promising compound for AD treatment [67].
Figure 5 shows the chemical structure of catechins and flavanones (compounds 5–22) that inhibit BACE1 with IC50 range of 0.75 to 39.20 µM.
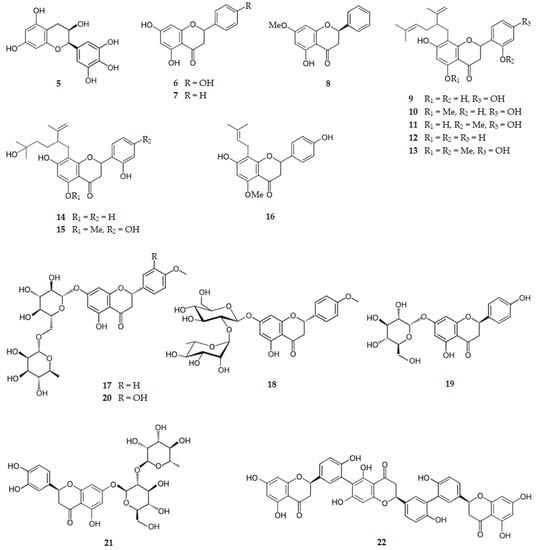
Figure 5.
Structure of catechins and flavanones that inhibit β-secretase.
2.1.2. Chalcones and Dihydrochalcones
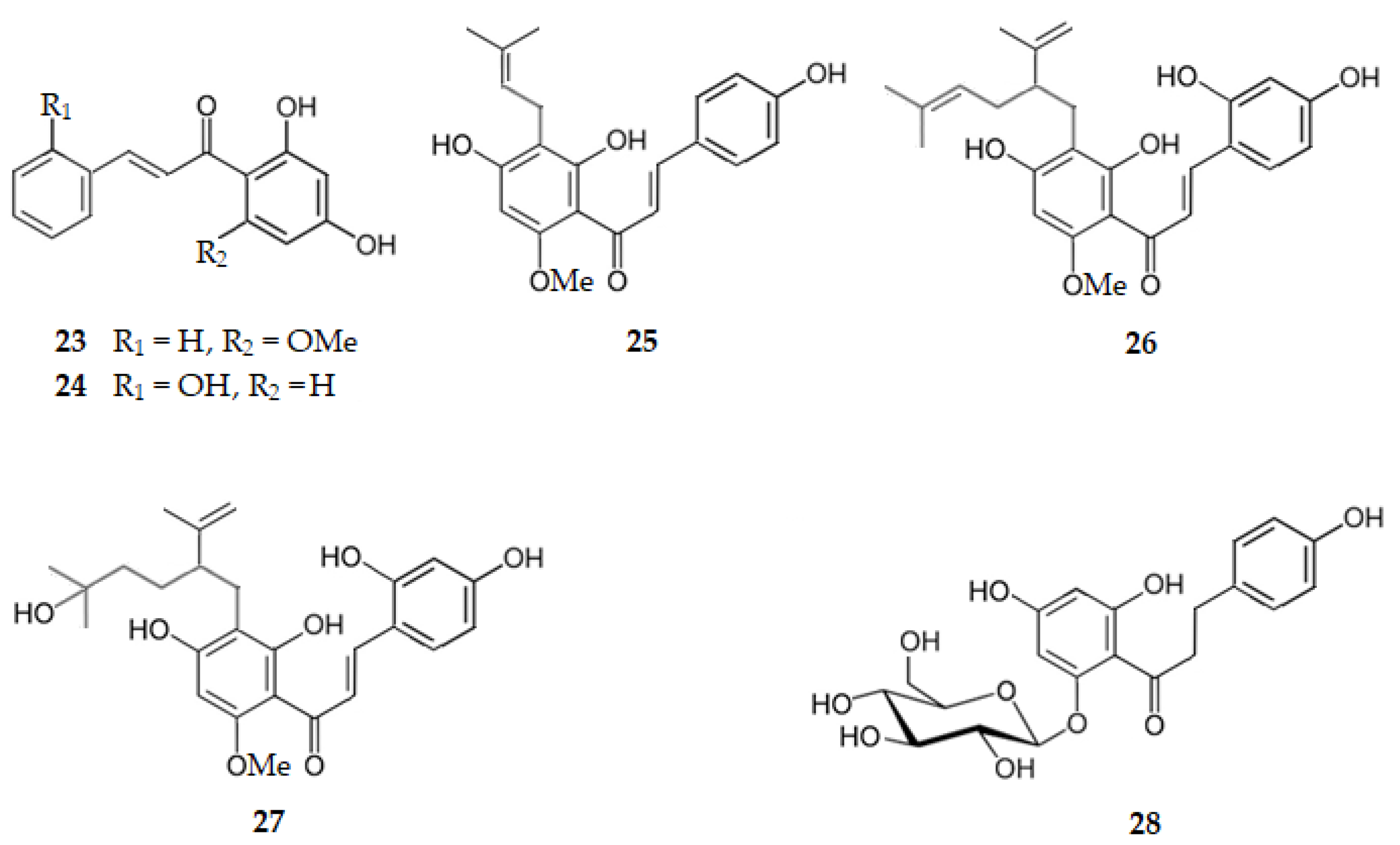
Boesenbergia rotunda (L.) Mansf., a plant commonly used as a food ingredient, as well as in traditional medicine, also known as fingerroot and Chinese ginger, contains cardamonin, the 2′,4′-dihydroxy-6′-methoxychalcone (23), which presents a BACE1 IC50 of 4.35 µM [61]. 2,2′,4′-Trihydroxychalcone (24), isolated from the plant Glycyrrhiza glabra L. (licorice), exhibits a BACE1 IC50 of 2.50 µM [51]. Xanthohumol, the 2′,4,4′-trihydroxy-6′-methoxy-3′-(3-methylbut-2-en-1-yl)chalcone (25), isolated from the dichloromethane fraction of an extract of Sophora flavescens, is another chalcone with activity against BACE1 (IC50 = 7.19 µM) [68]. From the same plant, S. flavescens, kuraridin, the 2,2′,4,4′-tetrahydroxy-3′-[5-methyl-2-(prop-1-en-2-yl)hex-4-en-1-yl]-6′-methoxychalcone (26), and Kuraridinol, the 2,2′,4,4′-tetrahydroxy-3′-[5-hydroxy-5-methyl-2-(prop-1-en-2-yl)hexyl]-6′-methoxychalcone (27), were isolated from the ethyl acetate fraction, exhibiting BACE1 IC50 values of 6.03 and 7.10 µM, respectively [68]. Phlorizin, the 4,4′,6′-trihydroxy-2′-2′-(β-d-glucopyranosyloxy)dihydrochalcone (28), isolated from Malus domestica Borkh. (cv. Anna), is a dihydrochalcone that inhibits BACE1 with an IC50 of 2.70 μM [69]. Thus, chalcones and dihydrochalcones are very active in their inhibition of BACE1 activity, regardless of the presenting prenyl groups, lavandulyl groups, hydrated or not, and glycosyl groups.
Figure 6 shows the chemical structure of chalcones and dihydrochalcones (compounds 23–28) that inhibit BACE1 with IC50 range of 2.50 to 7.19 µM.
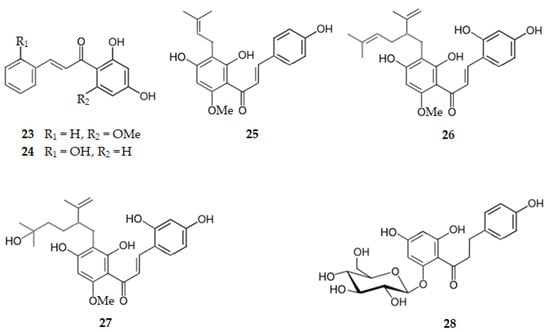
Figure 6.
Structure of chalcones and dihydrochalcones that inhibit β-secretase.
2.1.3. Flavones
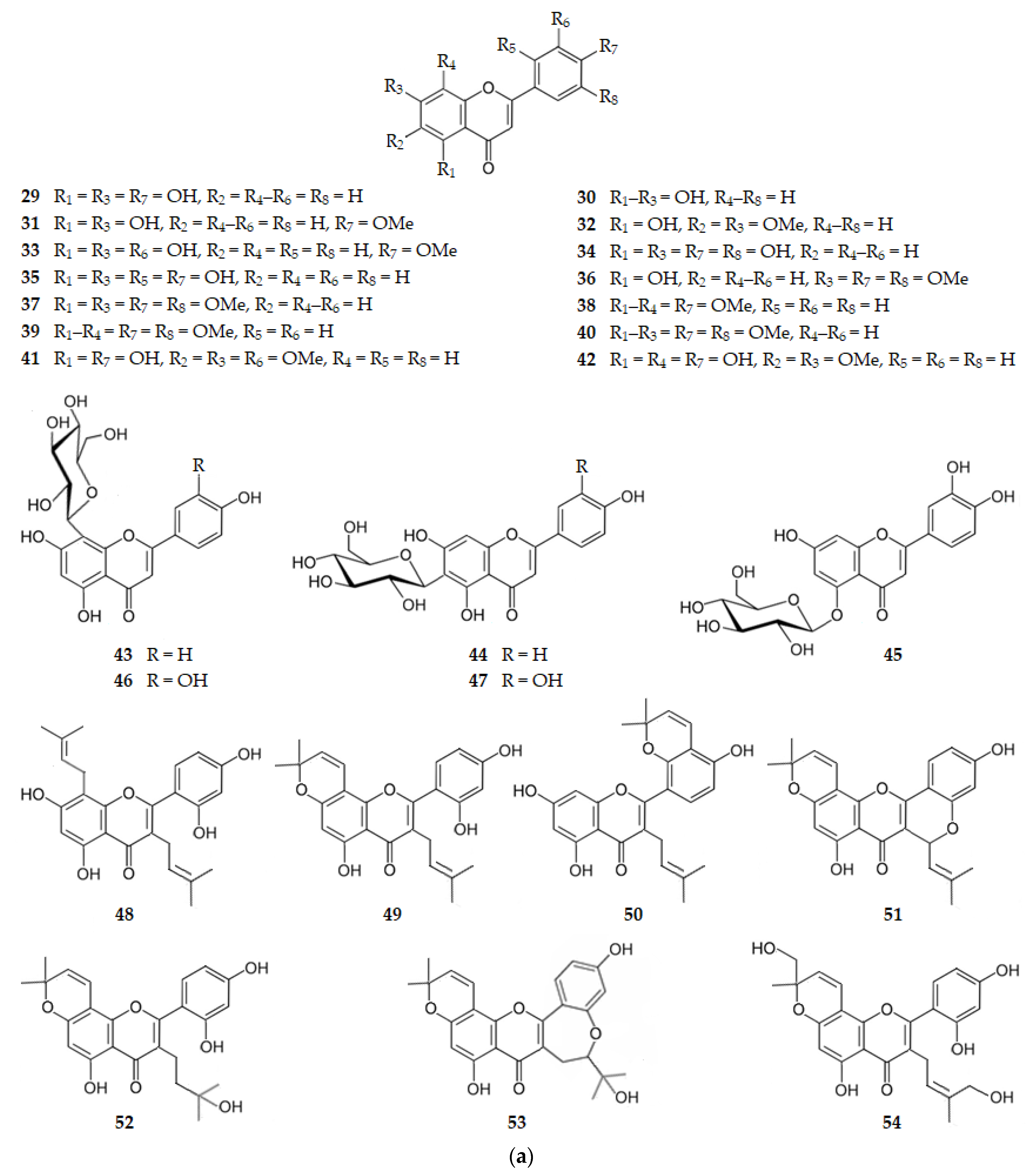
Apigenin, the 4′,5,7-trihydroxyflavone (29), which exists in oregano, mint, rosemary sage, celery, bell pepper, garlic, French peas, thyme, Tabernaqemontana pandaqcaqui and Phagnalon saxatile (L.) Cass., exhibits a BACE1 IC50 of 38.50 μM [49,50,52,53]. For baicalein, the 5,6,7-trihydroxyflavone (30), existing in Scutellaria baicalensis, also with three hydroxy groups, but all of them on ring A, the BACE1 inhibition decreases (IC50 = 69.18 μM) [70]. For acacetin, the 5,7-dihydroxy-4′-methoxyflavone (31), extracted from Agastache rugosa (Fisch. and C.A. Mey.) O. Kuntze (Lamiacae), where the hydroxy group of C4′ of ring B of apigenin is replaced by a methoxy group, there is also a decrease in BACE1 inhibition (IC50 = 88.50 μM) [72].
Mosloflavone, the 5-hydroxy-6,7-dimethoxyflavone (32), where the OH-6 and OH-7 present in baicalein (30) are replaced by methoxy groups, exhibits a small increase in BACE1 inhibition (IC50 = 43.65 μM) [70] when compared to baicalein. This flavone exists in Desmos dumosus and Phonus arborescens. When acacetin (31) bears a hydroxy group on C-3′ on ring B, this 3′,5,7-trihydroxy-4′-methoxyflavone (33) has the trivial name diosmetin. It exists on the Caucasian vetch which occurs in the region of North Caucasus [110]. There is an increase in BACE1 inhibition of 33 (IC50 = 43.65 μM) when comparing with acacetin (31) [70]. Luteolin, the 3′,4′,5,7-tetrahydroxyflavone (34), exhibits a BACE1 IC50 of 13.75 μM [74], showing the importance of the two hydroxy groups on C-3′ and C-4′ of ring B for hydrophilic interactions. It is interesting to notice that norartocarpetin (35), the 2′,4′,5,7-tetrahydroxyflavone, existing on Morus lhou and regioisomer of luteolin, exhibits a decrease in BACE1 inhibition (IC50 = 60.60 μM) [52], as the two hydroxy groups on ring B are at meta position and not ortho position as happens on luteolin. If the hydroxy group at C3′ of diosmetin (33) is replaced by a methoxy group, as well as the one at C7, the resulting 5-hydroxy-3′,4′,7-trimethoxyflavone (36) is a natural product isolated from the medicinal plant Lippia nodiflora [111]. This flavone is a very potent inhibitor of BACE1 (IC50 = 2.14 μM) [70], showing the importance of the methoxy groups on the ring A of the flavone. 3′,4′,5,7-Tetramethoxyflavone (37), existing in Orthosiphon aristatus and Bauhinia championii, where all the hydroxy groups are replaced by methoxy groups, exhibit a BACE1 IC50 of 1.66 μM even higher than 36 [70], suggesting an increase in hydrophobic interactions when the methoxy groups are linked to C5 and C7. There is a huge decrease in the inhibitory activity against BACE1 when citrus peels flavones such as tangeretin, the 4′,5,6,7,8-pentamethoxyflavone (38), or nobiletin, the 3′,4′,5,6,7,8-hexamethoxyflavone (39), or sinensetin, the 3′,4′,5,6,7-pentamethoxyflavone (40) (IC50 values of 49.00, 59.00 and 63.00 μM, respectively) [77], are compared with 3′,4′,5,7-tetramethoxyflavone (37) owing to steric hindrance. The same happens when cirsilineol, the 4′,5-dihydroxy-3′,6,7-trimethoxyflavone (41), is considered. However, there are now increases in the hydrophilic interactions owing to the hydroxy group on the C-4′ (IC50 = 20.35 μM). Another flavone, extracted from Ocimum sanctum (leaves), is isothymusin, the 5,8,4′-trihydroxy-6,7-dimethoxyflavone (42), also bearing a hydroxy group at C8, which exhibits an increase in BACE1 inhibition (IC50 = 4.45 μM) [78].
Considering glycosylated flavones, vitexin, the 8-(β-d-glucopyranosyl)-4′,5,7-trihydroxyflavone (43), is a C-glycoside isolated from Vigna radiata L. that presents a BACE1 IC50 of 51.07 µM [79]. Isovitexin (44), the regioisomer of vitexin having the β-d-glucopyranosyl group linked to C6, is inactive (IC50 > 100 µM) [79]. The same happens when the β-d-glucopyranosyl group is linked to OH-5. Indeed, the 5-O-(β-d-glucopyranosyl)luteolin (45) is also inactive against BACE1 (IC50 = 969.75 µM) [74]. Orientin, the 8-(β-d-glucopyranosyl)-3′,4′,5,7-tetrahydroxyflavone (46), and isoorientin, the 6-(β-d-glucopyranosyl)-3′,4′,5,7-tetrahydroxyflavone (47), with the β-d-glucopyranosyl group, respectively on C8 and C6, are also weak inhibitors of BACE1 with IC50 of 15.95 and 20.88 µM, respectively [76].
Seven alkyl-substituted flavones from methanol extract of Morus lhou, namely kuwanon C (48), morusin (49), kuwanon A (50), cyclomorusin (51), morusinol (52), neocyclomorusin (53) and mormin (54), present BACE1 IC50 values of 3.40, 59.40, 5.30, 102.20, 135.90, 146.10 and 103.50 µM, respectively, suggesting that BACE1 inhibition is significantly influenced by the presence of two hydroxy groups at ring B and the isoprenyl group at C3 [52,82].
