Mycoremediation as a Potentially Promising Technology: Current Status and Prospects—A Review
Abstract
:1. Introduction
2. Classification of Fungi, Bacteria, and Microalgae Species and Their Remediation Performance
| Organism | Genus | Ref. |
|---|---|---|
| Cryptococcus | [18] | |
| Trichoderma | [19] | |
| Rhizopus | [20] | |
| Penicillium | [21] | |
| Mucor | [22] | |
| Fungi | Lasiodiplodia | [23] |
| Fusarium | [24] | |
| Drechslera | [24] | |
| Curvularia | [25] | |
| Aspergillus | [26] | |
| Selenastrum | [27] | |
| Nannochloropsis | [28] | |
| Synechocystis | [29] | |
| Chlorococcum | [30] | |
| Oscillatoria | [31] | |
| Microalgae/Cyanobacteria | Scenedesmus | [32] |
| Spirogyra | [33] | |
| Chlorella | [34] | |
| Spirulina | [35] | |
| Marinobacter | [28] | |
| Oleispira | [36] | |
| Cycloclasticus | [37] | |
| Thallassolituus | [38] | |
| Alcanivorax | [39] | |
| Bacteria | Pseuodmonas | [40] |
| Flavobacterium | [41] | |
| Enterobacter | [42] | |
| Bacillus | [43] | |
| Alcaligens | [44] |
2.1. Wood Rot Fungi
2.1.1. Brown Rot Fungi
2.1.2. White Rot Fungi
2.2. Leaf Decomposing Fungi
2.3. Endophytic Fungi
2.4. Mycorrhiza
2.5. Soil Fungi
3. Bioremediation (In Situ and Ex Situ)
3.1. Ex Situ Bioremediation
3.1.1. Slurry Phase
3.1.2. Solid Phase
Soil Biopiles
Composting
Land Farming
3.2. In Situ Bioremediation
3.2.1. Types of In Situ Bioremediation
Intrinsic Bioremediation
Enhanced (Engineered) In Situ Bioremediation
- Biosparging
- Bioaugmentation
- Bio-venting
- Bioslurping
- Phytoremediation
3.3. Merits and Demerits of Bioremediation
3.3.1. Merits of Bioremediation
3.3.2. Demerits of Bioremediation
4. Mycoremediation
Merits and Demerits of Mycoremediation
5. Comparative Analysis and Application of Bioremediation Technologies
- (i)
- Inspection of the PAH-contaminated site and its associated risk assessment involves examining the extent of PAH contamination based on their permissible levels.
- (ii)
- Selection of cost-effective, feasible, and environmentally friendly soil PAH degradation techniques. Based on recent research, Table 5 lists some of bioremediation techniques’ influencing parameters, merits, and demerits. To date, laboratory-scale treatment methods have been implemented successfully [145,146,147]. Several important factors must be considered when applying bioremediation techniques at the field scale, including (a) the physical and chemical properties of the contaminated soils, including their composition, temperature, water-to-soil ratio, environmental conditions, and oxygen availability [145]; (b) the activity, diversity, microbial community, resistance, and interaction; (c) the mass trajectories, toxicity, PAH concentration and interaction [146]. To optimize these parameters for field-scale applications, they must be adapted appropriately.
- (iii)
- A PAH-contaminated site requires a pretreatment and posttreatment assessment. This phase examines the biochemical conversion of PAH compounds after treatment, e.g., their removal or conversion to non-toxic compounds [147].
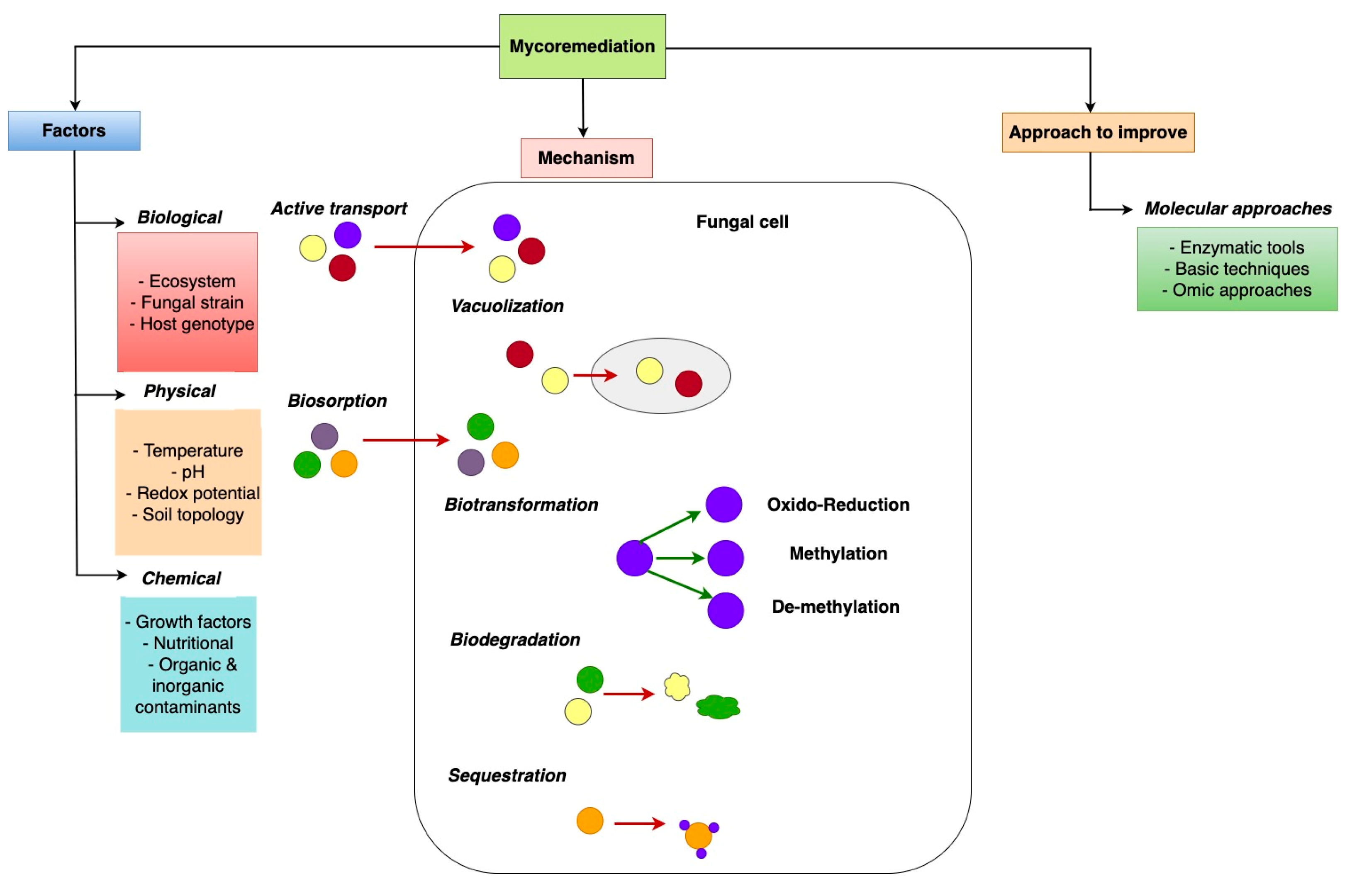
6. Mechanisms of Mycoremediation
- Avoidance reduces metal accumulation via absorption, precipitation, and biosorption, which lowers metal toxicity.
- Extrusion is the process of transporting contaminants out of the fungal biomass.
- Sequestration mechanisms involve synthesising intracellular chelating compounds and subsequent chelation in the fungal cells to dilute the contaminants.
- Biotransformation includes the reduction, oxidation, demethylation, methylation, and evaporation processes which convert toxic compounds and heavy metals (HMs) into less harmful forms.
6.1. Immobilisation Process
6.2. Mobilisation
6.3. Biosorption
6.4. Role of Fungal Enzymes in Mycoremediation
7. Factors Influencing Mycoremediation
7.1. Temperature
7.2. pH
7.3. Heavy Metals (HMs) Bonded with Hydrocarbon
8. Emerging Mycoremediation Processes
8.1. Myco-Nanotechnology
8.2. Molecular Approach to Improve Mycoremediation
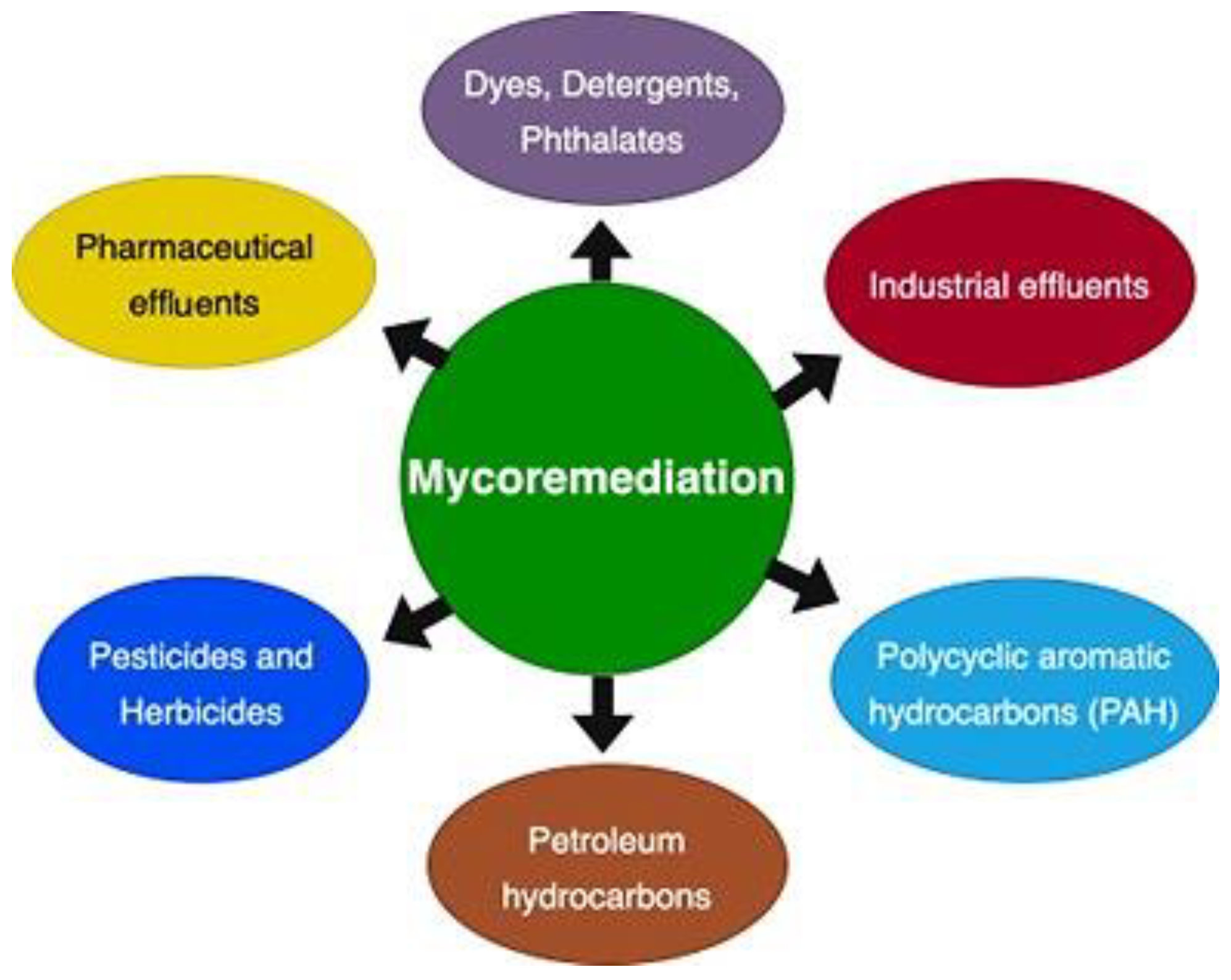
9. Emerging Mycoremediation Applications
9.1. Fungal Bioremediation of Industrial Effluents
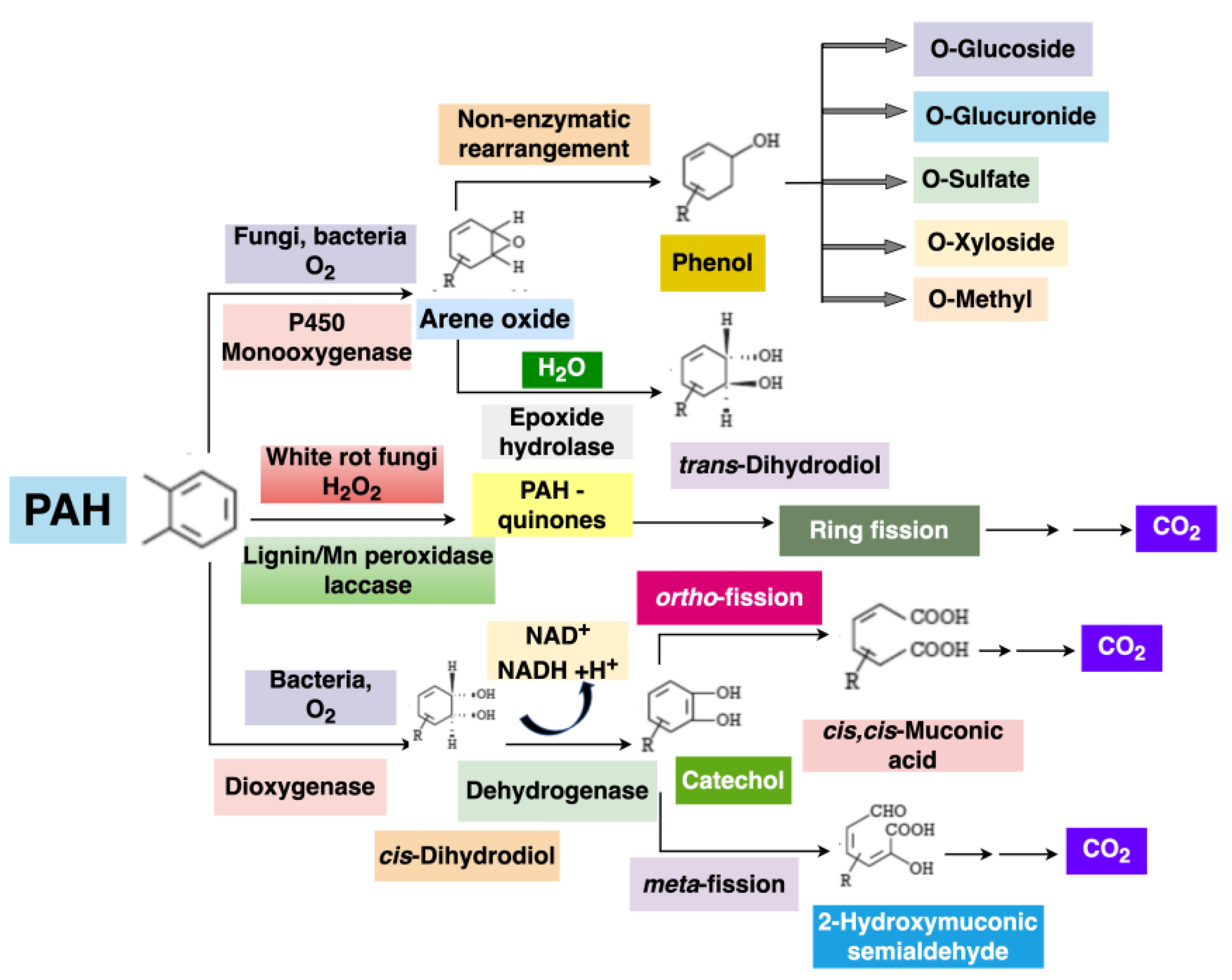
9.2. Polycyclic Aromatic Hydrocarbon (PAHs) by Mycoremediation
9.3. Mycoremediation of Pesticides
9.4. Mycoremediation of Pharmaceutical Effluents
9.5. Mycoremediation of Dye
10. Future Prospects
- To improve bioremediation applications, competitiveness, and practicability, it is necessary to screen new species of fungal and microbial consortia for the biodegradation of multiple contaminants with higher ecological adaptation.
- Mycoremediation is still in its infancy at the laboratory/greenhouse level, which limits its effectiveness in the field. Therefore, before commercialising this green technology, the mycoremediation abilities of each species must be evaluated in their natural environment.
- Increased study of high-throughput techniques (e.g., enzyme engineering, NGS, and microarray technologies) must be undertaken to make mycoremediation more economical and practically feasible.
- Finding different, more advantageous biological techniques (such as Phytoremediation, natural attenuation, etc.) and mechanisms for remediating stress caused by contaminants is necessary.
- Microbes’ genealogy and genetic modification must be investigated to better understand the remediation mechanisms.
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, V.; Majumder, C.; Agarwal, V. Sorption of Zn (II) ion onto the surface of activated carbon derived from eucalyptus bark saw dust from industrial wastewater: Isotherm, kinetics, mechanistic modeling, and thermodynamics. Desalin. Water Treat. 2012, 46, 332–351. [Google Scholar] [CrossRef]
- Gisbert, C.; Ros, R.; De Haro, A.; Walker, D.J.; Bernal, M.P.; Serrano, R.; Navarro-Aviñó, J. A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Biochem. Biophys. Res. Commun. 2003, 303, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Azin, E.; Moghimi, H. Efficient mycosorption of anionic azo dyes by Mucor circinelloides: Surface functional groups and removal mechanism study. J. Environ. Chem. Eng. 2018, 6, 4114–4123. [Google Scholar] [CrossRef]
- Noman, E.; Al-Gheethi, A.; Mohamed, R.M.S.R.; Talip, B.A. Myco-remediation of xenobiotic organic compounds for a sustainable environment: A critical review. Top. Curr. Chem. 2019, 377, 17. [Google Scholar] [CrossRef]
- Malik, A. Metal bioremediation through growing cells. Environ. Int. 2004, 30, 261–278. [Google Scholar] [CrossRef]
- Harms, H.; Schlosser, D.; Wick, L. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Leitão, A.L. Potential of Penicillium species in the bioremediation field. Int. J. Environ. Res. Public Health 2009, 6, 1393–1417. [Google Scholar] [CrossRef]
- Mani, D.; Kumar, C. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: An overview with special reference to phytoremediation. Int. J. Environ. Sci. Technol. 2014, 11, 843–872. [Google Scholar] [CrossRef]
- Asiriuwa, O.; Ikhuoria, J.; Ilor, E. Myco-remediation potential of heavy metals from contaminated soil. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 16–22. [Google Scholar]
- Hamba, Y.; Tamiru, M. Mycoremediation of heavy metals and hydrocarbons contaminated environment. Asian J. Nat. Appl. Sci. 2016, 5, 2. [Google Scholar]
- Sun, J.; Zou, X.; Ning, Z.; Sun, M.; Peng, J.; Xiao, T. Culturable microbial groups and thallium-tolerant fungi in soils with high thallium contamination. Sci. Total Environ. 2012, 441, 258–264. [Google Scholar] [CrossRef]
- Maheswari, S.; Murugesan, A. Remediation of arsenic in soil by Aspergillus nidulans isolated from an arsenic-contaminated site. Environ. Technol. 2009, 30, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Gentry, T.; Rensing, C. Pepper New approaches for bioaugmentation as a remediation technology. Crit. Rev. Environ. Sci. Technol. 2004, 34, 447–494. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, D.; Mondal, S.K.; Biswas, R.; Das, T.K.; Boujedaini, N.; Khuda-Bukhsh, A.R. Tolerance of arsenate-induced stress in Aspergillus niger, a possible candidate for bioremediation. Ecotoxicol. Environ. Saf. 2010, 73, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Pala, S.A.; Wani, A.H.; Boda, R.H.; Wani, B.A. Mushroom refinement endeavor auspicate non green revolution in the offing. Nusant. Biosci. 2014, 6, 173–185. [Google Scholar] [CrossRef]
- Lavelle, P.; Spain, A. Soil Ecology; Springer Science & Business Media: Cham, Switzerland, 2002. [Google Scholar]
- Krallish, I.; Gonta, S.; Savenkova, L.; Bergauer, P.; Margesin, R. Phenol degradation by immobilized cold-adapted yeast strains of Cryptococcus terreus and Rhodotorula creatinivora. Extremophiles 2006, 10, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Zafra, G.; Moreno-Montaño, A.; Absalón, E.; Cortés-Espinosa, D.V. Degradation of polycyclic aromatic hydrocarbons in soil by a tolerant strain of Trichoderma asperellum. Environ. Sci. Pollut. Res. 2015, 22, 1034–1042. [Google Scholar] [CrossRef]
- Hasan, I. Biodegradation of kerosene by Aspergillus niger and Rhizopus stolonifer. Appl. Environ. Microbiol. 2014, 2, 31–36. [Google Scholar]
- Govarthanan, M.; Fuzisawa, S.; Hosogai, T.; Chang, Y.-C. Biodegradation of aliphatic and aromatic hydrocarbons using the filamentous fungus Penicillium sp. CHY-2 and characterization of its manganese peroxidase activity. RSC Adv. 2017, 7, 20716–20723. [Google Scholar] [CrossRef]
- Dan, S.; Li, P.-j.; Frank, S.; Xiong, X.-z. Biodegradation of benzo [a] pyrene in soil by Mucor sp. SF06 and Bacillus sp. SB02 co-immobilized on vermiculite. J. Environ. Sci. 2006, 18, 1204–1209. [Google Scholar]
- Wang, C.; Liu, H.; Li, J.; Sun, H. Degradation of PAHs in soil by Lasiodiplodia theobromae and enhanced benzo [a] pyrene degradation by the addition of Tween-80. Environ. Sci. Pollut. Res. 2014, 21, 10614–10625. [Google Scholar] [CrossRef] [PubMed]
