Transformative Technology for FLASH Radiation Therapy
Featured Application
Abstract
1. Introduction
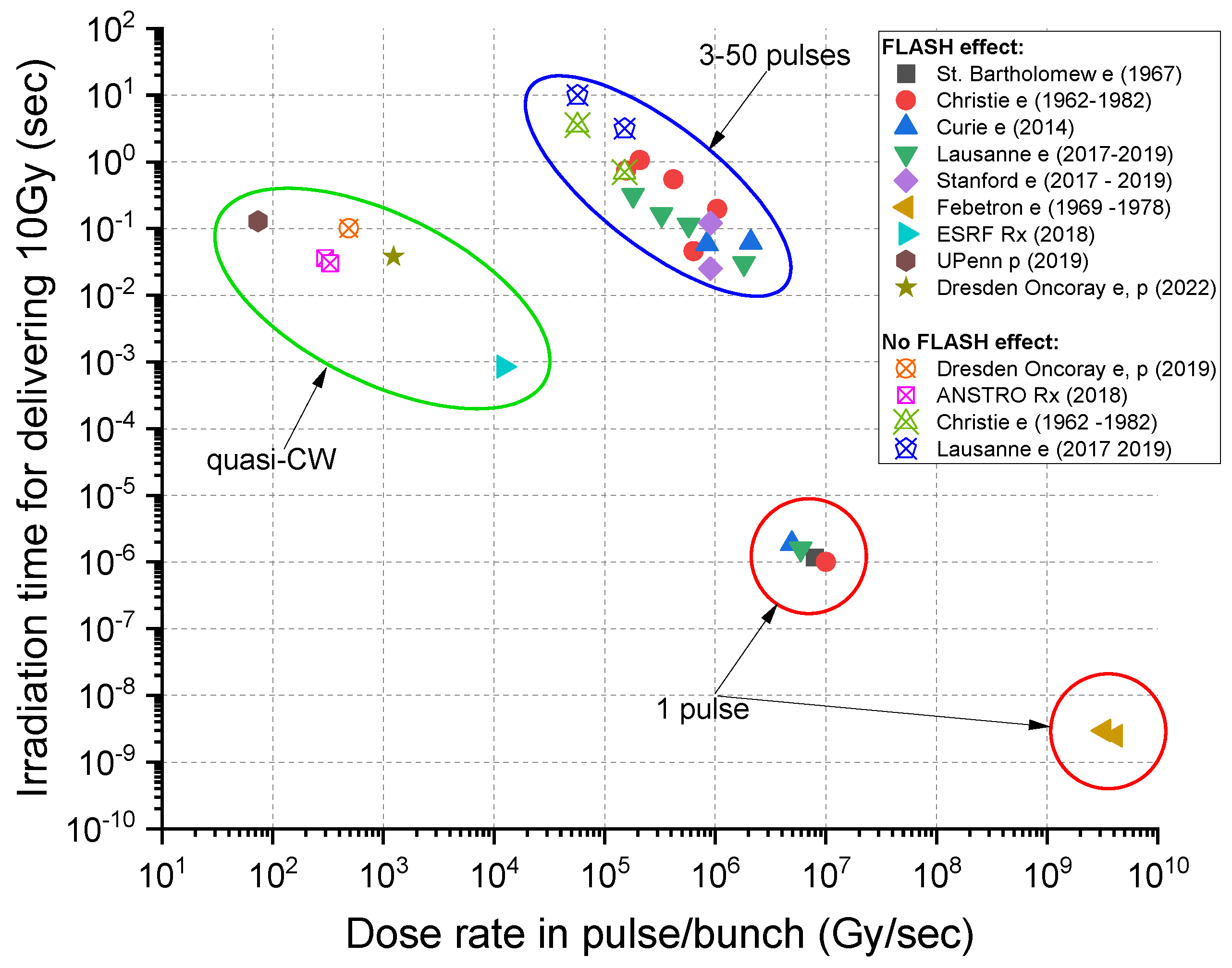
2. Beam Conditions for the FLASH Effect
2.1. FLASH with Electron Beams
- Study of pulmonary fibrosis from irradiation of the lung [1]: Severe to moderate fibrosis for the conventional average dose rate of 0.03 Gy/s, with a 17 Gy total dose. For an average dose rate of 40–60 Gy/s, the equivalent fibrosis occurred at a 30 Gy total dose.
- Study of neurocognitive impairment in mice from brain irradiation [14]: Severe neurocognitive degeneration at an average dose rate of 0.1 Gy/s, with a 10 Gy total dose. Reduced impairment starts at 30 Gy/s with no neurocognitive decline at 100 Gy/s, with an average dose rate for 10 Gy.
- Skin irradiation (mini-pig) [13]: Fibrosis and necrotic lesions were observed at an average dose rate of 0.08 Gy/s (22–37 Gy total dose), with only mild depigmentation at an average dose rate of 300 Gy/s (22–37 Gy total dose).
2.2. FLASH with Photon Beams
2.3. FLASH with Proton and Ion Beams
- FLASH effects—general
- a.
- Appear at average dose rates of >30 Gy/s, with the apparent optimal at 100 Gy/s;
- b.
- FLASH effect is likely highly dose-, tissue-, and end-point-dependent.
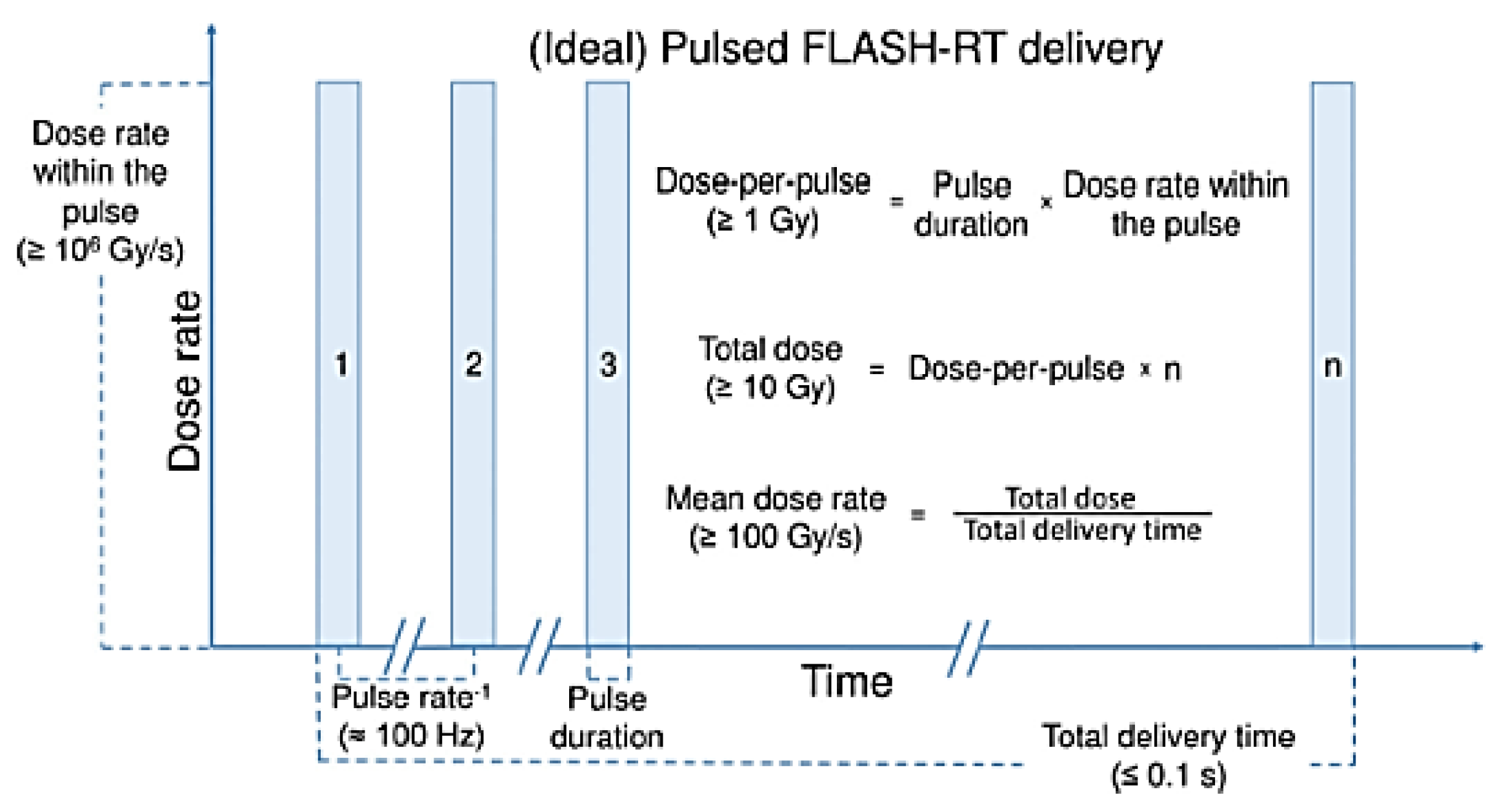
- Dependence of beam delivery on the beam structure and uniformity in dose deposition
- a.
- Typical dose delivery time for a consistent (electron) FLASH effect is ~100 ms (best <250 ms);
- b.
- Most positive FLASH studies used a modified pulsed clinical electron linac with a beam pulse length of ~microseconds and a repetition rate of 100–400 Hz;
- c.
- Instantaneous (within the pulse) minimum FLASH dose rate is 106 Gy/s (again, a characteristic of clinical electron linacs).
- Dosimetry and treatment-planning questions
- a.
- Observed volumetric dose-deposition dependence.
- b.
- Low-dose-rate areas not tolerated during FLASH—toxicity reappears?
- c.
- Bragg peak and pencil-beam scanning questions: do distal edge and penumbra effects and associated lower-dose rate beam “halos” create a problem?
- d.
- Can a relatively large target volume be uniformly irradiated by fractionated FLASH-compatible deliveries over a longer time frame?
- e.
- Instantaneous FLASH dose rate and delivery time for 10 Gy—is it consistent for all radiation types?
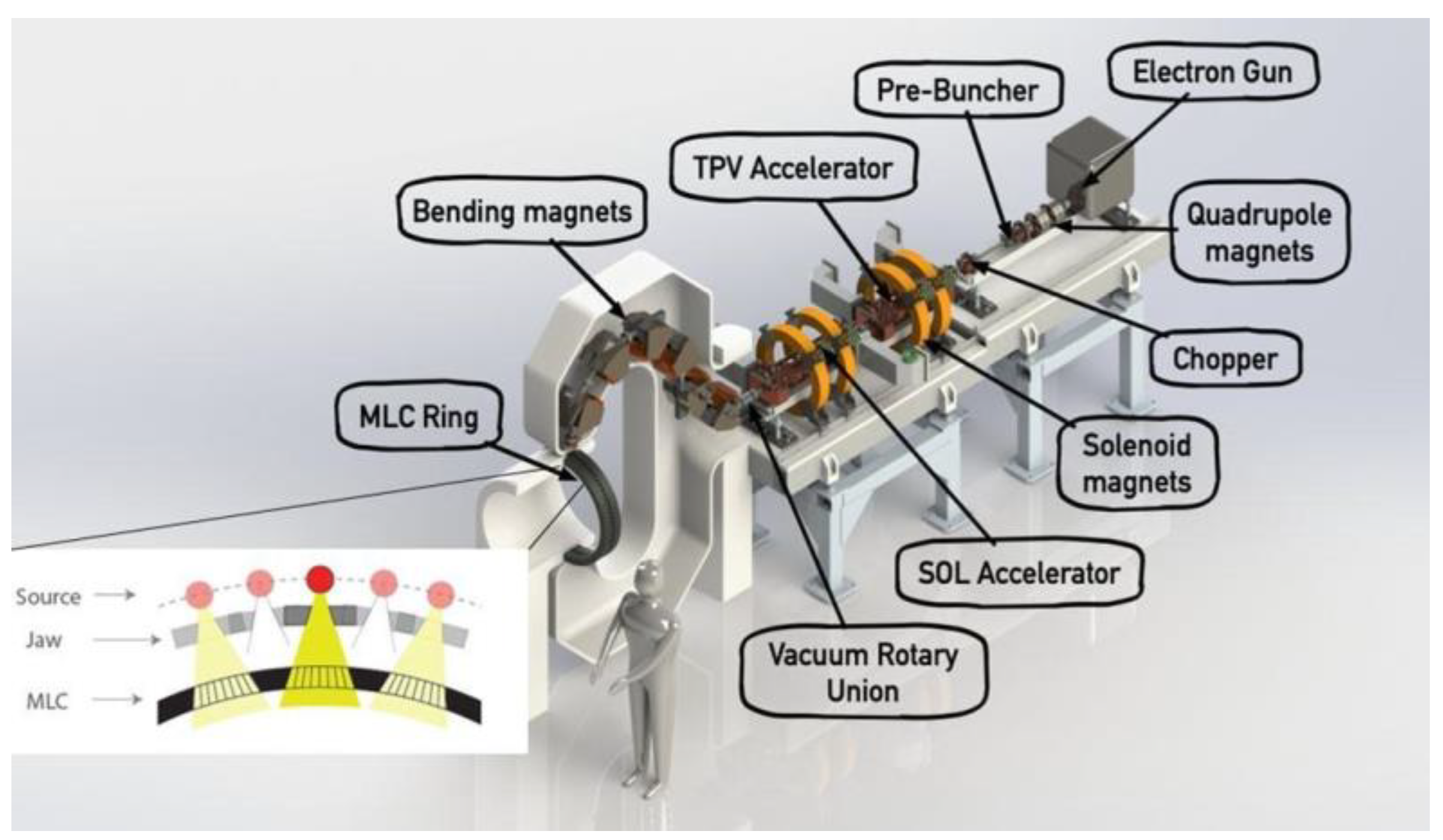
3. High-Gradient Ion Linacs for FLASH-RT Developed by Argonne National Laboratory and RadiaBeam
3.1. Linacs for Ion Beam Therapy
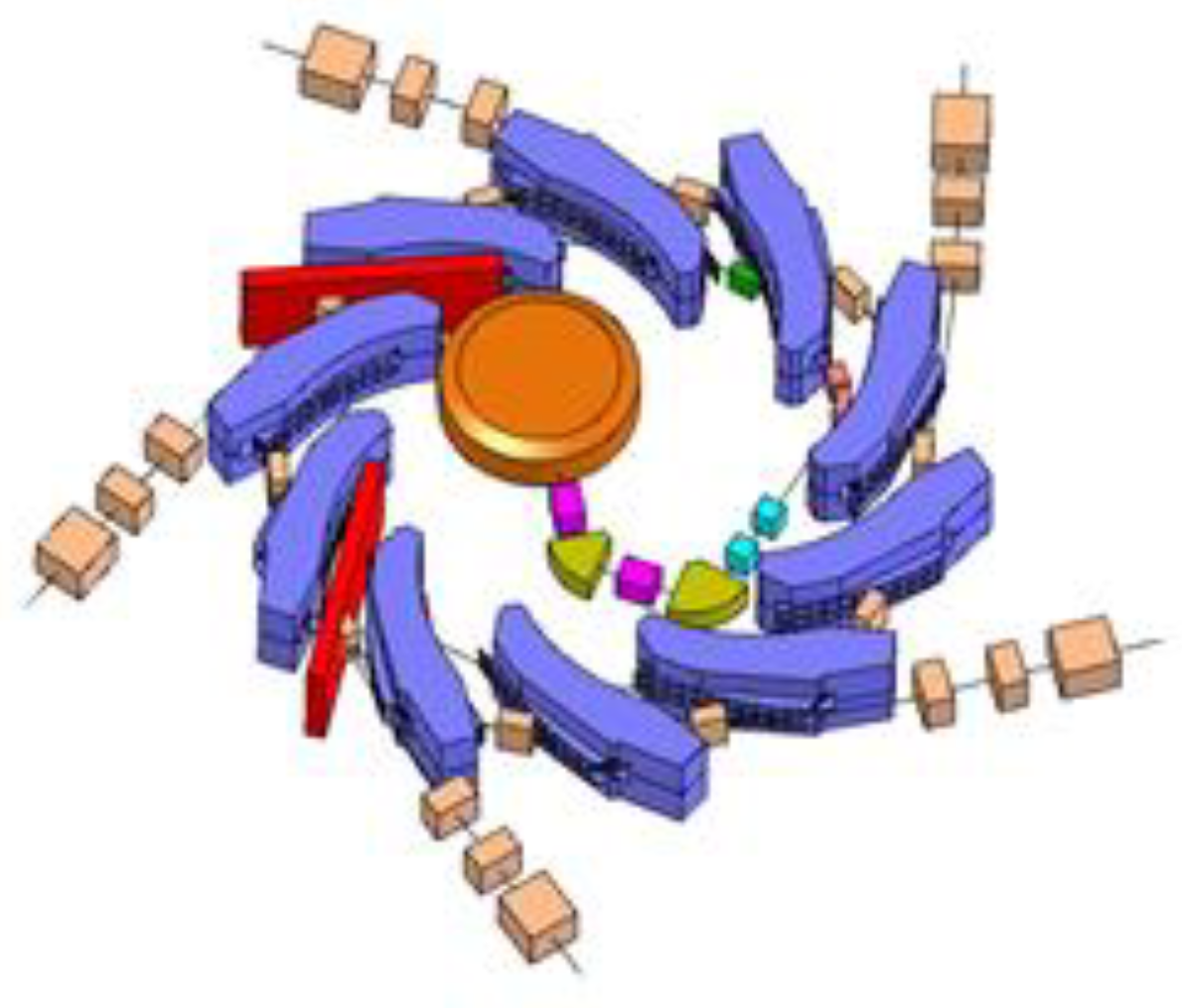
3.2. The ACCIL Ion Linac: General and FLASH Capabilities
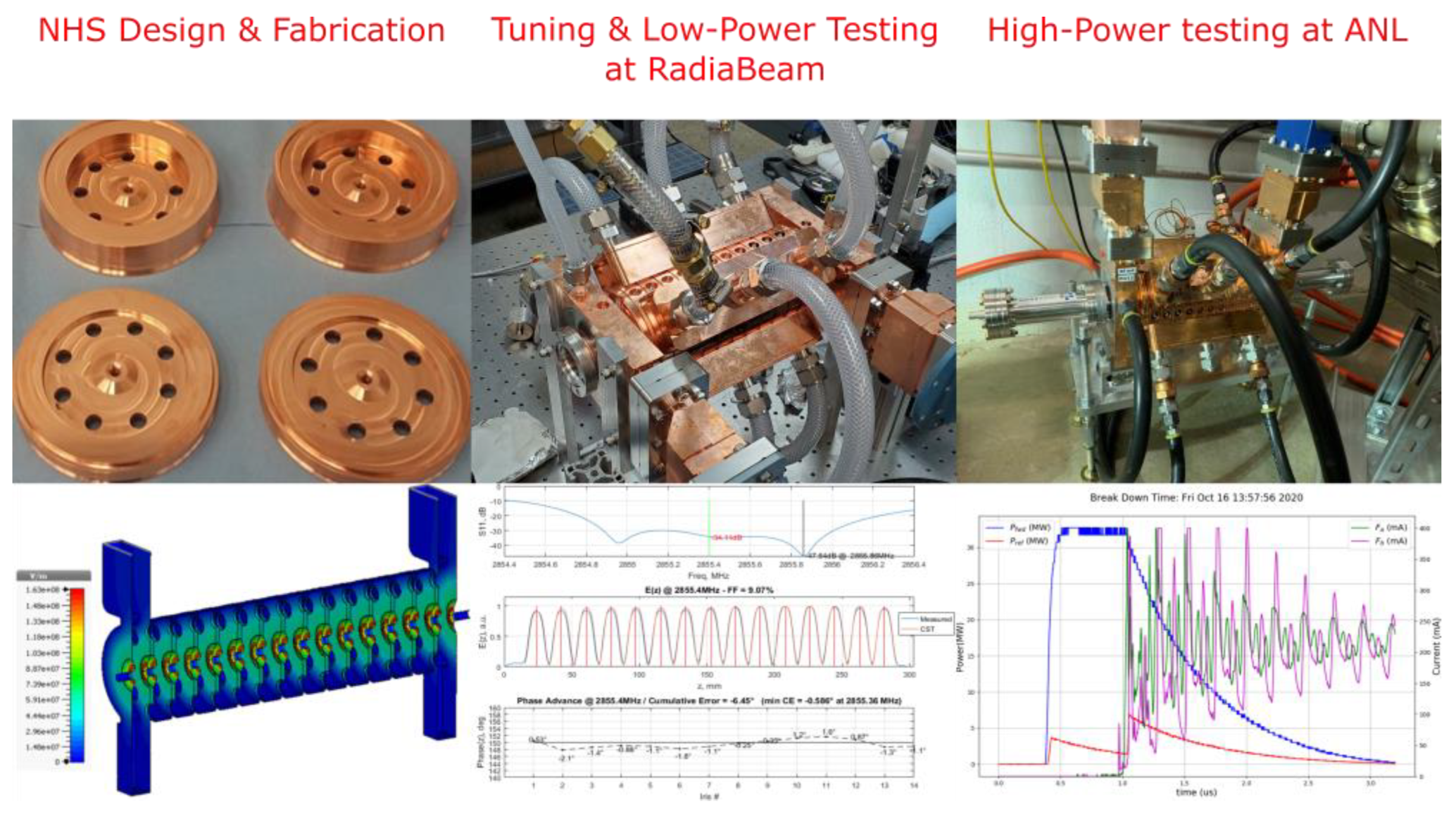
3.3. Enabling Technology: Low-Velocity High-Gradient Accelerating Structure Development
3.4. Future Developments for FLASH-RT
- Investigating and pushing the beam current limit of compact ion linacs;
- Increasing the repetition rate of high-gradient structures;
- Developing RF sources capable of delivering the required high pulsed power.
- Cancer therapy and radiobiology research with all ion beams up to neon;
- Radiography and tomography with ions lighter than carbon: proton, helium, etc.;
- Real-time MRI guidance during beam delivery, significantly enhancing the outcome of ion beam therapy;
- PET imaging using positron emitters (C-11, N-13, O-15, etc.) produced in the tumor for dose verification;
- FLASH ion therapy (FLASH IT) and other novel approaches.
4. Fixed-Field Gradient Accelerators for FLASH-RT
4.1. Scaling Fixed-Field Gradient Accelerator
4.2. Non-scaling FFGA for FLASH
4.2.1. Overview of Principle Design
4.2.2. The High-Energy Therapeutic Ring
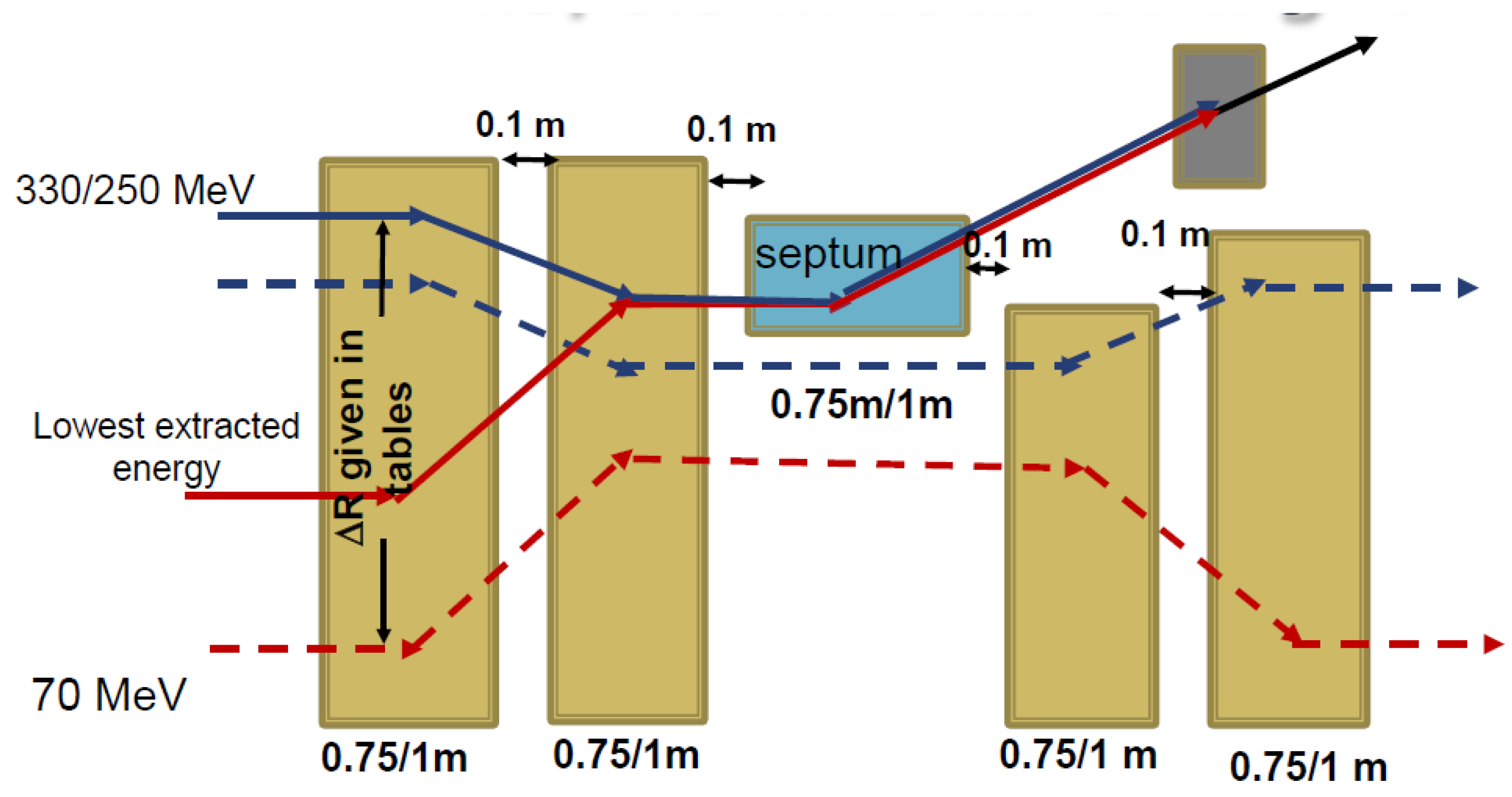
4.2.3. Variable-Energy Extraction
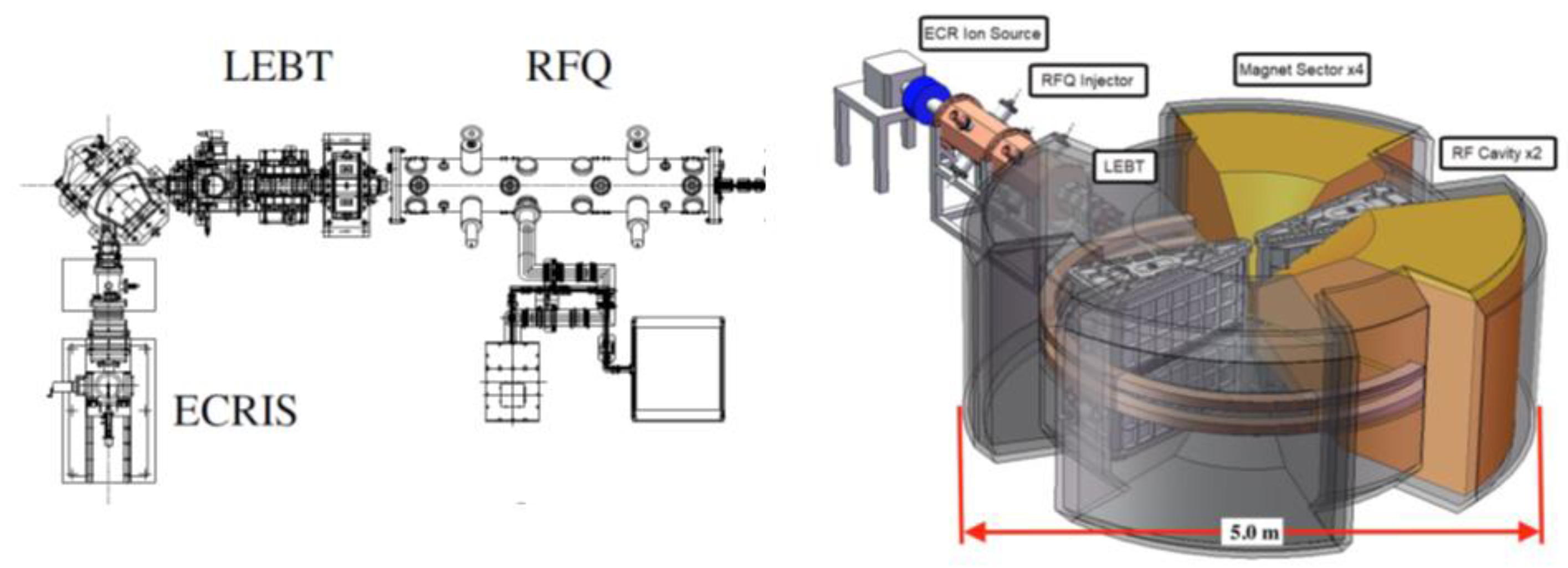
4.2.4. Source, LEBT, and RFQ, and Injector
4.2.5. Outline for the Non-Scaling FFGA for FLASH
5. FLASH Studies with Laser-Driven Particle Sources Developed at Lawrence Berkeley National Laboratory
5.1. Status of Laser-Driven Particle Sources
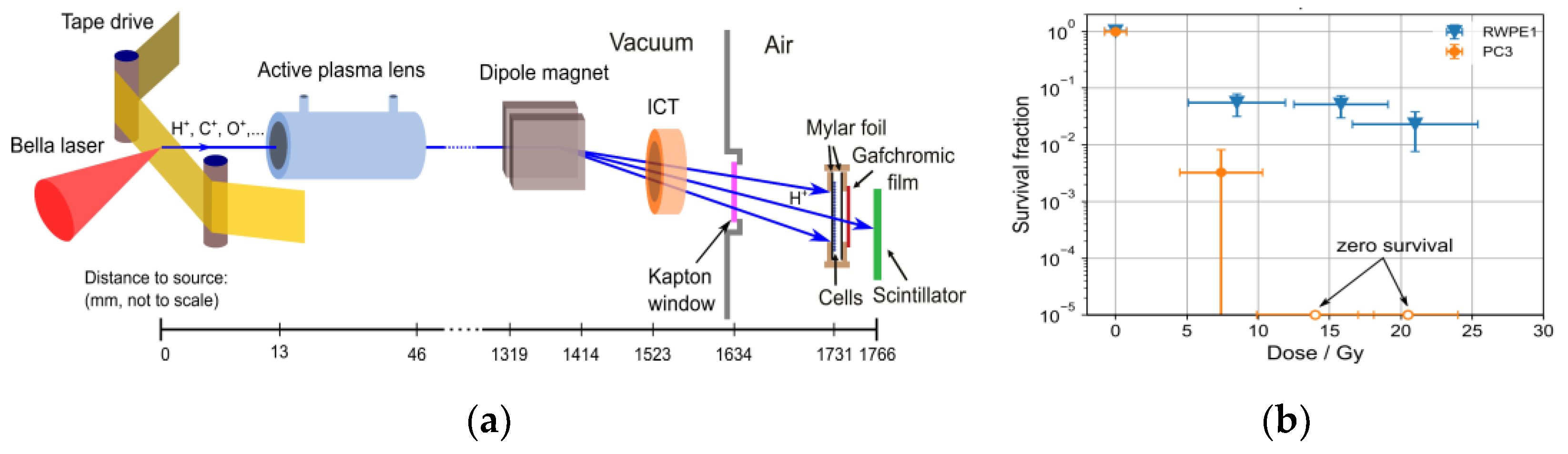
5.2. Laser-Driven Particle Sources for Preclinical Radiobiological Studies of the FLASH Effect
5.3. Potential of Laser-Driven Particle Sources for FLASH Radiation Therapy
5.4. Outlook for Laser-Driven FLASH
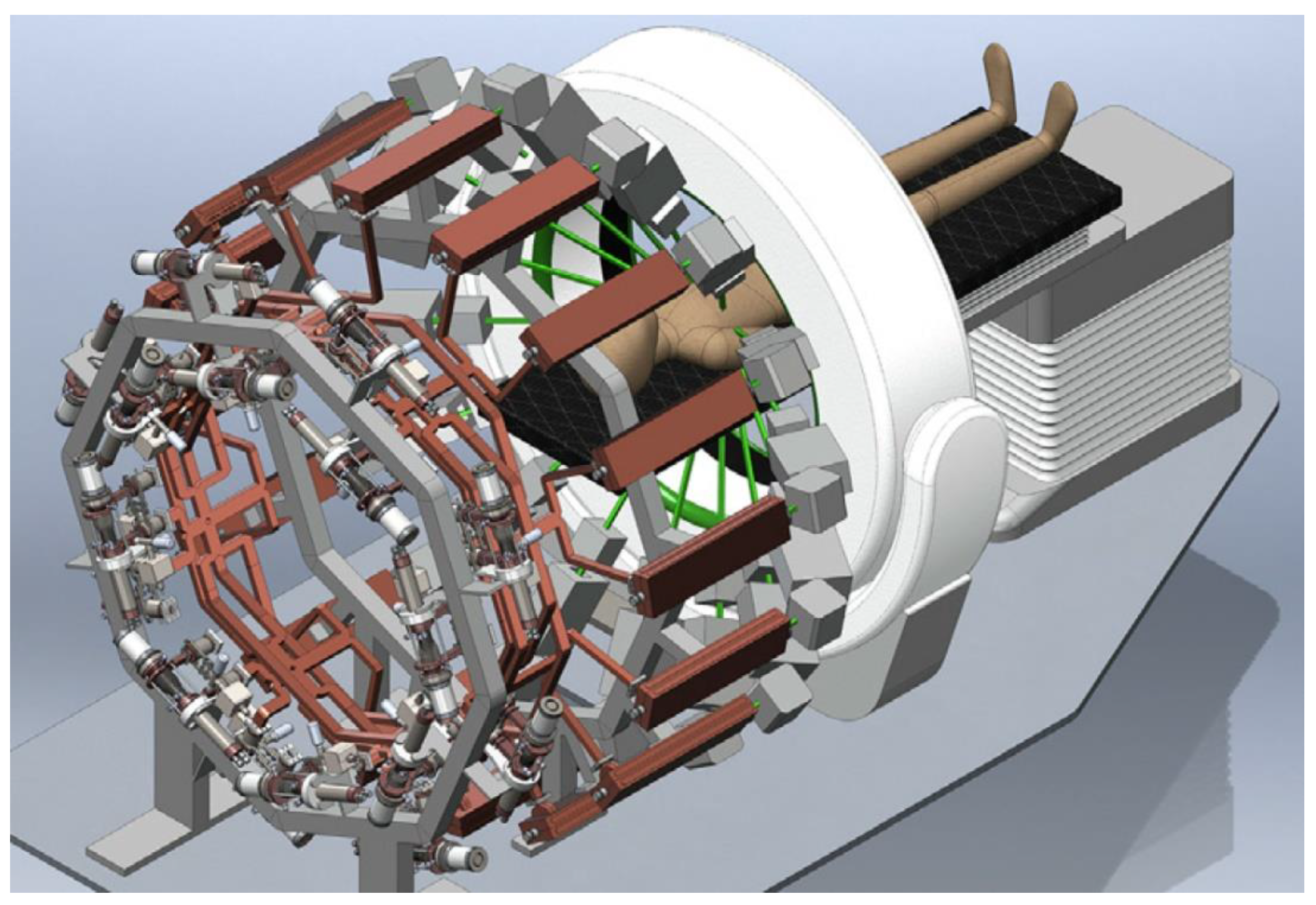