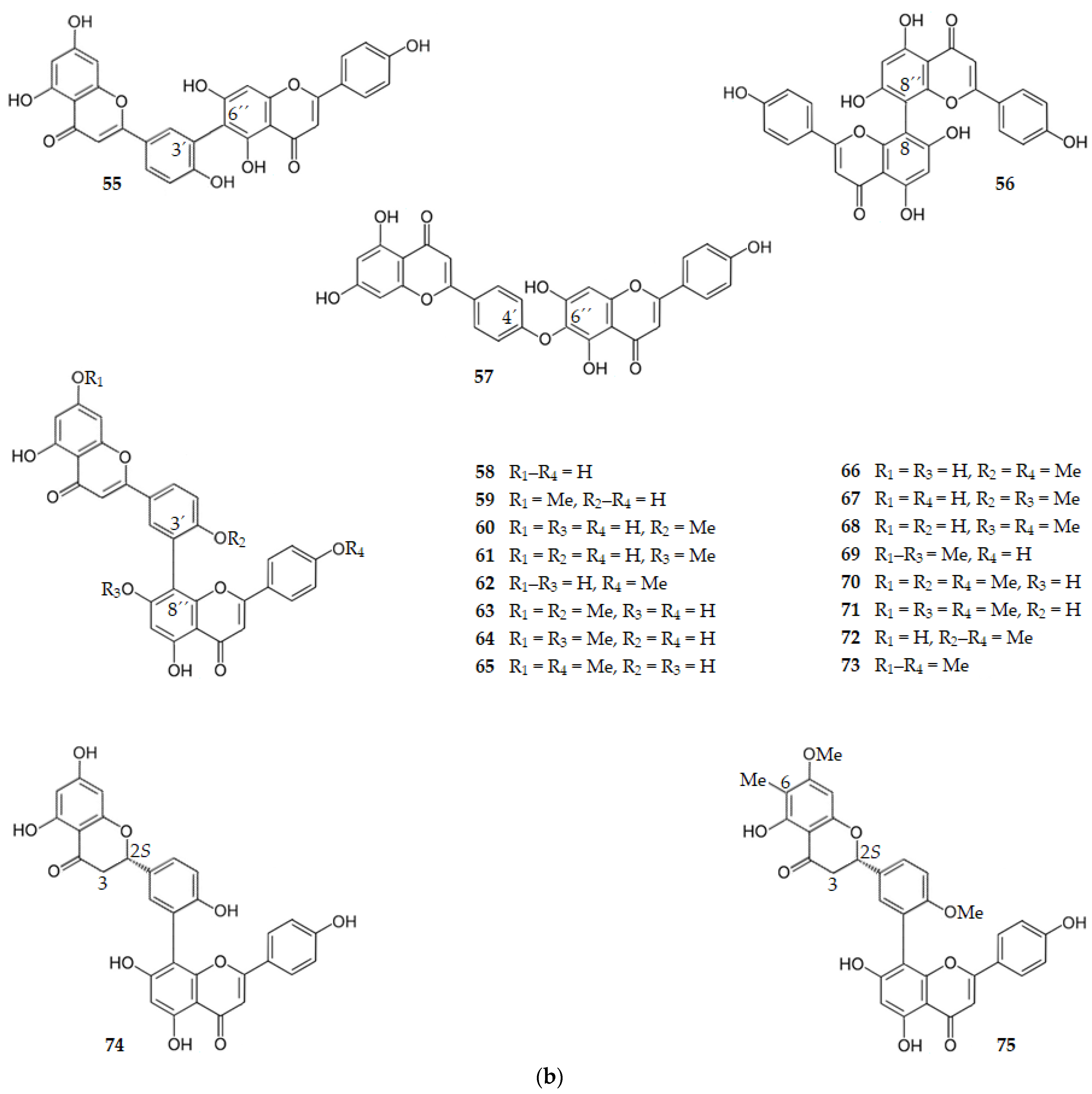
Several biflavones exhibit BACE1 inhibitory activity. Amentaflavone-type flavonoids, where the flavonoid moieties are connected through a C3′-C8″ bond, showed BACE1 inhibition. Robustaflavone (55), cupressuflavone (56), and hinokiflavone (57), biflavones where the two flavonoid moieties are connected through, respectively, C3′-C6″, C8-C8″ and C4′-C6″ bonds, are inactive [84]. Amentoflavone (58) and all the monomethoxy analogs of it, sequoiaflavone (59), bilobetin (60), sotetsuflavone (61), and podocarpusflavone A (62), showed a strong BACE1 inhibition (IC50 range of 0.99–2.02 µM). The dimethoxy amentoflavones ginkgetin (63), amentoflavone-7,7″-dimethyl ether (64), podocarpusflavone B (65), and isoginkgetin (66) showed a decrease in the BACE1 inhibition (IC50 range of 3.01–6.25 µM) when compared with their monomethoxy analogs. The other dimethoxy amentoflavones, 4′,7″-di-O-methylamentoflavone (67) and 7″,4″′-di-O-methylamentoflavone (68), were inactive, as well as the trimethoxy amentoflavones, 4′,7,7″-tri-O-methylamentoflavone (69), sciadopitysin (70), heveaflavone (71), and kayaflavone (72), and the tetramethoxy amentoflavone, 7,7″,4′,4″′-tetra-O-methylamentoflavone (73). The 2,3-dihydroamentoflavone (74) showed an increase in BACE1 inhibition (IC50 = 0.75 µM) whereas 2,3-dihydro-6-methylginkgetin (75) showed the strongest inhibitory activity (IC50 = 0.35 µM) [84].
Thus, it can be concluded that the amentaflavone-type biflavonoids, which have a scaffold consisting of two apigenin molecules linked by the C3′ of one molecule with the C8″ of the other apigenin molecule, have significant inhibitory activity against BACE1. The results suggest that more than two hydroxy groups at C7, C4′, C7″ and C4′″ are needed for the inhibitory activity of the biflavonoid. From the data obtained for the biflavones 74 and 75 it can be concluded that the presence of a flavanone moiety in these amentaflavone-type biflavonoids increases the BACE1 inhibition, even more so if there is a methyl group at C6 to increase the hydrophobic interactions with the enzyme [84].
Figure 7 shows the chemical structure of flavones (compounds 29–75) that inhibit BACE1 with an IC50 range of 0.35 to 146.10 µM, except for compounds 44, 45, 55–57 and 67–73 that were inactive.
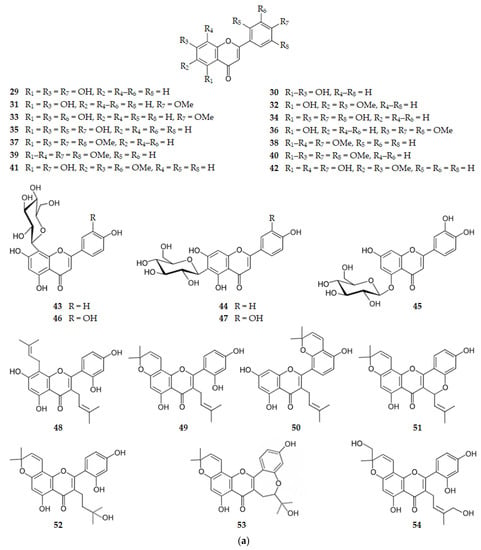
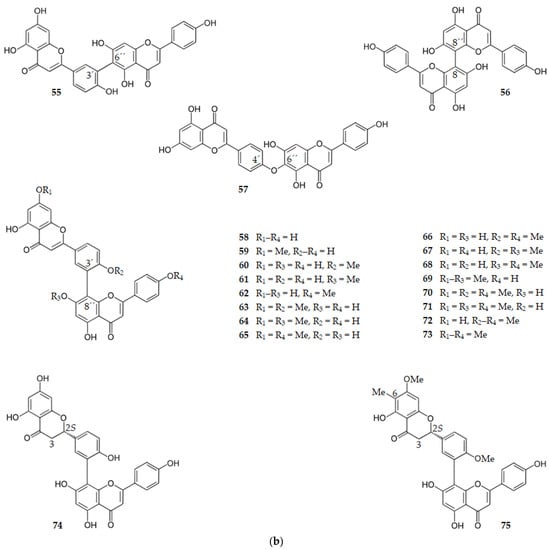
Figure 7.
(a) Structure of flavones that inhibit β-secretase and of the inactive flavones 44 and 45. (b) Structure of flavones that inhibit β-secretase and of the inactive flavones 55–57 and 67–73.
2.1.4. Flavonols
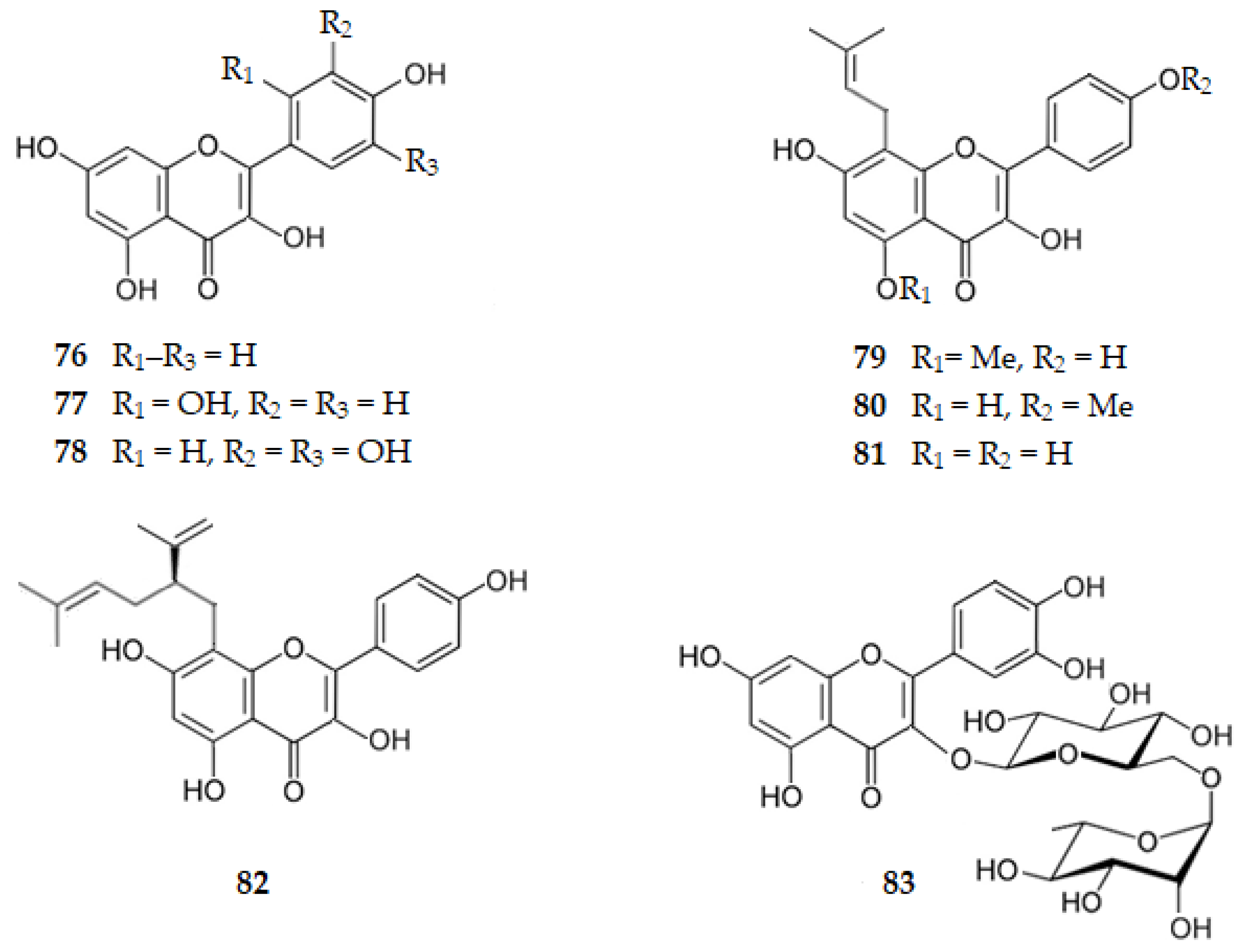
Kaempferol, the 3,4′,5,7-tetrahydroxyflavone (76), exhibits a BACE1 IC50 of 14.70 µM. Comparing with apigenin (29) (IC50 = 38.50 µM) it can be concluded that the hydroxy group on C3 is important for the hydrophilic binding to BACE1 of flavonols [52]. Quercetin, the 3,3′,4′,5,7-pentahydroxyflavone (1, Figure 3), isolated from several plants, such as Allium cepa L. and Malus pumila Mill., presents an IC50 of 5.40 μM [50,51,52] and morin, the 2′,3,4′,5,7-pentahydroxyflavone (77), isolated from Psidium guajava [53], has an IC50 of 21.70 μM [52], suggesting once more the significant role of the hydroxy group on C4′ for the hydrogen bonds. When there are two hydroxy groups on ring B they should be in the ortho position. Myricetin, the 3,3′,4′,5,5′,7-hexahydroxyflavone (78), which exists in Ficus auriculata, Ardisia sanguinolenta and many other plants, shows an increase in the inhibitory activity against BACE1 (IC50 = 2.40 μM) [52], suggesting that the six hydroxy groups improve the hydrogen binding to the enzyme.
Isolated from the plant Sophora flavescens, sophoflavescenol, the 3,4′,7-trihydroxy-5-methoxy-8-(3-methylbut-2-enyl)flavone (79), icaritin, the 3,5,7-trihydroxy-4′-methoxy-8-(3-methylbut-2-enyl)flavone (80), desmethylanhydroicaritin or 3,4′,5,7-tetrahydroxy-8-(3-methylbut-2-enyl)flavone (81), and kushenol C, the 2′,3,4′,5,7-pentahydroxy-8-[(2R)-5-methyl-2-prop-1-en-2-ylhex-4-enyl]flavone (82), presented BACE1 IC50 values of 10.98, 22.48, 1.51 [89] and 5.45 μM [68], respectively. These results suggest the importance of the hydroxy groups at C4′ and C5 when desmethylanhydroicaritin (81) is compared with icaritin (80) or sophoflavescenol (79). The results obtained for Kushenol C (82) and desmethylanhydroicaritin (81) indicate that the prenyl group, rather than the lavandulyl group, might make predominant contributions to BACE1 inhibition [68,89].
Rutin is the 3′,4′,5,7-tetrahydroxy-3-[α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyloxy]flavone (83), a glycosylated flavonol on C3 of several citrus fruits, which exhibits a BACE1 IC50 of 0.004 µM [90], confirming the importance of the hydroxy groups at C4′ and C5, as well as of a hydrophilic group at C3.
Figure 8 shows the chemical structure of the flavonols 76–83 that inhibit BACE1 with an IC50 range of 0.004 to 22.48 µM.
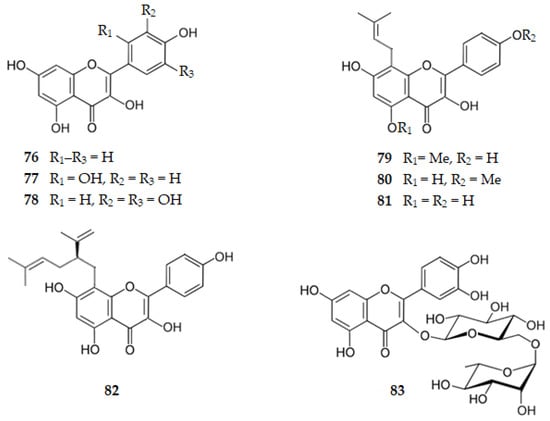
Figure 8.
Structure of flavonols that inhibit β-secretase.
2.2. Inhibition of NFTs Formation
2.2.1. Inhibition of GSK-3β
Flavanones
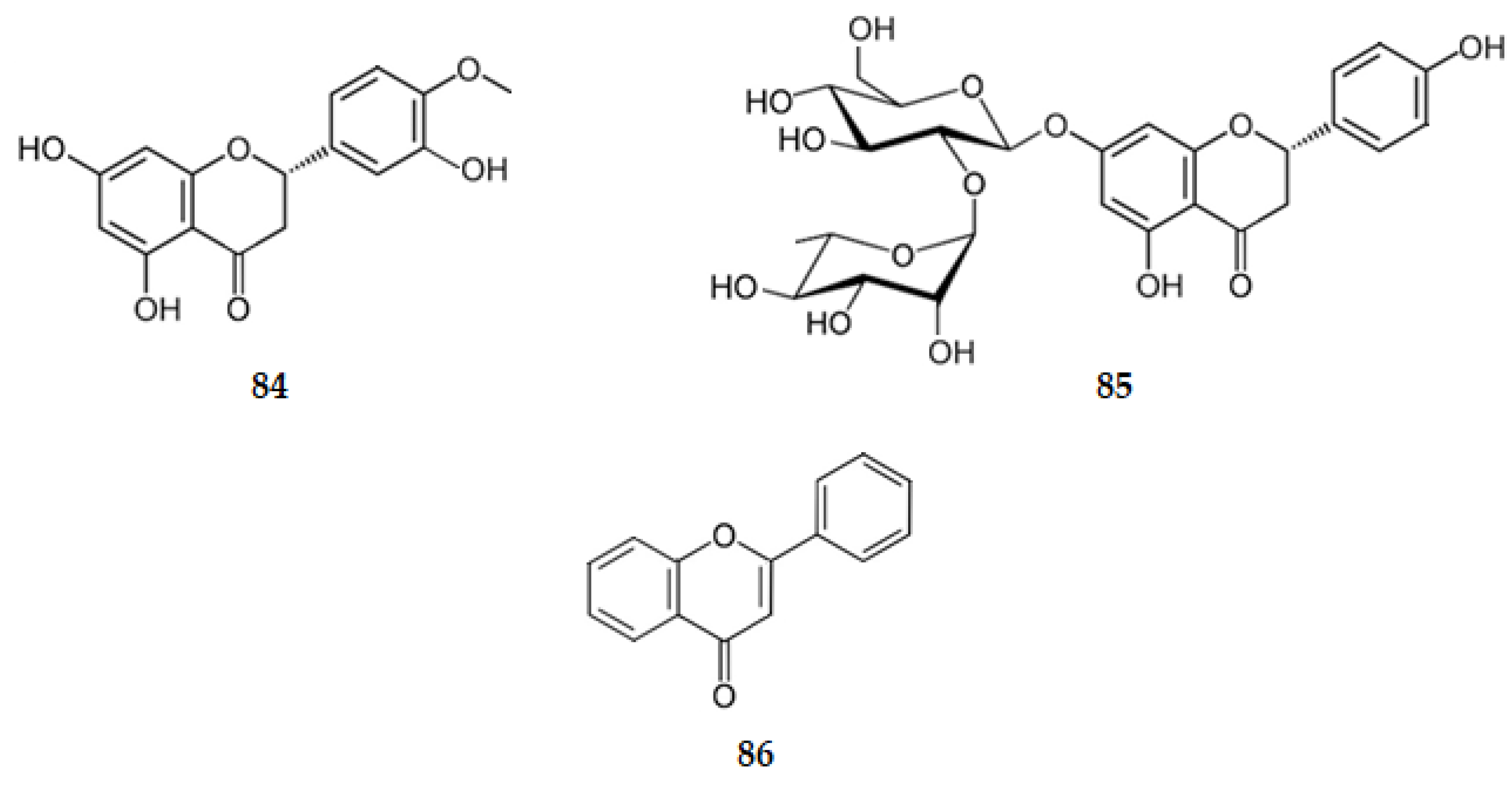
Naringenin (6) exhibits GSK-3β inhibition with an IC50 of 45.71 µM [52]. Hesperetin, the (2S)-3′,5,7-trihydroxy-4′-methoxyflavanone (84) (Figure 9), presents GSK-3β inhibition characterized by an IC50 of 26.92 µM [66]. However, the corresponding glycosylated flavanones naringin, the (2S)-4′,5-dihydroxy-7-[α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranosyloxy]flavan-4-one (85) (Figure 9), and hesperidin (20), existing also in several citrus fruits, have no effect on the inhibition of GSK-3β (IC50 > 300 µM) [66]. These results suggest that side group substitutions on the flavonoid scaffold affect the GSK-3β activity in a significant manner as the flavonoids with larger side groups exhibit lower inhibitory activity. The presence of bulkier groups leads to decreased interactions between enzymes and inhibitors [52,66].
