Laccase in Biorefinery of Lignocellulosic Biomass
Abstract
:1. Introduction
2. Pretreatment of Lignocellulosic Biomasses
2.1. Acidic Pretreatment for Lignocellulosic Biomasses
2.2. Alkali Pretreatment for Lignocellulosic Biomasses
2.3. Hydrothermal Pretreatment for Lignocellulosic Biomasses
2.4. Organosolv Pretreatment
2.5. Biological Pretreatment for Lignocellulosic Biomasses
3. Significance of Laccase in Biorefinery
3.1. Laccase Sources
3.1.1. Bacterial Laccase Production
3.1.2. Fungal Sources of Laccase
4. Bioprocesses for Laccase Production
4.1. Solid-State Fermentation
4.2. Submerged Fermentation
| Serial No. | Microorganisms | Bioprocess Type | Titers of Enzyme | Application | Reference |
|---|---|---|---|---|---|
| Bacteria | |||||
| 1. | Bacillus sp. PCH94 | SmF | 0.27 IU/mL | Lignin depolymerization | [51] |
| 2. | Aquisalibacillus elongatus | SmF | 8.02 U mL−1 | Biowaste delignification Biotreatment | [52] |
| 3. | Bacillus subtilis LP2 | SmF | 140.4 U/mg | Bioremediation process | [53] |
| 4. | Bacillus tequilensis SN4 MTCC 11828 | SmF | 18,356 nkats/ml | Degradation of residual lignin in kraft pulp | [54] |
| 5. | Bacillus safenis DSKK5 | SmF | 10.51 U/mL | - | [55] |
| 6. | Geobacillus thermocatenulatus MS5 | SmF | 1.52 U/mg | - | [56] |
| 7. | Pseudomonas aeruginosa | SmF | 0.038 U/ml | - | [57] |
| 8. | Pseudomonas desmolyticum NCIM 2112 | SmF | 0.012 ± 0.0003 U/mg | - | [58] |
| 9. | Pseudomonas putida F6 | SmF | 573 U/mg | - | [59] |
| 10. | Azospirillum lipoferum | SmF | 0.9 U/mg | - | [60] |
| 11. | Ceratorhiza hydrophila | SmF | 154 U/mL | Textile dyeing and printing processes | [61] |
| Fungi | |||||
| 12. | Trametes versicolor | SSF | 77.88 ± 5.62 U/g | Pre-industrial procedures to produce laccases | [62] |
| 13. | Pycnoporus sanguineus | SSF | 130.95 ± 2.20 U/g | Pre-industrial procedures to produce laccases | [62] |
| 14. | Trametes gibbosa An 360 | SmF | 55.83 ± 0.28 U/L | - | [63] |
| 15. | Vanderbylia fraxinea An 369 | SmF | 77.96 ± 1.60 U/L | - | [63] |
| 16. | Perenniporia pyricola Han 202 | SmF | 443.33 ± 15.49 U/L | - | [63] |
| 17. | Coriolopsis trogii Han 474 | SmF | 686.57 ± 16.49 U/L | - | [63] |
| 18. | Trametes versicolor Han 1504 | SmF | 162.04 ± 11.33 U/L | - | [63] |
| 19. | Pleurotus floridanus | SmF | 80.45 ± 0.132 U/mL | Bioprocess for de-oiled microalgal biomasses | [64] |
| 20. | Ganoderma leucocontextum | SmF | 855 U/L | - | [65] |
| 21. | Trichoderma harzianum S7113 | SmF | 391.38 ± 9.51 U/L | - | [49] |
| 22. | Trametes trogii | SSF | 2.1 U/g | - | [66] |
| 23. | Pycnoporus cinnabarinus | SmF | 280 U/L | Degradation of the disazo dye | [67] |
| 24. | Trametes pubescens | SmF | 333,000 U/L | - | [68] |
| 25. | Neurospora crassa | SmF | 10,000 U/L | Bioremediation of phenols | [69] |
| 26. | Trametes versicolor | SSF (Immersion, nylon sponge) | 229 | - | [70] |
| 27. | Trametes hirsuta | SSF (Tray, grape seeds) | 18,715 U/L | - | [71] |
5. Applications of Laccases
6. Laccase-Based Biological Pretreatment Lignocellulosic Biomasses
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singhania, R.R.; Patel, A.K.; Raj, T.; Chen, C.-W.; Ponnusamy, V.K.; Tahir, N.; Kim, S.-H.; Dong, C.-D. Lignin Valorisation via Enzymes: A Sustainable Approach. Fuel 2022, 311, 122608. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and Quantitative Analysis of Lignocellulosic Biomass Using Infrared Techniques: A Mini-Review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, N.; Aslam, S.; Hussain, N.; Bilal, M.; Iqbal, H.M.N. Transforming Lignin Biomass to Value: Interplay between Ligninolytic Enzymes and Lignocellulose Depolymerization. BioEnergy Res. 2022. [Google Scholar] [CrossRef]