6. High-Peak-Current Linear Induction Accelerator (LIA) for FLASH-RT Developed at Lawrence Livermore National Laboratory
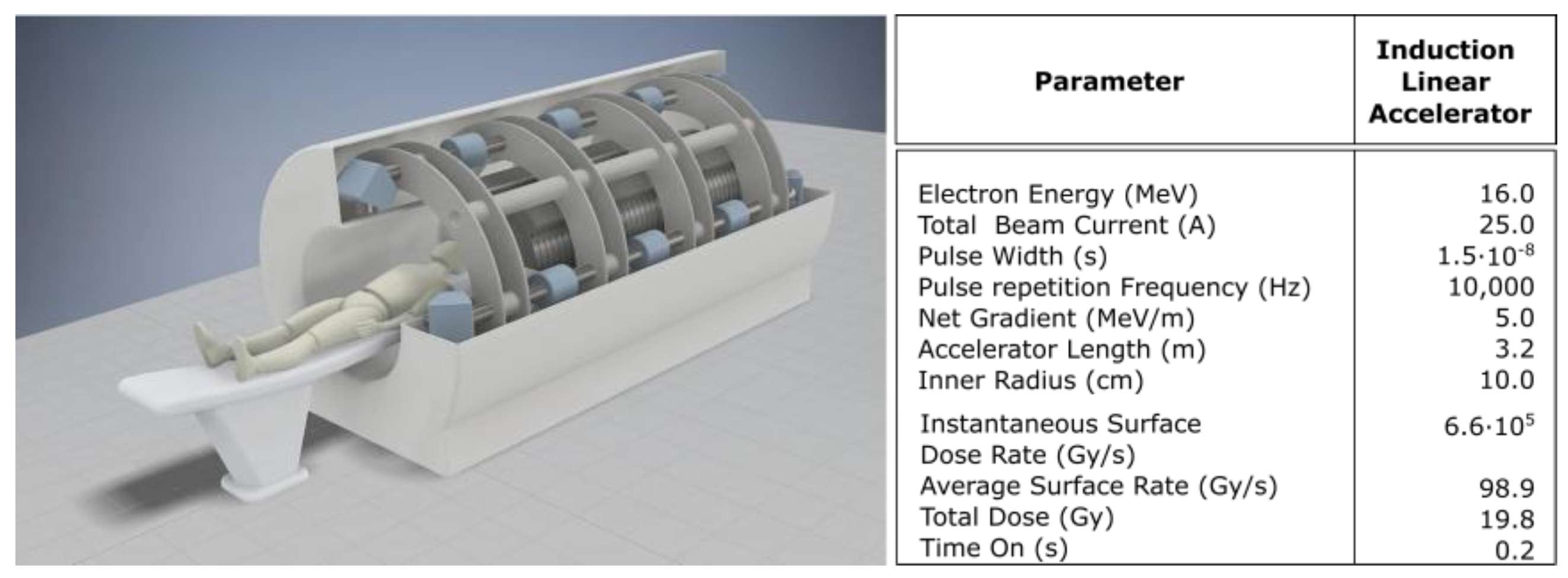
6.1. Illustrative Measurements from LIAs for FLASH-RT
6.2. Meeting the Criteria for FLASH-RT
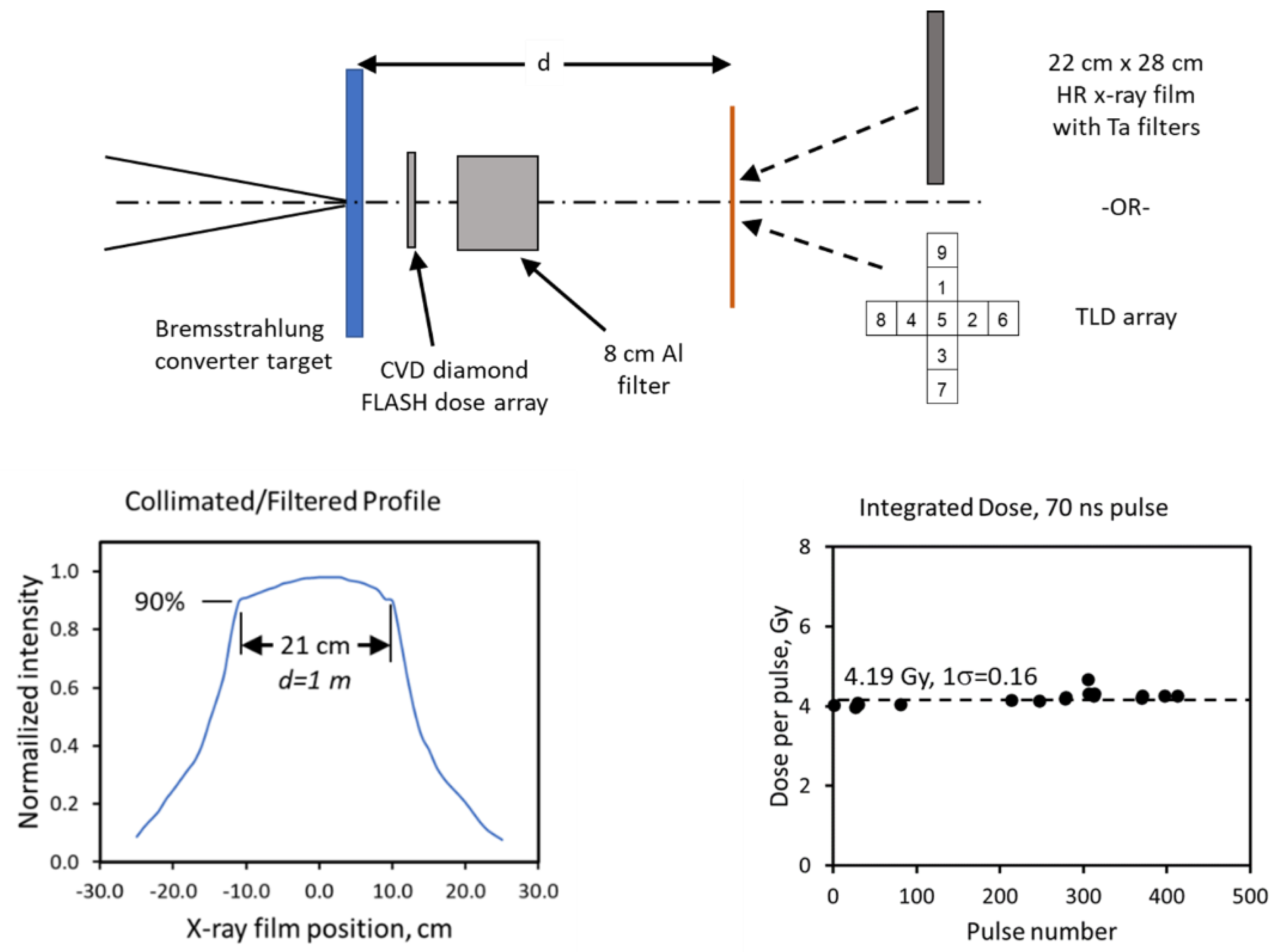
7. High-Current Electron Linear Accelerator for X-ray FLASH-RT Developed by RadiaBeam and UCLA
7.1. X-ray FLASH-RT
7.2. New Technology for X-ray FLASH-RT
8. Accelerator-Based Technology Developed at SLAC National Accelerator Laboratory and Stanford University
8.1. X-ray FLASH-RT with the PHASER
8.2. Very-High-Energy Electron (VHEE) FLASH-RT
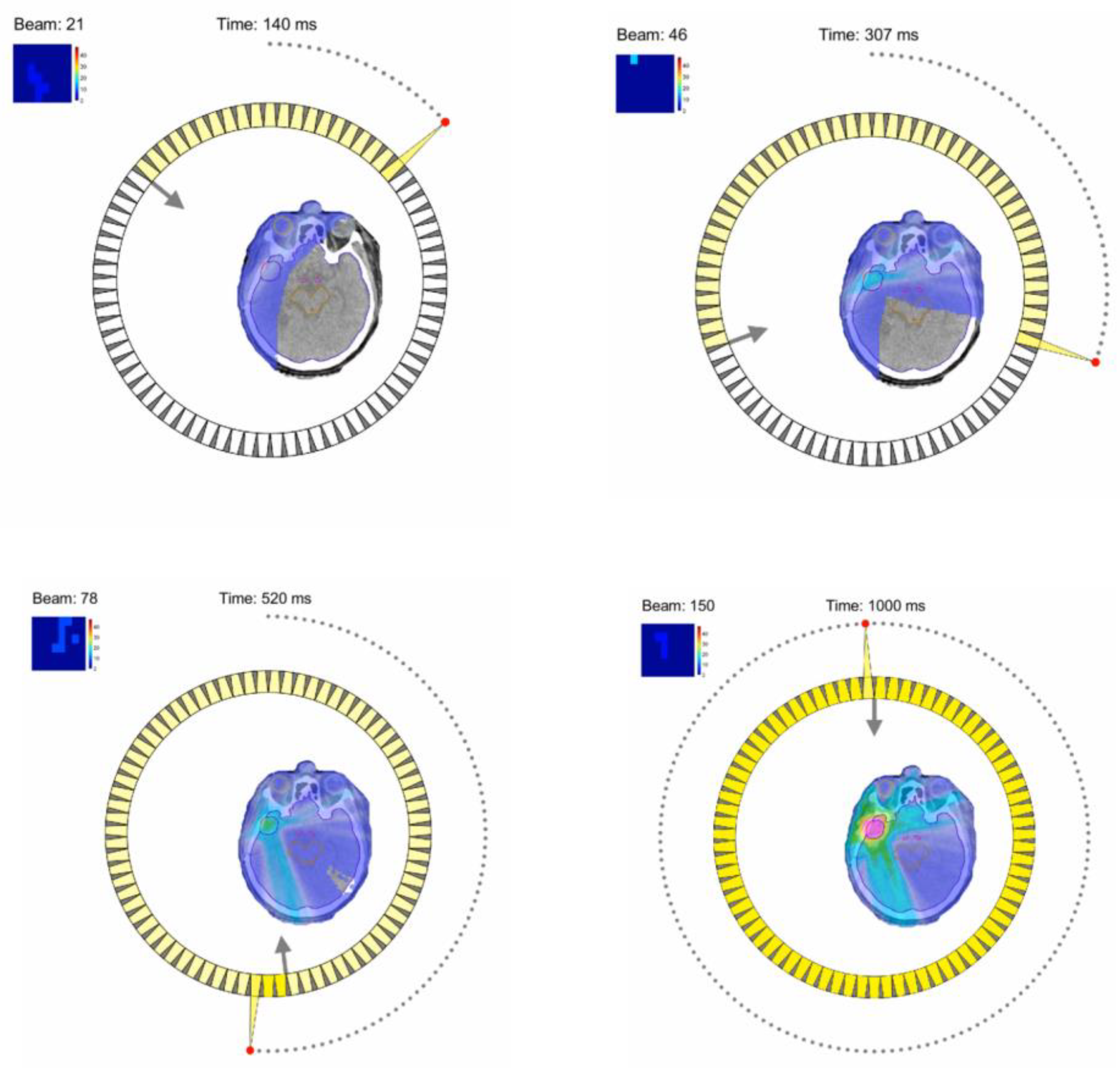
8.3. Fast 3D High-Speed Beam Scanner for Hadron FLASH-RT
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.-F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Gaide, O.; Herrera, F.; Sozzi, W.J.; Gonçalves Jorge, P.; Kinj, R.; Bailat, C.; Duclos, F.; Bochud, F.; Germond, J.-F.; Gondré, M.; et al. Comparison of ultra-high versus conventional dose rate radiotherapy in a patient with cutaneous lymphoma. Radiother. Oncol. 2022, 174, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Vozenin, M.-C.; Bourhis, J.; Durante, M. Towards clinical translation of FLASH radiotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Lin, H.; Choi, J.I.; Simone, C.B.; Kang, M. A Novel Proton Pencil Beam Scanning FLASH RT Delivery Method Enables Optimal OAR Sparing and Ultra-High Dose Rate Delivery: A Comprehensive Dosimetry Study for Lung Tumors. Cancers 2021, 13, 5790. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Lin, H.; Choi, J.I.; Press, R.H.; Lazarev, S.; Kabarriti, R.; Hajj, C.; Hasan, S.; Chhabra, A.M.; Ii, C.B.S.; et al. FLASH Radiotherapy Using Single-Energy Proton PBS Transmission Beams for Hypofractionation Liver Cancer: Dose and Dose Rate Quantification. Front. Oncol. 2022, 11, 813063. [Google Scholar] [CrossRef] [PubMed]
- Mascia, A.E.; Daugherty, E.C.; Zhang, Y.; Lee, E.; Xiao, Z.; Sertorio, M.; Woo, J.; Backus, L.R.; McDonald, J.M.; McCann, C.; et al. Proton FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases: The FAST-01 Nonrandomized Trial. JAMA Oncol. 2023, 9, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, E.C.; Mascia, A.; Zhang, Y.; Lee, E.; Xiao, Z.; Sertorio, M.; Woo, J.; McCann, C.; Russell, K.; Levine, L.; et al. FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases (FAST-01): Protocol for the First Prospective Feasibility Study. JMIR Res. Protoc. 2023, 12, e41812. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.J.; Hall, E.J.; Forster, D.W.; Storr, T.H.; Goodman, M.J. Survival of mammalian cells exposed to X rays at ultra-high dose-rates. Br. J. Radiol. 1969, 42, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Darafsheh, A.; Schuemann, J.; Dokic, I.; Lundh, O.; Zhao, T.; Ramos-Mendez, J.; Dong, L.; Petersson, K. Development of Ultra-High Dose-Rate (FLASH) Particle Therapy. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 252–262. [Google Scholar] [CrossRef]
- 1Esplen, N.; Mendonca, M.S.; Bazalova-Carter, M. Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy: A topical review. Phys. Med. Biol. 2020, 65, 23TR03. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front. Oncol. 2020, 9, 1563, Erratum in Front. Oncol. 2020, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J. Ultrahigh Dose-rate Radiotherapy: Next Steps for FLASH-RT. Clin. Cancer Res. 2019, 25, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.-F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother. Oncol. 2017, 124, 365–369. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Acharya, M.M.; Gonçalves Jorge, P.; Petit, B.; Petridis, I.G.; Fuchs, P.; Leavitt, R.; Petersson, K.; Gondré, M.; Ollivier, J.; et al. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma that Reduces Neurocognitive Side Effects in Mice. Clin. Cancer Res. 2021, 27, 775–784. [Google Scholar] [CrossRef]
- Karsch, L.; Pawelke, J.; Brand, M.; Hans, S.; Hideghéty, K.; Jansen, J.; Lessmann, E.; Löck, S.; Schürer, M.; Schurig, R.; et al. Beam pulse structure and dose rate as determinants for the flash effect observed in zebrafish embryo. Radiother. Oncol. 2022, 173, 49–54. [Google Scholar] [CrossRef]