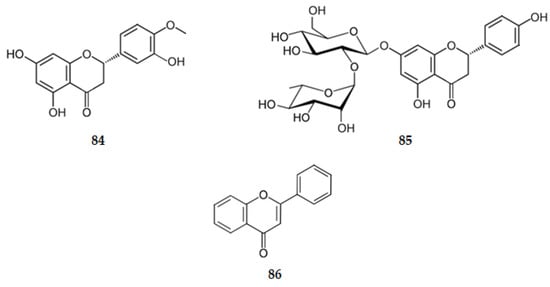
Figure 9.
Structure of the flavanones 84 and 85 and of flavone (86).
Flavones
Flavone (86) (Figure 9), the simplest compound of this class, with no hydroxy groups, presents no inhibitory activity of GSK-3β (IC50 > 100 µM) [66]. Very weak inhibitors of the enzyme are also nobiletin (39) (IC50 = 52.48 µM) and tangeritin (38) (IC50 > 100 µM) [66], which have multiple methoxy groups as substituents. These results show how the inhibitory activity of GSK-3β is influenced by the number of free hydroxy substituted groups on the flavonoids (hydroxy groups on A and B rings tend to form hydrogen bonds with the active site of the enzyme) [52,66].
The GSK-3β inhibitory activity of apigenin (29) and luteolin (34) is characterized by IC50 values of 1.91 and 1.51 µM, respectively, suggesting the importance of the hydroxy groups at C4′, C5 and C7 [66].
C-glycosylflavones at C6, such as isoorientin (47) and isovitexin (44) are weak inhibitors of GSK-3β, having IC50 values of 185.00 and 195.00 µM, respectively [80]. Orientin (46), the C-glycosylflavone at C8, is inactive against the enzyme (IC50 > 5000 µM). These results show that side bulky group substitutions on the flavonoid scaffold affect the GSK-3β activity in a significant manner, decreasing the interactions between the enzyme and inhibitors [80].
Flavonols
Quercetin (1) presents high GSK-3β inhibition (IC50 = 2.00 µM) [52] and morin (77), structurally related to 1, is also very active against GSK-3 [53] showing how important is the presence of the two hydroxy groups on the ring B. Kaempferol (76), with only one hydroxy group on ring B at C4′, exhibits an IC50 of 3.47 µM [66], suggesting that one of the two hydroxy groups on ring B should be on C4′.
Also, it can be concluded that flavones, such as luteolin (34) and apigenin (29), are more potent compared to flavan-4-ones such as hesperetin (84) and naringenin (6) [52,66].
2.3. Inhibition of Pro-Inflammatory Factors
2.3.1. Catechins, Flavanones and 3-Hydroxyflavanones
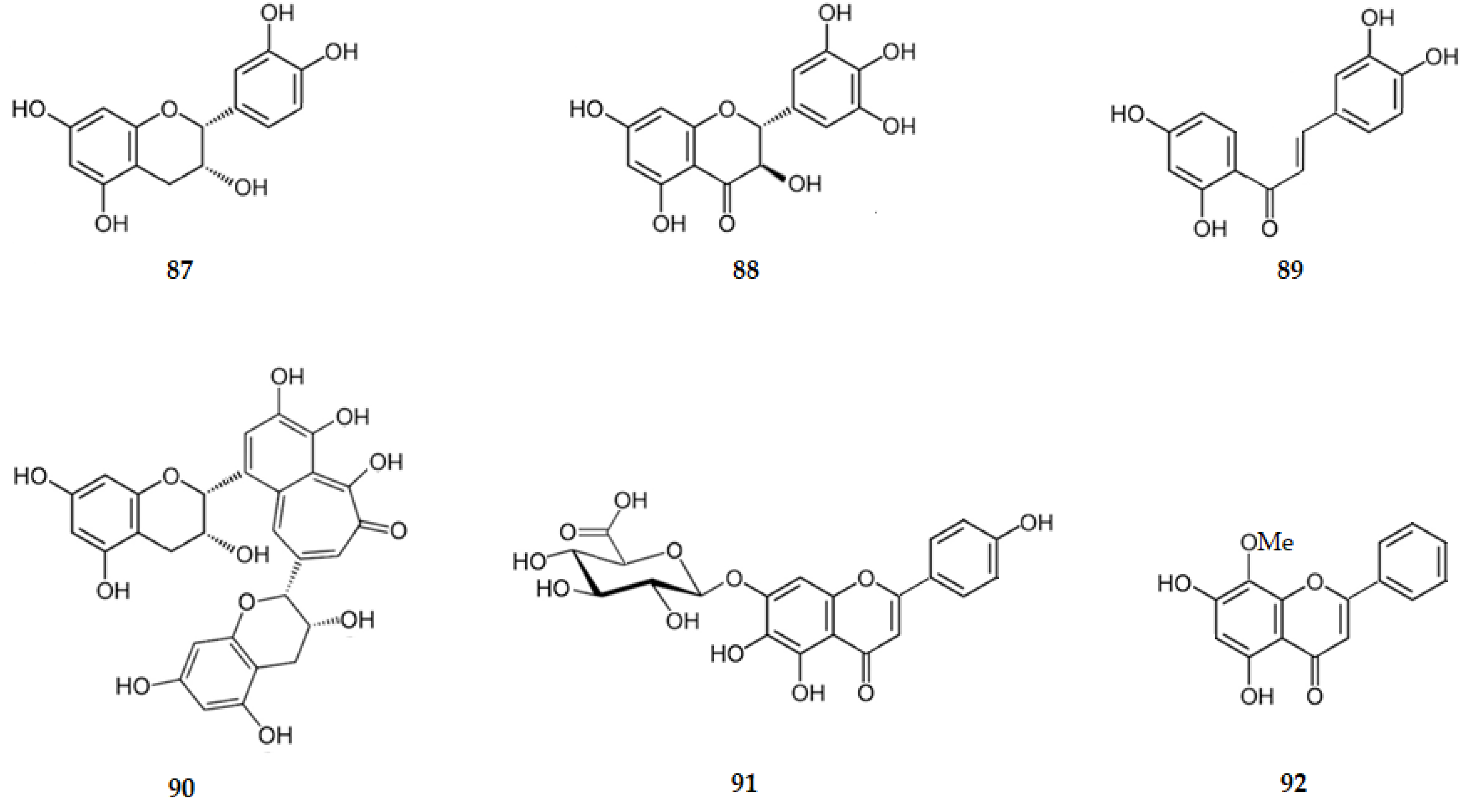
The neuroprotective action of EGCG (4, Figure 3) is due to its ability to inhibit neuroinflammation. EGCG reduces TNF-α, IL-1β, IL-6 and iNOS levels in Aβ-stimulated EOC13.31 microglia through mechanisms involving NF-κB [47]. Epicatechin (87) attenuates several pro-inflammatory mediators including TNF-α, NF-κB and iNOS [47]. The flavanone naringenin (6) inhibits iNOS and COX-2 expression and NO production in activated microglia [47,54]. Hesperetin (84) only induces a very weak anti-neuroinflammatory effect and doesn’t protect neurons from neuroinflammatory injury [54]. Glycosylated hesperetin, such as hesperidin (20), inhibits neuronal cells’ death by reducing the expression of pro-inflammatory factors as NF-κB, iNOS, and COX-2 [47], suggesting that the presence of the α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyloxy group at C7 is important for the inhibition of neuroinflammation. Sophoraflavanone G (9) reduces the expression of the genes: iNOS, COX-2, TNF-α, IL-6, and IL-1β [47]. Ampelopsin, the (2R,3R)-3,3′,4′,5,5′,7-hexahydroxyflavan-4-one (88), decreases hippocampal neuronal apoptosis, IL-1β, IL-6 and TNF-α [92].
2.3.2. Chalcones and Flavanols
Butein (89) exhibits one of the greatest inhibitory effect against NO production with an IC50 value of 10.9 μM [47].
Theaflavin (90) attenuates neuroinflammation by reducing pro-inflammatory factors as IL-6 or TNF-α [47].
2.3.3. Flavones
Several flavones have an inhibitory effect on the pro-inflammatory factors. Apigenin (29) decreases the production of pro-inflammatory IL-6 and TNF-α through mechanisms involving STAT1. Further evidence of the anti-inflammatory activity of this flavonoid was provided in investigations showing reduction in iNOS/NO and PGE2/COX-2 in activated microglia [47]. Luteolin (34) attenuates IL-6, TNF-α, COX-2 and iNOS gene expression in activated microglia, and inhibits cytokine-induced neuronal death [47,54,75]. This flavone inhibits the NO level with an IC50 value of 1.74 μM [76].
Studies in vitro revealed that diosmetin (33) decreases the production of pro-inflammatory mediators [47] and that scutellarin (91) also decreases the production of pro-inflammatory mediators as the expression of NF-κB [47]. Wogonin (92) suppresses the activity of NF-κB and the iNOS expression [75,94]. Baicalein (30) decreases the inflammatory processes and neuronal death by the inhibition of iNOS/NO [54]. These two flavones (92 and 30) inhibit the NO level with IC50 values of 45.3 and 66.4 μM, respectively [71].
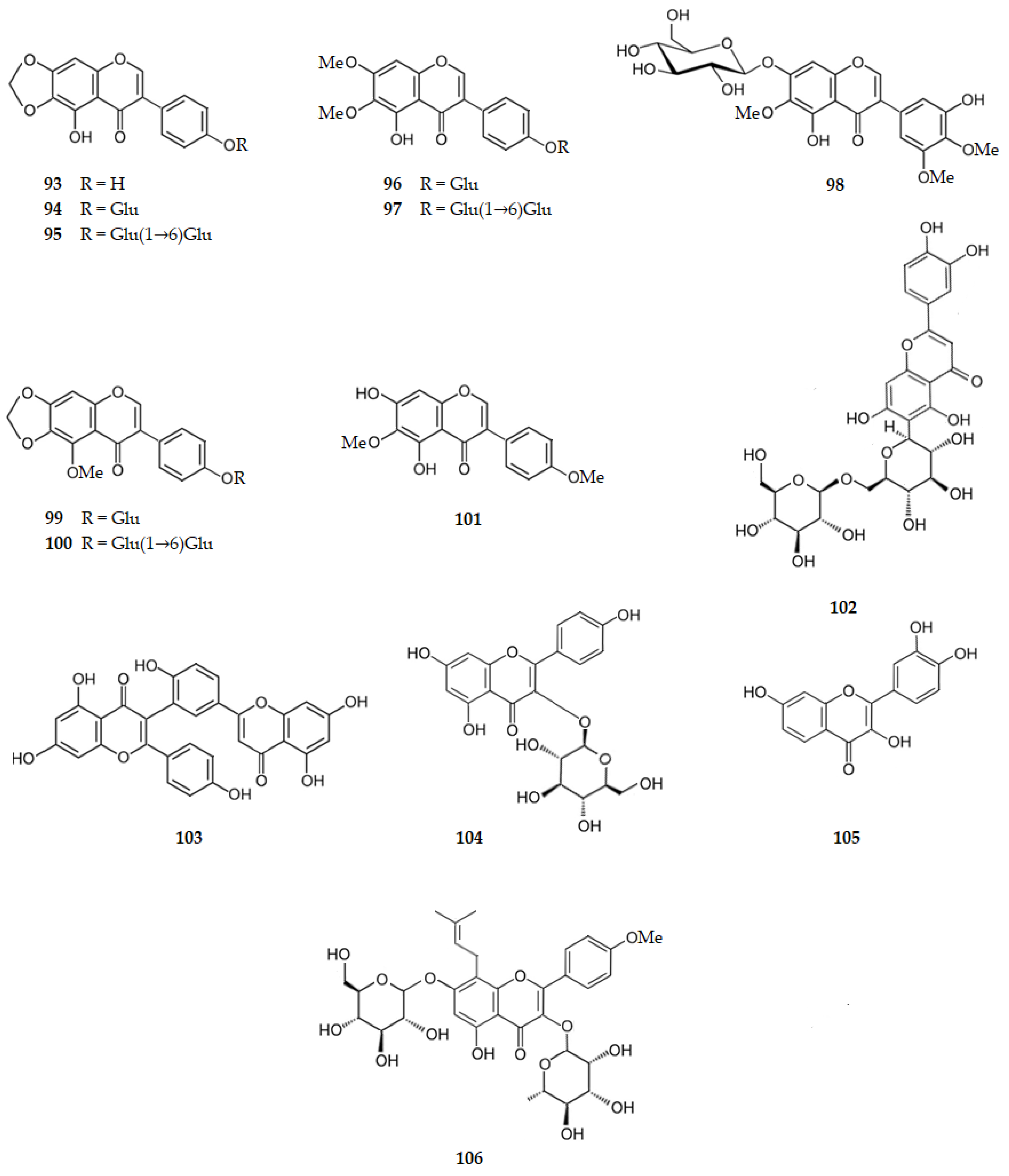
Considering the isoflavones genistein (2, Figure 3), or daidzein (3, Figure 3), they possess anti-inflammatory effects against activated microglial cell line inhibiting iNOS and IL-6 expression [47]. Nine isoflavones, isolated from Iris pseudopmila Tineo, inhibit the NO level with IC50 values ranging from 23.60 to 98.30 µM, such as: irilone, the 4′,5-dihydroxy-6,7-methylenedioxyisoflavone (93, 89.70 µM); 4′-O-(β-d-glucopyranosyl)irilone or 4′-O-(β-d-glucopyranosyl)-5-hydroxy-6,7-methylenedioxyisoflavone (94, 80.10 µM); 4′-O-[β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl]irilone or 5-hydroxy-4′-O-[β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl]-6,7-methylenedioxyisoflavone (95, 83.60 µM); 4′-O-(β-d-glucopyranosyl)-7-O-methyltectorigenin or 4′-O-(β-d-glucopyranosyl)-5-hydroxy-6,7-dimethoxyisoflavone (96, 56.90 µM); 4′-O-[β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl]-7-O-methyltectorigenin or 4′-O-[β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl]-5-hydroxy-6,7-dimethoxyisoflavone (97, 29.40 µM); iridin, the 3′,5-dihydroxy-4′,5′,6-trimethoxy-7-O-(β-d-glucopyranosyl)isoflavone (98, 67.50 µM); 4′-O-(β-d-glucopyranosyl)irisolone or 4′-O-(β- d-glucopyranosyl)-5-methoxy-6,7-methylenedioxyisoflavone (99, 98.30 µM); 4′-O-[β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl]irisolone or 5-methoxy-4′-O-[β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl]-6,7-methylenedioxyisoflavone (100, 55.10 µM) and irisolidone, the 5,7-dihydroxy-4′,6-dimethoxyisoflavone (101, 23.60 µM) [81]. Thus, comparing the IC50 values for 96 (IC50 = 56.90 µM) and 97 (IC50 = 29.40 µM), it is concluded that the presence of disaccharidyl moiety increases the activity against the NO production. The same occurred relatively to 99 (IC50 = 98.30 µM) and 100 (IC50 = 55.10 µM) [81].
Glycosilated flavones such as isoorientin (47), isovitexin (44), and 6″-O-(β-d-glucopyranosyl)isoorientin (102), isolated from Iris pseudopumila Tineo, exhibit inhibitory activity against NO production with IC50 values of 61.00, 66.70 and 71.90 µM, respectively [81].
Considering biflavonoids such as ginkgetin (63), bilobetin (60), taiwaniaflavone (103) and amentoflavone (58) they downregulate the expression of COX-2 and iNOS [85].