- Fillat, Ú.; Ibarra, D.; Eugenio, M.E.; Moreno, A.D.; Tomás-Pejó, E.; Martín-Sampedro, R. Laccases as a Potential Tool for the Efficient Conversion of Lignocellulosic Biomass: A Review. Fermentation 2017, 3, 17. [Google Scholar] [CrossRef]
- Dong, C.-D.; Tiwari, A.; Anisha, G.S.; Chen, C.-W.; Singh, A.; Haldar, D.; Patel, A.K.; Singhania, R.R. Laccase: A Potential Biocatalyst for Pollutant Degradation. Environ. Pollut. 2023, 319, 120999. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehra, R.; Muschiol, J.; Meyer, A.S.; Kepp, K.P. A Structural-Chemical Explanation of Fungal Laccase Activity. Sci. Rep. 2018, 8, 17285. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.; Baldrian, P.; Murugesan, K.; Chang, Y. Laccase-catalysed Oxidations of Naturally Occurring Phenols: From In Vivo Biosynthetic Pathways to Green Synthetic Applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Chandra, R. Ligninolytic Enzymes and Its Mechanisms for Degradation of Lignocellulosic Waste in Environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Leynaud Kieffer Curran, L.M.C.; Pham, L.T.M.; Sale, K.L.; Simmons, B.A. Review of Advances in the Development of Laccases for the Valorization of Lignin to Enable the Production of Lignocellulosic Biofuels and Bioproducts. Biotechnol. Adv. 2022, 54, 107809. [Google Scholar] [CrossRef]
- Fatma, S.; Hameed, A.; Noman, M.; Ahmed, T.; Shahid, M.; Tariq, M.; Sohail, I.; Tabassum, R. Lignocellulosic Biomass: A Sustainable Bioenergy Source for the Future. Protein Pept. Lett. 2018, 25, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Remzi Becer, C. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Solarte Toro, J.; Romero-García, J.; Martínez-Patiño, J.; Ruiz-Ramos, E.; Castro, E.; Cardona, C.A. Acid Pretreatment of Lignocellulosic Biomass for Energy Vectors Production: A Review Focused on Operational Conditions and Techno-Economic Assessment for Bioethanol Production. Renew. Sustain. Energy Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid Hydrolysis of Lignocellulosic Biomass: Sugars and Furfurals Formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef] [Green Version]
- Shekiro, J., III; Kuhn, E.M.; Nagle, N.J.; Tucker, M.P.; Elander, R.T.; Schell, D.J. Characterization of Pilot-Scale Dilute Acid Pretreatment Performance Using Deacetylated Corn Stover. Biotechnol. Biofuels 2014, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Ebrik, M.A.; Wyman, C.E. Sugar Yields from Dilute Sulfuric Acid and Sulfur Dioxide Pretreatments and Subsequent Enzymatic Hydrolysis of Switchgrass. Bioresour. Technol. 2011, 102, 8930–8938. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, R.; Tang, W.; Zheng, Y.; Meng, X. Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers. Fermentation 2022, 8, 558. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzyme Res. 2011, 2011, 787532. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Chen, C.-W.; Patel, A.K.; Dong, C.-D.; Singhania, R.R. Subcritical Water Pretreatment for the Efficient Valorization of Sorghum Distillery Residue for the Biorefinery Platform. Bioengineering 2023, 10, 38. [Google Scholar] [CrossRef]
- Martín, C.; Dixit, P.; Momayez, F.; Jönsson, L.J. Hydrothermal Pretreatment of Lignocellulosic Feedstocks to Facilitate Biochemical Conversion. Front. Bioeng. Biotechnol. 2022, 10, 846592. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Devin, I.; Chrusciel, L.; Brosse, N. Steam Explosion Pretreatment of Lignocellulosic Biomass: A Mini-Review of Theorical and Experimental Approaches. Front. Chem. 2021, 9, 705358. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gutierrez, J.M.; Verlinden, R.A.J.; van der Meer, P.C.; van der Wielen, L.A.M.; Straathof, A.J.J. Liquid Hot Water Pretreatment of Lignocellulosic Biomass at Lab and Pilot Scale. Processes 2021, 9, 1518. [Google Scholar] [CrossRef]