- Cecchi, D.D.; Therriault-Proulx, F.; Lambert-Girard, S.; Hart, A.; Macdonald, A.; Pfleger, M.; Lenckowski, M.; Bazalova-Carter, M. Characterization of an x-ray tube-based ultrahigh dose-rate system for in vitro irradiations. Med. Phys. 2021, 48, 7399–7409. [Google Scholar] [CrossRef] [PubMed]
- Bazalova-Carter, M.; Esplen, N. On the capabilities of conventional x-ray tubes to deliver ultra-high (FLASH) dose rates. Med. Phys. 2019, 46, 5690–5695. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Iordachita, I.; Wong, J.W. Ultrahigh dose-rate (FLASH) x-ray irradiator for pre-clinical laboratory research. Phys. Med. Biol. 2021, 66, 095006. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, Y.; Zhu, H.; Wang, J.; Xiao, D.; Zhou, Z.; Dai, T.; Zhang, Y.; Feng, G.; Li, J.; et al. First demonstration of the FLASH effect with ultrahigh dose rate high-energy X-rays. Radiother. Oncol. 2022, 166, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.M.L.; Donoghue, J.F.; Ventura, J.A.; Livingstone, J.; Bailey, T.; Day, L.R.J.; Crosbie, J.C.; Rogers, P.A.W. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci. Rep. 2018, 8, 12044. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Bouchet, A.; Jaccard, M.; Patin, D.; Serduc, R.; Aim, W.; Petersson, K.; Petit, B.; Bailat, C.; Bourhis, J.; et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother. Oncol. 2018, 129, 582–588. [Google Scholar] [CrossRef]
- Beyreuther, E.; Brand, M.; Hans, S.; Hideghéty, K.; Karsch, L.; Leßmann, E.; Schürer, M.; Szabó, E.R.; Pawelke, J. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation. Radiother. Oncol. 2019, 139, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.; McCauley, S.; Vairamani, K.; Speth, J.; Girdhani, S.; Abel, E.; Sharma, R.A.; Perentesis, J.P.; Wells, S.I.; Mascia, A.; et al. FLASH Proton Pencil Beam Scanning Irradiation Minimizes Radiation-Induced Leg Contracture and Skin Toxicity in Mice. Cancers 2021, 13, 1012. [Google Scholar] [CrossRef] [PubMed]
- Diffenderfer, E.S.; Sørensen, B.S.; Mazal, A.; Carlson, D.J. The current status of preclinical proton FLASH radiation and future directions. Med. Phys. 2022, 49, 2039–2054. [Google Scholar] [CrossRef]
- Tinganelli, W.; Sokol, O.; Quartieri, M.; Puspitasari, A.; Dokic, I.; Abdollahi, A.; Durante, M.; Haberer, T.; Debus, J.; Boscolo, D.; et al. Ultra-High Dose Rate (FLASH) Carbon Ion Irradiation: Dosimetry and First Cell Experiments. Int. J. Radiat. Oncol. 2021, 112, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, T.; Mein, S.; Walsh, D.W.; Schuhmacher, N.; Liew, H.; Cee, R.; Galonska, M.; Scheloske, S.; Schömers, C.; Weber, U.; et al. FLASH Dose Rate Helium Ion Beams: First In Vitro Investigations. Int. J. Radiat. Oncol. 2021, 111, 1011–1022. [Google Scholar] [CrossRef]
- Evans, B.T.; Cooley, J.; Wagner, M.M.; Yu, T.; Zwart, T. Demonstration of the FLASH Effect Within the Spread-out Bragg Peak After Abdominal Irradiation of Mice. Int. J. Part. Ther. 2022, 8, 68–75. [Google Scholar] [CrossRef]
- Darafsheh, A.; Hao, Y.; Zhao, X.; Zwart, T.; Wagner, M.; Evans, T.; Reynoso, F.; Zhao, T. Spread-out Bragg peak proton FLASH irradiation using a clinical synchrocyclotron: Proof of concept and ion chamber characterization. Med. Phys. 2021, 48, 4472–4484. [Google Scholar] [CrossRef] [PubMed]
- Darafsheh, A.; Hao, Y.; Zwart, T.; Wagner, M.; Catanzano, D.; Williamson, J.F.; Knutson, N.; Sun, B.; Mutic, S.; Zhao, T. Feasibility of proton FLASH irradiation using a synchrocyclotron for preclinical studies. Med. Phys. 2020, 47, 4348–4355. [Google Scholar] [CrossRef] [PubMed]
- Hamm, R.W.; Crandall, K.R.; Potter, J.M. Preliminary Design of a Dedicated Proton Therapy Linac. In Proceedings of the Conference Record of the 1991 IEEE Particle Accelerator Conference, San Francisco, CA, USA, 6–9 May 1991; Volume 4, pp. 2583–2585. [Google Scholar]
- Lu, X.; Li, Z.; Dolgashev, V.; Bowden, G.; Sy, A.; Tantawi, S.; Nanni, E.A. A proton beam energy modulator for rapid proton therapy. Rev. Sci. Instruments 2021, 92, 024705. [Google Scholar] [CrossRef] [PubMed]
- Degiovanni, A.; Amaldi, U. Proton and Carbon Linacs for Hadron Therapy. In Proceedings of the 27th Linear Accelerator Conference, Geneva, Switzerland, 31 August–5 September 2014; p. 6. [Google Scholar]
