Effect of Zr Modification on NH3-SCR Reaction Performance of Cu-Ce/SAPO-34 Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Experimental Conditions and Equipment
2.2.1. Catalytic Activity Test
2.2.2. Characterization of the Catalyst
3. Results and Discussion
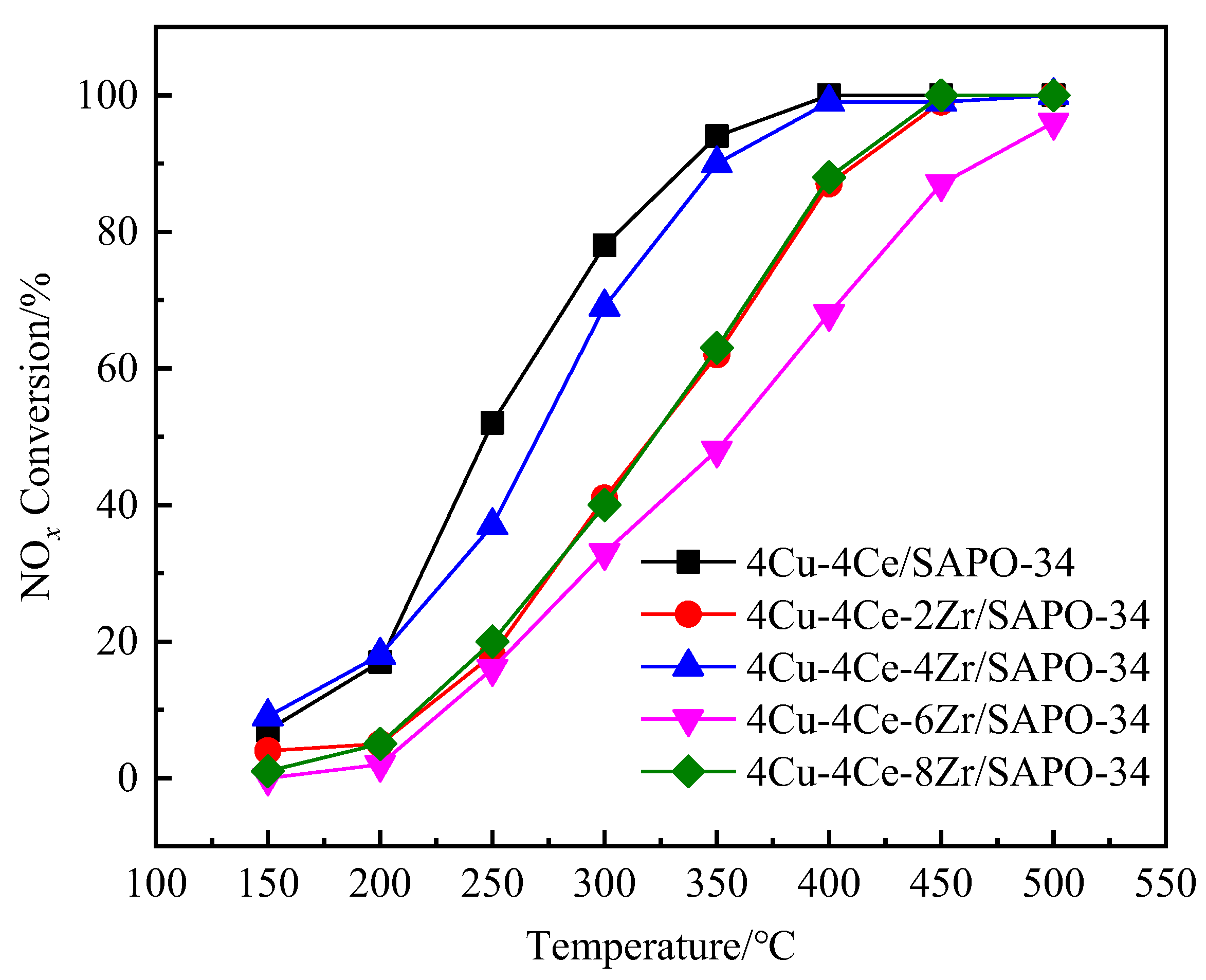
3.1. NH3-SCR Catalytic Activity
3.2. XRD
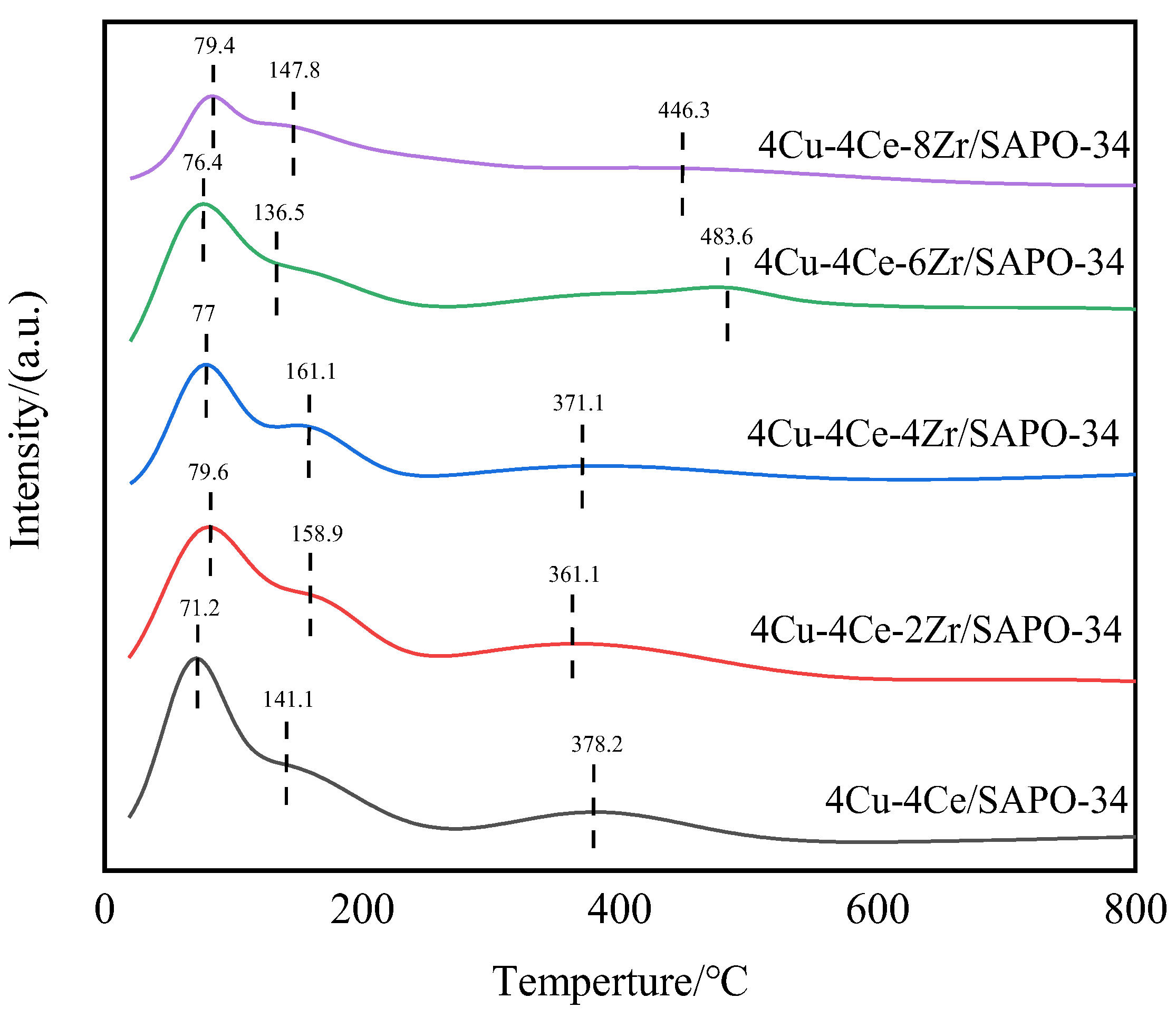
3.3. NH3-TPD
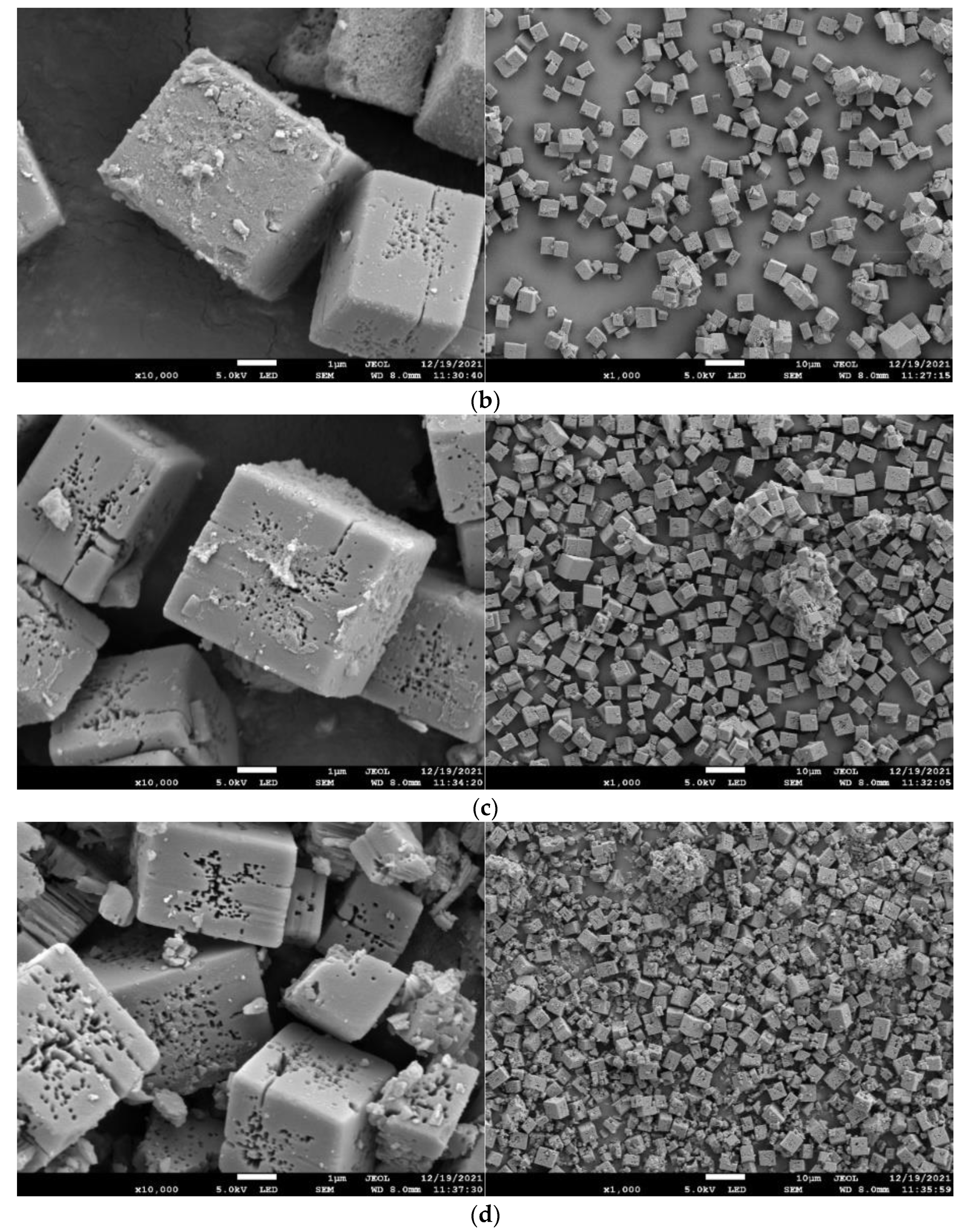
3.4. SEM
3.5. In Situ DRIFTS Measurements
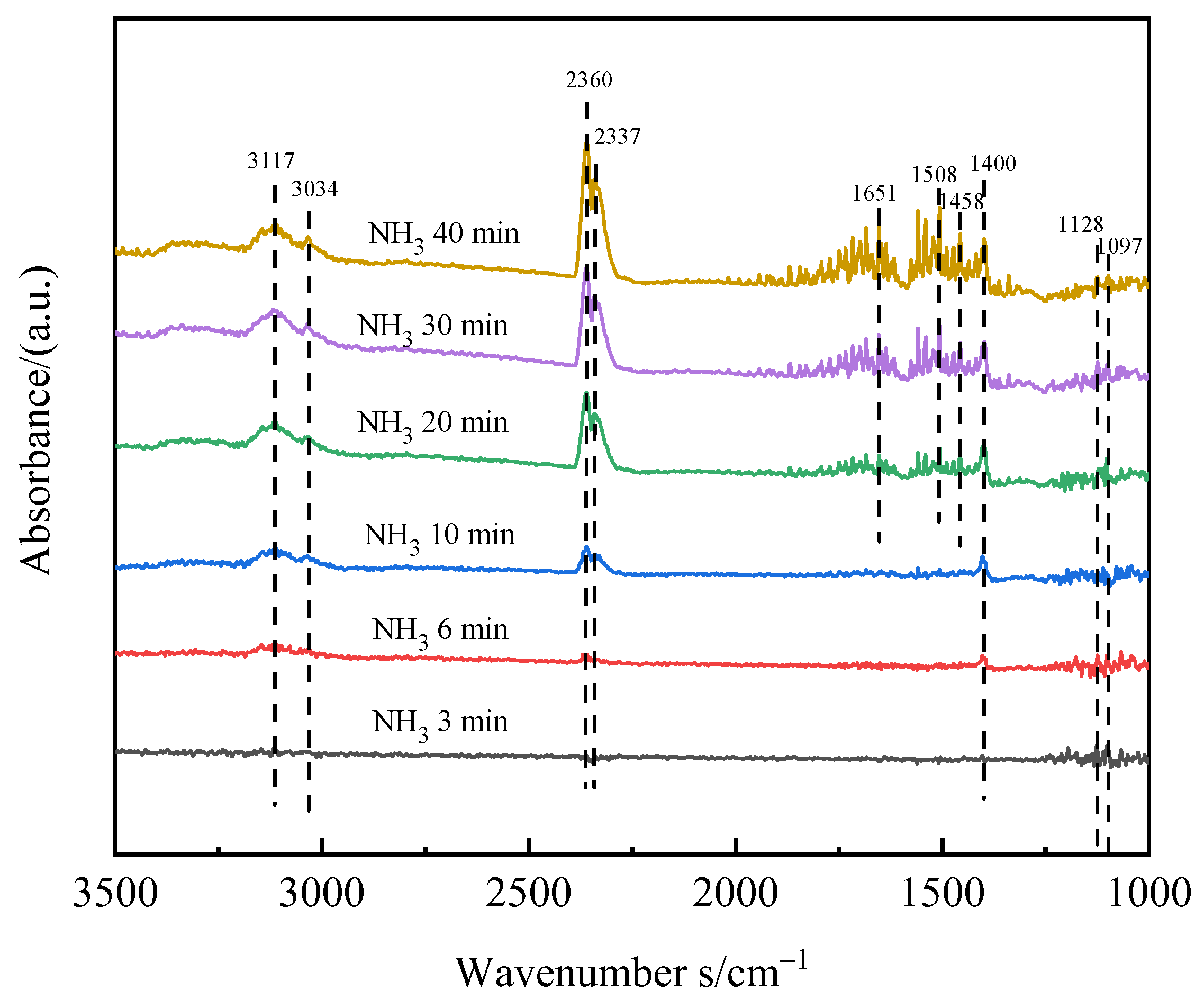
3.5.1. NH3 Adsorption Experiment
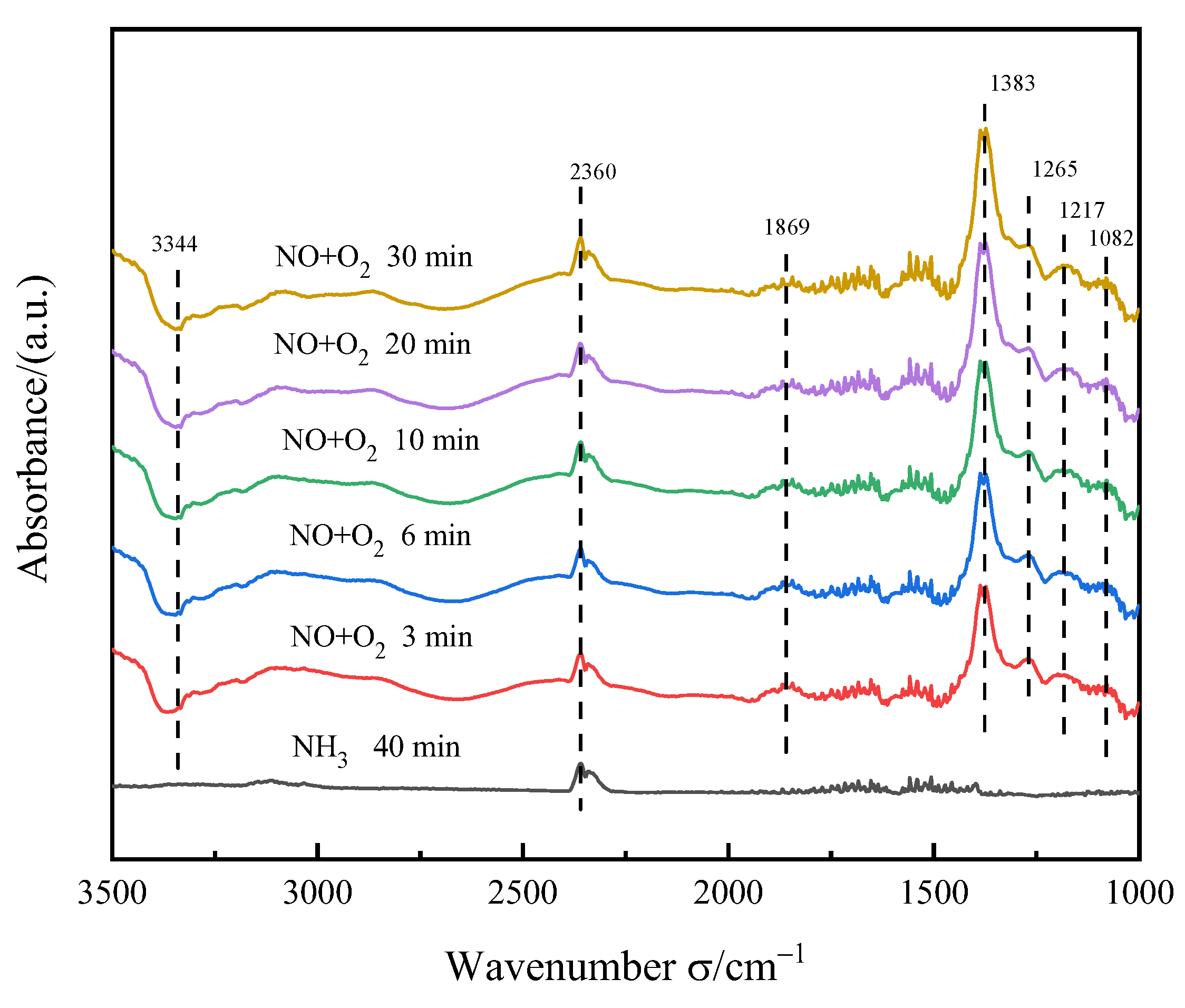
3.5.2. Transient Reaction of NO+O2 after NH3 Adsorption Saturation
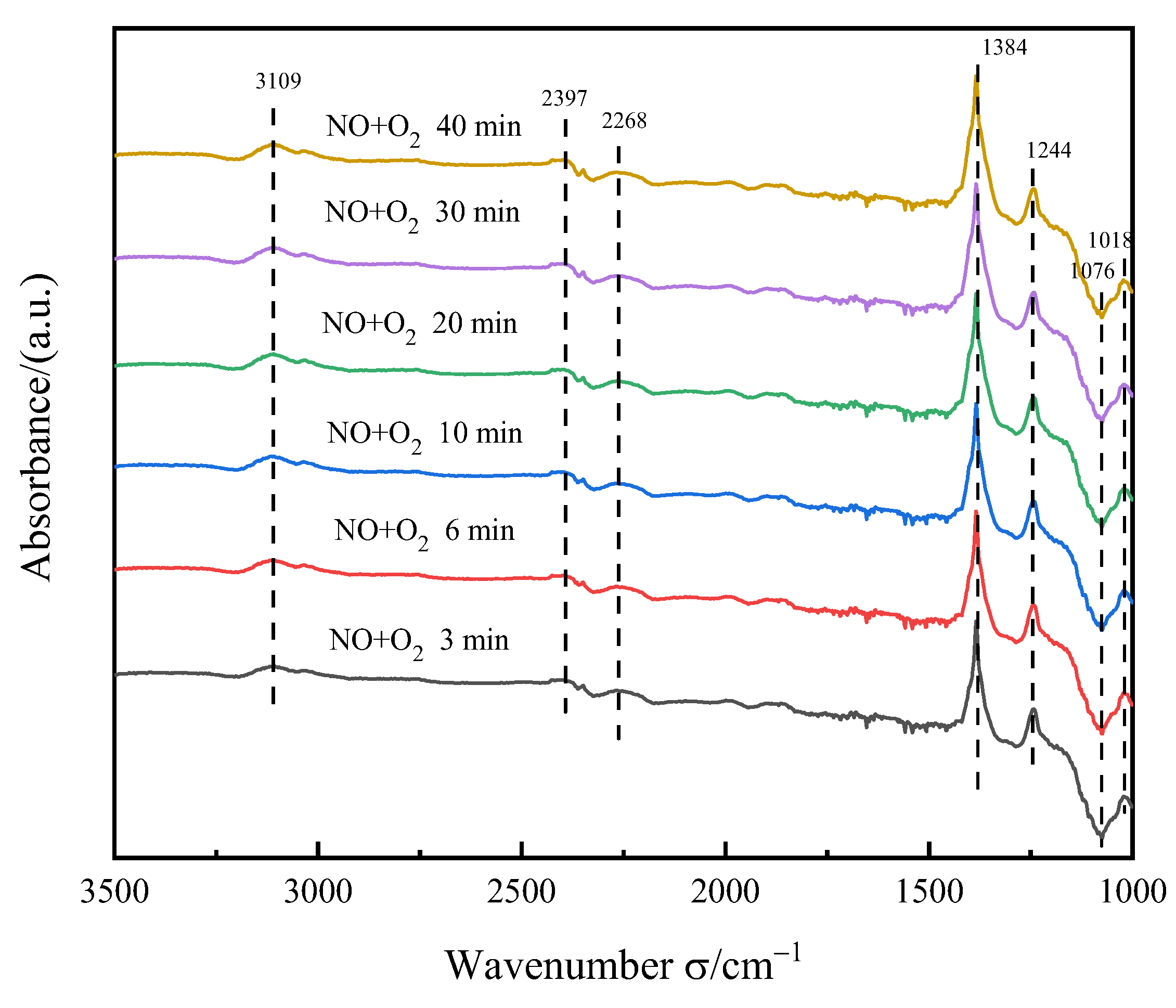
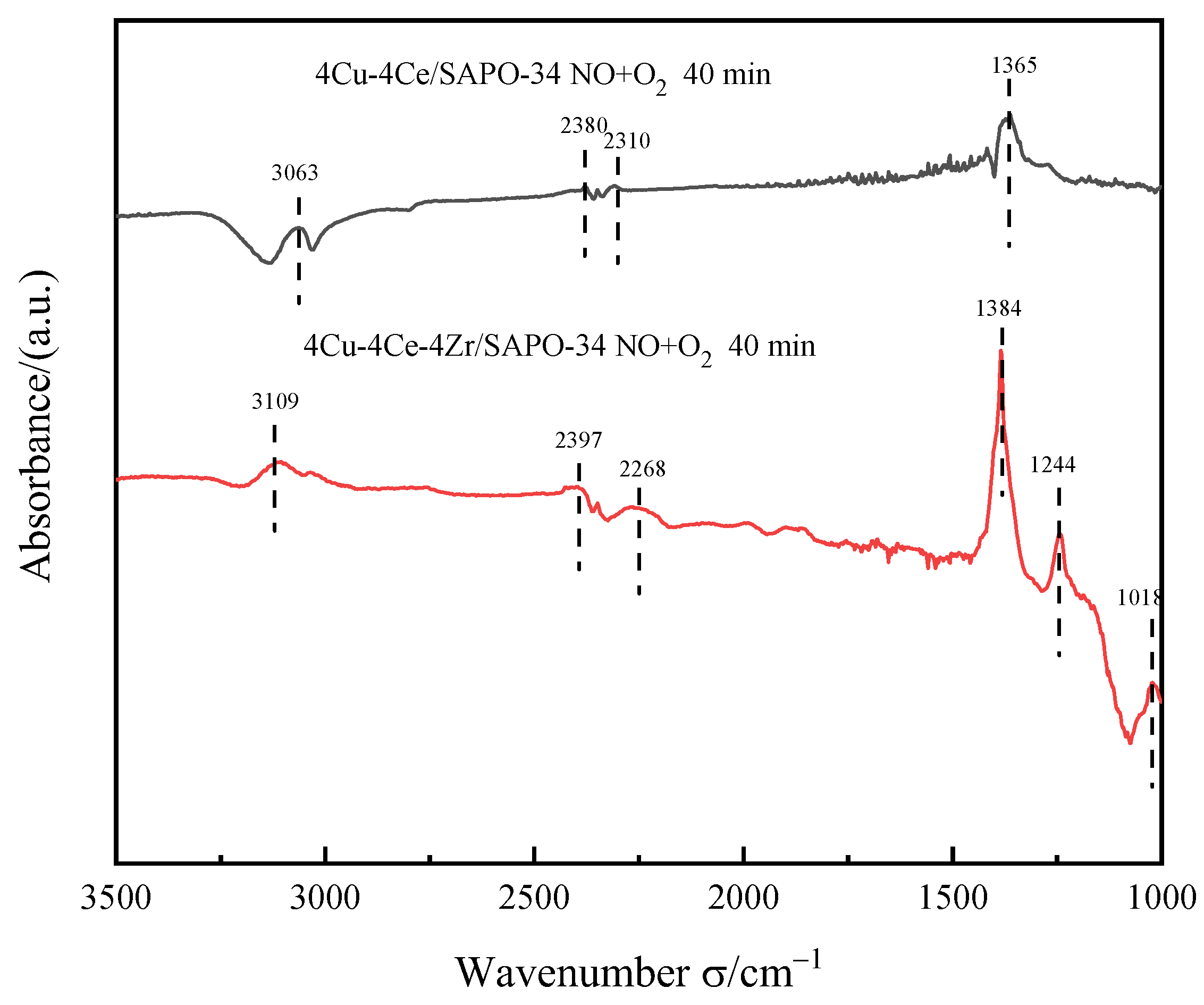
3.5.3. NO+O2 Adsorption Experiment
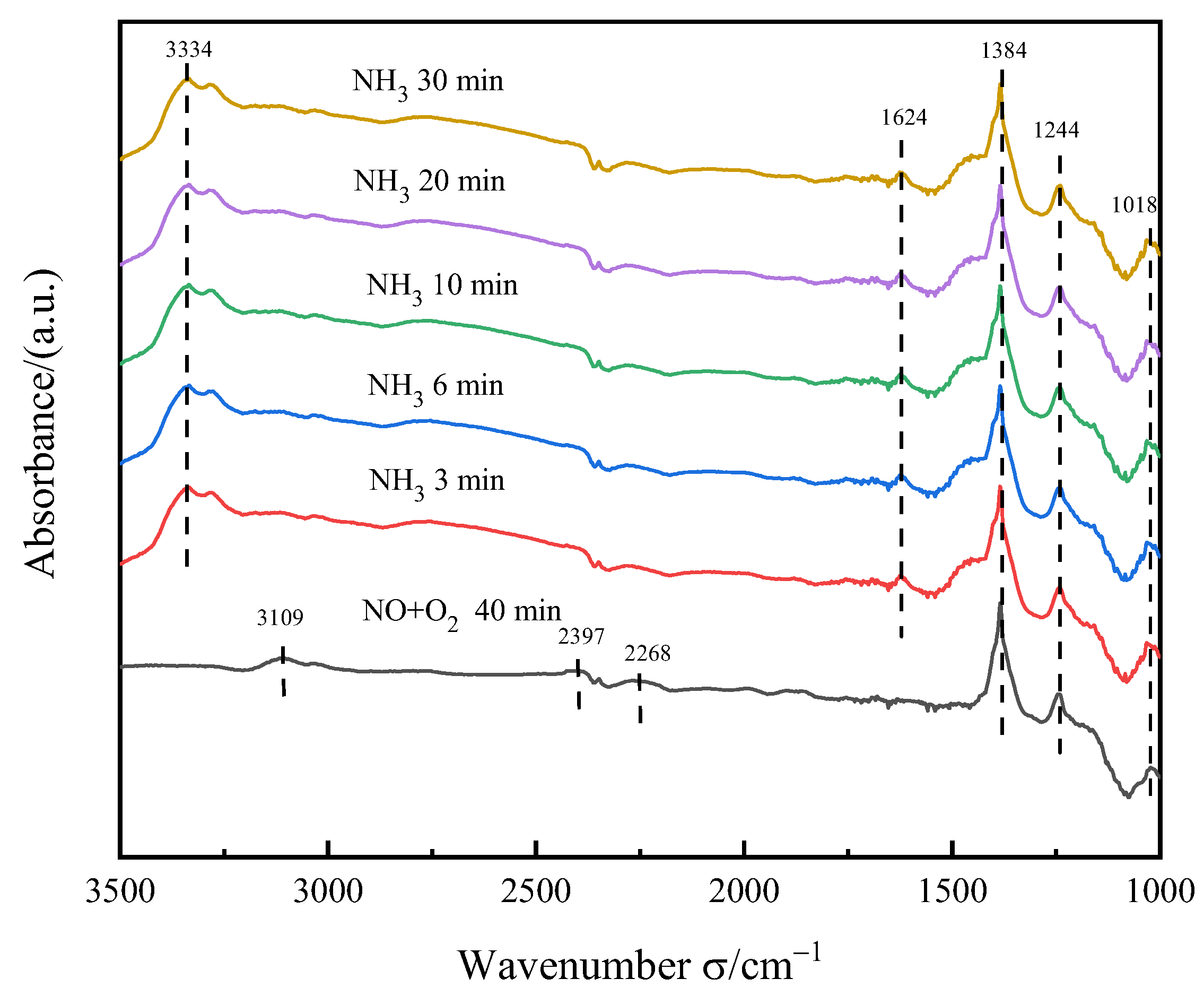
3.5.4. Transient Reaction of NH3 after NO+O2 Adsorption Saturation
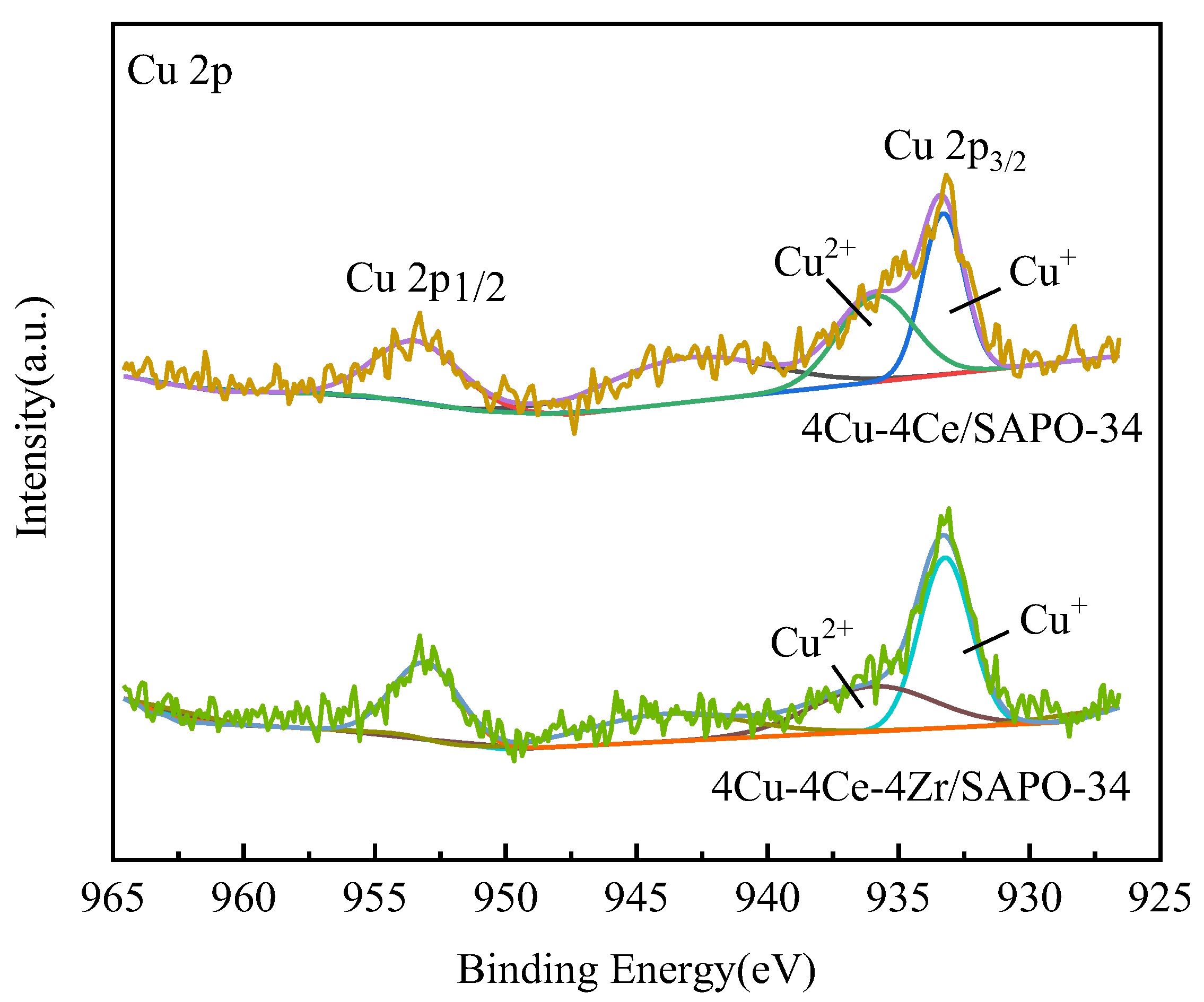
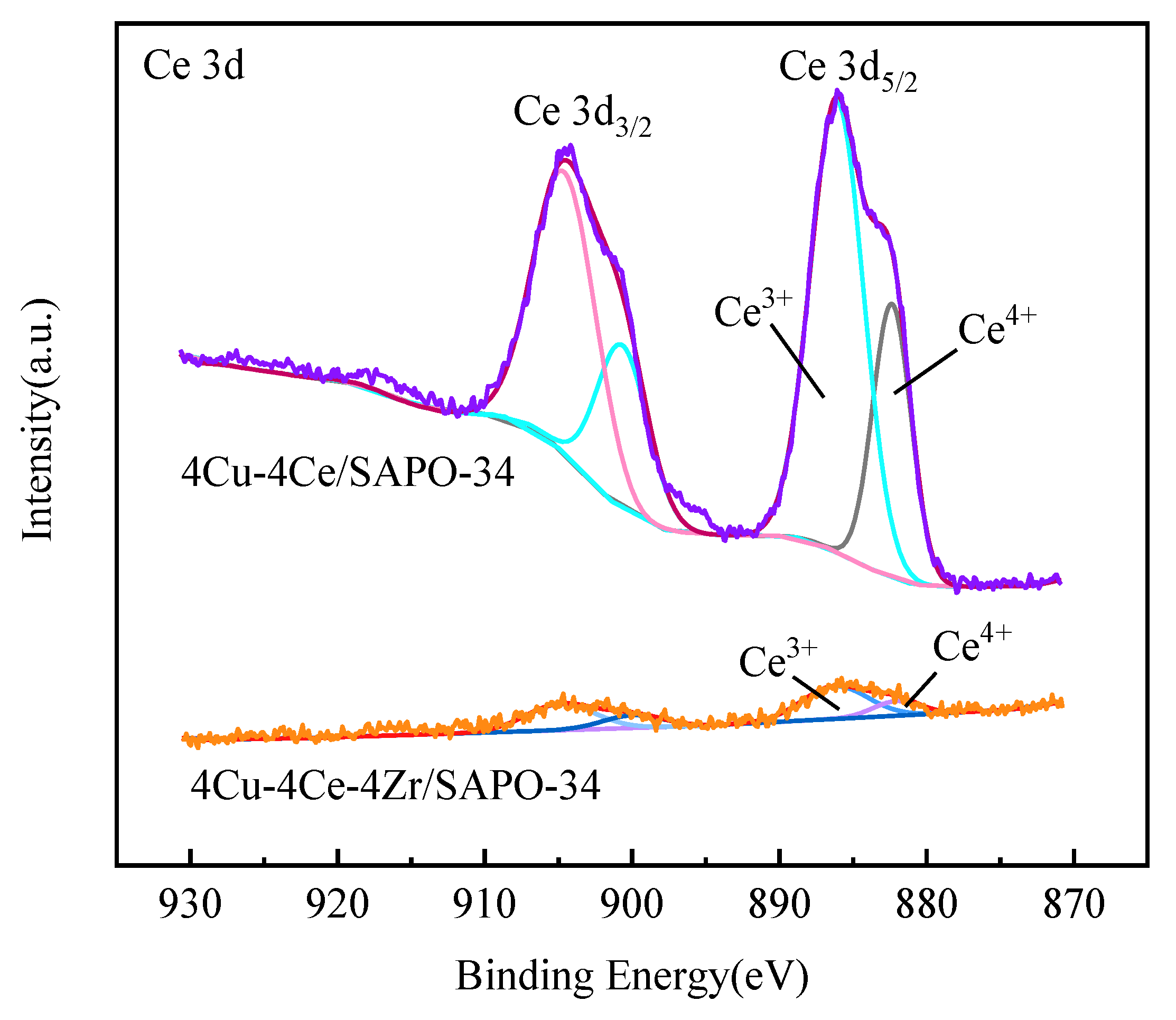
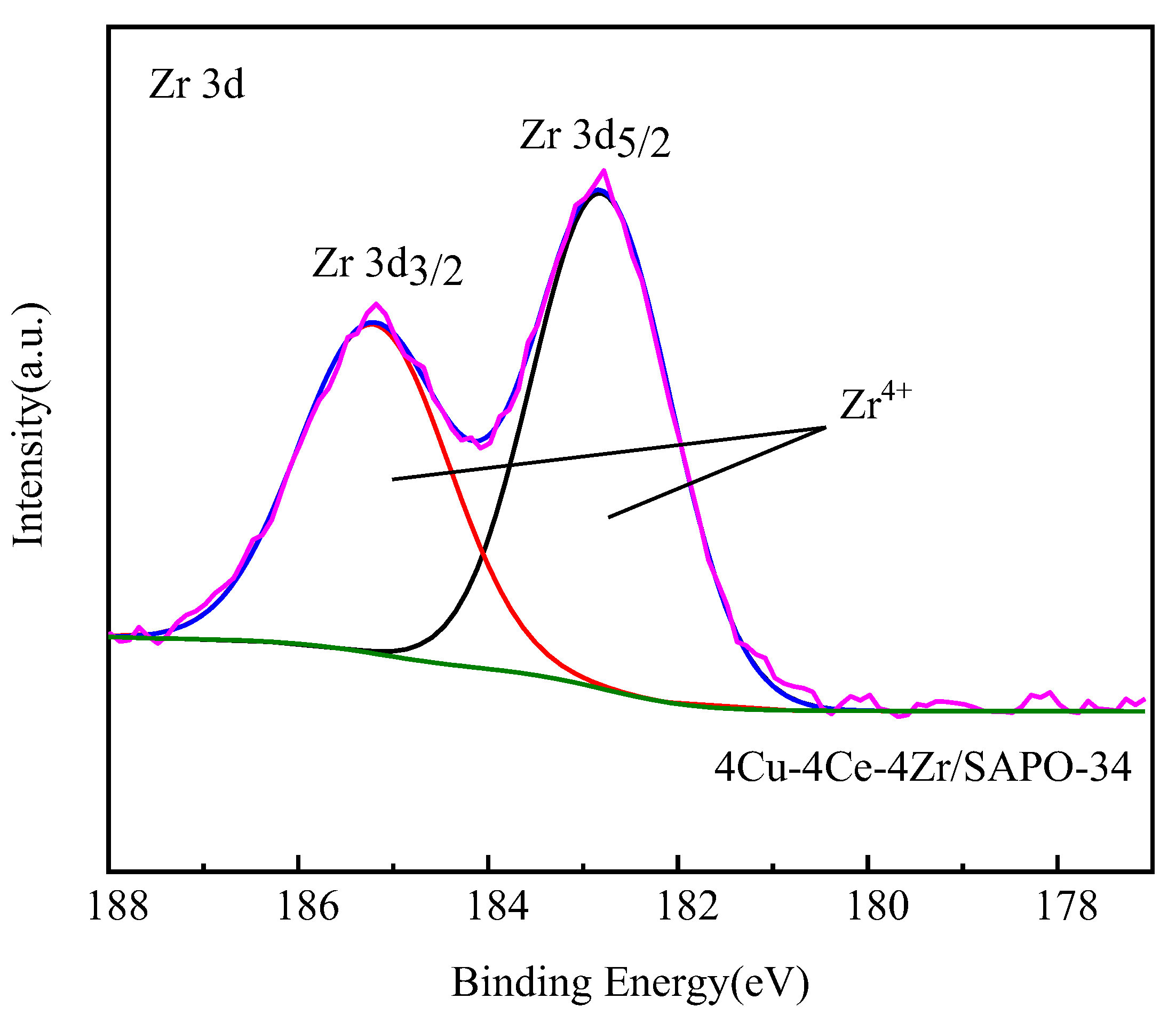
3.6. XPS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, D.; Hui, S.; Liu, C. Effect of the different types of titanium dioxide carrier on the DeNOX performance of V-Ti catalysts. Environ. Chem. 2014, 33, 5. [Google Scholar]
- Meng, L.; Fang, J.; Guan, X. Effect of Active Component Doping on the Denitration Properties of Manganese Based Catalyst at Low Temperature. Mater. Rev. 2017, 31, 35–39, 56. [Google Scholar]
- Wu, Y.; Liang, H.; Chen, X.; Chen, C.; Wang, X.; Dai, C.; Hu, L.; Chen, Y. Effect of preparation methods on denitration performance of V-Mo/TiO2 catalyst. J. Fuel Chem. Technol. 2020, 48, 189–196. [Google Scholar] [CrossRef]