- Obuekwe, C.O.; Badrudeen, A.M.; Al-Saleh, E.; Mulder, J.L. Growth and hydrocarbon degradation by three desert fungi under conditions of simultaneous temperature and salt stress. Int. Biodeterior. Biodegrad. 2005, 56, 197–205. [Google Scholar] [CrossRef]
- Balaji, V.; Arulazhagan, P.; Ebenezer, P. Enzymatic bioremediation of polyaromatic hydrocarbons by fungal consortia enriched from petroleum contaminated soil and oil seeds. J. Environ. Biol. 2014, 35, 521. [Google Scholar] [PubMed]
- Al-Hawash, A.B.; Zhang, X.; Ma, F. Removal and biodegradation of different petroleum hydrocarbons using the filamentous fungus Aspergillus sp. RFC-1. Microbiologyopen 2019, 8, e00619. [Google Scholar] [CrossRef]
- Chan, S.M.N.; Luan, T.; Wong, M.H.; Tam, N.F.Y. Removal and biodegradation of polycyclic aromatic hydrocarbons by Selenastrum capricornutum. Environ. Toxicol. Chem. Int. J. 2006, 25, 1772–1779. [Google Scholar] [CrossRef]
- Chernikova, T.N.; Bargiela, R.; Toshchakov, S.; Shivaraman, V.; Lunev, E.; Yakimov, M.; Thomas, D.; Golyshin, P. Hydrocarbon-degrading bacteria Alcanivorax and Marinobacter associated with microalgae Pavlova lutheri and Nannochloropsis oculata. Front. Microbiol. 2020, 11, 572931. [Google Scholar] [CrossRef]
- Patel, J.G.; Kumar, J.N.; Kumar, R.N.; Khan, S.R. Enhancement of pyrene degradation efficacy of Synechocystis sp., by construction of an artificial microalgal-bacterial consortium. Cogent Chem. 2015, 1, 1064193. [Google Scholar] [CrossRef]
- Semple, K.T.; Cain, R.; Schmidt, S. Biodegradation of aromatic compounds by microalgae. FEMS Microbiol. Lett. 1999, 170, 291–300. [Google Scholar] [CrossRef]
- Chavan, A.; Mukherji, S. Treatment of hydrocarbon-rich wastewater using oil degrading bacteria and phototrophic microorganisms in rotating biological contactor: Effect of N ratio. J. Hazard. Mater. 2008, 154, 63–72. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Hamouda, R.A.; Nizam, A.A. Biodegradation of crude oil by Scenedesmus obliquus and Chlorella vulgaris growing under heterotrophic conditions. Int. Biodeterior. Biodegrad. 2013, 82, 67–72. [Google Scholar] [CrossRef]
- Nweze, N.; Aniebonam, C. Bioremediation of petroleum products impacted freshwater using locally available algae. Bio-Res 2009, 7, 45477. [Google Scholar] [CrossRef]
- Kalhor, A.X.; Movafeghi, A.; Mohammadi-Nassab, A.D.; Abedi, E.; Bahrami, A. Potential of the green alga Chlorella vulgaris for biodegradation of crude oil hydrocarbons. Mar. Pollut. Bull. 2017, 123, 286–290. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Hamouda, R. Biodegradation of crude oil by some cyanobacteria under heterotrophic conditions. Desalination Water Treat. 2014, 52, 1448–1454. [Google Scholar] [CrossRef]
- Yakimov, M.; Giuliano, L.; Gentile, G.; Crisafi, E.; Chernikova, T.; Abraham, W.-R.; Lünsdorf, H.; Timmis, K.N.; Golyshin, P. Oleispira antarctica gen. nov., sp. nov., a novel hydrocarbonoclastic marine bacterium isolated from Antarctic coastal sea water. Int. J. Syst. Evol. Microbiol. 2003, 53, 779–785. [Google Scholar] [CrossRef]
- Kasai, Y.; Kishira, H.; Harayama, S. Bacteria belonging to the genus Cycloclasticus play a primary role in the degradation of aromatic hydrocarbons released in a marine environment. Appl. Environ. Microbiol. 2002, 68, 5625–5633. [Google Scholar] [CrossRef]
- Mahjoubi, M.; Cappello, S.; Souissi, Y.; Jaouani, A.; Cherif, A. Chapter Microbial Bioremediation of Petroleum Hydrocarbon–Contaminated Marine Environments; Intech Open: London, UK, 2018. [Google Scholar]
- Yakimov, M.M.; Timmis, K.N.; Golyshin, P.N. Obligate oil-degrading marine bacteria. Curr. Opin. Biotechnol. 2007, 18, 257–266. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Płaza, G.; Poliwoda, A.; Piotrowska-Seget, Z. Characterization of hydrocarbon-degrading and biosurfactant-producing Pseudomonas sp. P-1 strain as a potential tool for bioremediation of petroleum-contaminated soil. Environ. Sci. Pollut. Res. 2014, 21, 9385–9395. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Kim, D.-U.; Kim, D.; Kim, J. Flavobacterium petrolei sp. nov., a novel psychrophilic, diesel-degrading bacterium isolated from oil-contaminated Arctic soil. Sci. Rep. 2019, 9, 4134. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Arumugam, A.; Chandran, P. Optimization of Enterobacter cloacae (KU923381) for diesel oil degradation using response surface methodology (RSM). J. Microbiol. 2017, 55, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, J.; Lin, J.; Wang, W.; Li, S. Biodegradation of petroleum hydrocarbons by Bacillus subtilis BL-27, a strain with weak hydrophobicity. Molecules 2019, 24, 3021. [Google Scholar] [CrossRef]
- Durán, R.E.; Méndez, V.; Rodríguez-Castro, L.; Barra-Sanhueza, B.; Salvà-Serra, F.; Moore, E.; Castro-Nallar, E.; Seeger, M. Genomic and physiological traits of the marine bacterium Alcaligenes aquatilis QD168 isolated from Quintero Bay, Central Chile, reveal a robust adaptive response to environmental stressors. Front. Microbiol. 2019, 10, 528. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, N.; Mondal, R.; Kour, D.; Subrahmanyam, G.; Shabnam, A.A.; Khan, S.A.; Yadav, K.K.; Sharma, G.K.; Cabral-Pinto, M. Myco-remediation: A mechanistic understanding of contaminants alleviation from natural environment and future prospect. Chemosphere 2021, 284, 131325. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cui, L.; Song, X.; Cui, X.; Wei, Y.; Tang, L.; Mu, Y.; Xu, Z. Wood decay fungi: An analysis of worldwide research. J. Soils Sediments 2022, 22, 1688–1702. [Google Scholar] [CrossRef]
- Adenipekun, C.; Lawal, R. Uses of mushrooms in bioremediation: A review. Biotechnol. Mol. Biol. Rev. 2012, 7, 62–68. [Google Scholar]
- Martínez, Á.T.; Speranza, M.; Ruiz-Dueñas, F.; Ferreira, P.; Camarero, S.; Guillén, F.; Martínez, M.; Suárez, A.G.; Río Andrade, J.C. Biodegradation of lignocellulosics: Microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 2005, 8, 195–204. [Google Scholar]
- Kristanti, R.A.; Hadibarata, T.; Toyama, T.; Tanaka, Y.; Mori, K. Bioremediation of crude oil by white rot fungi Polyporus sp. S133. J. Microbiol. Biotechnol. 2011, 21, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, H.-Q. Biosorption of 2, 4-dichlorophenol by immobilized white-rot fungus Phanerochaete chrysosporium from aqueous solutions. Bioresour. Technol. 2007, 98, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Adenipekun, C.O.; Isikhuemhen, O. Bioremediation of engine oil polluted soil by the tropical white rot fungus, Lentinus squarrosulus Mont. (Singer). Pak. J. Biol. Sci. 2008, 11, 1634–1637. [Google Scholar] [CrossRef]
- Purnomo, A.S.; Mori, T.; Takagi, K.; Kondo, R. Bioremediation of DDT contaminated soil using brown-rot fungi. Int. Biodeterior. Biodegrad. 2011, 65, 691–695. [Google Scholar] [CrossRef]
- Winquist, E.; Björklöf, K.; Schultz, E.; Räsänen, M.; Salonen, K.; Anasonye, F.; Cajthaml, T.; Steffen, K.; Jørgensen, K.; Tuomela, M. Bioremediation of PAH-contaminated soil with fungi–From laboratory to field scale. Int. Biodeterior. Biodegrad. 2014, 86, 238–247. [Google Scholar] [CrossRef]
- Zafra, G.; Taylor, T.D.; Absalón, A.E.; Cortés-Espinosa, D.V. Comparative metagenomic analysis of PAH degradation in soil by a mixed microbial consortium. J. Hazard. Mater. 2016, 318, 702–710. [Google Scholar] [CrossRef]
- Anastasi, A.; Tigini, V.; Varese, G.C. The bioremediation potential of different ecophysiological groups of fungi. In Fungi as Bioremediators; Springer: Cham, Switzerland, 2013; pp. 29–49. [Google Scholar]
- Zavarzina, A.G.; Lisov, A.A.; Zavarzin, A.A.; Leontievsky, A.A. Fungal oxidoreductases and humification in forest soils. In Soil Enzymology; Springer: Cham, Switzerland, 2010; pp. 207–228. [Google Scholar]
- Arantes, V.; Jellison, J.; Goodell, B. Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl. Microbiol. Biotechnol. 2012, 94, 323–338. [Google Scholar] [CrossRef]
- Dunwell, J.M.; Khuri, S.; Gane, P.J. Microbial relatives of the seed storage proteins of higher plants: Conservation of structure and diversification of function during evolution of the cupin superfamily. Microbiol. Mol. Biol. Rev. 2000, 64, 153–179. [Google Scholar] [CrossRef]
- Vara, S. Mycoremediation of lignocelluloses. In Handbook of Research on Inventive Bioremediation Techniques; Springer: Cham, Switzerland, 2017; pp. 264–286. [Google Scholar]
- Abuhussein, A. Wastewater Refining and Reuse and City-Level Water Decision Making; Western Libraries: London, UK, 2018. [Google Scholar]
- Esther, F.; Tibor, C.; Gyula, O. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar]
- Coleman, D.C. From peds to paradoxes: Linkages between soil biota and their influences on ecological processes. Soil Biol. Biochem. 2008, 40, 271–289. [Google Scholar] [CrossRef]
- Das, M.; Royer, T.V.; Leff, L.G. Diversity of fungi, bacteria, and actinomycetes on leaves decomposing in a stream. Appl. Environ. Microbiol. 2007, 73, 756–767. [Google Scholar] [CrossRef]
- Purahong, W.; Wubet, T.; Lentendu, G.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Hofrichter, M.; Krüger, D.; Buscot, F. Life in leaf litter: Novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol. Ecol. 2016, 25, 4059–4074. [Google Scholar] [CrossRef] [PubMed]
- Rout, M.E. The plant microbiome. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; pp. 279–309. [Google Scholar]
- Roshchina, V.V. The Excretory Function of Higher Plants; Springer Science & Business Media: Cham, Switzerland, 2012. [Google Scholar]
- Kubartová, A.; Ranger, J.; Berthelin, J.; Beguiristain, T. Diversity and decomposing ability of saprophytic fungi from temperate forest litter. Microb. Ecol. 2009, 58, 98–107. [Google Scholar] [CrossRef]