2.3.4. Flavonols
Kaempferol (76) is a flavonol that inhibits neuroinflammation by reducing LPS-induced production of pro-inflammatory mediators in BV2 microglial cells through mechanisms involving NF-κB and p38 MAPK [47]. Astragalin, the 3-O-(β-d-glucopyranosyl)kaempferol (104), isolated from Iris pseudopumila Tineo, exhibits inhibitory activity against NO production with an IC50 value of 45.20 µM [81].
Quercetin (1), a similar flavonol to kaempferol with an extra hydroxy group at C-3′ (B ring), reduces iNOS-mediated NO production in LPS-stimulated BV-2 microglia through mechanisms involving suppression of NF-κB activation [47,53,54]. It lowers the expression of pro-inflammatory cytokines such as IL-6, TNF-α, IL-1β and COX-2 [47]. Rutin (83) reduces neuroinflammation, downregulating microgliosis and astrocytosis and reducing the expression of COX-2, IL-6, IL-1β, iNOS and NF-κB [47]. Fisetin is the 3,3′,4′,7-tetrahydroxyflavone (105), and shows one of the greatest inhibitory effects against NO production with an IC50 value of 13.5 μM [47]. Considering glycosylated flavonols, icariin (106) has an anti-inflammatory effect on primary rat microglial cultures activated by LPS. It reduces the release of NO and PGE2 in a dose dependent manner and down-regulates the expression of proinflammatory cytokines such as TNF-α, IL-1β and IL-6. Icariin also inhibites the protein expression of iNOS and COX-2 [96].
These results suggest that flavonoids may exert neuroprotective effects by inhibiting the activation of microglia which mediates inflammatory processes in the CNS. There is evidence indicating that flavonoids may act through four mechanisms: inhibition of the release of cytokines such as IL-1β and TNF-α; inhibition of iNOS induction and subsequent NO production; inhibition of the activation of NADPH oxidase and subsequent ROS generation; and downregulation of the activity of pro-inflammatory factors such as NF-κB [54]. Figure 10 shows the chemical structure of flavonoids 87–106 that inhibit the pro-inflammatory factors.
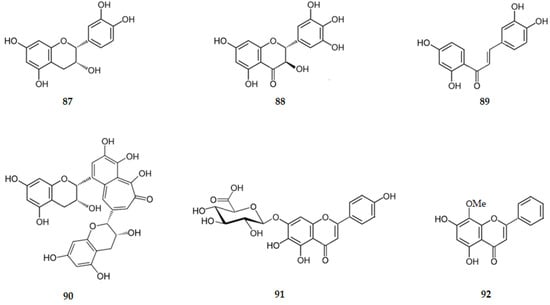
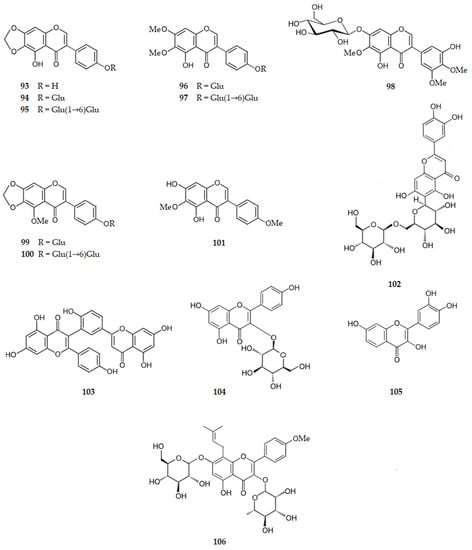
Figure 10.
Structure of some flavonoids that inhibit the pro-inflammatory factors. Legend: Glu–glucopyranosyl.
2.4. Inhibition of Acetylcholinesterase (AChE)
2.4.1. Catechins, Flavanones and 3-Hydroxyflavanones
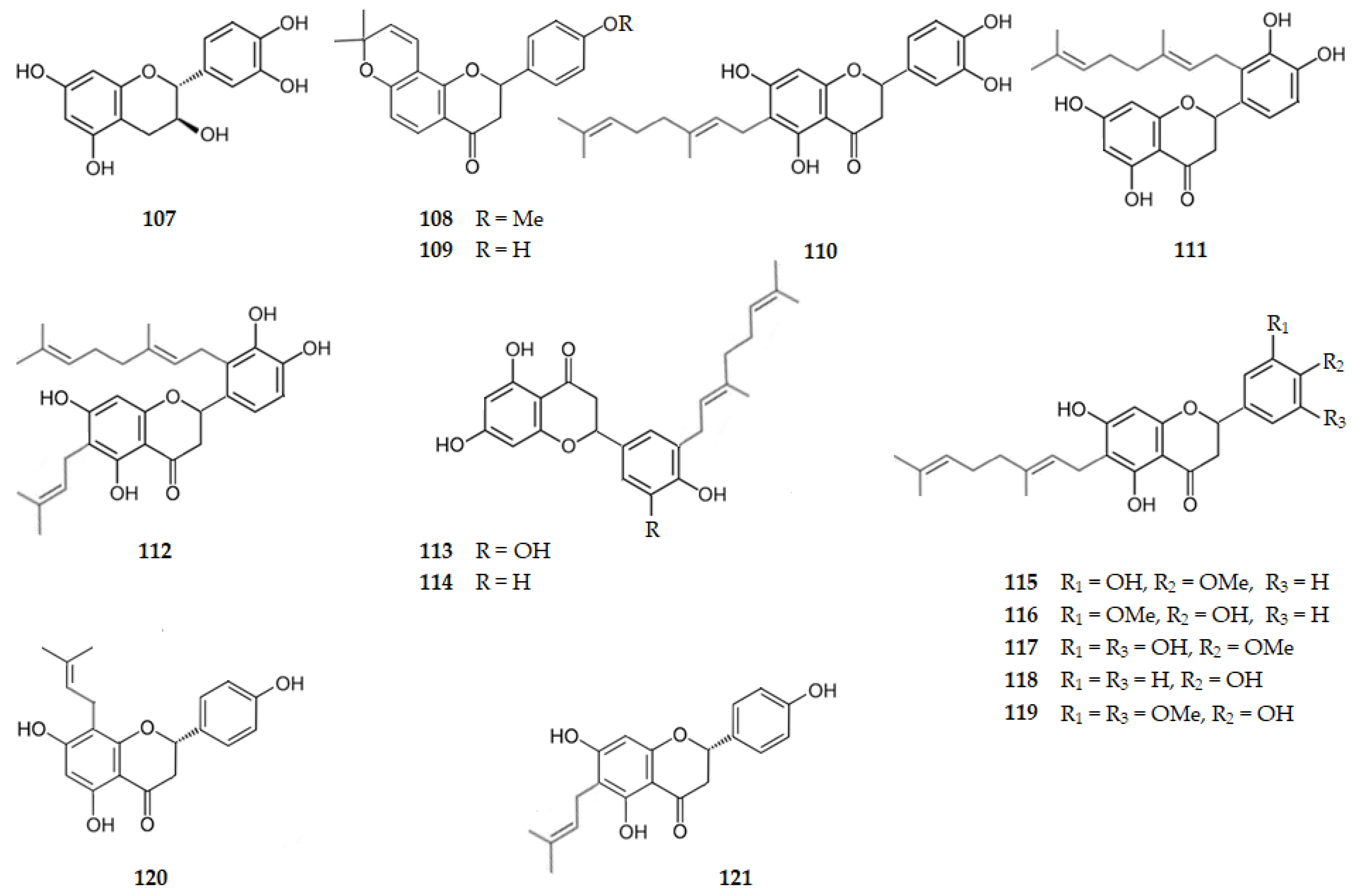
EGCG (4), extracted from the leaves of green tea (Camellia sinensis L.), is a very potent inhibitor of AChE (IC50 = 9.6 nM) [58] whereas catechin (107) is inactive [97], suggesting that the hydroxy group at C5′ increases the hydrogen bonding to the enzyme.
Naringenin (6) presents AChE inhibition, with an IC50 of 3.81 µM [60], pinostrobin (8) is inactive, having an IC50 > 236.79 µM [62], and naringin (85) has an IC50 of 26.40 µM [60], indicating that activity is increased by the hydroxy group at C4′ and decreased by alkylation at C7.
From the seeds of Millettia pachycarpa Benth, two compounds were isolated, namely: dorspoinsettifolin, the 4′-methoxy-6″,6″-dimethyldihydropyrano [2″,3″:7,8]flavanone) (108), that presents an AChE IC50 of 91.07 µM and 4′-hydroxyisolonchocarpin (4′-hydroxy-6″,6″-dimethyldihydropyrano [2″,3″:7,8]flavanone) (109), which was inactive, suggesting how important is for flavanones the methoxy group at C4′ for the AChE inhibition [98].
The glycosylated flavanones didymin (17), prunin (19) and poncirin (18), also present AChE inhibition with IC50 values of 2.13, 6.31 and 12.96 µM, respectively [59], or hesperidin (20) with an IC50 of 22.80 µM [66].
Five geranylated flavanones, isolated from Okinawa propolis, exhibit AChE IC50 values ranging from 7.23 to 15.70 µM, as follows: 6-geranyl-3′,4′,5,7-tetrahydroxyflavan-4-one (nymphaeol A, 110, 7.77 µM); 2′-geranyl-3′,4′,5,7-tetrahydroxyflavan-4-one (nymphaeol B, 111, 15.09 µM); 2′-geranyl-3′,4′,5,7-tetrahydroxy-6-(3-methylbut-2-enyl)flavan-4-one (nymphaeol C, 112, 15.70 µM); 5’-geranyl-3’,4’,5,7-tetrahydroxyflavan-4-one (isonymphaeol B, 113, 7.23 µM); and (2S)-3′-geranyl-4′,5,7-trihydroxyflavan-4-one (3′-geranylnaringenin, 114, 12.34 µM) [99]. These results might suggest a steric hindrance by the geranyl group (3,7-dimethylocta-2,6-dienyl) when it is linked to C2′. It should also be highlighted that pharmacokinetics and toxicological properties determination suggest that these propolis components might be beneficial in inflammation and AD treatment [99]. From Paulownia tomentosa Steud. another series of five geranylated flavanones were extracted, exhibiting AChE inhibition with IC50 range of 22.90 to 316.30 µM as follows: (2R,3R)-6-geranyl-3′,5,7-trihydroxy-4′-methoxyflavan-4-one (4′-O-methyldiplacone, 115, 92.40 µM); (2R,3R)-6-geranyl-4′,5,7-trihydroxy-3′-methoxyflavan-4-one (3′-O-methyldiplacone, 116, 109.20 µM); 6-geranyl-3′,5,5′,7-tetrahydroxy-4′-methoxyflavan-4-one (117, 22.90 µM); (2R,3R)-6-geranyl-4′,5,7-trihydroxyflavan-4-one (mimulone, 118, 91.50 µM); and 6-geranyl-4′,5,7-trihydroxy-3′,5′-dimethoxyflavan-4-one (119, 316.30 µM) [100]. These results suggest how important is the presence of two hydroxy groups on ring B for the hydrogen bonding of the flavanone to the enzyme. From Humulus lupulus L. (hops), three prenylated flavanones were isolated, namely isoxanthohumol (16), 8-prenilnaringenin or (2S)-4′,5,7-trihydroxy-8-(3-methylbut-2-en-1-yl)flavan-4-one (120), and 6-prenylnaringenin or 5,7,4′-trihydroxy-6-(3-methylbut-2-en-1-yl)flavan-4-one (121) that are inactive as inhibitors of AChE [64], suggesting the steric hinderance of the prenyl group.
From the fruits of Paulownia tomentosa Steud three geranylated 3-hydroxyflavanones were isolated, namely 4′-O-methyldiplacol or (2R,3R)-6-geraniol-3,3′,5,7-tetrahydroxy-4′-methoxyflavan-4-one (122), 3′-O-methyldiplacol or (2R,3R)-6-geraniol-3,4′,5,7-tetrahydroxy-3′-methoxyflavan-4-one (123), and 6-geranyl-3,3′,5,5′,7-pentahydroxy-4′-methoxyflavan-4-one (124) that inhibit AChE with IC50 values of 31.90, 48.50, and 15.60 µM, respectively [100]. Ampelopsin, the (2R,3R)-3′,4′,5,5′,7-pentahydroxyflavanonol (88), was isolated from Piper bavinum, and presented an IC50 of 59.47 µM [93]. These results show, once again, how important is the presence of two hydroxy groups on the ring B for the hydrogen bonding of the 3-hydroxyflavanone to the enzyme, as well as the presence of the geranyl group at C6 [100].
Figure 11 and Figure 12 show the chemical structure of flavonoids 107–124 that inhibit AChE with IC50 values ranging from 7.23 to 316.30 µM, except for compounds 107, 109, 120, and 121 that were inactive.
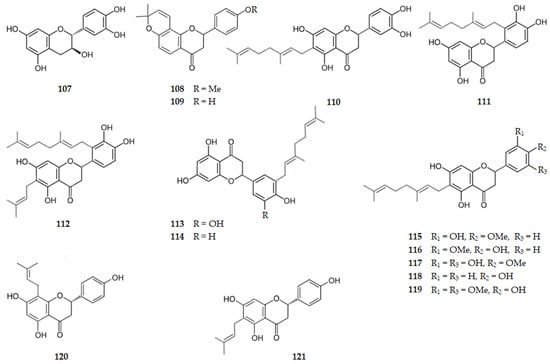
Figure 11.
Structure of some of the catechins and flavanones that inhibit AChE and of the inactive flavanones 109, 120 and 121.

Figure 12.
Structure of the AChE inhibitors 3-hydroxyflavanones 122–124.
2.4.2. Chalcones
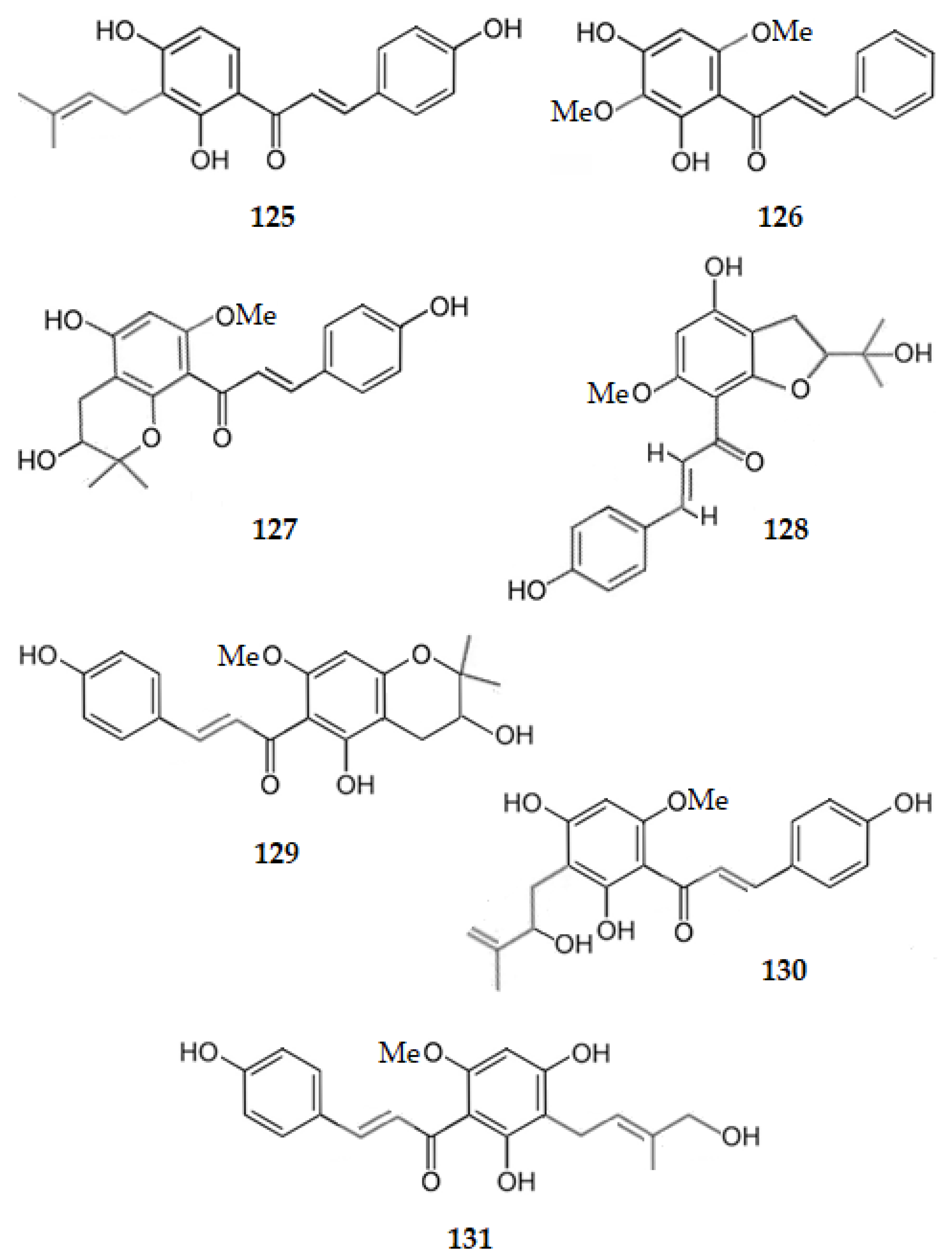
From the twigs of Dorstenia barteri, isobavachalcone (2′,4,4′-trihydroxy-3′-prenylchalcone) (125) and 2′,4′-dihydroxy-3′,6′-dimethoxychalcone (126) were isolated. Both the chalcones exhibited AChE inhibition with IC50 values of 18.34 and 20.15 µM, respectively [62]. From Humulus lupulus L six chalcones were isolated, namely: xanthohumol L, the 4′,4,5″-trihydroxy-2′-methoxy-6″,6″-dimethyldihydropyrano [2″,3″:5′,6′]chalcone (127); xanthohumol I, the (E)-1-[4-hydroxy-2-(2-hydroxypropan-2-yl)-6-methoxy-2,3-dihydro-1-benzofuran-7-yl]-3-(4-hydroxyphenyl)prop-2-en-1-one) (128); xanthohumol B, the 2′,4,5″-trihydroxy-6′-methoxy-6″,6″-dimethyldihydropyrano [2″,3″:3′,4′]chalcone) (129); xanthohumol D, the 2′,4,4′-trihydroxy-3-(2-hydroxybut-3-en-1-yl)-6′-methoxy-chalcone (130); 3-hydroxyxanthohumol or 2′,3,4′-trihydroxy-3′-(4-hidroxy-3-methylbut-2-en-1-yl)-6′-methoxychalcone (131), and xanthohumol (25). Only 3-hydroxyxanthohumol (131) and xanthohumol (25) weakly inhibit AChE with IC50 values of 51.25 and 71.34 µM, respectively. All the other compounds are inactive [64]. These results point out the importance of the two hydroxy groups at C2′ and C4′, as well as the prenyl group at C3′ and the methoxy group at C6′. Figure 13 shows the chemical structure of chalcones 125–131.