- Chen, G.; Fang, J.; Ma, T.; Wang, L. Effect of Different Precursor Solution on the Denitrification Performance of Mn/TiO2 Catalyst. Bull. Chin. Ceram. Soc. 2018, 37, 2274–2279. [Google Scholar]
- Wang, A.; Wang, Y.; Walter, E.D.; Washton, N.M.; Guo, Y.; Lu, G.; Peden, C.H.F.; Gao, F. NH3-SCR on Cu, Fe and Cu+Fe exchanged beta and SSZ-13 catalysts: Hydrothermal aging and propylene poisoning effects. Catal. Today 2019, 320, 91–99. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, S.; Qiu, L.; Gao, Y.; O’Hare, D.; Wang, Q. The synthesis of CuyMnzAl1-zOx mixed oxide as low-temperature NH3-SCR catalyst with enhanced catalytic performance. Dalton Trans. 2017, 47, 2992–3004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Huang, L.; Jiang, B.; Chen, M.; Zhang, J.; Hu, Y. Stability of Cu–Mn bimetal catalysts based on different zeolites for NOX removal from diesel engine exhaust. Chin. J. Catal. 2018, 39, 800–809. [Google Scholar] [CrossRef]
- Albert, K.B.; Fan, C.; Pang, L.; Chen, Z.; Ming, S.; Albert, T.; Li, T. The influence of chemical poisoning, hydrothermal aging and their co-effects on Cu-SAPO-34 catalyst for NOX reduction by NH3-SCR. Appl. Surf. Sci. 2019, 479, 1200–1211. [Google Scholar] [CrossRef]
- Cao, Y.; Zou, S.; Lan, L.; Yang, Z.; Xu, H.; Lin, T.; Gong, M.; Chen, Y. Promotional effect of Ce on Cu-SAPO-34 monolith catalyst for selective catalytic reduction of NOX with ammonia. J. Mol. Catal. A Chem. 2015, 398, 304–311. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, C.; Lin, J.; Yang, H.; Zhou, R. Promotional effects of cerium modification of Cu-USY catalysts on the low-temperature activity of NH3-SCR. Catal. Commun. 