An Overview on Management and Valorisation of Winery Wastes
Abstract
:1. Introduction
2. Winery Waste Composition and Source of Bio-Active Compounds
3. Grape Pomace as Antioxidant and Anti-Inflammatory Agents, Dietary Fibre, or as Ingredient in Food Products
4. Winery Waste for Water Treatment
5. Winery Wastes as Precursors for Sustainable Energy
6. Wine By-Products as Raw Materials for Biopolymers and Natural Reinforcing Fillers
7. Valorisation of Wine Lees
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Insights on Circular Economy. Available online: https://www.ingwb.com/en/insights/circular-economy?wt_ga=143714470482_626507333162&wt_gk=p_143714470482_circular%20economy&gclid=Cj0KCQjwy5maBhDdARIsAMxrkw37tEX7Vd5IvpEx-7DMhnxrJxirEjW9yPaPK8BjtUjrephvC2wGUdoaAlCdEALw_wcB (accessed on 24 October 2022).
- Circular Economy Introduction. Available online: https://ellenmacarthurfoundation.org/topics/circular-economy-introduction/overview (accessed on 24 October 2022).
- Impact of Dehydration on Retention of Bioactive Profile and Biological Activities of Different Grape (Vitis vinifera L.) Pomace Varieties|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S0377840118304887?token=3026434FD282031966E588085421274D043B897D3090076C70B767143227B1ECB1CEB34A51388155E7B0A68D0063A78A&originRegion=eu-west-1&originCreation=20221024064031 (accessed on 24 October 2022).
- Fia, G.; Bucalossi, G.; Gori, C.; Borghini, F.; Zanoni, B. Recovery of Bioactive Compounds from Unripe Red Grapes (Cv. Sangiovese) through a Green Extraction. Foods 2020, 9, 566. [Google Scholar] [CrossRef]
- Nakov, G.; Zlatev, Z.; Lazova-Borisova, I.; Lukinac, J. Management and valorization of agricultural wastes from wine production using statistical analysis to obtain novel food. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2021, 21, 381–386. [Google Scholar]
- Christ, K.L.; Burritt, R.L. Critical Environmental Concerns in Wine Production: An Integrative Review. J. Clean. Prod. 2013, 53, 232–242. [Google Scholar] [CrossRef]
- Merli, R.; Preziosi, M.; Acampora, A. Sustainability Experiences in the Wine Sector: Toward the Development of an International Indicators System. J. Clean. Prod. 2018, 172, 3791–3805. [Google Scholar] [CrossRef]
- Grape Production Worldwide. 2022. Available online: https://www.statista.com/statistics/237600/world-grape-production-in-2007-by-region/ (accessed on 24 October 2022).
- Navarro, P.; Sarasa, J.; Sierra, D.; Esteban, S.; Ovelleiro, J.L. Degradation of Wine Industry Wastewaters by Photocatalytic Advanced Oxidation. Water Sci. Technol. 2005, 51, 113–120. [Google Scholar] [CrossRef]
- Cortés, A.; Moreira, M.T.; Feijoo, G. Integrated evaluation of wine lees valorization to produce value-added products. Waste Manag. 2019, 95, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ahmedna, M. Functional Components of Grape Pomace: Their Composition, Biological Properties and Potential Applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Nakov, G.; Brandolini, A.; Hidalgo, A.; Ivanova, N.; Stamatovska, V.; Dimov, I. Effect of Grape Pomace Powder Addition on Chemical, Nutritional and Technological Properties of Cakes. LWT 2020, 134, 109950. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of Winery Waste vs. the Costs of Not Recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
- Chedea, V.; Dragulinescu, A.-M.; Tomoiaga, L.; Balaceanu, C.; Iliescu, M. Climate Change and Internet of Things Technologies—Sustainable Premises of Extending the Culture of the Amurg Cultivar in Transylvania—A Use Case for Târnave Vineyard. Sustainability 2021, 13, 8170. [Google Scholar] [CrossRef]
- Xia, L.; Xu, C.; Huang, K.; Lu, J.; Zhang, Y. Evaluation of phenolic compounds, antioxidant and antiproliferative activities of 31 grape cultivars with different genotypes. J. Food Biochem. 2019, 43, e12626. [Google Scholar] [CrossRef] [PubMed]
- Muhlack, R.; Potumarthi, R.; Jeffery, D. Sustainable Wineries through Waste Valorisation: A Review of Grape Marc Utilisation for Value-Added Products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for recycling and valorization of grape marc. Crit. Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Council Regulation (EC) No 479/2008 of 29 April 2008 on the Common Organisation of the Market in Wine, Amending Regulations (EC) No 1493/1999, (EC) No 1782/2003, (EC) No 1290/2005, (EC) No 3/2008 and Repealing Regulations (EEC) No 2392/86 and (EC) No 1493/1999 (Repealed). Available online: https://www.legislation.gov.uk/eur/2008/479/contents/adopted (accessed on 29 March 2023).