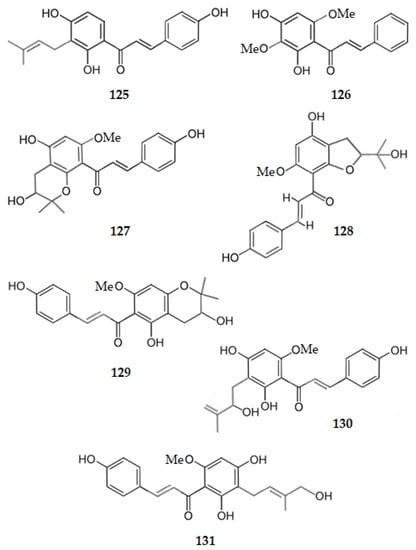
Figure 13.
Structure of chalcones 125–131, some of which inhibit AChE (125, 126 and 131).
2.4.3. Flavones
Apigenin (29) and acacetin (31) exhibit AChE inhibition with IC50 values of 34.43 µM [49] and 50.33 µM [73], respectively, suggesting that for flavones the presence of a methoxy group at C4′ decreases the AChE inhibition. From the roots of Scutellaria baicalensis (Chinese Skullcap) was extracted baicalein (30) with an IC50 of 0.61 µM [55]. Wogonin (5,7-dihydroxy-8-methoxyflavone) (92), also isolated from S. baicalensis, exhibits IC50 value < 10 µM [95], and luteolin (34) exhibits an IC50 of 9.27 µM [76].
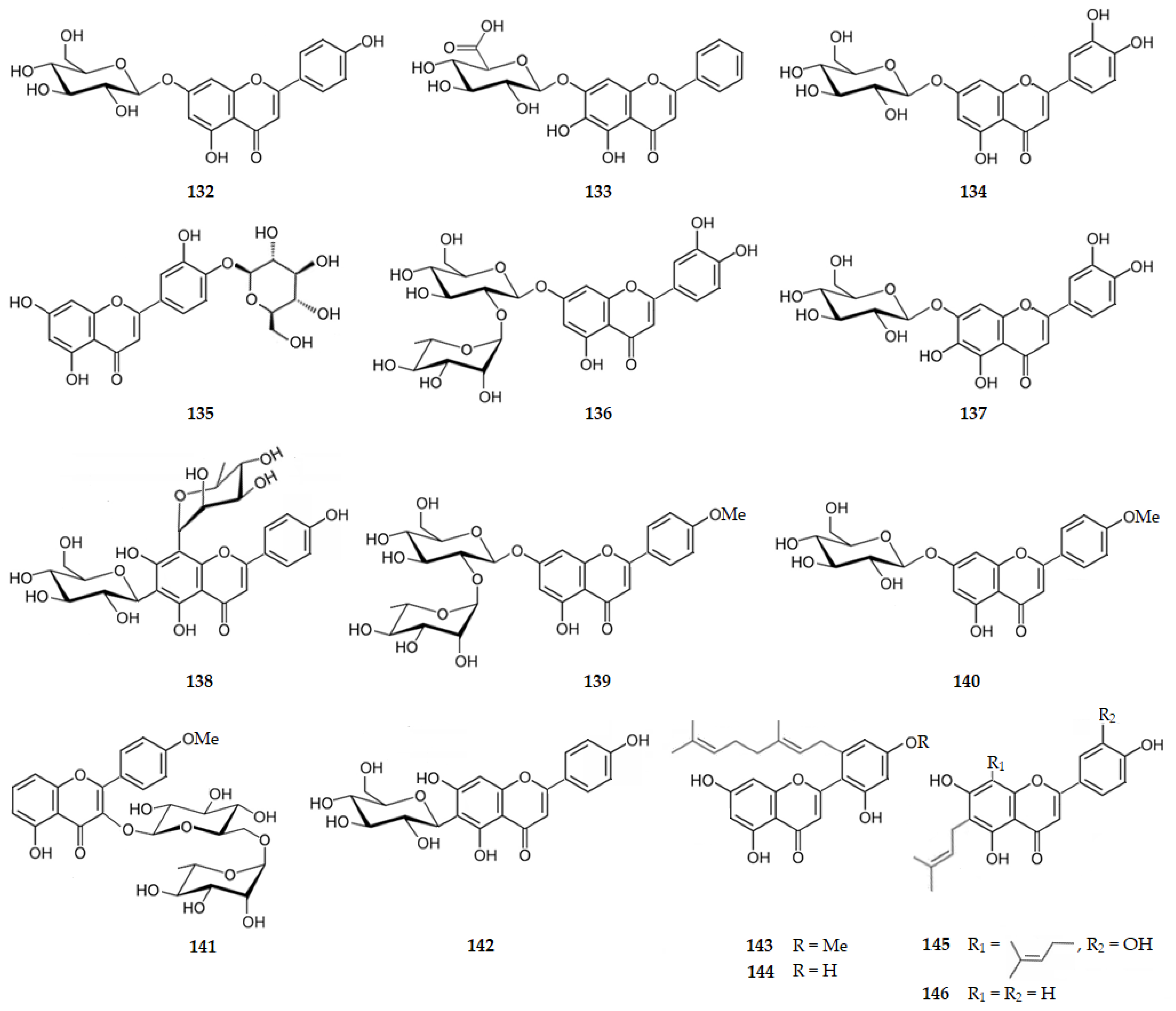
Regarding glycosylated flavones, 7-O-(β-d-glucopyranosyl)apigenin (132), isolated from Phagnalon saxatile (L.) Cass., is a much weaker inhibitor of AChE (IC50 = 237.74 µM) [101] when comparing with apigenin (29). Baicalin (133), the glycosylated form of baicalein at C7, exhibits an IC50 value much higher (204.10 µM) [102] than the aglycone (30). Cynaroside, the 7-O-(β-d-glucopyranosyl)luteolin (134), isolated from Drynariae rhizome [60], 4′-O-(β-d-glucopyranosyl)luteolin (135), isolated from Phagnalon saxatile (L.) Cass. [101], or veronicastroside (7-O-rutinosyl luteolin) (136) from Veronicastrum sibiricum var. japonicum [103], present IC50 of 17.13, 147.41 and 6.783.80 µM, respectively. Thus, these glycosylated luteolins are also weaker inhibitors of AChE than luteolin (34). Even C-glycosylation of luteolin at C6 and C8 decreases the AChE inhibition, as shown by orientin (46) and isoorientin (47), with IC50 values of 20.06 and 29.48 µM, respectively [76]. These results suggest that flavone glycosylation decreases the AChE inhibition. However, 7-O-glucosyl-6-hydroxyluteolin (137), isolated from Achillea millefolium, is a remarkable AChE inhibitor exhibiting an IC50 of 1.65 µM [55], being a more potent inhibitor than luteolin (34). The substitution of the proton by a hydroxy group at C6 makes the difference. Thus, 7-O-glucosyl-6-hydroxyluteolin (137) should be compared with baicalein (30) (IC50 = 0.61 µM) [55], showing the usual decrease in AChE inhibition due to the glycosylation. Violanthin, the 4′,5,7-trihydroxy-6-d-glucosyl-8-l-rhamnosylflavone (138), isolated from Piper bavinum, exhibiting an IC50 value of 79.80 µM [93], is another example. On the other hand, baicalin (133), the glycosylated form of baicalein at C7, exhibits an IC50 value much higher (204.10 µM) [102], suggesting that the AChE inhibition of 7-O-glucosyl-6-hydroxyluteolin (137) is also due to the hydrogen bonding with the hydroxy groups of C3′ and C4′, as well as of C6 or, in the case of violanthin, with the hydroxy groups of C4′. The decrease in activity when acacetin is glycosylated as in 7-O-(β-rhamnosyl)acacetin (139) and 7-O-(β-glucosyl)acacetin (140), which are inactive [104], and 3-O-[α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyl]acacetin (141), which exhibits an IC50 value of 165.04 µM [105], reinforces that glycosylation of flavones decreases AChE inhibition. Now, comparing vitexin (43), which is C-glycosylated apigenin at C8, with isovitexin (44) which is C-glycosylated apigenin at C6, and swertisin (7-O-methylisovitexin) (142), inhibiting AChE with IC50 values of 12.16, 6.24 [79] and 71.89 µM [106], respectively, it can be assumed that the steric hinderance of the glycoside is higher when it is linked to C8 of the flavone. Even when a methoxy group is linked to C8 the hydrogen bonding is weaker. The alkyl-substituted flavones 5′-geranyl-4′-methoxy-2′,5,7-trihydroxyflavone (143), 5′-geranyl-2′,4′,5,7-tetrahydroxyflavone (144), morusin (49), cyclomorusin (51), morusinol (52), neocyclomorusin (53) and kuwanon C, the 2′,4′5,7-tetrahydroxy-3,8-bis(3-methyl-2-buten-1-yl)flavone (48), isolated from the roots of Morus lhou, present AChE inhibition characterized by IC50 values of 10.95, 16.21, 36.40, 31.69, 173.49, 26.69 and 25.06 μM, respectively [83]. 6,8-Diprenyleriodictyol or 3′,4′,5,7-tetrahydroxy-6,8-bis(3-methylbut-2-en-1-yl)flavone (145) and 6-prenylapigenin or 4′,5,7-trihydroxy-6-(3-methyl-2-buten-1-yl)flavone (146), isolated from the leaves of Polygonum limbatum and the twigs of Dorstenia barteri Bureau var. multiradiata, exhibit IC50 values of 15.03 and 25.73 μM [62], respectively. Thus, the presence of a hydroxy group at C7 is important for the AChE inhibition. Comparing morusin (49) with morusinol (52), the structural difference consists in the hydration of the prenyl group linked to C3, producing a significant decrease in the activity to inhibit AChE. This result implies that hydrophobicity is important for the inhibition [83].
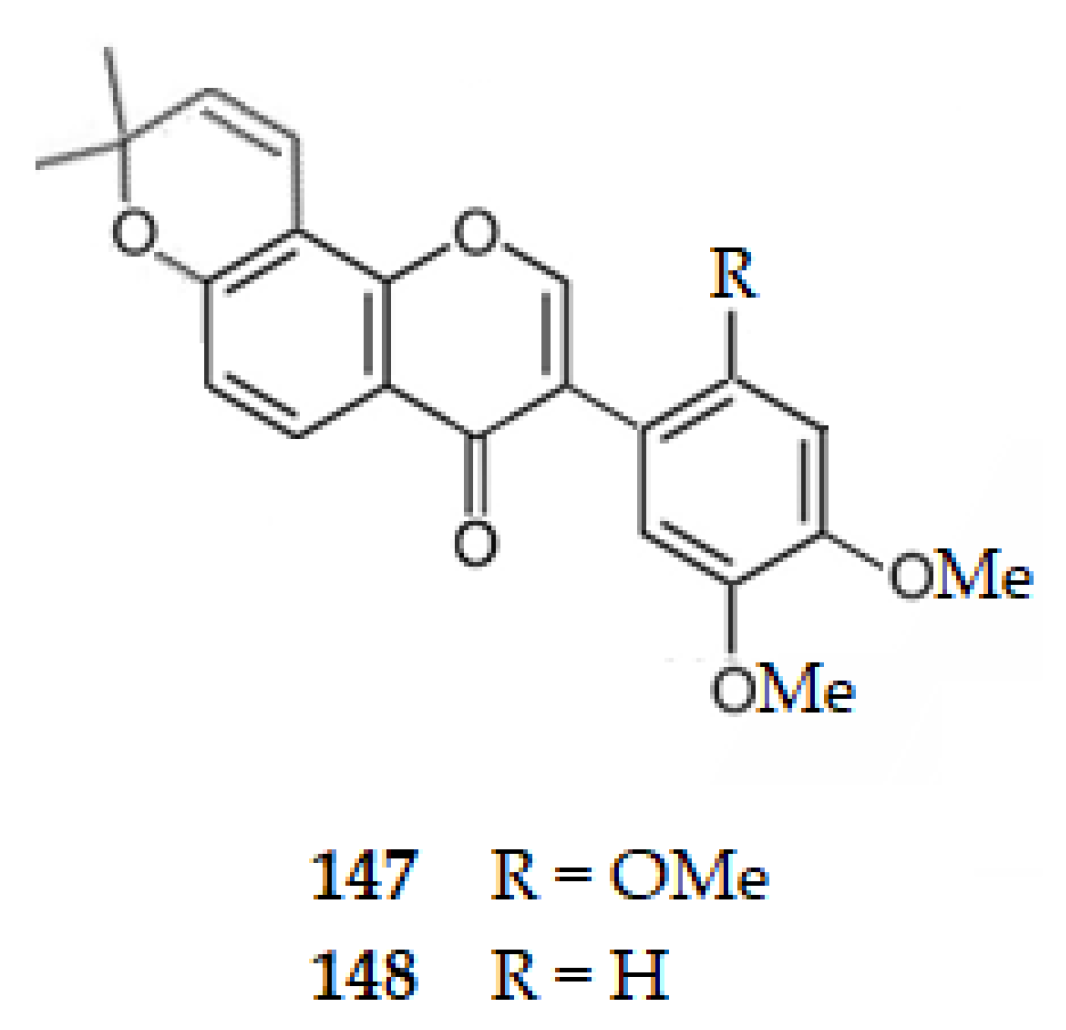
Considering isoflavones, genistein (2), extracted from the roots of Astragalus membranaceus (Fish.) Bge., exhibits an IC50 value of 167.00 μM, [57], a weaker AChE inhibition than the corresponding flavone, apigenin (29) (IC50 = 34.43 μM) [49]. Barbigerone (2′,4′,5′-trimethoxy-6,6-dimethylpyranoisoflavone) (147) and 4′,5′-dimethoxy-6,6-dimethylpyranoisoflavone (148), isolated from Millettia pachycarpa Benth, exhibit IC50 values of 121.60 and 131.17 μM, respectively, indicating that the loss of one methoxy group at ring B reduces the AChE inhibition [98].
Figure 14 and Figure 15 show the chemical structure of flavones 132–148 that inhibit AChE with IC50 values ranging from of 1.65 to 237.74 µM, except for compounds 136, 139 and 140 that were inactive.
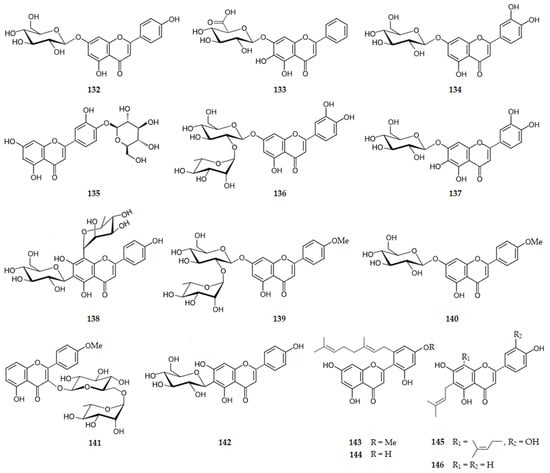
Figure 14.
Structure of flavones that inhibit AChE and of the inactive flavones 136, 139 and 140.
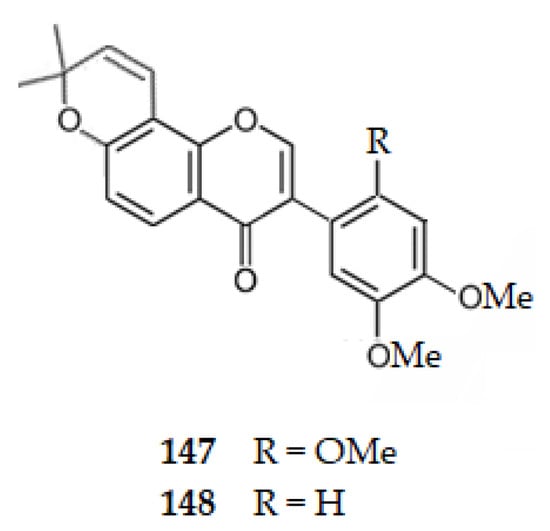
Figure 15.
Structure of isoflavones 147 and 148 that inhibit AChE.