- Purohit, J.; Chattopadhyay, A.; Biswas, M.K.; Singh, N.K. Mycoremediation of agricultural soil: Bioprospection for sustainable development. In Mycoremediation and Environmental Sustainability; Springer: Cham, Switzerland, 2018; pp. 91–120. [Google Scholar]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Finlay, R.D. Ecological aspects of mycorrhizal symbiosis: With special emphasis on the functional diversity of interactions involving the extraradical mycelium. J. Exp. Bot. 2008, 59, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- John, J. Assessment of Arbuscular Mycorrhizal Fungi in a Green Roof System; Dalhousie University: Halifax, UK, 2013. [Google Scholar]
- Lehmann, J.; Rillig, M.; Thies, J.; Masiello, C.; Hockaday, W.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Fritsche, W.; Scheibner, K.; Herre, A.; Hofrichter, M. Fungal Degradation of Explosives: TNT and Related Nitroaromatic Compounds; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Bamforth, S.M.; Singleton, I. Bioremediation of polycyclic aromatic hydrocarbons: Current knowledge and future directions. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2005, 80, 723–736. [Google Scholar] [CrossRef]
- Tan, Y.H. Behavioral Properties of Locally Isolated Acinetobacter Species in Degrading Hydrocarbon Chain in Crude Oil and Used Cooking Oil; UTAR: Penang, Malaysia, 2011. [Google Scholar]
- Passarini, M.R.; Rodrigues, M.V.; da Silva, M.; Sette, L.D. Marine-derived filamentous fungi and their potential application for polycyclic aromatic hydrocarbon bioremediation. Mar. Pollut. Bull. 2011, 62, 364–370. [Google Scholar] [CrossRef]
- Bhatt, P.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Chen, S. Biodegradation of allethrin by a novel fungus Fusarium proliferatum strain CF2, isolated from contaminated soils. Microorganisms 2020, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Dalecka, B.; Juhna, T.; Rajarao, G.K. Constructive use of filamentous fungi to remove pharmaceutical substances from wastewater. J. Water Process Eng. 2020, 33, 100992. [Google Scholar] [CrossRef]
- Hamad, M.T.M.H. Biodegradation of diazinon by fungal strain Apergillus niger MK640786 using response surface methodology. Environ. Technol. Innov. 2020, 18, 100691. [Google Scholar] [CrossRef]
- Ariste, A.F.; Batista-García, R.A.; Vaidyanathan, V.K.; Raman, N.; Vaithyanathan, V.K.; Folch-Mallol, J.L.; Jackson, S.A.; Dobson, A.D.; Cabana, H. Mycoremediation of phenols and polycyclic aromatic hydrocarbons from a biorefinery wastewater and concomitant production of lignin modifying enzymes. J. Clean. Prod. 2020, 253, 119810. [Google Scholar] [CrossRef]
- Singh, G.; Dwivedi, S. Decolorization and degradation of Direct Blue-1 (Azo dye) by newly isolated fungus Aspergillus terreus GS28, from sludge of carpet industry. Environ. Technol. Innov. 2020, 18, 100751. [Google Scholar] [CrossRef]
- Mostafa, A.A.-F.; Elshikh, M.S.; Al-Askar, A.A.; Hadibarata, T.; Yuniarto, A.; Syafiuddin, A. Decolorization and biotransformation pathway of textile dye by Cylindrocephalum aurelium. Bioprocess Biosyst. Eng. 2019, 42, 1483–1494. [Google Scholar] [CrossRef]
- Arunprasath, D.; Bala, B.D.; Sekar, G. Luxury of N-Tosylhydrazones in Transition-Metal-Free Transformations. Adv. Synth. Catal. 2019, 361, 1172–1207. [Google Scholar] [CrossRef]
- Coelho, E.; Reis, T.; Cotrim, M.; Mullan, T.; Corrêa, B. Resistant fungi isolated from contaminated uranium mine in Brazil shows a high capacity to uptake uranium from water. Chemosphere 2020, 248, 126068. [Google Scholar] [CrossRef]
- Sharma, G.K.; Jena, R.K.; Hota, S.; Kumar, A.; Ray, P.; Fagodiya, R.K.; Malav, L.C.; Yadav, K.K.; Gupta, D.K.; Khan, S.A.; et al. Recent development in bioremediation of soil pollutants through biochar for environmental sustainability. In Biochar Applications in Agriculture and Environment Management; Springer: Cham, Switzerland, 2020; pp. 123–140. [Google Scholar]
- Yuniati, M. Bioremediation of petroleum-contaminated soil: A Review. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Natural carriers in bioremediation: A review. Electron. J. Biotechnol. 2016, 23, 28–36. [Google Scholar] [CrossRef]
- Kostka, J.E.; Prakash, O.; Overholt, W.A.; Green, S.J.; Freyer, G.; Canion, A.; Delgardio, J.; Norton, N.; Hazen, T.C.; Huettel, M. Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the Deepwater Horizon oil spill. Appl. Environ. Microbiol. 2011, 77, 7962–7974. [Google Scholar] [CrossRef]
- Wu, M.; Li, W.; Dick, W.A.; Ye, X.; Chen, K.; Kost, D.; Chen, L. Bioremediation of hydrocarbon degradation in a petroleum-contaminated soil and microbial population and activity determination. Chemosphere 2017, 169, 124–130. [Google Scholar] [CrossRef]
- Nivetha, N.; Srivarshine, B.; Sowmya, B.; Rajendiran, M.; Saravanan, P.; Rajeshkannan, R.; Rajasimman, M.; Pham, T.H.T.; Shanmugam, V.; Dragoi, E.-N. A comprehensive review on bio-stimulation and bio-enhancement towards remediation of heavy metals degeneration. Chemosphere 2022, 312, 137099. [Google Scholar] [CrossRef] [PubMed]
- Maletić, S.P.; Dalmacija, B.D.; Rončević, S.D.; Agbaba, J.R.; Perović, S.D.U. Impact of hydrocarbon type, concentration and weathering on its biodegradability in soil. J. Environ. Sci. Health Part A 2011, 46, 1042–1049. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Zhao, J.; Li, F.; Gao, D.; Xing, B. Remediation of petroleum contaminated soils through composting and rhizosphere degradation. J. Hazard. Mater. 2011, 190, 677–685. [Google Scholar] [CrossRef]
- Wongsa, P.; Tanaka, M.; Ueno, A.; Hasanuzzaman, M.; Yumoto, I.; Okuyama, H. Isolation and characterization of novel strains of Pseudomonas aeruginosa and Serratia marcescens possessing high efficiency to degrade gasoline, kerosene, diesel oil, and lubricating oil. Curr. Microbiol. 2004, 49, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Mancera-López, M.; Esparza-García, F.; Chávez-Gómez, B.; Rodríguez-Vázquez, R.; Saucedo-Castaneda, G.; Barrera-Cortés, J. Bioremediation of an aged hydrocarbon-contaminated soil by a combined system of biostimulation–bioaugmentation with filamentous fungi. Int. Biodeterior. Biodegrad. 2008, 61, 151–160. [Google Scholar] [CrossRef]
- Potin, O.; Rafin, C.; Veignie, E. Bioremediation of an aged polycyclic aromatic hydrocarbons (PAHs)-contaminated soil by filamentous fungi isolated from the soil. Int. Biodeterior. Biodegrad. 2004, 54, 45–52. [Google Scholar] [CrossRef]
- Hoff, R.Z. Bioremediation: An overview of its development and use for oil spill cleanup. Mar. Pollut. Bull. 1993, 26, 476–481. [Google Scholar] [CrossRef]
- Vidali, M. Bioremediation. an overview. Pure Appl. Chem. 2001, 73, 1163–1172. [Google Scholar] [CrossRef]
- Philp, J.C.; Atlas, R. Bioremediation of contaminated soils and aquifers. Bioremed. Appl. Microb. Solut. Real-World Environ. Cleanup 2005, 1, 139–236. [Google Scholar]
- Prokop, G. Management of Contaminated Sites in Western Europe; Office for Official Publications of the European Communities: Brussels, Belgium, 2000. [Google Scholar]
- Pino-Herrera, D.O.; Pechaud, Y.; Huguenot, D.; Esposito, G.; van Hullebusch, E.D.; Oturan, M.A. Removal mechanisms in aerobic slurry bioreactors for remediation of soils and sediments polluted with hydrophobic organic compounds: An overview. J. Hazard. Mater. 2017, 339, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Hyman, M.; Dupont, R. Groundwater and Soil Remediation Process Design and Cost Estimating of Proven Technologies; American Society of Civil Engineers: Cambridge, MA, USA, 2001. [Google Scholar]
- Cunningham, C.; Philp, J. Comparison of bioaugmentation and biostimulation in ex situ treatment of diesel contaminated soil. Land Contam. Reclam. 2000, 8, 261–269. [Google Scholar]
- Singh, N.P.; Sharma, J.K.; Santal, A.R. Biotechnological approaches to remediate soil and water using plant–microbe interactions. Phytoremediat. Manag. Environ. Contam. 2016, 4, 131–152. [Google Scholar]
- Bulak, P.; Walkiewicz, A.; Brzezińska, M. Plant growth regulators-assisted phytoextraction. Biol. Plant. 2014, 58, 1–8. [Google Scholar] [CrossRef]
- Das, M.; Adholeya, A. Role of microorganisms in remediation of contaminated soil. Microorg. Environ. Manag. Microbes Environ. 2012, 1, 81–111. [Google Scholar]
- Verma, A. Bioremediation Techniques for Soil Pollution: An Introduction; IntechOpen: London, UK, 2021. [Google Scholar]
- Margesin, R.; Walder, G.; Schinner, F. Bioremediation assessment of a BTEX-contaminated soil. Acta Biotechnol. 2003, 23, 29–36. [Google Scholar] [CrossRef]
- Höhener, P.; Ponsin, V. In situ vadose zone bioremediation. Curr. Opin. Biotechnol. 2014, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gidarakos, E.; Aivalioti, M. Large scale and long term application of bioslurping: The case of a Greek petroleum refinery site. J. Hazard. Mater. 2007, 149, 574–581. [Google Scholar] [CrossRef]
- Kuiper, I.; Lagendijk, E.L.; Bloemberg, G.V.; Lugtenberg, B.J.J. Rhizoremediation: A beneficial plant-microbe interaction. Mol. Plant-Microbe Interact. 2004, 17, 6–15. [Google Scholar] [CrossRef]
- Vargas, A.; Soto, G.; Moreno, J.A.; Buitrón, G. Observer-based time-optimal control of an aerobic SBR for chemical and petrochemical wastewater treatment. Water Sci. Technol. 2000, 42, 163–170. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Zhou, Q.; Gu, G. Enhanced phosphorus biological removal from wastewater—Effect of microorganism acclimatization with different ratios of short-chain fatty acids mixture. Biochem. Eng. J. 2005, 27, 24–32. [Google Scholar] [CrossRef]
- Yergeau, E.; Arbour, M.; Brousseau, R.; Juck, D.; Lawrence, J.R.; Masson, L.; Whyte, L.G.; Greer, C.W. Microarray and real-time PCR analyses of the responses of high-arctic soil bacteria to hydrocarbon pollution and bioremediation treatments. Appl. Environ. Microbiol. 2009, 75, 6258–6267. [Google Scholar] [CrossRef]
- Goel, M.; Chovelon, J.-M.; Ferronato, C.; Bayard, R.; Sreekrishnan, T.R. The remediation of wastewater containing 4-chlorophenol using integrated photocatalytic and biological treatment. J. Photochem. Photobiol. B Biol. 2010, 98, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Deleu, M.; Paquot, M. From renewable vegetables resources to microorganisms: New trends in surfactants. Comptes Rendus Chim. 2004, 7, 641–646. [Google Scholar] [CrossRef]