- Silva, A.; Silva, V.; Igrejas, G.; Gaivão, I.; Aires, A.; Klibi, N.; Dapkevicius, M.E.; Valentão, P.; Falco, V.; Poeta, P. Valorization of Winemaking By-Products as a Novel Source of Antibacterial Properties: New Strategies to Fight Antibiotic Resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef]
- Rani, J.; Indrajeet, Y.; Rautela, A.; Kumar, S. Chapter 4—Biovalorization of winery industry waste to produce value-added products. In Biovalorisation of Wastes to Renewable Chemicals and Biofuels; Rathinam, N.K., Sani, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 63–85. ISBN 9780128179512. [Google Scholar] [CrossRef]
- Lelario, F.; Scrano, L.; De Franchi, S.; Bonomo, M.G.; Salzano, G.; Milan, S.; Milella, L.; Bufo, S.A. Identification and Antimicrobial Activity of Most Representative Secondary Metabolites from Different Plant Species. Chem. Biol. Technol. Agric. 2018, 5, 13. [Google Scholar] [CrossRef]
- Oliveira, M.; Duarte, E. Integrated Approach to Winery Waste: Waste Generation and Data Consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176. [Google Scholar] [CrossRef]
- Niculescu, V.-C.; Paun, N.; Ionete, R.-E. The Evolution of Polyphenols from Grapes to Wines; IntechOpen: London, UK, 2017; ISBN 978-953-51-3834-1. [Google Scholar]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.; Skotti, E. Novel Application and Industrial Exploitation of Winery By-Products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of Grape Pomace: An Approach That Is Increasingly Reaching Its Maturity—A Review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef]
- Nanni, A.; Messori, M. Effect of the Wine Wastes on the Thermal Stability, Mechanical Properties, and Biodegradation’s Rate of Poly(3-Hydroxybutyrate). J. Appl. Polym. Sci. 2021, 138, 49713. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.; Cabezudo, I.; da Silva-James, N.; Teles, A.; Cruz, A.; Mellinger-Silva, C.; Tonon, R.; Cabral, L.; Freitas, S. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.; Summo, C.; Caponio, F. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Souquet, J.-M.; Labarbe, B.; Le Guernevé, C.; Cheynier, V.; Moutounet, M. Phenolic Composition of Grape Stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Blackford, M.; Comby, M.; Zeng, L.; Dienes-Nagy, Á.; Bourdin, G.; Lorenzini, F.; Bach, B. A Review on Stems Composition and Their Impact on Wine Quality. Molecules 2021, 26, 1240. [Google Scholar] [CrossRef]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic Profile, Antioxidant Properties and Antimicrobial Activity of Grape Skin Extracts of 14 Vitis vinifera Varieties Grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Serra, A.; Matias, A.; Nunes, A.; Leitao, M.; Brito, D.; Bronze, R.; Silva, S.; Pires, A.; Crespo, M.; Romao, M.; et al. In Vitro Evaluation of Olive- and Grape-Based Natural Extracts as Potential Preservatives for Food. Innov. Food Sci. Emerg. Technol. 2008, 9, 311–319. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical Composition, Antioxidant and Antimicrobial Activity of Phenolic Compounds Extracted from Wine Industry by-Products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Baydar, N.; Ozkan, G. Tocopherol Contents of Some Turkish Wine By-Products. Eur. Food Res. Technol. 2006, 223, 290–293. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Hsu, W.-Y.; Simonne, A.; Lu, J.; Marshall, M.R. Antioxidant, Antibacterial, and Antibiofilm Properties of Polyphenols from Muscadine Grape (Vitis Rotundifolia Michx.) Pomace against Selected Foodborne Pathogens. J. Agric. Food Chem. 2014, 62, 6640–6649. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Salvador, A.A.; Smânia, A.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial Activity and Composition Profile of Grape (Vitis Vinifera) Pomace Extracts Obtained by Supercritical Fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and Antioxidant Activities of Grape (Vitis Vinifera) Seed Extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Cheng, V.; Bekhit, A.; McConnell, M.; Mros, S.; Zhao, J. Effect of Extraction Solvent, Waste Fraction and Grape Variety on the Antimicrobial and Antioxidant Activities of Extracts from Wine Residue from Cool Climate. Food Chem. 2012, 134, 474–482. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of Polyphenol-Rich Grape by-Products in Monogastric Nutrition. A Review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Roriz, C.L.; Heleno, S.A.; Calhelha, R.; Dias, M.I.; Pinela, J.; Rosales-Conrado, N.; León-González, M.E.; Ferreira, I.C.F.R.; Barros, L. Valorisation of Black Mulberry and Grape Seeds: Chemical Characterization and Bioactive Potential. Food Chem. 2021, 337, 127998. [Google Scholar] [CrossRef] [PubMed]
- Felhi, S.; Baccouch, N.; Ben Salah, H.; Smaoui, S.; Allouche, N.; Gharsallah, N.; Kadri, A. Nutritional Constituents, Phytochemical Profiles, in Vitro Antioxidant and Antimicrobial Properties, and Gas Chromatography–Mass Spectrometry Analysis of Various Solvent Extracts from Grape Seeds (Vitis vinifera L.). Food Sci. Biotechnol. 2016, 25, 1537–1544. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between Essential Oil Components and Antibiotics: A Review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial Capacity of Plant Polyphenols against Gram-Positive Bacteria: A Comprehensive Review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef]
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The Synergy and Mode of Action of Quercetin plus Amoxicillin against Amoxicillin-Resistant Staphylococcus epidermidis. BMC Pharmacol. Toxicol. 2016, 17, 39. [Google Scholar] [CrossRef]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic Interactions between Phenolic Compounds Identified in Grape Pomace Extract with Antibiotics of Different Classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef]
- Mattos, G.; Tonon, R.; Furtado, A.; Cabral, L. Grape By-Product Extracts against Microbial Proliferation and Lipid Oxidation: A Review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.T.V.; Maciel, G.M.; Silva, M.V.; Corrêa, V.G.; Peralta, R.M.; Haminiuk, C.W.I. Enrichment of waste yeast with bioactive compounds from grape pomace as an innovative and emerging technology: Kinetics, isotherms and bioaccessibility. Innov. Food Sci. Emerg. Technol. 2018, 45, 18–28. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Cueva, C.; Sanz-Buenhombre, M.; Guadarrama, A.; Moreno-Arribas, M.V.; Bartolomé, B. Dynamic gastrointestinal digestion of grape pomace extracts. Bioacessible phenolic metabolites and impact on human gut microbiota. J. Food Compos. Anal. 2018, 68, 41–52. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Bender, A.B.B.; Speroni, C.S.; Moro, K.I.B.; Morisso, F.D.P.; dos Santos, D.R.; da Silva, L.P.; Penna, N.G. Effects of micronization on dietary fiber composition, physicochemical properties, phenolic compounds, and antioxidant capacity of grape pomace and its dietary fiber concentrate. LWT 2020, 117, 108652. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef]