2.4.4. Flavonols
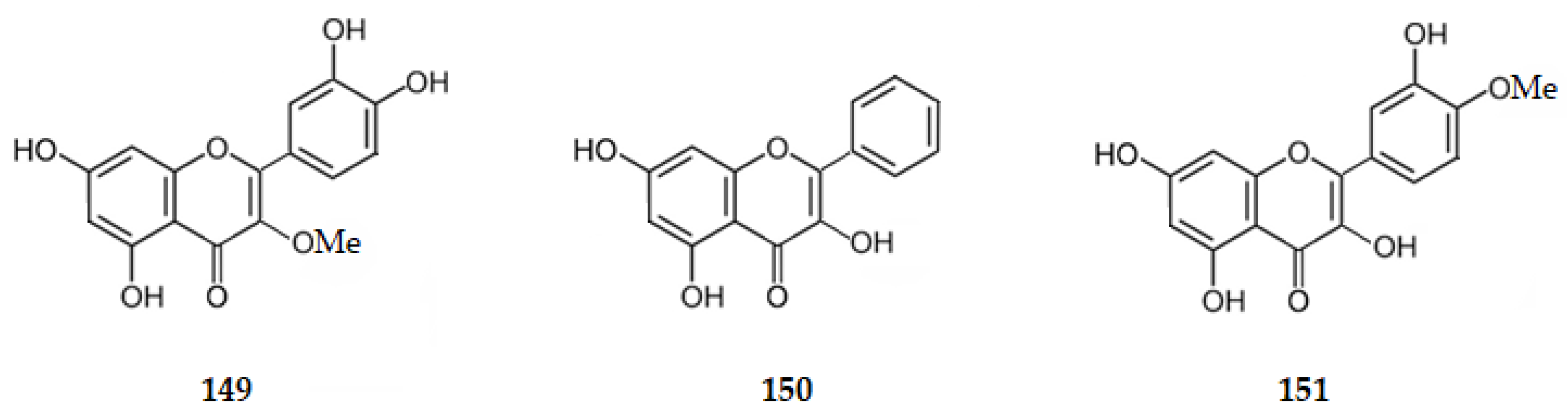
Quercetin (1) exhibits an IC50 value of 19.80 μM [55,56] and 3-methoxyquercetin (149), isolated from Agrimonia pilosa Ledeb., an IC50 value of 37.90 μM [55,56], being weaker inhibitors of AChE than the flavone luteolin (IC50 = 9.27 μM) [76]. Galangin (3,5,7-trihydroxyflavone) (150) [107], tamarixetin (3,3′,5,7-tetrahydroxy-4-methoxyflavone) (151), isolated from the buds of Cleistocalyx operculatus [86], kaempferol (76) [86], morin (77) [87] and myricetin (78) [88], exhibit IC50 values of 19.10, 22.30, 30.40, 210.00, and 157.11 μM, respectively, suggesting that there is a decrease in the AChE inhibition when the number of hydroxy groups on ring B increases.
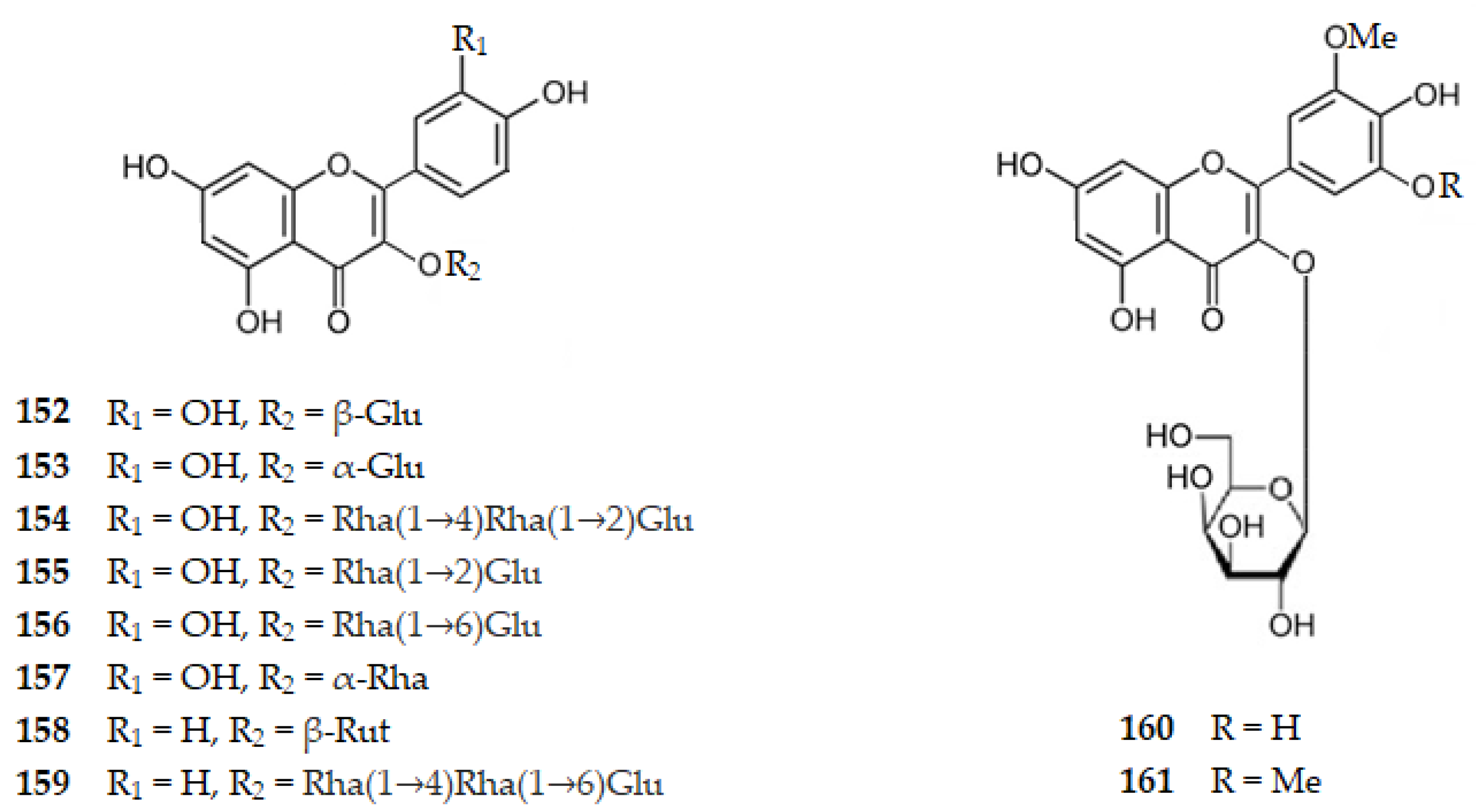
Considering glycosylated flavonols, several glycosylated quercetin (1) molecules were isolated from Gingko biloba namely 3-O-(β-d-glucopyranosyl)quercetin (152), 3-O-(α-d-glucopyranosyl)quercetin (153), 3-O-[α-l-rhamnopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranosyl]quercetin (154), 3-O-[α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranosyl]quercetin (155), and 3-O-[α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyl]quercetin (156) which present IC50 values of 124.73, 171.34, 148.81, 126.95, and 119.74 μM, respectively [105]. Quercitrin, the 3′,4′,5,7-tetrahydroxy-3-(α-l-rhamnopyranosyloxy)flavone (157), extracted from Agrimonia Pilosa, presents an IC50 value of 66.90 μM [55,56]. Rutin (83), another glycosylated quercetin, isolated from the leaves of Ouratea fieldingiana, also exhibits AChE inhibition with an IC50 value of 19.65 μM [91]. Thus, there is a decrease in the AChE inhibition for glycosylated quercetin molecules comparing with the aglycone. Glycosylated kaempferol (76) molecules as astragalin, the 3-O-(β-d-glucopyranosyl)kaempferol (104), isolated from Drinariae Rhizome [60], nicotiflorin, the 3-O-(β-d-rutinosyl)kaempferol (158), isolated from Ouratea fieldingiana leaves [91], or 3-O-[α-l-rhamnopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyl]kaempferol (159), isolated from Gingko biloba [105], present IC50 values of 18.24, 15.03, and 137.30 μM, respectively. Thus, glycosylated kaempferol molecules show an increase in AChE inhibitory activity when we compare them with the aglycone. Glycosylated myricetin (78) molecules were isolated from Cleistocalix operculatus (Roxb.) Merr and Perry plant, namely 3-O-(β-d-galactopyranosyl)-3′-O-methylmyricetin (160) or 3-O-(β-d-galactopyranosyl)-3′,5′-di-O-methylmyricetin (161) with IC50 values of 19.90 and 37.80 μM, respectively [86]. Thus, there is an increase in AChE inhibition when we compare the aglycone with glycosylated myricetin. These results suggest that, as the number of hydroxy groups on ring B increases, there is an interaction between them, not allowing hydrogen bonds with the enzyme. The glycosylated molecule allows the linkage with the enzyme by hydrogen bonding increasing the AChE inhibition. However, by steric hinderance multiglycosylated molecules decrease that activity.
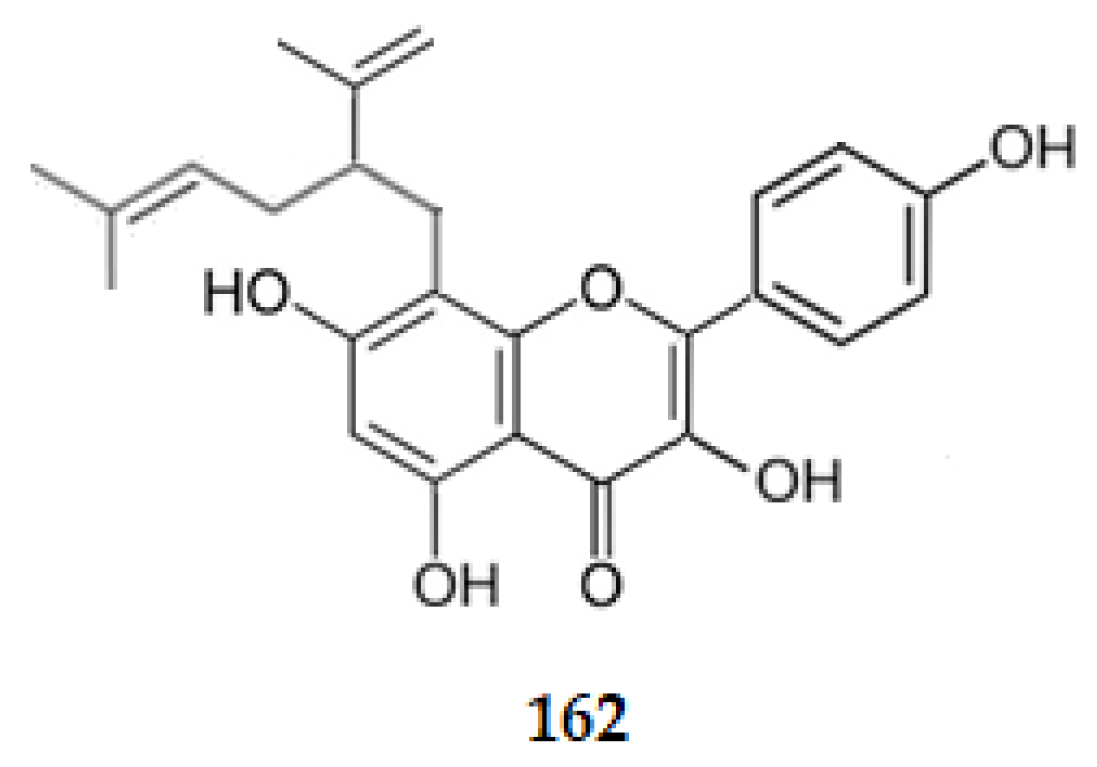
Considering prenyl or lavandulyl flavonoids, desmethylanhydroicaritin (81), icaritin (80), sophoflavescenol (79), and 8-lavandulylkaempferol, the 3,4′,5,7-tetrahydroxy-8-[5-methyl-2-(prop-1-en-2-yl)hex-4-enyl]flavone (162), isolated from Sophora flavescens, exhibit AChE inhibition with IC50 values of 6.67, 6.47, 8.37 and 5.16 μM, respectively [89]. Comparing these IC50 values with the one of kaempferol (30.40 μM) [86], it can be concluded that the prenyl or lavandulyl groups at C8 increase the AChE inhibition.
Figure 16, Figure 17 and Figure 18 show the chemical structure of flavonols 149–162 that inhibit AChE with IC50 ranging from 5.16 to 171.34 µM.
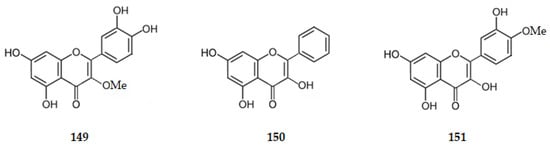
Figure 16.
Structure of flavonols 149–151 that inhibit AChE.
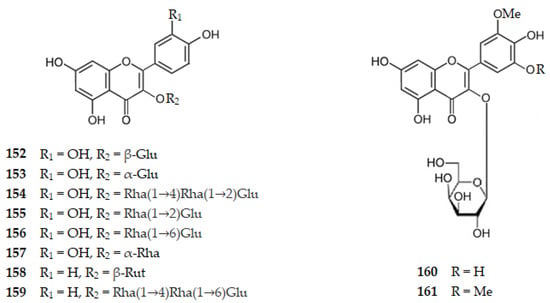
Figure 17.
Structure of glycosylated flavonols 152–161 that inhibit AChE. Legend: Glu–glucopyranosyl, Rha–Rhamnopyranosyl and Rut–rutinosyl.
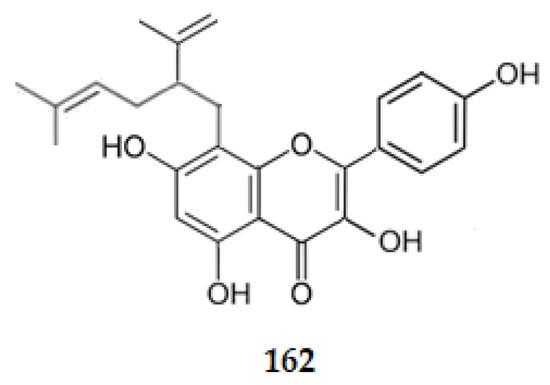
Figure 18.
Structure of lavandulylflavonol 162 that inhibits AChE.
3. Conclusions
Nowadays, 139 flavonoids, from natural sources, demonstrated as potential anti-AD agents: 4 (2.8%) flavanols, 30 (21.6%) flavanones, 4 (2.8%) 3-hydroxyflavanones, 10 (7.2%) chalcones and dihydrochalcones, 52 (37.4%) flavones, 13 (9.5%) isoflavones, and 26 (18.7%) flavonols. Considering flavones and flavonols, a total of 78 (56.1%) flavonoids active against AD was achieved. They are characterized by a 𝜋 system with various conjugated double bonds, and the molecule is stabilized in a planar configuration. The flavonoids discussed in this review exhibit LogP values ranging from −2.16 to 7.26, suggesting their potential to cross the BBB [112].
The most active flavonoid extracted from natural sources against BACE1 is rutin, the 3′,4′,5,7-tetrahydroxy-3-[α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyloxy]flavone (83), a glycosylated flavonol. On its scaffold it bears hydroxy groups on C5, C7 (on ring A) and on C3′ and C4′ (on ring B), as well as, linked to the oxygen of C3 α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside group, which allows formation of several hydrogen bonding. The difference of activity against BACE1 between myricetin (78) (IC50 = 2.40 µM) and rutin (83) (IC50 = 0.004 µM) shows, for the increase in BACE1 inhibition, how significant is the bond between the oxygen at C3 with a group that allows several hydrogen bonds as α-rhamnopyranosyl-(1→6)-β-d-glucopyranoside. It should also be highlighted that the two hydroxy groups on ring B are in the ortho position, showing the importance of these two hydroxy groups for hydrophilic interactions. Interestingly, the most active compounds against BACE1 of each group of flavonoids contain always on their scaffold three hydroxy substituents, one at C5 (or C2′ for chalcones), another at C7 (or C4′ for chalcones) and the last one at C4′ (or C4 for chalcones) as: (−)-epigallocatechin (5) belonging to the catechins’ group; sophoroflavanone G (9), kurarinone (10), and selagintriflavonoid A (22) in the flavanones’ group; phlorizin (28), representative of the dihydrochalcones’ group; and kuwanon C (48), amentoflavone (58), sequoiaflavone (59), podocarpusflavone A (62), 2,3-dihydroamentoflavone (74), myricetin (78), or desmethylanhydroicaritin (81), which are members of the flavone and flavonol groups.
Luteolin (34), a flavone, presents the highest activity against the enzyme GSK-3β (IC50 = 1.51 µM). This flavonoid also bears on its scaffold hydroxy groups on C5 and C7 (on ring A) and on C3′ and C4′ (on ring B). Glycosylation on ring A, as on isovitexin (44), orientin (46), or isoorientin (47) decreases the inhibition of GSK-3β, suggesting that side bulky group substitutions on the ring A of the flavonoid scaffold affect the GSK-3β activity in a significant manner, decreasing the interactions between the enzyme and inhibitors. It should also be noticed that flavonols bearing on its scaffold hydroxy groups on C5 and C7 (on ring A) and on C4′ (on ring B), such as kaempferol (76) (IC50 = 3.47 µM), present a decrease in the inhibition of GSK-3β. However, quercetin (1) (IC50 = 2.00 µM), with hydroxy groups at C3 and at C3′, C4′ C5 and C7 presents an inhibition of the same magnitude of luteolin (34).