- Tarabanko, N.; Baryshnikov, S.V.; Kazachenko, A.S.; Miroshnikova, A.V.; Skripnikov, A.M.; Lavrenov, A.V.; Taran, O.P.; Kuznetsov, B.N. Hydrothermal Hydrolysis of Microcrystalline Cellulose from Birch Wood Catalyzed by Al2O3-B2O3 Mixed Oxides. Wood Sci. Technol. 2022, 56, 437–457. [Google Scholar] [CrossRef]
- Zhang, Z.; Harrison, M.D.; Rackemann, D.W.; Doherty, W.O.S.; O’Hara, I.M. Organosolv Pretreatment of Plant Biomass for Enhanced Enzymatic Saccharification. Green Chem. 2016, 18, 360–381. [Google Scholar] [CrossRef] [Green Version]
- Chu, Q.; Tong, W.; Chen, J.; Wu, S.; Jin, Y.; Hu, J.; Song, K. Organosolv Pretreatment Assisted by Carbocation Scavenger to Mitigate Surface Barrier Effect of Lignin for Improving Biomass Saccharification and Utilization. Biotechnol. Biofuels 2021, 14, 136. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv Pretreatment of Lignocellulosic Biomass for Enzymatic Hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Chen, C.-W.; Sun, P.-P.; Patel, A.K.; Singhania, R.R.; Nargotra, P.; Dong, C.-D. Deep Eutectic Solvents as Promising Pretreatment Agents for Sustainable Lignocellulosic Biorefineries: A Review. Bioresour. Technol. 2022, 360, 127631. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, K.; Zhang, Y.; Wang, M.; Yong, C.; Chen, L.; Qu, P.; Huang, H.; Sun, E.; Pan, M. Lignocellulose Dissociation with Biological Pretreatment towards the Biochemical Platform: A Review. Mater. Today Bio 2022, 16, 100445. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Wu, C.-H.; Meng, C.-T.; Lin, C.-W. Decolorization of Azo Dye and Generation of Electricity by Microbial Fuel Cell with Laccase-Producing White-Rot Fungus on Cathode. Appl. Energy 2017, 188, 392–398. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose Degradation: An Overview of Fungi and Fungal Enzymes Involved in Lignocellulose Degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Zhuo, S.; Yan, X.; Liu, D.; Si, M.; Zhang, K.; Liu, M.; Peng, B.; Shi, Y. Use of Bacteria for Improving the Lignocellulose Biorefinery Process: Importance of Pre-Erosion. Biotechnol. Biofuels 2018, 11, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of Lignocellulose Using Ligninolytic Enzymes from White-Rot Fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.T.; Speranza, M.; Ruiz-Dueñas, F.J.; Ferreira, P.; Camarero, S.; Guillén, F.; Martínez, M.J.; Gutiérrez, A.; del Río, J.C. Biodegradation of Lignocellulosics: Microbial, Chemical, and Enzymatic Aspects of the Fungal Attack of Lignin. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2005, 8, 195–204. [Google Scholar]
- Chen, S.; Zhang, X.; Singh, D.; Yu, H.; Yang, X. Biological Pretreatment of Lignocellulosics: Potential, Progress and Challenges. Biofuels 2010, 1, 177–199. [Google Scholar] [CrossRef]
- Axelsson, L.; Franzén, M.; Ostwald, M.; Berndes, G.; Gopakumar, L.; Ravindranath, N.H. Perspective: Jatropha Cultivation in Southern India: Assessing Farmers’ Experiences. Biofuels 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Goradia, B.; Saxena, A. Bacterial Laccase: Recent Update on Production, Properties and Industrial Applications. 3 Biotech 2017, 7, 323. [Google Scholar] [CrossRef]
- Antošová, Z.; Sychrová, H. Yeast Hosts for the Production of Recombinant Laccases: A Review. Mol. Biotechnol. 2016, 58, 93–116. [Google Scholar] [CrossRef]