- Ostroumov, P.N.; Goel, A.; Mustapha, B.; Nassiri, A.; Plastun, A.S. Compact Carbon Ion Linac. In Proceedings of the 2016 North American Particle Accelerator Conference, Chicago, IL, USA, 14 October 2016; p. 3. [Google Scholar]
- Ungaro, D.; Degiovanni, A.; Stabile, P.; Sa, A. LIGHT: A Linear Accelerator for Proton Therapy. In Proceedings of the 2016 North American Particle Accelerator Conference, Chicago, IL, USA, 9–14 October 2016; p. 5. [Google Scholar]
- Jolly, S.; Owen, H.; Schippers, M.; Welsch, C. Technical challenges for FLASH proton therapy. Phys. Medica Eur. J. Med. Phys. 2020, 78, 71–82. [Google Scholar] [CrossRef]
- Benedetti, S.; Degiovanni, A.; Grudiev, A.; Wuensch, W.; Amaldi, U. RF design of a novel s-band backward travelling wave linac for proton therapy. In Proceedings of the 27th Linear Accelerator Conference, Geneva, Switzerland, 31 August–5 September 2014; p. 3. [Google Scholar]
- Kutsaev, S.V.; Agustsson, R.; Boucher, S.; Fischer, R.; Murokh, A.; Mustapha, B.; Nassiri, A.; Ostroumov, P.N.; Plastun, A.; Savin, E.; et al. High-gradient low- β accelerating structure using the first negative spatial harmonic of the fundamental mode. Phys. Rev. Accel. Beams 2017, 20, 120401. [Google Scholar] [CrossRef]
- Kutsaev, S.V.; Agustsson, R.; Boucher, S.; Chimalpopoca, O.; Meyer, D.; Murokh, A.; Mustapha, B.; Nassiri, A.; Smirnov, A.Y.; Smith, T.; et al. Test Results of a High-Gradient 2.856-GHz Negative Harmonic Accelerating Waveguide. IEEE Microw. Wirel. Components Lett. 2021, 31, 1098–1101. [Google Scholar] [CrossRef]
- Mustapha, B.; Abogoda, A.; Barcikowski, A.; Fischer, R.; Nassiri, A. Design of a High-Gradient S-Band Annular Coupled Structure. In Proceedings of the 3rd North American Particle Accelerator Conference (NAPAC2019), Lansing, MI, USA, 2–6 September 2019; p. WEPLM72. [Google Scholar]
- Matsuura, T.; Egashira, Y.; Nishio, T.; Matsumoto, Y.; Wada, M.; Koike, S.; Furusawa, Y.; Kohno, R.; Nishioka, S.; Kameoka, S.; et al. Apparent absence of a proton beam dose rate effect and possible differences in RBE between Bragg peak and plateau. Med. Phys. 2010, 37, 5376–5381. [Google Scholar] [CrossRef]
- Mustapha, B.; Aydogan, B.; Nolen, J.; Nassiri, A.; Noonan, J.; Pankuch, M.; Welsh, J.; Schulte, R.; Robb, J. Prospects for an advanced heavy ion therapy center in the Chicago area. AIP Conf. Proc. 2019, 2160, 050009. [Google Scholar] [CrossRef]
- Fornek, T.E. Advanced Photon Source Upgrade Project Preliminary Design Report; Argonne National Laboratory: Argonne, IL, USA, 2017; p. APSU-2.01-RPT-002. [Google Scholar]
- de Walle, J.V.; Abs, M.; Conjat, M.; Forton, E.; Henrotin, S.; Jongen, Y.; Kleeven, W.; Mandrillon, J.; Mandrillon, P.; Verbruggen, P. The S2C2: From Source to Extraction. In Proceedings of the Cyclotrons 2016, Zurich, Switzerland, 11–16 September 2016; p. 5. [Google Scholar]
- Yoshimoto, M.; Adachi, T.; Aiba, M.; Koba, K.; Machida, S.; Mori, Y.; Muramatsu, R.; Ohmori, C.; Sakai, I.; Sato, Y.; et al. Recent Beam Studies of the PoP FFAG Proton Synchrotron. In Proceedings of the PACS2001. Proceedings of the 2001 Particle Accelerator Conference (Cat. No.01CH37268), Chicago, IL, USA, 18–22 June 2001; IEEE: Chicago, IL, USA, 2001; Volume 1, pp. 51–53. [Google Scholar]
- Yonemura, Y.; Ikeda, N.; Matoba, M.; Aiba, M.; Machida, S.; Mori, Y.; Muto, A.; Nakano, J.; Ohmori, C.; Okabe, K.; et al. Development of FFAG Accelerator at KEK. In Proceedings of the 2005 Particle Accelerator Conference, Knoxville, TN, USA, 16–20 May 2005; IEEE: Knoxville, TN, USA, 2005; pp. 1943–1945. [Google Scholar]
- Antoine, S.; Autin, B.; Beeckman, W.; Collot, J.; Conjat, M.; Forest, F.; Fourrier, J.; Froidefond, E.; Lancelot, J.; Mandrillon, J.; et al. Principle design of a protontherapy, rapid-cycling, variable energy spiral FFAG. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers, Detect. Assoc. Equip. 2009, 602, 293–305. [Google Scholar] [CrossRef]
- Planche, T.; Fourrier, J.; Lancelot, J.; Méot, F.; Neuvéglise, D.; Pasternak, J. Design of a prototype gap shaping spiral dipole for a variable energy protontherapy FFAG. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers, Detect. Assoc. Equip. 2009, 604, 435–442. [Google Scholar] [CrossRef]
- Available online: https://indico.fnal.gov/event/2672/contributions/77834/attachments/48652/58457/FMeot1-090921.pdf (accessed on 9 September 2022).