- Nerantzis, E.; Tataridis, P. Integrated enology utilization of winery by-products into high added value products. J. Sci. Technol. 2006, 1, 79–89. [Google Scholar]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef]
- Boonchu, T.; Utama-ang, N. Optimization of extraction and microencapsulation of bioactive compounds from red grape (Vitis vinifera L.) pomace. J. Food Sci. Technol. 2015, 52, 783–792. [Google Scholar] [CrossRef]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; de Morais, S.M.; de Lima, A.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef]
- Kokkinomagoulos, E.; Kandylis, P. Sustainable Exploitation of By-Products of Vitivinicultural Origin in Winemaking. Proceedings 2020, 67, 5. [Google Scholar] [CrossRef]
- Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, Ș.O.; Pătrașca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef] [PubMed]
- de la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food. Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. The dose–response effect on polyphenol bioavailability after intake of white and red wine pomace products by Wistar rats. Food Funct. 2020, 11, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.L.; Iurian, S.; Puscas, C.; Silaghi-Dumitrescu, R.; Hanganu, D.; Bogdan, C.; Vlase, L.; Oniga, I.; Benedec, D. A Design of Experiments Strategy to Enhance the Recovery of Polyphenolic Compounds from Vitis vinifera By-Products through Heat Reflux Extraction. Biomolecules 2019, 9, 529. [Google Scholar] [CrossRef]
- Torre, E.; Iviglia, G.; Cassinelli, C.; Morra, M.; Russo, N. Polyphenols from grape pomace induce osteogenic differentiation in mesenchymal stem cells. Int. J. Mol. Med. 2020, 45, 1721–1734. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; De Diego, E.H.; Riomoros-Arranz, M.; Reynoso-Camacho, R.; Saura-Calixto, F.; Pérez-Jiménez, J. Effects of acute intake of grape/pomegranate pomace dietary supplement on glucose metabolism and oxidative stress in adults with abdominal obesity. Int. J. Food Sci. Nutr. 2020, 71, 94–105. [Google Scholar] [CrossRef]
- Urquiaga, I.; Troncoso, D.; Mackenna, M.J.; Urzúa, C.; Pérez, D.; Dicenta, S.; De la Cerda, P.M.; Amigo, L.; Carreño, J.C.; Echeverría, G.; et al. The Consumption of Beef Burgers Prepared with Wine Grape Pomace Flour Improves Fasting Glucose, Plasma Antioxidant Levels, and Oxidative Damage Markers in Humans: A Controlled Trial. Nutrients 2018, 10, 1388. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Léniz, A.; Martínez-Maqueda, D.; Amézqueta, S.; Fernández-Quintela, A.; Hereu, M.; Torres, J.L.; Portillo, M.P.; Pérez-Jiménez, J. Inter-Individual Variability in Insulin Response after Grape Pomace Supplementation in Subjects at High Cardiometabolic Risk: Role of Microbiota and miRNA. Mol. Nutr. Food Res. 2021, 65, 2000113. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Effects of grape pomace polyphenolic extract (Taurisolo®) in reducing tmao serum levels in humans: Preliminary results from a randomized, placebocontrolled, cross-over study. Nutrients 2019, 11, 139. [Google Scholar] [CrossRef]
- Taladrid, D.; de Celis, M.; Belda, I.; Bartolomé, B.; Moreno-Arribas, M.V. Hypertension- and glycaemia-lowering effects of a grape-pomace-derived seasoning in high-cardiovascular risk and healthy subjects. Interplay with the gut microbiome. Food Funct. 2022, 13, 2068–2082. [Google Scholar] [CrossRef] [PubMed]
- Rivera, K.; Salas-Pérez, F.; Echeverría, G.; Urquiaga, I.; Dicenta, S.; Pérez, D.; de la Cerda, P.; González, L.; Andia, M.E.; Uribe, S.; et al. Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease. Nutrients 2019, 11, 2135. [Google Scholar] [CrossRef] [PubMed]
- Sfaxi, I.; Charradi, K.; Limam, F.; El May, M.V.; Aouani, E. Grape seed and skin extract protects against arsenic trioxide induced oxidative stress in rat heart. Can. J. Physiol. Pharmacol. 2016, 94, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Guler, A.; Sahin, M.A.; Yucel, O.; Yokusoglu, M.; Gamsizkan, M.; Ozal, E.; Demirkilic, U.; Arslan, M. Proanthocyanidin prevents myocardial ischemic injury in adult rats. Med. Sci. Monit. 2011, 17, BR326–BR331. [Google Scholar] [CrossRef] [PubMed]
- Grujic-Milanovic, J.; Jacevic, V.; Miloradovic, Z.; Jovovic, D.; Milosavljevic, I.; Milanovic, S.D.; Mihailovic-Stanojevic, N. Resveratrol protects cardiac tissue in experimental malignant hypertension due to antioxidant, anti-inflammatory, and antiapoptotic properties. Int. J. Mol. Sci. 2021, 22, 5006. [Google Scholar] [CrossRef]
- Decean, H.; Fischer-Fodor, E.; Tatomir, C.; Perde-Schrepler, M.; Somfelean, L.; Burz, C.; Hodor, T.; Orasan, R.; Virag, P. Vitis vinifera seeds extract for the modulation of cytosolic factors BAX- and NF-kB involved in UVB-induced oxidative stress and apoptosis of human skin cells. Clujul Med. 2016, 89, 72. [Google Scholar] [CrossRef]
- Garrido, M.D.; Auqui, M.; Martí, N.; Linares, M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT Food Sci. Technol. 2011, 44, 2238–2243. [Google Scholar] [CrossRef]
- Ryu, K.S.; Shim, K.S.; Shin, D. Effect of grape pomace powder addition on TBARS and color of cooked pork sausages during storage. Korean J. Food Sci. Anim. Resour. 2014, 34, 200. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.J.; Jung, M.O.; Choi, J.S.; Jung, J.T.; Choi, Y.I.; Lee, J.K. Meat Quality and Storage Characteristics of Pork Loin Marinated in Grape Pomace. Korean J. Food Sci. Anim. Resour. 2017, 37, 726. [Google Scholar] [CrossRef]
- Selani, M.M.; Contreras-Castillo, C.J.; Shirahigue, L.D.; Gallo, C.R.; Plata-Oviedo, M.; Montes-Villanueva, N.D. Wine industry residues extracts as natural antioxidants in raw and cooked chicken meat during frozen storage. Meat Sci. 2011, 88, 397–403. [Google Scholar] [CrossRef]
- Shirahigue, L.D.; Plata-Oviedo, M.; De Alencar, S.M.; D’Arce, M.A.B.R.; De SouzaVieira, T.M.F.; Oldoni, T.L.C.; Contreras-Castillo, C.J. Wine industry residue as antioxidant in cooked chicken meat. Int. J. Food Sci. Technol. 2010, 45, 863–870. [Google Scholar] [CrossRef]