2018, 114, 60–64. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, X.; Ma, C.; Wang, X.; Wang, Z. Effect of a ZrO2 support on Cu/Fe2O3–CeO2/ZrO2 catalysts for NO removal by CO using a rotary reactor. Catal. Sci. Technol. 2018, 8, 5623–5631. [Google Scholar] [CrossRef]
- Xue, H.; Meng, T.; Liu, F.; Guo, X.; Wang, S.; Mao, D. Enhanced resistance to calcium poisoning on Zr-modified Cu/ZSM-5 catalysts for the selective catalytic reduction of NO with NH3. RSC Adv. 2019, 9, 38477–38485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wang, J.; Wang, J.; Wang, J.; Shen, M. Promotional effect of ion-exchanged K on the low-temperature hydrothermal stability of Cu/SAPO-34 and its synergic application with Fe/Beta catalysts. Front. Environ. Sci. Eng. 2021, 15, 30. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, D.; Yang, Y.; Jiang, X.; Tian, Y.; Ding, T.; Li, X. Influence of copper locations on catalytic properties and activities of Cu/SAPO-34 in C3H6-SCR. Ind. Eng. Chem. Res. 2021, 60, 6940–6949. [Google Scholar] [CrossRef]
- Salah Aldeen, O.D.A.; Mahmoud, M.Z.; Majdi, H.S.; Mutlak, D.A.; Uktamov, K.F.; Kianfar, E. Investigation of Effective Parameters Ce and Zr in the Synthesis of H-ZSM-5 and SAPO-34 on the Production of Light Olefins from Naphtha. Adv. Mater. Sci. Eng. 2022, 2022, 6165180. [Google Scholar] [CrossRef]
- Huang, F.; Cao, J.; Wang, L.; Wang, X.; Liu, F. Enhanced catalytic behavior for methanol to lower olefins over SAPO-34 composited with ZrO2. Chem. Eng. J. 2020, 380, 122626. [Google Scholar] [CrossRef]