- Saini, A.; Aggarwal, N.K.; Sharma, A.; Yadav, A. Actinomycetes: A Source of Lignocellulolytic Enzymes. Enzyme Res. 2015, 2015, 279381. [Google Scholar] [CrossRef] [Green Version]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial Sources, Production, Purification, and Potential Biotechnological Applications. Enzyme Res. 2011, 2011, 217861. [Google Scholar] [CrossRef] [Green Version]
- Arora, D.S.; Gill, P.K. Laccase Production by Some White Rot Fungi under Different Nutritional Conditions. Bioresour. Technol. 2000, 73, 283–285. [Google Scholar] [CrossRef]
- D’Souza, T.M.; Boominathan, K.; Reddy, C.A. Isolation of Laccase Gene-Specific Sequences from White Rot and Brown Rot Fungi by PCR. Appl. Environ. Microbiol. 1996, 62, 3739–3744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Góralczyk-Bińkowska, A.; Jasińska, A.; Długoński, A.; Płociński, P.; Długoński, J. Laccase Activity of the Ascomycete Fungus Nectriella pironii and Innovative Strategies for Its Production on Leaf Litter of an Urban Park. PLoS ONE 2020, 15, e0231453. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent Developments on Solid-State Fermentation for Production of Microbial Secondary Metabolites: Challenges and Solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef] [PubMed]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent Advances in Solid-State Fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Binod, P.; Sindhu, R.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Bioethanol Production from Rice Straw: An Overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef]
- Mitchell, D.A.; von Meien, O.F.; Luz, L.F.L.; Berovič, M. The Scale-up Challenge for SSF Bioreactors. In Solid-State Fermentation Bioreactors: Fundamentals of Design and Operation; Mitchell, D.A., Berovič, M., Krieger, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 57–64. ISBN 978-3-540-31286-4. [Google Scholar]
- Chmelová, D.; Legerská, B.; Kunstová, J.; Ondrejovič, M.; Miertuš, S. The Production of Laccases by White-Rot Fungi under Solid-State Fermentation Conditions. World J. Microbiol. Biotechnol. 2022, 38, 21. [Google Scholar] [CrossRef]
- Othman, A.M.; Mahmoud, M.; Abdelraof, M.; Abdel Karim, G.S.A.; Elsayed, A.M. Enhancement of Laccase Production from a Newly Isolated Trichoderma harzianum S7113 Using Submerged Fermentation: Optimization of Production Medium via Central Composite Design and Its Application for Hydroquinone Degradation. Int. J. Biol. Macromol. 2021, 192, 219–231. [Google Scholar] [CrossRef]
- Sukumaran, R.K.; Singhania, R.R.; Pandey, A. Microbial Cellulases-Production, Applications and Challenges. J. Sci. Ind. Res. 2005, 64, 832–844. [Google Scholar]
- Ambika; Kumar, V.; Chandra, D.; Thakur, V.; Sharma, U.; Singh, D. Depolymerization of Lignin Using Laccase from Bacillus sp. PCH94 for Production of Valuable Chemicals: A Sustainable Approach for Lignin Valorization. Int. J. Biol. Macromol. 2023, 234, 123601. [Google Scholar] [CrossRef]
- Rezaie, R.; Rezaei, S.; Jafari, N.; Forootanfar, H.; Khoshayand, M.R.; Faramarzi, M.A. Delignification and Detoxification of Peanut Shell Bio-Waste Using an Extremely Halophilic Laccase from an Aquisalibacillus elongatus Isolate. Extrem. Life Extreme Cond. 2017, 21, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Yasar, G.; Guven, U.G.; Guduk, E.; Aktas, F. Partial Purification and Characterization of the Novel Halotolerant and Alkalophilic Laccase Produced by a New Isolate of Bacillus Subtilis LP2. Biocatal. Biotransformation 2019, 37, 268–277. [Google Scholar] [CrossRef]