- Johnstone, C.; Schulte, R. A Review of Nonscaling CW FFAs for Proton and Ion Therapy Applications; Fermi National Accelerator Lab. (FNAL): Batavia, IL USA, 2019. [Google Scholar]
- Johnstone, C.; Kutsaev, S.V.; Lanza, R.; Boucher, S.; Johnson, R. High-Current Light-Ion Cyclotron for Applications in Nuclear Security and Radioisotope Production. IEEE Trans. Nucl. Sci. 2021, 68, 1072–1082. [Google Scholar] [CrossRef]
- Kutsaev, S.V.; Johnstone, C.; Adonyev, O.; Agustsson, R.; Ford, R.; Kashikhin, V. Electromagnetic and Engineering Design of a High-Current 15-MeV/u Cyclotron. IEEE Trans. Nucl. Sci. 2021, 68, 1083–1093. [Google Scholar] [CrossRef]
- Bulanov, S.; Esirkepov, T.; Khoroshkov, V.; Kuznetsov, A.; Pegoraro, F. Oncological hadrontherapy with laser ion accelerators. Phys. Lett. A 2002, 299, 240–247. [Google Scholar] [CrossRef]
- Emma, C.; van Tilborg, J.; Albert, F.; Labate, L.; England, J.; Gessner, S.; Fiuza, F.; Obst-Huebl, L.; Zholents, A.; Murokh, A.; et al. Snowmass 2021 Accelerator Frontier White Paper: Near Term Applications Driven by Advanced Accelerator Concepts. arXiv 2022, arXiv:2203.09094. [Google Scholar]
- Strickland, D.; Mourou, G. Compression of amplified chirped optical pulses. Opt. Commun. 1985, 56, 219–221. [Google Scholar] [CrossRef]
- Nakamura, K.; Mao, H.-S.; Gonsalves, A.J.; Vincenti, H.; Mittelberger, D.E.; Daniels, J.; Magana, A.; Toth, C.; Leemans, W.P. Diagnostics, Control and Performance Parameters for the BELLA High Repetition Rate Petawatt Class Laser. IEEE J. Quantum Electron. 2017, 53, 1–21. [Google Scholar] [CrossRef]
- Tajima, T.; Dawson, J.M. Laser Electron Accelerator. Phys. Rev. Lett. 1979, 43, 267–270. [Google Scholar] [CrossRef]
- Wilks, S.C.; Langdon, A.B.; Cowan, T.E.; Roth, M.; Singh, M.; Hatchett, S.; Key, M.H.; Pennington, D.; MacKinnon, A.; Snavely, R.A. Energetic proton generation in ultra-intense laser–solid interactions. Phys. Plasmas 2001, 8, 542–549. [Google Scholar] [CrossRef]
- Wang, X.; Zgadzaj, R.; Fazel, N.; Li, Z.; Yi, S.A.; Zhang, X.; Henderson, W.; Chang, Y.-Y.; Korzekwa, R.; Tsai, H.-E.; et al. Quasi-monoenergetic laser-plasma acceleration of electrons to 2 GeV. Nat. Commun. 2013, 4, 1988. [Google Scholar] [CrossRef]
- Leemans, W.P.; Gonsalves, A.J.; Mao, H.-S.; Nakamura, K.; Benedetti, C.; Schroeder, C.B.; Tóth, C.; Daniels, J.; Mittelberger, D.E.; Bulanov, S.S.; et al. Multi-GeV Electron Beams from Capillary-Discharge-Guided Subpetawatt Laser Pulses in the Self-Trapping Regime. Phys. Rev. Lett. 2014, 113, 245002. [Google Scholar] [CrossRef] [PubMed]
- Couperus, J.P.; Pausch, R.; Köhler, A.; Zarini, O.; Krämer, J.M.; Garten, M.; Huebl, A.; Gebhardt, R.; Helbig, U.; Bock, S.; et al. Demonstration of a beam loaded nanocoulomb-class laser wakefield accelerator. Nat. Commun. 2017, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, A.J.; Nakamura, K.; Daniels, J.; Benedetti, C.; Pieronek, C.; de Raadt, T.C.H.; Steinke, S.; Bin, J.H.; Bulanov, S.S.; van Tilborg, J.; et al. Petawatt Laser Guiding and Electron Beam Acceleration to 8 GeV in a Laser-Heated Capillary Discharge Waveguide. Phys. Rev. Lett. 2019, 122, 084801. [Google Scholar] [CrossRef] [PubMed]
- Leemans, W.P.; Geddes, C.G.R.; Faure, J.; Tóth, C.; van Tilborg, J.; Schroeder, C.B.; Esarey, E.; Fubiani, G.; Auerbach, D.; Marcelis, B.; et al. Observation of Terahertz Emission from a Laser-Plasma Accelerated Electron Bunch Crossing a Plasma-Vacuum Boundary. Phys. Rev. Lett. 2003, 91, 074802. [Google Scholar] [CrossRef] [PubMed]
- Powers, N.D.; Ghebregziabher, I.; Golovin, G.; Liu, C.; Chen, S.; Banerjee, S.; Zhang, J.; Umstadter, D. Quasi-monoenergetic and tunable X-rays from a laser-driven Compton light source. Nat. Photon. 2014, 8, 28–31. [Google Scholar] [CrossRef]
- Phuoc, K.T.; Corde, S.; Thaury, C.; Malka, V.; Tafzi, A.; Goddet, J.-P.; Shah, R.C.; Sebban, S.; Rousse, A. All-optical Compton gamma-ray source. Nat. Photon- 2012, 6, 308–311. [Google Scholar] [CrossRef]
- Snavely, R.A.; Key, M.H.; Hatchett, S.P.; Cowan, T.E.; Roth, M.; Phillips, T.W.; Stoyer, M.A.; Henry, E.A.; Sangster, T.C.; Singh, M.S.; et al. Intense High-Energy Proton Beams from Petawatt-Laser Irradiation of Solids. Phys. Rev. Lett. 2000, 85, 2945–2948. [Google Scholar] [CrossRef] [PubMed]
- Hatchett, S.P.; Brown, C.G.; Cowan, T.E.; Henry, E.A.; Johnson, J.S.; Key, M.H.; Koch, J.A.; Langdon, A.B.; Lasinski, B.F.; Lee, R.W.; et al. Electron, photon, and ion beams from the relativistic interaction of Petawatt laser pulses with solid targets. Phys. Plasmas 2000, 7, 2076–2082. [Google Scholar] [CrossRef]
- Steinke, S.; Bin, J.H.; Park, J.; Ji, Q.; Nakamura, K.; Gonsalves, A.J.; Bulanov, S.S.; Thévenet, M.; Toth, C.; Vay, J.-L.; et al. Acceleration of high charge ion beams with achromatic divergence by petawatt laser pulses. Phys. Rev. Accel. Beams 2020, 23, 021302. [Google Scholar] [CrossRef]
- Schreiber, J.; Bolton, P.R.; Parodi, K. Invited Review Article: “Hands-on” laser-driven ion acceleration: A primer for laser-driven source development and potential applications. Rev. Sci. Instruments 2016, 87, 071101. [Google Scholar] [CrossRef] [PubMed]
- Macchi, A.; Borghesi, M.; Passoni, M. Ion acceleration by superintense laser-plasma interaction. Rev. Mod. Phys. 2013, 85, 751–793. [Google Scholar] [CrossRef]
- Daido, H.; Nishiuchi, M.; Pirozhkov, A. Review of laser-driven ion sources and their applications. Rep. Prog. Phys. 2012, 75, 056401. [Google Scholar] [CrossRef] [PubMed]
- Higginson, A.; Gray, R.J.; King, M.; Dance, R.J.; Williamson, S.D.R.; Butler, N.M.H.; Wilson, R.; Capdessus, R.; Armstrong, C.; Green, J.S.; et al. Near-100 MeV protons via a laser-driven transparency-enhanced hybrid acceleration scheme. Nat. Commun. 2018, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Friedl, A.A.; Prise, K.M.; Butterworth, K.T.; Montay-Gruel, P.; Favaudon, V. Radiobiology of the FLASH effect. Med. Phys. 2022, 49, 1993–2013. [Google Scholar] [CrossRef]
- Bolton, P.; Parodi, K.; Schreiber, J. Applications of Laser-Driven Particle Acceleration; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-0-429-81709-0. [Google Scholar]
- Durante, M. New challenges in high-energy particle radiobiology. Br. J. Radiol. 2014, 87, 20130626. [Google Scholar] [CrossRef] [PubMed]
- Kroll, F.; Brack, F.-E.; Bernert, C.; Bock, S.; Bodenstein, E.; Brüchner, K.; Cowan, T.E.; Gaus, L.; Gebhardt, R.; Helbig, U.; et al. Tumour irradiation in mice with a laser-accelerated proton beam. Nat. Phys. 2022, 18, 316–322. [Google Scholar] [CrossRef]
- Yogo, A.; Sato, K.; Nishikino, M.; Mori, M.; Teshima, T.; Numasaki, H.; Murakami, M.; Demizu, Y.; Akagi, S.; Nagayama, S.; et al. Application of laser-accelerated protons to the demonstration of DNA double-strand breaks in human cancer cells. Appl. Phys. Lett. 2009, 94, 181502. [Google Scholar] [CrossRef]
- Kraft, S.; Richter, C.; Zeil, K.; Bussmann, M.; Beyreuther, E.; Bock, S.; Bussmann, M.; E Cowan, T.; Dammene, Y.; Enghardt, W.; et al. Dose-dependent biological damage of tumour cells by laser-accelerated proton beams. New J. Phys. 2010, 12, 085003. [Google Scholar] [CrossRef]
- Yogo, A.; Maeda, T.; Hori, T.; Sakaki, H.; Ogura, K.; Nishiuchi, M.; Sagisaka, A.; Kiriyama, H.; Okada, H.; Kanazawa, S.; et al. Measurement of relative biological effectiveness of protons in human cancer cells using a laser-driven quasimonoenergetic proton beamline. Appl. Phys. Lett. 2011, 98, 053701. [Google Scholar] [CrossRef]
- Bin, J.; Allinger, K.; Assmann, W.; Dollinger, G.; Drexler, G.A.; Friedl, A.A.; Habs, D.; Hilz, P.; Hoerlein, R.; Humble, N.; et al. A laser-driven nanosecond proton source for radiobiological studies. Appl. Phys. Lett. 2012, 101, 243701. [Google Scholar] [CrossRef]
- Doria, D.; Kakolee, K.F.; Kar, S.; Litt, S.K.; Fiorini, F.; Ahmed, H.; Green, S.; Jeynes, J.C.G.; Kavanagh, J.; Kirby, D.; et al. Biological effectiveness on live cells of laser driven protons at dose rates exceeding 109Gy/s. AIP Adv. 2012, 2, 011209. [Google Scholar] [CrossRef]
- Raschke, S.; Spickermann, S.; Toncian, T.; Swantusch, M.; Boeker, J.; Giesen, U.; Iliakis, G.; Willi, O.; Boege, F. Ultra-short laser-accelerated proton pulses have similar DNA-damaging effectiveness but produce less immediate nitroxidative stress than conventional proton beams. Sci. Rep. 2016, 6, 32441. [Google Scholar] [CrossRef] [PubMed]
- Manti, L.; Perozziello, F.; Borghesi, M.; Candiano, G.; Chaudhary, P.; Cirrone, G.; Doria, D.; Gwynne, D.; Leanza, R.; Prise, K.M.; et al. The radiobiology of laser-driven particle beams: Focus on sub-lethal responses of normal human cells. J. Instrum. 2017, 12, C03084. [Google Scholar] [CrossRef][Green Version]
- Bayart, E.; Flacco, A.; Delmas, O.; Pommarel, L.; Levy, D.; Cavallone, M.; Megnin-Chanet, F.; Deutsch, E.; Malka, V. Fast dose fractionation using ultra-short laser accelerated proton pulses can increase cancer cell mortality, which relies on functional PARP1 protein. Sci. Rep. 2019, 9, 10132. [Google Scholar] [CrossRef] [PubMed]
- Hanton, F.; Chaudhary, P.; Doria, D.; Gwynne, D.; Maiorino, C.; Scullion, C.; Ahmed, H.; Marshall, T.; Naughton, K.; Romagnani, L.; et al. DNA DSB Repair Dynamics following Irradiation with Laser-Driven Protons at Ultra-High Dose Rates. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rösch, T.F.; Szabó, Z.; Haffa, D.; Bin, J.; Brunner, S.; Englbrecht, F.S.; Friedl, A.A.; Gao, Y.; Hartmann, J.; Hilz, P.; et al. A feasibility study of zebrafish embryo irradiation with laser-accelerated protons. Rev. Sci. Instruments 2020, 91, 063303. [Google Scholar] [CrossRef]
- Han, J.; Mei, Z.; Lu, C.; Qian, J.; Liang, Y.; Sun, X.; Pan, Z.; Kong, D.; Xu, S.; Liu, Z.; et al. Ultra-High Dose Rate FLASH Irradiation Induced Radio-Resistance of Normal Fibroblast Cells Can Be Enhanced by Hypoxia and Mitochondrial Dysfunction Resulting From Loss of Cytochrome C. Front. Cell Dev. Biol. 2021, 9, 672929. [Google Scholar] [CrossRef] [PubMed]
- Bin, J.; Obst-Huebl, L.; Mao, J.-H.; Nakamura, K.; Geulig, L.D.; Chang, H.; Ji, Q.; He, L.; De Chant, J.; Kober, Z.; et al. A new platform for ultra-high dose rate radiobiological research using the BELLA PW laser proton beamline. Sci. Rep. 2022, 12, 1484. [Google Scholar] [CrossRef]
- Laschinsky, L.; Baumann, M.; Beyreuther, E.; Enghardt, W.; Kaluza, M.; Karsch, L.; Lessmann, E.; Naumburger, D.; Nicolai, M.; Richter, C.; et al. Radiobiological effectiveness of laser accelerated electrons in comparison to electron beams from a conventional linear accelerator. J. Radiat. Res. 2012, 53, 395–403. [Google Scholar] [CrossRef]
- Labate, L.; Andreassi, M.G.; Baffigi, F.; Basta, G.; Bizzarri, R.; Borghini, A.; Candiano, G.C.; Casarino, C.; Cresci, M.; Di Martino, F.; et al. Small-scale laser based electron accelerators for biology and medicine: A comparative study of the biological effectiveness. 2013, 8779, 87790O. Proceedings 2013, 8779, 87790O. [Google Scholar] [CrossRef]