- Marchiani, R.; Bertolino, M.; Ghirardello, D.; McSweeney, P.L.; Zeppa, G. Physicochemical and nutritional qualities of grape pomace powder-fortified semi-hard cheeses. J. Food Sci. Technol. 2016, 53, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, M.D.; Gil-Muñoz, R.; Gómez-Plaza, E.; Bautista-Ortín, A.B. Performance of purified grape pomace as a fining agent to reduce the levels of some contaminants from wine. Food Addit. Contam. Part A 2018, 35, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.; Smith, P.; Bindon, K. Application of insoluble fibres in the fining of wine phenolics. J. Agric. Food Chem. 2013, 61, 4424–4432. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, M.D.; Gómez-Plaza, E.; Molero, N.; Bautista-Ortín, A.B. Fining of red wines with pomace cell material: Effect on wine phenolic composition. Food Bioprocess Technol. 2017, 10, 1531–1539. [Google Scholar] [CrossRef]
- Ungureanu, G.; Patras, A.; Cara, I.G.; Sturza, R.; Ghendov-Mosanu, A. Innovative Recovery of Winemaking Waste for Effective Lead Removal from Wastewater. Agronomy 2022, 12, 604. [Google Scholar] [CrossRef]
- Liu, C.-C.; Kuang-Wang, M.; Li, Y.-S. Removal of Nickel from Aqueous Solution Using Wine Processing Waste Sludge. Ind. Eng. Chem. Res. 2005, 44, 1438–1445. [Google Scholar] [CrossRef]
- Farinella, N.V.; Matos, G.D.; Arruda, M.A.Z. Grape Bagasse as a Potential Biosorbent of Metals in Effluent Treatments. Bioresour. Technol. 2007, 98, 1940–1946. [Google Scholar] [CrossRef]
- Antunes, M.; Esteves, V.I.; Guégan, R.; Crespo, J.S.; Fernandes, A.N.; Giovanela, M. Removal of Diclofenac Sodium from Aqueous Solution by Isabel Grape Bagasse. Chem. Eng. J. 2012, 192, 114–121. [Google Scholar] [CrossRef]
- Demiral, H.; Güngör, C. Adsorption of Copper(II) from Aqueous Solutions on Activated Carbon Prepared from Grape Bagasse. J. Clean. Prod. 2016, 124, 103–113. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F.; Önal, Y. Conversion of Grape Industrial Processing Waste to Activated Carbon Sorbent and Its Performance in Cationic and Anionic Dyes Adsorption. J. Clean. Prod. 2015, 93, 84–93. [Google Scholar] [CrossRef]
- Zúñiga-Muro, N.M.; Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E.; Duran-Valle, C.J.; Ghalla, H.; Sellaoui, L. Recovery of Grape Waste for the Preparation of Adsorbents for Water Treatment: Mercury Removal. J. Environ. Chem. Eng. 2020, 8, 103738. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H. Nanomaterials for the Adsorptive Treatment of Hg(II) Ions from Water. Chem. Eng. J. 2019, 358, 264–282. [Google Scholar] [CrossRef]
- Al-Ghamdi, Y.O.; Alamry, K.A.; Hussein, M.A.; Marwani, H.M.; Asiri, A.M. Sulfone-Modified Chitosan as Selective Adsorbent for the Extraction of Toxic Hg(II) Metal Ions. Adsorpt. Sci. Technol. 2019, 37, 139–159. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of Pharmaceutical Compounds in Urban Wastewater: Removal, Mass Load and Environmental Risk after a Secondary Treatment—A Review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Sanderson, H.; Brain, R.A.; Johnson, D.J.; Wilson, C.J.; Solomon, K.R. Toxicity Classification and Evaluation of Four Pharmaceuticals Classes: Antibiotics, Antineoplastics, Cardiovascular, and Sex Hormones. Toxicology 2004, 203, 27–40. [Google Scholar] [CrossRef]
- Calisto, V.; Jaria, G.; Silva, C.P.; Ferreira, C.I.A.; Otero, M.; Esteves, V.I. Single and Multi-Component Adsorption of Psychiatric Pharmaceuticals onto Alternative and Commercial Carbons. J. Environ. Manag. 2017, 192, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Martins, M.; Rosca, M.; Rocha, V.; Lago, A.; Neves, I.C.; Tavares, T. Waste-Based Biosorbents as Cost-Effective Alternatives to Commercial Adsorbents for the Retention of Fluoxetine from Water. Sep. Purif. Technol. 2020, 235, 116139. [Google Scholar] [CrossRef]
- Zhang, N.; Hoadley, A.; Patel, J.; Lim, S.; Li, C. Sustainable Options for the Utilization of Solid Residues from Wine Production. Waste Manag. 2017, 60, 173–183. [Google Scholar] [CrossRef]
- Toscano, G.; Riva, G.; Duca, D.; Pedretti, E.; Corinaldesi, F.; Rossini, G. Analysis of the Characteristics of the Residues of the Wine Production Chain Finalized to Their Industrial and Energy Recovery. Biomass Bioenergy 2013, 55, 260–267. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A Comparative Review of Biochar and Hydrochar in Terms of Production, Physico-Chemical Properties and Applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Pala, M.; Kantarli, I.C.; Buyukisik, H.B.; Yanik, J. Hydrothermal Carbonization and Torrefaction of Grape Pomace: A Comparative Evaluation. Bioresour. Technol. 2014, 161, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; Kwong, C.W.; Broström, M.; Yoshikawa, K. Hydrothermal Treatment of Grape Marc for Solid Fuel Applications. Energy Convers. Manag. 2017, 145, 371–377. [Google Scholar] [CrossRef]
- Guardia, L.; Suárez, L.; Querejeta, N.; Pevida, C.; Centeno, T.A. Winery Wastes as Precursors of Sustainable Porous Carbons for Environmental Applications. J. Clean. Prod. 2018, 193, 614–624. [Google Scholar] [CrossRef]
- Molina, F.; Ruiz-Filippi, G.; Garcia, C.; Roca, E.; Lema, J. Winery Effluent Treatment at an Anaerobic Hybrid USBF Pilot Plant under Normal and Abnormal Operation. Water Sci. Technol. 2007, 56, 25–31. [Google Scholar] [CrossRef]
- Da Ros, C.; Cavinato, C.; Pavan, P.; Bolzonella, D. Winery Waste Recycling through Anaerobic Co-Digestion with Waste Activated Sludge. Waste Manag. 2014, 34, 2028–2035. [Google Scholar] [CrossRef]
- Liao, X.; Li, H. Biogas Production from Low-Organic-Content Sludge Using a High-Solids Anaerobic Digester with Improved Agitation. Appl. Energy 2015, 148, 252–259. [Google Scholar] [CrossRef]
- El Achkar, J.H.; Lendormi, T.; Hobaika, Z.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.-L. Anaerobic Digestion of Grape Pomace: Biochemical Characterization of the Fractions and Methane Production in Batch and Continuous Digesters. Waste Manag. 2016, 50, 275–282. [Google Scholar] [CrossRef]
- Guerini Filho, M.; Lumi, M.; Hasan, C.; Marder, M.; Leite, L.C.S.; Konrad, O. Energy Recovery from Wine Sector Wastes: A Study about the Biogas Generation Potential in a Vineyard from Rio Grande Do Sul, Brazil. Sustain. Energy Technol. Assess. 2018, 29, 44–49. [Google Scholar] [CrossRef]
- Nanni, A.; Parisi, M.; Colonna, M. Wine By-Products as Raw Materials for the Production of Biopolymers and of Natural Reinforcing Fillers: A Critical Review. Polymers 2021, 13, 381. [Google Scholar] [CrossRef]
- Polylactic Acid Market Size & Share|Global Report [2021–2028]. Available online: https://www.fortunebusinessinsights.com/polylactic-acid-pla-market-103429 (accessed on 6 February 2023).