- Sondhi, S.; Sharma, P.; George, N.; Chauhan, P.S.; Puri, N.; Gupta, N. An Extracellular Thermo-Alkali-Stable Laccase from Bacillus tequilensis SN4, with a Potential to Biobleach Softwood Pulp. 3 Biotech 2015, 5, 175–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Sharma, K.; Shenu, S.; Gakhar, S. Molecular Docking of Laccase Protein from Bacillus safensis DSKK5 Isolated from Earthworm Gut: A Novel Method to Study Dye Decolorization Potential. Water. Air. Soil Pollut. 2014, 225, 2175. [Google Scholar] [CrossRef]
- Rai, R.; Bibra, M.; Chadha, B.S.; Sani, R.K. Enhanced Hydrolysis of Lignocellulosic Biomass with Doping of a Highly Thermostable Recombinant Laccase. Int. J. Biol. Macromol. 2019, 137, 232–237. [Google Scholar] [CrossRef]
- Peter, J.K.; Vandana, P. Congo Red Dye Decolourization by Partially Purified Laccases From. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 105–115. [Google Scholar]
- Kalme, S.; Jadhav, S.; Jadhav, M.; Govindwar, S. Textile Dye Degrading Laccase from Pseudomonas Desmolyticum NCIM 2112. Enzyme Microb. Technol. 2009, 44, 65–71. [Google Scholar] [CrossRef]
- McMahon, A.M.; Doyle, E.M.; Brooks, S.; O’Connor, K.E. Biochemical Characterisation of the Coexisting Tyrosinase and Laccase in the Soil Bacterium Pseudomonas Putida F6. Enzyme Microb. Technol. 2007, 40, 1435–1441. [Google Scholar] [CrossRef]
- Diamantidis, G.; Effosse, A.; Potier, P.; Bally, R. Purification and Characterization of the First Bacterial Laccase in the Rhizospheric Bacterium Azospirillum Lipoferum. Soil Biol. Biochem. 2000, 32, 919–927. [Google Scholar] [CrossRef]
- Elsaba, Y.M.; El-Hennawi, H.M.; Ibrahim, M.M.; Wehaidy, H.R. Production of a Novel Laccase from Ceratorhiza Hydrophila and Assessing Its Potential in Natural Dye Fixation and Cytotoxicity against Tumor Cells. J. Genet. Eng. Biotechnol. 2023, 21, 14. [Google Scholar] [CrossRef]
- Backes, E.; Kato, C.G.; de Oliveira Junior, V.A.; Uber, T.M.; dos Santos, L.F.O.; Corrêa, R.C.G.; Bracht, A.; Peralta, R.M. Overproduction of Laccase by Trametes versicolor and Pycnoporus sanguineus in Farnesol-Pineapple Waste Solid Fermentation. Fermentation 2023, 9, 188. [Google Scholar] [CrossRef]
- Han, M.; Lin, L.; Guo, X.; An, M. Comparative Analysis of the Laccase Secretion Ability of Five White-Rot Fungi in Submerged Fermentation with Lignocellulosic Biomass. Available online: https://bioresources.cnr.ncsu.edu/ (accessed on 7 March 2023).
- Chenthamara, D.; Sivaramakrishnan, M.; Ramakrishnan, S.G.; Esakkimuthu, S.; Kothandan, R.; Subramaniam, S. Improved Laccase Production from Pleurotus Floridanus Using Deoiled Microalgal Biomass: Statistical and Hybrid Swarm-Based Neural Networks Modeling Approach. 3 Biotech 2022, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Ahmed, S. Optimization, Purification and Characterization of Laccase from Ganoderma Leucocontextum along with Its Phylogenetic Relationship. Sci. Rep. 2022, 12, 2416. [Google Scholar] [CrossRef]
- Levin, L.; Herrmann, C.; Papinutti, V.L. Optimization of Lignocellulolytic Enzyme Production by the White-Rot Fungus Trametes trogii in Solid-State Fermentation Using Response Surface Methodology. Biochem. Eng. J. 2008, 39, 207–214. [Google Scholar] [CrossRef]
- Schliephake, K.; Mainwaring, D.E.; Lonergan, G.T.; Jones, I.K.; Baker, W.L. Transformation and Degradation of the Disazo Dye Chicago Sky Blue by a Purified Laccase from Pycnoporus cinnabarinus. Enzyme Microb. Technol. 2000, 27, 100–107. [Google Scholar] [CrossRef]
- Galhaup, C.; Wagner, H.; Hinterstoisser, B.; Haltrich, D. Increased Production of Laccase by the Wood-Degrading Basidiomycete Trametes pubescens. Enzyme Microb. Technol. 2002, 30, 529–536. [Google Scholar] [CrossRef]
- Luke, A.K.; Burton, S.G. A Novel Application for Neurospora Crassa: Progress from Batch Culture to a Membrane Bioreactor for the Bioremediation of Phenols. Enzyme Microb. Technol. 2001, 29, 348–356. [Google Scholar] [CrossRef]