- Aghaei, E.; Haghighi, M.; Pazhohniya, Z.; Aghamohammadi, S. One-pot hydrothermal synthesis of nanostructured ZrAPSO-34 powder: Effect of Zr-loading on physicochemical properties and catalytic performance in conversion of methanol to ethylene and propylene. Microporous Mesoporous Mater. 2016, 226, 331–343. [Google Scholar] [CrossRef]
- Varzaneh, A.Z.; Towfighi, J.; Mohamadalizadeh, A. Comparative study of naphtha cracking over SAPO-34 and HZSM-5: Effects of cerium and zirconium on the catalytic performance. J. Anal. Appl. Pyrolysis 2014, 107, 165–173. [Google Scholar] [CrossRef]
- Tian, S.; Ji, S.; Lü, D.; Bai, B.; Sun, Q. Preparation of modified Ce-SAPO-34 catalysts and their catalytic performances of methanol to olefins. J. Energy Chem. 2013, 22, 605–609. [Google Scholar] [CrossRef]
- Bin, F.; Song, C.; Lv, G.; Song, J.; Wu, S.; Li, X. Selective catalytic reduction of nitric oxide with ammonia over zirconium-doped copper/ZSM-5 catalysts. Appl. Catal. B Environ. 2014, 150, 532–543. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Y.; Luo, H.; Liu, Z.; Tong, Z.; Tao, C.; Du, J. Ce regulated surface properties of Mn/SAPO-34 for improved NH3-SCR at low temperature. RSC Adv. 2020, 10, 40047–40054. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, C.; Zhao, Z.; Liu, Q.; Xu, G.; Wu, M.; Chen, J.; Li, J. A facile and controllable in situ sulfation strategy for CuCeZr catalyst for NH3-SCR. Appl. Catal. A Gen. 2020, 597, 117554. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, G.; Liu, Z.; Liu, Y. A Combined in-situ Diffuse Reflectance FTIR and On-line Mass Spectroscopy Study of Surface Acidity and Reactivity over a CuO/Al2O3 Catalyst. Acta Chim. Sin. 2008, 66, 1021–1027. [Google Scholar]