- Rivera, O.M.P.; Moldes, A.B.; Torrado, A.M.; Domínguez, J.M. Lactic Acid and Biosurfactants Production from Hydrolyzed Distilled Grape Marc. Process Biochem. 2007, 42, 1010–1020. [Google Scholar] [CrossRef]
- Liu, J.G.; Wang, Q.H.; Ma, H.Z.; Wang, S. Effect of Pretreatment Methods on L-Lactic Acid Production from Vinasse Fermentation. Adv. Mater. Res. 2010, 113–116, 1302–1305. [Google Scholar] [CrossRef]
- Bustos, G.; Moldes, A.B.; Cruz, J.M.; Domínguez, J.M. Formulation of Low-Cost Fermentative Media for Lactic Acid Production with Lactobacillus Rhamnosus Using Vinification Lees as Nutrients. J. Agric. Food Chem. 2004, 52, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Bustos, G.; de la Torre, N.; Moldes, A.B.; Cruz, J.M.; Domínguez, J.M. Revalorization of Hemicellulosic Trimming Vine Shoots Hydrolyzates Trough Continuous Production of Lactic Acid and Biosurfactants by L. pentosus. J. Food Eng. 2007, 78, 405–412. [Google Scholar] [CrossRef]
- Production of Fermentable Media from Vine-trimming Wastes and Bioconversion into Lactic Acid by Lactobacillus Pentosus—Bustos—2004—Journal of the Science of Food and Agriculture—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/jsfa.1922 (accessed on 16 February 2023).
- Rodríguez-Pazo, N.; Salgado, J.M.; Cortés-Diéguez, S.; Domínguez, J.M. Biotechnological Production of Phenyllactic Acid and Biosurfactants from Trimming Vine Shoot Hydrolyzates by Microbial Coculture Fermentation. Appl. Biochem. Biotechnol. 2013, 169, 2175–2188. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Rivas, S.; Moldes, A.B.; Domínguez, J.M. Simultaneous Lactic Acid and Xylitol Production from Vine Trimming Wastes. J. Sci. Food Agric. 2007, 87, 1603–1612. [Google Scholar] [CrossRef]
- Anderson, A.J.; Dawes, E.A. Occurrence, Metabolism, Metabolic Role, and Industrial Uses of Bacterial Polyhydroxyalkanoates. Microbiol. Rev. 1990, 54, 450–472. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Valentin, H.E. Diversity of Bacterial Polyhydroxyalkanoic Acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Solaiman, D.K.Y.; Ashby, R.D.; Foglia, T.A.; Marmer, W.N. Conversion of Agricultural Feedstock and Coproducts into Poly(Hydroxyalkanoates). Appl. Microbiol. Biotechnol. 2006, 71, 783–789. [Google Scholar] [CrossRef]
- Follonier, S.; Goyder, M.S.; Silvestri, A.-C.; Crelier, S.; Kalman, F.; Riesen, R.; Zinn, M. Fruit Pomace and Waste Frying Oil as Sustainable Resources for the Bioproduction of Medium-Chain-Length Polyhydroxyalkanoates. Int. J. Biol. Macromol. 2014, 71, 42–52. [Google Scholar] [CrossRef]
- Martinez, G.A.; Rebecchi, S.; Decorti, D.; Domingos, J.M.B.; Natolino, A.; Rio, D.D.; Bertin, L.; Porto, C.D.; Fava, F. Towards Multi-Purpose Biorefinery Platforms for the Valorisation of Red Grape Pomace: Production of Polyphenols, Volatile Fatty Acids, Polyhydroxyalkanoates and Biogas. Green Chem. 2015, 18, 261–270. [Google Scholar] [CrossRef]
- Dimou, C.; Kopsahelis, N.; Papadaki, A.; Papanikolaou, S.; Kookos, I.; Mandala, I.; Koutinas, A. Wine Lees Valorization: Biorefinery Development Including Production of a Generic Fermentation Feedstock Employed for Poly(3-Hydroxybutyrate) Synthesis. Food Res. Int. 2015, 73, 81–87. [Google Scholar] [CrossRef]
- Battegazzore, D.; Noori, A.; Frache, A. Natural Wastes as Particle Filler for Poly(Lactic Acid)-Based Composites. J. Compos. Mater. 2019, 53, 783–797. [Google Scholar] [CrossRef]
- Saccani, A.; Sisti, L.; Manzi, S.; Fiorini, M. PLA Composites Formulated Recycling Residuals of the Winery Industry. Polym. Compos. 2019, 40, 1378–1383. [Google Scholar] [CrossRef]
- Gowman, A.; Picard, M.; Rodriguez-Uribe, A.; Misra, M.; Khalil, H.; Thimmanagari, M.; Mohanty, A. Physicochemical Analysis of Apple and Grape Pomaces. Bioresources 2019, 14, 3210–3230. [Google Scholar] [CrossRef]
- Nanni, A.; Messori, M. Thermo-Mechanical Properties and Creep Modelling of Wine Lees Filled Polyamide 11 (PA11) and Polybutylene Succinate (PBS) Bio-Composites. Compos. Sci. Technol. 2020, 188, 107974. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From Winery Waste to Bioactive Compounds and New Polymeric Biocomposites: A Contribution to the Circular Economy Concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef]
- David, G.; Vannini, M.; Sisti, L.; Marchese, P.; Celli, A.; Gontard, N.; Angellier-Coussy, H. Eco-Conversion of Two Winery Lignocellulosic Wastes into Fillers for Biocomposites: Vine Shoots and Wine Pomaces. Polymers 2020, 12, 1530. [Google Scholar] [CrossRef]
- Nanni, A.; Messori, M. Effect of the Wine Lees Wastes as Cost-Advantage and Natural Fillers on the Thermal and Mechanical Properties of Poly(3-Hydroxybutyrate-Co-Hydroxyhexanoate) (PHBH) and Poly(3-Hydroxybutyrate-Co-Hydroxyvalerate) (PHBV). J. Appl. Polym. Sci. 2020, 137, 48869. [Google Scholar] [CrossRef]
- David, G.; Croxatto Vega, G.; Sohn, J.; Nilsson, A.E.; Hélias, A.; Gontard, N.; Angellier-Coussy, H. Using Life Cycle Assessment to Quantify the Environmental Benefit of Upcycling Vine Shoots as Fillers in Biocomposite Packaging Materials. Int. J. Life Cycle Assess 2021, 26, 738–752. [Google Scholar] [CrossRef]
- De Iseppi, A.; Marangon, M.; Vincenzi, S.; Lomolino, G.; Curioni, A.; Divol, B. A novel approach for the valorization of wine lees as a source of compounds able to modify wine properties. LWT 2021, 136, 110274. [Google Scholar] [CrossRef]
- Council Regulation (EEC) No 337/79 of 5 February 1979 on the Common Organization of the Market in Wine. Available online: https://op.europa.eu/en/publication-detail/-/publication/c908ef22-1fb5-49ee-bb41-ab65eb608bad/language-en (accessed on 29 March 2023).