- Oppelt, M.; Baumann, M.; Bergmann, R.; Beyreuther, E.; Brüchner, K.; Hartmann, J.; Karsch, L.; Krause, M.; Laschinsky, L.; Leßmann, E.; et al. Comparison study of in vivo dose response to laser-driven versus conventional electron beam. Radiat. Environ. Biophys. 2015, 54, 155–166. [Google Scholar] [CrossRef]
- Andreassi, M.G.; Borghini, A.; Pulignani, S.; Baffigi, F.; Fulgentini, L.; Koester, P.; Cresci, M.; Vecoli, C.; Lamia, D.; Russo, G.; et al. Radiobiological Effectiveness of Ultrashort Laser-Driven Electron Bunches: Micronucleus Frequency, Telomere Shortening and Cell Viability. Radiat. Res. 2016, 186, 245–253. [Google Scholar] [CrossRef]
- Babayan, N.; Grigoryan, B.; Khondkaryan, L.; Tadevosyan, G.; Sarkisyan, N.; Grigoryan, R.; Apresyan, L.; Aroutiounian, R.; Vorobyeva, N.; Pustovalova, M.; et al. Laser-Driven Ultrashort Pulsed Electron Beam Radiation at Doses of 0.5 and 1.0 Gy Induces Apoptosis in Human Fibroblasts. Int. J. Mol. Sci. 2019, 20, 5140. [Google Scholar] [CrossRef]
- Cavallone, M.; Rovige, L.; Huijts, J.; Bayart, É.; Delorme, R.; Vernier, A.; Gonçalves Jorge, P.; Moeckli, R.; Deutsch, E.; Faure, J.; et al. Dosimetric characterisation and application to radiation biology of a kHz laser-driven electron beam. Appl. Phys. B 2021, 127, 57. [Google Scholar] [CrossRef]
- Desrosiers, C.; Moskvin, V.; Bielajew, A.F.; Papiez, L. 150-250 MeV electron beams in radiation therapy. Phys. Med. Biol. 2000, 45, 1781–1805. [Google Scholar] [CrossRef] [PubMed]
- Kokurewicz, K.; Welsh, G.H.; Brunetti, E.; Wiggins, S.M.; Boyd, M.; Sorensen, A.; Chalmers, A.; Schettino, G.; Subiel, A.; DesRosiers, C.; et al. Laser-Plasma Generated Very High Energy Electrons (VHEEs) in Radiotherapy; SPIE: Bellingham, WA, USA, 2017. [Google Scholar]
- Labate, L.; Palla, D.; Panetta, D.; Avella, F.; Baffigi, F.; Brandi, F.; Di Martino, F.; Fulgentini, L.; Giulietti, A.; Köster, P.; et al. Toward an effective use of laser-driven very high energy electrons for radiotherapy: Feasibility assessment of multi-field and intensity modulation irradiation schemes. Sci. Rep. 2020, 10, 17307. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, K.; Guénot, D.; Svensson, J.B.; Petersson, K.; Persson, A.; Lundh, O. A focused very high energy electron beam for fractionated stereotactic radiotherapy. Sci. Rep. 2021, 11, 5844. [Google Scholar] [CrossRef]
- Pommarel, L.; Vauzour, B.; Mégnin-Chanet, F.; Bayart, E.; Delmas, O.; Goudjil, F.; Nauraye, C.; Letellier, V.; Pouzoulet, F.; Schillaci, F.; et al. Spectral and spatial shaping of a laser-produced ion beam for radiation-biology experiments. Phys. Rev. Accel. Beams 2017, 20, 032801. [Google Scholar] [CrossRef]
- Brack, F.-E.; Kroll, F.; Gaus, L.; Bernert, C.; Beyreuther, E.; Cowan, T.E.; Karsch, L.; Kraft, S.; Kunz-Schughart, L.A.; Lessmann, E.; et al. Spectral and spatial shaping of laser-driven proton beams using a pulsed high-field magnet beamline. Sci. Rep. 2020, 10, 9118. [Google Scholar] [CrossRef]
- Cirrone, G.A.P.; Petringa, G.; Catalano, R.; Schillaci, F.; Allegra, L.; Amato, A.; Avolio, R.; Costa, M.; Cuttone, G.; Fajstavr, A.; et al. ELIMED-ELIMAIA: The First Open User Irradiation Beamline for Laser-Plasma-Accelerated Ion Beams. Front. Phys. 2020, 8, 437. [Google Scholar] [CrossRef]
- Brüchner, K.; Beyreuther, E.; Baumann, M.; Krause, M.; Oppelt, M.; Pawelke, J. Establishment of a small animal tumour model for in vivo studies with low energy laser accelerated particles. Radiat. Oncol. 2014, 9, 57. [Google Scholar] [CrossRef]
- van Tilborg, J.; Steinke, S.; Geddes, C.G.R.; Matlis, N.H.; Shaw, B.H.; Gonsalves, A.J.; Huijts, J.V.; Nakamura, K.; Daniels, J.; Schroeder, C.B.; et al. Active Plasma Lensing for Relativistic Laser-Plasma-Accelerated Electron Beams. Phys. Rev. Lett. 2015, 115, 184802. [Google Scholar] [CrossRef]
- Linz, U.; Alonso, J. Laser-driven ion accelerators for tumor therapy revisited. Phys. Rev. Accel. Beams 2016, 19, 124802. [Google Scholar] [CrossRef]
- Masood, U.; Cowan, T.E.; Enghardt, W.; Hofmann, K.M.; Karsch, L.; Kroll, F.; Schramm, U.; Wilkens, J.J.; Pawelke, J. A light-weight compact proton gantry design with a novel dose delivery system for broad-energetic laser-accelerated beams. Phys. Med. Biol. 2017, 62, 5531–5555. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.M.; Masood, U.; Pawelke, J.; Wilkens, J.J. A treatment planning study to assess the feasibility of laser-driven proton therapy using a compact gantry design. Med. Phys. 2015, 42, 5120–5129. [Google Scholar] [CrossRef]
- Gotz, M.; Karsch, L.; Pawelke, J. A new model for volume recombination in plane-parallel chambers in pulsed fields of high dose-per-pulse. Phys. Med. Biol. 2017, 62, 8634–8654. [Google Scholar] [CrossRef]
- Geulig, L.D.; Obst-Huebl, L.; Nakamura, K.; Bin, J.; Ji, Q.; Steinke, S.; Snijders, A.M.; Mao, J.-H.; Blakely, E.A.; Gonsalves, A.J.; et al. Online charge measurement for petawatt laser-driven ion acceleration. Rev. Sci. Instruments 2022, 93, 103301. [Google Scholar] [CrossRef] [PubMed]
- Bin, J.H.; Ji, Q.; Seidl, P.A.; Raftrey, D.; Steinke, S.; Persaud, A.; Nakamura, K.; Gonsalves, A.; Leemans, W.P.; Schenkel, T. Absolute calibration of GafChromic film for very high flux laser driven ion beams. Rev. Sci. Instruments 2019, 90, 053301. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Karsch, L.; Dammene, Y.; Kraft, S.; Metzkes-Ng, J.; Schramm, U.; Schürer, M.; Sobiella, M.; Weber, A.; Zeil, K.; et al. A dosimetric system for quantitative cell irradiation experiments with laser-accelerated protons. Phys. Med. Biol. 2011, 56, 1529–1543. [Google Scholar] [CrossRef]
- Zeil, K.; Baumann, M.; Beyreuther, E.; Burris-Mog, T.; Cowan, T.E.; Enghardt, W.; Karsch, L.; Kraft, S.; Laschinsky, L.; Metzkes-Ng, J.; et al. Dose-controlled irradiation of cancer cells with laser-accelerated proton pulses. Appl. Phys. B 2013, 110, 437–444. [Google Scholar] [CrossRef]
- Esirkepov, T.; Borghesi, M.; Bulanov, S.V.; Mourou, G.; Tajima, T. Highly Efficient Relativistic-Ion Generation in the Laser-Piston Regime. Phys. Rev. Lett. 2004, 92, 175003. [Google Scholar] [CrossRef]
- Park, J.; Bulanov, S.S.; Bin, J.; Ji, Q.; Steinke, S.; Vay, J.-L.; Geddes, C.G.R.; Schroeder, C.; Leemans, W.P.; Schenkel, T.; et al. Ion acceleration in laser generated megatesla magnetic vortex. Phys. Plasmas 2019, 26, 103108. [Google Scholar] [CrossRef]
- Fiuza, F.; Stockem, A.; Boella, E.; Fonseca, R.A.; Silva, L.O.; Haberberger, D.; Tochitsky, S.; Mori, W.B.; Joshi, C. Ion acceleration from laser-driven electrostatic shocks. Phys. Plasmas 2013, 20, 056304. [Google Scholar] [CrossRef]
- Kiani, L.; Zhou, T.; Bahk, S.-W.; Bromage, J.; Bruhwiler, D.; Campbell, E.M.; Chang, Z.; Chowdhury, E.; Downer, M.; Du, Q.; et al. High Average Power Ultrafast Laser Technologies for Driving Future Advanced Accelerators. arXiv 2022, arXiv:2204.10774. [Google Scholar]
- Vozenin, M.; Herman, D. Suit Plenary Lecture - From Irradiation at Ultrahigh Dose Rate to FLASH Radiotherapy: New Radiobiology to Support Clinical Translation. arXiv 2022, arXiv:2204.10774. Presented at the 67th Annual International Research Society. [Google Scholar]
- Acharya, S.; Bhat, N.N.; Joseph, P.; Sanjeev, G.; Sreedevi, B.; Narayana, Y. Dose rate effect on micronuclei induction in human blood lymphocytes exposed to single pulse and multiple pulses of electrons. Radiat. Environ. Biophys. 2011, 50, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Briggs, R.J. (Eds.) Induction Accelerators; Springer: Heidelberg, Germany; New York, NY, USA, 2010; ISBN 978-3-642-13916-1. [Google Scholar]
- Barnard, J.J.; Bangerter, R.O.; Faltens, A.; Fessenden, T.J.; Friedman, A.; Lee, E.P.; Logan, B.G.; Lund, S.M.; Meier, W.; Sharp, W.M.; et al. Induction Accelerator Architectures for Heavy-Ion Fusion1Work Performed under the Auspices of the US Department of Energy at LLNL under Contract W-7405-ENG-48 and at LBNL by the Director, Office of Energy Research, Advanced Energy Projects Division, US D.O.E. under Contract DE-AC03-76SF00098.1. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1998, 415, 218–228. [Google Scholar]
- Smith, J.R.; Bailey, V.L.; Lackner, H.; Putnam, S.D. Performance of the Spiral Line Induction Accelerator; IEEE: New York, NY, USA, 1997. [Google Scholar]
- Sampayan, S.E.; Sampayan, K.C.; Caporaso, G.J.; Chen, Y.-J.; Falabella, S.; Hawkins, S.A.; Hearn, J.; Watson, J.A.; Zentler, J.-M. Megavolt bremsstrahlung measurements from linear induction accelerators demonstrate possible use as a FLASH radiotherapy source to reduce acute toxicity. Sci. Rep. 2021, 11, 17104. [Google Scholar] [CrossRef]
- Panofsky, W.K.H.; Bander, M. Asymptotic Theory of Beam Break-Up in Linear Accelerators. Rev. Sci. Instruments 1968, 39, 206–212. [Google Scholar] [CrossRef]
- Stanley, H., Jr. Charged Particle Beams; Reprinted; Dover Publications: New York, NY, USA, 2013; ISBN 978-0-486-49868-3. [Google Scholar]
- Uetomi, I.; Yamazaki, M.; Kobayashi, H.; Sato, I. Extended Theory of Beam Loading in Electron Linac. Jpn. J. Appl. Phys. 1993, 32, 2858–2864. [Google Scholar] [CrossRef]
- Multhauf, L.G.; Back, N.L.; Simmons, L.F.; Zentler, J.-M.; Scarpetti, R.D. The LLNL flash X-ray induction linear accelerator (FXR). Proceedings 2003, 4948, 622–633. [Google Scholar] [CrossRef]
- Nexsen, W.E.; Atkinson, D.P.; Barrett, D.M.; Chen, Y.-J.; Clark, J.C.; Griffith, L.V.; Kirbie, H.C.; Newton, M.A.; Paul, A.C.; Sampayan, S.; et al. The ETA-II induction linac as a high-average-power FEL driver. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers, Detect. Assoc. Equip. 1990, 296, 54–61. [Google Scholar] [CrossRef]
- Negre, J.P.; Rubbelynck, C. Application of fast CVD diamond photoconductor detectors to MeV X-ray metrology for the AIRIX flash radiographic facility. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers, Detect. Assoc. Equip. 2000, 451, 638–650. [Google Scholar] [CrossRef]
- Thumm, M. State-of-the-Art of High-Power Gyro-Devices and Free Electron Masers. J. Infrared Millim. Terahertz Waves 2020, 41, 1–140. [Google Scholar] [CrossRef]
- Fryar, C.D.; Kruszon-Moran, D.; Gu, Q.; Ogden, C.L. Mean Body Weight, Height, Waist Circumference, and Body Mass Index Among Adults: United States, 1999–2000 Through 2015–2016. Natl. Health Stat. Rep. 2018, 1–16. [Google Scholar]
- Durante, M.; Paganetti, H. Nuclear physics in particle therapy: A review. Rep. Prog. Phys. 2016, 79, 096702. [Google Scholar] [CrossRef] [PubMed]
- Svensson, R.; Brahme, A. Effective source size, yield and beam profile from multi-layered bremsstrahlung targets. Phys. Med. Biol. 1996, 41, 1353–1379. [Google Scholar] [CrossRef]
- Kutsaev, S.V. Advanced Technologies for Applied Particle Accelerators and Examples of Their Use (Review). Tech. Phys. 2021, 66, 161–195. [Google Scholar] [CrossRef]
- Kutsaev, S.; Agustsson, R.; Arodzero, A.; Berry, R.; Bezhanov, A.; Boucher, S.; Chimalpopoca, O.; Diego, A.; Faillace, L.; Gavryushkin, D.; et al. Compact X-Band electron linac for radiotherapy and security applications. Radiat. Phys. Chem. 2021, 185, 109494. [Google Scholar] [CrossRef]