- Fan, L.; Pandey, A.; Mohan, R.; Soccol, C.R. Use of various coffee industry residues for the cultivation of Pleurotus ostreatus in solid state fermentation. Acta Biotechnol. 2000, 20, 41–52. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T.; Vandenberghe, L.P.; Mohan, R. Biotechnological potential of agro-industrial residues. II: Cassava bagasse. Bioresour. Technol. 2000, 74, 81–87. [Google Scholar] [CrossRef]
- Webb, C.; Koutinas, W.R.; Wang, R. Developing a sustainable bioprocessing strategy based on a generic feedstock. Biomanufacturing 2004, 87, 195–268. [Google Scholar]
- Zervakis, G.; Papadopoulou, K.; Ehaliotis, C. Use of composts deriving from Mediterranean agro-industrial wastes in vegetable crops: Effects on disease suppression and plant growth. In Proceedings of the International Symposium on the Use of Composted Organic Wastes in Horticulture, Wageningen, The Netherlands, 11 April 2005. [Google Scholar]
- Krishna, C. Solid-state fermentation systems—An overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef]
- Revankar, M.S.; Desai, K.M.; Lele, S.S. Solid-state fermentation for enhanced production of laccase using indigenously isolated Ganoderma sp. Appl. Biochem. Biotechnol. 2007, 143, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Elisashvili, V.; Penninckx, M.; Kachlishvili, E.; Tsiklauri, N.; Metreveli, E.; Kharziani, T.; Kvesitadze, G. Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid-state fermentation of lignocellulosic wastes of different composition. Bioresour. Technol. 2008, 99, 457–462. [Google Scholar] [CrossRef]
- Rodríguez Pérez, S.; Oduardo, N.G.; Savón, R.B.; Boizán, M.F.; Augur, C. Decolourisation of mushroom farm wastewater by Pleurotus ostreatus. Biodegradation 2008, 19, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Rigas, F.; Papadopoulou, K.; Dritsa, V.; Doulia, D. Bioremediation of a soil contaminated by lindane utilizing the fungus Ganoderma australe via response surface methodology. J. Hazard. Mater. 2007, 140, 325–332. [Google Scholar] [CrossRef]
- Price, N.; Reed, J.; Palsson, B. Genome-scale models of microbial cells: Evaluating the consequences of constraints. Nat. Rev. Microbiol. 2004, 2, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.J.; Jones, K.C. Kinetic constraints on the in-situ remediation of soils contaminated with organic chemicals. Environ. Sci. Pollut. Res. 1995, 2, 244–252. [Google Scholar] [CrossRef]
- Sabean, J.; Scott, D.; Lee, K.; Venosa, A. Monitoring oil spill bioremediation using marsh foraminifera as indicators. Mar. Pollut. Bull. 2009, 59, 352–361. [Google Scholar] [CrossRef]
- Talley, J.W.; Sleeper, P.M. Roadblocks to the implementation of biotreatment strategies. Ann. N. Y. Acad. Sci. 1997, 829, 16–29. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.; Maiti, S.; Favas, P. Mycoremediation for mine site rehabilitation. In Bio-Geotechnologies for Mine Site Rehabilitation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 233–260. [Google Scholar]
- Amjad, A.; Di, G.; Mahar, A.; Ping, W.; Feng, S.; Ronghua, L.; Zhang, Z. Mycoremediation of potentially toxic trace elements—A biological tool for soil cleanup: A review. Pedosphere 2017, 27, 205–222. [Google Scholar]
- Jang, K.-Y.; Cho, S.-M.; Seok, S.-J.; Kong, W.-S.; Kim, G.-H.; Sung, J.-M. Screening of biodegradable function of indigenous ligno-degrading mushroom using dyes. Mycobiology 2009, 37, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Kumari, R. Bioremediation: A sustainable tool for environmental management–a review. Annu. Res. Rev. Biol. 2013, 3, 974–993. [Google Scholar]
- Yamada-Onodera, K.; Mukumoto, H.; Katsuyaya, Y.; Saiganji, A.; Tani, Y. Degradation of polyethylene by a fungus, Penicillium simplicissimum YK. Polym. Degrad. Stab. 2001, 72, 323–327. [Google Scholar] [CrossRef]
- Ulfig, K.; Przystas, W.; Płaza, G.; Miksch, K. Biodegradation of petroleum hydrocarbons by keratinolytic fungi. In Soil and Water Pollution Monitoring, Protection and Remediation; Springer: Cham, Switzerland, 2006. [Google Scholar]
- Njoku, K.; Yussuf, A.; Akinola, M.; Adesuyi, A.; Jolaoso, A.; Adedokun, A. Mycoremediation of petroleum hydrocarbon polluted soil by Pleurotus pulmonarius. Ethiop. J. Environ. Stud. Manag. 2016, 9, 865–875. [Google Scholar] [CrossRef]
- Durán, N.; Esposito, E. Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: A review. Appl. Catal. B: Environ. 2000, 28, 83–99. [Google Scholar] [CrossRef]
- Mai, C.; Schormann, W.; Majcherczyk, A. Degradation of acrylic copolymers by white-rot fungi. Appl. Microbiol. Biotechnol. 2004, 65, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, R. Mycoremediation an eco-friendly approach for the degradation of cellulosic wastes from paper industry with the help of cellulases and hemicellulase activity to minimize the industrial pollution. Int. J. Env. Eng. Manag. 2013, 4, 199–206. [Google Scholar]
- Malachová, K.; Pavlícková, Z.; Novotný, C.; Svobodová, K.; Lednická, D.; Musílková, E. Reduction in the mutagenicity of synthetic dyes by successive treatment with activated sludge and the ligninolytic fungus, Irpex lacteus. Environ. Mol. Mutagen. 2006, 47, 533–540. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Long, Y.; Kim, M.-J.; Kim, J.-J.; Kim, G.-H. Decolorization and degradation of synthetic dyes by Irpex lacteus KUC8958. J. Environ. Sci. Health Part A 2013, 48, 501–508. [Google Scholar] [CrossRef]
- Kulshreshtha, S.; Mathur, N.; Bhatnagar, P. Mycoremediation of paper, pulp and cardboard industrial wastes and pollutants. Fungi Bioremediators 2013, 1, 77–116. [Google Scholar]
- Baldrian, P. Interactions of heavy metals with white-rot fungi. Enzym. Microb. Technol. 2003, 32, 78–91. [Google Scholar] [CrossRef]
- García-Delgado, C.; D’Annibale, A.; Pesciaroli, L.; Yunta, F.; Crognale, S.; Petruccioli, M.; Eymar, E. Implications of polluted soil biostimulation and bioaugmentation with spent mushroom substrate (Agaricus bisporus) on the microbial community and polycyclic aromatic hydrocarbons biodegradation. Sci. Total Environ. 2015, 508, 20–28. [Google Scholar] [CrossRef]
- Christofi, N.; Ivshina, I. Microbial surfactants and their use in field studies of soil remediation. J. Appl. Microbiol. 2002, 93, 915–929. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Palanisami, T.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. In-situ remediation approaches for the management of contaminated sites: A comprehensive overview. Rev. Environ. Contam. Toxicol. 2016, 236, 1–115. [Google Scholar] [PubMed]
- Duan, L.; Naidu, R.; Thavamani, P.; Meaklim, J.; Megharaj, M. Managing long-term polycyclic aromatic hydrocarbon contaminated soils: A risk-based approach. Environ. Sci. Pollut. Res. 2015, 22, 8927–8941. [Google Scholar] [CrossRef] [PubMed]
- Kotoky, R.; Rajkumari, J.; Pandey, P. The rhizosphere microbiome: Significance in rhizoremediation of polyaromatic hydrocarbon contaminated soil. J. Environ. Manag. 2018, 217, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Premnath, N.; Mohanrasu, K.; Rao, R.G.R.; Dinesh, G.; Prakash, G.S.; Ananthi, V.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A crucial review on polycyclic aromatic Hydrocarbons-Environmental occurrence and strategies for microbial degradation. Chemosphere 2021, 280, 130608. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Ali, G.A.M.; Bashir, M.J.K.; Copty, N.K.; Abu Amr, S.S.; Abushammala, M.F.M.; Al Maskari, T. Recent advances of nanoremediation technologies for soil and groundwater remediation: A review. Water 2021, 13, 2186. [Google Scholar] [CrossRef]
- Dada, E.O.; Akinola, M.O.; Owa, S.O.; Dedeke, G.A.; Aladesida, A.A.; Owagboriaye, F.O.; Oludipe, E.O. Efficacy of vermiremediation to remove contaminants from soil. J. Health Pollut. 2021, 11, 210302. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Chakma, S. Remedial technologies for future waste management. In Hazardous Waste Management; Elsevier: Amsterdam, The Netherlands, 2022; pp. 305–322. [Google Scholar]
- Negi, B.B.; Das, C. Mycoremediation of wastewater, challenges, and current status: A review. Bioresour. Technol. Rep. 2023, 11, 101409. [Google Scholar] [CrossRef]
- Guo, M.; Gong, Z.; Li, X.; Allinson, G.; Rookes, J.; Cahill, D. Polycyclic aromatic hydrocarbons bioavailability in industrial and agricultural soils: Linking SPME and Tenax extraction with bioassays. Ecotoxicol. Environ. Saf. 2017, 140, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, A.; Dai, J.; Wang, N.; Wu, D. Accumulation and tolerance characteristics of cadmium in Chlorophytum comosum: A popular ornamental plant and potential Cd hyperaccumulator. Environ. Monit. Assess. 2012, 184, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Shukla, P. Alternative strategies for microbial remediation of pollutants via synthetic biology. Front. Microbiol. 2020, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Sayler, G.S.; Ripp, S. Field applications of genetically engineered microorganisms for bioremediation processes. Curr. Opin. Biotechnol. 2000, 11, 286–289. [Google Scholar] [CrossRef]
- Wu, C.; Li, F.; Yi, S.; Ge, F. Genetically engineered microbial remediation of soils co-contaminated by heavy metals and polycyclic aromatic hydrocarbons: Advances and ecological risk assessment. J. Environ. Manag. 2021, 296, 113185. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Perales-Garcia, A.; Hernandez-Carballo, J.; Martinez-Rabelo, F.; Hernández-Castellanos, B.; Barois, I.; Contreras-Ramos, S. Bioremediation of soil contaminated by hydrocarbons with the combination of three technologies: Bioaugmentation, phytoremediation, and vermiremediation. J. Soils Sediments 2019, 19, 1981–1994. [Google Scholar] [CrossRef]