The chalcone butein (89) exhibits one of the greatest inhibitory effects against NO production, one of the pro-inflammatory factors, with an IC50 value of 10.90 μM. It is interesting to note that this molecule, although not having the scaffold of flavones, allows also that the 𝜋 system is extended as there is an increase in two more conjugated double bonds, and the molecule will be stabilized in a planar configuration. On the other hand, it bears hydroxy groups on C3 (the corresponding C3′ of flavones), C4 (the corresponding C4′ of flavones), C2′ (the corresponding C5 of flavones) and C4′ (the corresponding C7 of flavones). The inhibition of production of NO increases from isovitexin (44) to isoorientin (47), suggesting the importance of having on the ring B hydroxy groups at C4′, and another one in the position ortho to it. Finally, the flavonol fisetin (105) with hydroxy groups on C3, C3′, C4′ and C7 presents an IC50 against NO production of 13.5 μM, suggesting that the closure of ring C with an oxygen on it is important for the inhibition of NO production. The flavonol quercetin (1) with hydroxy groups on C3, C3′, C4′, C5, and C7 has the ring C closed and has one more hydroxy group at C5 when compared with fisetin (105). In conclusion, quercetin (1) should be the flavonoid with the highest activity against NO production when compared to fisetin (105) by presenting a hydroxy group at C5. When compared with butein (89), quercetin presents the ring C closed by an atom of oxygen, thus favoring an increase in the hydrogen bonding.
When considering the natural flavonoids which inhibit AChE, EGCG, the (2R,3R)-3′,4′,5,5′,7-pentahydroxyflavan-3-yl 3,4,5-trihydroxybenzoate (4), extracted from the leaves of green tea (Camellia sinensis L.), is the most active flavonoid against AChE (IC50 = 9.6 nM).
Although for the other enzymes the scaffold of a flavonol is the most active one, for inhibiting AChE the more active scaffold is the one presented by catechins. It is interesting that, even for this different scaffold, the presence of hydroxy groups at C5 and C7 and at C3′, C4′ and C5′ increases the inhibition of AChE.
The results herein reported only concern the interaction of one flavonoid with the active site of one enzyme. However, as described above, AD has multiple pathogenic factors which may work together through interaction between genetic, molecular and cellular events. Thus, one possible successful strategy might be the use of MTDLs, that is to say, using a multitarget therapy. MTDLs is a therapy where only one active ingredient is administered [113], and so there is no risk of interaction between drugs, as in the case of mixing several compounds. Additionally, the prevision of pharmacokinetic and pharmacodynamics properties is simplified as there is only one single compound. Analyzing the scaffold of the several isolated flavonoids, which inhibit one of the mechanisms of AD, it is concluded that all the described mechanisms—Aβ plaques, NFTs, pro-inflammatory factors and AChE—are inhibited mostly by flavonoids with a planar core.
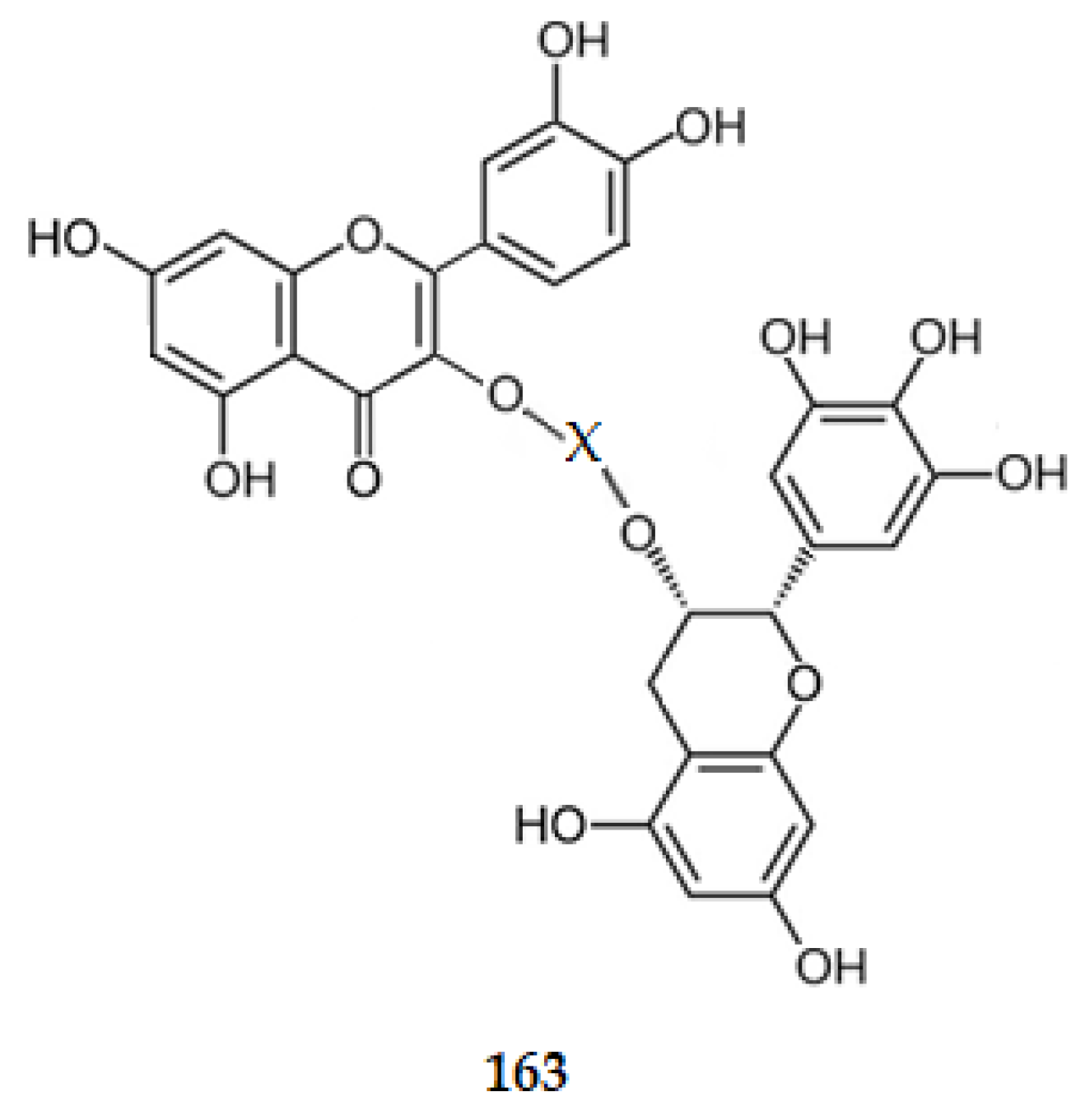
Even, for the AChE inhibition, nevertheless not being as planar as the scaffolds for flavone or flavonols, the catechin one presents two rings (A and B) which are completely planar. Ring C is a ring with three carbon atoms in a sp3 hybridization, and an oxygen atom. So, it is not as planar as rings A and B. The data obtained suggest that the AChE site adjusts better to a molecule with this scaffold. All these data suggest that a biflavonoid where two moieties, one from 3,3′,4′,5,7-pentadroxyflavone linked by C3 to the C3 of the other moiety, (2R,3R)-3′,4′,5,5′,7-pentahydroxyflavan-3-yl, i.e., linking quercetin (1) to EGCG (4). The linkage between the two C3 should be made directly or through a hydrophilic group (X), such as α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside (163) (Figure 19). However, as quercetin (1) presents a value of logP of 1.48 and EGCG (4) of 0.64, and have all the characteristics to be considered orally active compounds [114,115], a combination therapy, using a drug cocktail of the two compounds might be more advantageous [116].
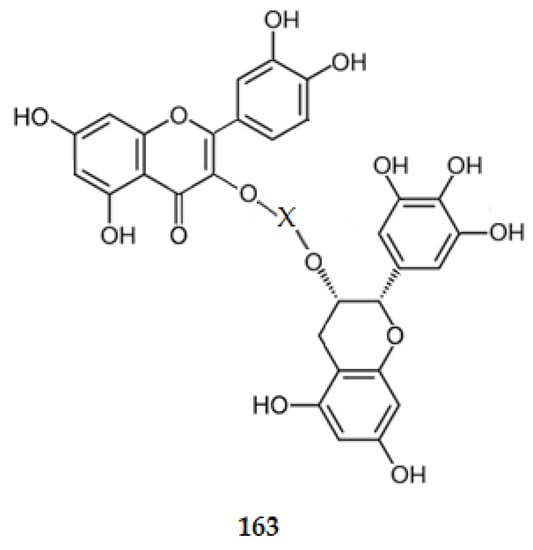
Figure 19.
Structure of a model of MTDL.
Considering the other flavonoids already isolated from natural sources, MTDL flavonoids inhibiting several pathways causing AD should be identified. As the pathways interact among them, future research should focus not only on identifying flavonoids that are active against only one, but on several pathways, encouraging researchers to follow this trend in their investigations, as well as to incorporate the bioavailability and metabolism of MTDL flavonoids into experimental design throughout all stages of pre-clinical research [117].
Author Contributions
Conceptualization, J.M. and E.L.; writing, J.M., E.L. and A.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Aβ: amyloid-beta; ACh, acetylcholine; AChE, acetylcholinesterase; AD, Alzheimer’s disease; APOE, apolipoprotein E; APP, amyloid precursor protein; BACE1, β-secretase (beta-site APP cleaving enzyme 1); BBB, blood-brain barrier; CIs, clinical indications; CNS, central nervous system; COX-2, cyclooxygenase-2; EOAD, early onset of AD; GSK-3β, glycogen synthase kinase-3 beta; IL, interleukin; LOAD, late onset of AD; LPS, lipopolysaccharide; logP, logarithm of the partition coefficient; MAPs, microtubule-associated proteins; MAPK, mitogen-activated protein kinase; MTs, microtubules; MTDLs, multitarget-directed ligands; NF-κB, nuclear factor-κB; NFTs, intracellular neurofibrillary tangles; NO, nitric oxide; iNOS, inducible NO synthase; PGE2, prostaglandin E2; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α.
References
- Russo, P.; Kisialiou, A.; Lamonaca, P.; Moroni, R.; Prinzi, G.; Fini, M. New drugs from marine organisms in Alzheimer’s disease. Mar. Drugs 2016, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer Dement. 2019, 15, 321–387. [Google Scholar]
- Van Cauwenberghe, C.; Van Broeckhoven, C.; Sleegers, K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. 2016, 18, 421–430. [Google Scholar] [CrossRef]
- Hauser, P.S.; Narayanaswami, V.; Ryan, R.O. Apolipoprotein E: From lipid transport to neurobiology. Prog. Lipid Res. 2011, 50, 62–74. [Google Scholar] [CrossRef]
- Liu, C.; Kanekiyo, T.; Xu, H.; Bu, G. Apoliprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Ver. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, j.; Basak, J.M.; Holtzman, D.M. The role of apoliprotein E in Alzheimer’s disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yamada, K.; Liddelow, S.A.; Smith, S.T.; Zhan, L.; Lun, W.; Tsai, R.M.; Spina, S.; Grinberg, L.T.; Rojas, J.C.; et al. ApoEε4 markedly exacerbates tau-mediated neurodegeneration in a mouse mode tauopathy. Nature 2017, 549, 523–527. [Google Scholar] [CrossRef]
- Isik, A.T. Late onset Alzheimer’s diseasae in older people. Clin. Interv. Aging 2010, 5, 307–311. [Google Scholar] [CrossRef]
- Bekris, L.M.; Yu, C.; Bird, T.D.; Tsuang, T.W. Genetics of Alzheimer’s disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 213–227. [Google Scholar] [CrossRef]
- Martins, M.; Silva, R.; Pinto, M.M.M.; Sousa, E. Marine natural products, multitarget therapy and repurposed agents in Alzheimer’s disease. Pharmaceuticals 2020, 13, 242. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B.; Singh, N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Macauley, S.L.; Holtzman, D.M. Recent advances from the bench toward the bedside in Alzheimer’s disease. EBioMedicine 2015, 2, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Takashima, A. Tau aggregation is a therapeutic target for Alzheimer’s disease. Curr. Alzheimer Res. 2010, 7, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Coman, H.; Nemes, B. New therapeutic targets in Alzheimer’s disease. Int. J. Gerontol. 2017, 11, 2–6. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment combinatoirs for Alzheimer’s disease: Current and future pharmacotherapy options. J. Alzheimer Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef]
- Fish, P.V.; Steadman, D.; Bayle, E.D.; Whiting, P. New approaches for the treatment of Alzheimer’s disease. Bioorgan. Med. Chem. Lett. 2019, 29, 125–133. [Google Scholar] [CrossRef]
- Kosik, K.S.; Joachim, C.L.; Selkoe, D.J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1986, 83, 4044–4048. [Google Scholar] [CrossRef]
- Naini, S.; Soussi-Yanicostas, N. Tau Hyperphosphorylation and oxidative stress, a critical vicious circle in neurodegenerative tauopathies? Oxidative Med. Cell. Longev. 2015, 2015, 151979. [Google Scholar]
- Desai, A.; Mitchison, T.J. Microtubule polymerization dynamics. Annual Rev. Cell Dev. Biol. 1997, 13, 83–117. [Google Scholar] [CrossRef]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef]
- Ballatore, C.; Brunden, K.R.; Huryn, D.M.; Trojanowski, J.Q.; Lee, V.M.-Y.; Smith, A.B., 3rd. Microtubule stabilizing agents as potential treatment for Alzheimer’s disease and related neurodegenerative tauopathies. J. Med. Chem. 2012, 55, 8979–8996. [Google Scholar] [CrossRef]
- White, J.A.; Banerjee, R.; Gunawardena, S. Axonal transport and neurodegeneration: How marine drugs can be used for the development of therapeutics. Mar. Drugs 2016, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Kolarova, M.; García-Sierra, F.; Bartos, A.; Ricny, J.; Ripava, D.; Ripava, D. Structure and pathology of tau protein in Alzheimer disease. Int. J. Alzheimers Dis. 2012, 2012, 731526. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.-L.; Yardin, C.; Terra, F. Tau protein kinases: Involvement in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Citron, M. Alzheimer’s disease: Strategies for disease modification. Nat. Rev. Drug Discov. 2010, 9, 387–398. [Google Scholar] [CrossRef]
- Li, G.; Yin, H.; Kuret, J. Casein kinase 1 delta phosphorylates tau and disrupts its binding to microtubules. J. Biol. Chem. 2004, 279, 15938–15945. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- Schain, M.; Kreisl, W.C. Neuroinflammation in neurodegenerative disorders–a review. Curr. Neuro/. Neurosci. Rep. 2017, 17, 25. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Cowan, M.; Petri, W.A. Microglia: Immune regulators of neurodevelopment. Front. Immunol. 2018, 9, 2576. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell. Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 44–468. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Cheng, J.; Hou, L. Drug development for Alzheimer’s disease: Microglia induced neuroinflammation as a target? Int. J. Mol. Sci. 2019, 20, E558. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Wang, X.; Liu, C.; Zhang, H.L. Pharmacological targeting of microglial activation: New therapeutic approach. Front. Cell. Neurosci. 2019, 13, 514. [Google Scholar] [CrossRef] [PubMed]
- Anglister, L.; Stiles, J.R.; Salpetert, M.M. Acetylcholinesterase density and turnover number at frog neuromuscular–junctions, with modeling of their role in synaptic function. Neuron 1994, 12, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s Disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Ren, Y.; Howes, M. Acetylcholinesterase inhibitors from plants and fungi. J. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Alvarez, A.; Perez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–889. [Google Scholar] [CrossRef]
- Alvarez, A.; Alarcón, R.; Opazo, C.; Campos, E.O.; Muñoz, F.J.; Calderón, F.H.; Dajas, F.; Gentry, M.K.; Doctor, B.P.; De Mello, F.G.; et al. Stable complexes involving acetylcholinesterase and amyloid-beta peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer’s fibrils. J. Neurosci. 1998, 18, 3213–3223. [Google Scholar] [CrossRef]
- Chen, J.J.; Genereux, J.C.; Wiseman, R.L. Endoplasmic reticulum quality control and systemic amyloid disease: Impacting protein stability from the inside out. IUBMB Life 2015, 67, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Freitas Silva, M.; Dias, K.S.T.; Gontijo, V.S.; Ortiz, C.J.C.; Viegas, C., Jr. Multi-target directed drugs as a modem approach for drug design towards Alzheimer’s disease: An update. Curr. Med. Chem. 2018, 25, 349–3525. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheímers Dement. 2019, 5, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, X.H.; Guan, J.; Ge, S.; Wu, M.B.; Lin, J.P. Advancement of multi-target drug discoveries and promising applications in the field of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 169, 200–223. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Gurib-FaKim, A.; Subratty, A.H. Antimicrobial activities and phytochemical profiles of endemic plants of Mauritius. Pharm. Biol. 2005, 43, 237–242. [Google Scholar] [CrossRef]
- Bakhtiari, M.; Panahi, Y.; Ameli, J.; Darvishi, B. Protective effect of flavonoids against Alzheimer’s disease related neural disfunctions. Biomed. Pharmacother. 2017, 93, 218–229. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
- Rauter Amélia, P.; Ennis, M.; Hellwich, K.H.; Herold Bernardo, J.; Horton, D.; Moss Gerard, P.; Schomburg, I. Nomenclature of flavonoids (IUPAC Recommendations 2017). Pure App. Chem. 2018, 9, 1429–1486. [Google Scholar] [CrossRef]
- Kaur, R.; Sood, A.; Lang, D.K.; Bhatia, S.; Al-Harrasi, A.; Aleya, L.; Behl, T. Potential of flavonoids as anti-Alzheimer’s agents: Bench to bedside. Environ. Sci. Pollut. Res. 2022, 29, 26063–26077. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sengupta, S.; Chakraborty, S. Scope of β-secretase (BACE1)– targeted therapy in Alzheimer’s disease: Emphasizing the flavonoid based natural scaffold for BACE1 inhibition. ACS Chem. Neurosci. 2020, 11, 3510–3522. [Google Scholar] [CrossRef]
- Naushad, M.; Durairajan, S.S.K.; Bera, A.K.; Senapati, S.; Li, M. Natural compounds with anti-BACE1 activity as promising therapeutic drugs for treating Alzheimer’s disease. Planta Medica 2019, 85, 1316–1325. [Google Scholar]
- Anand, P.; Singh, B. Flavonoids as lead compounds modulating the enzyme targets in Alzheimer’s disease. Med. Chem. Res. 2013, 22, 3061–3075. [Google Scholar] [CrossRef]