- Kutsaev, S.V.; Agustsson, R.; Arodzero, A.; Berry, R.; Boucher, S.; Diego, A.; Gavryushkin, D.; Hartzell, J.J.; Lanza, R.C.; Smirnov, A.Y.; et al. Linear accelerator for security, industrial and medical applications with rapid beam parameter variation. Radiat. Phys. Chem. 2021, 183, 109398. [Google Scholar] [CrossRef]
- Boucher, S.; Agustsson, R.; Kutsaev, S. Linear Accelerator for Generating High X-ray Doses. U.S. Patent WO2021113323A1, 6 October 2021. [Google Scholar]
- Lyu, Q.; Neph, R.; O’connor, D.; Ruan, D.; Boucher, S.; Sheng, K. ROAD: ROtational direct Aperture optimization with a Decoupled ring-collimator for FLASH radiotherapy. Phys. Med. Biol. 2021, 66, 035020. [Google Scholar] [CrossRef]
- Lempart, M.; Blad, B.; Adrian, G.; Bäck, S.; Knöös, T.; Ceberg, C.; Petersson, K. Modifying a clinical linear accelerator for delivery of ultra-high dose rate irradiation. Radiother. Oncol. 2019, 139, 40–45. [Google Scholar] [CrossRef]
- Kutsaev, S.V. Electron bunchers for industrial RF linear accelerators: Theory and design guide. Eur. Phys. J. Plus 2021, 136, 446. [Google Scholar] [CrossRef]
- Kutsaev, S.; Agustsson, R.; Boucher, S.; Kaneta, K.; Smirnov, A.Y.; Yu, V. Linear accelerator for demonstration of X-ray radio-therapy with flash effect. In LINAC; JACoW Publishing: Liverpool, UK, 2022. [Google Scholar]
- Gao, H.; Lin, B.; Lin, Y.; Fu, S.; Langen, K.; Liu, T.; Bradley, J. Simultaneous dose and dose rate optimization (SDDRO) for FLASH proton therapy. Med. Phys. 2020, 47, 6388–6395. [Google Scholar] [CrossRef] [PubMed]
- Maxim, P.G.; Tantawi, S.G.; Loo, B.W. PHASER: A platform for clinical translation of FLASH cancer radiotherapy. Radiother. Oncol. 2019, 139, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Tantawi, S.; Nasr, M.; Li, Z.; Limborg, C.; Borchard, P. Design and demonstration of a distributed-coupling linear accelerator structure. Phys. Rev. Accel. Beams 2020, 23, 092001. [Google Scholar] [CrossRef]
- Nasr, M.; Nanni, E.; Breidenbach, M.; Weathersby, S.; Oriunno, M.; Tantawi, S. Experimental demonstration of particle acceleration with normal conducting accelerating structure at cryogenic temperature. Phys. Rev. Accel. Beams 2021, 24, 093201. [Google Scholar] [CrossRef]
- Simmons, D.A.; Lartey, F.M.; Schüler, E.; Rafat, M.; King, G.; Kim, A.; Ko, R.; Semaan, S.; Gonzalez, S.; Jenkins, M.; et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother. Oncol. 2019, 139, 4–10. [Google Scholar] [CrossRef]
- Schüler, E.; Trovati, S.; King, G.; Lartey, F.; Rafat, M.; Villegas, M.; Praxel, A.J.; Loo, B.W.; Maxim, P.G. Experimental Platform for Ultra-high Dose Rate FLASH Irradiation of Small Animals Using a Clinical Linear Accelerator. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 195–203. [Google Scholar] [CrossRef]
- Lagzda, A.; Aitkenhead, A.; Corsini, R.; Farabolini, W.; Jones, R.; Kirkby, K.; MacKay, R.; Van Herk, M. Very-High Energy Electron (VHEE) Studies at CERN’s CLEAR User Facility. In Proceedings of the 9th International Particle Accelerator Conference, Vancouver, Canada, 29 April–4 May 2018. [Google Scholar]
- Rahman, M.; Ashraf, M.R.; Zhang, R.; Bruza, P.; Dexter, C.A.; Thompson, L.; Cao, X.; Williams, B.B.; Hoopes, P.J.; Pogue, B.W.; et al. Electron FLASH Delivery at Treatment Room Isocenter for Efficient Reversible Conversion of a Clinical LINAC. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 872–882. [Google Scholar] [CrossRef]
- Jaccard, M.; Durán, M.T.; Petersson, K.; Germond, J.-F.; Liger, P.; Vozenin, M.-C.; Bourhis, J.; Bochud, F.; Bailat, C. High dose-per-pulse electron beam dosimetry: Commissioning of the Oriatron eRT6 prototype linear accelerator for preclinical use. Med. Phys. 2018, 45, 863–874. [Google Scholar] [CrossRef]
- Lansonneur, P.; Favaudon, V.; Heinrich, S.; Fouillade, C.; Verrelle, P.; De Marzi, L. Simulation and experimental validation of a prototype electron beam linear accelerator for preclinical studies. Phys. Med. 2019, 60, 50–57. [Google Scholar] [CrossRef]
- Felici, G.; Barca, P.; Barone, S.; Bortoli, E.; Borgheresi, R.; De Stefano, S.; Di Francesco, M.; Grasso, L.; Linsalata, S.; Marfisi, D.; et al. Transforming an IORT Linac Into a FLASH Research Machine: Procedure and Dosimetric Characterization. Front. Phys. 2020, 8. [Google Scholar] [CrossRef]
- Moeckli, R.; Gonçalves Jorge, P.; Grilj, V.; Oesterle, R.; Cherbuin, N.; Bourhis, J.; Vozenin, M.C.; Germond, J.F.; Bochud, F.; Bailat, C. Commissioning of an ultra-high dose rate pulsed electron beam medical LINAC for FLASH RT preclinical animal experiments and future clinical human protocols. Med. Phys. 2021, 48, 3134–3142. [Google Scholar] [CrossRef]
- PMB-Alcen Announces the Launch of FLASHKNiFE, the FLASH Radiotherapy System Dedicated to Clinical Trials. Available online: https://www.pmb-alcen.com/en/news/pmb-alcen-announces-launch-flashknife-flash-radiotherapy-system-dedicated-clinical-trials (accessed on 9 September 2022).
- NOVAC 11—Soiort. Available online: https://www.soiort.com/novac-11/ (accessed on 9 September 2022).
- Palma, B.; Bazalova-Carter, M.; Hårdemark, B.; Hynning, E.; Qu, B.; Loo, B.W.; Maxim, P.G. Assessment of the quality of very high-energy electron radiotherapy planning. Radiother. Oncol. 2016, 119, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Schüler, E.; Eriksson, K.; Hynning, E.; Hancock, S.L.; Hiniker, S.M.; Bazalova-Carter, M.; Wong, T.; Le, Q.-T.; Loo, B.W., Jr.; Maxim, P.G. Very high-energy electron (VHEE) beams in radiation therapy—Treatment plan comparison between VHEE, VMAT, and PPBS. Med. Phys. 2017, 44, 2544–2555. [Google Scholar] [CrossRef] [PubMed]
- Papiez, L.; Desrosiers, C.; Moskvin, V. Very High Energy Electrons (50–250 MeV) and Radiation Therapy. Technol. Cancer Res. Treat. 2002, 1, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Bazalova-Carter, M.; Qu, B.; Palma, B.; Hårdemark, B.; Hynning, E.; Jensen, C.; Maxim, P.G.; Loo, B.W. Treatment planning for radiotherapy with very high-energy electron beams and comparison of VHEE and VMAT plans. Med. Phys. 2015, 42, 2615–2625. [Google Scholar] [CrossRef]
- Wuensch, W. The CHUV-CERN Facility for FLASH Treatment of Large, Deep-Seated Tumors: The DEFT (Deep Electron FLASH Therapy) Facility. In Proceedings of the FLASH Radiotherapy & Particle Therapy Conference, Barcelona, Spain, 1–3 December 2021. [Google Scholar]
- Han, Y.; Golfe, A.F.; Vallerand, C.; Bai, B.; Duchesne, P.; Prezado, Y.; Delorme, R.; Poortmans, P.; Favaudon, V.; Fouillade, C.; et al. Optics Design and Beam Dynamics simulation for a VHEE Radiobiology beam line at PRAE accelerator. J. Phys. Conf. Ser. 2019, 1350, 012200. [Google Scholar] [CrossRef]
- Farkas, Z.D.; Hoag, H.A.; Loew, G.A.; Wilson, P.B. SLED: A Method of Doubling SLAC’s Energy. In Proceedings of the 9th International Conference on High-Energy Accelerators, Stanford, CA, USA, 2–7 May 1974; pp. 576–583. [Google Scholar]
- Wilson, P.B.; Farkas, Z.D.; Ruth, R.D. SLED-II: A New Method of RF Pulse Compression. In Proceedings of the 15th International Linear Accelerator Conference, Albuquerque, NM, USA, 9–14 September 1990. [Google Scholar]
- Farkas, Z. Binary Peak Power Multiplier and its Application to Linear Accelerator Design. IEEE Trans. Microw. Theory Tech. 1986, 34, 1036–1043. [Google Scholar] [CrossRef][Green Version]
- Wang, J.W.; Tantawi, S.G.; Xu, C.; Franzi, M.; Krejcik, P.; Bowden, G.; Condamoor, S.; Ding, Y.; Dolgashev, V.; Eichner, J.; et al. Development for a supercompact X -band pulse compression system and its application at SLAC. Phys. Rev. Accel. Beams 2017, 20, 110401. [Google Scholar] [CrossRef]
- Franzi, M.; Wang, J.; Dolgashev, V.; Tantawi, S. Compact rf polarizer and its application to pulse compression systems. Phys. Rev. Accel. Beams 2016, 19, 062002. [Google Scholar] [CrossRef]
- Bai, M.; Barklow, T.; Bartoldus, R.; Breidenbach, M.; Grenier, P.; Huang, Z.; Kagan, M.; Lewellen, J.; Li, Z.; Markiewicz, T.W.; et al. C$^3$: A “Cool” Route to the Higgs Boson and Beyond. arXiv 2021, arXiv:2110.15800. [Google Scholar]
- Nanni, E.A.; Breidenbach, M.; Vernieri, C.; Belomestnykh, S.; Bhat, P.; Nagaitsev, S.; Bai, M.; Berg, W.; Barklow, T.; Byrd, J.; et al. C$^3$ Demonstration Research and Development Plan. arXiv 2022, arXiv:2203.09076. [Google Scholar]
- Martínez-Rovira, I.; Fois, G.; Prezado, Y. Dosimetric evaluation of new approaches in GRID therapy using nonconventional radiation sources. Med. Phys. 2015, 42, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Snively, E.; Deering, K.; Nanni, E. mm-Wave Linac Design for Next Generation VHEE Cancer Therapy Systems. In Proceedings of the 12th International Particle Accelerator Conference, Online, 24–28 May 2021. [Google Scholar] [CrossRef]
- Nanni, E.A.; Dolgashev, V.A.; Haase, A.; Neilson, J.; Tantawi, S.; Schaub, S.C.; Temkin, R.; Spataro, B. Prototyping high-gradient mm-wave accelerating structures. J. Phys. Conf. Ser. 2017, 874, 012039. [Google Scholar] [CrossRef]
- Othman, M. Others Initial Results of High-Gradient Breakdown Tests for W-Band Accelerating Structures. In Proceedings of the 10th International Particle Accelerator Conference, Melbourne, Australia, 19–24 May 2019; p. THPGW080. [Google Scholar]
- Snively, E.; Othman, M.; Deering, K.; Kuppusamy, S.; Wehner, G. High Gradient Mm-Wave Linac for VHEE Therapy. In Proceedings of the FLASH Radiotherapy & Particle Therapy Conference, Barcelona, Spain, 12 May 2021. [Google Scholar]
- Kutsaev, S.V.; Jacobson, B.; Smirnov, A.Y.; Campese, T.; Dolgashev, V.A.; Goncharik, V.; Harrison, M.; Murokh, A.; Nanni, E.; Picard, J.; et al. Nanosecond rf-Power Switch for Gyrotron-Driven Millimeter-Wave Accelerators. Phys. Rev. Appl. 2019, 11, 034052. [Google Scholar] [CrossRef]
- Othman, M.A.K.; Picard, J.; Schaub, S.; Dolgashev, V.A.; Lewis, S.M.; Neilson, J.; Haase, A.; Jawla, S.; Spataro, B.; Temkin, R.J.; et al. Experimental demonstration of externally driven millimeter-wave particle accelerator structure. Appl. Phys. Lett. 2020, 117, 073502. [Google Scholar] [CrossRef]
- Lewis, S.; Dolgashev, V.; Haase, A.; Kim, D.; Nanni, E.; Othman, M.; Simakov, E.; Sy, A.; Tantawi, S. Design of a High Gradient THz-Driven Electron Gun. In Proceedings of the 10th International Particle Accelerator Conference, Melbourne, Australia, 19–24 May 2019; p. TUPTS077. [Google Scholar]
- Holger, G. Dose Delivery System of the Varian ProBeam System with Continuous Beam. In Proceedings of the Workshop on Innovative Delivery Systems in Particle Therapy, Torino, Italy, 24 February 2017. [Google Scholar]
- Pedroni, E.; Bacher, R.; Blattmann, H.; Böhringer, T.; Coray, A.; Lomax, A.; Lin, S.; Munkel, G.; Scheib, S.; Schneider, U. The 200-MeV proton therapy project at the Paul Scherrer Institute: Conceptual design and practical realization. Med. Phys. 1995, 22, 37–53. [Google Scholar] [CrossRef]
- FlashForward Consortium | Varian. Available online: https://www.varian.com/about-varian/research/flashforward-consortium (accessed on 18 November 2022).