- Chirakkara, R.A.; Cameselle, C.; Reddy, K.R. Assessing the applicability of phytoremediation of soils with mixed organic and heavy metal contaminants. Rev. Environ. Sci. Bio/Technol. 2016, 15, 299–326. [Google Scholar] [CrossRef]
- Javed, F.; Hashmi, I. Vermiremediation–remediation of soil contaminated with oil using earthworm (Eisenia fetida). Soil Sediment Contam. Int. J. 2021, 30, 639–662. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Q.; Zhu, Q.; Zeng, J.; Ji, R.; Dumont, M.G.; Lin, X. Contributions of ryegrass, lignin and rhamnolipid to polycyclic aromatic hydrocarbon dissipation in an arable soil. Soil Biol. Biochem. 2018, 118, 27–34. [Google Scholar] [CrossRef]
- Sayara, T.; Sánchez, A. Bioremediation of PAH-contaminated soilsrocess enhancement through composting/compost. Appl. Sci. 2020, 10, 3684. [Google Scholar] [CrossRef]
- Rao, M.A.; Scelza, R.; Acevedo, F.; Diez, M.C.; Gianfreda, L. Enzymes as useful tools for environmental purposes. Chemosphere 2014, 107, 145–162. [Google Scholar] [CrossRef]
- Prigione, V.; Spina, F.; Tigini, V.; Giovando, S.; Varese, G.C. Biotransformation of industrial tannins by filamentous fungi. Appl. Microbiol. Biotechnol. 2018, 102, 10361–10375. [Google Scholar] [CrossRef] [PubMed]
- Shamim, S. Biosorption of Heavy Metals, Biosorption, Jan Derco and Branislav Vrana; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Janyasuthwiong, S.; Rene, E. Bioprecipitation—A Promising Technique for Heavy Metal Removal and Recovery from Contaminated Wastewater Streams. MOJCE 2017, 2, 52. [Google Scholar]
- Yadav, K.K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Khan, S.A. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Kumar, A.; Malyan, S.K.; Kumar, S.S.; Dutt, D.; Kumar, V. An assessment of trace element contamination in groundwater aquifers of Saharanpur, Western Uttar Pradesh, India. Biocatal. Agric. Biotechnol. 2019, 20, 101213. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of co-contaminated soil with heavy metals and pesticides: Influence factors, mechanisms and evaluation methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Kuyucak, N.; Volesky, B. Accumulation of cobalt by marine alga. Biotechnol. Bioeng. 1989, 33, 809–814. [Google Scholar] [CrossRef]
- Dua, M.; Singh, A.; Sethunathan, N.; Johri, A. Biotechnology and bioremediation: Successes and limitations. Appl. Microbiol. Biotechnol. 2002, 59, 143–152. [Google Scholar] [PubMed]
- Sindhura, P.; Vanamala, P.; Vasavilatha, T.; Gul, M.Z. Microbial and Phytoremediation-Based Removal of Polycyclic Aromatic Hydrocarbons (PAHs) in Soil Environments. In Bioremediation and Phytoremediation Technologies in Sustainable Soil Management; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 177–204. [Google Scholar]
- Singh, B.P.; Gupta, V.; Passari, A. Actinobacteria: Diversity and Biotechnological Applications: New and Future Developments. In Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Imam, A.; Suman, S.K.; Kanaujia, P.K.; Ray, A. Biological machinery for polycyclic aromatic hydrocarbons degradation: A review. Bioresour. Technol. 2022, 343, 126121. [Google Scholar] [CrossRef] [PubMed]
- Abdelhaleem, H.A.; Zein, H.S.; Azeiz, A.; Sharaf, A.N.; Abdelhadi, A.A. Identification and characterization of novel bacterial polyaromatic hydrocarbon-degrading enzymes as potential tools for cleaning up hydrocarbon pollutants from different environmental sources. Environ. Toxicol. Pharmacol. 2019, 67, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.D.; Hyder, M. Mycoremediation: A potential tool for sustainable management. J. Mycopathol. Res. 2019, 57, 25–34. [Google Scholar]
- Banerjee, A.; Jhariya, M.K.; Yadav, D.K.; Raj, A. Micro-remediation of metals: A new frontier in bioremediation. In Handbook of Environmental Materials Management; Springer: Cham, Switzerland, 2018; pp. 1–36. [Google Scholar]
- Deshmukh, R.; Khardenavis, A.A.; Purohit, H.J. Diverse metabolic capacities of fungi for bioremediation. Indian J. Microbiol. 2016, 56, 247–264. [Google Scholar] [CrossRef]
- Malyan, S.K.; Kumar, S.S.; Fagodiya, R.K.; Ghosh, P.; Kumar, A.; Singh, R.; Singh, L. Biochar for environmental sustainability in the energy-water-agroecosystem nexus. Renew. Sustain. Energy Rev. 2021, 149, 111379. [Google Scholar] [CrossRef]
- Urik, M.; Matus, P.; Korenkova, L. Mobilization and immobilization of potentially toxic metals and metalloids by filamentous fungi. Adv. Chem. Res. 2018, 42, 1–66. [Google Scholar]
- Okolie, C.U.; Chen, H.; Zhao, Y.; Tian, D.; Zhang, L.; Su, M.; Jiang, Z.; Li, Z.; Li, H. Cadmium immobilization in aqueous solution by Aspergillus niger and geological fluorapatite. Environ. Sci. Pollut. Res. 2020, 27, 7647–7656. [Google Scholar] [CrossRef]
- Gadd, G.M. Microbial influence on metal mobility and application for bioremediation. Geoderma 2004, 122, 109–119. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Lovley, D.R. Microbial detoxification of metals and radionuclides. Curr. Opin. Biotechnol. 2001, 12, 248–253. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Björkman, T.; Harman, G.E. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.L.; Massicotte, H.; Melville, L. Mycorrhizas: Anatomy and Cell Biology; NRC Research Press: Ottawa, ON, Canada, 2004. [Google Scholar]
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal recovery using oxalate chemistry: A technical review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393. [Google Scholar] [CrossRef]
- Lu, N.; Hu, T.; Zhai, Y.; Qin, H.; Aliyeva, J.; Zhang, H. Fungal cell with artificial metal container for heavy metals biosorption: Equilibrium, kinetics study and mechanisms analysis. Environ. Res. 2020, 182, 109061. [Google Scholar] [CrossRef]
- Dhankhar, R.; Hooda, A. Fungal biosorption–an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ. Technol. 2011, 32, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, M.; Binupriya, A.R.; Swaminathan, K.; Choi, J.G.; Yun, S.E. Bio-separation of toxic arsenate ions from dilute solutions by native and pretreated biomass of Aspergillus fumigatus in batch and column mode: Effect of biomass pretreatment. Bull. Environ. Contam. Toxicol. 2008, 81, 316–322. [Google Scholar] [CrossRef]
- Binupriya, A.; Sathishkumar, M.; Swaminathan, K.; Jeong, E.; Yun, S.; Pattabi, S. Biosorption of Metal Ions from Aqueous Solution and Electroplating Industry Wastewater by Aspergillus japonicushytotoxicity Studies. Bull. Environ. Contam. Toxicol. 2006, 77, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Ortiz, E.J.; Shakya, M.; Jain, R.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Sorption of zinc onto elemental selenium nanoparticles immobilized in Phanerochaete chrysosporium pellets. Environ. Sci. Pollut. Res. 2016, 23, 21619–21630. [Google Scholar] [CrossRef]
- Legorreta-Castañeda, A.J.; Lucho-Constantino, C.A.; Beltrán-Hernández, R.I.; Coronel-Olivares, C.; Vázquez-Rodríguez, G.A. Biosorption of water pollutants by fungal pellets. Water 2020, 12, 1155. [Google Scholar] [CrossRef]
- Schlüter, R.; Schauer, F. Biotransformation and Detoxification of Environmental Pollutants with Aromatic Structures by Yeasts. In Yeast Diversity in Human Welfare; Springer: Cham, Switzerland, 2017; pp. 323–369. [Google Scholar]
- Kaewdoung, B.; Sutjaritvorakul, T.; Gadd, G.M.; Whalley, A.J.; Sihanonth, P. Heavy metal tolerance and biotransformation of toxic metal compounds by new isolates of wood-rotting fungi from Thailand. Geomicrobiol. J. 2016, 33, 283–288. [Google Scholar] [CrossRef]
- Michelot, D.; Siobud, E.; Doré, J.-C.; Viel, C.; Poirier, F. Update on metal content profiles in mushrooms—Toxicological implications and tentative approach to the mechanisms of bioaccumulation. Toxicon 1998, 36, 1997–2012. [Google Scholar] [CrossRef] [PubMed]
- Taboski, M.A.; Rand, T.; Piórko, A. Lead and cadmium uptake in the marine fungi Corollospora lacera and Monodictys pelagica. FEMS Microbiol. Ecol. 2005, 53, 445–453. [Google Scholar] [CrossRef]
- Gorobets, S.; Gorobets, O.; Ukrainetz, A.; Kasatkina, T.; Goyko, I. Intensification of the process of sorption of copper ions by yeast of Saccharomyces cerevisiae 1968 by means of a permanent magnetic field. J. Magn. Magn. Mater. 2004, 272, 2413–2414. [Google Scholar] [CrossRef]
- Yesilada, O.; Birhanli, E.; Geckil, H. Bioremediation and decolorization of textile dyes by white rot fungi and laccase enzymes. In Mycoremediation and Environmental Sustainability; Springer: Cham, Switzerland, 2018; pp. 121–153. [Google Scholar]
- Goala, M.; Yadav, K.K.; Alam, J.; Adelodun, B.; Choi, K.S.; Cabral-Pinto, M.M.; Hamid, A.A.; Alhoshan, M.; Ali, F.A.A.; Shukla, A.K. Phytoremediation of dairy wastewater using Azolla pinnata: Application of image processing technique for leaflet growth simulation. J. Water Process Eng. 2021, 42, 102152. [Google Scholar] [CrossRef]
- Yadav, A.N.; Mishra, S.; Singh, S.; Gupta, A. Recent Advancement in White Biotechnology through Fungi: Volume 1: Diversity and Enzymes Perspectives; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Bharath, Y.; Gopalram, K. Mycoremediation of Garbage Contaminated Municipal Soil and Garage Contaminated Oil Soil Using Pleurotus Ostreatus–A Comparative Study. AIP Conf. Proc. 2023, 2427, 020066. [Google Scholar] [CrossRef]
- Bhargava, S.; Wenger, K.S.; Marten, M.R. Pulsed addition of limiting-carbon during Aspergillus oryzae fermentation leads to improved productivity of a recombinant enzyme. Biotechnol. Bioeng. 2003, 82, 111–117. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; Font, X.; Sánchez, A.; Gea, T.; Gabarrell, X.; Caminal, G. Co-composting as a management strategy to reuse the white-rot fungus Trametes versicolor after its use in a biotechnological process. Int. J. Environ. Waste Manag. 2013, 11, 100–108. [Google Scholar] [CrossRef]
- Danesh, Y.R.; Tajbakhsh, M.; Goltapeh, E.M.; Varma, A. Mycoremediation of Heavy Metals. Mycoremediation of heavy metals. In Fungi as Bioremediators; Springer: Cham, Switzerland, 2013; pp. 245–267. [Google Scholar]