- Zhang, Q.; Wang, X. Preparation and properties of Ce-Mn/ZSM-5 catalysts modified with different metals. J. Fuel Chem. Technol. 2019, 47, 1265–1272. [Google Scholar]
- Guo, J.; Yang, W.; Zhang, Y.; Gan, L.; Fan, C.; Chen, J.; Peng, Y.; Li, J. A multiple-active-site Cu/SSZ-13 for NH3-SCO: Influence of Si/Al ratio on the catalytic performance. Catal. Commun. 2020, 135, 105751. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, J.; Ning, P.; Song, Z.; Liu, X.; Wang, L.; Wang, J.; Wang, H.; Long, K. In situ DRIFTS investigation of NH3-SCR reaction over CeO2/zirconium phosphate catalyst. Appl. Surf. Sci. 2018, 435, 1037–1045. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.; He, B.; Yan, Z.; Wang, H.; Liu, L.; Wang, X. In situ DRIFTS investigation on CeOx catalyst supported by fly-ash-made porous cordierite ceramics for low-temperature NH3-SCR of NOX. Catalysts 2019, 9, 496. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Li, L.; Jin, L.; Wu, Y.; Liu, H.; Qin, Q.; Wei, X.; Liu, J.; Dong, L.; Li, B. Low temperature high activity of M (M = Ce, Fe, Co, Ni) doped M-Mn/TiO2 catalysts for NH3-SCR and in situ DRIFTS for investigating the reaction mechanism. Appl. Surf. Sci. 2020, 515, 146014. [Google Scholar] [CrossRef]
- Zhang, L.; Pierce, J.; Leung, V.L.; Wang, D.; Epling, W.S. Characterization of Ceria’s Interaction with NOX and NH3. J. Phys. Chem. C 2013, 117, 8282–8289. [Google Scholar] [CrossRef]
- Gao, C.; Yang, G.; Huang, X.; Yang, Q.; Li, B.; Wang, D.; Peng, Y.; Li, J.; Lu, C.; Crittenden, J. Key intermediates from simultaneous removal of NOx and chlorobenzene over a V2O5–WO3/TiO2 catalyst: A combined experimental and DFT study. Catal. Sci. Technol. 2021, 11, 7260–7267. [Google Scholar] [CrossRef]