- Calaga, R.; De Maria, R.; Barranco, J.; Giovannozzi, M.; Grudiev, A.; Tomas, R. Study of Multipolar RF Kicks from the Main Deflecting Mode in Compact Crab Cavities for LHC. Conf. Proc. C 2012, 1205201, 1873–1875. [Google Scholar]
- Snively, E. Deflector Cavity Design for Rapid 2-D Proton Beam Scanning. In Proceedings of the APS March Meeting, Denver, CO, USA, 2–6 March 2020. [Google Scholar]
- Dasu, S.; Nanni, E.A.; Peskin, M.E.; Vernieri, C.; Barklow, T.; Bartoldus, R.; Bhat, P.C.; Black, K.; Brau, J.; Breidenbach, M.; et al. Strategy for Understanding the Higgs Physics: The Cool Copper Collider. arXiv 2022, arXiv:2203.07646. [Google Scholar]





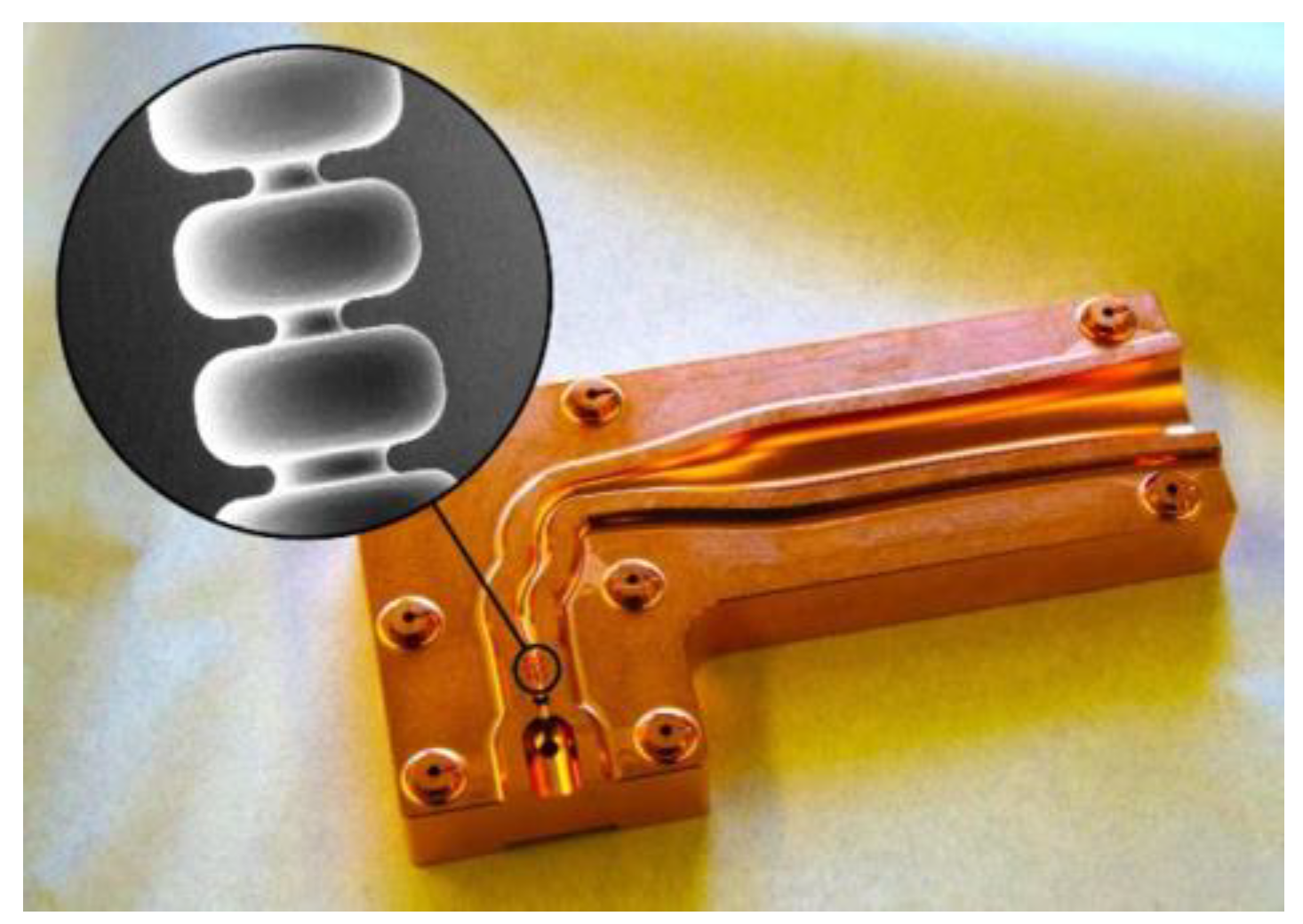
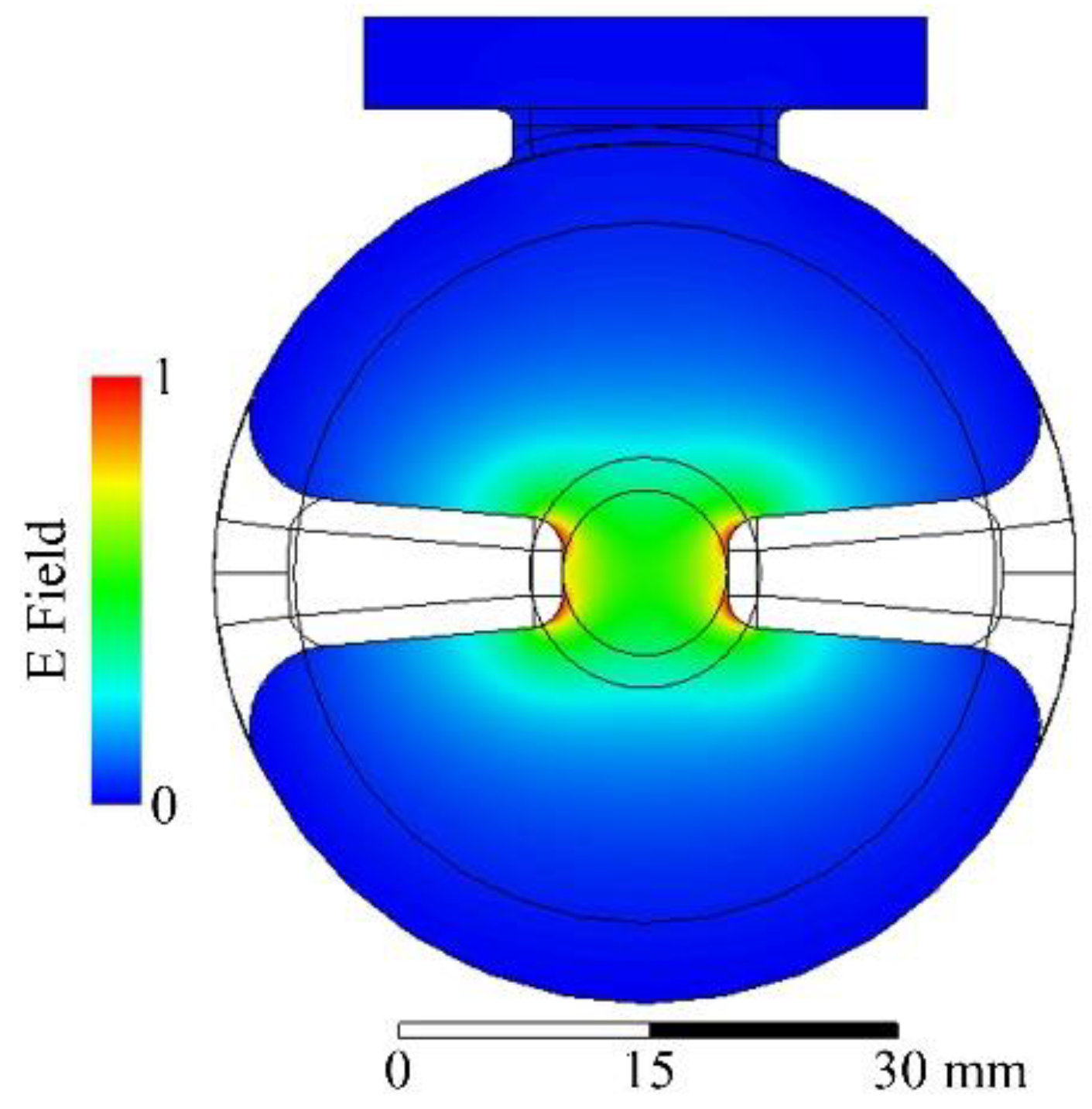
| Electron Beam | Min. for Observed FLASH | Optimal for FLASH |
|---|---|---|
| Average dose rate | 30 Gy/s | 100 Gy/s |
| Intrapulse dose rate | ~105 Gy/s | ≥106 Gy/s |
| Total dose | <10 Gy | ≥10 Gy—tissue dependent |
| Delivery time for 10 Gy | <1 s | 1 µs–10 ms |
| Dose Delivery Mode | Protons | Helium | Carbon |
|---|---|---|---|
| Conventional: 2.6 Gy/fraction Delivery time: 100 s | 2 × 109 p/s 0.4 nA | 5 × 108 He/s 0.2 nA | 1.7 × 108 C/s 0.2 nA |
| FLASH: ≥10 Gy/fraction Delivery Time: 100 ms | 1 × 1013 p/s 1.6 μA | 2.5 × 1012 He/s 0.8 μA | 0.8 × 1012 C/s 0.8 μA |
| Beam Parameter | Value |
|---|---|
| Dose per pulse | 1 Gy |
| Pulse length | 30 ns |
| Pulse repetition rate | 0.2 Hz |
| Instantaneous dose rate | 3 × 107 Gy/s |
| Mean dose rate | 0.2 Gy/s |
| System | ROAD | Conventional [18] |
|---|---|---|
| Energy [MeV] | 18 | 6 |
| Pulse length [µs] | 167 | 4 |
| Rep rate [Hz] | 150 | 250 |
| Duty cycle | 2.5% | 0.1% |
| Injected current [A] | 1.3 | 0.5 |
| Transmission | 25% | 25% |
| Peak current [A] | 0.325 | 0.125 |
| Average current [mA] | 8.14 | 0.125 |
| Dose rate factor [Gy/min/mA at 1 m] | 2000 | 120 |
| Dose rate, uncollimated, at 1 m [Gy/s] | 271 | 0.25 |
| Dose delivery efficiency | 25% | 25% |
| Dose rate, collimated, at 80 cm SAD [Gy/s] | 106.0 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulte, R.; Johnstone, C.; Boucher, S.; Esarey, E.; Geddes, C.G.R.; Kravchenko, M.; Kutsaev, S.; Loo, B.W., Jr.; Méot, F.; Mustapha, B.; et al. Transformative Technology for FLASH Radiation Therapy. Appl. Sci. 2023, 13, 5021. https://doi.org/10.3390/app13085021
Schulte R, Johnstone C, Boucher S, Esarey E, Geddes CGR, Kravchenko M, Kutsaev S, Loo BW Jr., Méot F, Mustapha B, et al. Transformative Technology for FLASH Radiation Therapy. Applied Sciences. 2023; 13(8):5021. https://doi.org/10.3390/app13085021
Chicago/Turabian StyleSchulte, Reinhard, Carol Johnstone, Salime Boucher, Eric Esarey, Cameron G. R. Geddes, Maksim Kravchenko, Sergey Kutsaev, Billy W. Loo, Jr., François Méot, Brahim Mustapha, and et al. 2023. "Transformative Technology for FLASH Radiation Therapy" Applied Sciences 13, no. 8: 5021. https://doi.org/10.3390/app13085021
APA StyleSchulte, R., Johnstone, C., Boucher, S., Esarey, E., Geddes, C. G. R., Kravchenko, M., Kutsaev, S., Loo, B. W., Jr., Méot, F., Mustapha, B., Nakamura, K., Nanni, E. A., Obst-Huebl, L., Sampayan, S. E., Schroeder, C. B., Sheng, K., Snijders, A. M., Snively, E., Tantawi, S. G., & Van Tilborg, J. (2023). Transformative Technology for FLASH Radiation Therapy. Applied Sciences, 13(8), 5021. https://doi.org/10.3390/app13085021