- Das, P.; Preethi, K.; Kiruba, A.A.; Nikhil, K.; Nayak, A. Flavonoids: An alternative pathway for the treatment of Alzheimer’s disease. Annals of Phytomedicine. 2021, 10, 240–251. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Aspects Med. 2012, 33, 83–97. [Google Scholar] [CrossRef]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Park, M. Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules 2007, 12, 2130. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, C.; Zhang, Y.; Tsao, R. On-line coupling pressurized liquid extraction with two-dimensional counter current chromatography for isolation of natural acetylcholinesterase inhibitors from Astragalus membranaceus. Phytochem. Anal. 2021, 32, 640–653. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, H.; Turdu, G. Traditional Chinese medicinal herbs as potential AChE inhibitors for anti-Alzheimer’s disease: A review. Bioorg. Chem. 2017, 75, 50–61. [Google Scholar] [CrossRef]
- Ali, M.Y.; Jannat, S.; Edraki, N.; Das, S.; Chang, W.K.; Kim, H.C.; Park, S.K.; Chang, M.S. Flavanone glycosides inhibit β-site amyloid precursor protein cleaving enzyme 1 and cholinesterase and reduce Aβ aggregation in the amyloidogenic pathway. Chem.-Biol. Interact. 2019, 309, 108707. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Zeng, F.; Shen, Y.; Wang, Y.-Y.; Zhang, N.; Geng, F. Biological isolation and structure identification of acetylcholinesterase enzyme inhibitors rom Drynariae rhizome. J. Anal. Methods Chem. 2020, 2020, 2971841. [Google Scholar] [CrossRef]
- Youn, K.; Jun, M. Biological evaluation and docking analysis of potent BACE1 inhibitors from Boesenbergia rotunda. Nutrients 2019, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Dzoyem, J.P.; Nkuete, A.H.L.; Ngameni, B.; Eloff, J.N. Anti-inflammatory and anticholinesterase activity of six flavonoids isolated from Polygonum and Dorstenia species. Arch. Pharm. Res. 2017, 40, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.M.; Ryu, Y.B.; Kim, H.Y.; Kim, D.; Hong, S.-G.; Lee, J.H.; Curtis-Long, M.J.; Jeong, S.H.; Park, J.-Y.; Park, K.H. BACE1 inhibitors effects of lavandulyl flavanones from Sophora flavescens. Bioorg. Med. Chem. 2008, 16, 6669–6674. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Jedrejek, D.; Senol, F.S.; Salmas, R.E.; Durdagi, S.; Kowalska, I.; Pecio, L.; Olezek, W. Molecular modeling and in vitro approaches towards cholinesterase inhibitory effect of some naural xanthohumol, naringenin, and acyl phloroglucinol derivatives. Phytomedicine 2018, 42, 25–33. [Google Scholar] [CrossRef]
- Lee, S.; Youn, K.; Lim, G.; Lee, J.; Jun, M. In silico docking and in vitro approaches towards BACE1 and cholinesterases. Molecules 2018, 23, 1509. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Rupasinghe, S.G.; Stefani, F.; Schuler, M.A.; Mejia, E.G. Citrus flavonoids luteolin, apigenin and quercetin inhibit glycogen synthase kinase-3β enzymatic activity by lowering the interaction energy within the binding cavity. J. Med. Food. 2011, 14, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Xy, P.; Zhang, G.; Cheng, F.; Chen, K.; Li, J.; Zhu, W.; Cao, D.; Xu, K.; Tan, G. Selagintriflavonoids with BACE1 inhibitory activity from the fern Selaginella doedrerleinii. Phytochemistry 2017, 134, 114–121. [Google Scholar] [CrossRef]
- Jung, H.A.; Yokozawa, T.; Kim, B.-W.; Jung, J.H.; Choi, J.S. Selective inhibition of prenylated flavonoids from Sophora flavescens against BACE1 and cholinesterases. Am. J. Chin. Med. 2010, 38, 415–429. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Hammam, W.E.; El-Tantawi, M.E.; Yassin, N.A.Z.; Kirollos, F.N.; Abdelhameed, M.F.; Abdelfattah, M.A.O.; Wink, M.; Sobeh, M. Apple leaves and their major secondary metabolite phlorizin exhibit distinct neuroprotective activities: Evidence from in vitro and in silico studies. Arab. J. Chem. 2021, 14, 103188. [Google Scholar] [CrossRef]
- Tran, T.-S.; Tran, T.-D.; Tran, T.-H.; Mai, T.-T.; Nguyen, N.-L.; Thai, K.-M.; Le, M.-T. Synthesis, in silico and in vitro evaluation of some derivatives for acetylcholinesterase and BACE-1 inhibitory activity. Molecules 2020, 25, 4064. [Google Scholar] [CrossRef]
- Huang, W.-H.; Lee, A.-R.; Yang, C.-H. Antioxidative and anti-inflammatory activities of polihydroxyflavonoids of Scutellaria baicalensis GEORGI. Biosci. Biotechnol. Biochem. 2006, 70, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Perumalsamy, H.; Kwon, H.W.; Na, Y.-E.; Ahn, Y.-J. Effects and possible mechanisms of action of acacetin on the behavior and eye morphology of Drosophila models of Alzheimer’s disease. Sci. Rep. 2015, 5, 16127. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, A.; Park, J.-H.; Choi, J.S.; Park, K.-S.; Hong, J.-P.; Park, H.-J. Structure determination and quantification of a new flavone glycoside with anti-acetylcholinesterase activity from the herbs of Elsholtzia ciliate. Nat. Prod. Res. 2019, 33, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Wagle, A.; Seong, S.H.; Shrestha, S.; Jung, H.A.; Choi, J.S. Korean Thistle (Cirsium japonicum var. maackii (Maxim) Matsum): A potential dietary supplement against diabetes and Alzheimer’s disease. Molecules 2019, 24, 649. [Google Scholar] [CrossRef]
- Devi, S.; Kumar, V.; Singh, S.K.; Dubey, A.K.; Kim, J.-J. Flavonoids: Potential candidates for the treatment of neurodegenerative disorders. Biomedicines 2021, 9, 99. [Google Scholar] [CrossRef]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, Y.M.; Park, H.J.; Sohn, H.S.; Jung, H.A. The effects of C-glycosilation of luteolin on its antioxidant, anti-Alzheimer’s disease, anti-diabetic, and anti-inflammatory activities. Arch. Pharm. Res. 2014, 37, 1354–1363. [Google Scholar] [CrossRef]
- Youn, K.; Yu, Y.; Lee, J.; Jeong, W.-S.; Chi-Tang Ho, C.-T.; Jun, M. Polymethoxyflavones: Novel β-secretase (BACE1) inhibitors from Citrus peel. Nutrients 2017, 9, 973. [Google Scholar] [CrossRef]
- Lata, R.; Azizur, R.; Mizanur, R.; Saurav, H. Extraction and in vitro screening of potential acetylcholinesterase, butyrylcholinesterase and BACE1 inhibitors from the leaves of Ocimum sanctum. Indo Am. J. P. Sci. 2017, 4, 2417–2424. [Google Scholar]
- Xu, H.; Zhou, Q.; Liu, B.; Cheng, K.-W.; Chen, F.; Wang, M. Neuroprotective potential of mung bean (Vigna radiata L.) polyphenols in Alzheimer’s disease: A review. J. Agr. Food Chem. 2021, 69, 11554–11571. [Google Scholar] [CrossRef]
- Liang, Z.; Li, Q.X. Discovery of selective, substrate-competitive, and passive membrane permeable glycogen synthase kinase- 3β inhibitors: Synthesis, biological evaluation, and molecular modeling of new C-glycosylflavones. ACS Chem. Neurosci. 2018, 9, 1166–1183. [Google Scholar] [CrossRef]
- Conforti, F.; Rigano, D.; Menichini, F.; Loizzo, M.R.; Senatore, F. Protection against neurodegenerative diseases of Iris pseudopumila extracts and their constituents. Fitoterapia 2009, 80, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.K.; Ryu, Y.B.; Curtis-Long, M.J.; Kim, J.Y.; Kim, D.; Lee, S.; Lee, W.S.; Park, K.H. Inhibition and structural reliability of prenilated flavones from the stem bark of Morus lhou on β-secretase (BACE-1). Bioorg. Med. Chem. Lett. 2011, 21, 2945–2948. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, W.S.; Kim, Y.S.; Curtis-Long, M.J.; Lee, B.W.; Ryu, Y.B.; Park, K.H. Isolation of cholinesterase-inhibiting flavonoids from Morus lhou. J. Agric. Food Chem. 2011, 59, 4589–4596. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Miki, K.; Kinoshita, K.; Koyama, K.; Juliawaty, L.D.; Achmad, S.A.; Hakim, E.H.; Kaneda, M.; Takahashi, K. β-Secretase (BACE-1) inhibitory effect of bioflavonoids. Bioorg. Med. Chem. Lett. 2010, 20, 4558–4560. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Tewari, D.; Mathew, B.; Aleya, L. Emerging signal regulating potential of small molecule biflavonoids to combat neuropathological insults of Alzheimer’s disease. Sci. Total Environ. 2020, 700, 134836. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Park, J.-S.; Choi, J.S. Antidiabetic complications and anti-Alzheimer activities of sophoflavescenol, a prenylated flavonol from Sophora flavescens, and its structure-activity relationship. Phytother. Res. 2011, 25, 709–715. [Google Scholar] [CrossRef]
- Min, B.S.; Cuong, T.D.; Lee, J.S.; Shin, B.S.; Woo, M.H.; Hung, T.M. Cholinesterase inhibitors from Cleistocalyx operculatus buds. Arch. Pharm. Res. 2010, 33, 1665–1670. [Google Scholar] [CrossRef]
- Remya, C.; Dileep, K.V.; Tintu, I.; Variyar, E.J.; Sadasivan, C. Design of potent inhibitors of acetylcholinesterase using morin as the starting compound. Front. Life Sci. 2012, 6, 107–117. [Google Scholar] [CrossRef]
- Athipornchai, A.; Ketpoo, P.; Saeeng, R. Acetylcholinesterase inhibitor from Tabernaemontana pandacaqui flowers. Nat. Prod. Commun. 2020, 15, 1–5. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Biophenols: Enzymes (β-secretase, cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 2018, 128, 118–129. [Google Scholar] [CrossRef]
- Frota, L.S.; Alves, D.R.; Freitas, L.S.; Lopes, F.F.S.; Marinho, M.M.; Marinho, E.S.; Morais, S.M. In vitro antioxidant and anticholinesterase activities of Ouratea fieldingiana (Gardner) Eng. leaf extract and correlation with its phenolics profile with an in silico study in relation to Alzheimer’s disease. J. Braz. Chem. Soc. 2022, 33, 446–455. [Google Scholar] [CrossRef]
- Martínez-Coria, H.; Mendoza-Rojas, M.X.; Arrieta-Cruz, I.; López-Valdés, H.E. Preclinical research of dihydromyricetin for brain aging and neurodegenerative diseases. Front. Pharmacol. 2019, 10, 1334. [Google Scholar] [CrossRef] [PubMed]
- Dung, V.D.; Cuong, T.D.; Chinh, N.M.; Quyen, D.; Kim, J.A.; Byeon, J.S.; Woo, M.H.; Choi, J.S.; Min, B.S. Compounds from the aerial parts of Piper bavinum and their anti-cholinesterase activity. Arch. Pharm. Res. 2015, 38, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.S.; Suk, K. The plant flavonoid wogonin suppress death of activated C6 rat glial cells by inhibiting nitric oxide production. Neurosci. Lett. 2001, 309, 67–71. [Google Scholar] [CrossRef]
- Huang, D.S.; Yu, Y.C.; Wu, C.-H.; Lin, J.Y. Protective effects of wogonin against Alzheimer’s disease by inhibition of amyloidogenic pathway. Evid.-Based Complement. Altern. Med. 2017, 2017, 3545169. [Google Scholar] [CrossRef]
- Angeloni, C.; Barbalace, M.C.; Hrelia, S. Icariin and its metabolites as potential protective phytochemicals against Alzheimer’s disease. Front. Pharmacol. 2019, 10, 271. [Google Scholar] [CrossRef]
- Mogana, R.; Adhikari, A.; Debnath, S.; Hazra, S.; Hazra, B.; Teng-Jin, K.; Wiart, C. The antiacetylcholinesterase and antileishmanial activities of Canarium patentinervium Miq. Biomed. Res. Int. 2014, 2014, 903529. [Google Scholar] [CrossRef]
- Tu, Y.; Wu, C.; Kang, Y.; Li, Q.; Zhu, C.; Li, Y. Bioactivity-guided identification of flavonoids with cholinesterase and β-amyloid peptide aggregation inhibitory effects from the seeds of Millettia pachycarpa. Bioorg. Med. Chem. Lett. 2019, 29, 1194–1198. [Google Scholar] [CrossRef]
- Shahinozzaman, M.; Taira, N.; Ishii, T.; Halim, M.A.; Hossain, M.A.; Tawata, S. Anti-inflammatory, anti-diabetic, and anti-Alzheimer’s effects of prenylated flavonoids from Okinawa propolis: An investigation by experimental and computational studies. Molecules 2018, 23, 2479. [Google Scholar] [CrossRef]
- Cho, J.K.; Ryu, Y.B.; Curtis-Long, M.J.; Ryu, H.W.; Yuk, H.J.; Kim, D.W.; Kim, H.J.; Lee, W.S.; Park, K.H. Cholinesterase inhibitors effects of geranylated flavonoids from Paulownia tomentosa fruits. Bioorg. Med. Chem. 2012, 20, 2595–2602. [Google Scholar] [CrossRef]
- Conforti, F.; Rigano, D.; Formisano, C.; Bruno, M.; Loizzo, M.R.; Menichini, F.; Senatore, F. Metabolite profile and in vitro activities of Phagnalon saxatile (L.) Cass. relevant to treatment of Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2010, 25, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Hu, X.; Pan, J.; Zhang, G. Inhibitory mechanism of baicalein on acetylcholinesterase: Inhibitory interaction, conformational change, and computational simulation. Foods 2022, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Kartal, M.; Tosun, F.; Sener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z. Naturforsch. C Biosci. 2007, 62, 829–832. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrink, S.; Trillk, J.; Gibbons, S.; Viljoen, A. Acacetin—A simple flavone exhibiting diverse pharmacological activities. Phytochem. Lett. 2019, 32, 56–65. [Google Scholar] [CrossRef]
- Ding, X.; Ouyang, M.-A.; Liu, X.; Wang, R.-Z. Acetylcholinesterase inhibitory activities of flavonoids from the leaves of Gingko biloba against Brown planthopper. J. Chem. 2013, 2013, 645086. [Google Scholar] [CrossRef]
- Ajayi, O.S.; Aderogba, M.A.; Obuotor, E.M.; Majinda, R.R.T. Acetylcholinesterase inhibitor from Anthocleista vogelii leaf rxtrcts. J. Ethnopharmacol. 2019, 231, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Balkis, A.; Tran, K.; Lee, Y.Z.; Ng, K. Screening flavonoids for inhibition of acetylcholinesterase identified baicalein as the most potent inhibitor. J. Agric. Sci. 2015, 7, 26–35. [Google Scholar] [CrossRef]
- Muralidharan, A.; Josyula, V.R.; Hariharapura, R.C. Exploring the potential of marine microbes in clinical management of Alzheimer’s disease: A road map for bioprospecting and identifying promising isolates. Life Sci. 2018, 208, 149–160. [Google Scholar] [CrossRef]
- Humidene, A.B.; Hanaki, M.; Murakami, K.; Irie, K.; Isoda, H.; Shigemori, H. Inhibitory activities of antioxidant flavonoids from Tamarix gallica on amyloid aggregation related to Alzheimer’s and type 2 diabetes diseases. Biol. Pharm. Bull. 2017, 40, 238–241. [Google Scholar] [CrossRef]
- Andreeva, O.A.; Ivashev, M.N.; Ozimina, I.L.; Masfikova, G.V. Diosmetin glycosides from Caucasian vetch: Isolation and study of biological activity. Pharm. Chem. J. 1998, 32, 28–30. [Google Scholar] [CrossRef]
- Sudha, A.; Srinivasan, P.; Kanimozhi, V.; Palanivel, K.; Kadalmani, B. Antiproliferative and apoptosis-induction studies of 5-hydroxy 3′,4′,7-trimethoxyflavone in human breast cancer cells MCF-7: An in vitro and in silico approach. J. Recept. Signal Transduct. Res. 2018, 38, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Uliassi, E.; Bolognesi, M.L. Two disease, one approach: Multitarget drug discovery in Alzheimer’s and neglected tropical diseases. Med. Chem. Comm. 2014, 5, 853–861. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Lima, E.; Medeiros, J. Marine organisms as alkaloid biosynthesizers of potential anti-Alzheimer agents. Mar. Drugs. 2022, 20, 75. [Google Scholar] [CrossRef]
- Hole, K.L.; Williams, R.J. Flavonoids as an intervention for Alzheimer’s Disease: Progress and hurdles towards defining a mechanism of action. Brain Plast. 2021, 6, 167–192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).