- Liang, H.; Gui, K.; Zha, X. DRIFTS study of γFe2O3 nano-catalyst for low-temperature selective catalytic reduction of NOX with NH3. Can. J. Chem. Eng. 2016, 94, 1668–1675. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; Petkov, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared spectra of surface nitrates: Revision of the current opinions based on the case study of ceria. J. Catal. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- Weng, X.; Dai, X.; Zeng, Q.; Liu, Y.; Wu, Z. DRIFT studies on promotion mechanism of H3PW12O40 in selective catalytic reduction of NO with NH3. J. Colloid Interface Sci. 2016, 461, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Morandi, S.; Prinetto, F.; Ghiotti, G.; Castoldi, L.; Lietti, L.; Forzatti, P.; Daturi, M.; Blasin-Aubé, V. The influence of CO2 and H2O on the storage properties of Pt-Ba/Al2O3 LNT catalyst studied by FT-IR spectroscopy and transient microreactor experiments. Catal. Today 2014, 231, 116–124. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, J.; Zheng, R.; Guo, L.; Yuan, J.; Zhang, S.; Gu, M. Insight into the reaction mechanism over PMoA for low temperature NH3-SCR: A combined In-situ DRIFTs and DFT transition state calculations. J. Hazard. Mater. 2021, 412, 125258. [Google Scholar] [CrossRef]
- Chen, L.; Si, Z.; Wu, X.; Weng, D. DRIFT study of CuO–CeO2–TiO2 mixed oxides for NOX reduction with NH3 at low temperatures. ACS Appl. Mater. Interfaces 2014, 6, 8134–8145. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Z.; Liu, Y.; Guo, G.; Dai, H.; Cui, S.; Deng, J. Support promotion effect on the SO2 and K+ co-poisoning resistance of MnO2/TiO2 for NH3-SCR of NO. J. Hazard. Mater. 