- Rhodes, C.J. Mycoremediation (bioremediation with fungi)–growing mushrooms to clean the earth. Chem. Speciat. Bioavailab. 2014, 26, 196–198. [Google Scholar] [CrossRef]
- Tavares, A.; Pereira, S.; Xavier, A. Biotechnological Applications of Trametes versicolor and their Enzymes. Curr. Biotechnol. 2017, 6, 78–88. [Google Scholar] [CrossRef]
- Ghosh, P.; Ghosh, U. Bioconversion of agro-waste to value-added product through solid-state fermentation by a potent fungal strain Aspergillus flavus PUF5. In Utilization and Management of Bioresources; Springer: Cham, Switzerland, 2018; pp. 291–299. [Google Scholar]
- Singh, M.; Srivastava, P.K.; Verma, P.C.; Kharwar, R.N.; Singh, N.; Tripathi, R.D. Soil fungi for mycoremediation of arsenic pollution in agriculture soils. J. Appl. Microbiol. 2015, 119, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Young, D.; Rice, J.; Martin, R.; Lindquist, E.; Lipzen, A.; Grigoriev, I.; Hibbett, D. Degradation of bunker C fuel oil by white-rot fungi in sawdust cultures suggests potential applications in bioremediation. PlOS ONE 2015, 10, e0130381. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Angayarkanni, J.; Das, A.; Palaniswamy, M. Mycoremediation of Benzo [a] pyrene by Pleurotus ostreatus isolated from Wayanad district in Kerala. India Int. J. Pharm. Bio. Sci. 2012, 2, 84–93. [Google Scholar]
- Pathak, A.P.; Rathod, M.; Gudda, R. Chapter Cultivation by Optimizing Substrate Dependent Growth of Selected Oyster Mushroom Species under Environmentally Controlled Conditions: A Review. In Biotechnology of Mushroom; Elsevier: Amsterdam, The Netherlands, 2015; p. 24. [Google Scholar]
- Dickson, U.J.; Coffey, M.; Mortimer, R.; Di Bonito, M.; Ray, N. Mycoremediation of petroleum contaminated soils progress, prospects and perspectives. Environ. Sci. Impacts 2019, 21, 1446–1458. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.C.; Weil, R. Elements of the Nature and Properties of Soils; Pearson: London, UK, 2004. [Google Scholar]
- Guerin, T.F. The differential removal of aged polycyclic aromatic hydrocarbons from soil during bioremediation. Environ. Sci. Pollut. Res. 2000, 7, 19–26. [Google Scholar] [CrossRef]
- Anderson, C.; Juday, G. Mycoremediation of petroleum: A literature review. J. Environ. Sci. Eng. A 2016, 5, 397–405. [Google Scholar]
- Kapahi, M.; Sachdeva, S. Mycoremediation potential of Pleurotus species for heavy metals: A review. Bioresour. Bioprocess. 2017, 4, 32. [Google Scholar] [CrossRef]
- D’Annibale, A.; Rosetto, F.; Leonardi, V.; Federici, F.; Petruccioli, M. Role of autochthonous filamentous fungi in bioremediation of a soil historically contaminated with aromatic hydrocarbons. Appl. Environ. Microbiol. 2006, 72, 28–36. [Google Scholar] [CrossRef]
- Purchase, D.; Scholes, L.; Revitt, D.; Shutes, R. Effects of temperature on metal tolerance and the accumulation of Zn and Pb by metal-tolerant fungi isolated from urban runoff treatment wetlands. J. Appl. Microbiol. 2009, 106, 1163–1174. [Google Scholar] [CrossRef]
- Pawar, R.M. The effect of soil pH on bioremediation of polycyclic aromatic hydrocarbons (PAHS). J. Bioremed. Biodegrad. 2015, 6, 291–304. [Google Scholar] [CrossRef]
- Zafar, S.; Aqil, F.; Ahmad, I. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour. Technol. 2007, 98, 2557–2561. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Suresh, S. Kinetic and equilibrium studies on the biosorption of reactive black 5 dye by Aspergillus foetidus. Bioresour. Technol. 2008, 99, 51–58. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace elements in soil-vegetables interface: Translocation, bioaccumulation, toxicity and amelioration-a review. Sci. Total Environ. 2019, 651, 2927–2942. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Effect of temperature on oil degradation by a psychrotrophic yeast in liquid culture and in soil. FEMS Microbiol. Ecol. 1997, 24, 243–249. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef]
- Villen-Guzman, M.; Gutierrez-Pinilla, D.; Gomez-Lahoz, C.; Vereda-Alonso, C.; Rodriguez-Maroto, J.; Arhoun, B. Optimization of Ni (II) biosorption from aqueous solution on modified lemon peel. Environ. Res. 2019, 179, 108849. [Google Scholar] [CrossRef] [PubMed]
- El-Rahim, W.M.A.; El-Ardy, O.A.M.; Mohammad, F.H. The effect of pH on bioremediation potential for the removal of direct violet textile dye by Aspergillus niger. Desalination 2009, 249, 1206–1211. [Google Scholar] [CrossRef]
- Meehan, C.; Banat, I.; McMullan, G.; Nigam, P.; Smyth, F.; Marchant, R. Decolorization of Remazol Black-B using a thermotolerant yeast, Kluyveromyces marxianus IMB3. Environ. Int. 2000, 26, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Gül, D.; Dönmez, G. Application of mixed fungal biomass for effective reactive dye removal from textile effluents. Desalination Water Treat. 2013, 51, 3597–3603. [Google Scholar] [CrossRef]
- Baldrian, P.; der Wiesche, C.I.; Gabriel, J.; Nerud, F.; Zadražil, F. Influence of cadmium and mercury on activities of ligninolytic enzymes and degradation of polycyclic aromatic hydrocarbons by Pleurotus ostreatus in soil. Appl. Environ. Microbiol. 2000, 66, 2471–2478. [Google Scholar] [CrossRef]
- Jouraeva, V.A.; Johnson, D.L.; Hassett, J.P.; Nowak, D.J.; Shipunova, N.A.; Barbarossa, D. Role of sooty mold fungi in accumulation of fine-particle-associated PAHs and metals on deciduous leaves. Environ. Res. 2006, 102, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Gong, J.-L.; Zeng, G.-M.; Niu, Q.-Y.; Zhang, H.-Y.; Niu, C.-G.; Deng, J.-H.; Yan, M. Adsorption of Cd (II) and Zn (II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem. Eng. J. 2010, 162, 487–494. [Google Scholar] [CrossRef]
- Das, M.T.; Kumar, S.S.; Ghosh, P.; Shah, G.; Malyan, S.K.; Bajar, S.; Thakur, I.S.; Singh, L. Remediation strategies for mitigation of phthalate pollution: Challenges and future perspectives. J. Hazard. Mater. 2021, 409, 124496. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Bai, J.; Wang, J.; Lu, Q.; Zhao, Q.; Cui, B.; Liu, X. Polycyclic aromatic hydrocarbons (PAHs) in wetland soils under different land uses in a coastal estuary: Toxic levels, sources and relationships with soil organic matter and water-stable aggregates. Chemosphere 2014, 110, 8–16. [Google Scholar] [CrossRef]
- Gauthier, P.T.; Norwood, W.P.; Prepas, E.E.; Pyle, G. Metal–PAH mixtures in the aquatic environment: A review of co-toxic mechanisms leading to more-than-additive outcomes. Aquat. Toxicol. 2014, 154, 253–269. [Google Scholar] [CrossRef]
- Di Gregorio, S.; Becarelli, S.; Siracusa, G.; Castiglione, M.R.; Petroni, G.; Masini, G.; Gentini, A.; de Lima e Silva, M.; Lorenzi, R. Pleurotus ostreatus spent mushroom substrate for the degradation of polycyclic aromatic hydrocarbons: The case study of a pilot dynamic biopile for the decontamination of a historically contaminated soil. J. Chem. Technol. Biotechnol. 2016, 91, 1654–1664. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Fatima, F.; Bajpai, P.; Pathak, N.; Singh, S.; Priya, S.; Verma, S.R. Antimicrobial and immunomodulatory efficacy of extracellularly synthesized silver and gold nanoparticles by a novel phosphate solubilizing fungus Bipolaris tetramera. BMC Microbiol. 2015, 15, 52. [Google Scholar] [CrossRef]
- Rai, M.R.M.; Yadav, A.Y.A.; Bridge, P.; Gade, A.G.A. Myconanotechnology: A new and emerging science. Appl. Mycol. 2009, 1, 258–267. [Google Scholar]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef]
- Malik, P.; Mukherjee, T. Hindawi Publishing Corporation. Chin. J. Biol 2014, 8, 162. [Google Scholar]
- Karman, S.B.; Diah, S.Z.M.; Gebeshuber, I.C. Raw materials synthesis from heavy metal industry effluents with bioremediation and phytomining: A biomimetic resource management approach. Adv. Mater. Sci. Eng. 2015, 2015, 185071. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Biological synthesis of metal nanoparticles. Hydrometallurgy 2006, 83, 132–140. [Google Scholar] [CrossRef]
- Chan, E.A.; Rendón-Barraza, C.; Wang, B.; Pu, T.; Ou, J.-Y.; Wei, H.; Adamo, G.; An, B.; Zheludev, N. Counting and mapping of subwavelength nanoparticles from a single shot scattering pattern. Nanophotonics 2023, 1, 2192–8614. [Google Scholar] [CrossRef]
- Kumar, A.; Chaturvedi, A.; Yadav, K.; Arunkumar, K.; Malyan, S.; Raja, P.; Kumar, R.; Khan, S.; Yadav, K.; Rana, K. Fungal phytoremediation of heavy metal-contaminated resources: Current scenario and future prospects. Recent advancement in white biotechnology through fungi. Perspect. Sustain. Environ. 2019, 437–461. [Google Scholar]
- Kumar, S.S.; Ghosh, P.; Malyan, S.K.; Sharma, J.; Kumar, V. A comprehensive review on enzymatic degradation of the organophosphate pesticide malathion in the environment. J. Environ. Sci. Health Part C 2019, 37, 288–329. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.-V.; Kamyab, H.; Khan, S.A.; Yadav, S.; et al. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches-A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef]
- Kumar, A.; Chaturvedi, A.K.; Surendran, U.; A Shabnam, A.; Singh, A.; Vinodakumar, S.; Tamuly, B.; Malyan, S.K.; Khan, S.A.; Cabral-Pinto, M.; et al. Mechanistic overview of metal tolerance in edible plants: A physiological and molecular perspective. Handb. Bioremed. 2021, 23–47. [Google Scholar]
- Deng, Z.; Cao, L.; Huang, H.; Jiang, X.; Wang, W.; Shi, Y.; Zhang, R. Characterization of Cd-and Pb-resistant fungal endophyte Mucor sp. CBRF59 isolated from rapes (Brassica chinensis) in a metal-contaminated soil. J. Hazard. Mater. 2011, 185, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Emuh, F. Mushroom as a purifier of crude oil polluted soil. Int. J. Sci. Nat. 2010, 1, 127–132. [Google Scholar]
- Singh, A.; Gauba, P. Mycoremediation: A treatment for heavy metal pollution of soil. J. Civ. Eng. Environ. Technol. 2014, 1, 59–61. [Google Scholar]