- Rodríguez Couto, S.; Moldes, D.; Liébanas, A.; Sanromán, A. Investigation of Several Bioreactor Configurations for Laccase Production by Trametes versicolor Operating in Solid-State Conditions. Biochem. Eng. J. 2003, 15, 21–26. [Google Scholar] [CrossRef]
- Rodríguez Couto, S.; López, E.; Sanromán, M.Á. Utilisation of Grape Seeds for Laccase Production in Solid-State Fermentors. J. Food Eng. 2006, 74, 263–267. [Google Scholar] [CrossRef]
- Frases, S.; Salazar, A.; Dadachova, E.; Casadevall, A. Cryptococcus Neoformans Can Utilize the Bacterial Melanin Precursor Homogentisic Acid for Fungal Melanogenesis. Appl. Environ. Microbiol. 2007, 73, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Balda, S.; Sharma, A.; Gupta, N.; Capalash, N.; Sharma, P. Deinking of Old Newsprint (ONP) Pulp with an Engineered Laccase: A Greener Approach for Paper Recycling. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Gupta, V.; Garg, S.; Capalash, N.; Gupta, N.; Sharma, P. Production of Thermo-Alkali-Stable Laccase and Xylanase by Co-Culturing of Bacillus sp. and B. Halodurans for Biobleaching of Kraft Pulp and Deinking of Waste Paper. Bioprocess Biosyst. Eng. 2015, 38, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Singhania, R.R.; Ruiz, H.A.; Awasthi, M.K.; Dong, C.-D.; Chen, C.-W.; Patel, A.K. Challenges in Cellulase Bioprocess for Biofuel Applications. Renew. Sustain. Energy Rev. 2021, 151, 111622. [Google Scholar] [CrossRef]
- Kim, S. Mushroom Ligninolytic Enzymes―Features and Application of Potential Enzymes for Conversion of Lignin into Bio-Based Chemicals and Materials. Appl. Sci. 2021, 11, 6161. [Google Scholar] [CrossRef]
- Yang, Y.; Song, W.-Y.; Hur, H.-G.; Kim, T.-Y.; Ghatge, S. Thermoalkaliphilic Laccase Treatment for Enhanced Production of High-Value Benzaldehyde Chemicals from Lignin. Int. J. Biol. Macromol. 2019, 124, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Murwanashyaka, J.N.; Pakdel, H.; Roy, C. Seperation of Syringol from Birch Wood-Derived Vacuum Pyrolysis Oil. Sep. Purif. Technol. 2001, 24, 155–165. [Google Scholar] [CrossRef]
- Burri, J.; Graf, M.; Lambelet, P.; Löliger, J. Vanillin: More than a Flavouring Agent—A Potent Antioxidant. J. Sci. Food Agric. 1989, 48, 49–56. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Benito-Garzón, L.; Fung, S.; Kohn, J.; Vázquez-Lasa, B.; San Román, J. Bioadhesive Functional Hydrogels: Controlled Release of Catechol Species with Antioxidant and Antiinflammatory Behavior. Mater. Sci. Eng. C 2019, 105, 110040. [Google Scholar] [CrossRef]
- Liu, D.; Yan, X.; Si, M.; Deng, X.; Min, X.; Shi, Y.; Chai, L. Bioconversion of Lignin into Bioplastics by Pandoraea Sp. B-6: Molecular Mechanism. Environ. Sci. Pollut. Res. 2019, 26, 2761–2770. [Google Scholar] [CrossRef]
- Farmer, T.J.; Comerford, J.W.; Pellis, A.; Robert, T. Post-Polymerization Modification of Bio-Based Polymers: Maximizing the High Functionality of Polymers Derived from Biomass. Polym. Int. 2018, 67, 775–789. [Google Scholar] [CrossRef]
- Nandhini, R.; Sivaprakash, B.; Rajamohan, N.; Vo, D.-V.N. Lignin and Polylactic Acid for the Production of Bioplastics and Valuable Chemicals. Environ. Chem. Lett. 2023, 21, 403–427. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, N. Microbial Laccase: A Robust Enzyme and Its Industrial Applications. Biologia 2020, 75, 1183–1193. [Google Scholar] [CrossRef]
- Ashrafi, S.D.; Rezaei, S.; Forootanfar, H.; Mahvi, A.H.; Faramarzi, M.A. The Enzymatic Decolorization and Detoxification of Synthetic Dyes by the Laccase from a Soil-Isolated Ascomycete, Paraconiothyrium Variabile. Int. Biodeterior. Biodegrad. 2013, 85, 173–181. [Google Scholar] [CrossRef]