2021, 416, 126117. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, Z.; Qiao, H.; Yu, H.; Hu, Y.; Chang, L.; Bao, W. Cerium stabilized Cu-SSZ-13 Catalyst for the Catalytic Removal of NOX by NH3. Ind. Eng. Chem. Res. 2016, 55, 1174–1182. [Google Scholar] [CrossRef]
- Wilken, N.; Nedyalkova, R.; Kamasamudram, K.; Li, J.; Currier, N.W.; Vedaiyan, R.; Yezerets, A.; Olsson, L. Investigation of the Effect of Accelerated Hydrothermal Aging on the Cu Sites in a Cu-BEA Catalyst for NH3-SCR Applications. Top. Catal. 2013, 56, 317–322. [Google Scholar] [CrossRef]
- He, P.; Shen, D.; Liu, G. NH3-SCR performance of modified SAPO-34 molecular sieve. J. Southeast Univ. (Nat. Sci. Ed.) 2017, 47, 513–520. [Google Scholar]
- Chen, D.; Yan, Y.; Guo, A.; Rizzotto, V.; Lei, H.; Qiao, Z.; Liang, H.; Jabłońska, M.; Jiang, X.; Jiang, J.; et al. Mechanistic insights into the promotion of low-temperature NH3-SCR catalysis by copper auto-reduction in Cu-zeolites. Appl. Catal. B Environ. 2023, 322, 122118. [Google Scholar] [CrossRef]
- Yan, Q.; Gao, Y.; Li, Y.; Vasiliades, M.A.; Chen, S.; Zhang, C.; Gui, R.; Wang, Q.; Zhu, T.; Efstathiou, A.M. Promotional effect of Ce doping in Cu4Al1OX–LDO catalyst for low-T practical NH3-SCR: Steady-state and transient kinetics studies. Appl. Catal. B Environ. 2019, 255, 117749. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Fu, Y.; Zhong, Y.; Luo, Z.; Cen, K. Preparation and characterization of CeO2/TiO2 catalysts for selective catalytic reduction of NO with NH3. Catal. Commun. 2010, 11, 465–469. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, X.; Yang, Y.; Cui, X.; Chen, T.; Wang, Y. M (M = Mn, Co, Cu)-CeO2 catalysts to enhance their CO catalytic oxidation at a low temperature: Synergistic effects of the interaction between Ce3+-Mx+-Ce4+ and the oxygen vacancy defects. Fuel 2022, 323, 124379. [Google Scholar] [CrossRef]
- Raveendra, G.; Li, C.; Liu, B.; Cheng, Y.; Meng, F.; Zhong, L. Synthesis of lower olefins from syngas over Zn/Al2O3–SAPO-34 hybrid catalysts: Role of doped Zr and influence of the Zn/Al2O3 ratio. Catal. Sci. Technol. 2018, 8, 3527–3538. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Liu, H.; Jia, W.; Yu, X.; Wang, S.; Zeng, X.; Sun, Y.; Wei, J.; Tang, X.; et al. Domino transformation of furfural to γ-valerolactone over SAPO-34 zeolite supported zirconium phosphate catalysts with tunable Lewis and Brønsted acid sites. Mol. Catal. 2021, 506, 111538. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wang, X.; Xing, L.; Cheng, X.; Zhang, X.; Li, H.; Liu, M. Effect of Zr Modification on NH3-SCR Reaction Performance of Cu-Ce/SAPO-34 Catalysts. Appl. Sci. 2023, 13, 4763. https://doi.org/10.3390/app13084763
Liu C, Wang X, Xing L, Cheng X, Zhang X, Li H, Liu M. Effect of Zr Modification on NH3-SCR Reaction Performance of Cu-Ce/SAPO-34 Catalysts. Applied Sciences. 2023; 13(8):4763. https://doi.org/10.3390/app13084763
Chicago/Turabian StyleLiu, Chongfei, Xuetao Wang, Lili Xing, Xingxing Cheng, Xingyu Zhang, Haojie Li, and Mengjie Liu. 2023. "Effect of Zr Modification on NH3-SCR Reaction Performance of Cu-Ce/SAPO-34 Catalysts" Applied Sciences 13, no. 8: 4763. https://doi.org/10.3390/app13084763