- Thenmozhi, R.; Arumugam, K.; Nagasathya, A.; Thajuddin, N.; Paneerselvam, A. Studies on mycoremediation of used engine oil contaminated soil samples. Adv. Appl. Sci. Res. 2013, 4, 110–118. [Google Scholar]
- Sousa, N.R.; Ramos, M.A.; Marques, A.P.; Castro, P.M. A genotype dependent-response to cadmium contamination in soil is displayed by Pinus pinaster in symbiosis with different mycorrhizal fungi. Appl. Soil Ecol. 2014, 76, 7–13. [Google Scholar] [CrossRef]
- Kacprzak, M.J.; Rosikon, K.; Fijalkowski, K.; Grobelak, A. The effect of Trichoderma on heavy metal mobility and uptake by Miscanthus giganteus, Salix sp. Phalaris arundinacea, and Panicum virgatum. Appl. Environ. Soil Sci. 2014, 2014, 506142. [Google Scholar] [CrossRef]
- Garon, D.; Sage, L.; Seigle-Murandi, F. Effects of fungal bioaugmentation and cyclodextrin amendment on fluorene degradation in soil slurry. Biodegradation 2004, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pundir, R.; Chary, G.; Dastidar, M. Application of Taguchi method for optimizing the process parameters for the removal of copper and nickel by growing Aspergillus sp. Water Resour. Ind. 2018, 20, 83–92. [Google Scholar] [CrossRef]
- Soutullo, S.; Sánchez, M.N.; Enríquez, R.; Olmedo, R.; Jiménez, M.J.; Heras, M.R. Comparative thermal study between conventional and bioclimatic office buildings. Build. Environ. 2016, 105, 95–103. [Google Scholar] [CrossRef]
- Sharma, B.; Shukla, P. Futuristic avenues of metabolic engineering techniques in bioremediation. Biotechnol. Appl. Biochem. 2022, 69, 51–60. [Google Scholar] [CrossRef]
- Okoli, A.S.; Blix, T.; Myhr, A.I.; Xu, W.; Xu, X. Sustainable use of CRISPR/Cas in fish aquaculture. Biosaf. Perspective. Transgenic Res. 2022, 31, 1–21. [Google Scholar]
- Zhang, J.; Zhang, H.; Li, S.; Li, J.; Yan, L.; Xia, L. Increasing yield potential through manipulating of an ARE1 ortholog related to nitrogen use efficiency in wheat by CRISPR/Cas9. J. Integr. Plant Biol. 2021, 63, 1649–1663. [Google Scholar] [CrossRef]
- Saritha, V.; Maruthi, Y.; Mukkanti, K. Potential fungi for bioremediation of industrial effluents. BioResources 2010, 5, 8–12. [Google Scholar]
- D’Souza, D.T.; Tiwari, R.; Sah, A.K.; Raghukumar, C. Enhanced production of laccase by a marine fungus during treatment of colored effluents and synthetic dyes. Enzym. Microb. Technol. 2006, 38, 504–511. [Google Scholar] [CrossRef]
- Leyval, C.; Joner, E.; Del Val, C.; Haselwandter, K. Potential of arbuscular mycorrhizal fungi for bioremediation. In Mycorrhizal Technology in agriculture: From Genes to Bioproducts; Springer: Berlin/Heidelberg, Germany, 2002; pp. 175–186. [Google Scholar]
- Azizi, A.B.; Liew, K.Y.; Noor, Z.M.Z.M.; Abdullah, N. Vermiremediation and mycoremediation of polycyclic aromatic hydrocarbons in soil and sewage sludge mixture: A comparative study. Int. J. Environ. Sci. Dev. 2013, 4, 565–568. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Sutherland, J.B. Bioremediation of polycyclic aromatic hydrocarbons by ligninolytic and non-ligninolytic fungi. In British Mycological Society Symposium Series; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Cerniglia, C.E. Fungal metabolism of polycyclic aromatic hydrocarbonsast, present and future applications in bioremediation. J. Ind. Microbiol. Biotechnol. 1997, 19, 324–333. [Google Scholar] [CrossRef]
- García-Delgado, C.; Alfaro-Barta, I.; Eymar, E. Combination of biochar amendment and mycoremediation for polycyclic aromatic hydrocarbons immobilization and biodegradation in creosote-contaminated soil. J. Hazard. Mater. 2015, 285, 259–266. [Google Scholar] [CrossRef]
- Fan, M.-Y.; Xie, R.-J.; Qin, G. Bioremediation of petroleum-contaminated soil by a combined system of biostimulation–bioaugmentation with yeast. Environ. Technol. 2014, 35, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Challa, S.; Dutta, T.; Neelapu, N.R.R. Fungal White Biotechnology Applications for Food Security: Opportunities and Challenges. In Recent Advancement in White Biotechnology Through Fungi; Springer International Publishing: Cham, Switzerland, 2019; pp. 119–148. [Google Scholar] [CrossRef]
- Uqab, B.; Mudasir, S.; Nazir, R. Review on bioremediation of pesticides. J. Bioremediat. Biodegrad. 2016, 7, 2. [Google Scholar]
- Spina, F.; Cecchi, G.; Landinez-Torres, A.; Pecoraro, L.; Russo, F.; Wu, B.; Cai, L.; Liu, X.; Tosi, S.; Varese, G. Fungi as a toolbox for sustainable bioremediation of pesticides in soil and water. Plant Biosystems-An International Journal Dealing with all. Asp. Plant Biol. 2018, 152, 474–488. [Google Scholar]
- Singh, B.; Kuhad, R. Biodegradation of lindane (γ-hexachlorocyclohexane) by the white-rot fungus Trametes hirsutus. Lett. Appl. Microbiol. 1999, 28, 238–241. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; López-Chuken, U.J.; Villarreal-Chiu, J.F. Microbial degradation of organophosphate pesticides: A review. Pedosphere 2018, 28, 190–208. [Google Scholar] [CrossRef]
- Spadaro, J.T.; Gold, M.H.; Renganathan, V. Degradation of azo dyes by the lignin-degrading fungus Phanerochaete chrysosporium. Appl. Environ. Microbiol. 1992, 58, 2397–2401. [Google Scholar] [CrossRef] [PubMed]


| Fungal Species | Remediation Methods | Pollutants | Experimental Conditions | Degradation Environment | Treatment (mg/L) | Removal /Uptake | Ref. |
|---|---|---|---|---|---|---|---|
| Fusarium proliferatum CF2 | Degradation | Allethrin (Insecticides) | Incubation conditions: 5 days, media—mineral slat at 26 °C, shaker speed—110 rpm and pH—6. | Aerobic | 50 mg/L | 95% | [78] |
| Tinea versicolor | Biosorption | Ketoprofen | 100 μL of fungi incubation were injected for 21 days at 25 °C under 150 rpm shaking speed. | Aerobic | 5 mg/L | 80% | [79] |
| Staphylococcus succinus HLJ-10, Aspergillus niger MK640786 | Degradation | Diazinon (Organophosphrous pesticides) | Culture conditions: T—30 °C, pH—5, shaker speed time: 7 days. | Aerobic | 25 mg/L | 91.8% | [80] |
| Pleurotus dryinus, Trametes hirsuta | Biosorption, and biotransformation | Phenol | 150 rpm media cylindrical woodchips and 4 g/L glucose at 27 °C. | Anerobic | Biorefinery wastewater | 94% and 100% | [81] |
| Aspergillusterreus | Adsorption and degradation | Azo dye | Incubation conditions: T—30 °C, and contact time 168 h | Aerobic | 100 mg/L | 98.4% | [82] |
| Cylindrocephalum aurelium | Biotransformation | Mordant Orange-1 | Incubation conditions: pH—3, agitation speed (100 rpm), in the dark for 30 days | Anerobic | 20,000 mg/L | 86% | [83] |
| Lasiodiplodia sp. | Degradation | Malachite green | Incubation conditions: pH—7, T—30 °C. | Aerobic | 50 mg/L | 96.9% | [84] |
| Talaromyces amestolkiae, Penicillium ludwigii, Penicillium citrinum, Gongronella butleri | Biosorption | Uranium | Incubation conditions: media—potato dextrose broth, shaker speed—horizontally at 150 rpm at 25 °C for 7 days. | Anerobic | 100 mg/L | 60% (11 species out 57) | [85] |
| Talaromyces islandicus | Uptake | Pb | Incubated for 5 days at 30 °C. | Aerobic | 100 mg/L | 89.14% | [86] |
| Chemical Class | Examples | Biodegradability |
|---|---|---|
| Polyaromatic hydrocarbons | Benzo(a)pyrene, anthracene, creosote | Aerobic |
| Petroleum hydrocarbons | Fuel oil | Aerobic |
| Ketones and esters | MEK, Acetone | Anaerobic and aerobic |
| Aromatic hydrocarbons | Toluene, benzene | Anaerobic and aerobic |
| Asbestos | Not biodegradable | |
| Corrosives | Caustics, inorganic acids | Not biodegradable |
| Radioactive materials | Cadmium, plutonium, uranium | Not biodegradable |
| Metals | Not degradable experimental biosorption | |
| Organic cyanides | Aerobic | |
| PCBs | Arochlors | Some evidence; not readily degradable |
| Chlorinated solvents | Anaerobic (reductive dichlorination), aerobic (methanotrophs) |
| Factors | Merits | Demerits |
|---|---|---|
| Natural Process | Applies a biological strategy that uses microorganisms to remediate polluted areas | The biological mechanism is very delicate and necessitates the presence of microorganisms with metabolic activity, favourable growth conditions, and appropriate nutrients. |
| Labour/Effort | It is easy and requires less labour. | It is difficult to transfer the mechanism from pilot-scale to large-scale application. |
| Cost-Effectiveness | Compared with more conventional methods for cleaning up toxic waste, it is a more affordable strategy. | Bioremediation techniques, such as reactor designs, can, however, be more costly than conventional methods. |
| Duration | A bioremediation treatment requires more time than other treatment options. Using little or no nutrient amendments can slow down the bioremediation process. | |
| Nutrient amendments | There is constant availability of nutrients (organic and inorganic wastes) that are readily applied to encourage the rapid growth of microbes. | The bioremediation process can be hampered by amendments and nutrients that are toxic to the microorganisms. |
| Ease of application | As bioremediation occurs on-site, it eliminates the need for waste to be moved off-site, protecting human health and the environment at the same time. | |
| Environmentally friendly | It is non-intrusive, meaning site users can continue to use the site without interruption. The method is environmentally friendly and sustainable. | Some biodegradation products have the potential to be more harmful than the original compounds in some cases, and persist in the environment. |
| Contaminant type | A wide range of biodegradable contaminants can be treated with this technique. | Not all substances undergo a complete and rapid decomposition, particularly inorganic contaminants. |
| Legislation and Guidelines | Regulators continue to disagree about the proper performance standards for bioremediation. |
| Techniques | Influencing Parameters | Merits | Demerits | Applicability | Duration | Ref. |
|---|---|---|---|---|---|---|