- Singh, G.; Dwivedi, S.K. Decolorization and Degradation of Direct Blue-1 (Azo Dye) by Newly Isolated Fungus Aspergillus terreus GS28, from Sludge of Carpet Industry. Environ. Technol. Innov. 2020, 18, 100751. [Google Scholar] [CrossRef]
- Şaşmaz, S.; Gedikli, S.; Aytar, P.; Güngörmedi, G.; Çabuk, A.; Hür, E.; Ünal, A.; Kolankaya, N. Decolorization Potential of Some Reactive Dyes with Crude Laccase and Laccase-Mediated System. Appl. Biochem. Biotechnol. 2011, 163, 346–361. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Y.; Guan, J.; Ding, Y.; He, Y.; Zhang, X.; Shukurov, N.; Romanholo Ferreira, L.F.; Liu, J.; Zhu, M. Biodecolorization and Ecotoxicity Abatement of Disperse Dye-Production Wastewater Treatment with Pycnoporus Laccase. Int. J. Environ. Res. Public. Health 2022, 19, 7983. [Google Scholar] [CrossRef]
- Wang, J.; Lu, L.; Feng, F. Improving the Indigo Carmine Decolorization Ability of a Bacillus amyloliquefaciens Laccase by Site-Directed Mutagenesis. Catalysts 2017, 7, 275. [Google Scholar] [CrossRef] [Green Version]
- Delorme, A.E.; Andanson, J.-M.; Verney, V. Improving Laccase Thermostability with Aqueous Natural Deep Eutectic Solvents. Int. J. Biol. Macromol. 2020, 163, 919–926. [Google Scholar] [CrossRef]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Cost Analysis in Laccase Production. J. Environ. Manag. 2011, 92, 2907–2912. [Google Scholar] [CrossRef]
- Bîtcan, I.; Petrovici, A.; Pellis, A.; Klébert, S.; Károly, Z.; Bereczki, L.; Péter, F.; Todea, A. Enzymatic Route for Selective Glycerol Oxidation Using Covalently Immobilized Laccases. Enzyme Microb. Technol. 2023, 163, 110168. [Google Scholar] [CrossRef]
- Ezike, T.C.; Udeh, J.O.; Joshua, P.E.; Ezugwu, A.L.; Isiwu, C.V.; Eze, S.O.O.; Chilaka, F.C. Substrate Specificity of a New Laccase from Trametes polyzona WRF03. Heliyon 2021, 7, e06080. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, W.-C.; Liu, L.; Zhu, J.-Q.; Li, X.; Li, B.-Z.; Yuan, Y.-J. Inhibition of Lignin-Derived Phenolic Compounds to Cellulase. Biotechnol. Biofuels 2016, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Tsai, M.-L.; Sharma, V.; Sun, P.-P.; Nargotra, P.; Bajaj, B.K.; Chen, C.-W.; Dong, C.-D. Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products. Environments 2023, 10, 6. [Google Scholar] [CrossRef]
- Aza, P.; de Salas, F.; Molpeceres, G.; Rodríguez-Escribano, D.; de la Fuente, I.; Camarero, S. Protein Engineering Approaches to Enhance Fungal Laccase Production in S. Cerevisiae. Int. J. Mol. Sci. 2021, 22, 1157. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Eugenio, M.E.; Tomás-Pejó, E. Laccases as versatile enzymes: From industrial uses to novel applications. J. Chem. Technol. Biotechnol. 2020, 95, 481–494. [Google Scholar] [CrossRef]
- de Freitas, E.N.; Alnoch, R.C.; Contato, A.G.; Nogueira, K.M.V.; Crevelin, E.J.; de Moraes, L.A.B.; Silva, R.N.; Martínez, C.A.; Polizeli, M.d.L.T.M. Enzymatic Pretreatment with Laccases from Lentinus sajor-caju Induces Structural Modification in Lignin and Enhances the Digestibility of Tropical Forage Grass (Panicum maximum) Grown under Future Climate Conditions. Int. J. Mol. Sci. 2021, 22, 9445. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zeng, G.; Tan, Z.; Jiang, M.; Li, H.; Liu, L.; Zhu, Y.; Yu, Z.; Wei, Z.; Liu, Y.; et al. Understanding Lignin-Degrading Reactions of Ligninolytic Enzymes: Binding Affinity and Interactional Profile. PLoS ONE 2011, 6, e25647. [Google Scholar] [CrossRef] [Green Version]
- Khanal, S.K.; Tarafdar, A.; You, S. Artificial Intelligence and Machine Learning for Smart Bioprocesses. Bioresour. Technol. 2023, 375, 128826. [Google Scholar] [CrossRef]