- Perez-Bibbins, B.; Torrado-Agrasar, A.; Salgado, J.M.; de Souza Oliveira, R.P.; Domínguez, J.M. Potential of lees from wine, beer and cider manufacturing as a source of economic nutrients: An overview. Waste Manag. 2015, 40, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.J.; Matias, A.A. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kopsahelis, N.; Dimou, C.; Papadaki, A.; Xenopoulos, E.; Kyraleou, M.; Kallithraka, S.; Kotseridis, Y.; Papanikolaou, S.; Koutinas, A.A. Refining of wine lees and cheese whey for the production of microbial oil, polyphenol-rich extracts and value-added co-products. J. Chem. Technol. Biotechnol. 2018, 93, 257–268. [Google Scholar] [CrossRef]
- Salgado, J.M.; Rodriguez, N.; Cortes, S.; Dominguez, J.M. Improving downstream processes to recover tartaric acid, tartrate and nutrients from vinasses and formulation of inexpensive fermentative broths for xylitol production. J. Sci. Food Agric. 2010, 90, 2168–2177. [Google Scholar] [CrossRef]
- De Iseppi, A.; Lomolino, M.; Marangon, M.; Curioni, A. Current and future strategies for wine yeast lees valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
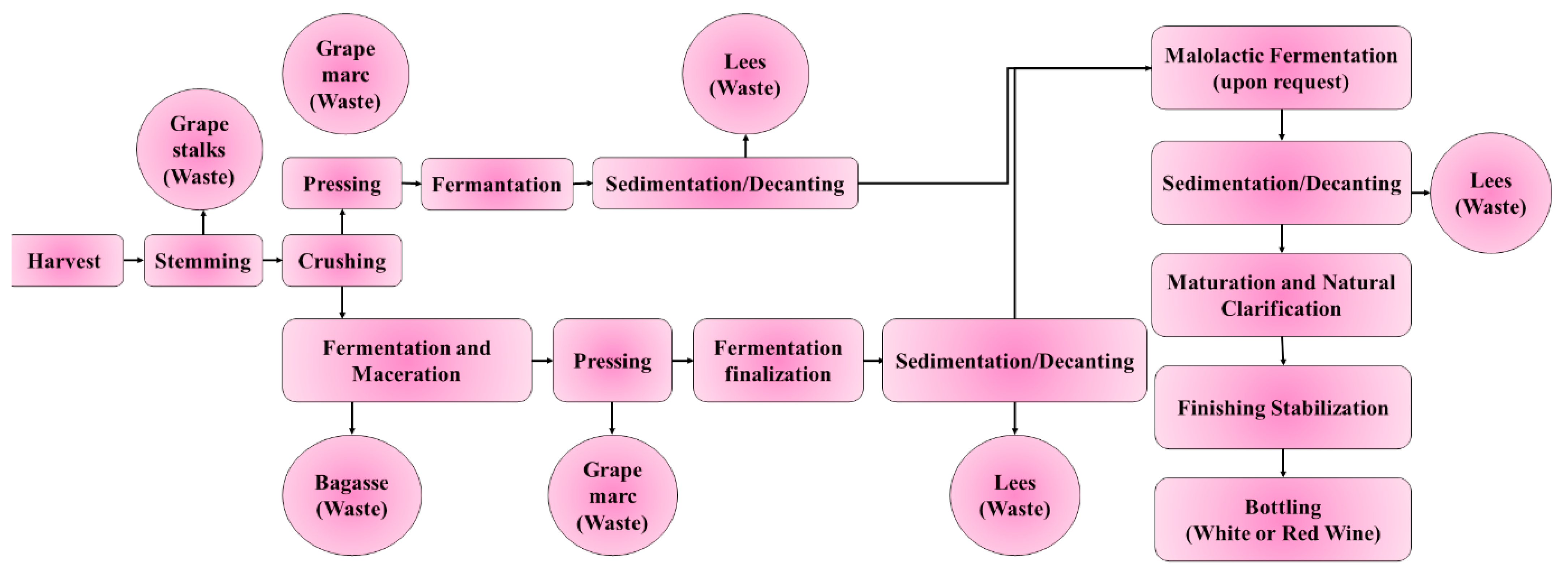
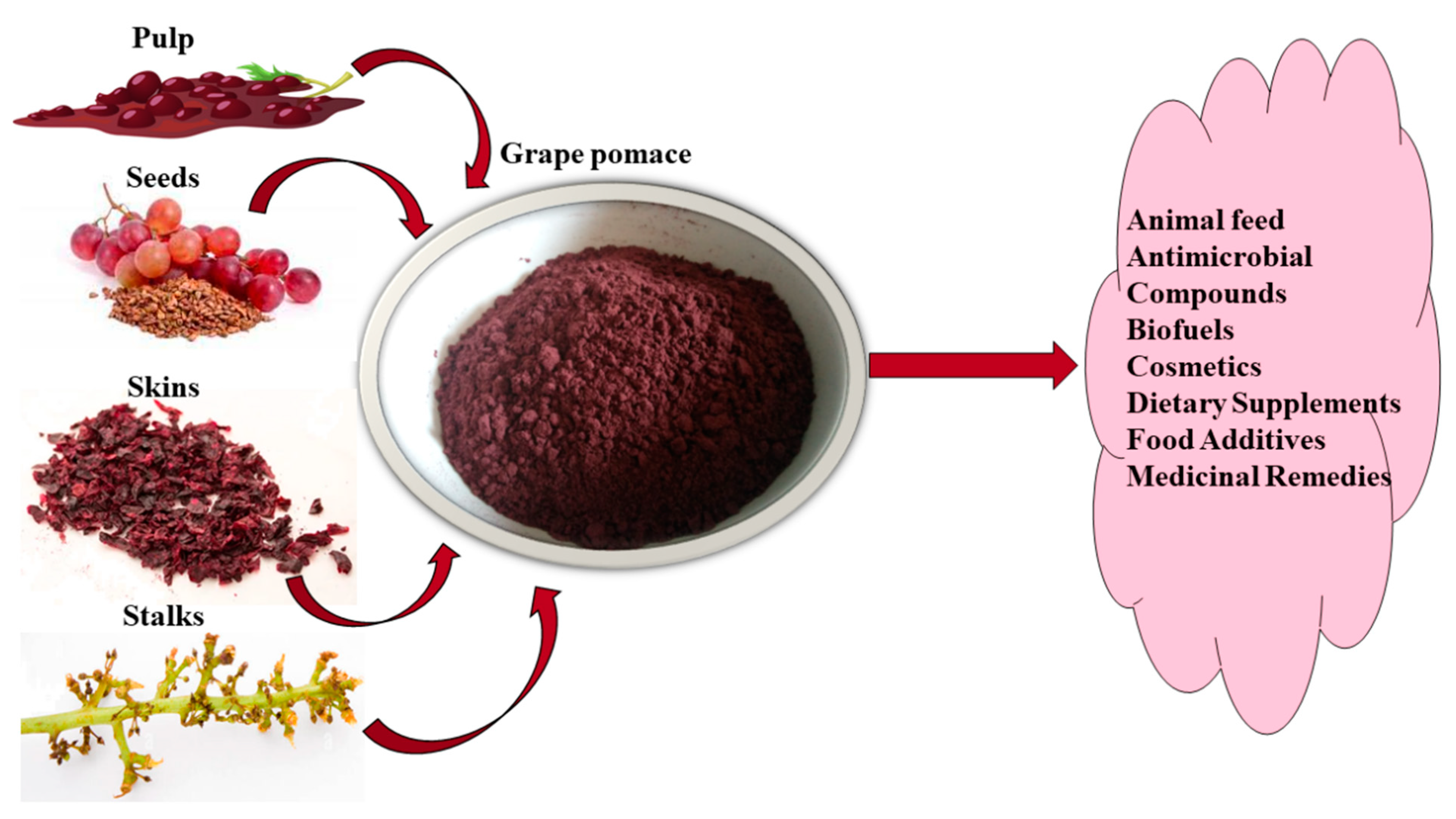

| Waste | Composition (w/w) | Percentage (%) | Ref. |
|---|---|---|---|
| Leaves | Anthocyanins | N/A | [20] |
| Flavonols | |||
| Organic acids | |||
| Tannins | |||
| Seeds | Essential oil | 16% | |
| Fibre | 40% | ||
| Protein | 11% | ||
| Phenolics | 7% | ||
| Stem | Insoluble residues | 71% | |
| Moisture content | 55–80% | ||
| Phenolics | 6% | ||
| Pomace (marc) | Cellulose | 27–75% | |
| Lignin | 17–24% | ||
| Moisture content | 50–70% | ||
| Protein | <4% | ||
| Lees | Dead yeast | N/A | |
| Grape pulp | |||
| Inorganic matter | |||
| Phenolics | |||
| Tartaric acid |
| Grape Variety | Country | By-Product | Extraction Method | Bio-Application | MIC Range (mg/L) | Ref | |
|---|---|---|---|---|---|---|---|
| Gram Negative | Gram Positive | ||||||
| Babic | Croatia | Skins | Ethanol/water (80:20) | B. cereus S. aureus | C. coli E. coli | 0.08–0.42 | [33] |
| Debit | 0.02–0.25 | ||||||
| Lain | 0.04–0.34 | ||||||
| Merlot | 0.13–0.44 | ||||||
| Plavina | 0.09–0.41 | ||||||
| Tmjac | 0.12–0.31 | ||||||
| Vranac | 0.16–0.23 | ||||||
| Arinto | Portugal | Seeds and skins | Water | B. cereus | E. coli | ND | [34] |
| Preto Martinho | Seeds | Ethanol/water (50:50) | B. cereus E. faecalis L. monocytogenes S. epidermidis S. aureus | K. pneumoniae | 0.001–0.010 | [35] | |
| Skins | B. cereus E. faecalis L. monocytogenes S. epidermidis S. aureus | - | 0.001–0.075 | ||||
| Stems | B. cereus E. faecalis L. monocytogenes S. epidermidis S. aureus | K. pneumoniae | 0.025–0.100 | ||||
| Emir | Turkey | Defatted seeds | Acetone/water: acetic acid (90:9:1) | B. cereus E. faecalis M. smegmatis S. aureus | A. hydrophyla E. aerogenes E. coli K. pneumoniae P. vulgaris P. aeruginosa | ND | [36] |
| Hasandede | |||||||
| Kalecic Karasi | |||||||