| Rhizoremediation | Soil type, texture, particle size, nutrients and organic matter content. | High production of biomass. Root exudation in the rhizosphere provides better nutrient uptake for rhizosphere microbiome. Efficient tolerance of plants towards PAHs. | Inability to determine an accurate degradation time for organic pollutants. Lack of field studies. | Small scale (long term) | Longer degradation time | [148] |
| Phytoremediation | Root zone, characteristics of plant species, characteristics of PAHs, characteristics of medium, environmental conditions. | Increased soil fertility through the release of organic matter. Suitable for large-scale applications. Environmental and eco-friendly. | Time consuming, particularly in moderately and highly contaminated sites due to slow growth rate and low production of biomass. | Large scale (long term) | Longer degradation time. | [149] |
| Genetically modified microorganism (GEMs) | Chemical structure, microbial population composition, environmental conditions. | Low-technology equipment is required. Depending on the soil condition, in situ and ex situ methods can be employed. Equipment requirements are minimal in comparison with other remediation technologies. It is possible to completely break down organic contaminants into non-toxic chemicals. | Less information available on risk assessment of GEMs. Treatment takes a longer time. A volatile organic compound (VOC) cannot be controlled effectively using the ex situ method. Physico-chemical characteristics and toxicity of soil are extremely sensitive to these parameters. Presence of incomplete breakdown of organic contaminants if the process is not well controlled, managed and monitored. | Large scale (long term) | Longer degradation time. | [150] |
| Nano-remediation | Remediation time, initial concentration of PAHs, dosage of nanomaterial. | Good surface-coating lability. Due to the large surface area, there is a high level of reactivity and a large number of active sites. Enables remediation in deeper soil. | Exposure of nanomaterials to both humans and the environment. | Large scale (long term) | Shorter degradation time | [151] |
| Vermiremediation | Earthworm’s life cycle (i.e., feeding, burrowing, metabolism, secretion). | Cost-effective remediation. Advantage of increasing earthworm biomass that can be harvested and used as livestock feed. | Earthworms may not be suitable as biomonitoring agents due to risk assessment. It is not suitable for cleaning up highly polluted soil. | Small scale (long term) | Very less | [152] |
| Electrokinetic remediation | Mixed nature of contaminant, electrolyte properties, voltage gradient, and soil heterogeneity. | Effective with low permeability soil. Low environmental impacts. | Not effective for all types of PAHs. Low solubility. Poor desorption ability. | Small scale (long term) | Longer degradation time | [153] |
| Mycoremediation | Temperature, pH, heavy metals, and redox potential. | It is economical, eco-friendly, and an effective strategy to combat the ever-increasing problem of soil and water pollution. | As a result, the process is often slow, and the proportion of contaminants removed rarely approaches 100%. | Small scale (long term) | Shorter degradation time | [154] |
| Pros | Cons | Remarks | Ref. | |
|---|---|---|---|---|
| Biotransformation | A faster fungal growth rate shortens the time required for transformation. The biocatalyst operates between 20 and 40 °C and has a pH range of 5.0 to 8.0 at ambient conditions. It requires minimal operational control and time-saving technology. | Biocatalyst operating parameters must be precise. Enzymes are an expensive system. A high concentration of product or substrate can inhibit certain biocatalytic reactions, thus halting biotransformation. | Biological catalysts are used in bio-transformations, which are organic reactions. | [165,166] |
| Biosorption | Cost-efficient production of biomass. Simple and customisable method to remediate a wide range of contaminants. Simultaneous removal of many HMs. | Expensive regeneration. Numerous kinds of adsorbents are required. Reactor saturation and clogging. | Multiple metals can be removed at once, which is environmentally friendly but expensive. | [167,168] |
| Precipitation | Removal of high pollution loads is mainly achievable. Excellent at removing metal sulphide. The simplest and least expensive wastewater treatment system. | Unfeasible for the removal of small amounts of pollutants. It is difficult to maintain an environment conducive to growth and development. | Pollutants are eliminated, and the end product is separated. | [168,169] |
| Natural attenuation | Economically feasible. Following other bioremediation treatments, it can be utilised as a “polish” treatment. Reducing environmental contaminants without human intervention. | Long-term and extensive performance monitoring required. Not a time-efficient method. Natural attenuation’s removal of one contaminant can also remove other advantageous components. | Utilised for the co-precipitation, dispersion, immobilisation, and reversible and irreversible sorption of inorganic components. | [170,171,172] |
| Surface sequestration | Fungi secrete an extracellular enzyme that converts complex material into a simpler form which is then absorbed by its cell wall, so facilitating the remediation of a broad spectrum of persistent pollutants (PAHs, insecticides, and pesticides). | Few candidates from the fungal kingdom demonstrate their effectiveness in bioremediation under field conditions. Extracellular enzyme activity is impeded by high glucose concentration and stimulated by reducing glucose concentration. | Comprises chelation, complexation, coordination, and physical adsorption. | [173,174] |
| Fungal Species | Enzymes Involved | Compound Degraded | Remarks | Ref. |
|---|---|---|---|---|
| Fusarium oxysporium | Endoglucanase | Transform silver | Grows on arid, temperate, and tundra soils. | [208] |
| Bjerkandera adusta | Lignin peroxidases | Xenobiotic compounds | Typically grows on decaying wood. | [209] |
| T. versicolor | Laccase | Toluene and benzene | Commonly grows in tilled layer. | [210] |
| Aspergillus flavus | Laccase | Dyes and surfactants | Legumes and cereals are excellent for promoting healthy growth. | [211] |
| Trametes pavonia, Penicillium verruculosum, Penicilliums piculisporus, Botryosphaeria laricina, Aspergillus glaucus | Ligninolytic enzymes | Herbicides and pesticides | Degradation | [212,213] |
| Fungal Species | Pollutants | Factor | Experiment Design | Remarks | Observation | Ref. |
|---|---|---|---|---|---|---|
| Rhodotorula mucilaginosa and Beauveria | Zn and Pb | Temperature, and time | Microcosm studies. Culture conditions: 21 days incubation, temperatures—4° and 30 °C, media—glycerol yeast extract agar (GYEA). | Accumulation efficiency: Rhodotorula mucilaginosa, Zn (2.50%), Pb (16.55%); Beauveria bassiana, Zn (0.64%), Pb (8.44%) | The growth of B. bassiana was not affected at a lower temperature (4 °C) and reached 74% (control), while at similar Zn concentrations at 30 °C, 62%, 70% and 88% were reached. | [222] |
| Penicillium freii and Aspergillus niger | PAHs (pHenanthrene, anthracene, flouranthene, and pyrene) | pH | pH range of soil microcosm (5.0–8.0). | At pH 7.5, anthracene, flouranthene, phenanthrene, and pyrene undergo 50% biodegradation. | Increasing the pH of Arthur Brower’s top soils increases their bioremediation potential. | [223] |
| Fungi with filaments (species of Rhizopus and Aspergillus) | Cd and Cr | Metal type, pH and fungus species | YMS culture medium incubated at pH 4.5 for 4 h at 25 °C was used for the biosorption assay. | Aspergillus sp.1 accumulated Cr (1.20 mg g−1), and Cd (2.72 mg g−1), while Rhizopus sp. accumulated significantly more Cr (4.33 mg g−1). | Level of tolerance of filamentous fungi to metals were observed in the order Cu > Cr > Cd > Co > Ni. However, there was no direct relationship between level of metal resistance and biosorption capacity in Aspergillus isolates. | [224] |
| Aspergillus foetidus | Azo dye (reactive black 5) | Temperature, dosage and pH | 0.1 MHCl/0.1 M NaOH/1.0 M was autoclaved with the fungal biomass for 1 h. Kinetics of biosorption of Azo reactive black (100 mg/L) onto fungal biomass prior to equilibrium were investigated. | At pH 2–3, Aspergillus foetidus showed a decolourisation efficiency of over 99%. The increase in temperature from 30 to 50 °C significantly increased the biosorption capacity. | The biosorption process was endothermic and spontaneous. Furthermore, the biosorption capacity is strongly dependent on the temperature and increases significantly with an increase in temperature from 30 to 50 °C | [225] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akpasi, S.O.; Anekwe, I.M.S.; Tetteh, E.K.; Amune, U.O.; Shoyiga, H.O.; Mahlangu, T.P.; Kiambi, S.L. Mycoremediation as a Potentially Promising Technology: Current Status and Prospects—A Review. Appl. Sci. 2023, 13, 4978. https://doi.org/10.3390/app13084978
Akpasi SO, Anekwe IMS, Tetteh EK, Amune UO, Shoyiga HO, Mahlangu TP, Kiambi SL. Mycoremediation as a Potentially Promising Technology: Current Status and Prospects—A Review. Applied Sciences. 2023; 13(8):4978. https://doi.org/10.3390/app13084978
Chicago/Turabian StyleAkpasi, Stephen Okiemute, Ifeanyi Michael Smarte Anekwe, Emmanuel Kweinor Tetteh, Ubani Oluwaseun Amune, Hassan Oriyomi Shoyiga, Thembisile Patience Mahlangu, and Sammy Lewis Kiambi. 2023. "Mycoremediation as a Potentially Promising Technology: Current Status and Prospects—A Review" Applied Sciences 13, no. 8: 4978. https://doi.org/10.3390/app13084978
APA StyleAkpasi, S. O., Anekwe, I. M. S., Tetteh, E. K., Amune, U. O., Shoyiga, H. O., Mahlangu, T. P., & Kiambi, S. L. (2023). Mycoremediation as a Potentially Promising Technology: Current Status and Prospects—A Review. Applied Sciences, 13(8), 4978. https://doi.org/10.3390/app13084978