- Sparlinek, L.; Leitner, V.; Kamm, B. Statistical Optimisation of Enzymatic Detoxification with Laccase from Trametes versicolor for Spent Sulphite Liquors Using a Novel In-Situ NMR Method. J. Biotechnol. 2018, 284, 63–67. [Google Scholar] [CrossRef]
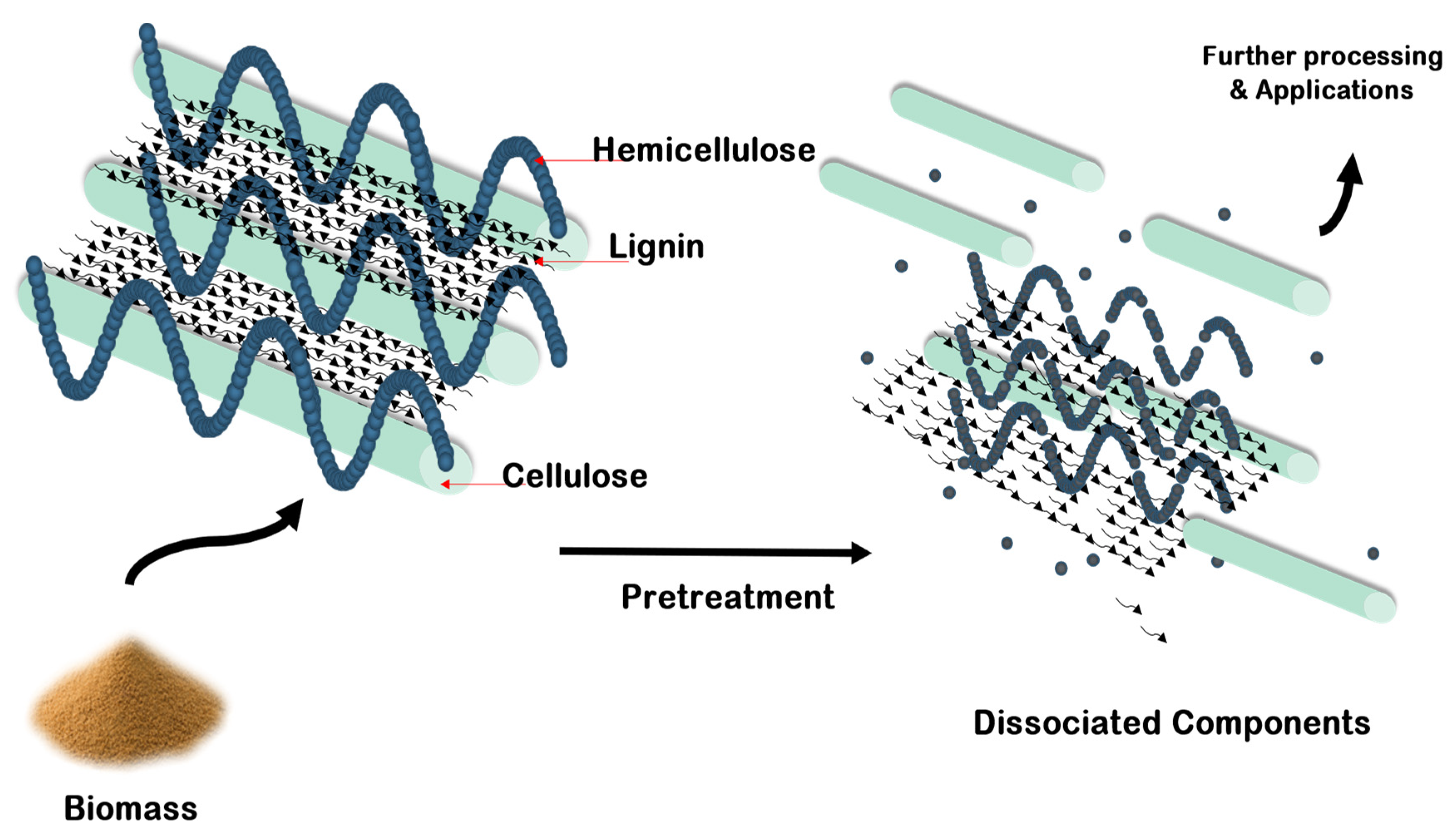
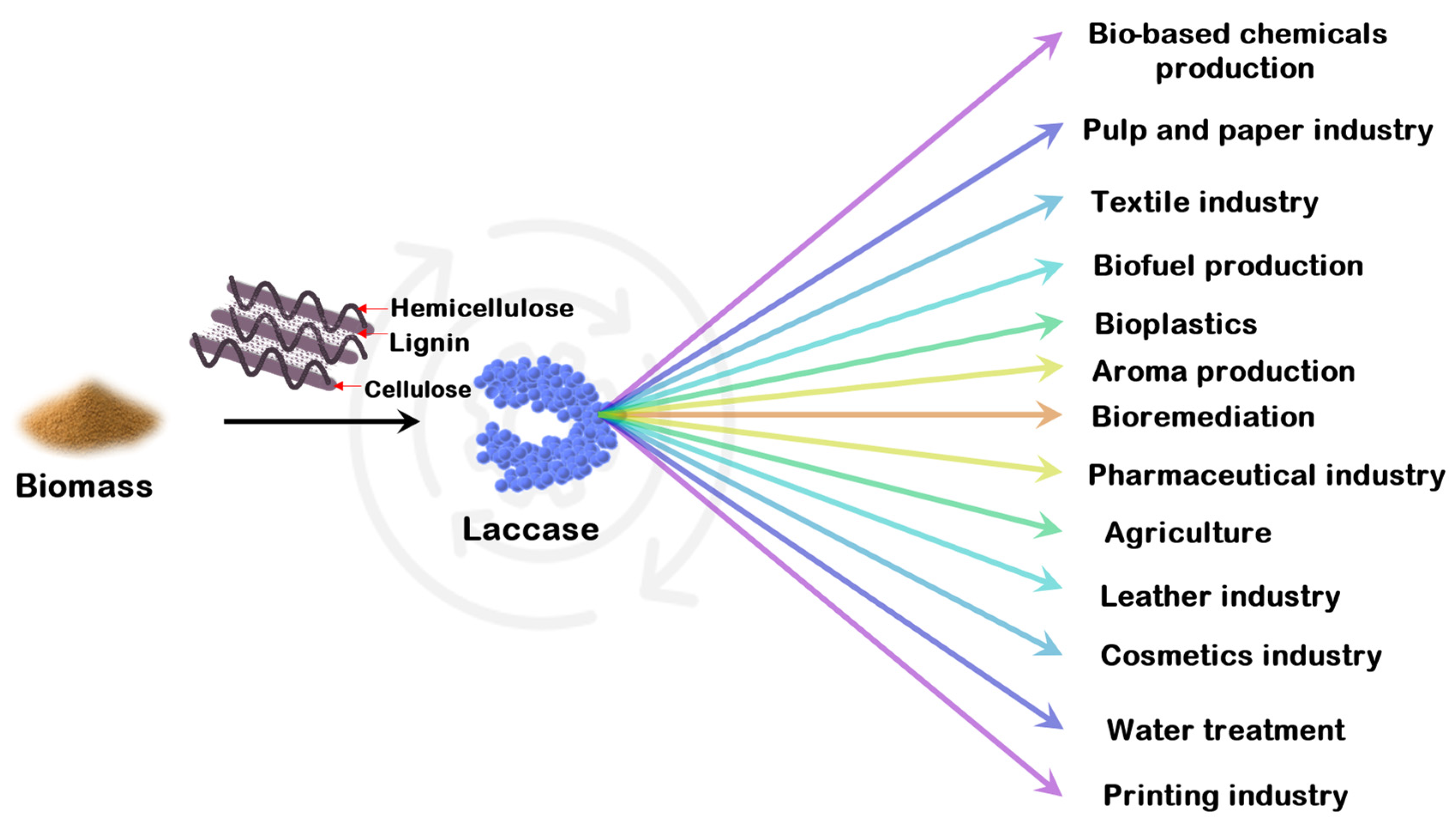
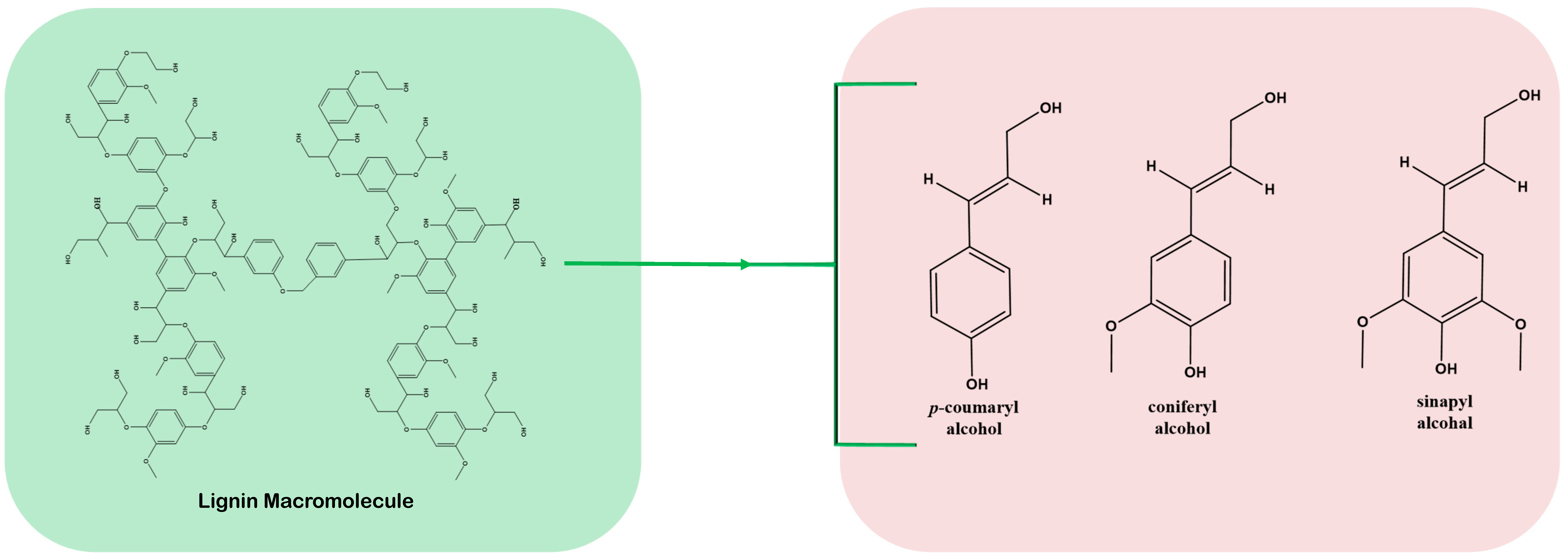
| Pretreatment Technique | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Biological Pretreatment | Most biological pretreatment methods utilize the ligninolytic enzyme system, which consists of oxidoreductases that can break down lignin, thereby enhancing biomass degradation. | Environmentally friendly and sustainable; can be conducted at low temperatures and pressures; generates fewer inhibitor compounds | Slow process; may require multiple stages; limited applicability to certain types of biomasses; high enzyme costs |
| Hydrothermal Pretreatment | Water at subcritical temperature acts as a catalyst as it changes into the hydronium ion, the pH becomes acidic and liquifies the cellulose and hemicellulose to an extent. | Environmentally friendly and sustainable; can be conducted without chemicals or solvents; can improve the accessibility of cellulose; can produce high sugar and ethanol yields | Requires a significant energy input; may generate significant amounts of inhibitor compounds; limited effect on lignin |
| Organosolv Pretreatment | Dissolves lignin and hemicellulose, leaving behind solid crystalline cellulose. | Can selectively extract lignin and hemicellulose; yields high-quality lignin that can be used for high-value products; can be conducted at low temperatures and pressures; generates fewer inhibitor compounds than acid pretreatments | Requires the use of organic solvents, which can be expensive and hazardous; may generate significant amounts of waste; can be energy-intensive; limited scalability |
| Dilute Acid Pretreatment | Uses dilute acid to selectively remove hemicellulose and increase the accessibility of cellulose. | More environmentally friendly than concentrated acid pretreatments; can selectively remove hemicellulose and increase the accessibility of cellulose; can produce high sugar and ethanol yields | Generates significant amounts of inhibitor compounds; requires a large amount of water and energy input; limited applicability to certain types of biomasses; may require multiple stages |
| Alkali Pretreatment | Alkali pretreatment changes the lignocellulosic structure by causing cellulose swelling, reducing crystallinity, and increasing polymerization. The internal surface area is enhanced due to the removal of acetyl groups and uronic acids from hemicellulose. | Can be effective at breaking down lignin and hemicellulose; can increase accessibility to cellulose; can produce high sugar and ethanol yields | May require significant amounts of water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, A.; Chen, C.-W.; Haldar, D.; Patel, A.K.; Dong, C.-D.; Singhania, R.R. Laccase in Biorefinery of Lignocellulosic Biomass. Appl. Sci. 2023, 13, 4673. https://doi.org/10.3390/app13084673
Tiwari A, Chen C-W, Haldar D, Patel AK, Dong C-D, Singhania RR. Laccase in Biorefinery of Lignocellulosic Biomass. Applied Sciences. 2023; 13(8):4673. https://doi.org/10.3390/app13084673
Chicago/Turabian StyleTiwari, Ashutosh, Chiu-Wen Chen, Dibyajyoti Haldar, Anil Kumar Patel, Cheng-Di Dong, and Reeta Rani Singhania. 2023. "Laccase in Biorefinery of Lignocellulosic Biomass" Applied Sciences 13, no. 8: 4673. https://doi.org/10.3390/app13084673