| Cabermet Franc | USA | Pomace | Acetone/water (80:20) | L. monocytogenes S. aureus | - | 4.7–75.0 | [37] |
| Chambourcin | 18.8–75.0 | ||||||
| Vidal Blanc | 15.6–250.0 | ||||||
| Viognier | 5.1–40.6 | ||||||
| Merlot | Brazil | Pomace | SFE-ethanol | B. cereus S. aureus | E. coli P. aeruginosa | 0.007–0.012 | [38] |
| SOX-hexane | - | ||||||
| Syrah | SFE-ethanol | B. cereus S. aureus B. cereus | - | - | |||
| SOX-hexane | 0.014 | ||||||
| Bangalore blue | India | Seeds | Acetone/water/acetic acid (90:9:1) | B. cereus B. coagulans B. subtilis S. aureus | E. coli P. aeruginosa | ND | [39] |
| Methanol/water/acetic acid (90:9:1) | |||||||
| Pinot Noir | New Zealand | Seeds | Acetone/water (50:50) | S. aureus | E. coli | 0.39–25.0 | [40] |
| Ethanol/water (50:50) | 0.78–25.0 | ||||||
| Methanol/water (50:50) | 0.19–25.0 | ||||||
| Skins | Acetone/water (50:50) | 0.39–25.0 | |||||
| Ethanol/water (50:50) | 0.78–25.0 | ||||||
| Methanol/water (50:50) | 12.5–25.0 | ||||||
| Pomace | Acetone/water (50:50) | 0/39–25.0 | |||||
| Ethanol/water (50:50) | 0.78–25.0 | ||||||
| Methanol/water (50:50) | 0.78–25.0 | ||||||
| Parameter/Compound Class | Component | Dry Matter Content | Ref. |
|---|---|---|---|
| Physico-chemical | Moisture content | 3.3 g/100 g | [51,56,57,58] |
| Ash | 1.7–9.1 g/100 g | ||
| Carbohydrates | 12.2–40.5 g/100 g | ||
| Fructose | 0.4–8.9 g/100 g | ||
| Glucose | 0.2–26.3 g/100 g | ||
| Lipids | 1.1–13.9 g/100 g | ||
| Proteins | 3.6–14.2 g/100 g | ||
| Fibre | 17.3–88.7 g/100 g | ||
| Bio-active substances | TPC 1 | 60.1 mg GAE/g 3 | |
| TAC 2 | 131.4 mg/100 g | ||
| Quercetin | 133.6 µg/g | ||
| Catechin | 1991.0 µg/g | ||
| Gallic acid | 615.0 µg/g | ||
| Procyanidin B2 | 1088.7 µg/g | ||
| Tannins | 13.9 mg CE/g 4 | ||
| Vitamin C | 26.3 mg AAE/g 5 | ||
| Vitamin E | 5.0 mg/kg | ||
| Minerals | Na | 87.0–244.0 mg/100 g | |
| K | 1184.0–2718.0 mg/100 g | ||
| Ca | 91.0–961.0 mg/100 g | ||
| Mg | 92.0–644.0 mg/100 g | ||
| Mn | 6.0–1356.0 mg/100 g | ||
| Fe | 5.0–5468.0 mg/100 g | ||
| Cu | 39.0–130.0 mg/100 g | ||
| Zn | 2.0–2254.0 mg/100 g | ||
| P | 4.0–3157.0 mg/100 g |
| Product | Grape Pomace Addition | Effects | Ref. |
|---|---|---|---|
| Pork burger | 0.06% grape pomace extract added to product weight | Positive: Increased lipid oxidation inhibition and enhanced colour stability | [74] |
| Pork sausages | 0.5 and 1% grape pomace incorporated into the recipe | Positive: Decreased lipid oxidationNegative: Induced colour change | [75] |
| Pork loin marinade | Pork loin was soaked in 0.5, 1.0, 2.0, 20.0, and 40.0% grape pomace solution | Positive: Inhibition of lipid oxidation and microbial growth | [76] |
| Chicken meat | Grape pomace extract adding up to TPC = 60 mg/kg (in meat) | Positive: Decreased lipid oxidationNegative: Induced colour and flavour change | [77] |
| Chicken meat | Grape pomace extract adding up to TPC of 10, 20, 40, and 60 mg/kg (in meat) | Positive: Decreased lipid oxidation | [78] |
| Waste | Removed Pollutant | Conditions | Adsorption Capacity | Ref. |
|---|---|---|---|---|
| Merlot grape marc | Pb | pH = 5.5 T = 22 °C | 40.00 mg/g | [83] |
| Sauvignon Blanc grape marc | 64.00 mg/g | |||
| Wine processing waste sludge (WPWS) | Ni | T = 50 °C | 66.55 µmol | [84] |
| Grape bagasse (GB) | Cd Pb | N/A | 54.00 mg/g 42.00 mg/g | [85,86] |
| Basic blue 9, Acid yellow 36 | N/A | <417.00 mg/g | [87,88] | |
| Hg | N/A | 46.00 mg/g | [89] |
| Waste | Bacteria | Treatment | Fermentation | Lactic Acid (g/dm3) | Yield (%) | Productivity (g/dm3 h) | Reference |
|---|---|---|---|---|---|---|---|
| Grape pomace | Lactobacillus pentosus | Acid hydrolysis | Batch fermentation | 7.20 | 70 | 0.48 | [109] |
| Wine lees | Lactobacillus casei | Alkaline hydrolysis coupled with microwaves | Batch fermentation | 17.50 | 70 | - | [110] |
| Lactobacillus rhamnosus | No treatment | Batch fermentation | 105.50 | 80 | 2.47 | [111] | |
| Vine shoots and wine lees | Lactobacillus pentosus | Acid hydrolysis and calcium carbonate detoxification | Batch fermentation | 15.50 | 70 | 3.10 | [112] |
| Vine shoots | Lactobacillus pentosus | Acid hydrolysis and calcium carbonate detoxification | Batch fermentation | 21.80 | 77 | 0.85 | [113] |
| Lactobacillus pentosus | Acid hydrolysis and delignification | Saccharification and fermentation | 43.00 | 68 | 0.25 | [114] | |
| Lactobacillus rhamnosus | Acid hydrolysis and calcium carbonate detoxification | Two-stage sequential batch fermentation | 31.50 | 93 | 1.31 | [115] |
| Biodegradable Polymer | Wine Waste | Filler Percent (%wt.) | Filler Treatment | Reference |
|---|---|---|---|---|
| Polylactic acid | Grape stalks | 30–50 | Untreated | [122] |
| Grape pomace | 5–20 | Untreated | [123] | |
| Polybutylene succinate | Grape pomace | 40–50 | Reactive extrusion with maleic anhydride-grafted polybutylene succinate | [124] |
| Wine lees | 10–30 | Reactive extrusion with silane (for the 20% formulation) | [125] | |
| Poly-3-hydoxybutyrate-co-3-hydroxyvalerate | Grape pomace | 5–20 | Both untreated and after polyphenols extraction | [126] |
| Vine shoots | 5–20 | Both untreated and after polyphenols extraction | [127] | |
| Wine lees | 10–30 | Reactive extrusion with silane (for the 20% formulation) | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculescu, V.-C.; Ionete, R.-E. An Overview on Management and Valorisation of Winery Wastes. Appl. Sci. 2023, 13, 5063. https://doi.org/10.3390/app13085063
Niculescu V-C, Ionete R-E. An Overview on Management and Valorisation of Winery Wastes. Applied Sciences. 2023; 13(8):5063. https://doi.org/10.3390/app13085063
Chicago/Turabian StyleNiculescu, Violeta-Carolina, and Roxana-Elena Ionete. 2023. "An Overview on Management and Valorisation of Winery Wastes" Applied Sciences 13, no. 8: 5063. https://doi.org/10.3390/app13085063
APA StyleNiculescu, V.-C., & Ionete, R.-E. (2023). An Overview on Management and Valorisation of Winery Wastes. Applied Sciences, 13(8), 5063. https://doi.org/10.3390/app13085063







